Summary
Interactions between epithelial cells are mediated by adherens junctions that are dynamically regulated during development. Here we show that the turnover of β-catenin is increased at cell interfaces that are targeted for disassembly during Drosophila axis elongation. The Abl tyrosine kinase is concentrated at specific planar junctions and is necessary for polarized β-catenin localization and dynamics. abl mutant embryos have decreased β-catenin turnover at shrinking edges, and these defects are accompanied a reduction in multicellular rosette formation and axis elongation. Abl promotes β-catenin phosphorylation on the conserved tyrosine 667 and expression of an unphosphorylatable β-catenin mutant recapitulates the defects of abl mutants. Notably, a phosphomimetic β-cateninY667E mutation is sufficient to increase β-catenin turnover and rescues axis elongation in abl deficient embryos. These results demonstrate that the asymmetrically localized Abl tyrosine kinase directs planar polarized junctional remodeling during Drosophila axis elongation through the tyrosine phosphorylation of β-catenin.
Introduction
The dynamic assembly and disassembly of adherens junctions is essential for tissue remodeling during development. Cell adhesion in epithelia is mediated by homophilic interactions between E-cadherin proteins on neighboring cells that are stabilized by association with the cytoplasmic α- and β-catenin proteins and with the actin cytoskeleton. The strength, organization, and dynamics of adherens junctions are modulated by a number of mechanisms, including trafficking to and from the plasma membrane, lateral clustering at the membrane, and the phosphorylation of junctional components (Halbleib and Nelson, 2006; Nishimura and Takeichi, 2009; Harris and Tepass, 2010). In particular, tyrosine phosphorylation has long been suspected to regulate cell adhesion, based on studies showing that an antibody to phosphotyrosine detects a strong enrichment of tyrosine phosphorylated epitopes at adherens junctions (Maher et al., 1985). Growth factor stimulation and E-cadherin engagement lead to an increase in tyrosine phosphorylation of several junctional proteins, including E-cadherin, β-catenin, and several tyrosine kinases and phosphatases (Daniel and Reynolds, 1997; Lilien and Balsamo, 2005; McLachlan and Yap, 2007). However, the physiological role of tyrosine phosphorylation in cell adhesion is not well understood.
A major component of adherens junctions that is phosphorylated on tyrosine is β-catenin. The tyrosine phosphorylation of β-catenin affects its subcellular localization and is required for the regulation of synaptic activity in neurons (Murase et al., 2002). In addition, tyrosine phosphorylation affects the interaction of β-catenin with growth factor receptors (Bonvini et al., 2001; Zeng et al., 2006), transcriptional regulators (Piedra et al., 2001; Coluccia et al., 2007; Kim et al., 2009), and other components of the adherens junction complex. Phosphorylation of β-catenin on tyrosine 142 by the Fyn, Fer, and cMet kinases reduces its affinity for α-catenin (Ozawa and Kemler 1998; Piedra et al., 2003; Brembeck et al., 2004; Tominaga et al., 2008), and phosphorylation of β-catenin on tyrosine 654 reduces its affinity for E-cadherin (Roura et al., 1999; Bonvini et al., 2001; Piedra et al., 2001; van Veelen et al., 2011). However, a β-catenin654E mutant that mimics constitutive phosphorylation at this residue can still mediate cell adhesion in culture (Tominaga et al., 2008; Shomori et al., 2009) and form epithelial structures in the mouse embryo (van Veelen et al., 2011). Therefore, the role of β-catenin phosphorylation at this residue in junctional assembly and dynamics is not known.
Abl is a conserved nonreceptor tyrosine kinase that is necessary for axon guidance and epithelial remodeling during development (Gertler et al., 1989; Koleske et al., 1998; Wills et al., 1999; Baum et al., 2001; Grevengoed et al., 2001, 2003; Fox and Peifer, 2007), and the constitutively active Bcr-Abl fusion causes chronic myeloid leukemia (Sawyers, 1999). Mouse embryos lacking the two Abl family kinases, Abl and Arg, are defective for neural tube closure (Koleske et al., 1998), and embryos mutant for the single Drosophila abl homolog have defects in epithelial morphogenesis in the embryo and eye (Grevengoed et al., 2001, 2003; Fox and Peifer, 2007; Xiong and Rebay, 2011). Abl family kinases have a large number of substrates that could influence cell shape and behavior, including regulators of Rho family GTPase signaling, as well as several proteins that directly regulate actin organization, such as Ena/VASP, cortactin, N-WASp and WAVE2/3 (Lanier and Gertler, 2000; Bradley and Koleske, 2009; Colicelli, 2010). The loss of Abl family kinases can influence cell migration and adhesion through the misregulation of Rho GTPase signaling and increased actomyosin contractility (Peacock et al., 2007; Zandy et al., 2007), or through the aberrant apical localization of Ena and F-actin (Grevengoed et al., 2003; Fox and Peifer, 2007). However, while it is clear that Abl has many important roles, in most cases it is not known which substrates are important for its different functions.
In the Drosophila embryo, cell rearrangements cause the germband epithelium to more than double in length from head to tail to form an elongated body axis (Zallen and Blankenship, 2008; Lye and Sanson, 2011). This process is driven by planar polarized actomyosin contractility (Bertet et al., 2004; Zallen and Wieschaus, 2004; Blankenship et al., 2006; Rauzi et al., 2008; Fernandez-Gonzalez et al., 2009). In addition, cell-cell junctions must be dynamically remodeled to translate spatially regulated forces into a permanent change in tissue organization. Adherens junctions are downregulated at cell contacts that display contractile activity during intercalation (Blankenship et al., 2006), in part through Rho-kinase-dependent exclusion of the Baz/Par-3 junctional stabilizing protein (Simoes et al., 2010) and localized clathrin-mediated endocytosis (Levayer et al., 2011). However, the mechanisms that control adherens junction dynamics to coordinate junctional disassembly with actomyosin contraction are not well understood.
Here we show that the Abl tyrosine kinase is enriched in specific planar junctions during axis elongation and is necessary for planar polarized β-catenin localization and dynamics. Embryos mutant for abl display reduced axis elongation and these defects are associated with a disruption of multicellular rosette formation. Abl promotes the phosphorylation of Drosophila β-catenin on tyrosine 667, which corresponds to tyrosine 654 in the mammalian protein, and this residue is necessary for rosette formation in vivo. Notably, a phosphomimetic β-catenin667E mutation is sufficient to enhance β-catenin dynamics and rescue axis elongation in abl-depleted embryos. These results demonstrate an essential role for β-catenin tyrosine 667 phosphorylation downstream of the Abl tyrosine kinase in polarized junctional remodeling during Drosophila axis elongation.
Results
Tyrosine kinase signaling is planar polarized in intercalating cells
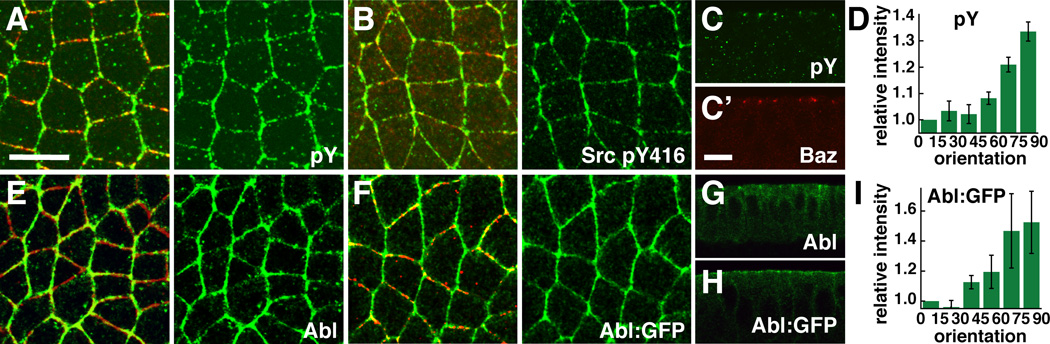
To identify the mechanisms that control polarized junctional remodeling during axis elongation, we looked for spatially regulated components of adherens junctions. As the planar polarized localization of junctional proteins is subtle (Blankenship et al., 2006), we speculated that posttranslational modifications of junctional proteins might reveal a more pronounced asymmetry. Consistent with this idea, we found that an antibody to phosphotyrosine (PY) detects increased tyrosine phosphorylation at junctions between anterior and posterior cells (AP junctions) in intercalating cells (Figure 1A,C,D). AP junctions are selectively disassembled during intercalation, suggesting that localized tyrosine kinase signaling could contribute to spatially regulated junctional remodeling during axis elongation.
Figure 1. Tyrosine kinase signaling is planar polarized in intercalating cells.
