Abstract
Objective
High sugar intake increases heart disease risk in humans. In animals, sugar intake accelerates heart failure development via increased reactive oxygen species (ROS). Glucose 6-phosphate dehydrogenase (G6PD) can fuel ROS production by providing NADPH for superoxide generation by NADPH oxidase. On the other hand, G6PD also facilitates ROS scavenging via the glutathione pathway. We hypothesized that high sugar intake would increase flux through G6PD to increase myocardial [NADPH] and ROS, and accelerate cardiac dysfunction and death.
Research Methods & Procedures
Six-week old TO-2 hamsters, a nonhypertensive model of genetic cardiomyopathy caused by a δ-sarcoglycan mutation, were fed a long-term diet of either high starch or high sugar (57% of energy from sucrose+fructose).
Results
After 24 weeks, δ-sarcoglycan deficient animals displayed expected decreases in survival and cardiac function associated with cardiomyopathy (ejection fraction: control=68.7±4.5%; TO-2 starch=46.1±3.7, p<0.05 TO-2 starch vs control; TO-2 sugar=58.0±4.2%, N.S. vs TO-2 starch or control; median survival: TO-2 starch=278 days, TO-2 sugar=318 days, P=0.133). Although we expected high sugar intake to exacerbate cardiomyopathy, surprisingly there was no further decrease in ejection fraction or survival with high sugar compared to starch in cardiomyopathic animals. Cardiomyopathic animals had systemic and cardiac metabolic abnormalities (elevated serum lipids and glucose, and decreased myocardial oxidative enzymes) which were unaffected by diet. High sugar intake increased myocardial superoxide, but [NADPH] and lipid peroxidation were unaffected.
Conclusions
A sugar enriched diet did not exacerbate ventricular function, metabolic abnormalities, or survival in heart failure despite an increase in NADPH and superoxide production.
Keywords: heart failure, reactive oxygen species, δ-sarcoglycan, diet, sugar, fructose, sucrose
Introduction
Clinical studies show that high sugar intake is associated with dyslipidemia and that high dietary glycemic load may increase the risk for coronary heart disease [1–5] ; however, little is known about the effects of sugar intake on the development and progression of heart failure. A recent epidemiological study found that hospitalization or death from new onset heart failure was not related to sugar intake over a 9 year follow-up in middle age and elderly women [6]. There is less information regarding effects of sugar intake in populations that are at high risk for developing heart failure, such as patients with hypertension, ischemic heart disease or inherited cardiomyopathies.
Recent studies from our group and others have found that high intake of fructose or sucrose accelerated the development of heart failure and mortality compared to a complex carbohydrate diet in rats with salt-induced hypertension [7;8], rats with volume overload [9], and mice with transverse aortic constriction (TAC) [10;11]. High fructose intake increases the generation of superoxide in myocardium, suggesting that cardiac function is decreased by accelerated formation of reactive oxygen species (ROS) and subsequent oxidative damage [11;12]. Support for this comes from the observation the accelerated development of heart failure by high fructose intake was prevented by tempol, a superoxide dismutase mimetic, in mice with aortic constriction [11]. There is greater oxidative stress in heart failure, as evidenced by increased lipid peroxidation products and a decreased ratio of reduced/oxidized glutathione (GSH/GSSG) [13]. Recent studies show glucose-6-phosphate dehydrogenase (G6PD) expression is increased in failing myocardium, and that superoxide generation is fueled by increased production of NADPH by G6PD in humans and animal models of heart failure [14–17]. Consumption of a high sugar diet may further increase flux through G6PD and accelerate production of NADPH and subsequent ROS production in heart failure [14–16;18;19].
Evidence for acceleration of cardiac pathology with high sugar intake comes from animal models that employed an increase in cardiac after load induced by aortic constriction or hypertension, which increases cardiac mass, myocardial oxygen consumption, and ROS generation. However, the majority of heart failure patients do not have this type of extreme uncontrolled hypertension. Thus, pressure overload models have limited relevance to many forms of human heart failure. In the present investigation, we examine the potential cardiotoxic effects of sugar intake in a model of heart failure that does not involve cardiac hypertrophy or an increase in myocardial workload and oxygen consumption. Studies were performed in cardiomyopathic hamsters which have a mutation in δ-sarcoglycan that results in progressive cardiac dysfunction and a shortened life span (~40–50 weeks compared to 2 to 3 years for normal strains). These animals have systemic and cardiac metabolic abnormalities (defects in cardiac mitochondria, and elevated circulating glucose and triglycerides and low insulin), and increased myocardial oxidative stress [13;18;20]. We hypothesized that long-term consumption of a high sugar diet would accelerate cardiac dysfunction due to greater oxidative stress via accelerated NADPH generation by G6PD. Dietary treatments were initiated at six weeks of age and survival was assessed out to 78 weeks. A subgroup was assessed at 29 weeks for cardiac function, and at 30 weeks for metabolic parameters, and myocardial ROS formation and oxidative damage. We hypothesized that in cardiomyopathic hamsters, a high sugar diet (57% of energy intake) would increase ROS formation and lipid peroxidation, impair cardiac and systemic metabolism, and decrease cardiac function and survival compared to the starch diet.
