Summary
The pathogenesis of intracranial arterial aneurysms (AA) has been debated for many years and still remains unclear, although these entities might pose life-threatening risks to the patient and understanding the disease is of utmost importance for choosing treatment concepts. Apart from the "classical" berry-type aneurysm, there are different other types of intracranial AA such as infectious, dissecting or giant, partially thrombosed aneurysms. From the clinician's perspective, the hypothesis that some of these intracranial aneurysms might be due to abluminal factors has been put forward for many years. Alterations of the vessel wall, either due to luminal or abluminal factors may be employed for an etiological classification of aneurysmal vasculopathies as will be discussed in this article. Moreover, regarding certain aneurysmal vasculopathies as an abluminal disease might alter current therapeutic strategies since therapy should not only aim at the intraluminal repair of the artery but may also target the vessel wall.
Key words: Aneurym, Vessel Wall, Dissection, Inflammation
Introduction
The pathogenesis of intracranial arterial aneurysms (AA) is complex and still not fully understood, while being of great importance to the choice of treatment of this disease. In 1997, Schievink proposed that "Intracranial arterial aneurysms (AA) are acquired lesions, that are most commonly located at the branching points of the major cerebral arteries coursing through the subarachnoid space at the base of the brain." 1 He pointed out that "Intracranial arteries have an attenuated tunica media and a lack of external elastica lamina" and that "intracranial AA have a thin tunica media or none, and the internal elastica lamina is either absent or severely fragmented" 1. These observations are generally correct, but do not attempt to distinguish among the diverse diseases that give rise to arterial aneurysms. The concept that the arterial blood stream first "expands" and then "bursts" an "aneurysmal herniation of the wall" is now considered too simple to explain the complex features of all arterial aneurysms thus necessitating a subclassification of different aneurysms. Different classification schemes have been based on aneurysm size (small vs. large vs. giant), location (posterior circulation vs. anterior circulation), clinical presentation (ruptured vs. unruptured), morphology (saccular vs. fusiform), or etiology (false or traumatic aneurysms, dissecting aneurysms, flow related aneurysms, infectious aneurysms). Each of these classifications has advantages, but a more thorough etiological understanding may be obtained from morphological and histological characteristics of the vessel wall (figure 1). This latter classification is based on the assumption, that an aneurysm can be regarded from two sides: the luminal and the abluminal part. Various shear stresses (flow, turbulences, jet effects and others) are known to produce AA. They represent "luminal" aneurysmal vasculopathies. Concerning the pathogenesis, a normal vessel wall abnormally triggered is postulated. Inflammatory, infectious, collagen diseases and dissective aneurysms, on the other hand point to "abluminal" aneurysmal vasculopathies 2. They suggest a primary involvement of the vessel wall potentially aggravated by shear stresses. In the following, we will distinguish between the luminal and the abluminal aneurysmal vasculopathies and their respective pathomechanisms and phenotypes.
Figure 1.
In this figure the same morphological type of aneurysm (fusiforme aneurysms) is depicted in four different patients. The term "fusiforme aneurysms" corresponds to an enlargement of the entire vessel circumference over a segment of the artery and is rather a morphological description than a pathomechanism. Although these aneurysms look similar, their pathomechanisms is completely different inferring different treatment strategies. In these examples, the four different underlying etiologies are: Aneurysm in the presence of atrial myxoma most likely resembling a neoplastic aneurysm in Frame A, acute transmural dissection with subarachnoid hemorrhage in Frame B, aneurismal dilatation present in familial candidosis in Frame C and fusiforme lumen of a partially thrombosed aneurysm following a healed dissecting process in frame D.
Luminal aneurysmal vasculopathies
Saccular aneurysms are the most frequently encountered form of aneurysm. They arise at arterial bifurcations and resemble berry-like outpouchings of the vessel wall. Their etiology and pathophysiology are complex and poorly understood. Genetic factors contribute to their development as underlined by (i) the familial forms of aneurysms and (ii) the many genetic diseases which manifest aneurysmal dilatation. Endogeneous factors such as aging, elevated blood pressure, blood shear stresses and anatomical variations of the circle of Willis as well as exogeneous risk factors such as cigarette smoking, drug abuse or contraceptive medication are thought to contribute to the formation and/or rupture of an aneurysm.
