Abstract
Background
REM sleep behavior disorder (RBD) is a parasomnia characterized by loss of muscle atonia during REM sleep that results in motor behaviors. Diagnosis of RBD involves a clinical interview in which history of dream enactment behaviors is elicited and a subsequent overnight polysomnography (PSG) evaluation to assess for REM sleep without atonia (RWA) and/or observe motor behaviors during REM sleep. Therefore, the nature of RBD diagnosis involves both subjective and objective measurements that attempt to qualify and quantify the different diagnostic sub-criteria.
Objectives
The primary aim of the current study was to identify and summarize the available clinical measurements that have been used for RBD assessment.
Methods
Two major online databases (MEDLINE and PsycInfo) were searched for articles developing, validating, or evaluating psychometric properties of the RBD diagnostic criteria or methods used for diagnosis. Studies of adult subjects (18+) that included sufficient psychometric data for validation were included.
Results
Fifty-eight studies were found to meet review criteria. The objective measurements for assessment of RBD reviewed included visual electromygraphic (EMG) scoring methods, computerized EMG scoring methods, cardiac 123I-MIBG scintigraphy, actigraphy, behavioral classification and video analysis. Subjective measurements of RBD included interviews and questionnaires.
Conclusion
Sleep history may be sufficient for diagnosis of RBD in some populations. However, PSG is necessary for a definitive diagnosis. EMG scoring methods vary in definition used and there is no single accepted approach to scoring muscle activity. Additional validation studies are required for establishing cutoff scores for the different methods. Questionnaires were shown to be appropriate screening tools, yet further validation in different population is necessary.
Introduction
Rapid eye movement (REM) sleep behavior disorder (RBD) is a parasomnia first identified and described in 1986 by Schenck, Mahowald and collogues1 in 5 older adults that exhibited abnormal muscle activity during REM sleep. REM sleep is normally characterized by minimal skeletal muscle activity i.e., muscle atonia, which manifests as low amplitude levels on the sub-mental electromyographic (EMG) channel in polysomnography (PSG) recording.2 RBD is characterized by REM sleep without atonia (RWA) i.e., abnormal increase of muscle activity during REM sleep evident by phasic and/or tonic muscle activity on the EMG channel. This loss of muscle atonia often (but not necessarily) results in vigorous and violent movements that are associated with vivid dreams and may result in injuries to the patient or the bed partner. RBD occurrences are typically described by patients as “acting our” their dreams. However, not all RBD involves dream-enactment behaviors and not every occurrence of vivid dreams are RBD. Previous research reported that up to 35% of RBD patients are not aware of dream-enactment behaviors.3
The prevalence rate of RBD in the general population is estimated to be between 0.38–0.5%.4,5 RBD has been shown to be significantly more prevalent in individuals with chronic neurological diseases such as multi-system atrophy (MSA)6, Parkinson’s disease (PD)3, progressive supranuclear palsy7, and narcolepsy8. A high percentage of idiopathic RBD (iRBD) patients eventually develop neurodegenerative diseases,9 and RBD has been recognized as a strong predictor of the development of synucleinopathies, including MSA and PD.10 Studies following iRBD patients reported that 38-65% developed a neurodegenerative disease within an average of 7 to 13 years after RBD onset.9,11,12
Muscle atonia during REM sleep results of interaction between multiple neuronal systems in the brain. Animal studies have demonstrated the involvement of structures in the brainstem (e.g., locus coeruleus-subcoeruleus complex, the raphe nucleus, and substantia nigra) and forebrain (e.g., hypothalamus, thalamus).13,14 These structures have been conceptualized in two distinct motor systems that allow for normal REM sleep; one system responsible for generation of the muscle atonia and the other responsible for the suppression of locomotor activity.14 Nonetheless, the exact nature of the interaction of these structures in the generation of muscle atonia is not fully understood and merits further study. It is hypothesized that dysfunction in one or more of these pathways is responsible for loss of muscle atonia during REM sleep and the pathogenesis of RBD. A detailed review of pathophysiology of RBD can be found in Boeve et al.14
Over the years a dynamic scientific debate has been unfolding in the literature regarding the appropriate diagnostic tools and definitions used for RBD assessment. In the heart of this debate is the question of making the diagnosis of RBD: Can it be made by subjective information alone (e.g., clinical history or questionnaires) or does it require objective measures (e.g., complex behaviors observed during REM during an overnight PSG)? RBD definition and diagnostic criteria evolved from being based only on clinical history15 to including specific PSG EMG findings16,17. Previous RBD diagnostic criteria referred to clinical history as minimal criteria16,18 indicating that full diagnostic criteria are only met when RBD is confirmed by PSG. EMG findings with no clinical history have been referred to as subclinical RBD.17 Current criteria according to the International Classification of Sleep Disorders, second edition (ICSD-II)17 is provided in Table 1.
Table 1.
RBD diagnostic criteria according to the International Classification of Sleep Disorders Second edition (ICSD-II)17
|
REM, rapid eye movement; RBD, REM sleep behavior disorder; PSG, polysomnography; EMG, electromyography; EEG, Electroencephalography;
In the background of the diagnosis debate is the growing number of studies attempting to define and quantify muscle activity during REM sleep in an effort to appropriately differentiate between normal and abnormal muscle activity levels. The scoring definition of the EMG channel in the American Academy of Sleep Medicine (AASM) scoring manual2, which evidence-based, was not able to provide a precise definition of what is considered excessive amounts of tonic and phasic EMG activity or a complete method of how muscle activity should be measured.
The aim of this review was to identify and summarize the available subjective and objective clinical tools that have been used for RBD assessment, identify their psychometric value, and report available evidence for their validity and reliability.
Methods
Search Strategy for Identification of Studies
Two major electronic bibliographical databases were searched: MEDLINE (1986 to October 1, 2010) and PsycINFO (1986 to October 1, 2010). The following search terms were used for MEDLINE: (“REM Sleep Behavior Disorder”[Mesh], OR “REM sleep behavior disorder”[All Fields], OR “REM behavior disorder”[All Fields], NOT Review[ptyp]). Search terms and key words used for PsycINFO included: DE=(“REM sleep”) AND DE=(“sleep disorders”) OR KW=(REM Sleep Behavior Disorder) or (REM behavior disorder).
After the articles identified by both electronic databases were combined and duplicate studies eliminated, each article’s type, title, and abstract were reviewed for inclusion and exclusion criteria (Table 2). Full-length articles were reviewed for studies that met criteria or those that could not be excluded based on the information provided in the title and abstract. Articles that were reviewed in full but did not meet criteria were excluded from the review.
Table 2.
Criteria for including or excluding papers from the review
| Inclusion | Exclusion |
|---|---|
|
|
Data extracted from studies included measures used (name and description), method used, and the study’s population characteristics (e.g., gender, age, clinical population). Validation and psychometric properties reported by studies were summarized. Values for reliability measures (e.g., kappa and Cronbach’s α) were interpreted according to suggested criteria19,20. Correlations were interpreted as small, medium, and large (.10, .30, and .50 respectively) according to accepted criteria21.
Results
Of the 615 studies that were identified through the electronic databases, 53 were included in this systematic review (see Figure 1 for additional details). Summary of the 53 studies’ characteristics is provided in Table 3.
Figure 1.
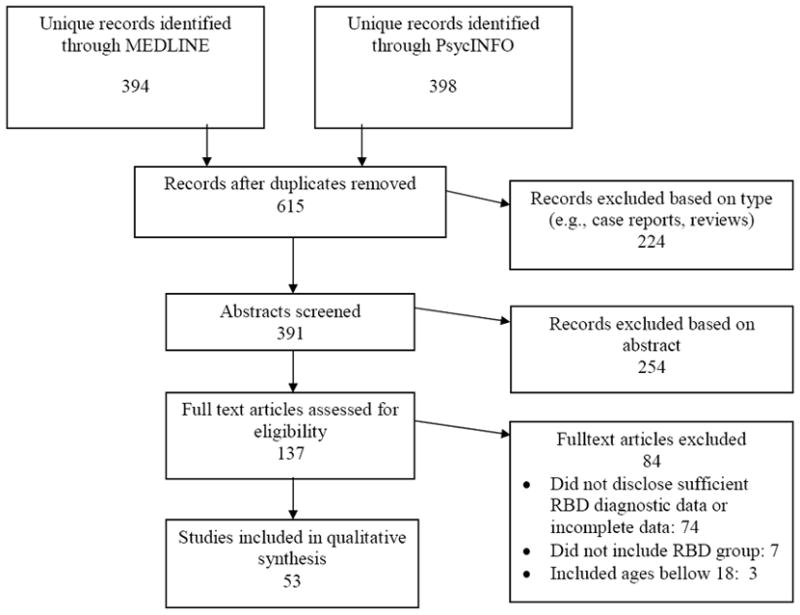
Literature review protocol
Table 3.
