Summary
Carotid blowout is a devastating complication in patients with head and neck cancer, commonly encountered as a delayed complication of radiation therapy. The clinical outcomes in patients with carotid blowout are discouraging; even transarterial embolization has been performed to control the acute massive bleeding. In recent years, covered stents have been reported as an alternative treatment producing favorable results.
In this study, 13 consecutive patients with acute carotid blowout syndrome were treated at our institute by covered-stent reconstruction between December 2005 and December 2007. The median posthemorrhagic survival period after reconstruction (187 days) was more than that reported in patients treated only with transarterial embolization (26 days). Though the estimated mortality was about 54%, those who survived showed favorable outcomes, and only one transit complication of acute in-stent thrombosis occurred. Thus, endovascular covered-stent reconstruction is a safe and effective approach to manage acute carotid blowout syndrome.
Keywords: carotid blowout, pseudoaneurysm, hemorrhage, head and neck cancer
Introduction
Carotid blowout (CB), first described in 19621, is still a devastating complication in patients with head and neck cancer, probably carrying 40% to 100% mortality and 60% neurological morbidity2,3. For patients with head and neck cancer who have undergone surgery and radiation therapy, acute carotid blowout syndrome (ACBS) is often presented as a prelude to mortality with unstable vital signs. Emergency surgery is not indicated because of the poor outcome 4. In the past, endovascular management of ACBS included transarterial embolization (TAE) and parent arterial occlusion (PAO) with detachable balloons3. However, dismal results were usually encountered. In a survey at our institute on the efficacy of TAE in controlling massive bleeding in 63 patients with head and neck cancer, the median posthemorrhagic survival period was only 26 days5. Furthermore, though endovascular PAO could quickly arrest hemorrhage, major ischemic neurological sequels occurred in up to 20% of cases, probably related to flawed test occlusion in an unstable hemodynamic status 6. There are several series highlighting the use of covered stents in ACBS and reporting favorable results7,8. Herein, we present our experiences of covered-stent reconstruction (CSR) to treat ACBS.Thirteen patients with post-treated head and neck cancer came to emergent angiogram due to acute massive bleeding and CSR was performed promptly.
This retrospective study aims to describe their clinical outcomes, complications, indications, and limitations.
Material and Methods
Thirteen patients with ACBS underwent 16 CSR procedures from December 2005 to December 2007. The underlying diseases included hypopharyngeal carcinoma (five patients), nasopharyngeal carcinoma (four), tongue carcinoma (two), radiation-induced sarcoma (one), and non-Hodgkin's lymphoma (one). Informed consent was obtained from the patients' families before the procedure. Twenty covered stents were used (17 Wallgraft; Boston Scientific, Ireland and 3 Fluency Plus; Angiomed, BARD, USA). Clopidogrel (225 to 300 mg) was administered via a nasogastric tube during the CSR procedure and a daily dose of 75 mg clopidogrel was recommended afterwards. Heparinization was performed using a 3000 units loading dose administered as an intravenous bolus immediately after successful CSR, followed by a 20,000 units per liter normal saline dose administered by slow infusion. Triple, double, and single stents were deployed in two, three, and eight patients, respectively (Table 1). The indication for CSR was a CB that could not be treated by TAE or PAO, including active bleeding from the common carotid artery (CCA), internal carotid artery (ICA), cervical carotid bifurcation (CCB), and proximal external carotid artery (ECA), if there were no contraindications. However, CSR was contraindicated in patients with a history of severe allergy to iodine-containing contrast agents, clopidogrel, or heparin. Patients with severe arterial tortuosity were considered inappropriate to receive CSR due to technical difficulties. The modified Rankin scale (mRS) was applied to evaluate the clinical activity of patients on admission and at discharge. Before treatment, there was one patient scoring 0; five, 1; three, 3; and four, 4.
Table 1.
