Abstract
Growing evidence suggests that oxytocin plays an important role in the regulation of energy balance and that central oxytocin administration induces weight loss in diet-induced obese (DIO) animals. To gain a better understanding of how oxytocin mediates these effects, we examined feeding and neuronal responses to oxytocin in animals rendered obese following exposure to either a high-fat (HFD) or low-fat diet (LFD). Our findings demonstrate that peripheral administration of oxytocin dose-dependently reduces food intake and body weight to a similar extent in rats maintained on either diet. Moreover, the effect of oxytocin to induce weight loss remained intact in leptin receptor-deficient Koletsky (fak/fak) rats relative to their lean littermates. To determine whether systemically administered oxytocin activates hindbrain areas that regulate meal size, we measured neuronal c-Fos induction in the nucleus of the solitary tract (NTS) and area postrema (AP). We observed a robust neuronal response to oxytocin in these hindbrain areas that was unexpectedly increased in rats rendered obese on a HFD relative to lean, LFD-fed controls. Finally, we report that repeated daily peripheral administration of oxytocin in DIO animals elicited a sustained reduction of food intake and body weight while preventing the reduction of energy expenditure characteristic of weight-reduced animals. These findings extend recent evidence suggesting that oxytocin circumvents leptin resistance and induces weight-loss in DIO animals through a mechanism involving activation of neurons in the NTS and AP, key hindbrain areas for processing satiety-related inputs.
Keywords: leptin, food intake
oxytocin is a peptide synthesized within both magnocellular and parvocellular neurons of the paraventricular nucleus (pPVN) as well as by magnocellular neurons of the supraoptic nucleus (SON) of the hypothalamus (19). Unlike magnocellular oxytocin neurons, which project to the neurohypophysis where they secrete oxytocin in the circulation, oxytocin-containing projections from the pPVN to hindbrain areas release oxytocin as a neuropeptide involved in the control of food intake and autonomic function (10). Whereas peripherally secreted oxytocin promotes uterine contraction during parturition and stimulates milk ejection during lactation (19), central release of oxytocin is implicated in both social behavior (maternal behavior, trust, emotion, social memory) (1, 18, 30) and energy homeostasis (2, 8, 31, 58, 63).
Several lines of evidence suggest that reduced oxytocin signaling is associated with obesity. Mice with haploinsufficiency of single-minded 1 (SIM1) (Sim1+/−), a gene essential for the formation of the PVN, have reduced expression of both oxytocin and melanocortin-4 receptor (Mc4r) in this hypothalamic structure (31, 32, 59) and are characterized by hyperphagic obesity, increased linear growth, and increased susceptibility to diet-induced obesity (DIO) (22, 31, 32, 59), and a similar phenotype is observed in humans with Sim1 haploinsufficiency (21, 23). Furthermore, the number and size of oxytocin neurons in the PVN are reduced in humans with Prader-Willi syndrome (PWS), a human genetic disorder characterized by severe hyperphagia and obesity (57). Reduced release of oxytocin from mouse PVN via synaptotagmin-4 (Syt4), an atypical modulator of synaptic exocytosis, also promotes the development of DIO (63). That oxytocin signaling is required for proper regulation of energy balance is further supported by evidence that mice deficient in either oxytocin (12) or oxytocin receptors (58) exhibit a late-onset obesity phenotype. Conversely, pharmacological studies demonstrate that either systemic (2, 3, 5) or intracerebroventricular (icv) (3, 5, 44, 50) administration of oxytocin dose-dependently reduces food intake in chow-fed rats, an effect prevented by icv pretreatment of an oxytocin-receptor antagonist in the latter model (45). Recent evidence also suggests that increased oxytocin signaling, via reduced expression of Syt4, a negative regulator of oxytocin release, prevents the development of DIO, while central administration of oxytocin is capable of inducing weight loss in DIO mice (63). These data collectively suggest that oxytocin plays an essential role in energy homeostasis, although the mechanisms underlying these effects remain to be established. Also unknown is whether chronic systemic administration of oxytocin is sufficient to cause weight loss in obese animals.
Growing evidence suggests that oxytocin neurons in the pPVN are part of a neurocircuit whereby hypothalamic leptin signaling is coupled to the satiety response of gut-derived signals such as cholecystokinin (CCK). Specifically, oxytocin is contained in neurons that project from the pPVN to hindbrain areas that respond to CCK and other meal-related inputs that lead to meal termination and hence determine meal size. Consistent with this hypothesis, oxytocin neurons in the pPVN that are activated by leptin (8) innervate hindbrain areas that regulate meal size (6, 8, 29), and leptin-induced anorexia requires oxytocin signaling (8). Delineating this neurocircuitry is a key priority, as most obese humans and rodents exhibit the combination of elevated circulating plasma leptin levels and reduced responsiveness to the effect of leptin to suppress food intake and induce weight loss (15, 16, 20, 52), a phenomenon commonly referred to as “leptin resistance”.
