Summary
Cerebral sinovenous thrombosis (CSVT) is an uncommon disorder that affects the dural venous sinus and cerebral vein. In our study, thirty-four patients were examined. Pre and/or post contrast-enhanced CT was done in 28 patients. MRI studies were done in 24 patients. 2-D TOF MR venography (MRV) and contrast-enhanced MRV (CEMRV) were done in 19 cases. Digital subtraction angiography (DSA) was done in 18 patients. Sixteen patients received systemic intravenous heparinization, and 12 received endovascular thrombolytic treatment with urokinase combined with anticoagulant therapy.
Neuroimages of CSVT can be acquired by direct visualization of the thrombus within the dural sinus or by parenchymal changes secondary to venous occlusion. As there are some pitfalls to MRI in the diagnosis of CSVT, the combination of MRI and MRV is now the gold standard in the diagnosis of CSVT. Usually, accuracy can be improved by applying 2-D TOF MRV and CE MRV. Furthermore, the source image of MRV is critical in differentiating between normal sinus variations and diseased ones. DSA is the best tool for demonstrating dynamic intracranial circulation in CSVT and mostly is used for endovascular treatment.
Systemic intravenous anticoagulant therapy with heparin is accepted as a first line treatment. Except for clinical manifestations after systemic heparinization, abnormal MR findings of parenchymal change can be used to determine when to initiate thrombolytic treatment. Endovascular therapy can be finished at the antegrade flow within the dural sinus and continuous anticoagulation is sufficient to facilitate clinical improvement.
Clinical suspicion and excellent neuroimaging are crucial in making the diagnosis of CSVT. Proper management with anticoagulants and/or endovascular thrombolytic therapy is mandatory in preventing propagation of the thrombosis and improving the clinical outcome.
Key words: dural sinus, thrombosis, MRI, CT, thrombolysis
Introduction
Cerebral sinovenous thrombosis (CSVT) is an uncommon disorder that affects the dural venous sinus and cerebral veins. The clinical presentation is nonspecific, however, and it can be lethal if it progresses rapidly and is left undiagnosed. In this study, we present imaging findings for CSVT in CT, MRI, MR venography (MRV) and digital subtraction angiography (DSA), correlating these findings with clinical stages and therapeutic outcomes with anticoagulant and endovascular thrombolytic treatment.
Materials and Method
Thirty-four patients (15 male, 19 female), aged two weeks to 54 years, were enrolled. Clinical symptoms included headache, blurred vision, nausea, vomiting, seizure and altered consciousness. The neurological signs were papilledema, focal neurological deficits, cranial nerve palsies and nystagmus. Preand/or postcontrast-enhanced CT were done for 28 patients. MRI studies, including axial section spinecho T1-and T2-weighted and FLAIR MR imaging, diffusion weighted imaging and coronal section T2-weighted MRI, were done in 24 patients. 2-D TOF and contrast-enhanced MR venography were performed in 19 cases, and DSA was performed in 18 patients.
Sixteen patients received anticoagulant therapy with systemic intravenous heparinization, with 5000 IU heparin as a loading dose, followed by continuous intravenous infusion of 500-1000 IU/hour for 3-5 days. The dosage was then adjusted by the index of APTT (1.5-2 X of control values). Endovascular intracranial thrombolytic treatment with urokinase in combination with anticoagulant therapy was performed in 12 patients. To start intracranial venous sinus treatment, a bolus of urokinase (200,000 units through a microcatheter) was given initially, followed by urokinase (80,000 units push slowly over 5 minutes) at intervals of 15 minutes. Follow-up intrasinus venograms were then done. The dosage was around 240,000-320,000 units per hour with a total of 0.6-1.5 million units within 2-4 hours. After thrombolysis, continuous systemic intravenous heparinization continued for 3-5 days.
Results
The involved intracranial venous structure included the superior sagittal sinus (24 cases), inferior sagittal sinus (nine cases), straight sinus (14 cases), transverse sinus (21 cases), sigmoid sinus (11 cases), cavernous sinus (four cases), internal cerebral vein and/or vein of Galen (ten cases), and cortical vein (12 cases).