Literature review results and population characteristics
| Authors | Pub. Year | RBD diagnosis Method developed (d)/evaluated(e)/used(u) | Notes | Population | N | Men/Women | Age: M-yrs(SD) M-yrs ± SEM |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lapierre&Montplaisir27 | 1992 | EMG scoring method d, e | Developed the RPSM | RBD | 5 | 3/2 | 58.6 (NS) |
| Normal Controls | 5 | 5/0 | 58.4 (NS) | ||||
| PLMS | 7 | (NS) | NS | ||||
|
| |||||||
| Kunz & Bes48 | 1999 | EMG scoring method (RPSM) u | Treatment study | RBD | 6 | 3/3 | 54 (NS) |
| Actigraphyu | |||||||
|
| |||||||
| Eisensehr et al.75 | 2001 | Clinical interview e | PD | 19 | 13/6 | 67.7 (9.4) | |
| EMG scoring u | Non-PD | 273 | 168/105 | 55 (16.0) | |||
|
| |||||||
| Takeuchi et al.49 | 2001 | EMG scoring method (RPSM) u | Treatment study | RBD | 15 | 14/1 | 63.5 (NS) |
|
| |||||||
| Gagnon et al.22 | 2002 | Clinical interview e | Used cutoff >20% for increase muscle tone for RBD diagnosis | PD | 33 | 21/12 | 62.9 (11.8) |
| EMG scoring method (RPSM) u | Matched Controls | 16 | 10/6 | 62.3 (6.9) | |||
|
| |||||||
| Garcia-Borreguero et al.50 | 2002 | Clinical Interview u | Treatment study | PD | 15 | 9/6 | 73.8 (4.9) |
| EMG scoring method (RPSM) u | RBD-PD | 5 | |||||
| nRBD-PD | 10 | ||||||
| Control group | 14 | 69.3 (4.9) | |||||
|
| |||||||
| Eisensehr et al.56 | 2003 | EMG scoring method d | Used cutoff >15% for long-lasting muscle activity for RBD diagnosis | Clinical RBD | 8 | 7/1 | 69.2 (7.6) |
| Sub-clinical RBD | 8 | 6/2 | 62.3 (13.5) | ||||
| Normal Controls | 11 | 9/2 | 61.6 (8.2) | ||||
| PD | 8 | 5/3 | 57.2 (6.6) | ||||
|
| |||||||
| Fantini et al.42 | 2003 | EMG scoring method (RPSM) u | Treatment study | RBD | 8 | 5/3 | 66.0 (6.8) |
|
| |||||||
| Vignatelli et al.74 | 2003 | Clinical interview e | Reliability of RBD diagnostic criteria | Neurological diseases | 10 | 5/5 | 54 (NS) |
|
| |||||||
| Kumru et al.45 | 2004 | Clinical Interview u | Park2-PD | 10 | 7/3 | 51.2 (11.6) | |
| EMG scoring method (RPSM) u | yRBD | 6 | 5/1 | 48.8 (12.0)* | |||
| nRBD | 4 | 2/2 | 54.8 (11.1)* | ||||
|
| |||||||
| Arnulf et al.7 | 2005 | EMG scoring method d | Proposed RWA Index | PSP | 15 | 7/8 | 68.0 (8.0) |
| Clinical interview e | PD | 15 | 7/8 | 67.0 (6.0) | |||
| Control Group | 15 | 7/8 | 67.0 (10.0) | ||||
|
| |||||||
| Consens et al.28 | 2005 | EMG scoring method (RPSM) u, e | Same population was used in both studies. | Neurodegenerative disease | 17 | 10/7 | 65.1 (9.8) |
| Questionnaire d,u | Normal Controls | 6 | 1/5 | 53 (4) | |||
| Clinical interview u | Computerized method used only in58 | ||||||
| Burns et al.58 | 2007 | Computerized EMG scoring d,e | |||||
|
| |||||||
| Iranzo et al.34 | 2005 | Clinical Interview u | RBD+MSA | 26 | 16/10 | 62.0 (7.1) | |
| EMG scoring method (RPSM) u | RBD+PD | 45 | 34/11 | 64.8 (7.8) | |||
| iRBD | 39 | 36/3 | 68.4 (5.9) | ||||
|
| |||||||
| Iranzo& Santamaria35 | 2005 | Clinical Interview u | OSA | 16 | 11/5 | 59.6 (7.7) | |
| EMG scoring method (RPSM) u | iRBD | 20 | 13/3 | 64.5 (5.1) | |||
| Control Group | 16 | 16/4 | 63.0 (9.8) | ||||
|
| |||||||
| Nightingale et al.76 | 2005 | Questionnaire u | Non-published questionnaire | Narcolepsy | 55 | NS | NS |
| Clinical Interview u | RBD | 20 | NS | 41.0 (NS) | |||
| EMG scoring method u | EMG method not disclosed | nRBD | 27 | NS | 44.0 (NS) | ||
| unknown | 8 | NS | NS | ||||
|
| |||||||
| Scaglione et al.71 | 2005 | Clinical Interview e | Reliability of RBD diagnostic criteria | PD | 195 | 114/81 | 64.5 (8.7) |
|
| |||||||
| Stiasny-Kolster et al.43 | 2005 | EMG scoring method (RPSM) u | iRBD | 6 | 4/2 | 62.8 (7.9)* | |
| sRBD | 13 | 8/5 | 41.7 (11.9)* | ||||
| Subclinical RBD | 11 | 6/5 | 47.8 (13.4) | ||||
|
| |||||||
| Gagnon et al.77 | 2006 | Clinical Interview u | AD | 15 | 7/8 | 70.2 (5.6) | |
| EMG scoring method (RPSM) u | Control Group | 15 | 11/4 | 67.9 (5.4) | |||
|
| |||||||
| Iranzo et al.36 | 2006 | Clinical Interview u | Symptomatic RBD | 20 | 19/1 | 74.2 (6.2) | |
| EMG scoring method (RPSM) u | Isolated RBD | 24 | 20/4 | 74.0 (6.8) | |||
|
| |||||||
| Mazza et al.41 | 2006 | EMG scoring method (RPSM) u | iRBD | 8 | 7/1 | 69.9 (8.2) | |
| Control Group | 9 | 67.4 (6.8) | |||||
|
| |||||||
| Dauvilliers et al.29 | 2007 | Clinical Interview u | Used a modified RPSM | Narcolepsy/Cataplexy | 16 | 11/5 | 59.0 (6.5) |
| EMG scoring method u,e | iRBD | 16 | 11/5 | 59.9 (7.9) | |||
| Normal Controls | 16 | 11/5 | NS | ||||
|
| |||||||
| De Cock et al.53 | 2007 | Clinical Interview u | Gd-PSP | 9 | 6/3 | 66.6 (10.1) | |
| EMG scoring method (RWA Index) u | PSP | 9 | 6/3 | 66.1 (8.8) | |||
| PD | 9 | 6/3 | 69.8 (7.2) | ||||
| Control group | 9 | 6/3 | 66.9 (9.5) | ||||
|
| |||||||
| De Cock et al.54 | 2007 | Clinical Interview u | PD+RBD | 65 | 69%men | 65.0 (9.0) | |
| EMG scoring method (RWA Index) u | PD-RBD | 35 | 60%men | 61.0 (13.0) | |||
|
| |||||||
| Frauscher et al.72 | 2007 | VPSG e | Evaluated motor behaviors during REM sleep | Parkinsonian Syndromes with RBD | 5 | 5/0 | 64.0 (8.7) |
| EMG scoring method (RPSM) u | Normal Controls | 5 | 5/0 | 64.0 (8.7) | |||
|
| |||||||
| Stiasny-Kolster et al.69 | 2007 | Questionnaire d,e | Developed the RBDSQ | RBD | 54 | 29/25 | 53.7 (15.8) |
| PSG u | Control Group 1 | 160 | 81/79 | 50.8 (15.5) | |||
| Control group 2 | 133 | 58/75 | 46.9 (12.3) | ||||
|
| |||||||
| Bliwise& Rye30 | 2008 | EMG scoring method d,e | Developed the PEM | RBD | 11 | 9/2 | 68.6 (10.6) |
| Control Group | 31 | 9/22 | 70.3 (9.3) | ||||
|
| |||||||
| Ferri et al.59 | 2008 | Computerized EMG scoring d,e | Developed the AI | iRBD | 21 | 18/3 | 70.1 (4.6) |
| Clinical Interview u | MSA+RBD | 10 | 4/6 | 63.6 (8.5) | |||
| Old Controls | 10 | 5/5 | 65.0 (5.0) | ||||
| Young Controls | 24 | 12/12 | 29.9 (3.5) | ||||
|
| |||||||
| Ferri et al.60 | 2008 | Computerized EMG scoring d,e | Developed the AI | Narcolepsy/Cataplexy with RBD | 17 | 13/4 | 41.0 (15.4) |
| Clinical Interview u | Narcolepsy/Cataplexy without RBD | 17 | 9/8 | 40.1 (15.4) | |||
| Normal Controls | 35 | 15/20 | 40.2 (16.7) | ||||
|
| |||||||
| Frauscher et al.32 | 2008 | EMG scoring method e | Used a modified RPSM | iRBD | 8 | 7/1 | 68.0 (4.1) |
| RBD+PD | 9 | 6/3 | 54.3 (8.7) | ||||
|
| |||||||
| Kumru et al.51 | 2008 | EMG scoring method (RPSM) u | Treatment study | PD+RBD | 11 | 8/3 | 62.1 (8.0) |
|
| |||||||
| Mattarozzi et al.46 | 2008 | Clinical Interview u | Narcolepsy/Cataplexy | 44 | |||
| EMG scoring method (RPSM) u | High freq. cataplectic attacks | 30 | 22/8 | 40.7 (15.0) | |||
| Low freq. cataplecticattacks | 14 | 6/8 | 36.4 (14.4) | ||||
|
| |||||||
| Mayer et al.61 | 2008 | Computerized EMG scoring d,e | iRBD | 28 | 17/11 | 51.4 (13.8) | |
| RBD+Narcolepsy/Cataplexy | 34 | 16/18 | 39.0 (12.6) | ||||
| Control Group | 25 | 16/6*** | 45.2 (16.3) | ||||
|
| |||||||
| Miyamoto et al.66 | 2008 | ClinicalInterviewu | iRBD | 31 | 23/8 | 66.3 (6.7) | |
| VPSG u | PD | 26 | 12/14 | 67.5 (6.3) | |||
| 123I-MIBG scintigraphye | DLB | 6 | 4/2 | 71.0 (5.9) | |||
| MSA | 10 | 6/4 | 64.7 (9.0) | ||||
| PSP | 13 | 10/3 | 70.7 (7.6) | ||||
| Control Group | 9 | 5/4 | 72.2 (7.7) | ||||
|
| |||||||
| Zhang et al.25 | 2008 | EMG scoring method e | RPSM and RWA | RBD | 55 | 44/11 | 65.8 (11.2) |
|
| |||||||
| Frauscher et al.33 | 2009 | EMG scoring method (RPSM) u | Severe RBD | 8 | 7/1 | 56.3 (12.3) | |
| Control Group | 8 | 7/1 | 56.3 (12.3) | ||||
|
| |||||||
| Iranzo et al.40 | 2009 | EMG scoring method (RPSM) u | iRBD | 11 | 9/2 | 73.2 (5.4) | |
|
| |||||||
| Limousin et al.52 | 2009 | Clinical Interview u | Parkin-PD | 11 | 3/8 | 49.0 (8.0) | |
| EMG scoring method (RWA Index) u | iPD | 11 | 3/8 | 59.0 (4.0) | |||
|
| |||||||
| Lin et al.38 | 2009 | EMG scoring method (RPSM) u | RBD | 70 | 45/25 | 66.0 (NS) | |
|
| |||||||
| Manni et al.73 | 2009 | VPSG e | Evaluated motor behaviors during REM sleep | iRBD | 12 | 11/1 | 67.6 (7.4) |
|
| |||||||
| Miyamoto et al.82 | 2009 | Questionnaire d,e | Developed the RBDSQ-J | iRBD | 52 | 36/16 | 66.4 (6.9) |
| PSG u | OSA | 55 | 44/11 | 63.1 (7.0) | |||
| Control Group | 65 | 37/28 | 64.6 (8.8) | ||||
|
| |||||||
| Miyamoto et al.65 | 2009 | VPSG u | iRBD with AHI<5 | 23 | NS | 65.2 (6.5) | |
| 123I-MIBG scintigraphye | iRBD with 5≤AHI<15 | 9 | NS | 66.0 (5.2) | |||
| iRBD with AHI≥15 | 15 | NS | 67.1 (8.1) | ||||
| OSA with nRBD | 16 | 14/2 | 59.8 (10.6) | ||||
|
| |||||||
| Sixel-Doring et al. 23 | 2009 | EMG scoring method (RWA Index) u | PSP | 20 | 14/6 | 71.0 (8.0) | |
| PD | 20 | 13/7 | 69.0 (5.0) | ||||
|
| |||||||
| Bliwise et al.31 | 2010 | Clinical Interview u | PD | 55 | 44/11 | 63.4 (10.7) | |
| EMG scoring method (PEM) e | |||||||
|
| |||||||
| Bugalho&Alvesda Silva84 | 2010 | Clinical Interview u | PD+RBD | 41 | 15/26 | 72.3 (7.0) | |
| Questionnaire (RBDSQ) u | PD-RBD | 34 | 17/17 | 72.8 (7.4) | |||
|
| |||||||
| Ferri et al.62 | 2010 | Computerized EMG scoring d,e | Developed the AI | iRBD-treated | 8 | 6/1*** | 67.6 (5.7) |
| Clinical Interview u | iRBD-untreated | 31 | 28/3 | 69.7 (4.9) | |||
| MSA | 10 | 4/6 | 63.6 (8.5) | ||||
