Introduction
Intracranial aneurysms are common lesions with an adult prevalence rate between one and five percent in autopsy studies 1. Fortunately, most aneurysms are small and an estimated 50 to 80 percent of all aneurysms do not rupture during the course of a person's lifetime 2. Intracranial aneurysms are considered to be sporadically acquired lesions, although a rare familiar form has been described3. Clinically, cerebral aneurysms can be silent or give rise to focal neurological symptoms or rupture leading to the dramatic event of subarachnoid haemorrhage.
Nowadays the gold standard technique for the detection of cerebral aneurysms is considered to be digital subtraction angiography (DSA) offering both dynamic and morphological information on the intracranial circulation. However, DSA is relatively expensive and not widely available. DSA also carries a potential risk of transient, reversible or permanent neurological and non neurological complications even though the actual risk is much lower than is perceived 4-13` In view of this, the ideal technique to evaluate cerebral aneurysms should be non invasive, easy and quick to perform, offering high spatial resolution and yielding optimal information on aneurysm location, size of its dome and neck, any parietal abnormality, collateral arteries originating from the dome and the relation between the parent artery and surrounding vascular and/or nervous structures.
Nowadays, the diagnostic work-up in patients with cerebral aneurysms or subarachnoid haemorrhage (SAH) includes several imaging techniques such as DSA, Computed Tomography Angiography (CTA) and Magnetic Resonance Angiography (MRA).
Worldwide neuroradiological experience during the past 15 years has led to a consensus on the major role of CTA in evaluating patients with intracranial aneurysms. CTA is a minimally invasive, less expensive technique than DSA and MRA, easy and quick to perform and often able to provide correct information on the presence, dimensions and anatomical location of cerebral aneurysms. Volumetric acquisition allows the neuroradiologist to obtain oblique, axial and para-axial views for better display of the aneurysm dome and neck 12-19.
The most important limit of CTA, widely accepted and common to most multislice CT scanners, is its dimensional constraints: most aneurysms with a dome diameter smaller than 3 mm (so-called "baby" aneurysms) can often be missed by CTA examination.
MRA, with different acquisition techniques (Time of flight - TOF or Phase Contrast - PC), is increasingly available, but its potential role remains limited by motion and vessels artefacts, sometimes even with contrast media injection. In addition, MRA is still considered a high cost procedure, strongly affected by the already known absolute and relative contraindications and some technical difficulties (particularly when dealing with SAH patients) 20-26.
In spite of these drawbacks, MRA has an important role in the follow-up of embolized cerebral aneurysms after endovascular treatment. In these cases, MRA sequences (TOF or PC), even without contrast media injection, are highly reliable in displaying major residual flow within the coil mesh or in the neck region of the aneurysm.
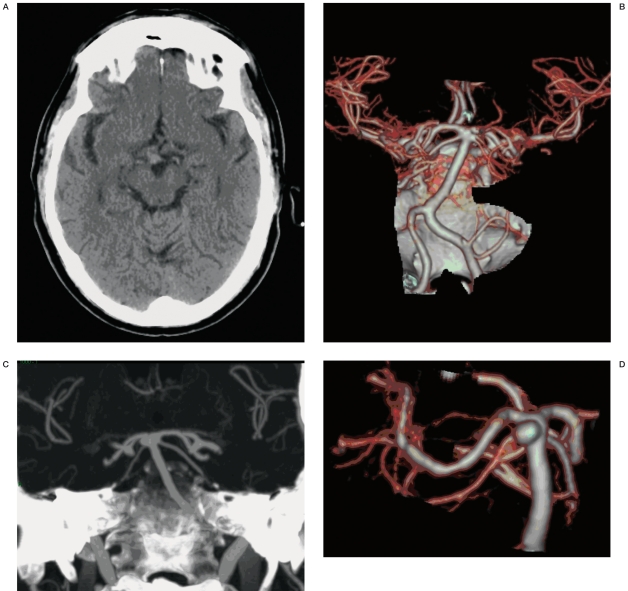
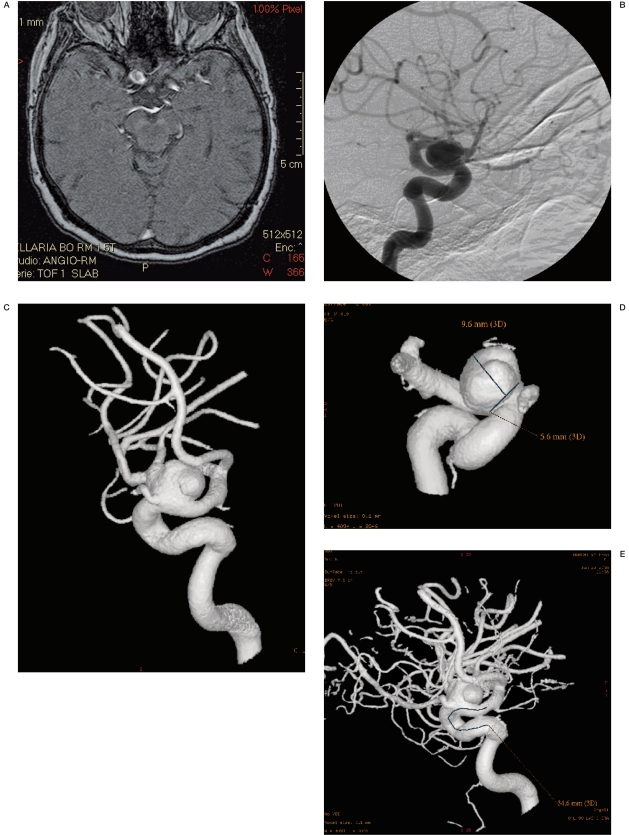
Figure 1 A-D.
CT and CTA (MIP and SSD) of a basilar aneurysm.
