Abstract
The movement of proteins between the cytoplasm and nucleus mediated by the importin superfamily of proteins is essential to many cellular processes, including differentiation and development, and is critical to disease states such as viral disease and oncogenesis. We recently developed a high-throughput screen to identify specific and general inhibitors of protein nuclear import, from which ivermectin was identified as a potential inhibitor of importin α/β-mediated transport. In the present study, we characterized in detail the nuclear transport inhibitory properties of ivermectin, demonstrating that it is a broad-spectrum inhibitor of importin α/β nuclear import, with no effect on a range of other nuclear import pathways, including that mediated by importin β1 alone. Importantly, we establish for the first time that ivermectin has potent antiviral activity towards both HIV-1 and dengue virus, both of which are strongly reliant on importin α/β nuclear import, with respect to the HIV-1 integrase and NS5 (non-structural protein 5) polymerase proteins respectively. Ivermectin would appear to be an invaluable tool for the study of protein nuclear import, as well as the basis for future development of antiviral agents.
Keywords: dengue virus, HIV, importin α/β1, ivermectin, nuclear import
Abbreviations: CLSM, confocal laser-scanning microscopy; DENV, dengue virus; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; GFP, green fluorescent protein; hCMV, human cytomegalovirus; Imp, importin; IN, integrase; LMB, leptomycin B; NES, nuclear export signal; NLS, nuclear localization signal; NS5, non-structural protein 5; PIC, pre-integration complex; PTHrP, parathyroid hormone-related protein; SRY, sex-determining region of the Y chromosome; sulfo-NHS-biotin, sulfo-N-hydroxysuccinimide biotin; SUMO, small ubiquitin-related modifier; SV40, simian virus 40; T-ag, large tumour antigen; Tat, transactivator of transcription; TRF1, telomeric repeat factor-binding protein 1; VSV-G, vesicular stomatitis virus glycoprotein
INTRODUCTION
The movement of proteins between the nucleus and cytoplasm is essential to key cellular processes such as differentiation and development, as well as being critical to disease states such as viral disease and oncogenesis [1–3]. Proteins with a molecular mass greater than ~45 kDa generally require an NLS (nuclear localization signal) to gain entry into the nucleus via the nuclear envelope-localized NPCs (nuclear pore complexes). NLSs are generally recognized by members of the Imp (importin) superfamily of proteins, NLS recognition commonly being through the Impα adaptor protein within the Impα/β1 heterodimer, or Impβ1 or homologues thereof directly [4–7]. Nuclear protein export is an analogous process, whereby NESs (nuclear export signals) are recognized by the exportin family of Impβ homologues.
With seven Impαs and >20 Impβs in humans and a wide variety of known NLS/NES sequences, the lack of specific inhibitors hampers analysis of the functional roles of these various transporters; currently, the exportin/CRM1 (chromosome region maintenance 1)-specific inhibitor LMB (leptomycin B) is the only widely accepted commercially available compound to inhibit nuclear transport. Although other inhibitory compounds are beginning to be developed [8–16], including compounds that are structurally related to LMB such as ratjadone, peptide-based inhibitors and several small-molecule inhibitors [17–21], these are not widely available and have not been extensively tested. Clearly, there is an urgent need for new and specific inhibitors of components of the mammalian cell nuclear transport machinery.
Previously we developed a high-throughput screening approach to identify inhibitors of viral protein nuclear import [22]. As a proof of concept, we targeted the interaction of the IN (integrase) protein from HIV-1 with its nuclear import receptor Impα/β. From this screening/cross-screening process, we identified several specific inhibitors of IN nuclear import, including mifepristone, but we also identified inhibitors that appeared to act on Impα/β-mediated nuclear import generally. One of these was ivermectin, a broad-spectrum anti-parasite medication used in humans most commonly to treat nematode infections such as onchocerciasis (river blindness) [23], as well as scabies [24] and lice [25]. In the present study, we investigated the effects of ivermectin treatment on the subcellular localization of numerous NLS-bearing cargo proteins, demonstrating that ivermectin is a potent inhibitor of Impα/β1-dependent transport, with no effect on proteins containing NLSs recognized by alternative nuclear import pathways. Importantly, it can be used to inhibit both HIV and DENV (dengue virus) infection, both of which rely on Impα/β1-dependent transport of IN and NS5 (non-structural protein 5) respectively [3,26] for efficient viral production, raising the intriguing possibility that drugs analogous to ivermectin could be potent broad-spectrum antiviral agents.
MATERIALS AND METHODS
Generation of GFP (green fluorescent protein)-fusion protein bacterial and mammalian expression plasmids
Bacterial or mammalian cell expression vectors encoding GFP-tagged IN, SV40 (simian virus 40) T-ag (large tumour antigen), DENV NS5, tumour-suppressor protein p53, hCMV (human cytomegalovirus) processivity factor UL44 and polymerase UL54, TRF1 (telomeric repeat factor-binding protein 1), SRY (sex-determining region of the Y chromosome), PTHrP (parathyroid hormone-related protein), histone H2B, the SUMO (small ubiquitin-related modifier)-conjugating E3 ligase UBC9, Tat (transactivator of transcription) protein from HIV-1 [27,28], and the chromatin remodelling factor aF10 [29] were generated using the Gateway cloning technology (Invitrogen) and vector pGFP-attC, for GFP-fusion protein expression in bacteria, or pDest53 (Invitrogen), for GFP-fusion protein expression in mammalian cells as described previously [30].