(A–D) Antibodies to phosphotyrosine (pY) (green, A and C) and phospho-Src (pY416) (green, B) detect increased staining at AP junctions, in addition to cytoplasmic vesicles. (D) Quantitation reveals an enrichment of pY at edges oriented at 90° relative to the AP axis (AP edges) (n=9 embryos, 97–224 edges/embryo). (E–I) Antibodies to Abl (green, E and G) and GFP (green, F and H) in stage 7 wild-type (WT) embryos. Anterior left, dorsal up. Cross sections shown in C, G, and H. Cells were costained with Baz (red in A,C,E,F) or α-catenin (red in B). (I) Quantitation of reveals an enrichment of Abl:GFP at AP edges (n=8 embryos, 88–247 edges/embryo). Each bar represents the mean intensity of edges in a 15° angular range. Error bars indicate the s.e.m. between embryos. Values were normalized to the mean intensity of edges parallel to the AP axis (0–15°). Bars, 10 µm. See also Figure S1.
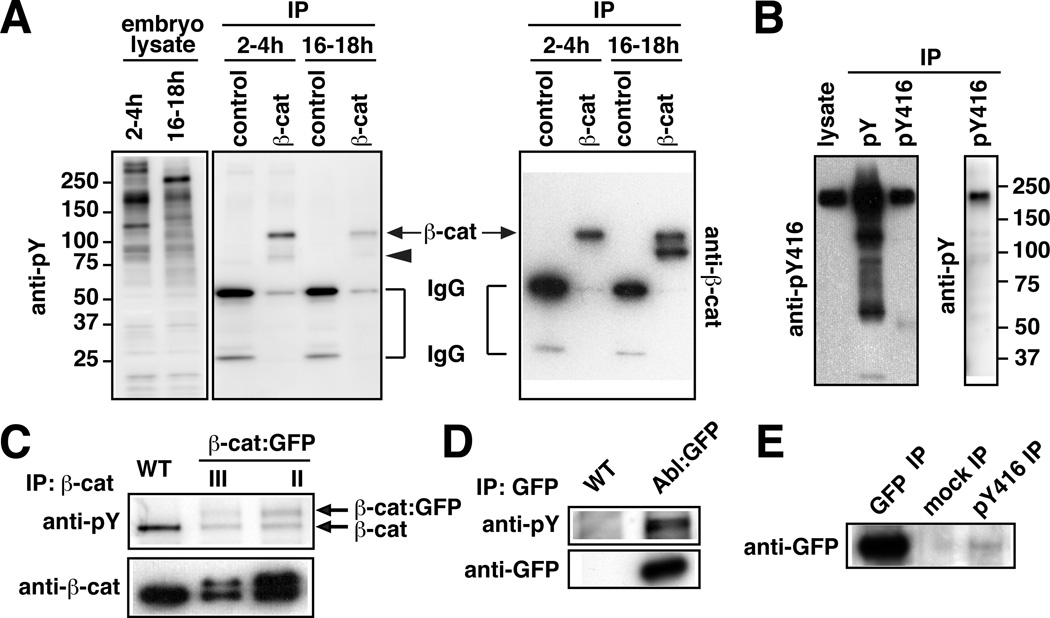
To identify the components of adherens junctions that are phosphorylated on tyrosine in intercalating cells, we immunoprecipitated junctional complexes with an anti-β-catenin antibody from lysates of staged embryos either during elongation (2–4 hr after egg laying) or after elongation is completed (16–18 hr after egg laying). The immunoprecipitated proteins contained a strong tyrosine-phosphorylated band at 110 kD and a weaker band at 80 kD (Figure 2A). The 110 kD band is similar in size to α-catenin and β-catenin. To determine whether this band represents αα-catenin or β-catenin, we immunoprecipitated β-catenin from lysates of embryos expressing β-catenin:GFP. Blotting with antibodies to phosphotyrosine detected a second band at 135 kD, the predicted molecular weight of β-catenin:GFP (Figure 2C). These results demonstrate that β-catenin is phosphorylated on tyrosine in the early Drosophila embryo. The 80 kD band was not identified, but is similar in molecular weight to the neural form of β-catenin and the C-terminal domain of E-cadherin (Loureiro and Peifer, 1998; Oda and Tsukita, 1999).
Figure 2. Abl and β-catenin are tyrosine phosphorylated in elongating embryos.
(A) Lysates from 2–4h and 16–18h WT embryos were incubated with anti-β-catenin (β-cat) or control mouse antibody. Eluted immunocomplexes and lysates were analyzed by anti-pY or anti-β-cat immunoblot, 110 kD (arrows) and 80 kD (arrowhead) bands are indicated. (B) Lysates from 2–4h embryos were incubated with anti-pY or anti-pY416 antibody. Eluted immunocomplexes and lysates were analyzed by anti-PY or pY416 immunoblot. (C) Lysates from 2–4h WT or embryos with β-cat:GFP inserted on chromosome III (lane 2) or II (lane 3) were incubated with anti-β-cat antibody. Eluted immunocomplexes were analyzed as in A. (D) Lysates from 2–4h WT or Abl:GFP embryos were incubated with anti-GFP antibody. Equivalent amounts of eluted immunocomplexes were analyzed by anti-pY or anti-GFP immunoblot. (E) Lysate from 2–4h Abl:GFP embryos was incubated with anti-GFP, anti-pY416, or control rabbit antibody. Equivalent amounts of eluted immunocomplexes were analyzed by anti-GFP immunoblot.
The Abl tyrosine kinase is asymmetrically localized in intercalating cells
In a parallel approach to identify asymmetrically localized tyrosine-phosphorylated proteins, we screened 34 phosphospecific antibodies generated to peptides from mammalian proteins. This screen led to the identification of three antibodies that detected an increased signal at AP junctions (Figure 1B; Figure S1). One antibody, generated to the phosphorylated human Src protein (anti-pY416), labeled a 180 kD band in western blots of lysates of 2–4 hr embryos (Figure 2B). However, the molecular weight of this band was greater than the predicted size of the two Drosophila Src proteins, Src42A and Src64B (~60 kD), suggesting that this antibody detects a different protein. We noticed that the sequence flanking Y416 in human Src has 75% similarity to Drosophila Abl, a nonreceptor tyrosine kinase with a molecular weight of 180 kD. In fact, we found that a functional Abl:GFP fusion protein (Fox and Peifer, 2007) was phosphorylated on tyrosine in the early embryo (Figure 2D) and was immunoprecipitated by the anti-pY416 antibody (Figure 2E). These studies indicate that this antibody cross-reacts with Drosophila Abl, although this antibody may also detect other proteins. Abl:GFP and the endogenous Abl protein were asymmetrically enriched at AP junctions in a planar polarized pattern (Figure 1E–I). Together, these results suggest that Abl is one of the proteins that contributes to asymmetric tyrosine kinase signaling in intercalating cells.
Abl is necessary for rosette formation and axis elongation
To ask if Abl is required for polarized cell behavior during axis elongation, we analyzed embryos that were maternally mutant for abl4, an early truncation mutation and a predicted null allele (Fox and Peifer, 2007), referred to here as abl mutants. Zygotic Abl expression was not detectable until later stages, suggesting that early embryonic development relies mainly on maternal Abl activity (Bennett and Hoffmann, 1992; Fox and Peifer, 2007; and data not shown). abl mutants display defects in cellularization, but these defects are cold-sensitive and are rarely present at 25°C (Grevengoed et al., 2003). We found that abl mutant embryos raised at 25°C displayed a significant reduction in axis elongation (Figure 3A,E,G). These defects were recapitulated by abl shRNA expression (Figure 7H) and were rescued by Abl:GFP, indicating that these defects are due to the loss of Abl (50% of abl mutant embryos completed elongation, n= 113, compared to 91% of abl mutants expressing Abl:GFP, n= 221).
Figure 3. abl mutants are defective for axis elongation and rosette formation.
(A–H) Cell behavior in time-lapse movies of WT and abl mutant embryos expressing β-catenin:GFP (n=5 WT and 8 abl movies). (A) Germband elongation was reduced in abl mutants (p=0.001). Germband length was normalized to the length at the onset of elongation (t=0), defined as the time in early stage 7 when the derivative of the elongation curve intersects zero. (B,C) The frequency of neighbor exchange in abl mutants was similar to WT (p=0.12), but the frequency of rosette formation was significantly reduced (p=0.0013). Only cells that lost a neighbor through neighbor exchange or rosette formation were counted as having participated in the event. (D) The average lifetime of vertices where 4 or more cells meet was 6.86+/−0.25 min (mean+/−s.e.m.) in WT (n=324 vertices in 3 embryos) and 4.21+/−0.27 min in abl mutants (n=238 vertices in 8 embryos, p<0.0001) (black circles/lines show the mean/standard deviation). (E,G) Stills from bright field movies of WT and abl embryos. Arrowheads indicate the posterior end of the germband, t=0 was the onset of dorsal pole cell displacement. (F,H) Stills from confocal movies of WT and abl embryos expressing β-catenin:GFP, time in min. (F) A multicellular cable (black line) contracts to form a rosette in WT. (H) Aligned cells in an abl mutant did not noticeably contract during the period of observation. Anterior left, dorsal up. See also Movies S1,S2. Bar, 5 µm.
