Abstract
In 1987, an outbreak of primary tuberculosis occurred in a Canadian aboriginal community of 350 people. The source case was a young woman who had been symptomatic for four months with smear positive cavitary pulmonary tuberculosis. Her 17 siblings and their families were frequent close contacts. Among the 626 persons surveyed in the community and environs, 35 additional active cases of tuberculosis were identified. The mean age of cases was 13 years and the median age 10 years. The method of diagnosis was bacteriological in 20 and radiological in 16. There were 257 positive tuberculin reactors of whom 120 had no previous record of a positive skin test. Isoniazid prophylaxis was recommended to all new reactors, close household contacts, reactors under the age of 35 years and reactors with lung scars. One late case was identified at one year of follow-up in a contact who had refused prophylaxis. The rates of infection and disease were higher in the family (65% and 46%, respectively) than in the community and environs (19% and 5.6%, respectively). This report illustrates the nature of a point source epidemic of primary tuberculosis in a susceptible community with a predictable reservoir of infection. The delay in diagnosis of the source case allowed numerous new infections to occur. However, prompt aggressive contact follow-up was successful in containing the epidemic. To prevent future outbreaks, the reservoir of infected persons must be identified and administered chemoprophylaxis.
Keywords: Aboriginal, Epidemic, Primary, Prophylaxis, Tuberculosis
RESUME:
En 1987, une épidémie de tuberculose de première atteinte est survenue dans une localité autochtone canadienne de 350 habitants. La source d’infection était une jeune femme qui présentait des symptômes depuis quatre mois et chez qui un frottis de crachats a confirmé une tuberculose pulmonaire cavitaire. Elle était fréquemment en contact avec ses 17 frères et soeurs et leurs families. Parmi les 626 personnes qui ont fait l’objet d’une enquête dans la localité et les environs, 35 cas supplémentaires de tuberculose active ont été diagnostiqués. L’âge moyen des personnes atteintes était de 13 ans et l’âge médian de 10 ans. La méthode diagnostique était bactériologique dans 20 cas et radiologique dans 16. Deux cent cinquantesept sujets ont présenté une réaction positive à la tuberculine, dont 120 pour la première fois. L’isoniazide a été prescrit à titre préventif à tous les nouveaux sujets à réaction tuberculinique positive, ainsi qu’aux proches de la première malade, aux sujets à réaction positive âgés de moins de 35 ans et aux sujets à réaction positive dont la radiographie pulmonaire présentait un voile. Un dernier cas a été identifié en cours de suivi, un an plus tard, chez un contact qui avait refusé le traitement prophylactique. Les taux d’infection et de maladie étaient plus élevés dans la famille de la première jeune femme diagnostiquée (65 % et 46 %, respectivement) que dans la localité et les environs (19 % et 5,6 %, respectivement). Le présent rapport illustre la nature de la source d’une épidémie de primo-infection tuberculeuse dans une collectivité réceptive comportant un réservoir prévisible d’agents infectieux. Le diagnostic tardif du premier contaminateur explique la survenue des nombreux cas nouveaux d’infection. Cependant, le dépistage énergique des contacts a permis de contenir l’épidémie avec succès. Pour prévenir les épidémies dans l’avenir, il est impératif de reconnaître le réservoir de personnes infectées et d’administrer une chimioprophylaxie.
Tuberculosis continues to be a major problem in the aboriginal population of Canada. During the 1970s the incidence of tuberculosis among Treaty native Canadians was 16 times greater than rates in nonaboriginal Canadians excluding the Inuit (1). Because Treaty native Canadians are registered with the government of Canada, accurate calculation of tuberculosis rates is possible. Since 1980, the rates of tuberculosis in Alberta have continued to fall, from 11.9 per 100,000 (1980) to 5.4 per 100,000 (1989), 5.5% per year. However, the rates in Treaty native Canadians have remained eight to 17 times higher than in other Albertans and 30 to 50 times higher than in other Albertans born in Canada.