On the basis of these observations, we hypothesized that by acting downstream of neuronal systems involved in leptin resistance, increased oxytocin signaling can circumvent this problem to induce weight loss in obese animals through activation of hindbrain neurons that regulate feeding. To test this hypothesis, we compared feeding and neuronal responses to either oxytocin or leptin in rats maintained on either a low-fat diet (LFD) or a high-fat diet (HFD) and examined the potential of oxytocin to induce weight loss in rats with DIO. Our findings demonstrate that oxytocin administration effectively induces weight loss irrespective of whether animals are obese and/or leptin signaling is intact.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley (SD) rats were obtained from Charles River Laboratories International, (Wilmington, MA), and adult male obese (fak/fak) Koletsky rats and lean (Fa/Fa) male littermates (Vassar College, Poughkeepsie, NY) were generated from serial back-crosses (N10 equivalent) of the fak mutation to the inbred rat strain LA/N. All animals were housed individually in Plexiglas cages in a temperature-controlled room under a 12:12-h light-dark cycle. Animals had ad libitum access to water and either a LFD containing 10% kcal from fat or a HFD containing 45% kcal from fat (Research Diets, 12450B; 12451, New Brunswick, NJ), unless otherwise stated. The current research protocols were approved both by the Institutional Animal Care and Use Committees of the Veterans Affairs Puget Sound Health Care System (VAPSHCS), the Department of Veterans Affairs Nebraska Western Iowa Health Care System, and the University of Washington in accordance with NIH Guidelines for the Care and Use of Animals.
Surgery
For experiments that required third ventricle (3V) administration of oxytocin, a separate cohort of animals was implanted with a cannula directed to the 3V as previously described (56). Briefly, animals under isoflurane anesthesia were placed in a stereotaxic apparatus with the incisor bar positioned 3.3 mm below the interaural line. A 26-gauge guide cannula (Small Parts, Miami Lakes, FL) was stereotaxically positioned 1 mm dorsal to the 3V (6.8 mm anterior to the interaural line; 0.05 mm lateral to the midline, and 6.2 mm ventral to the skull surface) and secured to the surface of the skull with dental cement and stainless steel screws. A 33-gauge obturator was inserted into the cannula to maintain patency. Animals were injected with buprenorphine hydrochloride (0.3 mg/kg sc; Reckett and Colman Pharmaceuticals, Richmond, VA) at the completion of surgery and were allowed to recover at least 7 days prior to verification of cannula placement. Cannula placement was verified before the start of experiments by measuring the drinking response following 3V injection of angiotensin at a dose of 10 ng/μl. All animals that drank at least 5 ml of water over a 30-min period were used in the subsequent data analysis.
Drugs
Oxytocin (Bachem, Torrance, CA) was initially dissolved in sterile water and diluted in saline. Recombinant rat leptin (Dr. A. F. Parlow, National Hormone & Peptide Program, CA) was dissolved in phosphate-buffered-saline (PBS) and adjusted to pH 7.9.
Body Composition
Determinations of lean body mass (LBM) and fat mass were made on LFD and HFD animals by quantitative magnetic resonance (QMR) using an EchoMRI 4-in-1 instrument (Echo Medical Systems, Houston, TX) at the VAPSHCS Rodent Metabolic and Behavioral Phenotyping Core at the VAPSHCS. Body composition measurements were also made on lean and obese Koletsky rats and DIO rats by using an EchoMRI-700TM instrument (Echo Medical Systems, Houston, TX) at the University of Washington Nutrition Obesity Research Center (NORC) Animal Studies Physiology Core.