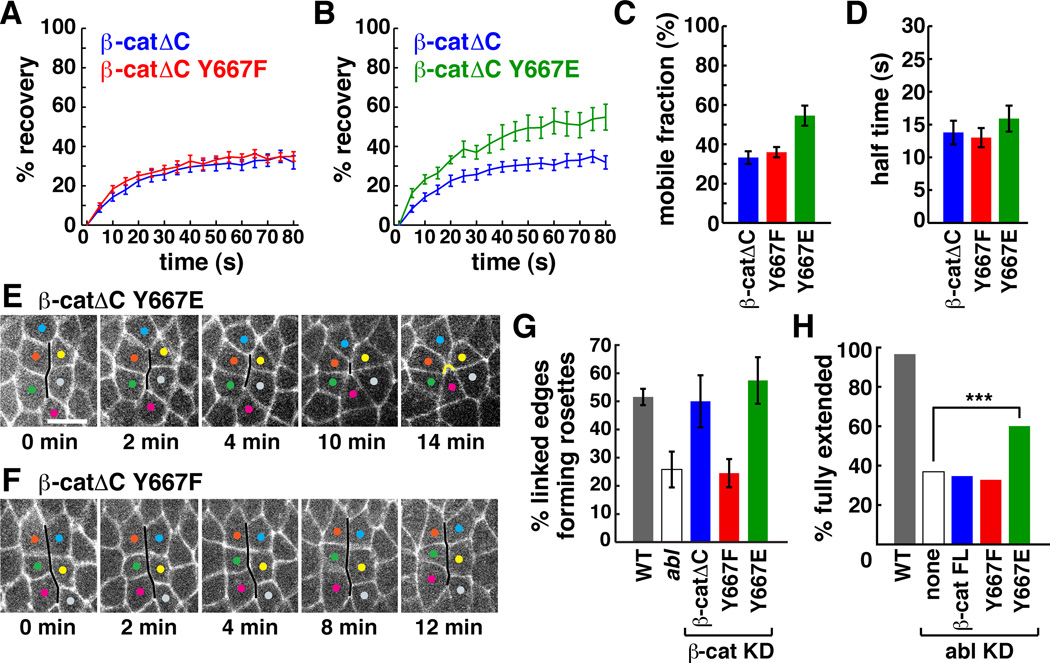
Figure 7. Phosphorylation of β-catenin on tyrosine 667 regulates β-catenin dynamics and rosette formation.
(A,B) The percentage of pre-bleach fluorescence after bleaching AP junctions in embryos expressing Venus-tagged β-catΔC, β-catΔC667E, or β-catΔC667F. (C) β-catΔC667E had increased mobility compared to β-catΔC (p=0.0009) and β-catΔC667F (p=0.0034). There was no difference in the mobility of β-catΔC and β-catΔC667F (p=0.5132) (n=21 β-catΔC, 18 β-catΔC667E, 23 β-catΔC667F). (D) The t½ was similar for all transgenes. (E, F) Stills from movies of β-catenin KD embryos expressing HA:β-catΔC667E or HA:β-catΔC667F. Cells labeled with Spider:GFP. A multicellular cable (black line) contracts to form a rosette in an embryo expressing β-catΔC667E (E) (new contacts, yellow line). Little contraction occurs in an embryo expressing β-catΔC667F (F). (G) Percentage of linked AP edges that joined a rosette of 5 or more cells in WT, abl, and β-cat KD embryos expressing the indicated β-catΔC transgenes. Fewer linked AP edges formed rosettes in abl (p=0.0421 vs. WT) and β-catΔC667F (p=0.0233 vs. β-catΔC and p=0.0149 vs. β-catΔC667E) (38–168 edges scored in 3–8 embryos/genotype). Error bars indicate the s.e.m. between embryos. (H) Percentage of fully extended embryos. 97% of Gal4 control embryos completed elongation (n=147 embryos) vs. 37% of abl KD embryos (n=527) (p<0.0001). 60% of abl KD embryos expressing full-length HA:β-catenin667E completed elongation (n=469) (p<0.0001 compared to abl KD), vs. 35% of abl KD embryos expressing full-length HA:β-catenin (n=239) (p=0.57) and 33% of abl KD embryos expressing full-length HA:β-catenin667F (n=511) (p=0.17). Bar, 5 µm. See also Figures S4,5.
Axis elongation in abl mutants was defective during the initial fast phase of axis elongation that is driven predominantly by cell intercalation (Figure 3A). Although a previous study did not observe intercalation defects in abl mutants (Fox and Peifer, 2007), we revisited this question using computational methods to quantify the two cell rearrangements that contribute to elongation, neighbor exchange and rosette formation (Experimental Procedures). We found that cell movements were correctly oriented in abl mutants and the frequency of neighbor exchange was similar to wild type (Figure 3B). However, abl mutants displayed a significant reduction in rosette formation (Figure 3C,F,H; Movies S1,S2). Consistent with this defect, vertices where four or more cells meet were shorter-lived in abl mutants compared to wild type (Figure 3D). These results suggest that the axis elongation defects in abl mutants are caused by a specific disruption of multicellular rosette formation.
abl mutants have normal actomyosin contractility
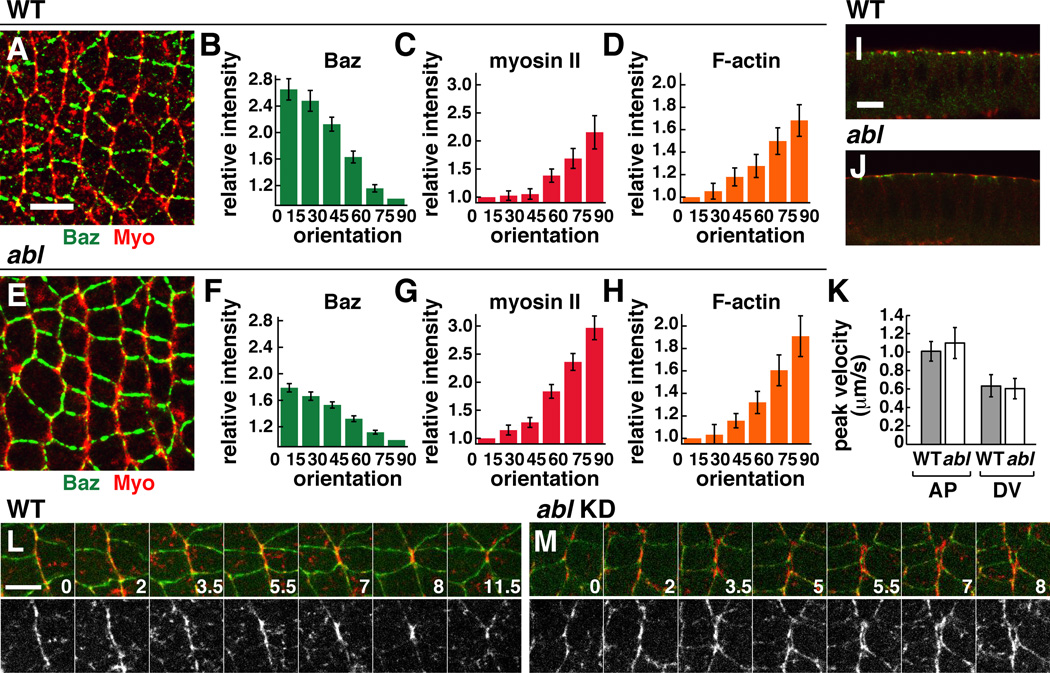
Rosette formation is driven by the contraction of oriented actomyosin cables that are mechanically integrated across multiple pairs of cells (Blankenship et al., 2006; Fernandez-Gonzalez et al., 2009). To ask if the rosette formation defects in abl mutants are due to a disruption of actomyosin contractility, we analyzed the distribution of myosin II and filamentous actin (F-actin). Apical myosin localization occurred normally in abl mutants (Figure 4I,J), consistent with previous results in mesodermal cells (Fox and Peifer, 2007). Myosin II and Factin were also enriched at AP junctions and were correctly organized into cables as in wild type (Figure 4A–H and data not shown). These results suggest that the axis elongation defects in abl mutants are not due to a failure to establish planar polarized actin and myosin localization.