| OSA | 5 | 4/1 | 63.4 (7.4) | ||||
| Young Controls | 25 | 12/13 | 29.9 (3.6) | ||||
| Aged Controls | 10 | 3/7 | 65.0 (5.0) | ||||
|
| |||||||
| Kim et al.39 | 2010 | Clinical Interview u | Treatment study | iRBD | 14 | 11/3 | 66.6 (4.5) |
| EMG scoring method (RPSM) u | iPD | 14 | 11/3 | 67.0 (4.1) | |||
| Control Group | 12 | 8/4 | 63.3 (5.7) | ||||
|
| |||||||
| Knudsen et al.57 | 2010 | Clinical Interview u | Narcolepsy+Cataplexy | 48 | 21/27 | 36.6 ± 2.4 | |
| EMG scoring method d | Muscle activity length | Narcolepsy | 15 | 7/8 | 28.73 ± 2.9 | ||
|
| |||||||
| Li et al.83 | 2010 | Questionnaire d | RBDQ-HK | Control Group | 107 | 62/45 | 55.3 (9.0) |
| RBD Group | 107 | 75/32 | 62.6 (15.5) | ||||
| iRBD | 51 | 80.4% male | 68.5 (11.2) | ||||
| sRBD | 29 | 79.3% male | 70.8 (8.6) | ||||
| RBD-like | 27 | 40.7% male | 42.5 (9.7) | ||||
|
| |||||||
| Montplaisir et al.24 | 2010 | EMG scoring method u, e | Modified version of the RPSM | iRBD | 80 | 62/18 | 62.7 (9.5) |
| Control Group | 80 | 62/18 | 61.3 (12.0) | ||||
|
| |||||||
| Naismith et al.68 | 2010 | Actigraphye | PD+RBD | 13 | 5/8 | 64.2 (4.3) | |
| Questionnaire (RBDSQ) u | PD-nRBD | 9 | 6/3 | 62.2 (10.7) | |||
|
| |||||||
| Nomura et al.67 | 2010 | 123I-MIBG scintigraphyu | PD+RBD | 18 | 5/13 | 71.3 (8.3) | |
| Clinical Interview u | PD+Subclinical RBD | 8 | 3/5 | 65.4 (8.6) | |||
| EMG scoring method (RPSM) u | PD-nRBD | 23 | 10/13 | 71.5 (7.2) | |||
|
| |||||||
| Postuma et al.37 | 2010 | Clinical Interview u | RBD Developed disease | 26 | 21/5 | 69.5 ± 1.5 | |
| EMG scoring method u | Modified version of the RPSM | RBD Did not develop disease | 26 | 21/5 | 66.7 ± 1.4 | ||
| Control Group | 26 | 21/5 | 68.9 ± 1.6 | ||||
, did not add up;
, calculated by this review; RBD, REM sleep behavior disorder; iRBD, idiopathic RBD; sRBD, symptomatic RBD; PSG, polysomnography; VPSG, video polysomnography; EMG, electromyography;PD, Parkinson’s disease; iPD idiopathic Parkinson’s disease; MSA, multi-system atrophy; DLB, dementia lewy-body; PSP, Progressive Supranuclear Palsy; OSA, obstructive sleep apnea; RWA, REM without atonia; RPSM, RBD PSG scoring method; RBDSQ, RBD screening questionnaire; PLMS, periodic limb movement during sleep;RPSM, RBD PSG Scoring Method; AI, Atonia Index; PEM, phasicelectromyographic metric; SRI, sleep related injury;RBDSQ, REM behavior disorder screening questionnaire; RBDSQ-J, REM behavior disorder screening questionnaire Japanese version; RBDQ-HK, REM behavior disorder questionnaire – Hong Kong; FLEP, frontal lobe epilepsy and parasomnias scale; SSQ, Stavanger sleepiness questionnaire;
Objective Measurements
RBD diagnosis may significantly differ based on whether diagnosis is made on the basis of RWA or dream enactment behavior observations. One study22 found that 8 of 19 PD patients (42%) presented with RWA but none presented with abnormal behaviors during REM sleep or dream enactment behaviors that were documented in the overnight PSG. Similarly, a second study23 reported that 17 of 20 progressive supranuclear palsy patients (85%) and 19 of 20 PD patients (95%) had RWA, but only 7 (35%) of the progressive supranuclear palsy patients and 13 (65%) of the PD patients had behavioral abnormalities observed on Video PSG during REM sleep. Comparable findings were reported in an iRBD population in which nearly a quarter of the patients did not present with abnormal behaviors during REM sleep on a single overnight recording.24 Finally, video as a diagnostic measure showed poor test-retest reliability over two consecutive nights.25 These findings indicate that behavioral abnormalities during REM sleep and dream enactment may not be present every night and requiring PSG documentation of abnormal behaviors emerging during REM sleep or dream enactment behaviors may miss a large number of RBDs that present only with loss of atonia.
Because some twitching and increase of phasic activity is considered normal in REM sleep,26 it is necessary to define a threshold indicating pathological loss of muscle atonia and/or excessive phasic muscle twitching. This review found several objective methods that attempted to quantify atonia for the purposes of RBD diagnosis.
Visual EMG Scoring Methods
Various visual scoring systems were used to quantify muscle activity during REM sleep. However, they differed in the length of epochs and mini-epochs, the definitions used for phasic and tonic activity, and the manner in which scores were calculated from these components. Furthermore, there was no consistency in the literature regarding the cutoff scores used. A summary of visual scoring definitions is provided in Tables 3 and 4.
Table 4.
EMG visual scoring methods
| EMG Channels | Epoch/Mini- epoch length (sec) |
Epoch scoring | Tonic Activity Definition | Tonic/Atonia EMG Scoring |
PhasicActivity Definition | Phasic EMG Scoring | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lapierre& Montplaisir27 | Submental | 20/2 | Tonic or Atonic: Tonic epoch if >50% of the epoch contained tonic chin EMG activity | NS | % Tonic/Atonic REM epochs | EMG activity bursts 0.1–5.0sec in length with amplitude x4 background EMG activity | % of REM mini-epochs containing phasic EMG events |
|
| |||||||
| Gagnon et al.22 | Submental | 20/NS | According to RPSM | EMG activity x2 the individual’s baseline atonia and >10μV | According to RPSM | NA | NA |
|
| |||||||
| Arnulf et al.7 | Chin and leg | NA | NA | muscle activity with amplitude at least of that observed during quiet wakefulness (per second) | RWA: Muscle activity duration divided by total REM sleep duration | NA | NA |
|
| |||||||
| Dauvilliers et al.29 | Submental | 20/2 | According to RPSM | Same as Gagnon et al.22 | According to RPSM | EMG activity bursts 0.3–5.0sec in length with amplitude x4 background activity | According to RPSM |
|
| |||||||
| Bliwise& Rye30 | Mentalis, Brachioradialis (R&L), Anterior Tibialis (R&L) | 30/2.5 | NA | NA | NA | PEM: Muscle activty ≥100-msec, with amplitude x4 background EMG and a return to baseline within a 2.5-sec interval | % of mini-epochs containing PEM activity |
| Bliwise et al.31 | |||||||
|
| |||||||
| Frauscher et al.32 | 13 different muscles | NS/3 | NA | NA | NA | EMG activity bursts 0.1–5.0sec in length with amplitude x2 background EMG activity | Mini-epochs scored: |
| 0-no phasic activity | |||||||
| 1-phasic activity | |||||||
| 3-artifact preventing scoring (excluded) | |||||||
| Phasic EMG = % of REM mini-epochs containing phasic EMG events | |||||||
|
| |||||||
| Montplaisir et al.24 | Submental | 20/2 | According to RPSM | Same as Gagnon et al.22 | According to RPSM | EMG events of 0.1–10.0-sec in length with amplitude > x4 background EMG activity | According to RPSM |
REM, rapid eye movement; RBD, REM sleep behavior disorder; EMG, electromyography; RPSM, RBD polysomnography scoring method; RWA, REM without atonia; PEM, phasicelectromyographic metric; NS, not specified; NA, not applicable;
The first attempt to systematically define and quantify motor events during REM sleep in RBD patients was undertaken by Lapierre and Montplaisir27 who introduced the RBD PSG scoring method. Using the RBD PSG scoring method, EMG activity yielded scores of percent phasic activity during REM sleep (also referred to as phasic density) and percent REM sleep atonia. The definition and criteria provided by Lapierre and Montplaisir did not specify an amplitude definition for tonic activity. Later, that same research group22 introduced an amplitude definition for tonic activity, however phasic activity was not used in that analysis. Over a decade after the RBD PSG scoring method was suggested, Consens et al.28 executed the first validation study for these methods. In that validation study an additional score was calculated as the average of both tonic and phasic components (called PSG RBD score). Dauvilliers et al.29 used a slightly different definition of phasic activity than the original RBD PSG scoring method (i.e., used 0.3-5.0 seconds as duration for activity burst as opposed to 0.1-5.0 seconds). Bliwise and colleagues30,31 suggested that EMG activity during REM sleep should be evaluated only using the phasic score (disregarding the tonic component). Frauscher et al.32 used a slightly different amplitude definition for phasic activity (amplitude of x2 the background compared to x4 in the RBD PSG scoring method) and accounted for artifact. Montplaisir et al.24 conducted a validation study using the tonic definition suggested by Gagnon et al.22 and changed the temporal definition for phasic activity to include EMG bursts of 0.1-10.0 seconds. It is evident that there is no uniformity in the methods used. However, the RBD PSG scoring method has been used extensively and available validation data for this method is summarized below.
RBD PSG scoring method
In the study by Lapierre and Montplaisir27 the RBD PSG scoring method was evaluated in 5 patients with RBD and 5 controls. Their analysis showed that RBD patients had more tonic and phasic activity during REM sleep with 94% of REM sleep time associated with tonic activity.