Indirect Calorimetry
Rats were acclimated to calorimetry cages prior to the study and data collection. Energy expenditure measures were obtained using a computer controlled indirect calorimetry system (Promethion; Sable Systems, Las Vegas, NV) located in the Animal Studies Core of NORC at the University of Washington. The calorimetry system consisted of 16 home cages with bedding, that were each equipped with water bottles and food hoppers connected to load cells for food and water intake monitoring. Eight cages apiece were contained in a pair of temperature- and humidity-controlled chambers maintained at 21.4 ± 0.01°C and 41 ± 0.2% relative humidity (Caron Products & Services, Marietta, OH). The air within the cages was sampled through microperforated stainless steel sampling tubes located in the inner bottom rim of the cages. Ambulatory activity and position were detected with XYZ beam arrays (BXYZ-R, Sable Systems) with a beam spacing of 1 cm. Respiratory gases were measured with an integrated fuel cell oxygen analyzer, spectrophotometric CO2 analyzer, and capacitive water vapor partial pressure analyzer (GA3, Sable Systems). The system used two GA-3 analyzers operating in parallel, devoted to eight cages apiece, to maximize throughput. Gas sensors were calibrated daily with 100% N2 as zero reference and with a span gas containing known concentrations of O2, CO2 with balance N2 (PraxAir, Tacoma WA). Promethion utilizes a pull-mode, negative pressure system. Two multichannel mass flow generators measured and controlled air flow (FR8, Sable Systems). The incurrent flow rate was set at 3,000 ml/min. Water vapor was continuously measured, and its dilution effect on O2 and CO2 was mathematically compensated for in the analysis stream (34). O2 consumption and CO2 production were measured for each rat for 1 min every 10-min interval. Incurrent air reference values were determined after measuring every four cages. Respiratory quotient (RQ) was calculated as the ratio of CO2 production over O2 consumption. Energy expenditure was calculated using the Weir equation: kcal/h = 60 × (0.003941 × V̇o2 +0.001106 × V̇co2) (61). To control for the influence of body size variation on TEE (11), group comparisons involving this outcome were adjusted for total body mass using analysis of covariance (ANCOVA), as recommended (25, 26). Ambulatory activity was determined simultaneously with the collection of the calorimetry data. Consecutive adjacent infrared beam breaks in the y-axes, i.e., the length of the cage, were scored as an activity count, and a tally was recorded every 10 min. Data acquisition and instrument control were coordinated by MetaScreen v. 1.6.2, and the obtained raw data was processed using ExpeData v. 1.4.3 (Sable Systems) using an analysis script detailing all aspects of data transformation. The script is available on request from the corresponding author.
Study Protocols
Determination of leptin-induced anorexia in DIO animals.
Animals were habituated to regular handling and sham injections for at least 1 wk prior to the commencement of studies. On the day of the study, 6-h-fasted animals received an intraperitoneal (ip) injection of either leptin (1.5 mg/kg LBM) or vehicle 1 h prior to dark cycle onset. Food was returned immediately prior to dark cycle onset and measured both 4 h and 18 h later, while body weight was recorded 18 h later.
Determination of oxytocin-induced anorexia in DIO animals.
A similar experimental paradigm was used to determine whether peripheral administration of oxytocin reduces food intake in lean and DIO rats. Based on the work of Arletti and colleagues (2, 3), 6-h-fasted rats received an ip injection of either vehicle or oxytocin at a dose of 125, 250, 500 or 1,000 μg/kg LBM immediately prior to dark cycle onset. The dose of oxytocin was adjusted on the basis of LBM rather than total body mass when lean and obese animals were compared, as LBM is better correlated to body water content and thus more likely to achieve comparable blood levels (7, 41). This approach resulted in doses in obese animals that were far lower than would have been the case had dosage been adjusted on the basis of weight. Food was returned immediately thereafter and intake measured both 4 h and 18 h later, while body weight was recorded 18 h later. Injections were performed in a crossover, within-subjects design with at least 72 h given between injections.
Determination of oxytocin-induced anorexia in leptin receptor-deficient animals.
To determine whether systemic oxytocin induces weight loss in an animal model of defective leptin signaling, obese leptin receptor-deficient Koletsky rats and their lean littermates followed a similar protocol as described above and received either oxytocin (1,000 μg/kg ip) or vehicle immediately prior to dark cycle onset. Food was returned immediately prior to dark cycle onset and measured both 4 h and 18 h later while body weight was recorded 18 h later.
Determination of CNS oxytocin-induced anorexia in DIO animals.
To determine whether central administration of oxytocin is sufficient to reduce food intake and body weight in rats maintained on either a LFD or a HFD, a separate cohort of animals was implanted with a cannula directed toward the 3V, as described earlier. Prior to the commencement of experiments, the cannula placement was verified, and animals were habituated to experimental conditions. Similar to the paradigm described above, 6-h-fasted animals received 3V injection of either vehicle or oxytocin at a dose of 1 μg immediately prior to dark cycle onset. Injections were performed over a 1-min period in a final injection volume of 1 μl. Food was returned immediately prior to dark cycle onset and measured at 4 h and 18 h later while body weight was recorded 18 h later.
Determination of oxytocin's chronic effects on food intake, body weight, and energy expenditure in DIO rats.
To determine whether chronic oxytocin administration causes weight loss in obese animals, rats maintained on a HFD for 4 wk were matched for body weight, food intake, and body adiposity and received either daily peripheral injections of oxytocin at 1,000 μg/kg LBM or vehicle just prior to dark cycle onset for 7 consecutive days. To determine whether body weight loss in DIO rats with oxytocin treatment was caused solely by reduced food intake or by increased energy expenditure as well, we placed animals into the Sable Systems indirect calorimeter system for measurement of energy expenditure during the dark cycle on the sixth day of oxytocin administration.