Summary of patient data.
| No | Sex/ Age(Y) |
Disease | Treatment protocol |
Site of bleeding/ endoleak/result |
Pretreatment status (mRS) |
Outcome (mRS) |
Survival period |
Complications | Cause of death |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M/58 | HPC | CSR*N | CCB/+/C | 1 | 1 | >24 mo | No | No |
| 2 | M/36 | HPC | CSR | ICA/+/P | 1 | 1 | >21 mo | No | No |
| 3 | M/61 | TC | CSRx2 | CCB/+/P | 3 | 6 | 72 d | No | MOF |
| 4 | M/50 | HPC | CSR | CCA/-/C | 0 | 1 | >16 d | No | No |
| 5 | M/48 | NHL | CSR*N | ECA/+/P | 4 | 6 | 3 d | No | Rebleeding |
| 6 | M/51 | HPC | CSR*N | CCB/+/C | 3 | 6 | 12 d | No | Rebleeding |
| 7 | M/43 | HPC | CSR*N | CCB/+/C | 4 | 6 | 21 d | No | Rebleeding |
| 8 | M/35 | NPC | CSRx3 | ICA/+/P | 4 | 6 | 59 d | No | MOF |
| 9 | M/50 | RIS | CSR*N | CCB/+/C | 1 | 1 | >9 mo | Occlusion of ICA |
No |
| 10 | M/43 | TC | CSRx2 | CCB/+/P | 4 | 6 | 18 d | No | MOF |
| 11 | M/47 | NPC | CSRx2*N | CCB/+/C | 1 | 1 | > 4 mo | No | No |
| 12 | M/38 | NPC | CSRx3*N | ICA/+/C | 1 | 1 | >30 d | Transit thrombus |
No |
| 13 | F/57 | NPC | CSR*N | ICA/+/C | 3 | 6 | 1 d | No | Rebleeding |
|
Y: age; M: male; F: female; HPC: hypopharyngeal cancer; TC: tongue cancer; NHL: non-Hogkin lymphoma; NPC: nasopharygeal carcinoma; RIS- radiation-induced sarcoma; CSR: covered stent reconstruction; N: n-butyl cyanoacrylate; "*": with; "x2": double stents placement; "x3": triple stents placement; CCB: cervical carotid bifurcation; ICA: internal carotid artery; CCA: common carotid bifurcation; ECA:proximal external carotid artery; "+": endoleak detected; "—": no endoleak; C: complete success; P: partial success; mRS: modified Rankin Scale; mo: months; d: day; No: no occurrence; MOF: multiple organ failure. | |||||||||
Angiography was performed to evaluate neck vessels, intracranial perfusion, and collaterals, and to detect causes of active bleeding such as contrast agent extravasations, stasis, or pseudoaneurysm. If the patient was indicated for CSR, we set bilateral femoral sheathes, the 10F for CSR and another 6F for the microcatheter. As endoleaks often occur in CSRs, we first cannulated microcatheters near the bleeding sites and then deployed self-expandable covered stents overcoming bleeding sites. Since the tip of the microcatheter was located in advance, a liquid mixture of 20% n-butyl-cyanoacrylate (NBCA) and 80% lipiodol oil was injected via the microcatheter to seal the bleeding sites and/or endoleaks after stent deployment. The microcatheters were withdrawn immediately after the NBCA injections. Follow-up angiography was done to verify the success of the procedure. End-to-end double or triple CSRs were manipulated if the bleeding or endoleak persisted. We defined complete success as CSR without visible bleeding and endoleak, and partial success as the cessation of bleeding with residual endoleak. We followed all patients by clinical inspections, progress notes, discharge summary and outpatient records.