Role of DSA in evaluating cerebral aneurysms
Cerebral angiography, pioneered by Egas Moniz in 1927 27,28 and highly evolved towards a digital technique, is still considered the gold standard for detecting vascular abnormalities of the brain and especially cerebral aneurysms. This is because DSA is still now the only available means of obtaining haemodynamic information. Some aspects of cerebral haemodynamics, such as collateral flow and flow direction, cannot be demonstrated by CTA or even by MRA 29,30. Rotational DSA, with 3D reconstructions, may improve diagnostic accuracy particularly if no aneurysms are demonstrated by 2D-DSA. 3D DSA offers much more detailed information on the spatial relations between the aneurysm and surrounding vessels and structures for evaluation of the cerebral circulation allowing better patient management planning.
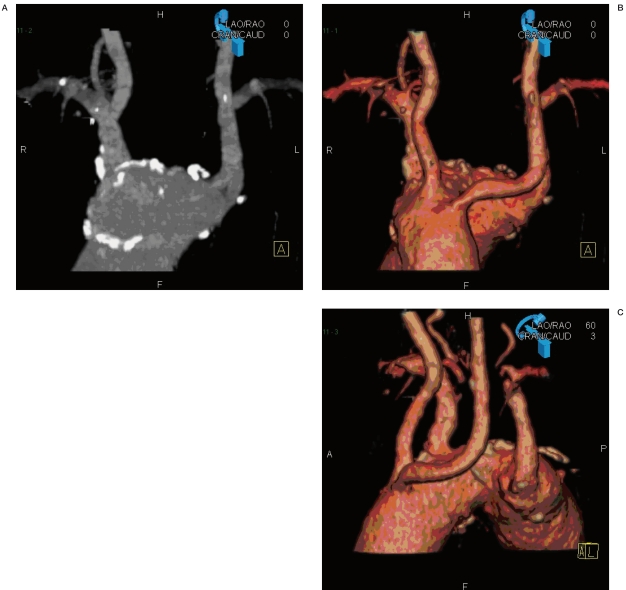
Figure 2 A-C.
Aortic arch in a patient with origin abnormalities of the supra-aortic vessels. CTA with MIP and SSD images completes the neuroradiological information for endovascular planning.
The 3D model allows observation and analysis from multiple directions to determine the working projection for interventional procedures. If the acquisition is made during a neuroradiological intervention session using latest generation DSA devices, the optimal working projection obtained from the model can be transferred back to the table side positioner control, thereby achieving subsequent acquisition at the same angle pushing a single button. Nowadays DSA remains strongly indicated in all those cases in which the diagnosis has not yet been established 2,3,30,31:
- SAH with a negative CTA;
- discrepancy between the distribution of SAH on CT scan and the display of the aneurysm on CTA;
- doubtful cases of CTA showing small aneu- rysms;
- CTA with suboptimal quality;
- to evaluate giant aneurysms to obtain haemodynamic information;
- to evaluate aneurysms very close to bone structures;
- to evaluate SAH supported by dissecting aneurysms;
- to evaluate multiple aneurysms;
- to better evaluate the correlations between the aneurysmal sac and the arteries surrounding or originating from the sac;
- last but not least, DSA is mandatory before endovascular treatment.
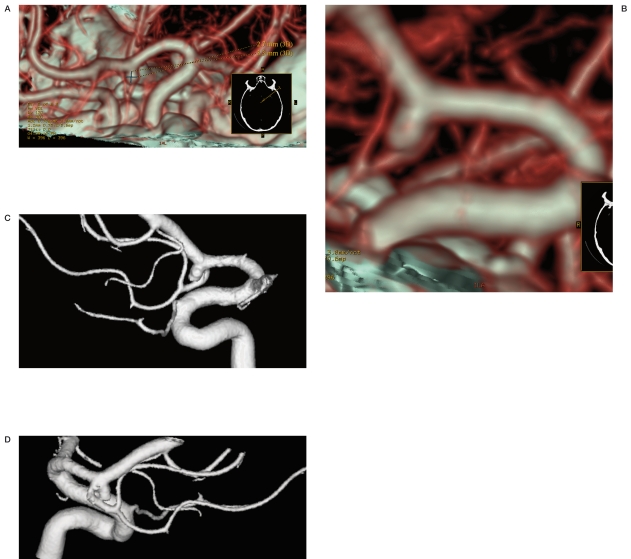
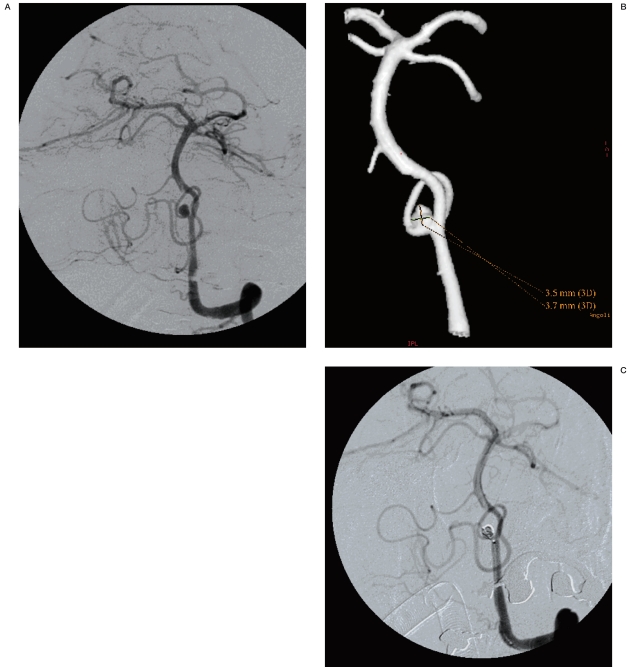
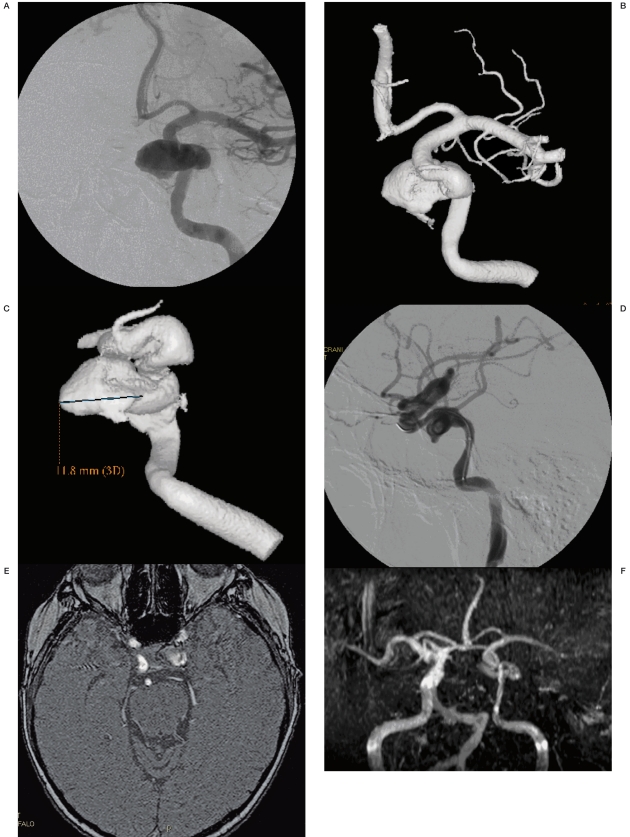
Figure 3 A-D.
