Abstract
Objective:
To determine the prevalence of resistance of Streptococcus pneumoniae to penicillin and other antimicrobial agents in metropolitan Toronto.
Design:
Consecutive pneumococcal isolates from different patients were obtained from two private community-based laboratories and from patients assessed in the emergency department of a tertiary-care teaching hospital in Toronto, Ontario between June and December 1993, and between March and October 1994. In vitro susceptibility testing was done by broth microdilution in accordance with National Committee for Clinical Laboratory Standards guidelines.
Results:
Twenty (7.3±3.1%) of 274 pneumococcal isolates were resistant to penicillin; six (30%) isolates had high-level resistance (minimal inhibitory concentration [mic] 2.0 μg/mL or greater); and 14 isolates had intermediate resistance (mic 0.1 to 1.0 μg/mL). Penicillin-resistant strains were also frequently resistant to tetracycline (55%), cotrimoxazole (50%), erythromycin (40%) and cefuroxime (35%). Resistant strains comprised several serotypes: 19F (six isolates), 9V (three), 23F (three), and one each of 6A, 6B, 14, and 19A; four isolates were nontypeable.
Conclusions:
There has been a recent emergence of penicillin-resistant S pneumoniae in southern Ontario. National and regional surveillance is warranted to determine the extent of the problem elsewhere in Canada.
Keywords: Antibiotic resistance, Streptococcus pneumoniae
Abstract
Objectif :
Déterminer la prévalence de la résistance de Streptococcus pneumoniae à la pénicilline et à d’autres antibiotiques dans le Toronto métropolitain.
Modèle :
Les isolats pneumococciques consécutifs de différents patients ont été obtenus à partir de deux laboratoires privés et chez des patients évalués aux urgences d’un hôpital universitaire de soins tertiaires à Toronto, en Ontario, entre juin et décembre 1993 et entre mars et octobre 1994. Les épreuves de sensibilité in vitro ont été effectuées par microdilution sur bouillon de culture, conformément aux directives du National Committee for Clinical Laboratory Standards.
Résultats :
Vingt (7,3±3,1 %) des 274 isolats pneumococciques se sont révélés résistants à la pénicilline, six (30 %) des isolats présentaient une résistance forte (concentration minimale inhibitrice [cmi], 2,0 μg/mL ou plus) et 14 isolats présentaient une résistance intermédiaire (cmi, 0,1 à 1,0 μg/mL). Souvent, les souches pénicillino-résistantes étaient également résistantes à la tétracycline (55 %), au cotrimoxazole (50 %), à l’érythromycine (40 %) et au céfuroxime (35 %). Les souches résistantes comprenaient plusieurs sérotypes : 19F (6 isolats), 9V (3), 23F (3) et un de chacun des sérotypes suivants : 6A, 6B, 14 et 19A. Quatre isolats se sont révélés non typables.
Conclusions :
On note une émergence récente de S. pneumoniae résistants à la pénicilline dans le Sud de l’Ontario. Une surveillance épidémiologique nationale et régionale s’impose afin de mesurer l’étendue du problème ailleurs au Canada.
Penicillin has long been considered to be the antibiotic of choice for the treatment of infections due to Streptococcus pneumoniae. In the past few years, though, there has been a worldwide increase in the prevalence of penicillin-resistant S pneumoniae (1–3). Although a strain of pneumococcus with reduced susceptibility to penicillin was first reported in Canada 20 years ago (4), since then there have been only sporadic reports of invasive infections due to resistant organisms in this country (5–7). Three large surveys of pneumococcal susceptibility in Canada found rates of resistance to penicillin of 2.4%, 1.3% and 1.5% in Alberta (8), Quebec (9) and Ontario (10), respectively. Two recent cases of invasive infection (meningitis, bacteremic pneumonia) due to penicillin-resistant strains of S pneumoniae seen at our hospital prompted us to conduct a pilot study to determine the prevalence of resistance of S pneumoniae to penicillin and other antimicrobial agents in metropolitan Toronto. We describe here one of the cases and the results of the prevalence survey.
CASE PRESENTATION
A 45-year-old male with human immunodeficiency virus infection and central nervous system lymphoma was admitted to hospital in November 1993 with a two-day history of fever and dyspnea. His medications on admission included trimethoprim-sulfamethoxazole, fluconazole and prednisone. Physical examination revealed cellulitis over the anterior aspect of his neck, and a chest x-ray revealed left lower lobe consolidation. Blood and sputum cultures grew S pneumoniae resistant to penicillin (minimal inhibitory concentration [mic] 4 μg/mL). He was admitted to the intensive care unit with respiratory failure. He was treated with vancomycin for two weeks and was discharged from hospital. However, he was readmitted one month later with recurrent fever and bacteremia due to penicillin-resistant S pneumoniae. He was retreated with vancomycin, but died several months later of causes unrelated to pneumococcal infection.