In addition to these "offensive" factors, there may also be "defective defense mechanisms" such as improper spontaneous self-repair of a vessel wall 3.
The roles of these different factors in a given individual are unknown. Therefore, the concept of individual host response must be considered when assessing the etiology of an aneurysm in a given patient. This individual host response is dependant on the individual life span and the regeneration capacity of arteries in normal or in pathological circumstances, at their branching or non-branching portions.
Offensive Pathomechanisms
Aging, hypertension, and/or smoking are thought to lead to general thickening of the intima of arteries, most pronounced distal and proximal to branching sites. These thickened intimal "pads" are inelastic and lead to an increased strain on more elastic parts of the vessel wall adjacent to these pads. Increased strain on the vessel leads to an increased activity of metalloproteinases and other extracellular matrix-degrading proteins, culminating in aneurysmal outpouching of the vessel wall. A vicious circle may then ensue as turbulent flow against the outpouching increases the strain on the vessel wall. Such hemodynamic stress may be especially significant at sites of congenital weakness of the vessel wall. For example, aneurysms often form in the vicinity of anatomical variations such as incomplete fusion of the basilar artery or unilateral absence of the horizontal segment of an anterior cerebral artery (A1). However, these theories are not able to explain why certain patients develop aneurysms, while others with similar "offensive exposures" do not. Therefore, individual defects in host response (e.g. a missing defensive line) may be present that finally lead to the formation of an aneurysm.
Pathomechanisms related to a defective defensive system
The construction and maintenance of blood vessels are the result of complex biological factors and events that involve repetitive steps and feedback to the vascular tree. The structural integrity of arteries is maintained and modified according to hemodynamic or metabolic demands (e.g. shear stress forces), which are genetically programmed and controlled. Vascular remodeling is an active and adaptive process of structural alteration and includes changes in cellular processes such as cell growth, cell death, cell migration, and production or degradation of the extracellular matrix 4. Shear stress forces activate specific (genetically programmed) steps that alter the balance of the mediators of remodeling (such as metalloproteinases, nitric oxide synthase, platelet-derived growth factor (PDGF), and transforming growth factor ß1). Aging decreases the capacity of the vessel wall to adapt to variations in the hemodynamic parameters, or to compensate for stressful events. This progressive aging process is an acquired vulnerability of the vessel wall, which will differ in males and females and differ with patient age. Congenital alterations in these programs could result in a variant, defective or absent reconstruction of the vessel wall. Similarly, we believe that the association of arterial variants with arterial aneurysms points to an incomplete maturation process of the arterial wall. The lack of cell selection that such pattern implies may preserve "weaker", i.e. less mature endothelial cells which will later develop arterial aneurysms when subject to secondary triggers such as hemodynamic stress (figure 2).
Figure 2.
Complex non-fusion of the anterior cerebral artery in the A1 segment with apparent duplication of the anterior communicating ramus and two associated aneurysms in statu nascendi. Anatomical variations such as these may point towards an immature vessel wall that may be more prone to aneurysm formation or to increased local shear stress forces on the vessels.
Before rupture, the aneurysm wall becomes unstable and undergoes morphological changes that start at an undefined time interval before rupture. These changes reflect the effect of risk factors that predispose to rupture as well as the maintenance and repair mechanisms trying to prevent rupture. As pointed out by Frosen in 2004, the factors distinguishing unruptured and ruptured SCAAs are: decellularization, apoptosis, and degeneration of wall matrix; de-endothelialization; thrombus organization; proliferation; and inflammatory infiltration 5.
Conclusions
In the individual patient, there is a complex interplay between the aggressive "offensive" factors and the host response. Formation of an aneurysm, therefore, indicates failure of the repair system at a given period in time, and/or the persistence of the abnormal triggering factors responsible for the failure of the remodeling processes. Therapy can then be targeted either toward augmenting the host's defensive system (not yet possible) or toward ameliorating local, aggressive "offensive" factors lead to the aneurysm. The ultimate goal is to stimulate the vascular remodeling system to repair the local injury to the vessel wall triggered by the offensive insult. Spontaneous resolution of berry-like aneurysms, seen after correction of abnormal flow dynamics, holds the promise of such therapy in the future 6 (figure 3).
Figure 3.