Materials and Methods
Experimental Design and Animal Treatment
All procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals (NIH publication No. 85-23), and were approved by the University of Maryland Animal Care and Use Committee. Six-week old male hamsters that were either normal healthy controls (Bio F1B strain) or cardiomyopathic due to a mutation in δ-sarcoglycan (Bio TO-2 strain) were purchased from Bio Breeders Inc (Watertown, MA). The healthy control animals were fed a standard high starch diet, and the cardiomyopathic animals were either maintained on the standard high starch diet or on a high sugar diet. The aim of this study was to assess the role of high sugar intake within the context of cardiomyopathy, therefore we did not include a high sugar diet group of healthy control F1B hamsters. Healthy controls (n=17) and cardiomyopathic animals (n=37 starch diet, n=30 high sugar diet) were assessed for mortality for 545 days. An additional subset (n=11–12/group) were assessed for cardiac function at 29 weeks and euthanized at 30 weeks of age for biochemical analysis.
Diets
Animals were fed ad libitum custom manufactured purified diets that derived 12% of energy from fat, 20% from protein (casein supplemented with L-cystine), and 68% from carbohydrate (Research Diets Inc, New Brunswick, NJ). The high starch diet (#D09103101) contained no sugar (12% of total energy from maltodextrin and 55% from corn starch). The high sugar diet (#D09103110, Research Diets) contained 5% of energy from corn starch, 5% from maltodextrin, 28.5% from fructose, and 28.5% from glucose.
Echocardiography
Cardiac function was assessed by echocardiography at 29 weeks of age (Vevo 770 High-Resolution Imaging Systems, Visual Sonics, Toronto) with a 15 MHz linear array transducer (model 716). Hamsters were anesthetized with 2% isoflurane in oxygen, shaved, and placed on a warming pad. Two-dimensional cine loops and guided M-mode frames were recorded from the parasternal short axis. Absolute wall thickness and relative wall thickness (RWT) were calculated as: (PWTd + AWTd) and (PWTd + AWTd) / EDD, where PWTd is the diastolic posterior wall thickness, AWTd is the diastolic anterior wall thickness, and EDD is the end diastolic diameter. Ejection fraction was calculated as: (EDV − ESV)/EDV × 100%, where EDV is the end diastolic volume and ESV is the end systolic volume. EDV and ESV were calculated as: 1.047 × EDD3 and 1.047 × ESD3 respectively, where ESD is the end systolic diameter.
Terminal Procedure
A subgroup was euthanized at 30 weeks of age for biochemical analysis. The procedure was performed from 3 to 5 hours into the light phase of the light-dark cycle. Nonfasted animals were anesthetized with 5% isoflurane, the chest was opened, and a 20–30 mg piece was cut out of the left ventricle (LV), freeze clamped, weighed, and stored for later [NADPH] measurement. The remainder of the heart was dissected in ice-cold phosphate buffered saline, and the LV was weighed and frozen in liquid nitrogen. Blood was drawn from the thorax and centrifuged to obtain serum and plasma. All samples were stored at −80°C for subsequent biochemical analysis.
Biochemical Measurements
Serum glucose, triglycerides and free fatty acid concentrations were assayed spectrophotometrically (Wako Diagnostics, Richmond, VA), and plasma insulin was measured with an enzyme-linked immunosorbent assay (Shibayagi Co., Shibukawa, Gunma, Japan). Whole tissue enzyme activities of citrate synthase (CS), medium-chain acyl-Coenzyme A dehydrogenase (MCAD), isocitrate dehydrogenase (ICDH), and G6PD were assayed in LV homogenates as previously described [11]. Myocardial tissue concentrations of the lipid peroxidation products, malondialdehyde (MDA) and 4-hydroxyalkenals (4HA), were measured LV homogenates (Oxford Biomedical Research, Oxford, MI).
Myocardial NADPH levels were measured using a commercial enzymatic spectrophotometric method (BioVision, CA, USA). Homogenized tissue was centrifuged, and the supernatant (200µl) was heated to 60°C for 30min to decompose all NADP+, leaving NADPH intact. Samples were cooled on ice, and 50µl of sample and 10µl NADPH developer was added into each well on a 96 well plate, incubated for 1hr and read at a 450nm.
Superoxide production was measured via lucigenin chemiluminescence.LV tissue was homogenized in MOPS (20mmol/L) – sucrose (250mmol/L) buffer (pH 7.4) containing 100mmol/L phenylmethylsulfonyl fluoride, 10µg/mL aprotinin, 10µg/mL leupeptin, and 200mmol/L pepstatin. This buffer system preserves intact cellular organelles in tissue homogenates [21]. Myocardial homogenate (20µL) was added to lucigenin (5µM) and Krebs solution buffered with 10mmol/L HEPES-NaOH (pH 7.4) to a final volume of 1mL, and incubated at 37°C, as previously described [14]. The measurement was repeated after adding an NADPH regenerating system consisting of glucose-6-phosphate (200µmol/L) and NADP+ (100µmol/L) to each reaction.
Statistics
Values are shown as mean ± standard error. The three groups were compared using a one-way analysis of variance with the Holm-Sidak correction. Kaplan-Meier analysis was used to assess survival. P<0.05 was considered significant.