Cell culture and transfection
HeLa (human cervical adenocarcinoma) cells were cultured in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% (v/v) FBS (fetal bovine serum), 1 mM L-glutamate, 1 mM penicillin/streptomycin and 20 mM Hepes at 37°C in 5% CO2. At 24 h before transfection, cells were seeded on to glass coverslips (15 mm×15 mm). Lipofectamine™ 2000 (Invitrogen) was used according to the manufacturer's instructions to transfect DNA into the HeLa cells. Where appropriate, cells were treated with ivermectin at a final concentration of 25 μM for 1 h before imaging. Cells were imaged live 24 h after transfection by CLSM (confocal laser-scanning microscopy) (Bio-Rad 1024ES or Olympus FV1000) using a ×60 or ×100 oil-immersion objective as described previously [30,31]. Digitized images were analysed using the ImageJ version 1.43g public domain software (NIH) to determine the ratio of nuclear (Fn) to cytoplasmic (Fc) fluorescence (Fn/c) according to the formula: Fn/c=(Fn−Fb)/(Fc−Fb), where Fb is background autofluorescence [5,32,33]. Statistical analysis was performed using Welch's test and the GraphPad Prism 5.0 software.
Protein purification and Impα/β dimerization
GFP-tagged NS5 protein was purified from bacteria as a His6-tagged protein under denaturing conditions [34], whereas Imp proteins were purified from bacteria as GST-fusion proteins under native conditions as described in [34–36]. Where appropriate, Impα and Impβ were pre-dimerized at 13.6 μM for 15 min at room temperature (22°C) to generate the Impα/β heterodimer for binding studies.
Biotinylation of Imp proteins
Impα was biotinylated as described previously [34] using the sulfo-NHS-biotin (sulfo-N-hydroxysuccinimide biotin) reagent (Pierce). Briefly, 3.5 mg of Imp was incubated with 250 μl of sulfo-NHS-biotin (1 mg dissolved in 150 μl of water) on ice for 2 h. Unbound biotin was removed via a PD-10 column (GE Healthcare) and the resulting biotinylated protein was concentrated in an Amicon-30 concentration device.
AlphaScreen-based binding assay
The AlphaScreen assay was performed in triplicate in 384-well white opaque plates (PerkinElmer) [34]. Briefly, 2 μl of 375 nM (30 nM final concentration) His6-tagged protein was added to each well, followed by 20 μl of Imp at the appropriate concentration (generally 0–60 nM), prepared by serial dilution in PBS, and incubation for 30 min at room temperature. All subsequent additions and incubations were made under subdued lighting because of the photosensitivity of the beads. A 1 μl volume of a 1:10 dilution (in PBS) of the acceptor beads and 1 μl of 2.5% BSA were added simultaneously and incubated for 90 min at room temperature. A 1 μl volume of a 1:10 dilution of the donor beads was then added to give a final sample volume of 25 μl and the mixture was incubated at room temperature for 2 h. The assay was quantified on a PerkinElmer FusionAlpha plate reader, triplicate values were averaged, and titration curves (three-parameter sigmoidal fit) were plotted using the Sigmaplot graphing program. Values in the ‘hooking zone’, where quenching of the signal has occurred through the presence of too much of either binding partner, were excluded from the final plot as previously [30,34,37]. These were used to determine the optimal Imp concentrations for the library screen.
HIV infectivity assay
HeLa cells plated on to 24-well plates at 40–50% confluence were infected with 200 ng (capsid equivalent)/well of VSV-G (vesicular stomatitis virus glycoprotein)-pseudotyped NL4-3.Luc.R-E- HIV in DMEM containing 50% (v/v) FBS and incubated at 4°C for 2 h to synchronize infection, followed by incubation at 37°C. At 2 h (for mifepristone) or 6 h (for ivermectin) after infection, virus was removed, the cells were washed with PBS, and duplicate wells were treated with DMEM/10% (v/v) FBS containing ivermectin at 25 or 50 μM or mifepristone at 100 and 200 μM. At 8 h after infection, medium was removed, and cells were washed with PBS and fresh DMEM/10% (v/v) FBS, followed by incubation at 37°C. At 48.5 h after infection, medium was removed, cells were harvested and lysed, and lysates were assayed for luciferase activity (Steady-Glo reagent; Promega) and protein concentration (Bradford assay), according to the manufacturer's instructions.
DENV infectivity assay
Vero cells cultured in DMEM/10% (v/v) FBS were pre-treated for 4 h with 25 or 50 μM ivermectin, or 25 μM mifepristone, and then infected with DENV-2 (New Guinea C) at an MOI (multiplicity of infection) of 4 as described in [31,38,39], and the cells were maintained in DMEM/2% (v/v) FBS. At various times after infection, the culture medium was collected and virus titres, calculated as plaque-forming units/ml, were determined using plaque assays in C6/36 cells as described previously [31,38,39].