Spontaneous resolution of an ophthalmic artery aneurysm that was associated with a high-flow shunting lesion of the ophthalmic artery that that spontaneously regressed after occlusion of the shunt. This example testifies for the repair system of the (otherwise healthy) vascular system after removing locally aggressive factors.
Abluminal Aneurysmal Vasculopathies
Structural vessel wall diseases and dissections point to "abluminal" aneurysmal vasculopathies 7. They suggest a primary involvement of the vessel wall potentially aggravated by shear stresses and may be responsible for a different subsets of aneurysms 8-10. In the past decades, a mechanistic point of view on aneurysms and their pathogenesis lured the clinician to think, that, although there are different kinds of aneurysms, the common final pathophysiology and, therefore, the treatment might be similar in all different kinds of aneurysms 4. Subsequently, repair of the lumen occulted the possibilities of vessel wall repair. Flow related AA associated with brain AVM treated by correction of the shunt and thus of the abnormal stresses, however, is an example of physiopathological management of a specific group of AA. Similarly, infectious AA treated with antibiotics and spontaneous repair of some dissecting AA further point to the role played by the vessel wall and its repair capacities. The specific group of "partially thrombosed AA" constitutes a peculiar group within the AA encountered in neurological practice and will be discussed in more detail. There are reports dating as far back as 1980 that, from the clinician's perspective, put forward the hypothesis that such giant or partially thrombosed aneurysms might be due to abluminal factors 11,12, however, this hypothesis was not confirmed until recently, when a study conducted by Zhao and coworkers, "biologically" confirmed these early hypotheses: Partially thrombosed aneuryms are the result of a process that is mediated from the "outside of the vessel" by the vasa vasorum of the affected vessels 13.
Vasa Vasorum
Vasa vasorum or vasa nutricia constitute a normal arterial network within the adventitia that are important for supplying nutrients and oxygen to both the adventitia and media. Whereas the presence of vasa vasorum within the proximal segments of the intracranial internal and the vertebral artery piercing the dura is confirmed 14, reports on intracranial arteries are contradictory 15,16. A growing body of evidence suggests that in intracranial atherosclerosis, vasa vasorum are present more distally on the carotid and vertebrobasilar systems. Furthermore, the absence of vasa vasorum in intracranial arteries in neonates and children as well as the increased density of vasa vasorum in proximal segments of atherosclerotic intracranial arteries indicate that vasa vasorum in proximal segments of intracranial arteries in adults are acquired and reactive in nature and express an angiogenetic potential 16. The supply to vasa vasorum is not known in detail yet some clinical situations suggest that the network receives contributions from branches arising from the main trunk itself 14.
The 5-Lipoxygenase Pathway
Zhao and colleagues 13 defined one potential mechanism responsible for the formation of arterial aneurysms, i.e. the 5-lipoxygenase (5-LO), a key enzyme in leukotriene production that is expressed in leukocytes, macrophages and mast cells 17. Once these cells are activated, 5-LO generates different forms of leukotrienes that are potent mediators of inflammation by further activating macrophages and recruiting monocytes and T-cells 18. One of the leukotrienes, LTD4 binds to endothelial cells of the vasa vasorum which leads to an increase of leukocyte extravasation. This adventitial inflammation leads to a weakening of the media by release of proinflammatory factors that invade the media and lead to a dilation that subsequently results in aneurysm formation 13. It is important to note, that the weakening of the artery does not occur from the inside of the vessel but instead is being caused from the outside, i.e. by abluminal factors, namely the 5-LO pathway.
The role of the vasa vasorum in the formation of aneurysm is clearly stressed by Zhao and coworkers in their study: Leukotrienes stimulate the production of macrophage inflammatory proteins (MIPs) that participate in the recruitment of leukocytes and the release of factors such as proteases that degrade the extracellular matrix, the elastic lamina of the vascular wall, and, finally, the integrity of the vessel lumen 19,20. In addition, neoangiogenesis of vasa vasorum is encountered in close proximity to 5-LO activated macrophages indicating that the inflammatory process of the adventitia might lead to a vicious circle by which an increase of vasa vasorum is encountered which in turn leads to a more easy supply of inflammatory cells to the vessel wall 13,21. Activation of the proinflammatory 5-LO pathway in combination with hypercholesterolemia has also been linked to the formation of atherosclerosis as a chronic inflammatory disorder of the vessel wall 18,22. These data therefore link the hyperlipidemia-dependent vessel wall inflammation of atherosclerosis to the formation of arterial aneurysms via the same pathogenetic route in which the vasa vasorum play a crucial role 23 (figure 4). The possibility of a subadventitial, i.e. intramural hematoma resulting from these vasa vasorum is implicit from these observations.