Results
Survival and Morphometric Parameters
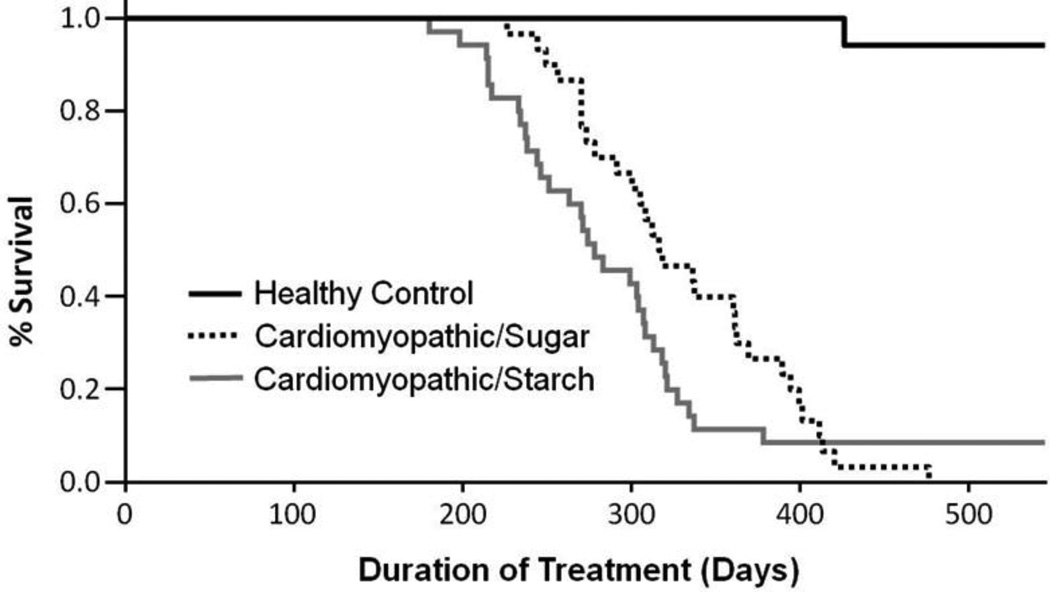
There were no differences in body mass between the two diets, but the high sugar diet increased combined fat mass by 29% (p<0.05; Table 1). High sugar had no effect on LV mass, but it resulted in 17% lower right ventricular and 60% lower atrial masses. Cardiomyopathic hamsters had the expected high rate of mortality compared to healthy control animals (Figure 1). We expected to see an increase in mortality in response to high sugar intake, however there was not a significant difference in mortality for the high sugar diet compared to the high starch diet (Median survival: 278 days for the cardiomyopathic starch group vs 318 for the high sugar group, p=0.133).
Table 1.
Morphometric measurements at 30 weeks of age.
| Parameter | Healthy Control |
Cardiomyopathic Starch |
Cardiomyopathic Sugar |
|---|---|---|---|
| LV Mass (mg) | 417±10 | 339±11* | 328±9* |
| LV Mass/Tibia Length (mg/mm) | 15.7±0.3 | 13.5±0.4* | 13.1±0.3* |
| LV Mass/Body Mass (mg/g) | 2.47±0.09 | 2.62±0.05 | 2.49±0.06 |
| RV Mass (mg) | 104±5 | 86±5* | 71±3*# |
| Combined Atrial Mass (mg) | 23.4±1.9 | 66.1±15.1* | 26.5±3.3# |
| Tibia Length (mm) | 26.5±0.2 | 25.1±0.2* | 25.0±0.2* |
| Terminal Body Mass (g) | 170±5 | 130±4* | 132±3* |
| Liver Mass (g) | 6.82±0.20 | 4.99±0.19* | 5.32±0.09* |
| Retroperitoneal Fat (g) | 2.00±0.11 | 0.82±0.08* | 1.20±0.12*# |
| Epididymal Fat (g) | 3.29±0.14 | 1.64±0.09* | 2.13±0.11*# |
| Subcutaneous Fat (g) | 4.23±0.29 | 1.75±0.12* | 2.09±0.13* |
| Combined Fat Mass (g) | 9.51±0.53 | 4.20±0.28* | 5.42±0.35*# |
LV, left ventricle; RV, right ventricle;
P <0.05 vs. Control;
P <0.05 vs. Starch; n=11–12.
Figure 1.
Survival of hamsters from initiation of the diet at 6 weeks of age. Cardiomyopathy resulted in the expected decrease in lifespan, but there was no effect of diet (p=0.133 high sugar vs. starch diets) (n=17 healthy controls, n=37 cardiomyopathic starch diet, n=30 cardiomyopathic high sugar diet).