A later study28 validating the RBD PSG scoring method used a questionnaires, clinical interview, and PSG. An EMG quantification protocol that was nearly identical to the RBD PSG scoring method was used (Table 4). Also, the PSG RBD score was calculated as the average of the tonic and the phasic components from the RBD PSG scoring method. The analysis showed that the PSG RBD score was mildly, yet significantly associated with the clinical impression of RBD and with the questionnaire scores. The tonic component was significantly associated with the clinical impression, and both tonic and phasic components significantly correlated with the questionnaire. A receiver operator curve (ROC) analysis using the clinical impression as a gold standard of indicator of disease revealed a PSG RBD cutoff score of 10% with a sensitivity of 89% and specificity of 57%.
Zhang et al.25 evaluated several different diagnostic criteria for PSG including the PSG RBD 10% cutoff suggested28, a clinical decision of RWA (subjective decision by a clinician), and video analysis. All of the diagnostic measures excluding video analysis revealed generally good diagnostic agreement over two consecutive nights of evaluation with reported sensitivity ranging 80-88.9% for all three measures. Combining the 10% cutoff with the video analysis showed sensitivity of 96.3% on night 1 and of 98.1% on night 2. Combining the clinical decision of RWA and the video analysis showed a sensitivity of 94.4% on night 1 and 98.1% on night 2.
Dauvilliers et al.29 used a slightly modified RBD PSG scoring method (see Table 4) in patients with narcolepsy in comparison to iRBD and a healthy control group. A cutoff of 20% of REM sleep time with tonic activity was used for indication of RWA. Their analysis revealed that both the iRBD and narcolepsy groups had significantly less REM sleep with atonia compared to the control group and that narcoleptics had significantly higher REM sleep atonia compared to the iRBD group. Both iRBD and narcolepsy groups had higher phasic EMG compared to controls. Conducting a ROC analysis using the data from the iRBD and control groups, the cutoff score of 20% RWA showed a sensitivity of 87.5% and a specificity of 81.3%. A cutoff score of 15% phasic EMG showed 87.5% sensitivity and 93.8% specificity.
Several studies also reported that the RBD PSG scoring method showed good test-retest reliability with strong associations and no significant differences between separate nights of evaluation.25,28
The RBD PSG scoring method has been extensively used in RBD populations including both iRBD and symptomatic RBD (sRBD is RBD associated with other chronic diseases)33-38, and in studies with only iRBD patients39-43 or sRBD44,45. The RBD PSG scoring method was used to show differences between clinical sub-populations of PD37,45, and narcolepsy/cataplexy46. Studies included the RBD PSG scoring method measurements in evaluating different clinical aspects and characteristics of RBD patients33,34,36,37,39-41,43 and in comparison to other clinical populations34,35. RBD PSG scoring method was also used to show that loss of atonia during REM sleep increases over time with the disease duration.40
Several studies used the RBD PSG scoring method in evaluation of treatment effectiveness. Clonazepam was shown to successfully treat symptoms of RBD47 and the study by Lapierre and Montplaisir27 showed significant reductions in RBD PSG scoring method measures with clonazepam treatment. However, a second study25 did not observe any differences between clonazepam treated and untreated RBD patients. Studies using melatonin as a treatment for RBD showed significant decreases in the tonic measure but not in the phasic measure.48,49 The RBD PSG scoring method was used in evaluation of levadopa treatment for PD which showed no effect for PD-RBD patients but significant increase in the RBD PSG scoring method measures for PD patients with no RBD.50 A different study42 assessing pramipexole for iRBD showed significant decrease in the RBD PSG scoring method tonic measure. However, use of pramipexole by PD patients did not result in any significant changes of the RBD PSG scoring method measures.51
RBD PSG scoring method has been consistently reported to discriminate well between iRBD and healthy control groups24,27,29,35,37,41,43, mixed RBD and control groups33,37, and iRBD versus OSA35. No RBD PSG scoring method differences were reported between iRBD and sRBD36. In neurodegenerative diseases the findings are mixed. One study34 reported that PD patients with RBD and iRBD patients do not differ on the RBD PSG scoring method measures. A different study37 reported that these groups differed but only on the tonic measure. One study50 reported no significant differences between PD patients with RBD compared to PD without RBD (this study assessed RBD using clinical history). One study34 reported that iRBD versus MSA, and MSA versus PD, differ only on the tonic measure.
Frauscher et al.32 used the phasic component from the RBD PSG scoring method with minor differences (Table 4) to find the combination of muscles (EMG recorded from 13 different muscles) that would yield highest rates of phasic EMG activity in patients with RBD. Highest rates of phasic EMG activity were recorded from the mentalis (41.5%), flexor digitorumsuperficialis (29.1%), and extensor digitorumbrevis (22.7%). Combining these three muscles detected 82.06% of phasic EMG activity, while using recordings from the mentalis muscle alone (the most commonly used and studied muscle for recording EMG activity) would have detected only 54.69% of the phasic EMG activity.
A recent study by Montplaisir et al.24 attempted to validate and set cutoff scores for the RBD PSG scoring method using a slightly modified definition (Table 4). Along with tonic and phasic measurement, this study also included leg movement index. Using ROC analysis a cutoff score of 30% for tonic EMG resulted with 73.8% sensitivity and 90% specificity and a cutoff score of 15% for phasic EMG resulted with 80% sensitivity and 87.5% specificity. Also, a leg movement index cutoff score of 24 had a sensitivity of 76.3% and specificity of 75.0%. When combining the phasic and tonic EMG in an either/or manner into the ROC analysis the sensitivity and specificity were 88.9% and 82.5%, respectively. Adding the leg movement index into this analysis with the same “either or” logic, the sensitivity improved to 93.8% but sensitivity decreased to 64.5%.
Other Visual Methods
The definitions for tonic activity suggested by Arnulf et al.7 are used to provide a RWA index (Table 4). While this measure has not been systematically validated, it has been used in several clinical populations including PD and progressive supranuclear palsy.23,52,53 This measure has been shown to discriminate between patients with progressive supranuclear palsy or PD and a control group7,53, and between PD patients with and without RBD54. Also, progressive supranuclear palsy patients were shown to have lower RWA compared to PD.23
Bliwise and colleagues 30,31 used their phasic-electromyographic metric (PEM) (Table 4) that was previously developed55 in a non-RBD population. In one study30, PEM appropriately differentiated between patients with RBD history compared to elderly controls even though dream enactment behaviors were observed in 1 of 11 patients. Furthermore, PEM appropriately differentiated between RBD and elderly controls when using only the final REM sleep period of the night. However, PEM was not able to distinguish between PD patients with and without a clinical history of RBD.31 Bliwise and Rye30 quantified PEM from 5 EMG channels and while all sites differentiated between the groups, mentalis and brachioradialis showed largest effect size. PEM also showed good rater agreement between a highly trained scorer and 2 medical students trained in conducting PEM but with no previous knowledge of background in PSG scoring.
Two studies56,57 used definitions of muscle activity that considered the length of the activity (scored as short or long activity) rather than considering the activity as phasic or tonic (Table 5). As these methods were not systematically validated and no other studies using these methods were identified, thus these studies are not reviewed further.
Table 5.
Visual methods quantifying duration of muscle activity
| EMG Channels used for RBD | Muscle Activity Scoring Definition | Additional Definitions | |
|---|---|---|---|
|
| |||
| Eisensehr et al.56 | Mental, Submental, both anterior tibialis, both musculibrachioradiales (Chin and extremities) | Short-lasting muscle activity: if at least 10 short lasting muscle activities (an increase in muscle activity of less than 0.5-sec) occurred during an epoch | Muscle activity: EMG amplitude increase of ≥50% compared to the immediately preceding atonic EMG baseline |
| Long-lasting muscle activity: if persistently increased muscle activity (each at least 0.5-sec) lasted at least 1 second in an epoch | |||
|
| |||
| Knudsen, Gammeltoft, & Jennum57 | Anterior tibialis (Left) | Short muscle activation: 0.1-0.49-sec | Muscle Activity: an amplitude greater than threshold that is x2 of baseline EMG activity |
| Long muscle activation: 0.5-15.0-sec | Threshold muscle activity: dynamic computerized calculation of a smoothed version of baseline EMG activity before the occurrence of a given muscle activity | ||
RBD, REM sleep behavior disorder; EMG, electromyography;
Computerized EMG Scoring Methods
The major drawbacks associated with visual methods for scoring EMG activity are that they are extremely time consuming and may introduce error due to the subjective nature of visually scoring amplitude. To overcome these limitations, several researchers have attempted to computerize the scoring of the EMG channel. Five studies58-62 using 3 different computerized scoring methods were identified. Three studies59,60,62 were using/improving the same method. Each of the other two studies58,61 developed and used a unique method (Table 6).
Table 6.
Computerized EMG scoring methods
| Name of Method or Metric | EMG Channels analyzed | Epoch/Mini-epoch length (sec) | Calculation/Definitions | Formulas/Metric Calculation | Additional Definition | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Burns et al.58 | STREAM | Submental | 30 /3 | Variance (σ) of the EMG signal during all 3-s mini-epochs |
|
Threshold for normal EMG background activity during REM sleep was defined as x4 the 5th percentile of the variance observed during all NREM | |
| STREAM = % of REM mini-epochs with mean σ above the threshold | |||||||
|
| |||||||
| Ferri, Manconi, &Plazzi et al.59 | Atonia Index (AI), amp, dur, int | Submental | 30/1 | amp: Averagerectifiedamplitude (amp) foreach mini-epoch was calculated and separated into 20classes (amp≤1μV, 1< amp≤2μV, … 18< amp≤19μV, and amp>19μV) | Sleep Atonia Index = amp ≤ 1/(100 − 1 < amp ≤ 2). | Noise correction: subtracting from each averaged mini-epoch the minimum value found in the 60 mini-epochs surrounding it. | |
| Ferri, Franceschini, & Zucconi et al.60 | dur: number of sequences of consecutive mini-epochs exceeding the value of 2μV per hour of sleep and separated into 20durclasses (dur=1-sec, dur=2-sec, … dur=19-sec, and dur>19-sec) | ||||||
| Ferri et al.62 | int: intervals (int) between the EMG events (amp and dur) separated int classes | ||||||
|
| |||||||
| Mayer et al.61 | Mentalis | EMG amplitude was derived from the difference of the upper and lower envelope of the mentalis muscle recording (smoothing over 0.025-sec). | Motor activity: muscle activity above threshold | ||||
| Mean muscle tone per second were derived from amplitude and summarized statistically for each sleep stage. | Long-Lasting muscle activity: ≥0.5-sec | ||||||
| Threshold was defined by smoothing the amplitude over 200-sec for NREM and multiplied x2 for REM. | Short-Lasting muscle activity: <0.5-sec | ||||||
| Tonic Amplitude: basic level of EMG that can be measured in a phase without clear cut EMG activation as an amplitude. | |||||||
REM, rapid eye movement; RBD, REM sleep behavior disorder; NREM, non REM sleep; EMG, electromyography; AI, atonia index; STREAM, supra-threshold REM EMG activity metric
The computer logarithm developed by Burns et al.58 was tested on the same groups of 23 subjects used in the RBD PSG scoring method validation study by Consens et al.28 This computerized method computes the supra-threshold REM EMG activity metric (STREAM). The STREAM was strongly and significantly correlated with the PSG EMG score according to the RBD PSG scoring method. A STREAM cutoff score of 15% showed 100% sensitivity and 71% specificity.