PATIENTS AND METHODS
Consecutive S pneumoniae isolates from different patients were obtained between June and December 1993, and between March and June 1994. Isolates were obtained from Med-Chem Laboratories and Flemingdon Medical Laboratory, two private community-based laboratories providing services to family physicians and nursing homes in metropolitan Toronto, and from patients assessed in the emergency department of a tertiary-care teaching hospital in Toronto. All isolates were identified as S pneumoniae based on colonial morphology, Gram stain characteristics, optochin susceptibility and bile solubility. In vitro susceptibility testing was done by a broth microdilution procedure, following National Committee for Clinical Laboratory Standards guidelines (11). An intermediate level of resistance to penicillin was defined as mic 0.1 to 1.0 μg/mL; high-level resistance was defined as an mic 2.0 μg/mL or greater (11).
Pneumococci were serotyped by the National Reference Centre for Streptococcus (Edmonton, Alberta). Genomic dna of resistant isolates was also examined by pulsed field gel electrophoresis (pfge). Genomic dna from pneumococcal isolates was prepared in agar plugs as previously described (12) and digested with SmaI (Boehringer-Mannheim). Digested dna was electrophoresed using the contour-clamped homogeneous electric field apparatus (CHEF-DRII, BioRad, California). Electrophoresis was carried out for 20 h using ramped pulse times beginning with 0.2 s and ending with 25 s, at an applied voltage of 6 V/cm. The gels were stained with ethidium bromide and photographed under ultraviolet illumination. Isolates were considered to represent different strains if the pfge band patterns differed by at least three bands.
RESULTS
A total of 274 pneumococcal isolates was available for testing; 175 isolates were from the two private laboratories and 99 isolates were from patients assessed in the hospital emergency department. Twenty (7.3%; 95% ci ±3.1%) penicillin-resistant S pneumoniae isolates were detected, six (30%) of which had high-level resistance and 14 with intermediate resistance (Table 1). Fourteen resistant strains were eye, ear or sputum isolates from pediatric out-patients and six strains (two from sputum and one each from blood, cerebrospinal fluid, bronchoalveolar washings and the eye) were from adults seen in the emergency department. Penicillin-susceptible strains were generally susceptible to the other antimicrobial agents tested. However, penicillin-resistant S pneumoniae were also frequently resistant to ceftazidime (55%), tetracycline (55%), trimethoprim-sulfamethoxazole (50%), erythromycin (40%), cefuroxime (35%) and ceftriaxone (25%) (Table 1). Isolates were uniformly susceptible to only vancomycin and imipenem.
TABLE 1.
Antimicrobial susceptibility of 274 isolates of Streptococcus pneumoniae
| Antimicrobial agent | Penicillin-susceptible isolates (n=254) | Penicillin-resistant isolates (n=20) | ||
|---|---|---|---|---|
| MIC90 (μg/mL)* | % resistant | MIC90 (μg/mL)* | % resistant | |
| Penicillin | ≤0.06 | 0 | 2.0 | 100 |
| Cefuroxime | ≤0.06 | 0 | 8.0 | 35 |
| Ceftriaxone | ≤0.06 | 0 | 2.0 | 25 |
| Cefotaxime | ≤0.06 | 0 | 1.0 | 5 |
| Ceftazidime | 0.5 | 0.8 | >4.0 | 55 |
| Imipenem | ≤0.06 | 0 | 0.25 | 0 |
| Erythromycin | ≤0.5 | 0.8 | >4.0 | 40 |
| Clindamycin | ≤0.5 | 0 | >4.0 | 15 |
| Tetracycline | ≤2.0 | 2.8 | >8.0 | 55 |
| Ciprofloxacin | 1.0 | 0.8 | 1.0 | 5 |
| Chloramphenicol | 4.0 | 0.8 | 4.0 | 10 |
| Trimethoprim-sulfamethoxazole | 4.0 | 9.1 | >4.0 | 50 |
| Vancomycin | 1.0 | 0 | 1.0 | 0 |
MIC90 Minimal inhibitory concentration of antimicrobial agent required to inhibit growth of 90% of the isolates tested
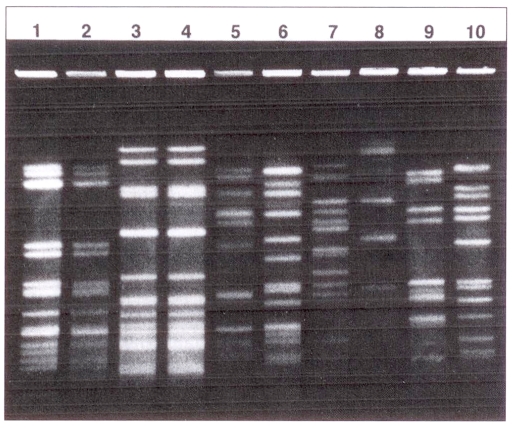
The penicillin-resistant pneumococci were serotyped as follows: 19F (six isolates), 9V (three), 23F (three), and one each of 6A, 6B, 14, 19A; four were nontypeable. The pfge results of a representative sample of penicillin-resistant isolates are shown in Figure 1. Different serotypes had clearly distinguishable pfge patterns. However, whereas all three serotype 9V isolates had identical patterns and a nontypeable strain shared an identical pattern with a serotype 23F isolate, the other 23F serotypes had distinct profiles and each of the 19F serotype isolates had a unique pattern.