Functional and Metabolic parameters
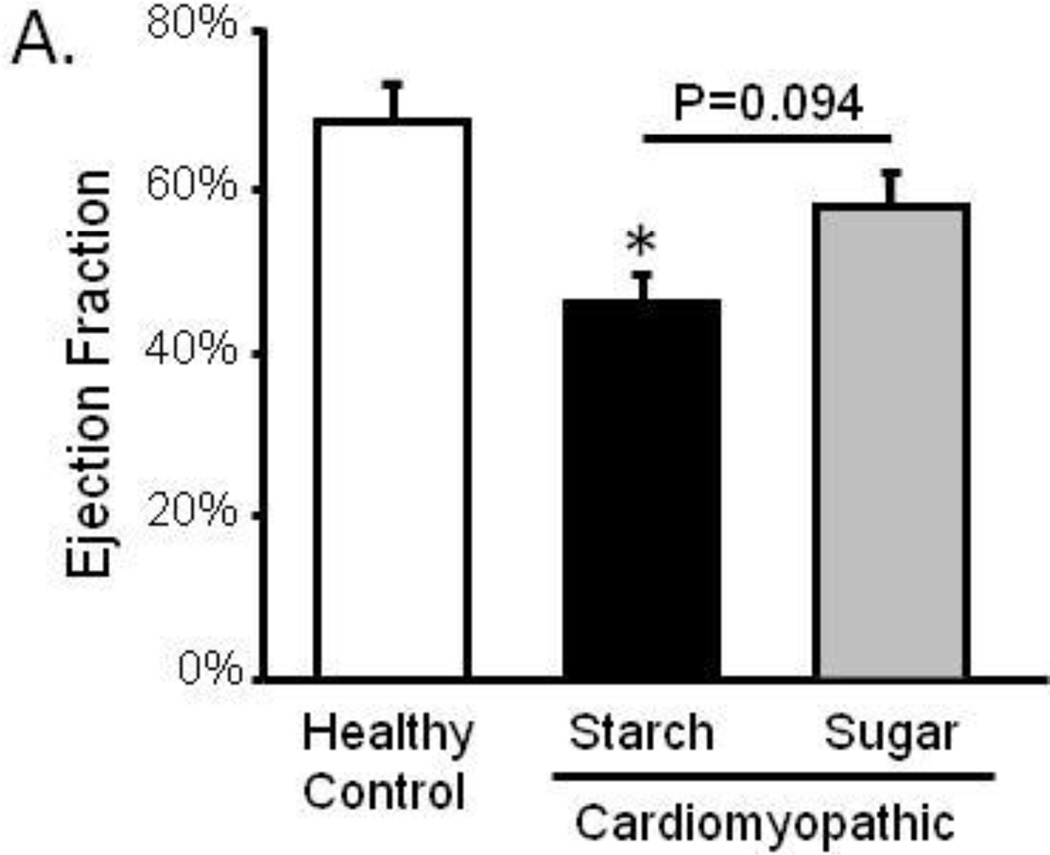
Ventricular dysfunction and wall thinning were evident in cardiomyopathic animals with high starch (Figure 2; Table 2). Ejection fraction, the percentage of blood that is pumped out of the heart with each beat, was decreased from 68.7% to 46.1% in cardiomyopathy indicative of ventricular dysfunction. Contrary to our hypothesis which predicted that high sugar intake would result in a further decrease in ejection fraction compared to starch, the high sugar diet did not decrease ejection fraction in cardiomyopathic animals and may increase ejection fraction compared to starch (p=0.094 for trend).
Figure 2. Echocardiography.
Left ventricular ejection fraction at 29 weeks of age; *P <0.05 vs. Control; n=11–12.
Table 2.
Echocardiography at 29 weeks of age.
| Parameter | Healthy Control |
Cardiomyopathic Starch |
Cardiomyopathic Sugar |
|---|---|---|---|
| ESV/Tibia Length (µL/mm) | 1.58±0.35 | 3.39±0.43* | 2.72±0.55 |
| EDV/Tibia Length (µL/mm) | 4.69±0.66 | 6.08±0.65 | 5.79±0.96 |
| AWT/Tibia Length (µm/mm) | 142±3 | 117±5* | 121±4* |
| Relative Wall Thickness | 0.748±0.032 | 0.591±0.042* | 0.590±0.029* |
| Heart Rate (BPM) | 338±16 | 323±13 | 344±8 |
ESV, end systolic volume; EDV, end diastolic volume; AWT, absolute wall thickness; BPM, beats per minute;
P <0.05 vs. Control; n=11–12.
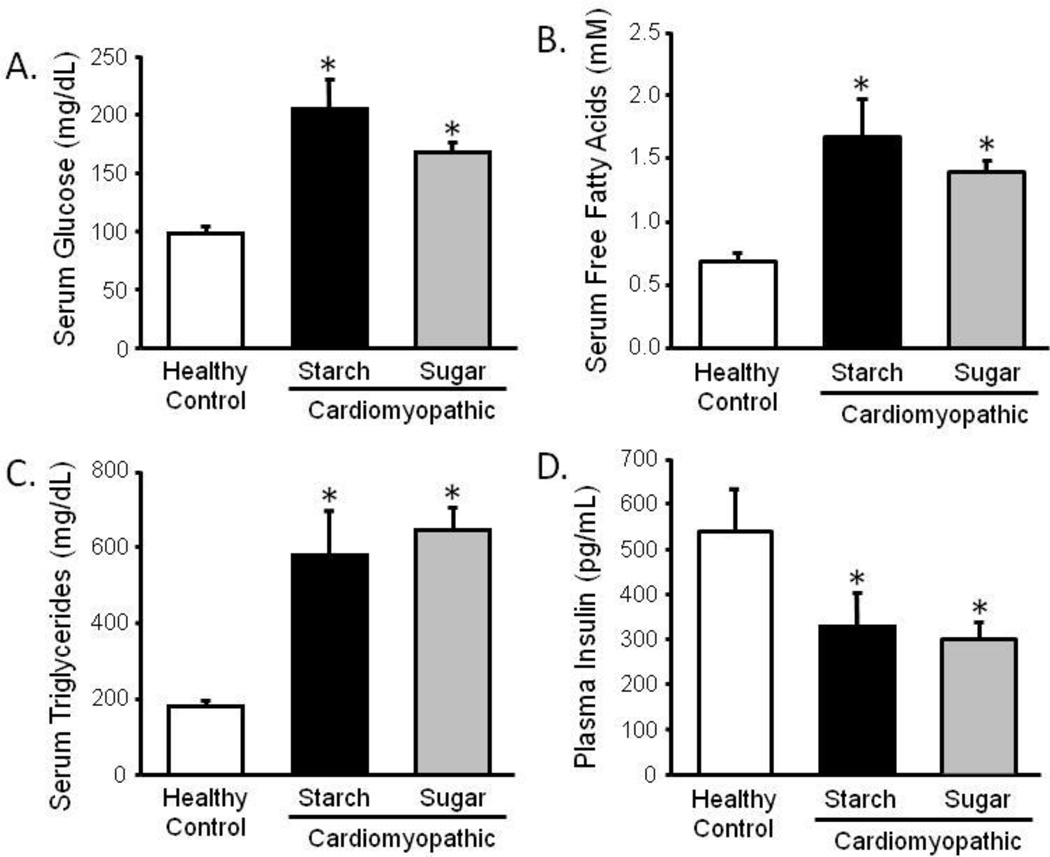
Cardiomyopathic hamsters had high circulating glucose (cardiomyopathy: 206mg/dL vs control: 95mg/dL, p<0.05), triglycerides (cardiomyopathy: 581mg/dL vs control: 184mg/dL, p<0.05), and free fatty acids (cardiomyopathy: 1.67mM vs control: 0.68mM, p<0.05), and this corresponded with low plasma insulin (cardiomyopathy: 329pg/mL vs control: 540pg/mL, p<0.05) (Figure 3). However, none of these parameters were significantly affected by sugar intake.