Eilertsen (2) has defined an epidemic of primary tuberculosis as “the occurrence of new cases of tuberculosis in excess of the average for the period, with one or few sources of infection”. Previous reviews of tuberculosis epidemics have not described outbreaks occurring among Treaty native Canadians (2–4). In this paper the authors describe a single point source epidemic which occurred in an aboriginal community in northern Alberta in the summer of 1987. Thirty-six cases were identified, of which 34 were new infections.
PATIENTS AND METHODS
Alberta Tuberculosis Services and University of Alberta Hospital records and x-rays of active tuberculosis cases were reviewed. All chest x-rays of active cases were independently reviewed by a radiologist. Bacteriological data were provided by the Provincial Laboratory of Public Health in Edmonton. Results of tuberculin skin testing were supplied by the local provincial Health Unit and Medical Services Branch, northern Alberta region. The community involved was visited and representative members of the affected family interviewed.
DESCRIPTION OF THE EPIDEMIC
The community:
The epidemic occurred in a rural community with a population of 350 persons (hereafter designated as ‘the community’). Most of the community members are Treaty native Canadians who belong to seven or eight large families, interrelated by marriage. The population is approximately one-half children and one-half adults. Detailed population statistics are not available because of outstanding land claims. The resident population numbers fluctuate seasonally. Hunting and trapping are the major economic activities of the community. Housing consists of small one-story frame dwellings heated by wood stoves and ventilated in summer with open doors and windows. There is no indoor plumbing.
Tuberculosis control activities in the community had consisted of annual tuberculin skin testing of school children in grades one and nine until September 1985, when testing was discontinued because reactors were so infrequent. No routine tuberculin skin testing had been done among adults since 1981. There had been no routine use of Calmette-Guérin bacillus (BCG) vaccination since 1963. Although tuberculosis cases were common in the 1950s and 1960s, the only recorded case of tuberculosis after 1969 was in a patient with tuberculous osteomyelitis in 1977. The last smear positive pulmonary case was identified in 1959.
The community is located 99 km from a town of 6355 persons (hereafter designated as ‘the town’). In addition, there is a second community located 14 km from the first, in which reside 184 persons of non-Treaty status who frequently visit the first community.
Index case:
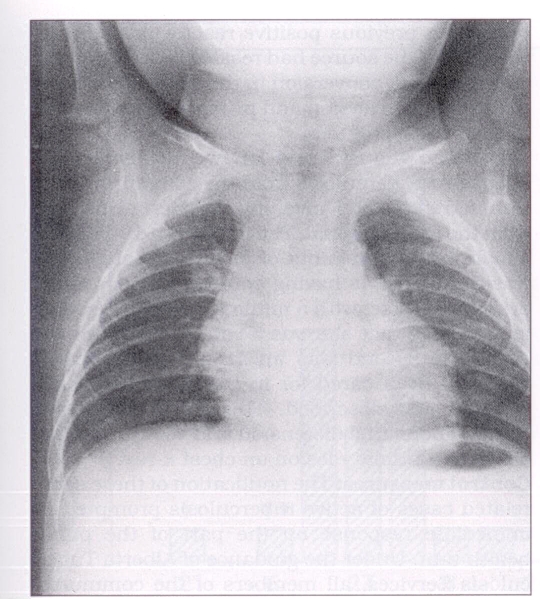
In May 1987, a four-month-old native Canadian male was admitted to the town hospital for investigation of cough. He was treated with erythromycin because two cases of pertussis had recently been diagnosed in the town. A skin test with five tuberculin units (TU) of purified protein derivative (PPD) was positive with 10 mm induration. A chest x-ray showed right paratracheal lymph node enlargement and a small patch of consolidation in the right upper lobe (Figure 1). Treatment for active tuberculosis was initiated July 21, 1987.
Figure 1).