Figure 1).
Restriction endonuclease analysis of genomic DNA of penicillin-resistant Streptococcus pneumoniae isolates obtained by pulsed field gel electrophoresis after digestion with SmaI. Lanes 1 and 2, serotype 9V isolates from two different patients; lane 3, nontypeable isolate; lane 4, serotype 23F; lanes 5–7, serotype 19F isolates from three different patients; lanes 8–10, serotypes 6A, 6B and 19A, respectively
DISCUSSION
The recent increase in the prevalence of penicillin-resistant S pneumoniae in the metropolitan Toronto region found in this survey is in marked contrast to results obtained in a 1988 study (10) of a similar community-based population when only eight of 551 (1.5%) penicillin-resistant strains were detected (P<0.0001). Furthermore, none of the previously identified pneumococcal isolates had demonstrated high-level penicillin resistance, whereas 30% of the resistant strains in this survey had high-level resistance.
The recent increase in prevalence is also reflected by notifications of invasive pneumococcal disease due to penicillin-resistant strains to the Ontario Pneumococcal Study Group (13) in 1993–94.
The results of serotyping and molecular typing by pfge suggest that multiple clones of penicillin-resistant pneumococci are appearing simultaneously in the metropolitan Toronto region. Similar results have been suggested by a cross-Canada survey of penicillin-resistant isolates (14). Moreover, this preliminary experience with these typing methods suggests that for S pneumoniae, pfge is more discriminatory than is serotyping; this observation would have to be confirmed by evaluating a larger number of isolates.
The emergence of penicillin-resistant S pneumoniae has major health care implications. Microbiology laboratories must be able to detect rapidly and accurately penicillin resistance in pneumococcal isolates. Although drug-resistant strains of pneumococci now appear to be much more prevalent in several parts of Canada, regional variations may exist. If the results of this survey are confirmed elsewhere in Canada, empirical antimicrobial treatment of a variety of infectious diseases (including pneumonia, bronchitis, otitis media, sinusitis and meningitis) will have to be modified. Treatment options may be limited because penicillin-resistant strains are also frequently resistant to other beta-lactam antibiotics (15,16). Moreover, alternative antimicrobial agents for the treatment of pneumococcal infections may not be as effective as penicillin would be for susceptible strains (17,18). Because invasive infections due to resistant strains are associated with considerable morbidity and mortality, greater use of pneumococcal vaccine should be promoted, particularly for those at high risk for severe pneumococcal infections (19). Continuous surveillance of S pneumoniae antimicrobial susceptibility is required to determine the extent of resistance to penicillin and alternative agents, so that appropriate therapy and control measures may be instituted.
Acknowledgments
We thank Med-Chem and Flemingdon Medical Laboratories for providing pneumococcal isolates; the National Reference Centre for Streptococcus (Edmonton, Alberta) for serotyping isolates; and C McGowan-Schulze for secretarial services.