Figure 3.
Serum glucose (a), free fatty acids (b), and triglycerides (c), and plasma insulin (d) taken at 30 weeks of age in the nonfasted state. *P <0.05 vs. Control; #P <0.05 vs. Starch; n=9–12.
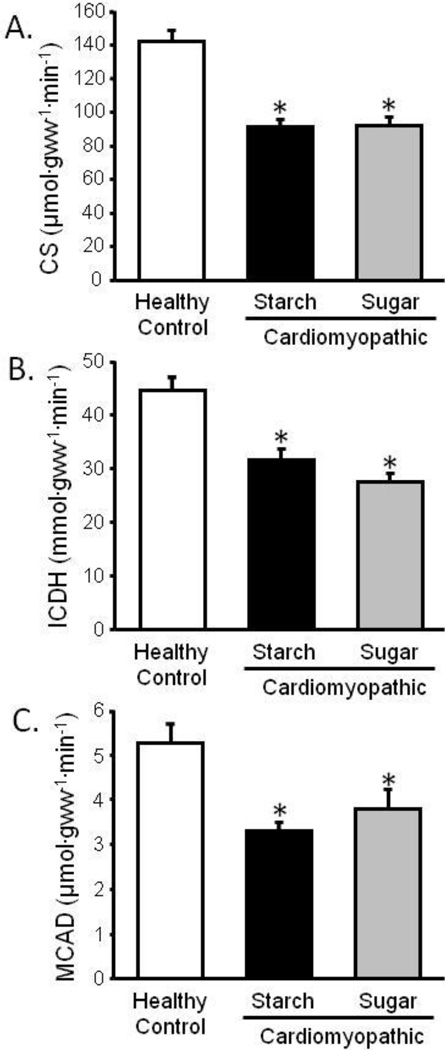
Heart failure is accompanied by mitochondrial dysfunction and low myocardial ATP levels [22;23], and disrupted mitochondria and decreased respiration rates have been reported in cardiomyopathic hamsters [24]. We observed a decrease in the activity of the mitochondrial oxidative enzymes CS (36% decrease), MCAD (37% decrease), and ICDH (29% decrease) in cardiomyopathic compared to control hamsters, with no significant differences between diets (Figure 4).
Figure 4. Metabolic Enzyme Activity.
Left ventricular citrate synthase (CS) (a), isocitrate dehydrogenase (ICDH) (b), and medium-chain acyl coenzyme-A dehydrogenase (MCAD) enzyme activity (c); *P <0.05 vs. Control; n=11–12.