CTA with SSD post-processing and 3D DSA equally showing a right MCA bifurcation aneurysm.
Angiographic examination fails to disclose an aneurysm in ten to 20 percent of cases of subarachnoid haemorrhage. When the results of angiography are negative, angiography is usually repeated in one to six weeks 2,32. Without an identified aneurysm the cause of subarachnoid haemorrhage remains unknown. Hypertensive rupture of a small artery or vein is one proposed mechanism. Other mechanisms may be spasm of the vessel harbouring the aneurysm, presence of haematoma and permanent or temporary thrombosis of the aneurysm. In these cases the question is whether we are dealing with a false negative finding. In particular, an aneurysm should be suspected in cases with a large amount of blood and in cases of focal haemorrhage, particularly at the level of the interhemispheric fissure.
The principal drawback of DSA is its potential risk. The combined transient and reversible neurologic complication rate of cerebral angiography has been reported to be as low as 0.4% and as high as 12.2% 4-11. The reported permanent neurologic complication rate varies from 0% to 5.4%. Limiting the review to prospective studies of 1,000 or more procedures reveals a combined transient and reversible neurologic complication rate between 0.4% and 2.3% (mean, 1.3%), a permanent neurologic complication rate between 0.1% and 0.5% (mean, 0.3%), and a mean overall rate of 1.6% 5-7,10-12. In our experience, complications were calculated in a series of 6000 vessels studied in 2154 patients disclosing 0.1 transient neurological deficits and 0.05 permanent neurological deficits. It is important for each neuroradiology team to assess its own incidence of complications rather than relying percentages published in the literature and refer to their own rates when giving patients information for the purposes of informed consent 13.
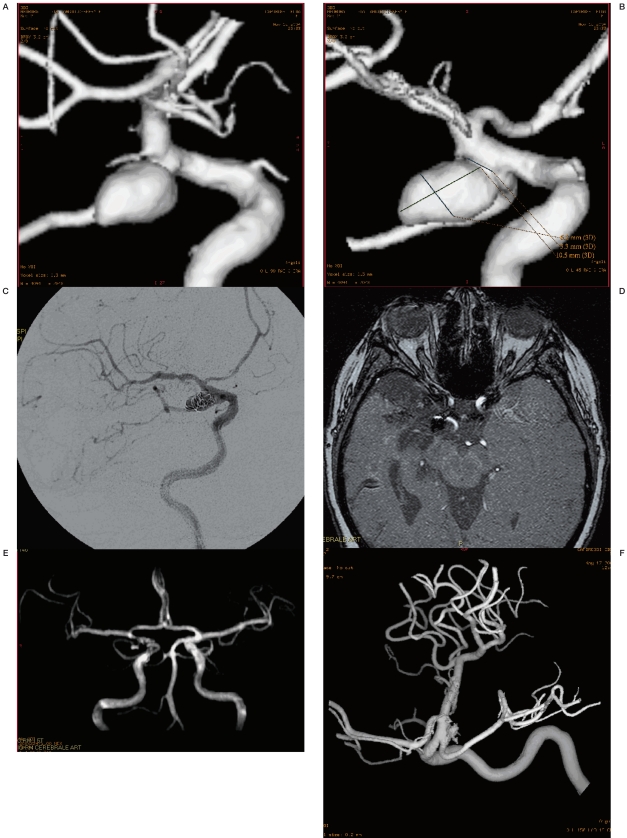
Figure 4 A-D.
A right MCA bifurcation aneurysm surgically treated on the basis of the CTA imaging alone.
The increasing use of rotation angiography and 3D DSA also increases the radiation dose which has to be considered another pitfall of the technique 33-35. In conclusion, DSA, possibly with rotational acquisition and 3D reconstruction, is a very useful diagnostic tool and still represents the gold standard for the detection of brain aneurysms.
Role of CTA in evaluating cerebral aneurysms
CTA has been significantly improved by the introduction of four- to 64-section spiral CT scanners, which offer rapid acquisition of isotropic data sets. A variety of techniques have been proposed for post-processing of the resulting images. The most widely used techniques are multiplanar reformation (MPR), thin-slab maximum intensity projection and volume rendering. Sophisticated segmentation algorithms, vessel analysis tools based on a centreline approach and automatic lumen boundary definition are emerging techniques; bone removal with thresholding or subtraction algorithms has been introduced. These techniques increasingly provide a quality of vessel analysis comparable to that achieved with intra-arterial three-dimensional rotational angiography. The technological progress in 3D imaging, such as maximum intensity projection (MIP) and volume rendering (VR) technique, appears to be very helpful in detecting intracranial aneurysms, particularly around the circle of Willis.