Chest x-ray, index case, four-month-old male: right upper lobe patchy consolidation
Source case:
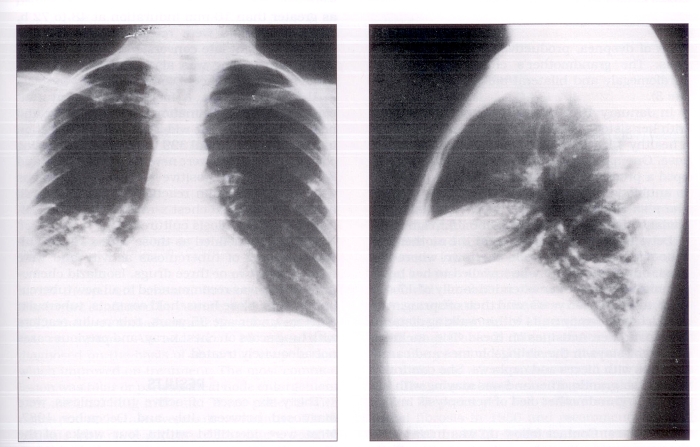
The diagnosis of tuberculosis in the infant prompted a search for a source case by public health authorities. The child’s 25-year-old mother had been unwell with cough and weight loss for several months. Her chest x-ray revealed a cavitary lesion with an air fluid level in the right lower lobe and consolidation in the right middle lobe (Figure 2). Sputum specimens showed a large number of acid-fast bacilli and cultures were positive for Mycobacterium tuberculosis at three weeks. Mother and child were transferred to the University of Alberta Hospital for further management on August 9, 1987.
Figure 2).
Chest x-ray, source case, 25-year-old female: right lower lobe cavity, right middle lobe consolidation
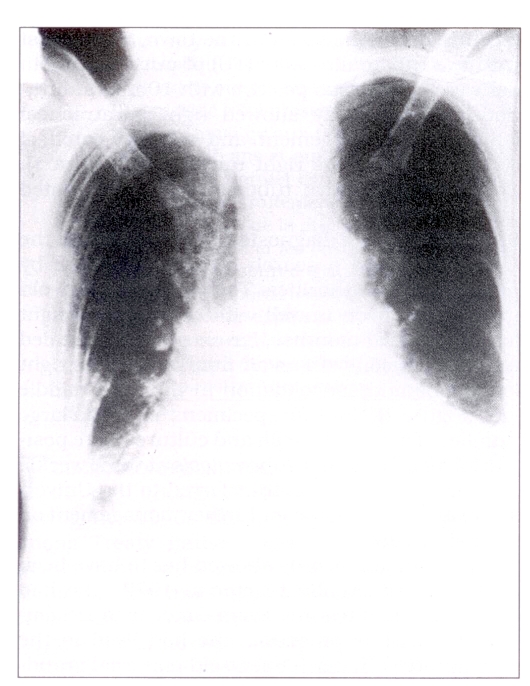
The mother’s records showed her to have been a negative tuberculin reactor in 1977. She had had no interval testing. From October to December 1986, while pregnant, she had lived in the community with her 94-year-old maternal grandmother, who at the time had been ill with symptoms of dyspnea, productive cough and hemoptysis. The grandmother’s chest x-ray showed cardiomegaly and bilateral basal infiltrates (Figure 3).
Figure 3).
Chest x-ray, 94-year-old grandmother: bilateral basal infiltrates, cardiomegaly
In January 1987, the mother returned to live with her sister’s family and in February delivered a healthy 4 kg (8 1b, 12 oz) male infant, the index case. One month post partum, the mother developed a productive cough which failed to respond to antibiotic therapy. Over the next four months she also experienced fever, night sweats, occasional hemoptysis and a 22 kg (48 1b) weight loss.
Between February and August the mother had little contact with people in the town where she lived, but on weekends she travelled to her home community to visit her extended family of 17 siblings, aged 16 to 45 years, and their offspring, who lived in small family units within walking distance of one another. Activities on these visits included overnight stays in the siblings’ homes and camping trips with nieces and nephews. She continued to visit her grandmother and was staying with her when the grandmother died of hemoptysis in May 1987.