RESULTS AND DISCUSSION
Ivermectin inhibits the nuclear import of multiple Impα/β1 cargo proteins
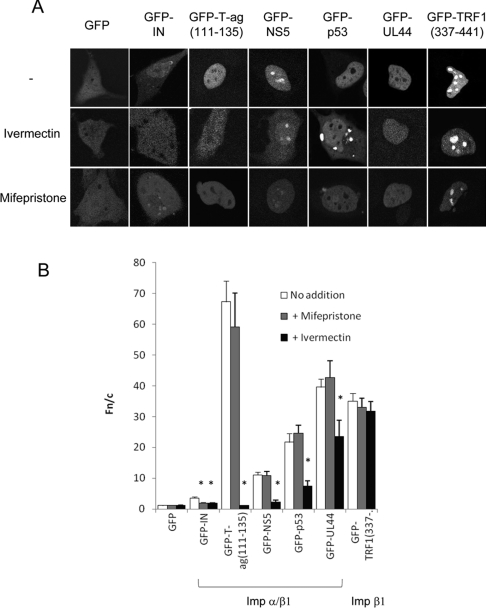
We recently used high-throughput screening to identify several compounds, including mifepristone, as specific inhibitors of IN recognition by Impα/β1 [22]. In addition, several compounds, including ivermectin, were identified that were also able to inhibit recognition by Impα/β1 of SV40 T-ag [4], raising the possibility that ivermectin may specifically inhibit Impα/β1-dependent nuclear import in general, and thereby represent a valuable tool to study nuclear transport. To build on these preliminary observations, a number of nuclear-localizing proteins were expressed as GFP-fusion proteins in HeLa cells and treated with or without ivermectin or mifepristone as a control (Figure 1 and Table 1). These included IN and T-ag, as well as the Impα/β-recognized proteins DENV NS5, p53 and hCMV UL44, and the Impβ1-recognized TRF1. As expected [22], IN nuclear accumulation was significantly inhibited in the presence of both ivermectin and mifepristone, whereas T-ag nuclear accumulation was inhibited by ivermectin but not mifepristone. Importantly, all of the other cargo proteins were unaffected by mifepristone treatment, consistent with mifepristone being a highly specific inhibitor of IN nuclear accumulation. Significantly, nuclear accumulation of all of the cargo proteins containing Impα/β1-recognized NLSs was reduced (P<0.01) in the presence of ivermectin, whereas no such effect was observed for TRF1, which is transported to the nucleus in a manner dependent on Impβ1 alone. These results imply that ivermectin is a broad-spectrum inhibitor of Impα/β1-mediated nuclear import, through an effect on the Impα/β1 heterodimer.
Figure 1. Ivermectin inhibits Impα/β1- but not Impβ1-dependent nuclear import, whereas mifepristone specifically inhibits IN nuclear accumulation.
(A) Typical CLSM images of HeLa cells expressing the indicated GFP-fusion proteins 24 h after transfection, treated with or without 25 μM ivermectin or 50 μM mifepristone as indicated for 1 h before imaging. (B) Results (mean±S.E.M., n>89) for quantitative analysis of images such as those in (A) to determine the nuclear to cytoplasmic fluorescence ratio (Fn/c); *P<0.01.
Table 1. Summary of data for the effects of ivermectin or mifepristone on nuclear import of a range of cargoes imported by different nuclear import pathways.
Peptide fragments are indicated by parentheses, indicating the residues of the peptide; otherwise proteins were full-length. NT, not tested. AF10 is transported into the nucleus independently of Imps through direct interactions with nucleoporins [29]. Tat has been reported to be recognized directly by Impβ1 [27], but shown to have an Imp-independent nuclear import mechanism in vitro [28].
| Protein/peptide fragment | Import pathway | Ivermectin | Mifepristone |
|---|---|---|---|
| GFP | – | No effect | No effect |
| GFP–AF10-(696–794) | – | No effect | NT |
| GFP–ppUL44 | Impα/β | Inhibits | No effect |
| GFP–p53 | Impα/β | Inhibits | No effect |
| GFP–UL54-(1145–1161) | Impα/β | Inhibits | NT |
| GFP–T-ag-(111–135) | Impα/β | Inhibits | No effect |
| GFP–IN | Impα/β | Inhibits | Inhibits |
| GFP–NS5 | Impα/β and Impβ1 | Inhibits | No effect |
| GFP–TRF1-(337–441) | Impβ1 | No effect | No effect |
| GFP–SRY | Impβ1 and calmodulin | No effect | NT |
| GFP–PTHrP-(66–94) | Impβ1 | No effect | NT |
| GFP–Tat-(46–64) | Impβ1 (?) | No effect | NT |
| GFP–H2B | Multiple Impβs | No effect | NT |
| GFP–UBC9 | Imp13 | No effect | NT |
Ivermectin does not affect nuclear accumulation of cargo proteins containing NLSs recognized by other Imps
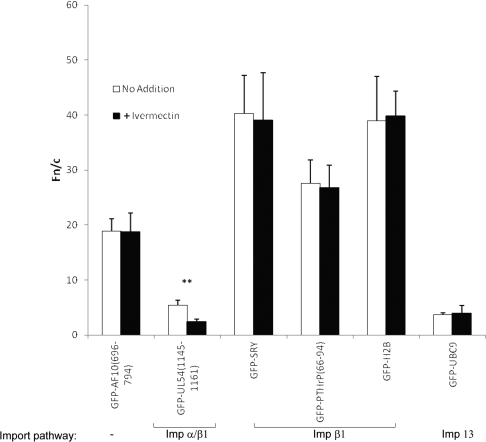
To confirm the specificity of ivermectin action, various GFP-fusion proteins containing NLSs recognized by a variety of Imps were expressed in HeLa cells and treated with/without ivermectin for 1 h before imaging. Results (Figure 2 and Table 1) indicate that ivermectin only inhibited the nuclear accumulation of hCMV UL54, which contains classical Impα/β1-recognized NLSs [40,41]. In contrast, no effect was seen on SRY or PTHrP, which both contain NLSs recognized by Impβ1 [6,42,43], consistent with that observed for TRF1. Interestingly, histone H2B, which contains at least two NLSs and is thought to be imported into the nucleus by multiple different Impβ homologues [44–46] was also not affected by ivermectin, implying that ivermectin does not affect these various nuclear import pathways. Likewise, the SUMO-conjugating enzyme UBC9, which is imported into the nucleus through the action of Imp13 [47], was not affected by ivermectin. These results (summarized in Table 1) indicate that ivermectin is specific for Impα/β1-recognized nuclear import cargoes, and has no effect on any of the other nuclear import pathways tested, including that mediated by Impβ1 alone.