Indices of Oxidative Stress
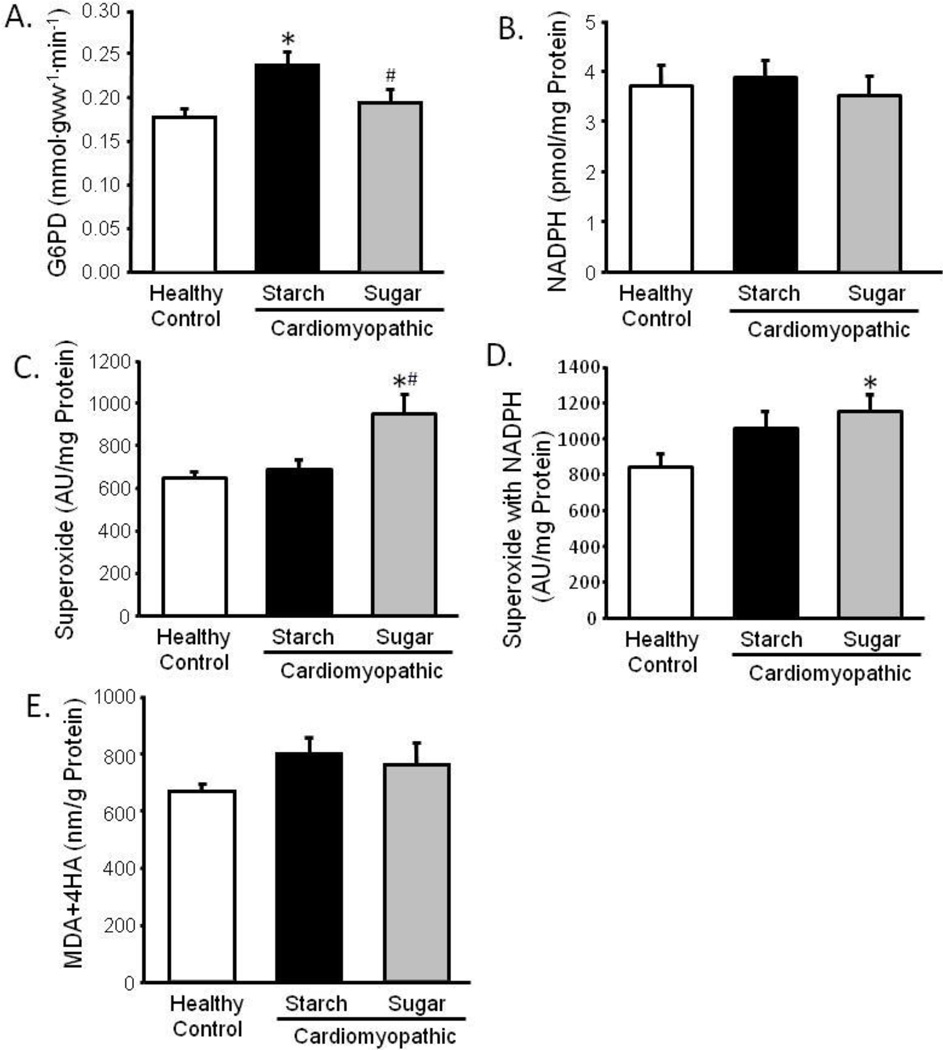
Cardiomyopathic hamsters fed the high starch diet had elevated myocardial G6PD activity (35% increase) compared to normal hamsters, however this was not observed with high sugar intake (Figure 5). The high sugar diet increased superoxide production by 39%, but there were no differences in myocardial NADPH concentration or lipid peroxidation products among the three groups.
Figure 5. Indices of Oxidative Stress.
Left ventricular glucose-6-phosphate dehydrogenase (G6PD) activity (a), NADPH content (b), superoxide production (c), superoxide production with NADPH included in the reaction mix (d), and lipid peroxidation products, malondialdehyde and 4-hydroxyalkenals (MDA+4HA) (e); *P <0.05 vs. Control; #P <0.05 vs. Starch; n=11–12.
Discussion
Previous studies in pressure overload models of heart failure demonstrated that high intake of sugar accelerated the development of cardiac hypertrophy, contractile dysfunction, and death. Since this type of extreme afterload-induced heart failure has limited clinical relevance, it is important to also investigate if this effect occurs in a heart failure model that does not involve hypertension and the subsequent increase in cardiac workload. Here we used the well described cardiomyopathic TO-2 hamsters, and found that a high sugar diet increased myocardial superoxide production, but did not affect [NADPH] or lipid peroxidation products. Surprisingly, the high sugar diet did not exacerbate LV dysfunction or accelerate mortality. This was independent of systemic and cardiac metabolic abnormalities (elevated serum lipids and glucose, and decreased myocardial mitochondrial oxidative enzyme activities) which were unaffected by diet.
High sugar intake can accelerate heart failure, however this finding is not consistent [25]. In salt-sensitive hypertensive rats, high sugar intake increased fetal gene expression, apoptosis, contractile dysfunction, and mortality [7;8]. Similarly, mortality, cardiac hypertrophy, and fetal gene expression were increased by high sugar intake in mice with TAC-induced pressure overload [10;11]. In a volume overload model generated by an arterial-venous shunt in rats, a high fructose diet exacerbated chamber dilation and systolic dysfunction [9]. On the other hand, in a rat abdominal aortic banded model of heart failure, high sucrose intake attenuated LV hypertrophy and did not affect cardiac function or fetal gene expression compared to a high starch diet [26]. Finally, ischemia-reperfusion models have shown a benefit of fructose feeding [27;28], as in both in vivo and isolated perfused hearts there is protection from ischemia-reperfusion injury by high sugar intake despite increased lipid peroxidation and oxidative stress. These studies indicate that the effects of high sugar intake are complex and require further study.
Patients with cardiac dysfunction often show signs of metabolic disturbances that may be affected by diet [29]. We observed metabolic defects in cardiomyopathic hamsters on the high starch diet, including high circulating glucose, triglycerides, and free fatty acids, as well as decreased insulin, which were not exacerbated by the high sugar diet. High sugar intake can result in metabolic dysfunction, however there were already metabolic defects in cardiomyopathic hamsters regardless of diet. The decreased plasma insulin in cardiomyopathic hamsters may indicate β-cell dysfunction, and was observed previously in young cardiomyopathic hamsters [30]. A previous report indicated that β-sarcoglycan deficient mice exhibited decreased tolerance to glucose, and that this corresponded with decreased fat pad mass [20]. In the present work, the combined fat pad mass was largely decreased in cardiomyopathic hamsters. However, with high sugar intake there was a partial-preservation of fat mass which may be beneficial [31]. These results indicate that although cardiomyopathic hamsters exhibited metabolic defects, these defects were not exacerbated by high sugar intake.