Other cases:
Contact follow-up was initiated on the assumption that the mother was the likely source case. Examination of the first sibling family revealed a previous positive reactor in the sister with whom the source had resided, a recent tuberculin skin test conversion in the sister’s husband, and recent conversion and primary x-ray changes in their three children.
Between August 7 and 13, two further cases of suspected tuberculosis from the community were transferred to the University of Alberta Hospitals from the town hospital. The first was a 19-year-old female with complaints of fever and cough. She was diagnosed as having sputum smear positive primary disease, with a minimal lesion. Questioning revealed that she was a contact of the source case. The second was an 18-month-old female who had been cared for in the home of the 19-year-old just described. This infant had fever, cough, abdominal distension and vomiting, in addition to a primary lesion on chest x-ray.
Control measures:
The notification of these seven related cases of active tuberculosis prompted an immediate response on the part of the public health unit. Under the guidance of Alberta Tuberculosis Services, all members of the community without record of a previous positive tuberculin reaction received an intradermal tuberculin test with 5 TU PPD. Of 297 community members assessed, 53 were previous positive reactors and 46 were new reactors. A positive reaction was defined as greater than 10 mm induration at 48 to 72 h. Negative reactors were retested at eight to 12 weeks and eight late conversions were identified. Tuberculin testing was also carried out among residents of the adjacent community and contacts in the distant town. A mobile chest x-ray unit carried out 216 examinations of both positive and negative reactors. The wider contact investigation included an additional 329 people in surrounding communities. Sixty were new positive reactors and 84 were previous positive reactors. All persons with large tuberculin reactions, symptoms of ill health or abnormal chest x-rays had sputum collected for tuberculosis cultures.
Cases were defined as those with x-ray or culture evidence of tuberculosis activity and were treated with two or three drugs. Isoniazid chemoprophylaxis was recommended to all new tuberculin reactors, close household contacts, tuberculin reactors under age 35 years, tuberculin reactors with lung scars on chest x-ray, and previous cases not adequately treated.
RESULTS
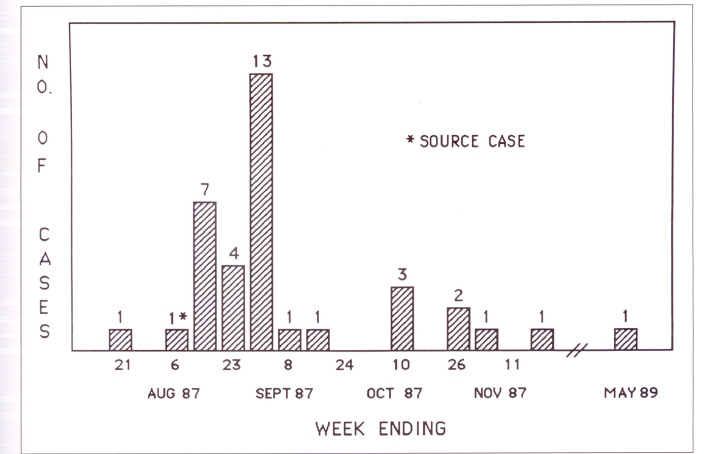
Thirty-six cases of active tuberculosis were diagnosed between July and December 1987. Most were identified within four weeks of the diagnosis of the source case (Figure 4). Eight were diagnosed by the microscopic finding of acid-fast bacilli in sputum smears. Subsequently, all of these were confirmed by culture, and an additional 12 who were initially smear negative were positive on culture. An additional 16 cases were diagnosed on the basis of abnormal chest x-rays which improved on treatment. The most common lesion was hilar or paratracheal node enlargement with minimal lung infiltrate. Three patients with nodes and atelectasis were culture negative. Three patients with normal x-rays were culture positive (Table 1). Several individuals initially diagnosed as ‘suspect tuberculosis’ on the basis of minimal x-ray change were reclassified as ‘no disease’, when culture and tuberculin skin tests remained negative and x-rays stable. One case was redefined after full treatment as tuberculin conversion only, because independent radiological interpretation did not confirm chest x-ray abnormality.