Imaging findings of CSVT were categorized by visualization of the thrombus in the dural sinus or cerebral veins, and the parenchymal changes secondary to venous outflow occlusion. The CT finding of intravascular thrombus presented as cord sign and/or dense triangular sign on non-contrast enhanced CT (Figure 1A) in 16 cases. The triangular defect (empty delta sign) from enhanced dura surrounding the thrombus can be demonstrated on contrast-enhanced CT (Figure 1B) in ten cases. On MR imaging, the signal intensity of venous thrombosis was affected by the degree of residual flow and the age of the thrombus. In the acute stage (first 3-5 days, 7 cases), the thrombus was isointense on T1WI (Figure 4A) and hypointense on T2WI. In the sub-acute stage (515 days, 12 cases), the thrombus appeared hyperintense on T1WI and T2WI (Figure 1C-E). In later stages (>15 days, five cases), there was progressive heterogeneous signal loss. By the combination of 2D TOF MR venography (Figure 1F, 2A, 3D) and Gadolinium-enhanced MRV (Figure 2B) with the source image (Figure 2C-D), absence or incomplete filling of dural sinuses were detected in 17 cases. DSA was performed in 12 cases and the imaging findings included absence or incomplete filling of dural sinuses or cerebral vein (Figures 2E-F, 3E-F), engorged or tortuous collateral veins, reversal of normal venous flow direction, and regional or global delayed venous flow.
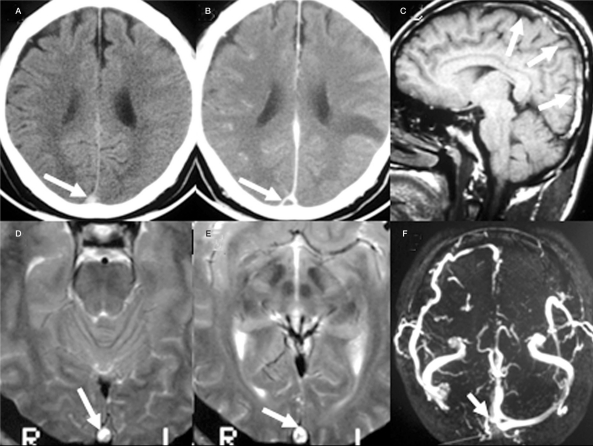
Figure 1.
The intravascular thrombus (arrow) within the superior sagittal sinus is elucidated as hyperdensity on non-contrast CT (A), the triangular defect (empty delta sign) on contrast enhancing CT (B), heterogeneous hyperintensity on sagittal T1WI (C) and axial T2WI(D,E) MR images. The 2D TOF MRV shows absence of right transverse sinus with incomplete filling of right sinus confluence (F).
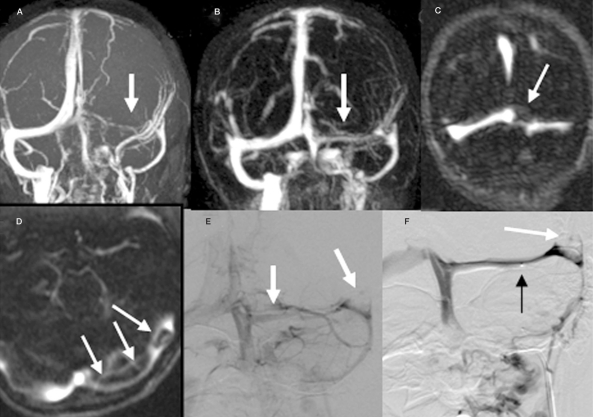
Figure 2.
The 2D TOF (A) and contrast enhancing MR venography (B) show decreased caliber of left transverse sinus (arrow). The source images of CEMRV (C, D) detect filling defects from the thrombus within the sinus (arrow). The venous phase of the vertebral angiogram (E) shows the small caliber of the left transverse sinus. During intracranial venous thrombolytic treatment (F), the venous sinus is reopened with residual thrombus (white arrow). The tip of the micro-catheter is in position (black arrow).
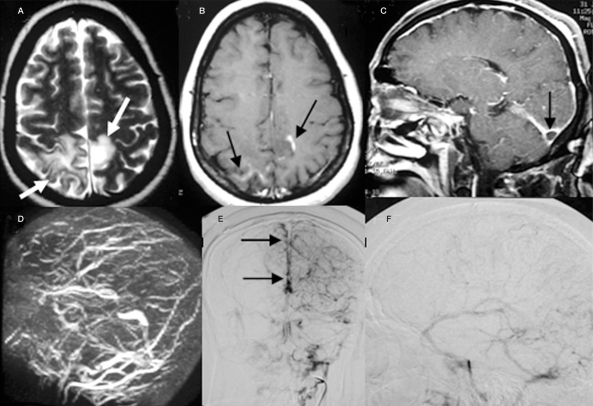
Figure 3.
The axial section T2WI (A) and post-Gd enhancing (B) MR images show stage III parenchymal change with hyperintensity surrounded with mild edema (arrow) and faint contrast enhancement (black arrow) over both posterior and parieto-occipital regions. The empty delta sign (arrow) within the right transverse sinus on contrast enhancing sagittal MRI is evident (C). Poor visualization of the superior sagittal and both transverse sinuses on 2D TOF MRV is noted (D). The PA (E) and lateral view (F) in the venous phase of the carotid angiogram show the absence of both transverse sinuses and a filling defect (black arrow) caused by an intraluminal thrombus.