The computerized method developed by Mayer et al.61(Table 6) was based on previous visual definitions56 (Table 5) of long- and short-lasting muscle activity. This computerized method was evaluated on three groups: RBD with narcolepsy/cataplexy, iRBD, and a control group. No significant differences in mean muscle tone were noted between the groups. However, there were significant differences in long- and short-lasting muscle activity between clinical RBD groups (not subclinical RBD) and the control group. No differences were found between RBD with narcolepsy/cataplexy and iRBD patients.
The computerized method by Ferri and colleagues59,60,62 was developed without the use of pre-established rules and definitions for muscle tone. Rather, they initially inspected the amplitude of muscle activity visually and allowed the data to guide their analysis. A sleep atonia index was calculated (Table 6). This metric was shown to differentiate between iRBD, MSA and control groups.59 However, this method did not differentiate between narcolepsy/cataplexy patients with and without RBD60 and between untreated RBD patients and those treated with clonazepam62. They proposed an arbitrary sleep atonia index threshold of 0.7 to support RBD diagnosis. A later introduction of a correction for noise in the amplitude signal improved the ability of the sleep atonia index to differentiate between RBD populations and controls.62 After the correction, two new sleep atonia index thresholds were proposed. A cutoff of sleep atonia index I<0.9 for iRBD showed a 74.3% sensitivity and 91.4% specificity. An sleep atonia index I<0.8 for MSA patients had sensitivity and specificity of 100%.62
Other Objective Measurements for RBD Diagnosis
One of the arguments for the necessity of objective measurements in the diagnosis of RBD is that some diseases may clinically present as pseudo-RBD (for review of deferential diagnosis see Schenck and Mahowald63). This is true in some cases of nocturnal seizures63 and in some patients with severe obstructive sleep apnea (OSA)35. Additionally, loss of muscle atonia was also reported in patients who are using antidepressant medications and do not have RBD.64 Miyamoto et al.65 evaluated the use of 123I-MIBG scintigraphy (an imaging technique that relies on radioactive decay processes and uses internal radionuclides to create two-dimensional images) for differentiating between iRBD patients with no OSA, RBD patients with moderate and severe OSA, and OSA patients with no RBD. Their analysis showed the expected reduction in cardiac uptake of the radioactive compound in patients with iRBD compared to those with OSA. No differences were observed between iRBD patients and RBD patients with OSA. They concluded that 123I-MIBG scintigraphy can differentiate between actual RBD patients with severe OSA and OSA patients presenting as pseudo-RBD. Cardiac 123I-MIBG scintigraphy was also used to compare iRBD, PD, MSA, Dementia with Lewy bodies (DLB), and progressive supranuclear palsy patients. 66 123I-MIBG scintigraphy was able to discriminate between iRBD compared to MSA, progressive supranuclear palsy, and control groups, but to a lesser extent between iRBD and PD. This study reported iRBD, PD and DLB had similar findings. A different study67 using 123I-MIBG scintigraphy in PD population reported that PD patients with RBD had significantly lower values compares to PD patients with subclinical RBD and PD with no RBD.
Naismith et al.68 used actigraphy to evaluate nocturnal symptoms of patients with probable RBD according to a questionnaire69 reviewed below. PD patients classified as probable RBD had significantly more wake bouts recorded by actigraphy compared to PD patients who were not classified as RBD. There are several significant limitations to this study (e.g., no objective assessment of sleep disorders, no PSG confirmation of RBD) and further validation studies are necessary before including actigraphy as a viable RBD assessment tool. Actigraphy was also used in the study of melatonin treatment for RBD48. Although melatonin treatment showed significant reduction in RWA, no differences were observed in the actigraphy measures.
Behavior Classification and Video Analysis
Behaviors exhibited during RBD episodes vary in duration, scope, intensity, and complexity. Abnormal behaviors observed in RBD range from excessive muscle twitching, jerking, and gesturing to vocalizations such as talking, swearing, whistling, yelling and complex dream-enactment behaviors such as kicking, running, crawling, and punching.3,63,70 Earlier versions of the ICSD16included classification of severity. However, such classification was never systematically validated and this classification was abandoned in the subsequent ICSD-II17. Although several studies in this review reported observations of abnormal behaviors during REM sleep22,23,35,42,45,53,54,60,71,72, several studies also categorized behaviors observed on video-synchronized PSG (VPSG) either by intensity 34,45,72, complexity22,42,72,73, or type25,33,72. This review did not identify any video analysis or behavioral classification system that was evaluated for its reliability and validity.
Subjective Measurements
There is a large array of subjective measurements including structured, semi-structured, and unstructured clinical interviews as well as questionnaires.
Clinical Interviews
Two studies by the same research group71,74 evaluated the inter-observer reliability of the ICSD-R16 criterion B, C1, C2, and C3 (see Table 1) that compose the minimal criteria for RBD. Scaglione et al.71 observed a 91% overall agreement of RBD diagnosis between the different raters with substantial IR (Kappa = 0.81). Inter-observer reliability was moderate to substantial for sub criteria. The second study74 exhibited 83% agreement for the diagnosis of RBD with a moderate inter-observer reliability (Kappa = 0.65). For single criterion, C1 and C2 had highest and nearly perfect inter-observer reliability while criteria B and C3 had only moderate IR.
Eisensehr et al.75 evaluated the psychometric properties of a clinical interview for diagnosing RBD. This study reviewed PSG recordings of 292 patients (including 19 patients with PD) who underwent an overnight VPSG due to sleep complaints. A clinical diagnosis of RBD was given when the minimal criteria18 were fulfilled. Clinical evaluation was confirmed by VPSG if a 30-second epoch of REM sleep was associated with documented complex movements and tonic muscle activity that was present for more than 50% of that epoch. Results revealed that 4 of 19 PD patients (21%) had RBD diagnosed based on interview but that 9 PD patients (47%) had RBD according to VPSG. Only 4 of 273 non-PD patients (1.8%) met the clinical RBD diagnosis which was also confirmed by VPSG. These findings reveal a sensitivity of 33% and specificity of 90% for the use of clinical interview in PD but 100% sensitivity and 99.6% specificity in non-PD population.
Gagnon et al.22 attempted to determine the frequency of RBD in the PD population using both clinical history and PSG. A clinical diagnosis of RBD was given when the minimal criteria18 were fulfilled during a structured clinical interview. RBD diagnosis was confirmed by PSG if more than 20% of REM sleep was associated with increased EMG muscle tone. Five of 29 PD patients (17%) had RBD according to the minimal diagnostic criteria and were also confirmed by the PSG criteria. However, 4 additional PD patients who did not report clinical history of RBD met the PSG criteria. These results revealed 56% sensitivity for the clinical diagnosis of RBD in PD.
A study by De Cock et al.54 that interviewed 100 PD patients with their spouses for RBD reported that 60 patients met clinical diagnosis of RBD based on history alone. VPSG was performed on 36 of these patients and RBD was confirmed in 35 (97.2%). Fifteen of the 40 PD patients who did not present with clinical history also underwent VPSG. RBD was suggested by VPSG in 6 (40%) of these patients. While not reported, the findings by suggest a sensitivity of 87.2% and a specificity of 90.9% for the clinical interview.
Nightgale et al.76 assessed the frequency of RBD in 78 narcolepsy patients using a non-published questionnaire. Of the 55 patients who responded and were interviewed by phone, 20 patients (36%) reported a history of RBD. While only 13 of the 55 patients had PSG recordings available, there was no significant difference in increase muscle tone during REM sleep between patients with and without clinical history of RBD. This study did not report the EMG quantification method.
Arnulf et al.7 used clinical evaluation and RWA measure (Table 4). RBD suggested by clinical history was found in 2 patients (13%) with progressive supranuclear palsy and in 3 patients (20%) with PD. However, RWA was observed in 4 (27%) of progressive supranuclear palsy patients and 4 (27%) of PD patients. A study77 assessing the frequency of RBD in Alzheimer disease (AD) using clinical evaluation and RWA measure reported that RBD was not clinically presented in any of the AD patients, however PSG recording revealed that 4 patients of 15 (26.7%) had RWA that exceeded 2 SD above the mean RWA of the control group. Only 1 patient of these 4 presented with complex movements during REM sleep.
Questionnaires
The gold standard of RBD diagnosis is a clinical interview by an interviewer specializing in sleep medicine and confirmation using Video PSG. However, such diagnosis is time consuming, expensive, and may not be feasible under some circumstances (e.g., large epidemiological studies, rural areas). Therefore, there is a clinical and research utility for easily administered screening measures sufficiently sensitive to recognize RBD. There are several studies that used either unpublished 5,71,78 or non-validated 28,58,79-81 questionnaires for RBD. Only 3 questionnaires69,82,83 have been developed and validated for RBD.
REM Behavior Disorder Screening Questionnaire (RBDSQ)
The RBDSQ69 was developed in an attempt to provide a simple pen and paper RBD screening tool. The RBDSQ attempted to follow the major features of RBD according to the ICSD-II17 and was created in German and English. The RBDSQ is comprised of 10 items with 13 yes/no questions (maximum score possible =13) which address frequency, dream content, nocturnal movements, injuries to self or bed partner, types of motor behaviors during the night, nocturnal awakenings, sleep disruption, and the presence of a neurological disease. The RBDSQ was evaluated in RBD patients and 2 separate control groups. The RBDSQ showed good internal consistency (Cronbach’s α = 0.885). Mean RBDSQ for the RBD patients was not significantly different between RBD patients with narcolepsy and without narcolepsy. Mean RBDSQ was significantly different between the RBD group and control group 1. A different study84 that used the RBDSQ as well as a clinical interview reported that PD patients meeting minimal diagnosis for RBD scored higher on the RBDSQ than PD patients with no clinical RBD. Using ROC analysis a RBDSQ cutoff score of 5 showed a sensitivity of 96% and a specificity of 56% (Table 7). However, when comparing to subjects from the general population (control group 2) the RBDSQ had a specificity of 92% with 93% correct diagnosis.69
Table 7.