Figure 4).
Active cases by week of diagnosis
TABLE 1.
Radiological diagnosis of cases†
| + | − | |
|---|---|---|
| Normal | 3 | 1* |
| Nodes (paratracheal or hilar) | 1 | 1 |
| Nodes plus infiltrate | 11 | 9 |
| Nodes plus atelectasis | — | 3 |
| Infiltrate | 3 | 2 |
| Infiltrate plus cavity | 1 | — |
| Apical fibrosis plus infiltrate | 1 | — |
| Total | 20 | 16 |
Hilar adenopathy, original diagnosis not confirmed.
Independent radiology review.
Culture positive;
Culture negative
An examination of the 36 confirmed cases demonstrates a mean age of 13 years and a median age of 10 years (Table 2). Nine patients were younger than five years old; 12 were between six and 14 years old; and 12 were between 15 and 22 years old. The source case was 25 years old and her father 71 years old. The father’s record showed apical fibrosis in 1956 and recommendation for treatment in 1974; however, treatment was not confirmed. At the time of the epidemic his x-ray was stable and sputum culture negative. He refused prophylaxis. In May 1989, during hospitalization with bronchitis and a new left lower lobe peribronchial infiltrate, his sputum culture grew M tuberculosis. The source case may also have had reactivation disease, but had been a negative reactor when last tested in 1977 and had a lesion in the lower lung zone, unusual for reactivation disease. In the 20 instances of culture positive disease, the organism identified was M tuberculosis, sensitive to all drugs tested.
TABLE 2.
Active tuberculosis cases
| Total number of cases | 36 | |
| Sex: | Female | 13 |
| Male | 23 | |
| Age: | Range | 6 months to 71 years |
| Mean | 12.9 years | |
| Median | 10 years | |
Although there exists a Canadian Federal Medical Services policy to give BCG immunization to Treaty native Canadians at birth, because the provincial health agency contracted to provide health services does not administer BCG, none of the active cases had records of having received BCG. None had a previous history of active tuberculosis.
Contact with the presumptive source of the epidemic, the 25-year-old mother of the index case, was documented in 30 of the 35 active cases. Duration of contact ranged from a few hours in eight contacts to more than a day in 14. There were three cases in which contact could not be established. Two had had contact with the 19-year-old woman who was briefly smear positive and who was possibly a secondary source.
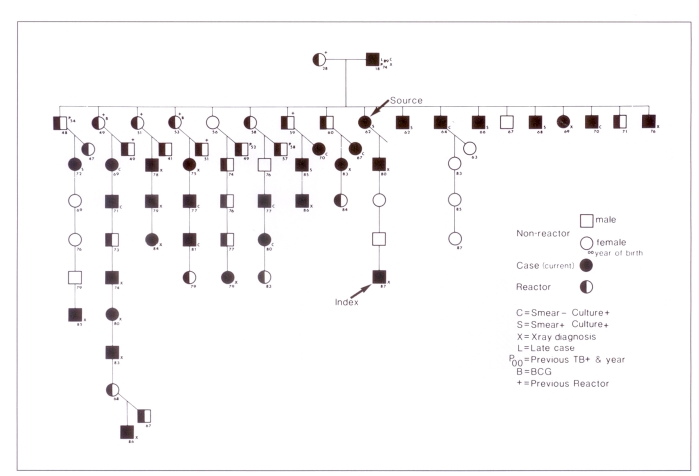
The group with the highest prevalence of infection and disease was the family membership of 69 persons (Figure 5), in which 45 persons were new positive reactors (infection rate 65%) (Table 3). Thirty-one of the active cases were from the family (attack rate 46%). These rates compare with an infection rate of 16.9% (106 of 626 at risk) and an attack rate of 5.6% (35 of 626 at risk) in members of the surrounding communities. Four older family members had records of previous tuberculosis, adequately treated. The older siblings were previous positive reactors with no disease.