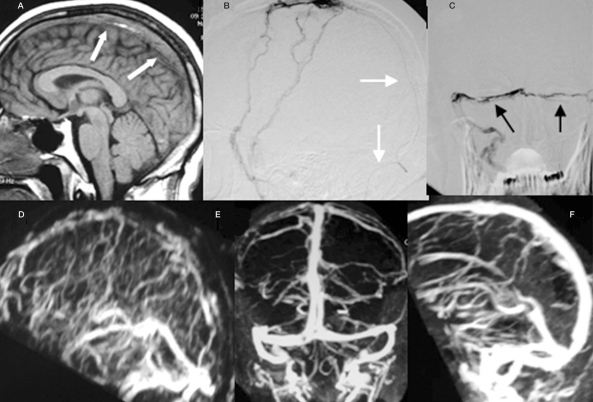
Figure 4.
The T1 weighted MR image (A) shows diffuse intraluminal thrombus within the superior sagittal sinus (arrow). During retrograde transvenous thrombolytic treatment, the micro-catheter (arrow) is demonstrated on lateral view (B) with the presence of filling defects caused by the thrombi (black arrow) within both transverse sinuses (C). The patient received subsequent heparinization for 4 days and was improved clinically. The follow-up PA (E) and lateral (F) view of the CEMRV at two weeks shows a more patent venous sinus when compared to the lateral view of MR venography done the next day of treatment (D).
According to the study of Fong Tsai 1, the abnormal parenchymal change on CT and/or both T2WI and FLAIR MR findings were staged as follows: stage I -no parenchymal change (6 cases), stage II -brain swelling, sulci effacement and mass effect, no signal change (5 cases), stage III - increased intensity of signal change as mild to moderate edema (Figure 3A-B) (8 cases), stage IV - severe edema, with or without hemorrhage (10 cases ) and stage V - massive edema and/or hemorrhage (five cases).
The overall neurological outcomes following the CSVT, either with or without treatment, were: having no significant neurological deficits in 21 cases, some sequential neurological deficits in 11 cases, recurrence in three cases, and death in three cases.
Discussion
CSVT can be detected with neuroimaging studies by direct visualization of the thrombus within the dural sinus or by parenchymal changes secondary to venous occlusion 2. Non-contrast enhancing CT (NECT) studies can disclose a hyperdense thrombus (Figure 1A) in the thrombosed dural sinus or vein, and the cortical or subcortical hemorrhage adjacent to an occluded sinus due to venous congestion and extravasation. In the post-contrast CT imaging study, the engorged dural cavernous space, meningeal venous tributaries and collateral venous channels are enhanced. In addition, the so-call "empty delta sign", formed by a relatively hypodense thrombus in the occluded sinus surrounded by the enhanced dura, appears as a triangular filling defect (Figure 1B). According to previous studies, failure to diagnosis CSVT or underestimation of sinus involvement and venous infarcts were reported in 16-40% of patients 3.
There are some situations that mimic venous sinus thrombosis in NECT. In neonates, the combination of decreased density in unmyelinated brain, physiologic polycythemia and slower venous flow, normally makes the falx and dural sinus appear denser, which mimics the dense triangular sign3. Also, subarachnoid or subdural hemorrhage along the edges of the tentorium cerebelli is difficult to distinguish from sinus thrombosis 3,4.
In the imaging findings in MRI, the signal intensity of venous thrombosis is affected by the degree of residual flow and the age of the thrombus 5. In the acute stage, the deoxyhemoglobin within the thrombus appears as isointensity on T1WI (Figure 4A) and hypointensity on T2WI. In the subacute stage, the thrombus shows hyperintensity on T1WI and T2WI (Figure 1C-D) from the methemoglobin blood content. The later appearance of the thrombus is due to progressive heterogeneous signal loss. Owing to the different breakdown stages of the thrombosis together with factors of variable velocity of blood in normal sinuses, flow-related enhancement, and dephasing effects, there exists overlap of signal intensity between normal sinus and sinus thrombosis. The hypointense appearance of the thrombus on T2-weighted images in early stages can be mistaken for normal flow voids 5. As there are some pitfalls in MRI in the diagnosis of CSVT, a combination of MRI and MR venography is now the gold standard in the diagnosis of CSVT 6. Usually, the absence or incomplete filling of dural sinuses can be detected by 2D TOF and phase contrast MR venography. However, accuracy can be further improved by applying Gadolinium-enhanced MRV6 (Figures 2B, 4D-F). In some cases, it is hard to distinguish the diffuse intraluminal thrombus from congenital hypoplasia of the venous sinus simply based on the decreased caliber of the venous sinus noted on MR venography. At this moment, the source image for MRV is critical in differentiating between normal sinus variation and the disease 6 (Figure 2C-D). DSA has been the best tool for demonstrating dynamic intracranial circulation in CSVT. Nowadays, DSA is performed mostly for endovascular treatment (Figure 4B-C) rather than for its diagnostic role in CSVT due to the notable accuracy of MR imaging studies.