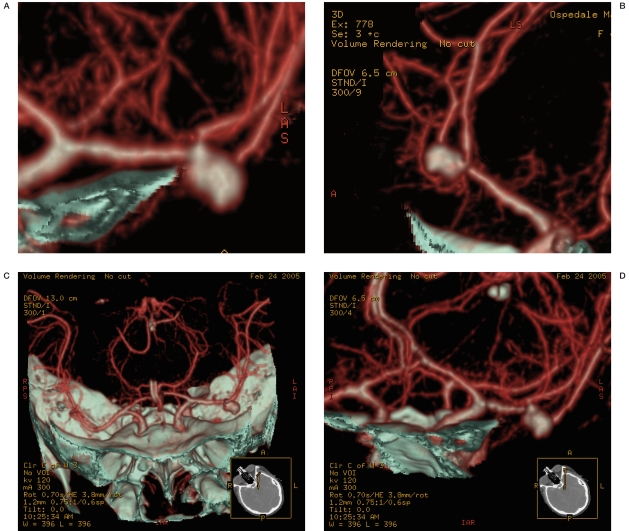
Figure 5 A-E.
An aneurysm of the left pericallosal artery and the fronto-orbital branch originating from the neck of the aneurysm. Foetal origin of the left PCA.
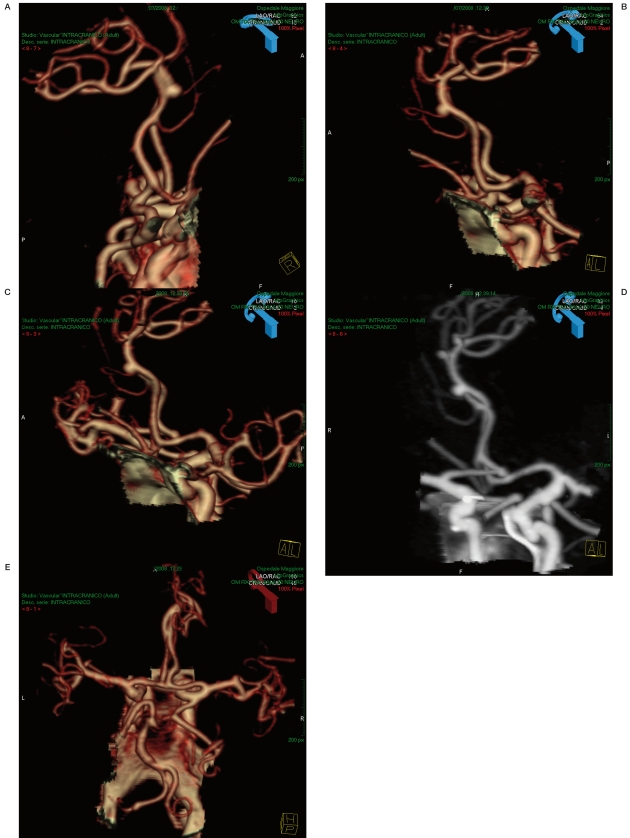
Figure 6 A-C.
Showing a left PICA aneurysm (DSA with 3D image post-processing allowing measurement of the aneurysm diameter). Next step: endovascular treatment.
In 1992 Aoki et Al 36 demonstrated the accuracy of 3D CTA in detecting 3mm diameter aneurysms in 15 patients. This is an important diagnostic goal since autopsy studies have shown the critical aneurysm size to be around 4 mm 63. It is well known that the natural history of all aneurysms is to enlarge and then to rupture, making it essential to identify and monitor aneurysms with a non invasive technique before rupture occurs.
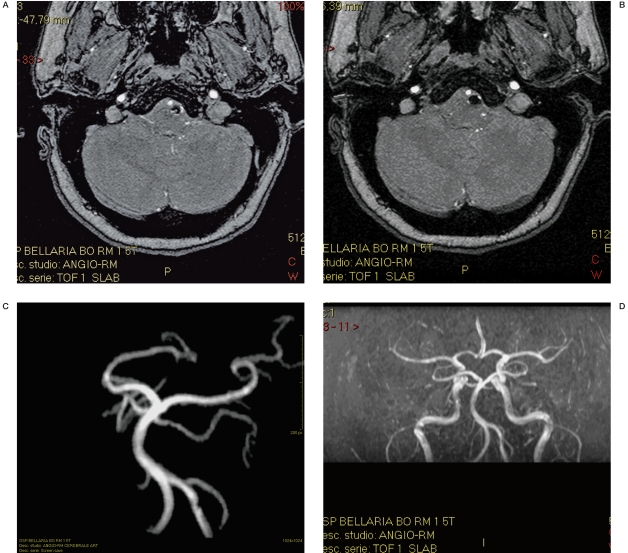
Figure 7 A-D.
Same case as in figure 6. MRA follow-up after 3 months shows the absence of the signal in the coil mesh and patency of the PICA.
Katz 62 correlated the number of vessels visible by CTA (using MIP post-processed images), MRA and DSA at the level of the circle of Willis showing the same sensitivity and specificity in vessel detection. Other reports 37,38 also correlated CTA with MRA and DSA showing CTA to be a reliable alternative to MRA evaluating circle of Willis aneurysms.
The experience of the group of Zouaoui 40 in 120 cases found a sensitivity and specificity of CTA with MIP and SSD reconstructions comparable to DSA in terms of the number of aneurysms detected, the visibility of the aneurysm neck and relations with surrounding structures. Alberico et Al showed the difficulty of demonstrating 2 mm aneurysms of the posterior communicating artery due to their proximity to the internal carotid artery, whereas there were no problems in evaluating other aneurysms such as those of the anterior communicating artery and middle cerebral artery 41. In his experience, the sensitivity and specificity of CTA detecting 5 mm or smaller aneurysm has been between 90% and 98%. Velthuis et Al reported a CTA sensitivity between 97% and 91% in two different observers evaluating 128 patients with SAH; the lack of sensitivity was related to the aneurysm of the posterior communicating artery, the ICA, the ophthalmic artery and the PICA 42. Young et Al 43 identified in operator inexperience and small aneurysms the most frequent cause of pitfalls in the spiral CT evaluation of intracranial aneurysms.
Recently, Hanley et Al compared the accuracy of the DSA, CTA and MRA at estimating the volume of cerebral aneurysms, emphasizing the tendency of the CTA to overestimate volume, MRA to underestimate and DSA to both under and overestimate equally 44.