Results
Sixteen CSRs were performed in 38 angiographic procedures in 13 patients. The median posthemorrhagic survival after CSRs was 187 days (range, 1-727 days) to the end of December 2007. The mortality was 38% (5/13) at 30 days and 54% (7/13) at 90 days. Eight out of thirteen (62%) patients got complete success and the remaining five (38%) patients with partial successes. Among the eight patients with complete success, the median post-hemorrhagic survival was 204 days (range 1 to 727 days) and survival rate was 63% (5/8) at discharge. All the five surviving patients (patients 1, 4, 9, 11, 12) from the complete success group (survival period, 30 days to 24 months) showed favorable outcome, mRS scores of one at discharge. The other three patients died within the first 30 days (survival periods, 1 to 12 days). Only one out of five patients survived from partial success group. The other four had survival periods ranging from 18 to 72 days. Immediate and delay complications of CSRs occurred but no obvious sequel left after appropriate managements. Acute in-stent thrombosis of ICA occurred in one patient (patient 12) with transit focal weakness, but he was successfully treated by glycoprotein IIb/IIIa inhibitor (Aggrastat; Merck & Co., Inc., USA) without neurological deficit. Stent migration was detected 2 weeks later in the same patient and double CSRs were manipulated successfully. Another patient (patient 9) with tortuous ICA underwent CSR. He was found with occlusion of the affected ICA 2 months later in the follow-up computed tomography. Fortunately, no obvious neurological deficit left.
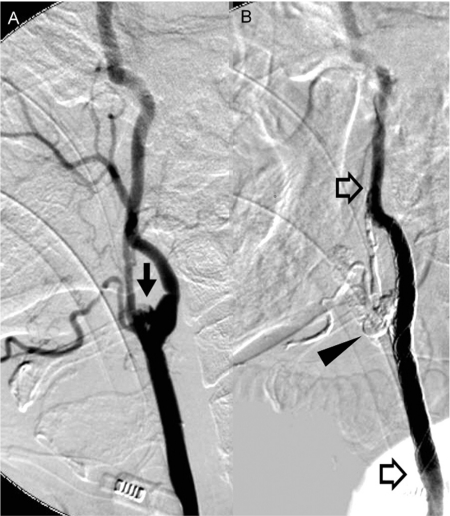
Figure 1.
Acute massive bleeding was caused by a pseudoaneurysm (arrow) from carotid bifurcation in a 50-year-old male (patient 9) with radiation-induced sarcoma. It was successfully treated by a CSR (open arrows) with NBCA injection (black arrowhead).
Active bleeding from the CCA was reported in one patient, ICA in four, CCB in seven, and proximal ECA in one. Endoleaks were found in 12 (92%) patients. There was only one patient (patient 4) whose CCA bleeding was treated by one covered stent without endoleak. After CSR treatments, six patients scored 1 and seven patients scored 6 on the mRS at 72 days. Those who had good mRS scores (0 and 1) before CSR got favorable outcome (scoring 1). However, all patients with poor mRS scores (3 and 4) before treatment died within 72 days. Among the seven cases of mortality, rebleeding of unknown origin was recorded in four patients. Two out of the four patients (patients 5 and 13) were reassessed by angiography, but no discernible rebleeding sources reported. The other two died in other institutes without angiographic records.
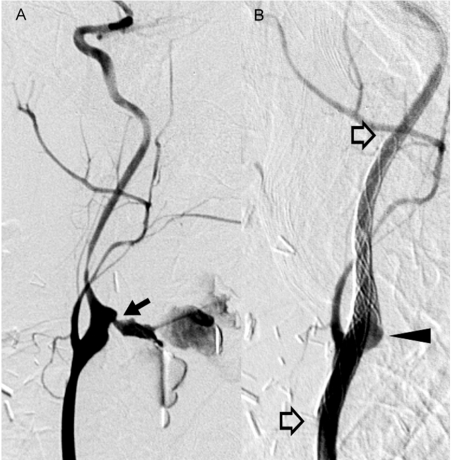
Figure 2.
Acute carotid blowout directly from the bifurcation (arrow) was found in a 43-year-old male (patient 10) with tongue cancer. Though bleeding ceased after double CSRs (open arrows), endoleak persisted. The patient died 18 d later from multiple organ failure.