Questionnaire summary
| Authors | Name | Language | Population | Cutoff score | Sensitivity % | Specificity % | PPV % | NPV % | AUC |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Stiasny-Kolster et al.69 | RBDSQ | English & German | RBD vs. Control group 1 | 5 | 96.0 | 56.0 | 66.0 | NS | 0.87 |
| RBD vs. Control group 2 | 5 | NS | 92.0 | 93.0 | NS | NS | |||
|
| |||||||||
| Miyamoto et al.82 | RBDSQ-J | Japanese | iRBD vs. Healthy controls | 4.5 | 88.5 | 96.9 | 97.9 | 91.4 | 0.97 |
| iRBD vs. OSA | 4.5 | 88.5 | 90.9 | 90.2 | 89.3 | 0.93 | |||
|
| |||||||||
| Li et al.83 | RBDQ-HK | Chinese | Overall RBD group vs. Control group | 18/19 | 82.2 | 86.9 | 86.3 | 83.0 | 0.90 |
| iRBD | 18/19 | 78.4 | 86.9 | 74.1 | 89.4 | 0.89 | |||
| sRBD | 18/19 | 79.3 | 86.9 | 62.2 | 93.9 | 0.88 | |||
| RBD-like | 20/21 | 92.6 | 86.9 | 64.1 | 98.0 | 0.94 | |||
PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve; RBDSQ, REM behavior disorder screening questionnaire; RBDSQ-J, REM behavior disorder screening questionnaire Japanese version; RBDQ-HK, REM behavior disorder questionnaire – Hong Kong; RBD, REM behavior disorder; iRBD, idiopathic RBD; sRBD, symptomatic RBD; OSA, obstructive sleep apnea; NFLE, nocturnal frontal lobe epilepsy; NS, not specified;
The RBDSQ was used in the study of actigraphy and RBD in PD.68 This study showed that RBDSQ and wake bouts calculated by the actigraphy were positively and significantly correlated (r = 0.45) with a medium effect size, and that PD patients scoring above the RBDSQ cutoff of 5 had more awakenings during nights than those scoring bellow the suggested cutoff.
The RBDSQ69 was subsequently translated into Japanese (RBDSQ-J) by Miyamoto et al82 and was administered to PSG-confirmed iRBD patients, obstructive sleep apnea (OSA) patients, and a healthy control group. Test-retest reliability using Cronbach’s α coefficient was conducted with 16 of the 52 iRBD patients and showed good test-retest reliability (Cronbach’s α = 0.84). Internal consistency was also good (Cronbach’s α = 0.87). Mean RBDSQ-J was significantly different between the iRBD group, healthy controls, and the OSA group. There was also a significant difference in the RBDSQ-J mean score between men and women in all three groups, with men consistently scoring higher than women. Finally, conducting a ROC analysis with a cutoff score of 4.5 revealed high levels of sensitivity and specificity when compared with healthy controls (Table 7).
REM sleep behavior disorder questionnaire Hong Kong (RBDQ-HK)
The RBDQ-HK83 was developed, tested, and validated in Chinese based on the ICSD-II17. The RBDQ-HK was developed in an attempt to obtain more information on the currency, frequency, and severity of RBD symptoms. The RBDQ-HK is composed of 13 questions and each question is answered on two scales: lifetime occurrence (don’t know, no, yes) and recent 1-year frequency (occurred in the last year, once or few times per year, once or few times per month, 1-2 times per week, and 3+ times per week). Questions 1 & 2 cover currency and frequency of dreams and nightmares, questions 3-5 ask about the dream content, questions 6 &7 ask about vocalizations during sleep, questions 8&9 discuss motor behaviors during sleep, questions 10-12 ask about injuries during sleep, and question 13 asks about sleep disruption. Questions are weighted differently and total score can range from 0 to 100 points. Although an English version is published, it seems that it was not translated systematically, was not validated, and appears to have culture specific phrases (e.g., chased by a ghost) that may not be appropriate in non-Chinese cultures.
After the piloting stage, the RBDQ-HK was validated in a group of PSG-confirmed RBD patients sub-classified as iRBD, sRBD, RBD-like disorders (symptoms due to psychotropic medications), and a control group free of RBD. The mean RBDQ-HK score of the overall RBD group was significantly higher than the mean score of the control group. There were no significant differences in mean score between the 3 RBD sub groups. Factor analysis using varimax rotation resulted in a significant two-factor solution explaining 59.1% of the variance. Based on item loading, factor 1 was dream related and factor 2 was behavioral manifestations. Internal consistency was excellent for the overall scale (0.90) and good for both factor 1 (0.86) and factor 2 (0.85). No differences in mean scores were found between the 2 test retest RBDQ-HK administrations. ROC analysis revealed a cutoff score for the total scale of 18/19 with moderate sensitivity and specificity (Table 7). A higher cutoff score (20/21) was indicated for the RBD-like group. ROC analysis was also conducted using only factor 2 (behavioral manifestations) and revealed a cutoff score for factor 2 of 7/8 with 87.9% sensitivity and 81.3% specificity, with a positive predictive value of 82.5% and a negative predictive value of 87.0%.
Other Questionnaires used for RBD Evaluation
Several studies assessed for RBD using questionnaires that were either not originally developed for RBD diagnosis79-81, not published78, or not validated5,28. Thus, these measures are not reviewed in detail.
Discussion
The diagnosis of RBD is composed of two major components: abnormal muscle activity during REM sleep and history of abnormal behaviors during REM sleep or actual dream enactment behaviors. This review found several measurements, objective and subjective, that were developed to aid the assessment of these components.
EMG Scoring Method
An EMG scoring method is necessary as abnormal behavior during REM sleep and/or dream enactment behavior does not occur every night and a single PSG may miss such behaviors.24 Studies have shown a lack of test-retest reliability when using only video recording for diagnosis.25 Furthermore, although abnormal behaviors during REM and dream enactment behaviors do not occur every night, the presence of abnormal EMG tone during REM sleep, a hallmark of RBD, is more consistent and showed good test retest reliability. 25,28
Several attempts were made to develop a method for scoring EMG for increased muscle tone during REM sleep. Of the visual scoring methods, the most commonly used is the RBD PSG scoring method. Several studies have used a modified version of the RBD PSG scoring method22,24,24,29,32 and there is a striking inconsistency between the different methods in definitions, equipment, sites of recording, and cutoff scores, and reporting of the metrics (e.g., atonia, tonic, phasic, PSG EMG score). The RBD PSG scoring method has been used successfully in different clinical populations. Overall, this method has shown good reliability, validity, sensitivity to change, and discriminant capability.24,25,27,28,58 To date, there is no agreed upon cutoff score and several scores were used for indicating abnormal increase of muscle tone. Additional studies are necessary for establishing a single cutoff score for this method.
Several other visual EMG scoring methods were proposed that are significantly different from the RBD PSG scoring method.7,30,31,56,57 Some of these methods represent crucial theoretical disagreements with the RBD PSG scoring method. More specifically, some question the use of tonic EMG as a valid measurement of abnormal muscle activity and propose a measure that they believe to better resemble brain activation related to RBD30,31. Other researchers56,57 question the use of both tonic and phasic EMG and suggest definitions that focus on the duration of the muscle activity. Although these methods were developed with a different theoretical orientation, they are not widely used and require further validation.
Three different computerized EMG scoring methods59,61 were developed to overcome the disadvantages associated with visual EMG scoring methods (i.e., very time consuming and introduce measurement error). However, only one method59 was used in additional evaluations 60,62. As the field is heavily focused on EMG abnormalities during REM sleep, there is an apparent need for further validation studies of computerized EMG scoring methods that hold a promise to introduce uniformity and consistency in RBD assessment.
Other Objective Methods
Cardiac 123I-MIBG scintigraphy was used by some studies either for discriminating RBD from other disorders that might impede correct diagnosis65 or for discriminating between different neurological populations that have been shown to be highly related to RBD66,67. These studies report excellent discriminative ability of this measure. However, this diagnostic tool is costly, invasive, and not widely available thus limited in its applicability to a wider population.
There is a need for more studies validating the use of actigraphy as a measure of RBD as this was conducted in only one study68. Also, behaviors observed during RBD occurrences are widely reported in studies but here is no consistency in the categorizations of the behaviors. No validation studies were identified for any classification or categorizations system of behaviors observed during REM sleep.
Clinical Interviews
Several studies have assessed the reliability and validity of clinical interviews. Clinical interviews were shown to have good reliability71,74. However, the validity evidence for clinical interviews is less straight forward. RBD frequency rates are very different when using interview as opposed to PSG.7,22,77 Furthermore, clinical interviews were reported to have nearly perfect discriminant validity in the general population75, but in PD populations, disciminant validity appears to be much lower22,54,75. These inconsistent validity findings support the insistence of the scientific ICSD-II17 to include objective measurements in the diagnosis of RBD. Nonetheless, there is no doubt that clinical interviews will remain the first line in RBD assessment.
Questionnaires
This review yielded only 2 questionnaires that were developed specifically for RBD.69,83 The RBDSQ69 was developed in English and German and was later translated into Japanese82 showed good reliability measures, validity, and discriminant capabilities. The RBDQ-HK83 was developed in Chinese but was not systematically translated into English. The RBDQ-HK showed good psychometric properties for reliability and validity. Nonetheless, additional validation studies are necessary for the use of these measures in different clinical populations such as PD, progressive supranuclear palsy, and MSA.
Conclusion
Various methods have been utilized in the diagnosis of RBD including a variety of subjective and objective methods. Although a detailed sleep history may be sufficient in some populations for diagnosis of RBD, a definite diagnosis could be achieved only with a PSG recording. Several questionnaires were developed for RBD and appear to provide a good screening tool for RBD. With that said, the current gold standard for appropriate diagnosis of clinical RBD include history of abnormal movements, sleep-related injuries, or dream-enactment behaviors that are supported with PSG findings indicating the loss of REM sleep muscle atonia and/or excessive phasic muscle twitching, or actual observation of RBD occurrences.
Practice points.
Clinical interview may be sufficient to uncover RBD in some populations. However, diagnostic accuracy is better achieved with the combined use of clinical history and video PSG recording.
Muscle atonia during REM sleep is most commonly assessed by the submental EMG channel in an overnight PSG recording. There are several methods for quantifying EMG and the most widely used visual quantification method is the RBD PSG scoring method detailed by Lapierre and Montplaisir27.
There is no consistency and agreement in the literature regarding the EMG quantification methods.
There have been attempts to develop automated methods for quantifying EMG however, these methods are not widely used and need further validation. Nonetheless, automated methods are crucial for wide use of EMG quantification as visual analysis is time consuming and increases error.
Clinical interviews are the first line in RBD assessment.
Subjective measurements are commonly used for assessing RBD and include structured, semi-structured, and unstructured clinical interviews as well as standardized questionnaires. Clinical interviews show good reliability but their validity is dependant on population used.
The current gold standard for appropriate diagnosis of clinical RBD includes history of abnormal movements, sleep-related injuries, or dream-enactment behaviors that are supported with PSG findings indicating the intermittent or sustained loss of muscle atonia or actual observation of RBD occurrences.
Research agenda.
While visual methods of scoring EMG have been widely used, there has been no consistency methods and definition. Many studies used only slight differences in the methods however these are likely to result in inequality of the metric. It is necessary to reach an agreement regarding the methods and the definitions such that reliable and valid comparisons between studies could be done.
There are major drawbacks associated with visual methods for scoring EMG activity (i.e., time consuming and increased error). While some computerized methods have been suggested, more validation research is necessary using different clinical population to achieve more wide use of these methods. There are no PSG software packages with EMG quantification capabilities. It is recommended that industry be engaged in this process so that EMG quantification could be easily done and thus more widely utilized.