Figure 4.
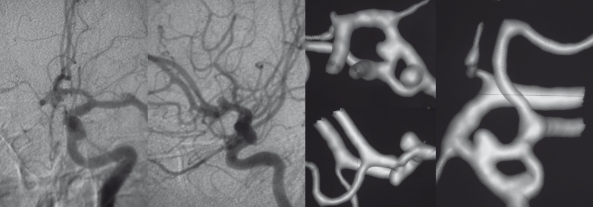
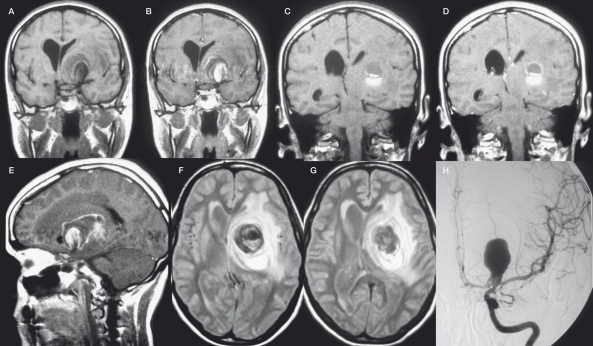
Pathogenesis of intracerebral giant artery aneurysm formation. Frame A demonstrates a model of the vasa vasorum and the 5-lipoxygenase pathway participation in leukocyte recruitment, arterial remodelling and, finally intracerebral arterial giant aneurysm formation. Macrophages reach the adventitia via vasa vasorum, that arise from the parent vessel themselves (see frames B-G). These adventitial macrophages express 5-lipoxygenase (5-LO) and, subsequently generate leukotrienes which in turn activate (1) T cells, (2) other macrophages, (3) proliferation of vasa vasorum and (4) monocytes and lead to an increased extravasation of leukocytes from the vasa vasorum to the adventita. These activated leukocytes release proinflammatory factors (such as metalloproteinases) that damage the media by degradation of the extracellular matrix (ECM) and the elastic lamina that lead to a focally weakened parent vessel wall from which subsequently the aneurysmal lumen may form (5) (13). We complement this biological cascade with the traditional clinical observation of subadventitial hematomas in so-called "partially thrombosed aneurysms": Repeated subadventitial bleeding from the vasa vasorum (6) lead to an onion-shaped intramural hematoma of different ages, which can be perceived in the example of a giant aneurysm of the basilar tip in a 60 year old woman (Frames B-E) who presented with a partial third cranial nerve palsy along with a contralateral moderate hemiparesis. Frames B and C were taken on admission and show the "partially thrombosed" aneurysm with "clots" of different ages represented by crescent-shaped signal alterations in the vessel wall. Frame D was taken one year later and demonstrate a new crescent-shaped peripheral hematoma in the outer vessel wall (arrow). Digital subtraction angiography (DSA) in 3D (Frame E) shows the lumen of the aneurysm, however, the greatest part (i.e. the subadventitial hematoma) of the aneurysm is not visualized, since DSA can only depict intraluminal portions of the vessels. Frames F and G schematically explain the increase in size as being due to recurrent hemorrhages into the aneurysm vessel wall from the vasa vasorum leading to onion-shaped thrombus formations within the adventita (12). (BA: basilar artery, PCA: posterior cerebral artery, SCA: superior cerebellar artery).
Pathogenesis of giant aneurysms
From a clinical point of view, there are different intracranial pathological conditions which have been described for some time and which now could be explained by these findings. Amongst those entities are, so-called giant or "partially thrombosed" AA.