In CSVT patient, venous outflow obstruction results in venous congestion. Thus there is a decrease in cerebral blood flow and in cerebral perfusion pressure 2,7; eventually, venous infarction combined with hemorrhage follow8. The abnormal MR findings of parenchymal changes can be used to determine when to initiate thrombolytic treatment1.
In the acute CSVT attack, anticoagulant treatment is used for restoring circulation9. Systemic intravenous anticoagulant therapy with heparin has been accepted as a first line treatment6,9. When patients show clinical deterioration after 24 hours of heparin therapy or neuroimaging studies reveal rapid progression of thrombosis and diffuse brain swelling, with or without hemorrhage (stage II or III of brain parenchymal change), endovascular treatment is done 1. Endovascular intravenous thrombolytic therapy with Urokinase or recombinant tissue plasminogen activator (rt-PA) directly infused into the thrombosed dural sinus is performed by way of a retrograde transvenous approach. The endpoint of thrombolytic treatment is set at the antegrade flow within the dural sinus instead of at the total absence of thrombus within the dural sinus by angiography. If thrombolytic treatment is too aggressive, intracranial hemorrhage will be complicated. After endovascular therapy, the re-establishment of antegrade flow with continuous anticoagulation is sufficient to facilitate clinical improvement10. Usually, the venous sinus will once again patent on follow-up MRV (Figure 4E-F) after subsequent heparinization.
The most effective way to ensure dural sinus revascularization is mechanical disruption of the thrombus followed by thrombolysis. Endovascular treatment can be accomplished by using either a guide-wire, catheter, or balloon thrombectomy6 with fibrinolysis or transluminal balloon angioplasty with or without stenting2,6.
Conclusions
Clinical suspicion and excellent neuroimaging are crucial in making the diagnosis of CSVT. Proper management with anticoagulant and/or endovascular thrombolytic therapy is important in preventing propagation of the thrombosis and improving the outcome. A combination of thrombectomy and balloon assisted thrombolysis will enhance the result of the thrombolytic effect.
References
- 1.Tsai FY, Wang AM, et al. MR Staging of Acute Dural Sinus Thrombosis: Correlation with Venous Pressure and Implication for Treatment and Prognosis. 1995;16:1021–1029. [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai FY, Kostanian V, et al. Cerebral Venous Congestion as Indication for Thrombolytic Treatment. Cardiovasc Intervent Radiol. 2007;30:675–87. doi: 10.1007/s00270-007-9046-1. [DOI] [PubMed] [Google Scholar]
- 3.Shroff M, deVeber G. Sinovenous Thrombosis in Children. Neuroimag Clin N Am. 2003;13:115–138. doi: 10.1016/s1052-5149(02)00064-3. [DOI] [PubMed] [Google Scholar]
- 4.Kriss VM. Hyperdense posterior falx in the neonate. Pediatr Radiol. 1998;38:17–19. doi: 10.1007/s002470050472. [DOI] [PubMed] [Google Scholar]
- 5.Mark MP. Magnetic Resonance Imaging of Brain and Spine. Philadelphia: Lippincott Williams and Wilkins; 2002. pp. 962–967. [Google Scholar]
- 6.Lee SK, terBrugge Cerebral Venous Thrombosis in Adult: The Role of Imaging Evaluation and Management. Neuroimag Clin N Am. 2003;13:139–152. doi: 10.1016/s1052-5149(02)00095-3. [DOI] [PubMed] [Google Scholar]
- 7.Rother J, Waggie K, et al. Experimental Cerebral Venous Thrombosis: Evaluation using Magnetic Resonance Imaging. J Cereb Blood Flow Metab. 1996;16:1353–1361. doi: 10.1097/00004647-199611000-00033. [DOI] [PubMed] [Google Scholar]
- 8.Nakase H, Kakizaki T, et al. Use of Local Cerebral Blood Flow Monitoring to Predict Brain Damage after Disturbance to Vein Occlusion Model by Photochemical Dye. Neurosurgery. 1995;37:280–285. doi: 10.1227/00006123-199508000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Bousser MG. Cerebral Venous Thrombosis: Nothing, Heparin, or Local Thrombolysis? Stroke. 1999;30:484–489. [Google Scholar]
- 10.Lee SF, Kim BS, Terbrugge K. Clinical Presentation, Imaging and Treatment of Cerebral Venous Thrombosis (CVT) Interventional Neuroradiology. 2002;8:5–14. doi: 10.1177/159101990200800102. [DOI] [PMC free article] [PubMed] [Google Scholar]