There is a necessity for a vibrant discussion on what levels of REM sleep without atonia should be considered pathological.
Additional research is necessary on the differences between REM sleep without atonia with and without dream enactment behaviors. The question still remains if there is either research or clinical utility for differentiation between the “pre-clinical” and “clinical” RBD.
While the current RBD questionnaires appear to be valid and reliable tools for suggesting RBD, it is necessary to validate these questionnaires in different clinical populations.
Acknowledgments
Supported by: NIA AG08415, UL1RR031980 (CTRI), the UCSD Stein Institute for Research on Aging and the Department of Veterans Affairs Center of Excellence for Stress and Mental Health (CESAMH).
References
- *1.Schenck CH, Bundlie SR, Ettinger M, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- 2.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The Scoring of Sleep and Associated Events: Rules, Technical Specifications and Terminology. Chicago: AASM; 2007. [Google Scholar]
- 3.Iranzo A, Santamaria J, Tolosa E. The clinical and pathophysiological relevance of REM sleep behavior disorder in neurodegenerative diseases. Sleep Medicine Reviews. 2009;13:385–401. doi: 10.1016/j.smrv.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Ohayon MM, Caulet M, Priest RG. Violent behavior during sleep. Journal of Clinical Psychiatry. 1997;58:369–376. [PubMed] [Google Scholar]
- 5.Chiu HFK, Wing YK, Lam LCW, et al. Sleep-related injury in the elderly: an epidemiological study in Hong Kong. Sleep. 2000;23:513–517. [PubMed] [Google Scholar]
- 6.Plazzi G, Corsini R, Provini F, et al. REM sleep behavior disorders in multiple system atrophy. Neurology. 1997;48:1094–1097. doi: 10.1212/wnl.48.4.1094. [DOI] [PubMed] [Google Scholar]
- 7.Arnulf I, merino-andreu m, Bloch F, et al. REM sleep behavior disorder and REM sleep without flatonia in patients with progressive supranuclear palsy. Sleep. 2005;28:349–354. [PubMed] [Google Scholar]
- 8.Schenck CH, Mahowald MW. Motor dyscontrol in narcolepsy: Rapid eye movement (REM) sleep without atonia and REM sleep behavior disorder. Annals of Neurology. 1992;32:3–10. doi: 10.1002/ana.410320103. [DOI] [PubMed] [Google Scholar]
- *9.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathoc rapid eye movement sleep behavior disorder. Neurology. 1996;46:388–393. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- *10.Boeve BF, Silber MH, Ferman TJ, Lucas JA, Parisi JE. Association of REM Sleep Behavior Disorder and Neurodegenerative Disease May Reflect an Underlying Synucleinopathy. Movement Disorders. 2001;16:622–630. doi: 10.1002/mds.1120. [DOI] [PubMed] [Google Scholar]
- 11.Schenck CH, Bundlie SR, Mahowald M. REM Sleep Behavior Disorder: Delayed emergence of Parkinsonism and/or dementia in 65% of older men initially diagnosed with idiopathic REM sleep behavior disorder and an analysis of the minimum and maximum tonic and/or phasic EMG abnormalities found during REM sleep. Sleep. 2003;26(Suppl.):A316. [Google Scholar]
- 12.Iranzo A, Molinuevo JL, Santamaria J. Sixty-four percent of patients with idiopathic REM sleep behavior disorder developed a neurological disorder after a mean clinical follow-up of seven years. Sleep. 2008;31:A280. [Google Scholar]
- 13.Gagnon JF, Postuma RB, Mazza S, Doyon J, Montplaisir J. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. 2006;5:424–432. doi: 10.1016/S1474-4422(06)70441-0. [DOI] [PubMed] [Google Scholar]
- *14.Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130:2770–2788. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 15.Diagnostic Classification Steering Committee TMJC. International classification of sleep disorders: Diagnostic and coding manual. Rochester, MN: American Sleep Disorders Association; 1990. [Google Scholar]
- 16.Diagnostic Classification Steering Committee. International classification of sleep disorders: Diagnostic and Coding Manual. Rochester: American Academy of Sleep Medicine; 2001. Revised. [Google Scholar]
- 17.American Academy of Sleep Medicine. The international classification of sleep disorders. 2. Westchester: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 18.American Academy of Sleep. The international classification of sleep disorders, revised. Rochester, MN: American Sleep Disorders Association; 1997. [Google Scholar]
- 19.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 20.Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment. 1994;6:284–290. [Google Scholar]
- 21.Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 22.Gagnon JF, Bedard MA, Fantini ML, et al. REM sleep behavior disorder and REM sleep without atonia in Parkinson’s disease. Neurology. 2002;59:585–589. doi: 10.1212/wnl.59.4.585. [DOI] [PubMed] [Google Scholar]
- 23.Sixel-Doring F, Schweitzer M, Mollenhauer B, Trenkwalder C. Polysomnographic findings, video-based sleep analysis and sleep perception in progressive supranuclear palsy. Sleep Medicine. 2009;10:407–415. doi: 10.1016/j.sleep.2008.05.004. [DOI] [PubMed] [Google Scholar]
- *24.Montplaisir J, Gagnon JF, Fantini ML, et al. Polysomnographic diagnosis of idiopathic REM sleep behavior disorder. Movement Disorders. 2010;25:2044–2051. doi: 10.1002/mds.23257. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Lam SP, Ho CKW, et al. Diagnosis of REM Sleep Behavior Disorder by Video-Polysomnographic Study: Is One Night Enough? Sleep. 2008;31:1179–85. [PMC free article] [PubMed] [Google Scholar]
- 26.Chase MH, Morales FR. Subthreshold excitatory activity and motoneuron discharge during REM periods of active sleep. Science. 1983;221:1195–8. doi: 10.1126/science.6310749. [DOI] [PubMed] [Google Scholar]
- *27.Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992;42:1371–1374. doi: 10.1212/wnl.42.7.1371. [DOI] [PubMed] [Google Scholar]
- *28.Consens FB, Chervin RD, Koeppe RA, et al. Validation of a polysomnographic score for REM sleep behavior disorder. Sleep. 2005;28:993–997. doi: 10.1093/sleep/28.8.993. [DOI] [PubMed] [Google Scholar]
- 29.Dauvilliers Y, Romprq S, Gagnon JF, Vendette M, Petit D, Montplaisir J. REM sleep characteristics in narcolepsy and REM sleep behavior disorder. Sleep. 2007;30:844–9. doi: 10.1093/sleep/30.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bliwise DL, Rye DB. Elevated PEM (phasic electromyographic metric) rates identify rapid eye movement behavior disorder patients on nights without behavioral abnormalities. Sleep. 2008;31:853–7. doi: 10.1093/sleep/31.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bliwise DL, Trotti LM, Greer SA, Juncos JJ, Rye DB. Phasic muscle activity in sleep and clinical features of Parkinson disease. Annals of Neurology. 2010;68:353–359. doi: 10.1002/ana.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frauscher B, Iranzo A, Hogl B, et al. Quantification of electromyographic activity during REM sleep in multiple muscles in REM sleep behavior disorder. Sleep. 2008;31:724–31. doi: 10.1093/sleep/31.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frauscher B, Gschliesser V, Brandauer E, Ulmer H, Poewe W, Hogl B. The relation between abnormal behaviors and REM sleep microstructure in patients with REM sleep behavior disorder. Sleep Medicine. 2009;10:174–181. doi: 10.1016/j.sleep.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Iranzo A, Santamaria J, Rye DB, et al. Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and PD. Neurology. 2005;65:247–52. doi: 10.1212/01.wnl.0000168864.97813.e0. [DOI] [PubMed] [Google Scholar]
- 35.Iranzo A, Santamaria J. Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep. 2005;28:203–206. doi: 10.1093/sleep/28.2.203. [DOI] [PubMed] [Google Scholar]
- 36.Iranzo A, Molinuevo JL, Santamarea J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. The Lancet Neurology. 2006;5:572–577. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 37.Postuma RB, Gagnon JF, Rompre S, Montplaisir JY. Severity of REM atonia loss in idiopathic REM sleep behavior disorder predicts Parkinson disease. Neurology. 2010;74:239–44. doi: 10.1212/WNL.0b013e3181ca0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin FC, Lai CL, Huang P, Liu CK, Hsu CY. The rapid eye movement sleep behavior disorder in Chinese Taiwanese patients. Psychiatry and Clinical Neurosciences. 2009;63:557–562. doi: 10.1111/j.1440-1819.2009.01998.x. [DOI] [PubMed] [Google Scholar]
- 39.Kim YK, Yoon IY, Kim JM, et al. The implication of nigrostriatal dopaminergic degeneration in the pathogenesis of REM sleep behavior disorder. European Journal of Neurology. 2010;17:487–492. doi: 10.1111/j.1468-1331.2009.02854.x. [DOI] [PubMed] [Google Scholar]
- 40.Iranzo A, Ratti PL, Casanova-Molla J, Serradell M, Vilaseca I, Santamaria J. Excessive muscle activity increases over time in idiopathic REM sleep behavior disorder. Sleep. 2009;32:1149–53. doi: 10.1093/sleep/32.9.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazza S, Soucy JP, Gravel P, et al. Assessing whole brain perfusion changes in patients with REM sleep behavior disorder. Neurology. 2006;67:1618–22. doi: 10.1212/01.wnl.0000242879.39415.49. [DOI] [PubMed] [Google Scholar]
- 42.Fantini ML, Gagnon JF, Filipini D, Montplaisir J. The effects of pramipexole in REM sleep behavior disorder. Neurology. 2003;61:1418–1420. doi: 10.1212/wnl.61.10.1418. [DOI] [PubMed] [Google Scholar]
- 43.Stiasny-Kolster K, Doerr Y, Moller JC, et al. Combination of ‘idiopathic ’REM sleep behaviour disorder and olfactory dysfunction as possible indicator for {alpha}-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain. 2005;128:126–37. doi: 10.1093/brain/awh322. [DOI] [PubMed] [Google Scholar]
- 44.Bonakis A, Howard RS, Ebrahim IO, Merritt S, Williams A. REM sleep behaviour disorder (RBD) and its associations in young patients. Sleep Medicine. 2009;10:641. doi: 10.1016/j.sleep.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Kumru H, Santamaria J, Tolosa E, et al. Rapid eye movement sleep behavior disorder in parkinsonism with parkin mutations. Annals of Neurology. 2004;56:599–603. doi: 10.1002/ana.20272. [DOI] [PubMed] [Google Scholar]