It was in 1980 that Schubiger et al suggested that the formation of intracranial giant artery aneurysms could be due to a chronic dissection process associated to recurrent subadventitial hemorrhage from the vasa vasorum 11. Yasargil reported that on surgical exploration of giant intracranial aneurysms a tremendous network of fine vessels covering the aneurysm can be seen24. They argued that these small vessels might represent the increased amount of vasa vasorum at the aneurysmal wall level and that they may be responsible for the curvilinear enhancement that one can appreciate on CT after contrast injection. Subsequently, it was found out, that subarachnoid hemorrhage in partially thrombosed AA did not occur from the aneurysm itself but instead from the vessel wall with formation of fresh clot both inside and outside the vessel wall 12 (figure 4). Postmortem microscopic studies of 18 cases with giant intracranial aneurysms demonstrated that the wall of such AA consisted of fibrous tissue, often with calcifications, loss of the elastic lamina and muscularis and a large number of small vessels within the wall corresponding to the vasa vasorum 14. In a more recent report Iihara et al noted growth of a giant partially thrombosed aneurysm after complete occlusion of the parent vessel in a patient 25. Although there was no angiographically demonstrated evidence of filling of a lumen, the aneurysm continued to enlarge, requiring surgery. During the operation, the presence of markedly developed vasa vasorum was recognized on the parent artery and neck of the aneurysm 25. The common finding of these studies is, that recurrent bleeding from the vasa vasorum can result in an increase in size of the aneurysm and in further proliferation of neomembranes and new vessels. The increase in size of a giant aneurysm is therefore not due to intraluminal factors (i.e. change in hemodynamics, weak vessel wall leading to centrifugal growth of the aneurysm) but due to the apposition of new layers of thrombus at the periphery 26. Similarly, thrombosis of the so called partially thrombosed aneurysm seen on imaging studies does not correspond to intraluminal thrombus but is likely to refer to recurrent intramural hematoma similar to intramural hematoma seen on dissections of large arteries 27. Intracranial giant arterial aneurysms can therefore be regarded as a proliferative disease of the vessel wall induced by extravascular activity 28,29 (figure 5).
Figure 5.
This patient became symptomatic with an intraparenchymal bleeding that was observed posterior and superior to a mass lesion in the left basal ganglia. The mass lesion extended to the basal cisterns and could be identified as a partially thrombosed carotid bifurcation aneurysm. T1 weighted images pre-contrast (A, C) and post contrast (B,D,E) demonstrate that the site of the bleeding is distant from the perfused part of the aneurysm that is located more anterior and inferior. There is partial rim enhancement, and T2 weighted images (F, G) demonstrate an extensive perifocal edema both testifying for the inflammatory component of this lesion.
The primary cause for the dissection is unknown, however, one might presume, that atherosclerosis can - via a chronic inflammatory response - lead to a thinning of the media and cause vascular rupture within the adventitia, the bleed stops the further dissection of the media, otherwise an acute transmural dissection with subarachnoid hemorrhage occurs 19,30,31 (figure 6). The increase in number and size of the vasa vasorum suits well to this concept. Since the biological phenomenon is persisting, the effects may be still active which explains why some cases of dissection continue to evolve even after occlusion of the vessel as a possible therapy.
Figure 6.
Dissecting aneurysmal vasculopathies: Whereas in "classic" dissecting aneurysms a transmural dissection leads to an acute SAH, partially thrombosed aneurysms are characterized by recurrent subacute and non-transmural dissections that result in repeated subadventitial bleeding and progressive enlargement of the aneurysm. If SAH in these types of aneurysms was present, the neck of the aneurysm is most often involved, presumably due to a transmural dissection at this site. Arterial dissection might therefore generate a wide range of intracranial arterial aneurysms depending on the impact of extrinsic factors (inflammation, hemodynamic forces, etc.), the vessel wall integrity and the intrinsic repair systems.
Conclusions
Adventitial inflammatory reactions and vasa vasorum seem to play an important role in intracranial giant aneurysm formation. This biological contribution retrospectively confirms the empirical use of steroids and other anti-inflammatory drugs 32 in acutely symptomatic socalled "partially thrombosed" aneurysms. This reading of the pathophysiology has important therapeutic implications since therapy should not only aim at the intraluminal repair of the artery. One should also consider a treatment regimen that is able to cross the vessel wall to reach the vasa vasorum.
Drug eluting stents placed proximal to the lesion and targeted to the origin of the vasa vasorum could be considered as a potential future option. "Intelligent" MRI contrast agents could also be used (i.e. macrophage marking) to detect vasa vasorum proliferation and weakening of the vessel wall in vivo 33-35.