Discussion
ACBS has been defined as an episode of acute transoral or transcervical hemorrhage or exposure of a part of the carotid artery in patients who has undergoing curative or palliative surgery for cervical malignancy6. Several etiological factors of CB have been implicated, including radiation therapy, radical resection, flap necrosis with carotid exposure, wound infection, pharyngocutaneous fistula, and recurrent or persistent carcinoma4,8. Since the late 1980s, the gold standard for detecting CB has been angiography, and its advent has not only helped in the detection of the site of origin of acute hemorrhage, but has also facilitated treatment9. CB has been categorized into threatened, impending, and acute types to facilitate clinical management6. Threatened CB is characterized by inevitable hemorrhage in the immediate future if no action is taken; impending CB, by an episode of hemorrhage that resolves spontaneously or with packing or pressure; acute CB, with hemorrhage that cannot be stopped by packing or pressure.
ACBS is often a premortem event with discouraging results of treatments in the past. Emergency surgery was not preferred due to the poor outcome 4, which usually complicated by local wound infection, flap necrosis, hemodynamic instability, profound hypotension, global cerebral ischemia, and consumptive coagulopathy secondary to extreme blood loss7. TAE was considered an effective treatment to control massive bleeding restricted the side branches of carotid arteries; it was not feasible in case of direct blowout from CCA, CCB, or ICA. In a survey5 at our institute on the efficacy of TAE in controlling massive bleeding in 63 patients with head and neck cancer, the median posthemorrhagic survival period of patients receiving TAE was 26 days, while patients who received angiography alone survived for only 8 days. The use of endovascular detachable balloons for PAO was initially described in 198410. The treatment was applied to a limited number of patients who had favorable intracranial collaterals. Although hemorrhage could be quickly arrested, immediate and delayed major neurological sequels have been reported in up to 20% of the patients 6,8. Test occlusion was inherently flawed as it failed to identify the subset of patients who developed delayed hemodynamic ischemia after permanent occlusion of the affected ICA6.
Once covered stents with smaller-caliber delivery systems were developed to permit a percutaneous approach and reduce the procedural time, the idea was quickly applied to treat ACBS7,9. We preferred CSR to PAO as almost all patients were exsanguinating with unstable hemodynamic status on the angiography table. Reconstitution of the arterial wall rather than acute arrest of the parent artery could control intracranial perfusion and prevent global cerebral ischemia. However, endoleaks, probably related to rebleeding, were usually encountered in CSR. Type I endoleaks 11 were found in 92% (12/13) of the patients in our study. CSR was usually performed at carotid bifurcation where was an anatomically paradoxical dilatation and branching. The incomplete stent apposition 12 to the inner wall of the carotid bulb left a potential gap for an endoleak, which could lead to potentially life-threatening rebleeding in ACBS. There was no standard treatment to treat endoleaks at the carotid bifurcation. Maldonado et Al 13 reported a liquid adhesive glue such as NBCA was applied to seal the endoleak. If NCBA could not be injected, double or triple CSRs were manipulated. Even if endoleaks persisted after double or triple CSRs, cessation of bleeding was always achieved in the study. Clinical observation or angiographic follow-up was done thereafter. Local contaminated or infected wounds are usually a concern in these patients. Therefore, meticulous precaution in ensuring good aseptic technique during stent deployment was required. With the advancement of infectious control, local infection or contamination did not seem to be a contraindication for CSR in an inevitably acute condition 7,8,9. Polymicrobial antibiotic prophylaxis, such as sulbactam ampicillin,was advocated8,14,15.
CSR provided immediate hemostasis in ACBS, especially in combination with complete obliteration of the endoleak. The procedure was effective in all patients with good clinical conditions (mRS 0 and 1) and gave favorable outcome (mRS 1). In summary, CSRs improved clinical outcome to levels never before encountered at our institute, especially for patients with good clinical conditions before treatment. However, further studies and larger series are needed as a smaller series was assessed in this study.
Conclusions
Endovascular CSR improves the management of ACBS. Patients in good clinical condition suffering from ACBS are the best candidates for CSR with complete obliteration of endoleak.