Figure 5).
The family of a source case of an epidemic of primary tuberculosis in a Canadian aboriginal community in 1987
TABLE 3.
Contact follow-up in an epidemic of primary tuberculosis in a Canadian aboriginal community in 1987
| Family* | Community | Town | Elsewhere | Total (%) | ||
|---|---|---|---|---|---|---|
| Number assessed | (69) | 297 | 259 | 70 | 626 | |
| Tuberculin skin test | ||||||
| Previous positive | (11) | 53 | 66 | 18 | 137 | (21.8%) |
| New positive | (45) | 46 | 44 | 16 | 106 | (16.9%) |
| Late conversions | (0) | 8 | 7 | 0 | 15 | (2.4%) |
| Negative | (13) | 187 | 140 | 33 | 360 | (57.5%) |
| Not done | 3 | 2 | 3 | 8 | (1.3%) | |
| Number of x-rays taken | (69) | 93 | 91 | 32 | 216 | |
| Number of active cases | (32) | 36 | 0 | 0 | 36 | |
| Number recommended isoniazid | (24) | 60 | 84 | 28 | 172 | |
| Number accepted isoniazid | (22) | 41 | 55 | 18 | 114 | |
| Infection rate | 65% | 18% | 19% | 23% | 19% | |
| Disease attack rate | 46% | 5.6% | 0% | 0% | 5% | |
These subjects were also part of the ‘community’
Younger family members were more likely to have large positive reactions, and significant numbers had evidence of primary infection.
Treatment of active cases consisted of a two drug regimen of isoniazid and rifampin administered twice weekly under supervision for nine months (5,6). Only hospitalized patients had their initial course of treatment on a daily basis. The source case received pyrazinamide and streptomycin in addition to isoniazid and rifampin in the initial six weeks. Two patients required substitution of ethambutol for rifampin due to an elevated aspartate transaminase, and extended treatment to 15 months. Four patients experienced nausea and vomiting without a rise in aspartate transaminase, but only two of these required replacement of rifampin with ethambutol.
Isoniazid chemoprophylaxis was recommended to 172 contacts of the source and accepted by 114. Isoniazid was discontinued in eight contacts because of side effects, in seven because of noncompliance, and in 10 close contacts whose tuberculin reactivities remained negative at three months. Positive reactors refusing isoniazid were followed for two years.
DISCUSSION
This report exemplifies a ‘point source epidemic’ (7), which occurs when a previously uninfected population is exposed to a single source case resulting in ‘community contamination’ (3). The prolonged cough, cavitary lesion and direct smear positive sputum of the 25-year-old mother are factors associated with a high degree of infectivity (8–10). Although seven other active cases with direct smear positive sputum were identified, they were unlikely sources of further spread because their numbers of organisms were small and their symptoms were minimal and of short duration. Although no infectious tuberculosis had been identified in the community since 1960, it is probable that cases inadequately treated in the past may have excreted M tuberculosis intermittently. The grandmother with hemoptysis may have infected the 25-year-old woman, whose pregnant state may have increased her likelihood of reactivation or immediate progression to active disease (11). Prolonged close contact with large numbers of the immediate family resulted in 31 cases of primary disease and 45 new infections. The rates of infection (65%) and disease (46%) were higher in the family than in the community and its environs and higher than in other communities previously reported (Table 4) (2, 12–16).
TABLE 4.