References
- 1.Schievink WI. Intracranial aneurysms. N Engl J Med. 1997;2:28–40. doi: 10.1056/NEJM199701023360106. [DOI] [PubMed] [Google Scholar]
- 2.Krings T, Piske RL, Lasjaunias PL. Intracranial arterial aneurysm vasculopathies: targeting the outer vessel wall. Neuroradiology. 2005;47:931–7. doi: 10.1007/s00234-005-1438-9. [DOI] [PubMed] [Google Scholar]
- 3.Krings T, Geibprasert S, Pereira V, et al. Aneurysms. In: Naidich T, editor. Neuroradiology of the Brain and Spine. New York: Elsevier; 2008. in press. [Google Scholar]
- 4.Lasjaunias P. From aneurysm to aneurysmal vasculopathies. Operative Techniques in Neurosurgery. 2000;3:160–5. [Google Scholar]
- 5.Frosen J, Piippo A, Paetau A, et al. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004;35:2287–93. doi: 10.1161/01.STR.0000140636.30204.da. [DOI] [PubMed] [Google Scholar]
- 6.Hans FJ, Krings T, Reinges MH, et al. Spontaneous regression of two supraophthalmic internal cerebral artery aneurysms following flow pattern alteration. Neuroradiology. 2004;46:469–73. doi: 10.1007/s00234-004-1204-4. [DOI] [PubMed] [Google Scholar]
- 7.Zhao WY, Krings T, Alvarez H, et al. Management of spontaneous haemorrhagic intracranial vertebrobasilar dissection: review of 21 consecutive cases. Acta Neurochir. 2007;149:585–96. doi: 10.1007/s00701-007-1161-x. [DOI] [PubMed] [Google Scholar]
- 8.Inci S, Spetzler RF. Intracranial aneurysms and arterial hypertension: a review and hypothesis. Surg Neurol. 2000;53:530–42. doi: 10.1016/s0090-3019(00)00244-5. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson GG. Physical factors in the initiation, growth, and rupture of human intracranial saccular aneurysms. J Neurosurg. 1972;37:666–77. doi: 10.3171/jns.1972.37.6.0666. [DOI] [PubMed] [Google Scholar]
- 10.Stehbens WE. Pathology and pathogenesis of intracranial berry aneurysms. Neurol Res. 1990;12:29–34. doi: 10.1080/01616412.1990.11739909. [DOI] [PubMed] [Google Scholar]
- 11.Schubiger O, Valavanis A, Hayek J. Computed tomography in cerebral aneurysms with special emphasis on giant intracranial aneurysms. J Comput Assist Tomogr. 1980;4:24–32. doi: 10.1097/00004728-198002000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Schubiger O, Valavanis A, Wichmann W. Growth-mechanism of giant intracranial aneurysms; demonstration by CT and MR imaging. Neuroradiology. 1987;29:266–71. doi: 10.1007/BF00451765. [DOI] [PubMed] [Google Scholar]
- 13.Zhao L, Moos MP, Grabner R, et al. The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nat Med. 2004;10:966–73. doi: 10.1038/nm1099. [DOI] [PubMed] [Google Scholar]
- 14.Berenstein A, Lasjaunias P, TerBrugge KG. Surgical Neuroangiography. Vol. 2.1. Berlin: Springer; 2004. [Google Scholar]
- 15.Aydin F. Do human intracranial arteries lack vasa vasorum? A comparative immunohistochemical study of intracranial and systemic arteries. Acta Neuropatho. 1998;96:22–8. doi: 10.1007/s004010050856. [DOI] [PubMed] [Google Scholar]
- 16.Connolly ES, Jr., Huang J, Goldman JE, Holtzman RN. Immunohistochemical detection of intracranial vasa vasorum: a human autopsy study. Neurosurgery. 1996;38:789–93. [PubMed] [Google Scholar]
- 17.Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220:568–75. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- 18.Spanbroek R, Grabner R, Lotzer K, et al. Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proc Natl Acad Sci U S A. 2003;100:1238–43. doi: 10.1073/pnas.242716099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longo GM, Xiong W, Greiner TC, et al. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–32. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daugherty A, Cassis LA. Mechanisms of abdominal aortic aneurysm formation. Curr Atheroscler Rep. 2002;4:222–7. doi: 10.1007/s11883-002-0023-5. [DOI] [PubMed] [Google Scholar]