CTA could offer valid information for endovascular planning, concerning aortic arch showing any origin abnormalities in the supra-aortic vessels. CTA could be adequate for correct surgical planning, related also to the degree of neurosurgeons' confidence in CTA images, in many aneurysms, such as the M1 or A1 segment, the MCA bifurcation and in many cases of the anterior communicating artery aneurysms, whereas it was inadequate for certain aneurysms of the siphon, basilar trunk or posterior communicating artery.
Advances in technology will yield better quality images in the future with high spatial resolution and less operator dependence.
The advantages of CTA can be summarised as follows:
1. Relatively low invasiveness.
2. Higher accessibility (with reference to DSA and MRA) and an increasingly homogeneous territorial distribution.
3. Fast to perform (including the post-processing).
4. Good reliability in strategic planning.
The limits of CTA can be summarised as follows:
1. Difficulty in detecting small aneurysms (dome diameter smaller than 3mm);
2. Impossibility of dynamic evaluation of cerebral blood flow;
3. Difficulty on evaluating aneurysms very close to bone structures;
4. Difficulties on evaluating aneurysms of the posterior circle.
Role of MRA in evaluating cerebral aneurysms
MR angiography is a well established non- invasive tool to explore cerebral vessels. It is generally performed without contrast administration and this may be considered one of its advantages over DSA and CTA. It requires less technical training and may be easily repeated for follow-up purposes.
However, the limited spatial resolution offered by MRA decreases its accuracy in evaluating small aneurysms (< 3-5 mm) and the difficulties in monitoring critical patients make it unsuitable in these cases. That is why its role in the diagnosis of berry aneurysms has decreased. In spite of all efforts made during the past 15 years 22,45-48, MRA cannot yet replace DSA for the diagnosis of aneurysm in the acute phase of subarachnoid haemorrhage and CTA has already been considered superior to MRA for this purpose.
Two MRA techniques are generally used: the Time-Of-Flight technique - TOF, based on the "inflow effect" and the Phase Contrast technique - PC, based on motion-related phase changes.
TOF effects result in an increased signal intensity as fully magnetized spins enter the slice of tissue being imaged, while stationary spins are saturated. With TOF sequences, spin saturation at low flow velocities may determine inappropriate detection of aneurysms with slow flow, which can be inconsistently depicted or missed. In addition, slow flow components of giant aneurysms or partially thrombosed aneurysms may be missed, while incomplete background suppression may determine a shine through effect of tissues with short T1, which can be misinterpreted as vascular structures 45-48.
In PC images, spins moving through regions of magnetic field variations determined by the application of a bipolar gradient undergo a phase shift, depending on flow velocity, the strength of the field gradient and the time interval between positive and negative gradient application. Using phase contrast sequences which allow a better detection of slow flow, image quality is inferior to TOF sequences. More-over, as phase shift and signal intensity are proportional to flow velocity, slow flow structures may be partially missed or inconsistently detected.
Figure 8 A-F.
Right siphon aneurysm and foetal origin of the ipsilateral PCA. 3D DSA allowing the measurement of the aneurysm, then endovascular treatment, MRA follow-up after 3 months and the post-processed images (MIP and 3D) show the presence of a flow signal in the coil mesh.
Figure 9 A-F.
Other patient with a huge right siphon aneurysm. MRA, 3D DSA allowing measurement of the aneurysm.
Figure 10 A-F.
A huge partially thrombosed left siphon aneurysm. DSA and 3D DSA allowing measurement of the aneurysm. Stent positioning and 1 month MRA follow-up showing a reduction of blood flow in the aneurysm.
Image post-processing, both for TOF and PC sequences, is generally performed with the MIP (maximum intensity projection) technique, showing on a bi-dimensional image only the high-signal components of voxels being examined, with the double risk of missing vascular structures with low signal and assigning an inappropriately low signal to small structures 46-48.
Other methods, such as MPR (multi-planar reconstruction), seem to have some advantages over the MIP technique. With MPR images are post-processed in thin slices with different spatial orientation and surface-rendering (SR), in which a 3D-isosurface is generated on an intensity profile determined with an operator-dependent threshold,.
MRA may also be performed with contrast administration 47. With first-pass dynamic contrast enhancement MRA, image acquisition is performed at first pass after contrast administration, when T1 of blood becomes as low as 30-60 ms for up to 20 s.
The short interval between arterial and venous enhancement limits its intracranial applications, but excellent background suppression make images of great value for proper depiction of brain aneurysms. With contrast enhancement 3D-TOF, image acquisition is performed at the steady state when blood T1 falls to 200400 ms for about 30 min.
Although spatial resolution is higher, background suppression may not be as excellent and the aneurysm lumen may not be as adequately depicted as with first-pass MRA.
Generally, the most widely used technique is 3D-TOF followed by MIP or volume rendering post-processing, because spatial resolution is good, the duration of scan times acceptable and signal loss for turbulent flow is minimal. The latest contrast enhancement technique is first-pass MRA, which allows a better visualization of small aneurysms.
MRA is used for the evaluation of patients at high risk for aneurysms, such as the relatives of patients with brain aneurysms, patients with polycystic kidney and patients with cranial nerve palsy, sometimes only as a preliminary investigation before DSA, and for the follow-up of patients submitted to aneurysm occlusion with Guglielmi detachable coils.
Role of MRA in the follow-up of embolized brain aneurysms
Embolization of saccular cerebral aneurysms requires numerous follow-up controls to monitor coil compaction after treatment or to detect the neck flow-related signal due to regrowth of the whole sac or to the coil mesh gathering.
DSA has long been used in the follow-up of cerebral aneurysms after embolization to disclose any residual aneurysm after treatment or long-term lesion recurrence, both of which may be responsible for bleeding.
Because angiography is an invasive procedure poorly tolerated by patients, effective reliable alternative monitoring techniques have been sought especially in view of the repeated follow-up controls required in aneurysm patients.
3D TOF sequences without paramagnetic contrast administration are the MRA scans most commonly used in the follow-up of embolized cerebral aneurysms as they offer reliable views of the intracranial vessels 52-61.