It has been proposed that an increase in NADPH generation by G6PD is a major contributor to ROS production in heart failure [14;15;19]. Studies in patients and dogs with heart failure indicated that increased G6PD expression was associated with increased [NADPH] and ROS production that was prevented by G6PD inhibition. We therefore predicted that G6PD activity and [NADPH] would be increased in genetic cardiomyopathy. We further predicted that if high sugar intake increases the flux of glucose-6-phosphate through G6PD, then high sugar intake would elevate [NADPH] and further increase superoxide production. However, although high sugar intake increased superoxide production, [NADPH] was unaffected, suggesting that changes in [NADPH] and G6PD do not play a significant role in this model of cardiomyopathy.
The clinical implications of this study should be interpreted with caution. Although high sugar intake did not exacerbate cardiac dysfunction in a hamster model of cardiomyopathy, it should be stressed that this may or may not be the case in humans, particularly in patients with acquired forms of heart failure resulting from hypertension and/or ischemic heart disease. Although the effects of sugar intake on the progression and development of heart failure in humans has not been studied per se, high sugar intake does increase the risk of heart disease in humans, and this may lead to heart failure [1;3]. Studies should examine the effects of diet on the progression of heart failure in humans.
Conclusion
We determined that high sugar intake did not exacerbate ventricular function, metabolic abnormalities, or survival. The results further indicate that NADPH and superoxide production do not play major roles in this model. These results warrant further investigation of sugar intake effects on humans with similar forms of genetic cardiomyopathy.
Acknowledgments
this work was supported by the National Institutes of Health [grant numbers HL074237, HL072751-06].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: none declared.
References
- 1.Liu S, Willett WC, Stampfer MJ, Hu FB, Franz M, Sampson L, et al. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr. 2000 Jun;71(6):1455–1461. doi: 10.1093/ajcn/71.6.1455. [DOI] [PubMed] [Google Scholar]
- 2.Welsh JA, Sharma A, Abramson JL, Vaccarino V, Gillespie C, Vos MB. Caloric sweetener consumption and dyslipidemia among US adults. JAMA. 2010 Apr 21;303(15):1490–1497. doi: 10.1001/jama.2010.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, et al. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006 Nov 9;355(19):1991–2002. doi: 10.1056/NEJMoa055317. [DOI] [PubMed] [Google Scholar]
- 4.Chess DJ, Stanley WC. Role of diet and fuel overabundance in the development and progression of heart failure. Cardiovasc Res. 2008 Jul 15;79(2):269–278. doi: 10.1093/cvr/cvn074. [DOI] [PubMed] [Google Scholar]
- 5.Beulens JW, de Bruijne LM, Stolk RP, Peeters PH, Bots ML, Grobbee DE, et al. High dietary glycemic load and glycemic index increase risk of cardiovascular disease among middle-aged women: a population-based follow-up study. J Am Coll Cardiol. 2007 Jul 3;50(1):14–21. doi: 10.1016/j.jacc.2007.02.068. [DOI] [PubMed] [Google Scholar]
- 6.Levitan EB, Mittleman MA, Wolk A. Dietary glycemic index, dietary glycemic load, and incidence of heart failure events: a prospective study of middle-aged and elderly women. J Am Coll Nutr. 2010 Feb;29(1):65–71. doi: 10.1080/07315724.2010.10719818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma N, Okere IC, Duda MK, Johnson J, Yuan CL, Chandler MP, et al. High fructose diet increases mortality in hypertensive rats compared to a complex carbohydrate or high fat diet. Am J Hypertens. 2007 Apr;20(4):403–409. doi: 10.1016/j.amjhyper.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Sharma N, Okere IC, Barrows BR, Lei B, Duda MK, Yuan CL, et al. High-sugar diets increase cardiac dysfunction and mortality in hypertension compared to low-carbohydrate or high-starch diets. J Hypertens. 2008 Jul;26(7):1402–1410. doi: 10.1097/HJH.0b013e3283007dda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouchard-Thomassin AA, Lachance D, Drolet MC, Couet J, Arsenault M. A high-fructose diet worsens eccentric left ventricular hypertrophy in experimental volume overload. Am J Physiol Heart Circ Physiol. 2011 Jan;300(1):H125–H134. doi: 10.1152/ajpheart.00199.2010. [DOI] [PubMed] [Google Scholar]
- 10.Chess DJ, Lei B, Hoit BD, Azimzadeh AM, Stanley WC. Deleterious effects of sugar and protective effects of starch on cardiac remodeling, contractile dysfunction, and mortality in response to pressure overload. Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1853–H1860. doi: 10.1152/ajpheart.00544.2007. [DOI] [PubMed] [Google Scholar]