Tissue Collection and Processing
To determine whether peripherally administered oxytocin activates neurons in hindbrain areas that express oxytocin receptors (37), elicit c-Fos-positive(+)-like immunoreactivity (cFLI, a marker of neuronal activation) in response to central administration of oxytocin (46), and are involved in the regulation of meal size (46, 64), we measured cFLI(+) cells in the nucleus of the solitary tract (NTS) and area postrema (AP), key hindbrain areas for satiety perception. SD rats maintained on a LFD or a HFD were fasted for 6 h and received an ip injection of either oxytocin (1,000 μg/kg LBM) or vehicle. Animals were returned to their cages following injections and continued to have their food withheld to prevent the potential confounding effects of differences of intake on hindbrain c-Fos expression. Ninety minutes following injection of vehicle or oxytocin, animals were anesthetized with ketamine cocktail [ketamine hydrochloride (71.4 mg/kg), xylazine (3.57 mg/kg), and acepromazine (1.1 mg/kg) in a 3 ml/kg injection volume] and transcardially exsanguinated with PBS followed by perfusion with 4% paraformaldehyde in 0.1 M PBS. Brains were removed, stored overnight in fresh fixative at 4°C and subsequently transferred to 0.1 M PBS containing 25% sucrose for 48 h. Brains were then frozen by submersion for 20–30 s in isopentane chilled with dry ice.
Immunohistochemical Staining and Quantification
Coronal cryostat sections (14 μm) of the hindbrain were mounted on slides and stored at −80°C. c-Fos staining was performed on anatomically matched sections throughout the NTS and AP, as has been published previously (8). Briefly, slides were washed at room temperature with 10 mM PBS followed by a blocking buffer (5% normal goat serum in 10 mM PBS) for 90 min, followed by additional buffer washes. The primary antibody was rabbit polyclonal anti-c-Fos (Calbiochem, San Diego CA) diluted 1:5,000 in 0.1% BSA in 10 mM PBS, and the secondary antibody was goat anti-rabbit IgG-Cy3 (Jackson ImmunoResearch Laboratories, West Grove, PA) diluted 1:200 in 0.1% BSA in 10 mM PBS. Control sections incubated with normal rabbit serum did not show staining.
Slides were analyzed with a Zeiss Axioplan fluorescence microscope, and all the measurements were made with a ×20 objective lens. Identification of anatomic landmarks was assisted by staining cell nuclei with Hoechst 33258 (Sigma-Aldrich, St. Louis, MO), which was added to the mounting medium and observed with a conventional DAPI filter set. Digital RGB images of the fluorescent preparations were acquired with a Nikon Eclipse E-800 (Melville, NY) with a QImaging Retiga 1300i Fast 1394 high-performance digital CCD camera (Burnaby, BC, Canada) plus the Image-Pro Express imaging system (Media Cybernetics, Bethesda, MD) and were exported to Photoshop CS2 (Adobe, Tucson, AZ). Measurements of cFLI expression in the NTS were pooled from three sections separated by 240 μm (bregma − 14.066 to −13.406) according to the rat brain atlas (48). In each NTS section analyzed, the number of neurons that had cFLI(+) immunofluorescence in the nucleus was recorded bilaterally. The total number of NTS cFLI(+) neurons across the three anatomically matched sections was analyzed across the treatment groups. Unlike the NTS, the data for the AP were sampled and pooled from only two sections separated by 240 μm (bregma − 14.066 to −13.736 mm) (48). The number of cFLI(+) cells in the NTS or the AP was derived from the cumulative number of cFLI(+) cells between the treatments across three sections for the NTS or two sections for the AP, respectively.
Plasma Assays
Blood samples in rats fed the LFD and HFD were collected immediately prior to transcardial perfusion by cardiac puncture in chilled serum separator tubes (SST-amber; Becton-Dickinson, Franklin Lakes, NJ) for measurement of serum oxytocin and plasma leptin. Whole blood was centrifuged at 6,000 rpm for 1.5-min at 4°C, serum was removed, and it was aliquoted and stored at −80°C for subsequent analysis. Plasma immunoreactive leptin levels were measured using a Millipore kit (Millipore, Billerica, MA), and serum oxytocin levels were determined by ELISA (Phoenix Pharmaceuticals, Burlingame, CA) using the Mouse Metabolic Phenotyping Diabetes and Energy Balance Core at the University of Washington.