Figure 2. Ivermectin is a broad-spectrum Impα/β1 inhibitor that does not affect other nuclear import pathways.
HeLa cells transfected to express the indicated GFP-fusion proteins were treated with or without 25 μM ivermectin for 1 h before live-cell imaging 24 h after transfection. Results (mean±S.E.M., n>68) were determined as described in Figure 1(B); **P<0.001.
Ivermectin inhibits infection by HIV-1 and DENV which rely on Impα/β1-mediated nuclear transport
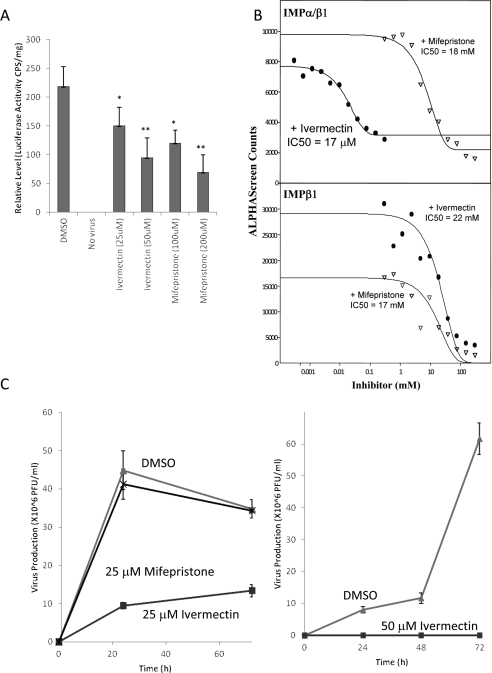
Nuclear import of viral proteins is critical to the life cycle of many viruses, including many RNA viruses that replicate exclusively in the cytoplasm such as DENV, respiratory syncytial virus and rabies [2,3,31,48,49]. In the case of HIV, the virus generates a PIC (pre-integration complex), consisting of the newly transcribed viral cDNA and several HIV (e.g. IN) and host proteins. The PIC is then transported into the nucleus most likely through the action of IN [26], subsequent to which IN integrates the viral cDNA into the host cell genome, which is essential for productive infection [50]. Owing to these critical nuclear functions of IN, it is likely that inhibition of IN nuclear import will impede productive HIV infection. To test this formally, HeLa cells were infected with 200 ng/well VSV-G-pseudotyped NL4-3.Luc.R-E- HIV and the infection was synchronized at 4°C for 2 h. Duplicate wells were then treated with ivermectin for 2 h or mifepristone for 6 h and viral infectivity was measured by relative luciferase activity 48 h after infection (Figure 3A). Strikingly, compared with DMSO control wells, treatment with ivermectin at concentrations as low as 25 μM treatment for as little as 2 h was able to significantly reduce virus production; under these conditions, there is essentially no observable toxicity induced by the various treatments (LD50 values for ivermectin and mifepristone in 50% confluent HeLa cells incubated for 24 h with each compound were 150 μM and 33 mM respectively; the assay was performed using the Invitrogen Multitox Fluor Multiplex Cytotoxicity Assay). This is consistent with ivermectin being able to generally inhibit Impα/β1-mediated nuclear import, which is essential for HIV infection and the first demonstration that inhibitors of nuclear import can have potent antiviral activity. Mifepristone also significantly inhibited HIV infectivity (Figure 3A), as expected, consistent with its ability to specifically inhibit IN nuclear import activity.
Figure 3. Ivermectin can inhibit HIV-1 and DENV infection.