Figure 4. Abl is not required for planar polarized actomyosin localization and contractility.
(A–J) Localization of myosin II (red) and Baz/Par-3 (green) in WT (A,I) and abl mutant (E,J) embryos at stage 7. Quantitation of planar polarity in WT (B–D) and abl (F–H). Values were normalized to the mean intensity of edges oriented at 0–15° (for myosin II and F-actin) or at 75–90° (for Baz), where 0° is parallel to the AP axis. n=7–12 embryos, 77–247 edges/embryo. Planar polarity is significantly decreased in abl mutants for Baz (p=0.00017), and is only slightly increased for myosin (p=0.036) and F-actin (p=0.33). (K) Peak retraction velocities after ablation in WT (n=10 AP, 6 DV ablations) and abl (n=6 AP, 7 DV ablations). (L,M) Stills from time-lapse movies in WT and abl knockdown (KD) embryos. E-cadherin:GFP (green), Myo:mCherry (red). Time in min. Anterior left, dorsal up. Cross sections shown in I,J. Bars, 10 µm in A–J, 5 µm in L,M. See also Figure S2 and Movies S3,S4.
To test whether actomyosin networks are functional in abl mutants, we analyzed the cellular response to laser ablation, a property that depends on actomyosin contractility (Rauzi et al., 2008; Fernandez-Gonzalez et al., 2009). The wild-type response to laser ablation is planar polarized, with faster peak retraction velocities produced by ablation of linked AP edges associated with multicellular cables, and slower peak retraction velocities after ablation of DV edges that have lower levels of myosin (Figure 4K) (Rauzi et al., 2008; Fernandez-Gonzalez et al., 2009). The extent and polarization of the response to ablation occurred normally in abl mutants (Figure 4K), indicating that actomyosin networks in abl mutants generate mechanical forces that are wild-type in intensity and orientation. However, when we followed the behavior of actomyosin cables in time-lapse movies of abl shRNA-expressing embryos, we found that they behaved differently from wild type. Cortical myosin structures were closely apposed at AP junctions in wild-type embryos (Figure 4L, Movie S3). By contrast, gaps between cortical myosin in neighboring cells were often observed in abl mutants, accompanied by disruption of E-cadherin (Figures 4M, S2, Movie S4). These apparent breaks between cells that are normally tightly adherent raise the possibility that Abl may regulate cell adhesion in intercalating cells.
Abl regulates planar polarized β-catenin localization and dynamics
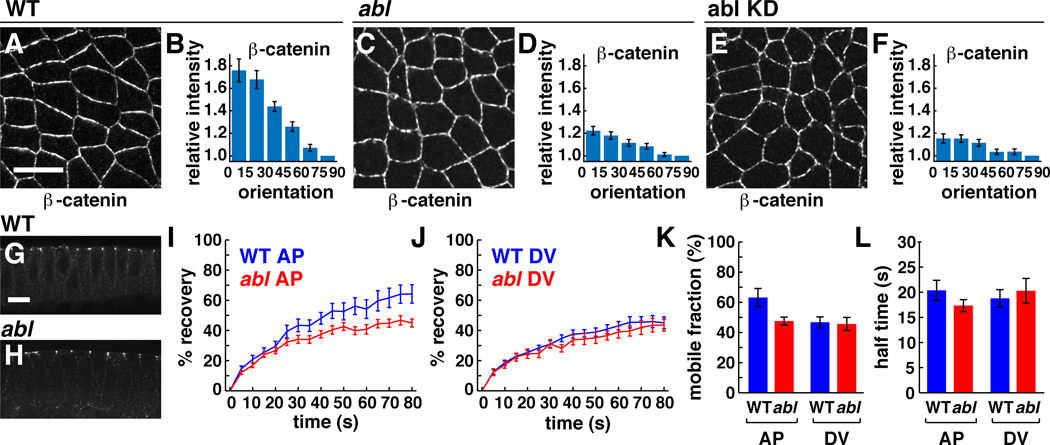
Adherens junctions are planar polarized in intercalating cells, with higher levels of E-cadherin and β-catenin at junctions between dorsal and ventral cells (DV junctions) (Blankenship et al., 2006). To ask if Abl is required for junctional planar polarity, we analyzed the localization of junctional proteins in abl mutants. We found that the apicolateral localization of β-catenin occurred normally (Figure 5G,H), but the planar polarized enrichment of β-catenin at DV junctions was significantly reduced in abl mutants (Figure 5A–D) and in embryos expressing abl shRNA (Figure 5E,F). A similar disruption was observed in the planar polarized localization of E-cadherin (Figure S3). Baz/Par-3 was still planar polarized in abl mutants, although to a lesser extent than in wild type (Figure 4E,F). These results demonstrate that Abl is required for the planar polarized localization of β-catenin and E-cadherin.
Figure 5. Abl is required for the planar polarized localization and dynamics of β-catenin.
(A–F) β-catenin localization in WT (A, B), abl mutant (C, D), and abl KD embryos (E,F). Values were normalized to the mean intensity of edges perpendicular (75°–90°) to the AP axis. β-catenin planar polarity was significantly reduced in abl mutant (p=0.0002) and abl KD embryos (p=0.000017) (n=7 WT, 12 abl mutant, 3 abl KD embryos, 76–169 edges/embryo). Anterior left, dorsal up. Cross sections shown in G,H. (I,J) The percentage of pre-bleach fluorescence observed after bleaching β-catenin:GFP at AP or DV junctions. (K) In WT, β-catenin displayed a higher mobile fraction at AP junctions (63+/−6%, n=19 edges) (mean+/−s.e.m.) than at DV junctions (47+/−4%, n=25) (p=0.023). This difference was abolished in abl mutants (48+/−3% at AP junctions, n=25, 46+/−4% at DV junctions, n=20) (p=0.317). (L) The time to recover half maximal fluorescence (t½) was similar for all edges. Bars, 10 µm. See also Figure S3.
To ask if the defects in junctional localization in abl mutants are due to a disruption of junctional dynamics, we analyzed β-catenin turnover using fluorescence recovery after photobleaching (FRAP). A 1.0 × 1.0 µm region was bleached at the cell cortex in embryos expressing a functional β-catenin:GFP fusion (McCartney et al., 2001) and the recovery of fluorescence was analyzed over time. In wild-type embryos, β-catenin displayed significantly increased mobility at AP junctions (63%) compared to DV junctions (47%, p=0.023) (Figure 5I–K). The planar polarized increase in β-catenin turnover was abolished in abl mutants, and all junctions displayed the lower turnover characteristic of DV junctions in wild type (Figure 5I–K). By contrast, the time required to recover the half maximal fluorescence (t½) was unaffected in abl mutants (Figure 5L). These results demonstrate that Abl is required for the planar polarized dynamics of β-catenin in intercalating cells.
Abl promotes β-catenin phosphorylation on tyrosine 667
Next we asked if Abl regulates β-catenin dynamics directly by phosphorylating β-catenin, or indirectly through effects on the actin cytoskeleton or other effector pathways. Abl can phosphorylate mammalian β-catenin in vitro, and this phosphorylation is associated with decreased binding to N-cadherin and Axin and increased nuclear localization and transcriptional activity (Coluccia et al., 2007; Rhee et al., 2007). We found that expression of Abl induced high levels of β-catenin tyrosine phosphorylation in cultured Drosophila S2R+ cells (Figure 6B). To identify the sites that are phosphorylated on β-catenin, we expressed β-catenin mutants that substituted unphosphorylatable amino acids at one or more of its conserved tyrosines (Figure 6A). Drosophila β-catenin contains 17 tyrosines, of which 11 are conserved in mammals. A β–catenin variant lacking the C-terminal 163 amino acids (β-catΔC) was still tyrosine phosphorylated when coexpressed with Abl, although at lower levels than the full-length protein (Figure 6B). Since embryos expressing exogenous or endogenous β-catΔC protein are wild-type for junctional planar polarity and axis elongation (Figure S4 and data not shown), we focused on conserved tyrosines in the remainder of the protein. The tyrosine phosphorylation of β-catΔC was similar in mutants that have mutations in tyrosines 40 and 150, tyrosine 573, or tyrosines 262, 314, 339, 341, and 497 (Figure 6B). However, β-catΔC phosphorylation was strongly reduced when tyrosine 667 was mutated to phenylalanine (Figure 6B,C), indicating that tyrosine 667 is the primary site on β-catenin that is regulated by Abl in this assay.