- 46.Mattarozzi K, Bellucci C, Campi C, et al. Clinical, behavioural and polysomnographic correlates of cataplexy in patients with narcolepsy/cataplexy. Sleep Medicine. 2008;9:425–433. doi: 10.1016/j.sleep.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Gagnon JF, Postuma RB, Montplaisir J. Update on the pharmacology of REM sleep behavior disorder. Neurology. 2006;67:742–747. doi: 10.1212/01.wnl.0000233926.47469.73. [DOI] [PubMed] [Google Scholar]
- 48.Kunz D, Bes F. Melatonin as a therapy in REM sleep behavior disorder patients: an open-labeled pilot study on the possible influence of melatonin on REM-sleep regulation. Movement Disorders. 1999;14:507–511. doi: 10.1002/1531-8257(199905)14:3<507::aid-mds1021>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 49.Takeuchi N, Uchimura N, Hashizume Y, et al. Melatonin therapy for REM sleep behavior disorder. Psychiatry Clin Neurosci. 2001;55:267–269. doi: 10.1046/j.1440-1819.2001.00854.x. [DOI] [PubMed] [Google Scholar]
- 50.Garcia Borreguero D, Caminero AB, De La Llave Y, et al. Decreased phasic EMG activity during rapid eye movement sleep in treatment naive Parkinson’s disease: Effects of treatment with levodopa and progression of illness. Movement Disorders. 2002;17:934–941. doi: 10.1002/mds.10233. [DOI] [PubMed] [Google Scholar]
- 51.Kumru H, Iranzo A, Carrasco E, et al. Lack of effects of pramipexole on REM sleep behavior disorder in Parkinson disease. Sleep. 2008;31:1418. [PMC free article] [PubMed] [Google Scholar]
- 52.Limousin N, Konofal E, Karroum E, et al. Restless legs syndrome, rapid eye movement sleep behavior disorder, and hypersomnia in patients with two parkin mutations. Movement Disorders. 2009;24:1970–1976. doi: 10.1002/mds.22711. [DOI] [PubMed] [Google Scholar]
- 53.De Cock VC, Lannuzel A, Verhaeghe S, et al. REM sleep behavior disorder in patients with guadeloupean parkinsonism, a tauopathy. Sleep. 2007;30:1026–1032. doi: 10.1093/sleep/30.8.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Cock VC, Vidailhet M, Leu S, et al. Restoration of normal motor control in Parkinson’s disease during REM sleep. Brain. 2007;130:450. doi: 10.1093/brain/awl363. [DOI] [PubMed] [Google Scholar]
- 55.Bliwise DL, He L, Ansari FP, Rye DB. Quantification of electromyographic activity during sleep: a phasic electromyographic metric. J Clin NeuroPhysiol. 2006;23:59–67. doi: 10.1097/01.wnp.0000192303.14946.fc. [DOI] [PubMed] [Google Scholar]
- 56.Eisensehr I, Linke R, Tatsch K, et al. Increased muscle activity during rapid eye movement sleep correlates with decrease of striatal presynaptic dopamine transporters. IPT and IBZM SPECT imaging in subclinical and clinically manifest idiopathic REM sleep behavior disorder, Parkinson’s disease, and controls. Sleep. 2003;26:507–512. doi: 10.1093/sleep/26.5.507. [DOI] [PubMed] [Google Scholar]
- 57.Knudsen S, Gammeltoft S, Jennum PJ. Rapid eye movement sleep behaviour disorder in patients with narcolepsy is associated with hypocretin-1 deficiency. Brain. 2010;133:568–479. doi: 10.1093/brain/awp320. [DOI] [PubMed] [Google Scholar]
- 58.Burns JW, Consens FB, Little RJ, Angell KJ, Gilman S, Chervin RD. EMG variance during polysomnography as an assessment for REM sleep behavior disorder. Sleep. 2007;30:1771–1778. doi: 10.1093/sleep/30.12.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferri R, Manconi M, Plazzi G, et al. A quantitative statistical analysis of the submentalis muscle EMG amplitude during sleep in normal controls and patients with REM sleep behavior disorder. Journal of Sleep Research. 2008;17:89–100. doi: 10.1111/j.1365-2869.2008.00631.x. [DOI] [PubMed] [Google Scholar]
- 60.Ferri R, Franceschini C, Zucconi M, et al. Searching for a marker of REM sleep behavior disorder: submentalis muscle EMG amplitude analysis during sleep in patients with narcolepsy/cataplexy. Sleep. 2008;31:1409–1417. [PMC free article] [PubMed] [Google Scholar]
- 61.Mayer G, Kesper K, Ploch T, et al. Quantification of tonic and phasic muscle activity in REM sleep behavior disorder. Journal of Clinical Neurophysiology. 2008;25:48–55. doi: 10.1097/WNP.0b013e318162acd7. [DOI] [PubMed] [Google Scholar]
- 62.Ferri R, Rundo F, Manconi M, et al. Improved computation of the atonia index in normal controls and patients with REM sleep behavior disorder. Sleep Medicine. 2010;11:947–949. doi: 10.1016/j.sleep.2010.06.003. [DOI] [PubMed] [Google Scholar]
- * 63.Schenck CH, Mahowald MW. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep. 2002;25:120–138. doi: 10.1093/sleep/25.2.120. [DOI] [PubMed] [Google Scholar]
- 64.Winkelman JW, James L. Serotonergic antidepressants are associated with REM sleep without flatonia. Sleep. 2004;27:317–321. doi: 10.1093/sleep/27.2.317. [DOI] [PubMed] [Google Scholar]
- 65.Miyamoto T, Miyamoto M, Suzuki K, et al. Comparison of severity of obstructive sleep apnea and degree of accumulation of cardiac 123I-MIBG radioactivity as a diagnostic marker for idiopathic REM sleep behavior disorder. Sleep Medicine. 2009;10:577–580. doi: 10.1016/j.sleep.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 66.Miyamoto T, Miyamoto M, Suzuki K, Nishibayashi M, Iwanami M, Hirata K. 123I-MIBG cardiac scintigraphy provides clues to the underlying neurodegenerative disorder in idiopathic REM sleep behavior disorder. Sleep. 2008;31:717–723. doi: 10.1093/sleep/31.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nomura T, Inoue Y, Hogl B, et al. Relationship between 123I-MIBG scintigrams and REM sleep behavior disorder in Parkinson’s disease. Parkinsonism and Related Disorders. 2010;13:683–685. doi: 10.1016/j.parkreldis.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 68.Naismith SL, Rogers NL, MacKenzie J, Hickie IB, Lewis SJG. The relationship between actigraphically defined sleep disturbance and REM sleep behaviour disorder in Parkinson’s Disease. Clinical Neurology and Neurosurgery. 2010;112:420–423. doi: 10.1016/j.clineuro.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 69.Stiasny-Kolster K, Mayer G, Schafer S, Moller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire - a new diagnostic instrument. Movement Disorders. 2007;22:2386–2393. doi: 10.1002/mds.21740. [DOI] [PubMed] [Google Scholar]
- * 70.Schenck CH, Hurwitz TD, Mahowald MW. Symposium: Normal and abnormal REM sleep regulation: REM sleep behaviour disorder: an update on a series of 96 patients and a review of the world literature. J Sleep Res. 1993;2:224–231. doi: 10.1111/j.1365-2869.1993.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 71.Scaglione C, Vignatelli L, Plazzi G, et al. REM sleep behaviour disorder in Parkinson’s disease: a questionnaire-based study. Neurol Sci. 2005;25:316–321. doi: 10.1007/s10072-004-0364-7. [DOI] [PubMed] [Google Scholar]
- 72.Frauscher B, Gschliesser V, Brandauer E, et al. Video analysis of motor events in REM sleep behavior disorder. Movement Disorders. 2007;22:1464–1470. doi: 10.1002/mds.21561. [DOI] [PubMed] [Google Scholar]
- 73.Manni R, Terzaghi M, Glorioso M. Motor-behavioral episodes in REM sleep behavior disorder and phasic events during REM sleep. Sleep. 2009;32:241–245. doi: 10.1093/sleep/32.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bologna G. Interobserver reliability of ICSD-R criteria for REM sleep behaviour disorder. Journal of Sleep Research. 2003;12:255–257. doi: 10.1046/j.1365-2869.2003.00359.x. [DOI] [PubMed] [Google Scholar]
- 75.Eisensehr I, Lindeiner H, Jager M, Noachtar S. REM sleep behavior disorder in sleep-disordered patients with versus without Parkinson’s disease: is there a need for polysomnography? J Neurol Sciences. 2001;186:7–11. doi: 10.1016/s0022-510x(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 76.Nightingale S, Orgill JC, Ebrahim IO, De Lacy SF, Agrawal S, Williams AJ. The association between narcolepsy and REM behavior disorder (RBD) Sleep Medicine. 2005;6:253–258. doi: 10.1016/j.sleep.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 77.Gagnon JF, Petit D, Fantini ML. REM sleep behavior disor-der and REM sleep without flatonia in probable Alzheimer disease. Sleep. 2006;29:1321–1325. doi: 10.1093/sleep/29.10.1321. [DOI] [PubMed] [Google Scholar]
- 78.Onofrj M, Thomas A, D’Andreamatteo G, et al. Incidence of RBD and hallucination in patients affected by Parkinson’s disease: 8-year follow-up. Neurological Sciences. 2002;23:91–94. doi: 10.1007/s100720200085. [DOI] [PubMed] [Google Scholar]
- 79.Manni R, Terzaghi M, Repetto A. The FLEP scale in diagnosing nocturnal frontal lobe epilepsy, NREM and REM parasomnias: data from a tertiary sleep and epilepsy unit. Epilepsia. 2008;49:1581–1585. doi: 10.1111/j.1528-1167.2008.01602.x. [DOI] [PubMed] [Google Scholar]
- 80.Gjerstad MD, Boeve B, Wentzel-Larsen T, Aarsland D, larsen JP. Occurrence and clinical correlates of REM sleep behaviour disorder in patients with Parkinson’s disease over time. Journal of Neurology, Neurosurgery & Psychiatry. 2008;79:387–391. doi: 10.1136/jnnp.2007.116830. [DOI] [PubMed] [Google Scholar]
- 81.Forsaa EB, larsen JP, Wentzel-Larsen T, et al. A 12-Year Population-Based Study of Psychosis in Parkinson Disease. Archives of Neurology. 2010;67:996–1001. doi: 10.1001/archneurol.2010.166. [DOI] [PubMed] [Google Scholar]
- 82.Miyamoto T, Miyamoto M, Iwanami M, et al. The REM sleep behavior disorder screening questionnaire: validation study of a Japanese version. Sleep Medicine. 2009;10:1151–1154. doi: 10.1016/j.sleep.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 83.Li SX, Wing YK, Lam SP, et al. Validation of a new REM sleep behavior disorder questionnaire (RBDQ-HK) Sleep Medicine. 2010;11:43–48. doi: 10.1016/j.sleep.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 84.Bugalho P, da Silva JA, Neto B. Clinical features associated with REM sleep behavior disorder symptoms in the early stages of Parkinson’s disease. Journal of Neurology. 2011;258:50–55. doi: 10.1007/s00415-010-5679-0. [DOI] [PubMed] [Google Scholar]