Statistical Analyses
All results are expressed as means ± SE. Comparisons between multiple groups were made using a two-way analysis of variance with a Fisher's least significant difference post hoc test for comparisons between groups. For two-group comparisons, a two-sample, unpaired Student's t-test was used. Analyses were performed using the statistical program SYSTAT (Systat Software, Point Richmond, CA). To control for the influence of body size variation on TEE (11), group comparisons involving this outcome were adjusted for total body mass by using analysis of covariance (ANCOVA), as suggested (25, 26). ANCOVA was performed with the univariate general linear model module in PASW statistics (v. 17; IBM, Chicago, IL). Differences were considered significant at P < 0.05.
RESULTS
Effect of Peripheral Leptin Administration on Food Intake in DIO Animals
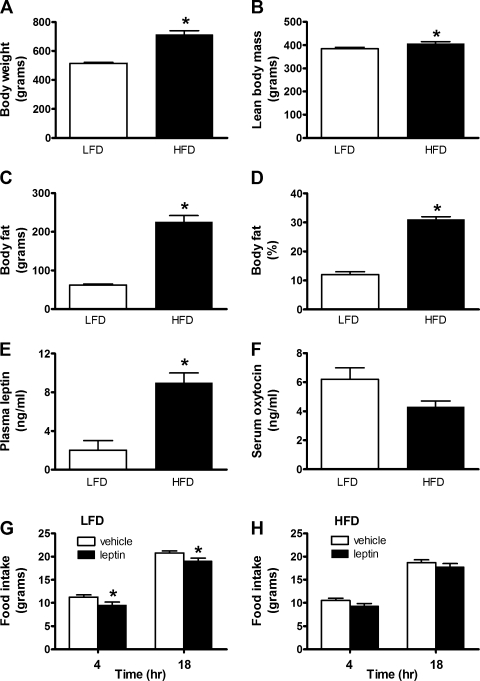
Rats that consumed the HFD for ∼4 mo gained significantly more body weight than those consuming the LFD over the same time interval (n = 17–20/group; Fig. 1A). This increase in body weight was due largely to a marked increase of body adiposity (Fig. 1, B–D). Whereas plasma leptin levels were significantly elevated (n = 10/group) in animals maintained on HFD (n = 7–8/group) relative to LFD controls (n = 10/group), serum oxytocin levels tended to be lower in these animals (Fig. 1, E and F). To directly assay leptin sensitivity in these animals, we examined the effect of a peripheral dose of leptin (1.5 mg/kg LBM) administered 1 h prior to dark cycle onset to reduce food intake in both groups (n = 17/group). We found that food intake was significantly reduced at both 4 h and 18 h after leptin administration in LF-fed rats (P < 0.05), but this effect was absent in HF-fed rats (P = NS; Fig. 1, G and H). These data are consistent with previous evidence (16, 60) of leptin resistance in rodent models of DIO.
Fig. 1.
High-fat diet (HFD) animals are obese, hyperleptinemic, and leptin resistant. Body weight (A), lean body mass (LBM; B), body fat (C), %body fat (D), plasma leptin (E), and serum oxytocin levels (F) in animals maintained on either a LFD or HFD for 4 mo. *P < 0.05 vs. LFD. Food intake at 4 h and 18 h in either LFD (E) or HFD rats (F) following ip injection of vehicle or leptin (1.5 mg/kg body wt) immediately prior to dark cycle onset. Data represent means ± SE. *P < 0.05 vs. vehicle.
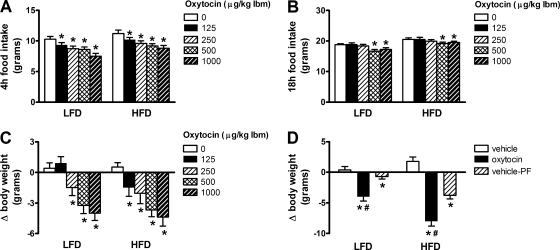
We next examined whether peripheral administration of oxytocin reduces food intake and body weight in these DIO, leptin-resistant rats. Consistent with previous reports (2, 3, 50), we found that peripheral administration of oxytocin dose-dependently reduced food intake in animals on the LFD (n = 20/group; Fig. 2A). Relative to vehicle-treated controls, 4-h intake was reduced by a single injection of oxytocin at doses at or above 125 μg/kg LBM (P < 0.05 for each). After 18 h, food intake remained reduced by 11 and 12% at the 500 and 1,000 μg/kg LBM dose, respectively (P < 0.05; Fig. 2A). In DIO animals (n = 19/group), oxytocin dose-dependently reduced food intake in a manner similar to that observed in animals fed the LFD (Fig. 2B). Relative to vehicle-treated controls, oxytocin significantly reduced food intake at 4 h at doses of 125 μg/kg LBM and higher (P < 0.05 for each) and significantly reduced food intake at 18 h at the 500 and 1,000 μg/kg LBM dose (P < 0.05; Fig. 2B). These data show that, unlike leptin, peripheral administration of oxytocin effectively reduces food intake in animals rendered obese following exposure to a HFD.