Using digital angiography as a reference, MRA has a 90-95% sensitivity rate in the diagnosis of residual/recurrent aneurysms larger than 3-4 mm 20. This is the threshold size below which MRA is no longer diagnostic with a significant drop in sensitivity for aneurysms measuring less than 3 mm in diameter.
Diagnosis of residual aneurysm or recurrence after treatment is mainly based on an analysis of MRA signal intensity.
MRA distinguishes the different components of an embolized aneurysmal sac, the coil mesh being depicted as a hypointense signal in the angiographic sequences whereas possible residue or recurrences show a hyperintense flow signal.
According to Raffi et Al 23 and Anzalone et Al54, this feature makes MRA more reliable than digital subtraction angiography in which pathological filling of contrast medium can be masked by the radiopaque coils.
Major technical advances now offer the possibility of using 3D TOF sequences which give an excellent panoramic view of the intracranial vessels on a par with images produced by state-of-the-art rotational angiograms. Multiple views allow detailed display of the origin of the treatment aneurysm and its neck which is particularly advantageous when 3D rotational angiography is unavailable.
Comparing the reliability of DSA and MRA, the angiographic finding of no contrast medium within a well-packed coil mesh and in the anatomical region of the aneurysm neck, fitted with MRA findings of exclusion from the circulation with no flow signal within the aneurysm.
Nowadays, the main aim of follow-up of embolized brain aneurysms is to obtain MRA images with a sufficient spatial resolution to diagnose and distinguish clinically significant (> 3-5 mm in diameter) aneurysm residue or recurrence at risk for bleeding, from clinically not significant changes (< 3-5 mm in diameter).
Conclusions
Diagnostic imaging evaluation in patients with cerebral aneurysms includes DSA, CTA and MRA. DSA is still considered the gold standard for detecting cerebral aneurysms, but its invasiveness and the potential risk of complications make it unsuitable as a first-choice examination. CTA is a relatively safe, fast, accessible and reliable technique increasingly used for intracranial aneurysm detection. MRA is a very important technique, above all in the follow-up of embolized aneurysms.
References
- 1.Wiebers DO, Whisnant JP, Huston J, III, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–10. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 2.Connolly ES, Solomon RA. Management of unruptured aneurysms. In: Le Roux PD, Winn HR, Newell DW, editors. Management of cerebral aneurysms. Philadelphia: Saunders; 2004. pp. 271–85. [Google Scholar]
- 3.Schievink WI. Intracranial aneurysms. N Engl J Med. 1997;336:28–40. doi: 10.1056/NEJM199701023360106. [DOI] [PubMed] [Google Scholar]
- 4.Waugh JR, Sacharias N. Arteriographic complications in the DSA era. Radiology. 1992;182:243–246. doi: 10.1148/radiology.182.1.1727290. [DOI] [PubMed] [Google Scholar]
- 5.Grzyska U, Freitag J, Zeumer H. Selective cerebral intra-arterial DSA: complication rate and control of risk factors. Neuroradiology. 1990;32:296–299. doi: 10.1007/BF00593048. [DOI] [PubMed] [Google Scholar]
- 6.Dion JE, Gates PC, Fox AJ, et al. Clinical events following neuroangiography: a prospective study. Stroke. 1987;18:997–1004. doi: 10.1161/01.str.18.6.997. [DOI] [PubMed] [Google Scholar]
- 7.Earnest F, Forbes G, Sandok BA, et al. Complications of cerebral angiography: prospective assessment of risk. AJR Am J Roentgenol. 1984;142:247–253. doi: 10.2214/ajr.142.2.247. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberg RL, Bank WO, Hedgcock MW. Neurologic complications of angiography in patients with critical stenosis of the carotid artery. Neurology. 1980;30:892–895. doi: 10.1212/wnl.30.8.892. [DOI] [PubMed] [Google Scholar]
- 9.Faught E, Trader SD, Hanna GR. Cerebral complications of angiography for transient ischemia and stroke: prediction of risk. Neurology. 1979;29:4–15. doi: 10.1212/wnl.29.1.4. [DOI] [PubMed] [Google Scholar]
- 10.Heiserman JE, Dean BL, Hodak JA, et al. Neurologic complications of cerebral angiography. Am J Neuroradiol. 1994;15:1401–1407. [PMC free article] [PubMed] [Google Scholar]
- 11.Willinsky RA, Taylor SM, TerBrugge K, et al. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology. 2003;227:522–8. doi: 10.1148/radiol.2272012071. [DOI] [PubMed] [Google Scholar]
- 12.Zappoli F, Lavaroni A, Fabris G, et al. L'angiographie numerique dans l'etude des aneurysmes intracraniens. Radiologie J CEPUR. 1991;11:21–22. [Google Scholar]
- 13.Leonardi M, Cenni P, Simonetti L, et al. Retrospective Study of Complications Arising during Cerebral and Spinal Diagnostic Angiography from 1998 to 2003. Interventional Neuroradiology. 2005;11:213–221. doi: 10.1177/159101990501100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flohr TG, Stierstorfer K, Ulzheimer S, et al. Image reconstruction and image quality evaluation for a 64-slice CT scanner with z-flying focal spot. Med Phys. 2005;32:2536–2547. doi: 10.1118/1.1949787. [DOI] [PubMed] [Google Scholar]
- 15.Napoli A, Fleischmann D, Chan FP, et al. Computed tomography angiography: state-of-the-art imaging using multidetector-row technology. J Comput Assist Tomogr. 2004;28:32–45. doi: 10.1097/01.rct.0000120859.80935.10. [DOI] [PubMed] [Google Scholar]