Figure 6. Abl promotes the phosphorylation of β-catenin on tyrosine 667.
(A) Schematic of β-catenin transgenes. β-catΔC is a deletion of aa 681–843, equivalent to the armXM19 allele. β-catΔCS10/150A and β-catΔCS12 have an additional deletion of 35–87 or 549–606 aa, respectively. All tyrosine (Y) residues in β-catΔC and Y to phenylalanine (F) or alanine (A) mutations are indicated. Asterisks indicate conserved tyrosines. (B) S2R+ cells were transfected with Venus-tagged β-catenin plasmids with or without Abl:HA. Asterisk indicates β-catΔC667F. (C) S2R+ cells were transfected with Venus-tagged β-catΔC, β-catΔC667F, or β-catΔC667E plasmids with or without Abl:HA. Cell lysates were incubated with anti-GFP antibody to detect Venus, and eluted immunocomplexes were analyzed by anti-PY or anti-GFP immunoblot. Upper panel shows protein levels. (D,E) Lysates from 3–6h embryos expressing HA-tagged β-catΔC, β-catΔC667F, or β-catΔC667E transgenes at 20°C were immunoprecipitated with anti-E-cadherin (D) or anti-HA (E) antibody and eluted immunocomplexes were analyzed by anti-β-catenin, anti-E-cadherin, or anti-α-catenin immunoblot. See also Figure S4.
Phosphorylation of β-catenin on tyrosine 667 increases β-catenin turnover
Since Abl is necessary for increased β-catenin turnover at AP junctions, we tested whether phosphorylation of β-catenin on tyrosine 667 is sufficient to increase β-catenin turnover. We performed FRAP experiments to analyze the dynamics of Venus-tagged β-catΔC proteins that contain a Y667F mutation that prevents tyrosine phosphorylation at this site, or a Y667E mutation that introduces a negatively charged amino acid that resembles tyrosine phosphorylation. We found that β-catΔC667E displayed significantly increased mobility at AP junctions compared to β-catΔC and β-catΔC667F (Figure 7A–D). β-catΔC667F dynamics were similar to β-catΔC, perhaps because the endogenous β-catenin recruits proteins that mediate junctional turnover. These results indicate that the phosphorylation of a single tyrosine 667 in β-catenin is sufficient to increase β-catenin dynamics in intercalating cells.
Tyrosine 667 in Drosophila β-catenin corresponds to tyrosine 654 in the mammalian protein. Phosphorylation of tyrosine 654 in mammalian β-catenin has been shown to reduce the association between β-catenin and E-cadherin (Roura et al., 1999; Bonvini et al., 2001; Piedra et al., 2001; van Veelen et al., 2011), suggesting a mechanism by which tyrosine phosphorylation could lead to β-catenin dissociation from the cortex. To investigate this possibility, we immunoprecipitated junctional complexes from embryos that express phosphodefective or phosphomimetic β-catΔC transgenes (Figure 6D,E). Immunoprecipitation of E-cadherin pulled down similar levels of all three β-catΔC transgenes (Figure 6D), and immunoprecipitation of the different β-catΔC transgenes pulled down similar levels of E-cadherin and α-catenin (Figure 6E). These results indicate that phosphorylation of β-catenin on tyrosine 667 does not prevent its association with E-cadherin and α-catenin in the early Drosophila embryo.
In an alternative model, phosphorylation of mammalian β-catenin on tyrosines 142, 489 or 654 have been shown to promote β-catenin nuclear localization and the activation of Wnt target genes (Piedra et al., 2001; Brembeck et al., 2004; Rhee et al., 2007; van Veelen et al., 2011). To test whether phosphorylation of Drosophila β-catenin activates its transcriptional activity in the Wingless signaling pathway, we analyzed the striped expression of Engrailed, a gene whose pattern of expression relies on Wingless activity. We observed no change in Engrailed expression in embryos that were defective for abl or embryos that expressed β-catenin mutant transgenes (Figure S5), consistent with previous findings that abl mutants do not have obvious segmentation defects in the embryonic cuticle (Grevengoed et al., 2001). Together, these results indicate that phosphorylation of Drosophila β-catenin on tyrosine 667 does not affect junctional complex formation or Wingless signaling.
β-catenin tyrosine phosphorylation is necessary for rosette formation and rescues axis elongation in abl mutants
To ask if the phosphorylation of β-catenin on tyrosine 667 is important for cell behavior during axis elongation, we analyzed the activity of β-catΔC667F and β-catΔC667E transgenes in embryos in which endogenous β-catenin was depleted by injection of dsRNA to the C-terminal domain. In wild-type embryos, 52% of linked AP edges associated with multicellular cables contracted to form rosettes, and this number was significantly reduced in abl mutants (Figure 7H). Expression of β-catΔC or β-catΔC667E transgenes rescued cell adhesion and restored wild-type levels of rosette formation in β-catenin knockdown embryos (Figure 7E,G). By contrast, the β-catΔC667F transgene rescued cell adhesion but did not support rosette formation, recapitulating the defects of abl mutants (Figure 7F,G). These results demonstrate that tyrosine 667 is necessary for the role of β-catenin in rosette formation.
These results are consistent with a model in which phosphorylation of β-catenin mediates the effect of Abl on polarized cell behavior. This model predicts that expression of phosphomimetic β-catenin should rescue the axis elongation defects in abl mutants. To investigate this possibility, we expressed β-catenin mutant transgenes in embryos in which Abl was depleted by expression of an abl shRNA. We found that expression of the phosphomimetic β-catenin667E transgene significantly rescued axis elongation in abl knockdown embryos, while expression of β-catenin and β-catenin667F had no effect (Figure 7H). The rescue was incomplete, perhaps due to differences in the level of transgene expression relative to the endogenous protein, structural differences between glutamic acid and phosphotyrosine, or contributions of other Abl effector pathways. These results demonstrate that a mutation that mimics constitutive phosphorylation of β-catenin on tyrosine 667 can circumvent the requirement for Abl in axis elongation.
Discussion
Epithelial morphogenesis during development requires the dynamic assembly and disassembly of adherens junctions. Here we show that the Abl tyrosine kinase and the phosphorylation state of a single tyrosine in β-catenin regulate junctional dynamics and multicellular reorganization during Drosophila axis elongation. The Abl tyrosine kinase is asymmetrically concentrated at junctions that display increased contractile activity and Abl is necessary for planar polarized β-catenin localization and dynamics. The axis elongation defects in abl mutants are associated with a specific disruption of multicellular rosette formation, providing strong evidence that rosette behaviors are necessary for elongation. Abl can promote the phosphorylation of β-catenin on tyrosine 667 in culture, and an unphosphorylatable β-catenin667F variant is functional for adhesion but fails to mediate rosette formation in vivo. Conversely, a phosphomimetic β-catenin667E variant displays increased turnover at adherens junctions and rescues the axis elongation defects of abl-deficient embryos. These results support a model in which the Abl tyrosine kinase regulates polarized cell rearrangements during Drosophila axis elongation by directing the tyrosine phosphorylation and dynamics of β-catenin.
Adherens junctions are asymmetrically localized in intercalating cells (Blankenship et al., 2006). Here we show that the planar polarized localization and dynamics of β-catenin require the Abl tyrosine kinase. Previous studies have shown that junctional planar polarity requires the exclusion of the junctional stabilizing protein Baz/Par-3 from AP edges by Rho-kinase (Simoes et al., 2010), as well as myosin-dependent endocytosis of E-cadherin by the clathrin machinery (Levayer et al., 2011). However, β-catenin planar polarity was severely disrupted despite the continued presence of asymmetrically localized myosin II and Par-3 in abl mutants, demonstrating that Abl is also required for adherens junction organization. Abl could provide the spatial cue that targets adherens junctions at AP edges for endocytosis, or Abl could regulate junctional dynamics independently of these known mechanisms. Abl is required for planar polarity in the Drosophila eye and wing, and can phosphorylate Dishevelled in vitro on a residue that is required for Dishevelled localization and activity in vivo (Singh et al., 2010). Together with our work, these results demonstrate that Abl plays a conserved role in regulating planar cell polarity. Abl is activated by the EGF and PDGF growth factor receptors and Src, the Eph and Robo receptors, cadherins and integrins, and binding to adaptor proteins (Colicelli, 2010). These upstream regulators of Abl activity are candidates for detecting the global spatial cues that initiate planar polarized junctional organization during epithelial morphogenesis.