Fig. 2.
Peripheral oxytocin reduces food intake and body weight in diet-induced obese (DIO) rats. Food intake and body weight change in rats maintained on a LFD (A, C) vs. HFD (B, C) following ip injection of vehicle or 125, 250, 500, or 1,000 μg/kg LBM dose of oxytocin administered immediately prior to dark cycle onset. D: body weight change in LFD and HFD fed rats that received ip administration of vehicle or oxytocin (1,000 μg/kg LBM) or vehicle and were pair-fed to intake of oxytocin-treated rats. Data represent means ± SE. *P < 0.05 vs. vehicle, #P < 0.05 vs. vehicle-PF.
The effect of oxytocin to reduce food intake was also accompanied by significant reductions of body weight in animals on both the LFD and HFD (Fig. 2C); indeed, weight loss associated with the lowest dose of oxytocin was actually enhanced in DIO animals relative to lean controls (P < 0.05). This enhancement of oxytocin-induced weight loss is unlikely to be explained by differences in circulating oxytocin, as plasma levels were similar in both lean and DIO animals 90 min following injection of oxytocin (1,000 μg/kg LBM) (51 ± 10 vs. 58 ± 18 ng/ml, P = NS) relative to vehicle-treated controls (6.2 ± 0.8 vs. 4.3 ± 0.4 ng/ml, P = NS).
In addition, we found that, in DIO rats, ip oxytocin caused significant weight loss at doses that failed to reduce food intake, suggesting that oxytocin can lower body weight via effects that are both dependent on and independent of reduced food intake. As a first step to investigate this possibility, we measured weight loss in vehicle-treated rats that were pair-fed to the intake of those that received oxytocin. We found that, irrespective of diet, vehicle-treated animals that were pair-fed to oxytocin-treated animals exhibited less weight loss than animals receiving oxytocin (P < 0.05; Fig. 2D).
Effect of Peripheral Oxytocin Administration on Food Intake in Leptin Receptor-Deficient Animals
To verify that oxytocin can reduce food intake in rats that are unable to respond to leptin, we next examined whether the anorexic effects of oxytocin remained intact in leptin receptor-deficient obese Koletsky rats, a genetic model of defective leptin signaling. Adult male Koletsky rats (mean body wt 644 g) and their lean littermates (mean body wt 378 g) were treated with either oxytocin (1,000 μg/kg ip) or its vehicle (n = 6/group). Although the effect of oxytocin to reduce food intake at 4 h and 24 h in lean Koletsky rats did not reach statistical significance, it did reduce body weight gain in these animals (Fig. 3, A and C). In contrast, peripheral administration of oxytocin at the same dose reduced food intake in obese Koletsky rats at 4 h and 18 h by 35 and 19%, respectively (P < 0.05 for each), effects accompanied by a significant reduction in body weight gain (Fig. 3, B and C). Taken together, these data indicate that oxytocin-induced food intake inhibition is intact in animals with both genetic and acquired leptin resistance.
Fig. 3.
Oxytocin reduces food intake and body weight in leptin receptor-deficient Koletsky rats. Food intake and body weight change in both lean (Fa/Fa) (A, C) and obese leptin receptor-deficient (fak/fak) Koletsky rats (B, C) following an ip injection of either vehicle or oxytocin (1,000 μg/kg body wt) administered immediately prior to dark cycle onset. Data represent means ± SE. *P < 0.05 vs. vehicle.
Effect of Peripheral Oxytocin Administration on cFLI Expression in Hindbrain Areas of DIO Rats
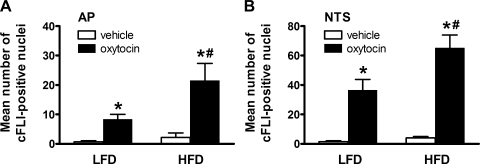
To determine if the anorexic effect of systemically administered oxytocin activates neurons in the same brain areas as that following central administration of oxytocin, we measured cFLI induction in both the NTS and AP. Animals maintained on either a LFD (n = 10/group) or a HFD (n = 7–8/group) received either a peripheral injection of oxytocin (1,000 μg/kg LBM) or vehicle (Fig. 4). Compared with ip vehicle, oxytocin increased the mean number of nuclei that were positive for cFLI within both the AP and NTS of animals in each diet group (Fig. 5, A and B). Moreover, whereas we observed no difference in the number of c-Fos-positive cells detected among vehicle-treated controls, the ability of systemic oxytocin to increase the number of cFLI(+) cell numbers was enhanced in both the AP and NTS of animals maintained on a HFD relative to the LFD (Fig. 5, A and B; P < 0.05).