REFERENCES
- 1.Jacobs MR, Koornhof JG, Robins-Browne RM, et al. Emergence of multiply resistant pneumococci. N Engl J Med. 1978;299:735–40. doi: 10.1056/NEJM197810052991402. [DOI] [PubMed] [Google Scholar]
- 2.Applebaum PC. Antimicrobial resistance in Streptococcus pneumoniae: An overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 3.Breiman RF, Butler JC, Tenover FC, Elliott JA, Facklam RR. Emergence of drug-resistant pneumococcal infections in the United States. JAMA. 1994;271:1831–5. [PubMed] [Google Scholar]
- 4.Dixon JMS. Pneumococcus with increased resistance to penicillin. Lancet. 1974;ii:474. doi: 10.1016/s0140-6736(74)91865-0. [DOI] [PubMed] [Google Scholar]
- 5.Kibsey PC. A case of multiply-resistant Streptococcus pneumoniae – Alberta. Can Dis Wkly Rep. 1986;12:13–4. [Google Scholar]
- 6.Lapointe JR, Joncas JH. Penicillin resistant and multi-resistant pneumococcal strains – Quebec. Can Dis Wkly Rep. 1982;8:133–5. [Google Scholar]
- 7.Burdge DR, Woo VC, Ritchie PMA. Bacteremic pneumonia caused by penicillin-resistant pneumococci: case report and review with a Canadian perspective. Can J Infect Dis. 1992;3:185–8. doi: 10.1155/1992/963907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon JMS, Lipinski AE, Graham MEP. Detection and prevalence of pneumococci with increased resistance to penicillin. Can Med Assoc J. 1977;117:1159–61. [PMC free article] [PubMed] [Google Scholar]
- 9.Jetté LP, Lamothe F, the Pneumococcus Study Group Surveillance of invasive Streptococcus pneumoniae infection in Quebec, Canada, from 1984 to 1986: serotype distribution, antimicrobial susceptibility, and clinical characteristics. J Clin Microbiol. 1989;27:1–5. doi: 10.1128/jcm.27.1.1-5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzulli T, Simor AE, Jaeger R, Fuller S, Low DE. Comparative in vitro activities of several new fluoroquinolones and b-lactam antimicrobial agents against community isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1990;34:467–9. doi: 10.1128/aac.34.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. 3rd edn. Villanova: NCCLS; 1993. Approved Standard M7-A3. [Google Scholar]
- 12.Murray BE, Singh KV, Heath JD, Sharma BR, Weinstock GM. Comparison of genomic DNA of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J Clin Microbiol. 1990;28:2059–63. doi: 10.1128/jcm.28.9.2059-2063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Low DE, Gregson D, Kanchana MV, et al. The rapid emergence of penicillin-resistant Streptococcus pneumoniae (PRSP) in Ontario. 34th Interscience Conference on Antimicrobial Agents and Chemotherapy; Orlando, Florida. 1994; (Abst C22) [Google Scholar]
- 14.Kanchana MV, Matsumara S, Willey B, et al. Genetic characterization of penicillin-resistant Streptococcus pneumoniae (PRSP) from across Canada. 62nd Conjoint Meeting on Infectious Diseases; Montreal, Quebec. 1994; (Abst PS-10) [Google Scholar]
- 15.Simberkoff MS, Lukaszewski M, Cross A, et al. Antibiotic-resistant isolates of Streptococcus pneumoniae from clinical specimens: a cluster of serotype 19A organisms in Brooklyn, New York. J Infect Dis. 1986;153:78–82. doi: 10.1093/infdis/153.1.78. [DOI] [PubMed] [Google Scholar]
- 16.Haglund LA, Istre GR, Pickett DA, Welch DF, Fine DP, the Pneumococcus Study Group Invasive pneumococcal disease in central Oklahoma: emergence of high-level penicillin resistance and multiple antibiotic resistance. J Infect Dis. 1993;168:1532–6. doi: 10.1093/infdis/168.6.1532. [DOI] [PubMed] [Google Scholar]
- 17.Viladrich PF, Gudiol F, Linares J, et al. Evaluation of vancomycin for therapy of adult pneumococcal meningitis. Antimicrob Agents Chemother. 1991;35:2467–72. doi: 10.1128/aac.35.12.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sloas MM, Barrett FF, Chesney PJ, et al. Cephalosporin treatment failure in penicillin- and cephalosporin- resistant Streptococcus pneumoniae meningitis. Pediatr Infect Dis J. 1992;11:662–6. [PubMed] [Google Scholar]
- 19.Canadian Task Force on the Periodic Health Examination Periodic health examination, 1991 update: 2. Administration of pneumococcal vaccine. Can Med Assoc J. 1991;144:665–71. [PMC free article] [PubMed] [Google Scholar]