- 16.Luccichenti G, Cademartiri F, Pezzella FR, et al. 3D reconstruction techniques made easy: knowhow and pictures. Eur Radiol. 2005;15:2146–2156. doi: 10.1007/s00330-005-2738-5. [DOI] [PubMed] [Google Scholar]
- 17.Napel S, Marks MP, Rubin GD, et al. CT angiography with spiral CT and maximum intensity projection. Radiology. 1992;185:607–610. doi: 10.1148/radiology.185.2.1410382. [DOI] [PubMed] [Google Scholar]
- 18.Calhoun PS, Kuszyk BS, Heath DG, et al. Three-dimensional volume rendering of spiral CT data: theory and method. Radio-Graphics. 1999;19:745–764. doi: 10.1148/radiographics.19.3.g99ma14745. [DOI] [PubMed] [Google Scholar]
- 19.Venema HW, Hulsmans FJH, den Heeten GJ. CT angiography of the circle of Willis and intracranial internal carotid arteries: maximum intensity projection with matched mask bone elimination-feasibility study. Radiology. 2001;218:893–898. doi: 10.1148/radiology.218.3.r01mr30893. [DOI] [PubMed] [Google Scholar]
- 20.Arlart I. Magnetic Resonance Angiography. Berlin: Springer-Verlag; 2002. p. 199. [Google Scholar]
- 21.Adams W, Laitt R, Jackson A. The role of MR angiography in the pre-treatment assessment of intracranial aneurysms: a comparative study. Am J Neuroradiol. 2000;21:618–1628. [PMC free article] [PubMed] [Google Scholar]
- 22.Sales S, Daniele D. MR Angiography in the Diagnosis of Brain Aneurysms. Rivista di Neuroradiologia. 2002;15:531–536. [Google Scholar]
- 23.Raffi L, Simonetti L, Leonardi M, et al. Follow Up Study of Embolized Brain Aneurysms. Rivista di Neuroradiologia. 2003;16:595–608. [Google Scholar]
- 24.Michardiere R, Bensalem D, Martin D, et al. Comparison of MRA and angiography in the follow up of intracranial aneurysms treated with GDC. J Neuroradiol. 2001;28:75–83. [PubMed] [Google Scholar]
- 25.Nome T, Bakke SJ, Makstad PH. MR angiography in the follow up of coiled cerebral aneurysms after treatment with Guglielmi detachable coils. Acta Radiol. 2002;43:10–14. doi: 10.1080/028418502127347574. [DOI] [PubMed] [Google Scholar]
- 26.Weber W, Yousry TA, et al. Non invasive follow up of GDC-treated saccular aneurysms by MR angiography. Eur Radiol. 2001;11:1792–7. doi: 10.1007/s003300000741. [DOI] [PubMed] [Google Scholar]
- 27.Moniz E. L'angiographie cérébrale, ses applications et résultats en anatomic, physiologie te clinique (Cerebral angiography, its applications and results in anatomy, physiology, and clinic) Paris: 1934. [Google Scholar]
- 28.Duarte G, Goulão A. Egas Moniz, the Pioneer of Cerebral Angiography. Interventional Neuroradiology. 1997;3:107. doi: 10.1177/159101999700300201. [DOI] [PubMed] [Google Scholar]
- 29.Bederson JB, Awad IA, Wiebers DO, et al. Recommendations for the management of patients with unruptured intracranial aneurysms: a statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke. 2000;31:2742–50. doi: 10.1161/01.str.31.11.2742. [DOI] [PubMed] [Google Scholar]
- 30.Brisman JL, Joon K, et al. Cerebral Aneurysms. N Engl J Med. 2006;9:928–939. doi: 10.1056/NEJMra052760. [DOI] [PubMed] [Google Scholar]
- 31.The International Study of Unruptured Intracranial Aneurysm Investigators. Unruptured intracranial aneurysms - risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339:1725–33. doi: 10.1056/NEJM199812103392401. [DOI] [PubMed] [Google Scholar]
- 32.van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain. 2001;124:249–78. doi: 10.1093/brain/124.2.249. [DOI] [PubMed] [Google Scholar]
- 33.Norbash AM, Busick D, Marks MP. Techniques for reducing interventional neuroradiologic skin dose: tube position rotation and supplemental beam filtration. Am J Neuroradiol. 1996;17:41–49. [PMC free article] [PubMed] [Google Scholar]
- 34.Huda W, Peters KR. Radiation-induced temporary epilation after a neuroradiologically guided embolization procedure. Radiology. 1994;193:642–44. doi: 10.1148/radiology.193.3.7972801. [DOI] [PubMed] [Google Scholar]
- 35.Hatakeyama Y, Kakeda S, Ohnari N, et al. Reduction of radiation dose for cerebral angiography using flat panel detector of direct conversion type: a vascular phantom study. Am J Neuroradiol. 2007;28:645–50. [PMC free article] [PubMed] [Google Scholar]
- 36.Aoki S, Sasaki Y, Machida T, et al. Cerebral aneurysm: detection and delineation using 3-D-CT angiography. Am J Neuroradiol. 1992;13:1115–1120. [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison MJ, Johnson BA, Gardner GM, et al. Preliminary results on the management of unruptured intracranial aneurysms with magnetic resonance angiography and computed tomographic angiography. Neurosurgery. 1997;40:947–55. doi: 10.1097/00006123-199705000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Cenni P, Agati R, Simonetti L, et al. L'angio-TC nel riscontro e nell'analisi delle caratteristiche delle lesioni aneurismatiche dei pazienti con ESA. Rivista di Neuroradiologia. 2000;13:711–720. [Google Scholar]
- 39.Cenni P, Agati R, Leonardi M, et al. Ruolo dell'angio-TC nello studio degli aneurismi intracranici e del vasospasmo. Rivista di Neuroradiologia. 2001;14:37–66. [Google Scholar]
- 40.Zouaoui A, Sahel M, Marro B, et al. Three-dimensional Computed Tomographic Angiography in Detection of Cerebral Aneurysms in Acute Subarachnoid Hemorrhage. Neurosurgery. 1997;41:125–130. doi: 10.1097/00006123-199707000-00026. [DOI] [PubMed] [Google Scholar]