These results demonstrate that the phosphorylation of β-catenin on tyrosine 667 enhances β-catenin turnover at the plasma membrane and is necessary for multicellular rosette formation and axis elongation in Drosophila. It will be interesting to determine whether β-catenin tyrosine phosphorylation is a general feature of dynamic junctions during morphogenesis. Abl-dependent β-catenin phosphorylation could facilitate junctional disassembly by reducing the amount or clustering of junctional proteins at the plasma membrane, or by reducing their association with junctional complexes on neighboring cells or with the actin cytoskeleton. Alternatively, tyrosine phosphorylation of β-catenin could promote the endocytosis of junctional complexes, as β-catenin tyrosine phosphorylation promotes N-cadherin endocytosis during synaptic remodeling in neurons (Tai et al., 2007). In addition, the increase in junctional dynamics in response to Abl activity could generate a pool of β-catenin that is recycled to sites where strong adhesion is required or where new adhesions are assembled to maintain epithelial continuity. While phosphorylation of mammalian β-catenin on tyrosine 654 decreases its affinity for E-cadherin or N-cadherin (Roura et al., 1999; Rhee et al., 2002, 2007), other studies find that mammalian β-catenin phosphorylated on tyrosine 654 can still form functional adhesion complexes (Tominaga et al., 2008; Shomori et al., 2009; van Veelen et al., 2011). We do not detect a change in the ability of phosphomimetic β-catenin667E to coimmunoprecipitate with E-cadherin or α-catenin, localize to adherens junctions, or mediate adhesion. Tyrosine phosphorylation could also affect the interaction of β-catenin with other proteins that associate with its C-terminal Arm repeats, such as the c-erbB-2 EGF receptor (Hoschuetzky et al., 1994; Shibata et al., 1996; Bonvini et al., 2001) or the Sec10 exocyst complex subunit that promotes E-cadherin recycling (Langevin et al., 2005).
The specific role of Abl in rosette formation suggests a requirement for β-catenin tyrosine phosphorylation in cells where mechanical tension is highest (Fernandez-Gonzalez et al., 2009). Cell intercalation requires the active recycling of adhesive complexes at the plasma membrane (Shaye et al., 2008; Levayer et al., 2011, and this work), but at the same time exposes cells to increased mechanical force, which has been shown to induce junctional assembly (Liu et al., 2010). An interesting possibility is that Abl-dependent β-catenin dynamics could simultaneously facilitate regulated junctional disassembly and promote persistent adhesion to allow cells to withstand the polarized forces that drive cell rearrangement. Mechanical forces lead to an increase in the size and tyrosine phosphorylation of cell-matrix adhesions (Geiger and Bershadsky, 2001) and influence the localization and dynamics of adherens junction proteins (Gomez et al., 2011), and ectopic forces can enhance β-catenin tyrosine phosphorylation (Whitehead et al., 2008). The tyrosine phosphorylation of β-catenin downstream of Abl could provide a mechanism that transduces mechanical signals to stabilize dynamic adhesions at sites of increased mechanical activity.
Experimental procedures
Flies and genetics
Oregon-R was the wild-type control unless otherwise specified. Embryos were raised and scored at 25°C. Germline clones were generated with the FLP-DFS system (Chou and Perrimon, 1996). Larvae of the following genotypes were heat-shocked and crossed to abl4/+ males:
hs-FLP38; abl4 FRT2A/ovoD1 FRT2A
hs-FLP38; β-cat:GFP/+; abl4 FRT2A/ovoD1 FRT2A
hs-FLP38; Abl:GFP/+; abl4 FRT2A/ovoD1 FRT2A
All progeny were maternally mutant and half are predicted to be zygotically mutant. See Supplemental Information for details about transgene construction.
Immunohistochemistry
Antibodies were mouse Arm/β-catenin (1:25, Developmental Studies Hybridoma Bank, DSHB), guinea pig Baz/Par-3 (1:1000) (Blankenship et al., 2006), rabbit Abl (1:100; gift of D. Van Vactor), rat HA (1:500, Roche), rabbit myosin II heavy chain (Zip) (1:1250, gift of C. Field), mouse PY (1:250, PY20 Santa Cruz), rabbit GFP (1:100, Torrey Pines), rabbit phospho-Src Family (pY416) (1:100, Cell Signaling), and rat DE-cadherin (1:25, DSHB). For antibodies to Abl, GFP, and DE-cadherin, embryos were fixed 1 hr in 4% PFA (Electron Microscopy Sciences) in PBS/heptane and manually devitellinized. For other antibodies, embryos were boiled 10 s in 0.03% Triton X-100/0.4% NaCl, cooled on ice, and devitellinized in heptane/methanol. Secondary antibodies conjugated to Alexa-488, Alexa-568, or Alexa-647 and rhodamine-conjugated phalloidin (Molecular Probes) were used at 1:500. Embryos were mounted in Prolong Gold (Invitrogen) and imaged on a Zeiss LSM510 META confocal microscope with a PlanNeo 40×/1.3NA objective. 1.2 µm Z slices were acquired at 0.6 µm steps. Maximum intensity projections of 1.8–3 µm in the apical junctional domain were analyzed for planar polarity as described (Simoes et al., 2010) by measuring the mean pixel intensity for all edges in a 50 µm × 50 µm field and calculating average values for edges in 15° angular bins using SIESTA, a tool for Scientific ImagE SegmenTation and Analysis (Fernandez-Gonzalez and Zallen, 2011).
Immunoprecipitation and cell transfection
Drosophila S2R+ cells were cultured in Schneider’s medium with 10% fetal bovine serum at 25C. Transfections were performed with CellFectin (Invitrogen) using the manufacturer’s protocol. pUASp-β-catenin:Venus plasmids were cotransfected with pAc5.1/V5-HisB-GAL4 with or without pUASp-Abl:HA. Cells were lysed 24 hr after transfection in lysis buffer [50 mM Tris-HCl (pH=7.5), 2 mM EDTA, 150 mM NaCl, 10% glycerol, 1% Nonidet P-40, 1 mM phenylmethylsulphonyl fluoride, 10 µg/ml leupeptin, 1 µg/ml pepstatin, 10 µg/ml aprotinin, 2 mM Sodium Orthovanadate, Phosphatase inhibitor cocktail 2 (Sigma, 1:200)]. For embryo lysates, embryos were dechorionated 2 min in bleach and lysed in lysis buffer. Lysates were centrifuged (16,000xg) 20 min at 4°C and supernatants were incubated 30 min with antibody and then with Protein G- or Protein A- Sepharose 4B (Amersham) for 3 hr at 4°C. Immunocomplexes were washed 3x in lysis buffer, eluted with SDS-sample buffer and analyzed by SDS-PAGE and immunoblot (see Supplemental Information). Western blots are representative of 3–5 experiments.
Time-lapse imaging
Embryos were dechorionated 2 min in 50% bleach, washed in water, and mounted in halocarbon oil 27 (Sigma) between a coverslip and an oxygen-permeable membrane (YSI). The ventrolateral germband was imaged with a Perkin Elmer Ultraview RS5 spinning disk confocal using Metamorph software and PlanNeo 40×/1.3NA, PlanApo 63×/1.4NA, or PlanApo 100×/1.4NA oil-immersion objectives (Zeiss). Maximum intensity projections of a 1–4 µm region containing the junctional signal were generated for analysis (Supplemental Information). For FRAP, embryos expressing full-length β-catenin:GFP, β-catΔC:Venus, β-catΔC667F:Venus, or β-catΔC667E:Venus were imaged on a Zeiss LSM510 META confocal with a PlanApo 63×/1.3NA objective at zoom 5. Three 1.1 µm-thick optical slices were acquired at 0.55 µm steps and maximum intensity projections were analyzed. Fluorescence intensity in the bleached region was measured by custom Matlab routines. For laser ablation, an N2 Micropoint laser (Photonics Instruments) tuned to 365 nm was used to ablate cell interfaces labeled with β-catenin:GFP and vertices were tracked and analyzed as described (Fernandez-Gonzalez et al., 2009).
dsRNA- and shRNA-mediated knockdown
For β-catenin knockdown, dsRNA was generated and injected into embryos as described (Simoes et al., 2010) (see Supplemental Information). Transgenic flies expressing abl shRNA from the UASp promoter (GL00234) (Ni et al., 2011) were crossed to matαtub67;15 females (gift of D. St. Johnston) to drive germline expression. The F2 progeny were analyzed and controls were the F2 progeny of wild-type males crossed to matαtub67;15 females.
Cell behavior analysis
Time-lapse movies were analyzed computationally through several image processing steps in Matlab. Errors were corrected manually with an interactive user interface. For axis elongation measurements (Figure 3A), a contiguous group of cells present in all time points was approximated as an ellipse by calculating the inertia tensor for the collection of centers of mass of the cells. The length of the group was the span of the ellipse parallel to the AP axis. Embryos were temporally registered by setting t=0 as the time in early stage 7 when the derivative of the elongation curve intersects zero. The registration of mutant embryos was adjusted (on the order of single minutes) to maximize the overlap for several geometric and topological measurements. Overlap was quantified as the least cumulative squared distance relative to the average value for all embryos.