(A) HeLa cells were infected with 200 ng (capsid protein-equivalent) of VSV-G-pseudotyped NL4-3.Luc.R-E- HIV, treated with or without the indicated agents (concentration in parentheses, μM) 2 h after infection for 6 h, then medium was removed, and cells were harvested for measurement of luciferase reporter activity. LD50 values for ivermectin and mifepristone in 50% confluent HeLa cells incubated for 24 h with each compound were 150 μM and 33 mM respectively. Results are means±S.E.M. for an average of four repeats; *P<0.05, **P<0.01. (B) AlphaScreen binding inhibition curves for DENV NS5 and the indicated Imps. Assays were performed as described in the Materials and methods section using 30 μM His6-tagged NS5 protein and 30 μM biotinylated Impα/β1, in the presence of the indicated concentrations of ivermectin, mifepristone or DMSO (vehicle) control. (C) Vero cells were treated with or without 25 μM (left) or 50 μM (right) ivermectin or mifepristone (as indicated) for 3 h before infection with DENV-2. Cell culture medium was collected and viral titres were analysed at various times after infection by plaque assay. PFU, plaque-forming units.
In the case of DENV, it has been shown previously that, despite its critical role in viral replication, which occurs in the cytoplasm, a majority of DENV NS5 protein is found in the nucleus during certain parts of the virus infectious life cycle and that mutation of critical residues in the Impα/β1-recognized NLS severely inhibits virus production [3]. As a first step towards investigating the use of ivermectin as an anti-DENV treatment, inhibition of NS5 binding to Impα/β1 was first examined using our established AlphaScreen protein–protein-binding assay. Ivermectin was found to strongly inhibit the binding of Impα/β1 to NS5 (IC50=17 μM, Figure 3B), but not of Impβ1 alone to NS5 (IC50>22 mM; it should be noted that NS5 contains a secondary NLS recognized by Impβ1 alone that, in contrast with the Impα/β1-recognized NLS, is not essential for NS5 nuclear accumulation) [3]. This indicates that ivermectin is able specifically to disrupt the interaction between NS5 and Impα/β1. Mifepristone, in contrast, showed no significant effect on the binding of NS5 to either Imp as expected.
To test whether ivermectin can inhibit DENV infection, Vero cells were treated with or without ivermectin for 3 h before infection with DENV-2 (Figure 3C). Ivermectin, in contrast with mifepristone, almost completely abolished virus production when utilized at 50 μM, and significantly reduced virus production at 25 μM, consistent with the critical role nuclear import plays in the DENV life cycle [3]. It should be noted that the present study was intended to prove the principle that an inhibitor of viral protein nuclear import could have antiviral properties, and is not in any way proposing that ivermectin should be used at 25 μM or anything near that to treat viral disease. In this context, it should also be remembered that optimization experiments or rigorous dose-response analysis have not been performed; however, it does show that inhibitors of nuclear transport can be potent antiviral agents and provides a platform for further development of antivirals in the future.
In summary, the results of the present study show that ivermectin is a novel inhibitor of nuclear protein import specifically mediated by Impα/β1; the nuclear accumulation of all Impα/β1-recognized cargoes tested to date can be inhibited by short treatments with ivermectin under conditions that do not lead to cytotoxicity, with no effect on nuclear import mediated by other Imps such as Impβ1 alone or Imp13. Our recent work demonstrating that ivermectin inhibits binding of Impα2 to IN and NS5 even in the absence of Impβ1 in the AlphaScreen assay (S. M. Heaton, K. M. Wagstaff and D. A. Jans, unpublished work) strongly implies that ivermectin's mode of action is likely to be through binding to the NLS-binding pocket of Impα, thereby preventing it from recognizing NLS-containing cargo proteins, rather than alternative mechanisms such as interfering with Impα/β heterodimerization. This is in stark contrast with small-molecule nuclear import inhibitors directed at Impβ1 that prevent binding to RanGTP/cargo release [10,14], which are mostly unsuitable for live-cell work because of uptake and precipitation issues and not highly efficient in inhibiting nuclear import in all cells. Most importantly, in the present study, we have demonstrated for the first time that inhibitors of nuclear import such as ivermectin can be potent antiviral agents, able to significantly inhibit the production of HIV-1 and DENV in infected cell systems.
Apart from the importance of the observations of the present study in terms of the potential use of ivermectin in the future for research purposes, the results imply that nuclear import of specific viral proteins is clearly a viable target for the development of urgently needed antivirals to tackle a number of the world's major diseases. Compounds that are specific in inhibiting viral protein nuclear import, such as mifepristone as shown in the present study, loom as exciting possibilities in this context, and are the focus of future work in this laboratory.
AUTHOR CONTRIBUTION
Kylie Wagstaff designed and executed the majority of experiments (except as noted) and wrote, drafted and edited the paper before submission. Haran Sivakumaran performed the HIV infectivity assay (Figure 3A). Steven Heaton performed some of the DENV infectivity assays (Figure 3C, left-hand panel). David Harrich supervised/provided technical expertise on the HIV infectivity assays. David Jans supervised/provided technical expertise on all aspects of the experimental procedures and critically evaluated/edited the paper before submission.
ACKNOWLEDGEMENTS
We thank Cassandra David for routine tissue culture performed for the present study. Some of the confocal imaging used in the present study was performed at the Monash Micro Imaging facility, Monash University, Clayton.