- 11.Chess DJ, Xu W, Khairallah R, O'Shea KM, Kop WJ, Azimzadeh AM, et al. The antioxidant tempol attenuates pressure overload-Induced cardiac hypertrophy and contractile dysfunction in mice fed a high-fructose diet. Am J Physiol Heart Circ Physiol. 2008 Oct 17;295(6):H2223–H2230. doi: 10.1152/ajpheart.00563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mellor K, Ritchie RH, Meredith G, Woodman OL, Morris MJ, Delbridge LM. High-fructose diet elevates myocardial superoxide generation in mice in the absence of cardiac hypertrophy. Nutrition. 2010 Jul;26(7–8):842–848. doi: 10.1016/j.nut.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Nishizawa T, Iwase M, Kanazawa H, Ichihara S, Ichihara G, Nagata K, et al. Serial alterations of beta-adrenergic signaling in dilated cardiomyopathic hamsters: possible role of myocardial oxidative stress. Circ J. 2004 Nov;68(11):1051–1060. doi: 10.1253/circj.68.1051. [DOI] [PubMed] [Google Scholar]
- 14.Gupte RS, Vijay V, Marks B, Levine RJ, Sabbah HN, Wolin MS, et al. Upregulation of glucose-6-phosphate dehydrogenase and NAD(P)H oxidase activity increases oxidative stress in failing human heart. J Card Fail. 2007 Aug;13(6):497–506. doi: 10.1016/j.cardfail.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Gupte SA, Levine RJ, Gupte RS, Young ME, Lionetti V, Labinskyy V, et al. Glucose-6-phosphate dehydrogenase-derived NADPH fuels superoxide production in the failing heart. J Mol Cell Cardiol. 2006 Aug;41(2):340–349. doi: 10.1016/j.yjmcc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Rajasekaran NS, Connell P, Christians ES, Yan LJ, Taylor RP, Orosz A, et al. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell. 2007 Aug 10;130(3):427–439. doi: 10.1016/j.cell.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serpillon S, Floyd BC, Gupte RS, George S, Kozicky M, Neito V, et al. Superoxide production by NAD(P)H oxidase and mitochondria is increased in genetically obese and hyperglycemic rat heart and aorta before the development of cardiac dysfunction. The role of glucose-6-phosphate dehydrogenase-derived NADPH. Am J Physiol Heart Circ Physiol. 2009 Jul;297(1):H153–H162. doi: 10.1152/ajpheart.01142.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi K, Kimata H, Obata K, Matsushita A, Fukata A, Hashimoto K, et al. Xanthine oxidase inhibition improves left ventricular dysfunction in dilated cardiomyopathic hamsters. J Card Fail. 2008 Apr;14(3):238–244. doi: 10.1016/j.cardfail.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Matsui R, Xu S, Maitland KA, Hayes A, Leopold JA, Handy DE, et al. Glucose-6 phosphate dehydrogenase deficiency decreases the vascular response to angiotensin II. Circulation. 2005 Jul 12;112(2):257–263. doi: 10.1161/CIRCULATIONAHA.104.499095. [DOI] [PubMed] [Google Scholar]
- 20.Groh S, Zong H, Goddeeris MM, Lebakken CS, Venzke D, Pessin JE, et al. Sarcoglycan complex: implications for metabolic defects in muscular dystrophies. J Biol Chem. 2009 Jul 17;284(29):19178–19182. doi: 10.1074/jbc.C109.010728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohazzab KM, Wolin MS. Sites of superoxide anion production detected by lucigenin in calf pulmonary artery smooth muscle. Am J Physiol. 1994 Dec;267(6 Pt 1):L815–L822. doi: 10.1152/ajplung.1994.267.6.L815. [DOI] [PubMed] [Google Scholar]
- 22.Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007 Mar 15;356(11):1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 23.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005 Jul;85(3):1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 24.Hoppel CL, Tandler B, Parland W, Turkaly JS, Albers LD. Hamster cardiomyopathy. A defect in oxidative phosphorylation in the cardiac interfibrillar mitochondria. J Biol Chem. 1982 Feb 10;257(3):1540–1548. [PubMed] [Google Scholar]
- 25.Mellor KM, Ritchie RH, Davidoff AJ, Delbridge LM. Elevated dietary sugar and the heart: experimental models and myocardial remodeling. Can J Physiol Pharmacol. 2010 May;88(5):525–540. doi: 10.1139/y10-005. [DOI] [PubMed] [Google Scholar]
- 26.Duda MK, O'Shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, et al. Low-carbohydrate/high-fat diet attenuates pressure overload-induced ventricular remodeling and dysfunction. J Card Fail. 2008 May;14(4):327–335. doi: 10.1016/j.cardfail.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joyeux-Faure M, Rossini E, Ribuot C, Faure P. Fructose-fed rat hearts are protected against ischemia-reperfusion injury. Exp Biol Med (Maywood ) 2006 Apr;231(4):456–462. doi: 10.1177/153537020623100411. [DOI] [PubMed] [Google Scholar]
- 28.Jordan JE, Simandle SA, Tulbert CD, Busija DW, Miller AW. Fructose-fed rats are protected against ischemia/reperfusion injury. J Pharmacol Exp Ther. 2003 Dec;307(3):1007–1011. doi: 10.1124/jpet.103.055970. [DOI] [PubMed] [Google Scholar]
- 29.Jakob SM, Stanga Z. Perioperative metabolic changes in patients undergoing cardiac surgery. Nutrition. 2010 Apr;26(4):349–353. doi: 10.1016/j.nut.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Missihoun C, Zisa D, Shabbir A, Lin H, Lee T. Myocardial oxidative stress, osteogenic phenotype, and energy metabolism are differentially involved in the initiation and early progression of delta-sarcoglycan-null cardiomyopathy. Mol Cell Biochem. 2009 Jan;321(1–2):45–52. doi: 10.1007/s11010-008-9908-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reue K, Phan J. Metabolic consequences of lipodystrophy in mouse models. Curr Opin Clin Nutr Metab Care. 2006 Jul;9(4):436–441. doi: 10.1097/01.mco.0000232904.82038.db. [DOI] [PubMed] [Google Scholar]