Attack rates in community tuberculosis epidemics
| Year | Site (reference) | Number at risk | Infection rate (%) | Attack rate (%) |
|---|---|---|---|---|
| 1945 | Netherlands (11) | 1530 | 24.0 | 6.8 |
| 1956 | Bergen, Norway (2) | 10,963 | 0.4 | 0.2 |
| 1957 | Ontario (12) | 2,300 | 1.7 | — |
| 1960 | Saskatchewan (13) | 127 | — | 23.0 |
| 1963 | Northwest Territories (14) | 345 | — | 23.0 |
| 1979 | Sandwell, UK (15) | 3764 | 2.9 | 0.4 |
| 1987 | Community and environs | 626 | 19 | 5.6 |
| 1987 | Family | 69 | 65 | 46 |
The means of tuberculosis control in the elimination phase has been addressed in a strategic plan by the Centers for Disease Control (17). The first step in the plan for elimination is a recommendation for more stringent application of existing control methods: the early identification of cases, supervision of appropriate medication to achieve cure, and provision of prophylaxis to contacts and others at high risk of reactivation.
In this report, once the source case was identified, prompt and extensive follow-up of all contacts and administration of chemotherapy to active cases and prophylaxis to those infected with no evidence of disease, were central to the containment of this primary tuberculosis epidemic. None of the 114 contacts given isoniazid chemoprophylaxis developed tuberculosis. Among contacts who refused isoniazid, follow-up identified one case 20 months later.
BCG, advocated in populations where infection rates are greater than 1% per year, is recommended for Treaty native Canadians at birth. The degree of protection offered by BCG against development of tuberculosis ranges in several major prospective studies from 80 to 0% (18). Recently, several retrospective studies have examined BCG efficacy and have found it to be about 60% (19). Had BCG been given in the present community, the number of cases and converters might have been reduced by 50%. The background of BCG exposure might have confounded the decision to recommend prophylaxis. In exposure circumstances, BCG history should be ignored and isoniazid prophylaxis provided.
In countries of low incidence such as Canada, passive case finding is standard practice. There is, however, a place for active case finding in minority groups in whom rates of tuberculosis remain high (ie, immigrants from high risk countries and aboriginal Canadians). In such communities, the cost effectiveness of chest x-ray and sputum culture surveys must be assessed (20).
Whether school tuberculin surveys are of value as a means of detecting clusters of primary infection heralding an undetected source case, is debated. At present, the government of Alberta recommends that such skin test surveys be done in communities in which infection rates are greater than 1% or active case rates greater than 50 per 100,000 population. The decision to discontinue testing in this small, closed community seemed appropriate after 13 years of negative school surveys, during which time no source case had been identified. However, the reservoir of infection in the elderly had not been dealt with in a preventive manner. In Alaska, where tuberculosis rates in remote aboriginal communities resemble those in Alberta, universal tuberculin skin testing is maintained at school entry and intermittently to grade 11 (21). Clusters of reactors might have served to provoke an earlier search for a background source of infection.
CONCLUSION
The present description of a point source epidemic of tuberculosis in a small, relatively closed community with a very large population of children who had no previous exposure to tuberculosis, and in a small population of elderly individuals with extensive tuberculosis experience, is not unexpected in the elimination phase of tuberculosis.
The core of North American tuberculosis control practices includes passive case finding, prompt supervised treatment, and aggressive contact follow-up with prophylaxis for those infected with no disease. In selected communities of identifiable minorities known to have high rates of tuberculosis, there remains a place for active case finding by chest x-ray, sputum culture screening and tuberculin skin test surveys of school populations.
Acknowledgments
The authors acknowledge the efforts of Tuberculosis Services of Alberta, the Peace River Health Unit, station 84 of the University of Alberta Hospital, and the Tuberculosis Division of the Provincial Laboratory of Public Health in Edmonton for their prompt, effective response to this epidemic and their cooperation with the present documentation. The authors also thank Dr William Beamish, Department of Radiology, University of Alberta, for independent review of all chest roentgenograms.