FUNDING
This work is supported by the National Health and Medical Research Council [project grant number 606409 and fellowship number APP1002486] and the Australian Research Council [fellowship number DP110104437].
References
- 1.Hogarth C. A., Calanni S., Jans D. A., Loveland K. L. Importin α mRNAs have distinct expression profiles during spermatogenesis. Dev. Dyn. 2006;235:253–262. doi: 10.1002/dvdy.20569. [DOI] [PubMed] [Google Scholar]
- 2.Moseley G. W., Filmer R. P., DeJesus M. A., Jans D. A. Nucleocytoplasmic distribution of rabies virus P-protein is regulated by phosphorylation adjacent to C-terminal nuclear import and export signals. Biochemistry (Moscow) 2007;46:12053–12061. doi: 10.1021/bi700521m. [DOI] [PubMed] [Google Scholar]
- 3.Pryor M. J., Rawlinson S. M., Butcher R. E., Barton C. L., Waterhouse T. A., Vasudevan S. G., Bardin P. G., Wright P. J., Jans D. A., Davidson A. D. Nuclear localization of dengue virus nonstructural protein 5 through its importin α/β-recognized nuclear localization sequences is integral to viral infection. Traffic. 2007;8:795–807. doi: 10.1111/j.1600-0854.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 4.Forwood J. K., Jans D. A. Nuclear import pathway of the telomere elongation suppressor TRF1: inhibition by importin α. Biochemistry (Moscow) 2002;41:9333–9340. doi: 10.1021/bi025548s. [DOI] [PubMed] [Google Scholar]
- 5.Forwood J. K., Lam M. H., Jans D. A. Nuclear import of Creb and AP-1 transcription factors requires importin-β1 and Ran but is independent of importin-α. Biochemistry (Moscow) 2001;40:5208–5217. doi: 10.1021/bi002732+. [DOI] [PubMed] [Google Scholar]
- 6.Lam M. H., Briggs L. J., Hu W., Martin T. J., Gillespie M. T., Jans D. A. Importin β recognizes parathyroid hormone-related protein with high affinity and mediates its nuclear import in the absence of importin α. J. Biol. Chem. 1999;274:7391–7398. doi: 10.1074/jbc.274.11.7391. [DOI] [PubMed] [Google Scholar]
- 7.Lam M. H., Thomas R. J., Loveland K. L., Schilders S., Gu M., Martin T. J., Gillespie M. T., Jans D. A. Nuclear transport of parathyroid hormone (PTH)-related protein is dependent on microtubules. Mol. Endocrinol. 2002;16:390–401. doi: 10.1210/mend.16.2.0775. [DOI] [PubMed] [Google Scholar]
- 8.Ambrus G., Whitby L. R., Singer E. L., Trott O., Choi E., Olson A. J., Boger D. L., Gerace L. Small molecule peptidomimetic inhibitors of importin α/β mediated nuclear transport. Bioorg. Med. Chem. 2010;18:7611–7620. doi: 10.1016/j.bmc.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cansizoglu A. E., Lee B. J., Zhang Z. C., Fontoura B. M., Chook Y. M. Structure-based design of a pathway-specific nuclear import inhibitor. Nat. Struct. Mol. Biol. 2007;14:452–454. doi: 10.1038/nsmb1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hintersteiner M., Ambrus G., Bednenko J., Schmied M., Knox A. J., Meisner N. C., Gstach H., Seifert J. M., Singer E. L., Gerace L., Auer M. Identification of a small molecule inhibitor of importin β mediated nuclear import by confocal on-bead screening of tagged one-bead one-compound libraries. ACS Chem. Biol. 2010;5:967–979. doi: 10.1021/cb100094k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou Y., McGuinness D. E., Prongay A. J., Feld B., Ingravallo P., Ogert R. A., Lunn C. A., Howe J. A. Screening for antiviral inhibitors of the HIV integrase–LEDGF/p75 interaction using the AlphaScreen luminescent proximity assay. J. Biomol. Screening. 2008;13:406–414. doi: 10.1177/1087057108317060. [DOI] [PubMed] [Google Scholar]
- 12.Kosugi S., Hasebe M., Entani T., Takayama S., Tomita M., Yanagawa H. Design of peptide inhibitors for the importin α/β nuclear import pathway by activity-based profiling. Chem. Biol. 2008;15:940–949. doi: 10.1016/j.chembiol.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Mata M. A., Satterly N., Versteeg G. A., Frantz D., Wei S., Williams N., Schmolke M., Peña-Llopis S., Brugarolas J., Forst C. V., et al. Chemical inhibition of RNA viruses reveals REDD1 as a host defense factor. Nat. Chem. Biol. 2011;7:712–719. doi: 10.1038/nchembio.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soderholm J. F., Bird S. L., Kalab P., Sampathkumar Y., Hasegawa K., Uehara-Bingen M., Weis K., Heald R. Importazole, a small molecule inhibitor of the transport receptor importin-β. ACS Chem. Biol. 2011;6:700–708. doi: 10.1021/cb2000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wigle T. J., Herold J. M., Senisterra G. A., Vedadi M., Kireev D. B., Arrowsmith C. H., Frye S. V., Janzen W. P. Screening for inhibitors of low-affinity epigenetic peptide–protein interactions: an AlphaScreen-based assay for antagonists of methyl-lysine binding proteins. J. Biomol. Screening. 