For neighbor exchange and rosette formation (Figure 3B,C), only cells in the corrected region for at least 50 time points (12.5 min) after t=0 were analyzed. Shrinking edges were scored as participating in neighbor exchange (also known as a T1 process) if a vertex was formed by the collapse of a single edge and no other shrinking edges collapsed into the same vertex. A shrinking edge was scored as participating in a rosette if at least two shrinking edges collapsed into the same vertex and both edges disappeared before the vertex resolved. For vertex lifetime measurements (Figure 3D), all vertices where at least 4 cells meet that could be tracked for ≥10 min after vertex formation were analyzed. Vertices were considered resolved at the first time point when the two cells that shared the original edge no longer share a vertex for the following ten time points (2.5 min). To analyze rosette formation in injected embryos (Figure 7G), all linked AP edges (AP edges oriented at 75–90° relative to the AP axis that were connected to at least one other AP edge) were identified computationally at a single time point in mid-stage 7 and then analyzed manually for 20–30 min using the criteria above. For uninjected WT and abl mutant embryos in this plot, linked AP edges were tracked automatically and results for t=8–12 min were averaged.
Statistical analysis
Mean values were compared using Student's t test. For movies, the test statistic was the last value of the movie for the cell behavior analysis and t=80s for the FRAP experiments. For planar polarity, the mean values in the 0–15° bin for each image were compared for β-catenin and Ecadherin and in the 75–90° bin for myosin II and F-actin. The chi square test was used in Figure 7H.
Supplementary Material
Acknowledgments
We thank Sérgio Simoes for advice on embryo injection and time-lapse imaging, Rodrigo Fernandez-Gonzalez for advice on FRAP, laser ablation and statistical analysis, Ori Weitz for computational methods, Todd Blankenship and Nick Tolwinski for discussions, and Leah Greenspan and Justina Sanny for excellent technical assistance. We are grateful to David van Vactor for antibodies, Mike Buszczak for plasmids, and Peter Besmer, Rodrigo Fernandez-Gonzalez, Alan Hall, Karen Kasza, Emily Marcinkevicius, Sérgio Simoes, Athea Vichas and Richard Zallen for comments on the manuscript. We thank the TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947) for transgenic RNAi fly stocks. This work was supported by a W. M. Keck Foundation Distinguished Young Scholar in Medical Research Award, a Burroughs Wellcome Fund Career Award in the Biomedical Sciences, and NIH/NIGMS R01 grant GM079340 to JAZ. JAZ is an Early Career Scientist of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baum B, Perrimon N. Spatial control of the actin cytoskeleton in Drosophila epithelial cells. Nat Cell Biol. 2001;3:883–890. doi: 10.1038/ncb1001-883. [DOI] [PubMed] [Google Scholar]
- Bennett R, Hoffmann F. Increased levels of the Drosopila Abelson tyrosine kinase in nerves and muscles: subcellular localization and mutant phenotypes imply a role in cell-cell interactions. Development. 1992;116:953–966. doi: 10.1242/dev.116.4.953. [DOI] [PubMed] [Google Scholar]
- Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- Blankenship J, Backovic S, Sanny J, Weitz O, Zallen J. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11:459–470. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Bonvini P, An WG, Rosolen A, Nguyen P, Trepel J, Garcia de Herreros A, Dunach M, Neckers LM. Geldanamycin abrogates ErbB2 association with proteasome-resistant β-catenin in melanoma cells, increases β-catenin-E-cadherin association, and decreases β-catenin-sensitive transcription. Cancer Res. 2001;61:1671–1677. [PubMed] [Google Scholar]
- Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9-2 in the switch between β-catenin’s adhesive and transcriptional functions. Genes Dev. 2004;18:2225–2230. doi: 10.1101/gad.317604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley WD, Koleske AJ. Regulation of cell migration and morphogenesis by Abl-family kinases: emerging mechanisms and physiological contexts. J Cell Sci. 2009;122:3441–3454. doi: 10.1242/jcs.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TB, Perrimon N. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics. 1996;144:1673–1679. doi: 10.1093/genetics/144.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colicelli J. ABL tyrosine kinases: evolution of function, regulation, and specificity. Sci Signal. 2010;3:re6. doi: 10.1126/scisignal.3139re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coluccia AM, Vacca A, Dunach M, Mologni L, Redaelli S, Bustos VH, Benati D, Pinna LA, Gambacorti-Passerini C. Bcr-Abl stabilizes β-catenin in chronic myeloid leukemia through its tyrosine phosphorylation. EMBO J. 2007;26:1456–1466. doi: 10.1038/sj.emboj.7601485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Reynolds AB. Tyrosine phosphorylation and cadherin/catenin function. Bioessays. 1997;19:883–891. doi: 10.1002/bies.950191008. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Simoes SM, Roper JC, Eaton S, Zallen JA. Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell. 2009;17:736–743. doi: 10.1016/j.devcel.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Zallen JA. Oscillatory behaviors and hierarchical assembly of contractile structures in intercalating cells. Phys Biol. 2011;8:045005. doi: 10.1088/1478-3975/8/4/045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox DT, Peifer M. Abelson kinase (Abl) and RhoGEF2 regulate actin organization during cell constriction in Drosophila. Development. 2007;134:567–578. doi: 10.1242/dev.02748. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A. Assembly and mechanosensory function of focal contacts. Curr Opin Cell Biol. 2001;13:584–592. doi: 10.1016/s0955-0674(00)00255-6. [DOI] [PubMed] [Google Scholar]
- Gertler FB, Bennett RL, Clark MJ, Hoffmann FM. Drosophila abl tyrosine kinase in embryonic CNS axons: a role in axonogenesis is revealed through dosage-sensitive interactions with disabled. Cell. 1989;58:103–113. doi: 10.1016/0092-8674(89)90407-8. [DOI] [PubMed] [Google Scholar]
- Gomez GA, McLachlan RW, Yap AS. Productive tension: force-sensing and homeostasis of cell-cell junctions. Trends Cell Biol. 2011;21:499–505. doi: 10.1016/j.tcb.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Grevengoed EE, Fox DT, Gates J, Peifer M. Balancing different types of actin polymerization at distinct sites: roles for Abelson kinase and Enabled. J Cell Biol. 2003;163:1267–1279. doi: 10.1083/jcb.200307026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevengoed EE, Loureiro JJ, Jesse TL, Peifer M. Abelson kinase regulates epithelial morphogenesis in Drosophila. J Cell Biol. 2001;155:1185–1198. doi: 10.1083/jcb.200105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- Hoschuetzky H, Aberle H, Kemler R. β-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol. 1994;127:1375–1380. doi: 10.1083/jcb.127.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Kugler MC, Wei Y, Kim KK, Li X, Brumwell AN, Chapman HA. Integrin α3β1-dependent β-catenin phosphorylation links epithelial Smad signaling to cell contacts. J Cell Biol. 2009;184:309–322. doi: 10.1083/jcb.200806067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleske AJ, Gifford AM, Scott ML, Nee M, Bronson RT, Miczek KA, Baltimore D. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21:1259–1272. doi: 10.1016/s0896-6273(00)80646-7. [DOI] [PubMed] [Google Scholar]
- Langevin J, Morgan MJ, Sibarita JB, Aresta S, Murthy M, Schwarz T, Camonis J, Bellaiche Y. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev Cell. 2005;9:365–376. doi: 10.1016/j.devcel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Lanier LM, Gertler FB. From Abl to actin: Abl tyrosine kinase and associated proteins in growth cone motility. Curr Opin Neurobiol. 2000;10:80–87. doi: 10.1016/s0959-4388(99)00058-6. [DOI] [PubMed] [Google Scholar]
- Levayer R, Pelissier-Monier A, Lecuit T. Spatial regulation of Dia and Myosin-II by RhoGEF2 controls initiation of E-cadherin endocytosis during epithelial morphogenesis. Nat Cell Biol. 2011;13:529–540. doi: 10.1038/ncb2224. [DOI] [PubMed] [Google Scholar]
- Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of β-catenin. Curr Opin Cell Biol. 2005;17:459–465. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci USA. 2010;107:9944–9949. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro J, Peifer M. Roles of Armadillo, a Drosophila catenin, during central nervous system development. Curr Biol. 1998;8:622–632. doi: 10.1016/s0960-9822(98)70249-0. [DOI] [PubMed] [Google Scholar]
- Lye CM, Sanson B. Tension and epithelial morphogenesis in Drosophila early embryos. Curr Top Dev Biol. 2011;95:145–187. doi: 10.1016/B978-0-12-385065-2.00005-0. [DOI] [PubMed] [Google Scholar]
- Maher PA, Pasquale EB, Wang JYJ, Singer SJ. Phosphotyrosine-containing proteins are concentrated in focal adhesions and intercellular junctions in normal cells. Proc Natl Acad Sci USA. 1985;82:6576–6580. doi: 10.1073/pnas.82.19.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney BM, McEwen DG, Grevengoed E, Maddox P, Bejsovec A, Peifer M. Drosophila APC2 and Armadillo participate in tethering mitotic spindles to cortical actin. Nat Cell Biol. 2001;3:933–938. doi: 10.1038/ncb1001-933. [DOI] [PubMed] [Google Scholar]
- McLachlan RW, Yap AS. Not so simple: the complexity of phosphotyrosine signaling at cadherin adhesive contacts. J Mol Med. 2007;85:545–554. doi: 10.1007/s00109-007-0198-x. [DOI] [PubMed] [Google Scholar]
- Murase S, Mosser E, Schuman EM. Depolarization drives β-catenin into neuronal spines promoting changes in synaptic structure and function. Neuron. 2002;35:91–105. doi: 10.1016/s0896-6273(02)00764-x. [DOI] [PubMed] [Google Scholar]
- Ni JQ, Zhou R, Czech B, Liu LP, Holderbaum L, Yang-Zhou D, Shim HS, Tao R, Handler D, Karpowicz P, Binari R, Booker M, Brennecke J, Perkins LA, Hannon GJ, Perrimon N. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat Methods. 2011;8:405–407. doi: 10.1038/nmeth.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Takeichi M. Remodeling of the adherens junctions during morphogenesis. Curr Top Dev Biol. 2009;89:33–54. doi: 10.1016/S0070-2153(09)89002-9. [DOI] [PubMed] [Google Scholar]
- Oda H, Tsukita S. Nonchordate classic cadherins have a structurally and functionally unique domain that is absent from chordate classic cadherins. Dev Biol. 1999;216:406–422. doi: 10.1006/dbio.1999.9494. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Kemler R. Altered cell adhesion activity by pervanadate due to the dissociation of α-catenin from the E-cadherin.catenin complex. J Biol Chem. 1998;273:6166–6170. doi: 10.1074/jbc.273.11.6166. [DOI] [PubMed] [Google Scholar]
- Peacock JG, Miller AL, Bradley WD, Rodriguez OC, Webb DJ, Koleske AJ. The Abl-related gene tyrosine kinase acts through p190RhoGAP to inhibit actomyosin contractility and regulate focal adhesion dynamics upon adhesion to fibronectin. Mol Biol Cell. 2007;18:3860–3872. doi: 10.1091/mbc.E07-01-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedra J, Martinez D, Castano J, Miravet S, Dunach M, de Herreros AG. Regulation of β-catenin structure and activity by tyrosine phosphorylation. J Biol Chem. 2001;276:20436–20443. doi: 10.1074/jbc.M100194200. [DOI] [PubMed] [Google Scholar]
- Piedra J, Miravet S, Castano J, Palmer HG, Heisterkamp N, Garcia de Herreros A, Dunach M. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate β-catenin Tyr-142 phosphorylation and β-catenin-α-catenin Interaction. Mol Cell Biol. 2003;23:2287–2297. doi: 10.1128/MCB.23.7.2287-2297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauzi M, Verant P, Lecuit T, Lenne P. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat Cell Biol. 2008;10:1401–1410. doi: 10.1038/ncb1798. [DOI] [PubMed] [Google Scholar]
- Rhee J, Buchan T, Zukerberg L, Lilien J, Balsamo J. Cables links Robo-bound Abl kinase to N-cadherin-bound β-catenin to mediate Slit-induced modulation of adhesion and transcription. Nat Cell Biol. 2007;9:883–892. doi: 10.1038/ncb1614. [DOI] [PubMed] [Google Scholar]
- Rhee J, Mahfooz NS, Arregui C, Lilien J, Balsamo J, VanBerkum MF. Activation of the repulsive receptor Roundabout inhibits N-cadherin-mediated cell adhesion. Nat Cell Biol. 2002;4:798–805. doi: 10.1038/ncb858. [DOI] [PubMed] [Google Scholar]
- Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36734–36740. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999;340:1330–1340. doi: 10.1056/NEJM199904293401706. [DOI] [PubMed] [Google Scholar]
- Shaye DD, Casanova J, Llimargas M. Modulation of intracellular trafficking regulates cell intercalation in the Drosophila trachea. Nat Cell Biol. 2008;10:964–970. doi: 10.1038/ncb1756. [DOI] [PubMed] [Google Scholar]
- Shibata T, Ochiai A, Kanai Y, Akimoto S, Gotoh M, Yasui N, Machinami R, Hirohashi S. Dominant negative inhibition of the association between β-catenin and cerbB-2 by N-terminally deleted β-catenin suppresses the invasion and metastasis of cancer cells. Oncogene. 1996;13:883–889. [PubMed] [Google Scholar]
- Shomori K, Ochiai A, Akimoto S, Ino Y, Shudo K, Ito H, Hirohashi S. Tyrosine-phosphorylation of the 12th armadillo-repeat of β-catenin is associated with cadherin dysfunction in human cancer. Int J Oncol. 2009;35:517–524. doi: 10.3892/ijo_00000363. [DOI] [PubMed] [Google Scholar]
- Simoes S, de M, Blankenship JT, Weitz O, Farrell DL, Tamada M, Fernandez-Gonzalez R, Zallen JA. Rho-kinase directs Bazooka/Par-3 planar polarity during Drosophila axis elongation. Dev Cell. 2010;19:377–388. doi: 10.1016/j.devcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Yanfeng WA, Grumolato L, Aaronson SA, Mlodzik M. Abelson family kinases regulate Frizzled planar cell polarity signaling via Dsh phosphorylation. Genes Dev. 2010;24:2157–2168. doi: 10.1101/gad.1961010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai C-Y, Mysore SP, Chiu C, Schuman EM. Activity-regulated N-cadherin endocytosis. Neuron. 2007;54:771–785. doi: 10.1016/j.neuron.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Tominaga J, Fukunaga Y, Abelardo E, Nagafuchi A. Defining the function of β-catenin tyrosine phosphorylation in cadherin-mediated cell-cell adhesion. Genes Cells. 2008;13:67–77. doi: 10.1111/j.1365-2443.2007.01149.x. [DOI] [PubMed] [Google Scholar]
- van Veelen W, Le NH, Helvensteijn W, Blonden L, Theeuwes M, Bakker ER, Franken PF, van Gurp L, Meijlink F, van der Valk MA, Kuipers EJ, Fodde R, Smits R. β-catenin tyrosine 654 phosphorylation increases Wnt signaling and intestinal tumorigenesis. Gut. 2011;60:1204–1212. doi: 10.1136/gut.2010.233460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead J, Vignjevic D, Futterer C, Beaurepaire E, Robine S, Farge E. Mechanical factors activate β-catenin-dependent oncogene expression in APC1638N/+ mouse colon. HFSP J. 2008;2:286–294. doi: 10.2976/1.2955566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills Z, Marr L, Zinn K, Goodman CS, Van Vactor D. Profilin and the Abl tyrosine kinase are required for motor axon outgrowth in the Drosophila embryo. Neuron. 1999;22:291–299. doi: 10.1016/s0896-6273(00)81090-9. [DOI] [PubMed] [Google Scholar]
- Xiong W, Rebay I. Abelson tyrosine kinase is required for Drosophila photoreceptor morphogenesis and retinal epithelial patterning. Developmental Dynamics. 2011;240:1745–1755. doi: 10.1002/dvdy.22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen J, Blankenship J. Multicellular dynamics during epithelial elongation. Semin Cell Dev Biol. 2008;19:263–270. doi: 10.1016/j.semcdb.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen J, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell. 2004;6:343–355. doi: 10.1016/s1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- Zandy NL, Playford M, Pendergast AM. Abl tyrosine kinases regulate cell-cell adhesion through Rho GTPases. Proc Natl Acad Sci U S A. 2007;104:17686–17691. doi: 10.1073/pnas.0703077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng G, Apte U, Micsenyi A, Bell A, Monga SP. Tyrosine residues 654 and 670 in β-catenin are crucial in regulation of Met–β-catenin interactions. Exp Cell Res. 2006;312:3620–3630. doi: 10.1016/j.yexcr.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.