REFERENCES
- 1.Enarson DA, Grzybowski S. Incidence of active tuberculosis in the native population of Canada. Can Med Assoc J. 1986;134:1149–52. [PMC free article] [PubMed] [Google Scholar]
- 2.Eilertsen E. Epidemics of primary tuberculosis and their significance. Acta Tuberc Scand. 1959;37:203–16. [PubMed] [Google Scholar]
- 3.Lincoln EM. Epidemics of tuberculosis. Adv Tuberc Res. 1965;14:157–201. [PubMed] [Google Scholar]
- 4.Davies JW. Epidemics of tuberculosis in Canada in the sixties. Can Med Assoc J. 1976;96:1156–60. [PMC free article] [PubMed] [Google Scholar]
- 5.Dutt AK, Stead WW. Short-course chemotherapy; the Arkansas experience. Chest. 1981;80:724–7. doi: 10.1378/chest.80.6.724. [DOI] [PubMed] [Google Scholar]
- 6.American Thoracic Society Treatment of tuberculosis and tuberculosis infection in adults and children. Am Rev Respir Dis. 1986;134:355–63. doi: 10.1164/arrd.1986.134.2.355. [DOI] [PubMed] [Google Scholar]
- 7.Last J, editor. A Dictionary of Epidemiology. New York: Oxford University Press; 1988. p. 42. [Google Scholar]
- 8.American Thoracic Society Control of tuberculosis. Am Rev Respir Dis. 1983;128:336–42. [PubMed] [Google Scholar]
- 9.Shaw JB, Wynn-Williams N. Infectivity of pulmonary tuberculosis in relation to sputum status. Am Rev Tuberc. 1954;69:724–32. doi: 10.1164/art.1954.69.5.724. [DOI] [PubMed] [Google Scholar]
- 10.Riley RL. Airborne infection. Am J Med. 1974;57:466–75. doi: 10.1016/0002-9343(74)90140-5. [DOI] [PubMed] [Google Scholar]
- 11.St Hill CA, Finn R, Denye V. Depression of cellular immunity in pregnancy due to a serum factor. Br Med J. 1973;3:513–4. doi: 10.1136/bmj.3.5879.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogewind F. Een tuberculose – Epidemic ten plattelande. Med T Geneesk. 1945;89:175. [Google Scholar]
- 13.Grzybowski S. A small epidemic of tuberculosis. Am Rev Tuberc. 1957;75:432–41. doi: 10.1164/artpd.1957.75.3.432. [DOI] [PubMed] [Google Scholar]
- 14.Barnett S. Tuberculosis control in Saskatchewan – Past and future. Lancet. 1961;81:167–70. [PubMed] [Google Scholar]
- 15.Moore PE. Puvalluttuq. An epidemic of tuberculosis at Eskimo Point, Northwest Territories. Presented at Annual Meeting of Canadian Tuberculosis Association; Toronto. 1963. [PMC free article] [PubMed] [Google Scholar]
- 16.Rao V, Joanes R, Kilbane P, Galbraith N. Outbreak of tuberculosis after minimal exposure to infection. Br Med J. 1980;281:187–9. doi: 10.1136/bmj.281.6234.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Public Health Service, Centers for Disease Control, Division of Tuberculosis Control A strategic plan for the elimination of tuberculosis in the United States. MMWR. 1989;38:S3. [Google Scholar]
- 18.Clemens J, Chuong J, Feinstein A. The BCG controversy – A methodological and statistical reappraisal. JAMA. 1983;249:2362–9. [PubMed] [Google Scholar]
- 19.Smith PG. Case control studies of the efficacy of BCG against tuberculosis. Tuberculosis and Respiratory Disease. Annual Congress of International Union Against Tuberculosis Proceedings; Singapore: Professional Postgraduate Services International. 1986. pp. 73–9. [Google Scholar]
- 20.US Public Health Service, Centers for Disease Control Screening for tuberculosis and tuberculous infection in high risk populations and the use of preventive therapy for tuberculosis infection in the United States: Recommendations of the Advisory Committee for the Elimination of Tuberculosis. MMWR. 1990;39:1–12. [Google Scholar]
- 21.Tuberculosis Policy Manual . Alaska: Division of Public Health, Department of Health and Social Services; 1990. [Google Scholar]