Fig. 4.
Peripheral oxytocin activates cFLI expression in key hindbrain areas. Representative images of cFLI in coronal sections of rat hindbrain as determined by immunofluorescence in animals maintained on a HFD and treated with either ip saline vehicle (A, C) or oxytocin (B, D) at a dose of 1,000 μg/kg LBM at the level of the area postrema (AP) and nucleus of the solitary tract. (NTS), respectively. A–D are all visualized at ×20 magnification.
Fig. 5.
Peripheral oxytocin activates Fos expression in key hindbrain areas. Mean number of c-Fos-positive(+)-like immunoreactivity (cFLI)-positive nuclei per section in the AP (A) and NTS (B) as determined by immunohistochemistry after ip injection of saline vehicle or oxytocin at a dose of 1,000 μg/kg LBM in rats maintained on a LFD or HFD. Data represent means ± SE. *P < 0.05 vs. vehicle; #P < 0.05 vs. LFD-oxytocin.
Effect of Central Administration of Oxytocin on Food Intake in DIO Rats
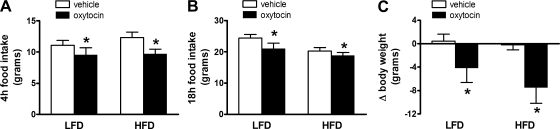
To determine whether central administration of oxytocin is sufficient to reduce food intake and body weight in DIO animals, separate cohorts of adult male SD rats were placed on either a LFD (n = 13/group) or a HFD (n = 5–8/group). After 3 mo, each animal received icv injection of oxytocin (1 μg) or vehicle. Consistent with previous reports (2, 3, 5), we found that 3V administration of oxytocin significantly reduced food intake at both 4 h and 18 h by 14 and 13%, respectively, in animals on the LFD, and this effect was accompanied by a significant reduction in body weight relative to vehicle-treated controls (P < 0.05; Fig. 6, A and C). Centrally administered oxytocin similarly reduced both food intake and body weight relative in DIO rats relative to vehicle-treated controls (63) (Fig. 6, B and C).
Fig. 6.
Central oxytocin administration reduces food intake in lean and DIO rats. Food intake and body weight change in rats maintained on a LFD (A, C) compared with a HFD (B, C) following 3rd ventricle (3V) administration of vehicle or oxytocin (1 μg) administered immediately prior to dark cycle onset. Data represent means ± SE. *P < 0.05 vs. vehicle.
Determination of Oxytocin's Chronic Effects on Weight Loss and Energy Expenditure in DIO Rats
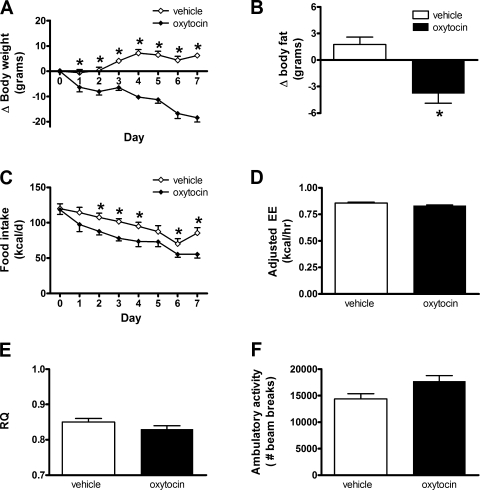
To determine whether chronic systemic administration of oxytocin causes sustained weight loss in the setting of DIO, 4-wk HFD-fed rats (mean body wt 571 ± 14 g, mean %body fat 17.9 ± 0.9%) were treated daily with peripheral injection of either oxytocin (1,000 μg/kg LBM) or vehicle for 7 consecutive days (n = 8/group). Consistent with our earlier findings, we found that, relative to vehicle-treated controls, oxytocin significantly reduced body weight on day 1, and this weight loss continued and persisted throughout the duration of the study (Fig. 7A). This effect was accompanied by a significant reduction in body fat content and a consistent, sustained effect of oxytocin to suppress food intake (Fig. 7, B and C). To determine whether oxytocin also increases energy expenditure in addition to its effects to suppress food intake, oxytocin- and vehicle-treated rats were placed in an indirect calorimeter during the dark cycle on the day 6 of oxytocin treatment. We found that, despite significant reductions of both food intake and body weight, which normally reduces the rate of energy expenditure and lowers RQ, these animals maintained levels of energy expenditure, RQ, and ambulatory activity no different from those of vehicle-treated controls (Fig. 7, D–F).