References
- 1.Borsanyi SJ. Rupture of the carotids following radical neck surgery in irradiated patients. Ear Nose Throat J. 1962;41:531–533. [PubMed] [Google Scholar]
- 2.Cohen J, Rad I. Contemporary management of carotid blowout. Curr Opin Otolaryngol Head Neck Surg. 2004;12:110–115. doi: 10.1097/00020840-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Lesley WS, Chaloupka JC, et al. Preliminary experience with endovascular reconstruction for the management of carotid blowout Syndrome. Am J Neuroradiol. 2003;24:975–981. [PMC free article] [PubMed] [Google Scholar]
- 4.Citardi MJ, Chaloupka JC, et al. Management of carotid artery rupture by monitored endovascular therapeutic occlusion (1988 -1994) Laryngoscope. 1995;105:1086–1092. doi: 10.1288/00005537-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Chou WC, Lu CH, et al. Transcutaneous arterial embolization to control massive tumor bleeding in head and neck cancer: 63 patients' experiences from a single medical center. Support Care Cancer. 2007;15:1185–1190. doi: 10.1007/s00520-007-0234-y. [DOI] [PubMed] [Google Scholar]
- 6.Chaloupka JC, Putman CM, et al. Endovascular therapy for the carotid blowout syndrome in head and neck surgical patients: diagnostic and managerial considerations. Am J Neuroradiol. 1996;17:843–852. [PMC free article] [PubMed] [Google Scholar]
- 7.Macdonald S, Gan J, et al. Endovascular treatment of acute carotid blow-out syndrome. J Vasc Interv Radiol. 2000;11:1184–1188. doi: 10.1016/s1051-0443(07)61361-x. [DOI] [PubMed] [Google Scholar]
- 8.Chang FC, Lirng JF, et al. Carotid Blowout Syndrome in Patients with Head-and-Neck Cancers: Reconstructive Management by Self-Expandable Stent-Grafts. Am J Neuroradiol. 2007;28:181–188. [PMC free article] [PubMed] [Google Scholar]
- 9.Warren FM, Cohen JI, et al. Management of carotid 'blowout' with endovascular stent grafts. Laryngoscope. 2002;112:428–433. doi: 10.1097/00005537-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Osguthorpe JD, Hungerford GD, et al. Transarterial carotid occlusion: case report and review of the literature. Arch Otolaryngol Head Neck Surg. 1984;110:694–696. doi: 10.1001/archotol.1984.00800360066017. [DOI] [PubMed] [Google Scholar]
- 11.Baum RA, Stavropoulos SW, et al. Endoleaks after endovascular repair of abdominal aortic aneurysm. J Vasc Interv Radiol. 2003;14:1111–1117. doi: 10.1097/01.rvi.0000085773.71254.86. [DOI] [PubMed] [Google Scholar]
- 12.Onizuka M, Kazekawa K, et al. The significance of incomplete stent apposition in patients undergoing stenting of internal carotid artery stenosis. Am J Neuroradiol. 2006;27:1505–1507. [PMC free article] [PubMed] [Google Scholar]
- 13.Maldonado TS, Rosen RJ, et al. Initial successful management of type I endoleak after endovascular aortic aneurysm repair with n-butyl cyanoacrylate adhesive. J Vasc Surg. 2003;38:664–670. doi: 10.1016/s0741-5214(03)00729-8. [DOI] [PubMed] [Google Scholar]
- 14.Weber RS, Raad I, et al. Ampicillin-sulbactam vs. clindamycin in head and neck oncological surgery: the need for Gram-negative coverage. Arch Otolaryngol Head Neck Surg. 1992;118:1159–63. doi: 10.1001/archotol.1992.01880110027007. [DOI] [PubMed] [Google Scholar]
- 15.Chang FC, Lirng JF, et al. Brain abscess formation: a delayed complication of carotid blowout syndrome treated by self-expandable stent-graft. Am J Neuroradiol. 2006;27:1543–1545. [PMC free article] [PubMed] [Google Scholar]