2010;15:62–71. doi: 10.1177/1087057109352902. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J. H., Chung T. D., Oldenburg K. R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screening. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 17.Flint S. J., Huang W., Goodhouse J., Kyin S. A peptide inhibitor of exportin1 blocks shuttling of the adenoviral E1B 55 kDa protein but not export of viral late mRNAs. Virology. 2005;337:7–17. doi: 10.1016/j.virol.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Kau T. R., Schroeder F., Ramaswamy S., Wojciechowski C. L., Zhao J. J., Roberts T. M., Clardy J., Sellers W. R., Silver P. A. A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell. 2003;4:463–476. doi: 10.1016/s1535-6108(03)00303-9. [DOI] [PubMed] [Google Scholar]
- 19.Kau T. R., Way J. C., Silver P. A. Nuclear transport and cancer: from mechanism to intervention. Nat. Rev. Cancer. 2004;4:106–117. doi: 10.1038/nrc1274. [DOI] [PubMed] [Google Scholar]
- 20.Meissner T., Krause E., Vinkemeier U. Ratjadone and leptomycin B block CRM1-dependent nuclear export by identical mechanisms. FEBS Lett. 2004;576:27–30. doi: 10.1016/j.febslet.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 21.Mutka S. C., Yang W. Q., Dong S. D., Ward S. L., Craig D. A., Timmermans P. B., Murli S. Identification of nuclear export inhibitors with potent anticancer activity in vivo. Cancer Res. 2009;69:510–517. doi: 10.1158/0008-5472.CAN-08-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagstaff K. M., Rawlinson S. M., Hearps A. C., Jans D. A. An AlphaScreen®-based assay for high-throughput screening for specific inhibitors of nuclear import. J. Biomol. Screening. 2011;16:192–200. doi: 10.1177/1087057110390360. [DOI] [PubMed] [Google Scholar]
- 23.Babalola O. E. Ocular onchocerciasis: current management and future prospects. Clin. Ophthalmol. 2011;5:1479–1491. doi: 10.2147/OPTH.S8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Victoria J., Trujillo R. Topical ivermectin: a new successful treatment for scabies. Pediatr. Dermatol. 2001;18:63–65. doi: 10.1046/j.1525-1470.2001.018001063.x. [DOI] [PubMed] [Google Scholar]
- 25.Strycharz J. P., Yoon K. S., Clark J. M. A new ivermectin formulation topically kills permethrin-resistant human head lice (Anoplura: Pediculidae) J. Med. Entomol. 2008;45:75–81. doi: 10.1603/0022-2585(2008)45[75:aniftk]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Hearps A. C., Jans D. A. HIV-1 integrase is capable of targeting DNA to the nucleus via an importin α/β-dependent mechanism. Biochem. J. 2006;398:475–484. doi: 10.1042/BJ20060466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Efthymiadis A., Briggs L. J., Jans D. A. The HIV-1 Tat nuclear localization sequence confers novel nuclear import properties. J. Biol. Chem. 1998;273:1623–1628. doi: 10.1074/jbc.273.3.1623. [DOI] [PubMed] [Google Scholar]
- 28.Truant R., Cullen B. R. The arginine-rich domains present in human immunodeficiency virus type 1 Tat and Rev function as direct importin β-dependent nuclear localization signals. Mol. Cell. Biol. 1999;19:1210–1217. doi: 10.1128/mcb.19.2.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai Y., Gao Y., Sheng Q., Miao S., Cui X., Wang L., Zong S., Koide S. S. Characterization and potential function of a novel testis-specific nucleoporin BS-63. Mol. Reprod. Dev. 2002;61:126–134. doi: 10.1002/mrd.1139. [DOI] [PubMed] [Google Scholar]
- 30.Wagstaff K. M., Glover D. J., Tremethick D. J., Jans D. A. Histone-mediated transduction as an efficient means for gene delivery. Mol. Ther. 2007;15:721–731. doi: 10.1038/sj.mt.6300093. [DOI] [PubMed] [Google Scholar]
- 31.Rawlinson S. M., Pryor M. J., Wright P. J., Jans D. A. CRM1-mediated nuclear export of dengue virus RNA polymerase NS5 modulates interleukin-8 induction and virus production. J. Biol. Chem. 2009;284:15589–15597. doi: 10.1074/jbc.M808271200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poon I. K., Oro C., Dias M. M., Zhang J., Jans D. A. Apoptin nuclear accumulation is modulated by a CRM1-recognized nuclear export signal that is active in normal but not in tumor cells. Cancer Res. 2005;65:7059–7064. doi: 10.1158/0008-5472.CAN-05-1370. [DOI] [PubMed] [Google Scholar]