- 41.Alberico RA, Patel M, Casey S, et al. Evaluation of the circle of Willis with three-dimensional CT angiography in patients with suspected intracranial aneurysms. Am J Neuroradiol. 1995;16:1571–1580. [PMC free article] [PubMed] [Google Scholar]
- 42.Velthuis BK, Van Leeuwen MS, Witkamp TD, et al. Computerized tomography angiography in patients with subarachnoid hemorrhage: from aneurysm detection to treatment without conventional angiography. J Neurosurg. 1999;91:761–767. doi: 10.3171/jns.1999.91.5.0761. [DOI] [PubMed] [Google Scholar]
- 43.Young N, Dorsch NWC, Kingston RJ. Pitfalls in the use of spiral CT for identification of intracranial aneurysms. Neuroradiology. 1999;41:93–99. doi: 10.1007/s002340050712. [DOI] [PubMed] [Google Scholar]
- 44.Hanley M, Zenzen WJ, Brown M, et al. Comparing the Accuracy of Digital Subtraction Angiography, CT Angiography and MR Angiography at Estimating the Volume of Cerebral Aneurysms. Interventional Neuroradiology. 2008;14:173. doi: 10.1177/159101990801400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dal Pozzo G. Compendio di Risonanza magnetica Cranio e Rachide. Torino: UTET; 2001. pp. 84–161. [Google Scholar]
- 46.Kobayashi H, Tsuji T, Ishii H, et al. Diagnosis of unruptured asymptomatic cerebral aneurysms by magnetic resonance angiography. J Clin Neurosci. 1997;4:197–200. doi: 10.1016/s0967-5868(97)90073-4. [DOI] [PubMed] [Google Scholar]
- 47.Anzalone N. Contrast-enhanced MRA of intracranial vessels. Eur Radiol. 2005;15:E3–10. doi: 10.1007/s10406-005-0160-3. [DOI] [PubMed] [Google Scholar]
- 48.Heverhagen JT, Bourekas E, Sammet S, et al. Time-of-flight magnetic resonance angiography at 7 Tesla. Invest Radiol. 2008;43:568–73. doi: 10.1097/RLI.0b013e31817e9b2c. [DOI] [PubMed] [Google Scholar]
- 49.Dumas L, Abdennebi A, Belin C, et al. Role of 3D Display MR Angiography in the Study of Intracranial Aneurysms. Interventional Neuroradiology. 1995;1:59. doi: 10.1177/159101999500100108. [DOI] [PubMed] [Google Scholar]
- 50.Gang G. The Quantification of Cerebral Blood Flow by Phase Contrast MRA: Basics and Applications. The Neuroradiology Journal. 2008;21:11. doi: 10.1177/197140090802100102. [DOI] [PubMed] [Google Scholar]
- 51.Park W, Han MH, Cha SH, et al. PC-Based 3D Reconstruction of MR Angiography in Evaluation of Intracranial Aneurysms. The Values of Pre-Treatment Planning for Embolization and Post-Treatment Follow-up. Interventional Neuroradiology. 2002;8:169. doi: 10.1177/159101990200800209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michardiere R, Bensalem D, Martin D, et al. Comparison of MRA and angiography in the follow up of intracranial aneurysms treated with GDC. J Neuroradiol. 2001;28:75–83. [PubMed] [Google Scholar]
- 53.Weber W, Yousry TA, et al. Non invasive follow up of GDC-treated saccular aneurysms by MR angiography. Eur Radiol. 2001;11:1792–7. doi: 10.1007/s003300000741. [DOI] [PubMed] [Google Scholar]
- 54.Anzalone N, Righi C, Simionato F, et al. Three-dimensional time-of-flight MR angiography in the evaluation of intracranial aneurysms treated with Guglielmi detachable coils. Am J Neuroradiol. 2000;21:746–752. [PMC free article] [PubMed] [Google Scholar]
- 55.Hayashi J, Asai H, Sugimoto M, et al. 2D Fast Spoiled Gradient Echo (2D-FSPGR) Gd-DTPA Enhanced Dynamic MR Angiography in Cerebral Aneurysms after Treatment with Platinum Detachable Coils. Interventional Neuroradiology. 2001;7:65. doi: 10.1177/15910199010070S110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stracke CP, Spuentrup E, Reinacher P, et al. Time Resolved 3D MRA. Applications for Interventional Neuroradiology. Interventional Neuroradiology. 2006;12:223. doi: 10.1177/159101990601200304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saguchi T, Murayama Y, Ishibashi T, et al. Efficacy of 3-D Reconstructed Time of Flight MRA Follow-up of the Embolized Cerebral Aneurysms. Interventional Neuroradiology. 2006;12:45. doi: 10.1177/15910199060120S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boulin A, Pierot L. Follow up of intracranial aneurysms treated with detachable coils: comparison of gadolinium enhanced 3D time-of-flight MR angiography and digital subtraction angiography. Radiology. 2001;219:108–113. doi: 10.1148/radiology.219.1.r01mr06108. [DOI] [PubMed] [Google Scholar]
- 59.Derdeyn CP, Graves VB, Tursky PA, et al. MR angiography of saccular aneurysms after treatment with Guglielmi detachable coils: preliminary experience. Am J Neuroradiology. 1997;18:279–286. [PMC free article] [PubMed] [Google Scholar]
- 60.Kähära VJ, Seppänem SK, Ryymin PS, et al. MR angiography with three-dimensional time-of-flight and targeted maximum-intensity-projection reconstructions in the follow up of intracranial aneurysms embolized with Guglielmi detachable coils. Am J Neuroradiol. 1999;20:1470–1475. [PMC free article] [PubMed] [Google Scholar]
- 61.Zizka J, Krajina A, Lojik K, et al. MR angiography in the noninvasive follow up of endovascularly treated intracranial aneurysms. Journal of Neuroradiology. 2002:1S1–1S248. [Google Scholar]
- 62.Katz DS, Jorgensen MJ, Rubin G. Detection and follow-up of important extra-arterial lesions with helical CT angiography. D. Clin Radiol. 1999;54:294–300. doi: 10.1016/s0009-9260(99)90557-3. [DOI] [PubMed] [Google Scholar]
- 63.Inagawa T, Hirano A. Ruptured intracranial aneurysms: an autopsy study of 133 patients. Surg Neurol. 1990;33:117–23. doi: 10.1016/0090-3019(90)90020-p. [DOI] [PubMed] [Google Scholar]