- 21.Kumamoto M, Nakashima Y, Sueishi K. Intimal neovascularization in human coronary atherosclerosis: its origin and pathophysiological significance. Hum Pathol. 1995;26:450–6. doi: 10.1016/0046-8177(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 22.Lotzer K, Spanbroek R, Hildner M, et al. Differential leukotriene receptor expression and calcium responses in endothelial cells and macrophages indicate 5-lipoxygenase-dependent circuits of inflammation and atherogenesis. Arterioscler Thromb Vasc Biol. 2003;23:32–6. doi: 10.1161/01.ATV.0000082690.23131.CB. [DOI] [PubMed] [Google Scholar]
- 23.Palinski W. Aneurysms: leukotrienes weaken aorta from the outside. Nat Med. 2004;10:896–8. doi: 10.1038/nm0904-896. [DOI] [PubMed] [Google Scholar]
- 24.Yasargil MG. Micorneurosurgery. Vols 1 and 2. Stuttgart: Thieme; 1984. Clinical considerations, surgery of the intracranial aneurysms and results. [Google Scholar]
- 25.Iihara K, Murao K, Sakai N, et al. Continued growth of and increased symptoms from a thrombosed giant aneurysm of the vertebral artery after complete endovascular occlusion and trapping: the role of vasa vasorum. Case report. J Neurosurg. 2003;98:407–13. doi: 10.3171/jns.2003.98.2.0407. [DOI] [PubMed] [Google Scholar]
- 26.Krings T, Alvarez H, Reinacher P, et al. Rupture mechanisms of partially thrombosed aneurysms. Interventional Neuroradiology. 2007;13:117–26. doi: 10.1177/159101990701300201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yonas H, Agamanolis D, Takaoka Y, et al. Dissecting intracranial aneurysms. Surg Neurol. 1977;8:407–15. [PubMed] [Google Scholar]
- 28.Stehbens WE. Apoptosis and matrix vesicles in the genesis of arterial aneurysms of cerebral arteries. Stroke. 1998;29:1478–80. doi: 10.1161/01.str.29.7.1478. [DOI] [PubMed] [Google Scholar]
- 29.Endo S, Nishijima M, Nomura H, et al. A pathological study of intracranial posterior circulation dissecting aneurysms with subarachnoid hemorrhage: report of three autopsied cases and review of the literature. Neurosurgery. 1993;33:732–8. doi: 10.1227/00006123-199310000-00026. [DOI] [PubMed] [Google Scholar]
- 30.Dollery CM, Owen CA, Sukhova GK, et al. Neutrophil elastase in human atherosclerotic plaques: production by macrophages. Circulation. 2003;107:2829–36. doi: 10.1161/01.CIR.0000072792.65250.4A. [DOI] [PubMed] [Google Scholar]
- 31.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 32.Cyrus T, Sung S, Zhao L, et al. Effect of low-dose aspirin on vascular inflammation, plaque stability, and atherogenesis in low-density lipoprotein receptor-deficient mice. Circulation. 2002;106:1282–7. doi: 10.1161/01.cir.0000027816.54430.96. [DOI] [PubMed] [Google Scholar]
- 33.Trivedi RA, JM UK-I, Graves MJ, et al. In vivo detection of macrophages in human carotid atheroma: temporal dependence of ultrasmall superparamagnetic particles of iron oxide-enhanced MRI. Stroke. 2004;35:1631–5. doi: 10.1161/01.STR.0000131268.50418.b7. [DOI] [PubMed] [Google Scholar]
- 34.Corot C, Petry KG, Trivedi R, et al. Macrophage imaging in central nervous system and in carotid atherosclerotic plaque using ultrasmall superparamagnetic iron oxide in magnetic resonance imaging. Invest Radiol. 2004;39:619–25. doi: 10.1097/01.rli.0000135980.08491.33. [DOI] [PubMed] [Google Scholar]
- 35.Litovsky S, Madjid M, Zarrabi A, Casscells SW, Willerson JT, Naghavi M. Superparamagnetic iron oxide-based method for quantifying recruitment of monocytes to mouse atherosclerotic lesions in vivo: enhancement by tissue necrosis factor-alpha, interleukin-1beta, and interferon-gamma. Circulation. 2003;107:1545–9. doi: 10.1161/01.cir.0000055323.57885.88. [DOI] [PubMed] [Google Scholar]