- 33.Poon I. K., Oro C., Dias M. M., Zhang J. P., Jans D. A. A tumor cell-specific nuclear targeting signal within chicken anemia virus VP3/apoptin. J. Virol. 2005;79:1339–1341. doi: 10.1128/JVI.79.2.1339-1341.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagstaff K. M., Jans D. A. Intramolecular masking of nuclear localization signals: analysis of importin binding using a novel AlphaScreen-based method. Anal. Biochem. 2006;348:49–56. doi: 10.1016/j.ab.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 35.Hubner S., Xiao C. Y., Jans D. A. The protein kinase CK2 site (Ser111/112) enhances recognition of the simian virus 40 large T-antigen nuclear localization sequence by importin. J. Biol. Chem. 1997;272:17191–17195. doi: 10.1074/jbc.272.27.17191. [DOI] [PubMed] [Google Scholar]
- 36.Xiao C. Y., Hubner S., Jans D. A. SV40 large tumor antigen nuclear import is regulated by the double-stranded DNA-dependent protein kinase site (serine 120) flanking the nuclear localization sequence. J. Biol. Chem. 1997;272:22191–22198. doi: 10.1074/jbc.272.35.22191. [DOI] [PubMed] [Google Scholar]
- 37.Wagstaff K. M., Fan J. Y., De Jesus M. A., Tremethick D. J., Jans D. A. Efficient gene delivery using reconstituted chromatin enhanced for nuclear targeting. FASEB J. 2008;22:2232–2242. doi: 10.1096/fj.07-099911. [DOI] [PubMed] [Google Scholar]
- 38.Gualano R. C., Pryor M. J., Cauchi M. R., Wright P. J., Davidson A. D. Identification of a major determinant of mouse neurovirulence of dengue virus type 2 using stably cloned genomic-length cDNA. J. Gen. Virol. 1998;79:437–446. doi: 10.1099/0022-1317-79-3-437. [DOI] [PubMed] [Google Scholar]
- 39.Pryor M. J., Gualano R. C., Lin B., Davidson A. D., Wright P. J. Growth restriction of dengue virus type 2 by site-specific mutagenesis of virus-encoded glycoproteins. J. Gen. Virol. 1998;79:2631–2639. doi: 10.1099/0022-1317-79-11-2631. [DOI] [PubMed] [Google Scholar]
- 40.Alvisi G., Ripalti A., Ngankeu A., Giannandrea M., Caraffi S. G., Dias M. M., Jans D. A. Human cytomegalovirus DNA polymerase catalytic subunit pUL54 possesses independently acting nuclear localization and ppUL44 binding motifs. Traffic. 2006;7:1322–1332. doi: 10.1111/j.1600-0854.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 41.Cimica V., Chen H. C., Iyer J. K., Reich N. C. Dynamics of the STAT3 transcription factor: nuclear import dependent on Ran and importin-β1. PLoS ONE. 2011;6:e20188. doi: 10.1371/journal.pone.0020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaur G., Jans D. A. Dual nuclear import mechanisms of sex determining factor SRY: intracellular Ca2+ as a switch. FASEB J. 2011;25:665–675. doi: 10.1096/fj.10-173351. [DOI] [PubMed] [Google Scholar]
- 43.Lam M. H., Hu W., Xiao C. Y., Gillespie M. T., Jans D. A. Molecular dissection of the importin β1-recognized nuclear targeting signal of parathyroid hormone-related protein. Biochem. Biophys. Res. Commun. 2001;282:629–634. doi: 10.1006/bbrc.2001.4607. [DOI] [PubMed] [Google Scholar]
- 44.Jakel S., Albig W., Kutay U., Bischoff F. R., Schwamborn K., Doenecke D., Gorlich D. The importin β/importin 7 heterodimer is a functional nuclear import receptor for histone H1. EMBO J. 1999;18:2411–2423. doi: 10.1093/emboj/18.9.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langer T. Nuclear transport of histone 2b in mammalian cells is signal- and energy-dependent and different from the importin α/β-mediated process. Histochem. Cell Biol. 2000;113:455–465. doi: 10.1007/s004180000147. [DOI] [PubMed] [Google Scholar]
- 46.Mosammaparast N., Guo Y., Shabanowitz J., Hunt D. F., Pemberton L. F. Pathways mediating the nuclear import of histones H3 and H4 in yeast. J. Biol. Chem. 2002;277:862–868. doi: 10.1074/jbc.M106845200. [DOI] [PubMed] [Google Scholar]
- 47.Mingot J. M., Kostka S., Kraft R., Hartmann E., Gorlich D. Importin 13: a novel mediator of nuclear import and export. EMBO J. 2001;20:3685–3694. doi: 10.1093/emboj/20.14.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fulcher A. J., Jans D. A. Regulation of nucleocytoplasmic trafficking of viral proteins: an integral role in pathogenesis? Biochim. Biophys. Acta. 2011;1813:2176–2190. doi: 10.1016/j.bbamcr.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghildyal R., Ho A., Wagstaff K. M., Dias M. M., Barton C. L., Jans P., Bardin P., Jans D. A. Nuclear import of the respiratory syncytial virus matrix protein is mediated by importin beta1 independent of importin α. Biochemistry (Moscow) 2005;44:12887–12895. doi: 10.1021/bi050701e. [DOI] [PubMed] [Google Scholar]
- 50.Tavassoli A. Targeting the protein–protein interactions of the HIV lifecycle. Chem. Soc. Rev. 2011;40:1337–1346. doi: 10.1039/c0cs00092b. [DOI] [PubMed] [Google Scholar]