Fig. 7.
Effect of chronic peripheral oxytocin administration on weight-loss and energy expenditure in DIO rats. Change in body weight (A), change in body fat (B), daily food intake (C), adjusted energy expenditure (EE; D), respiratory quotient (RQ; E), and ambulatory activity levels (F) as measured using indirect calorimetry in 4-wk HFD-fed rats treated daily with peripheral administration of vehicle or oxytocin (1,000 μg/kg LBM) for 7 days. Data represent means ± SE. *P < 0.05 vs. vehicle.
DISCUSSION
The primary goals of this study were to determine whether increased signaling by oxytocin, a downstream mediator of leptin action, is sufficient to induce anorexia in obese, leptin-resistant animals and whether this effect can be induced following systemic as well as central oxytocin administration. We found that, whereas leptin-induced inhibition of food intake was attenuated in DIO animals, administration of oxytocin dose-dependently reduced food intake and body weight irrespective of dietary fat content and in the presence of either genetic or diet-induced leptin resistance. In addition, oxytocin's anorexic effects could be elicited following either peripheral or central oxytocin administration, and this effect was accompanied by a loss of body weight that could not be fully explained by the reduction of food intake. Moreover, chronic oxytocin administration induced prolonged, sustained weight loss in rats with DIO and blocked the decrease of energy expenditure that normally accompanies weight loss. Combined with our finding that systemic oxytocin administration activates neurons in both the AP and NTS (as has previously been reported following central administration), we conclude that increased oxytocin signaling circumvents leptin resistance in DIO animals by maintaining activation of hindbrain areas that regulate energy balance. Therapeutic interventions that target this pathway may therefore have untapped potential in obesity treatment.
So far as we are aware, ours is the first report that acute peripheral administration of oxytocin dose-dependently reduces food intake and body weight not only in lean animals on a LFD, as previously described (2, 3, 5), but that the anorexic effects of oxytocin are not diminished in rats with obesity, hyperleptinemia, and leptin resistance induced by HFD feeding. Moreover, we found that chronic treatment with oxytocin systemically resulted in persistent, sustained weight loss in DIO rats. While oxytocin receptors are expressed in peripheral tissues, including the reproductive tract and kidney (19), and we cannot rule out a peripheral site of action (or a neural mechanism that requires activation of peripherally located afferent fibers), our data suggest that the anorexic effects of oxytocin are mediated centrally. This hypothesis is supported by evidence that oxytocin receptors are expressed in brain areas including the NTS (37) and that neurons in this brain area were activated following and peripheral administration of oxytocin. Our findings that the same hindbrain areas are activated by central administration of oxytocin at much lower doses (46) and that icv administration of oxytocin reduces food intake at doses that are not effective when administered peripherally (2, 3, 5) are consistent with a common mechanism of action regardless of whether oxytocin is given centrally or peripherally.
Indeed, recent work suggests that systemic administration of oxytocin activates oxytocin neurons in the supraoptic nucleus, as well as the magnocellular PVN and pPVN (13, 63), and induces the release of oxytocin from the PVN (63). These findings raise the interesting possibility that the oxytocin-mediated activation of pPVN oxytocin neurons induces release of oxytocin in the NTS, a mechanism that in some ways parallels leptin action. Unlike leptin, however, the question of whether circulating oxytocin plays a physiological role to regulate energy balance has yet to be investigated. Future studies will be aimed at studying the effects of systemic oxytocin to reduce food intake, body weight, and induce Fos in the NTS of animals with reduced oxytocin receptor expression in the NTS as one approach to determine whether systemic administration of oxytocin requires activation of oxytocin receptor-expressing neurons in the NTS.
Substantial literature suggests that oxytocin neurons in the hypothalamic pPVN are activated by leptin [either directly or via projections from melanocortin neurons in the arcuate nucleus (ARC)] and serve to couple forebrain leptin action to hindbrain areas that respond to meal-related satiety signals. In support of this hypothesis, leptin activates oxytocin-producing neurons in the pPVN (8), a subset of these pPVN oxytocin neurons sends projections to NTS regions that respond to CCK and control meal size (8), and oxytocinergic fibers in the NTS originate solely from pPVN neurons (49). Furthermore, the ability of leptin both to reduce food intake and to enhance the satiety and neuronal response to CCK appears to require oxytocin signaling, as it is attenuated by 3V pretreatment with an oxytocin receptor antagonist (8). Evidence that the melanocortin pathway is a component of this neurocircuit includes the finding that leptin activates POMC neurons in the ARC (14, 54), that leptin's ability to activate pPVN neurons is blocked by melanocortin-3/4 receptor antagonists (55), and that intact melanocortin signaling is required for leptin-induced anorexia (55). Like leptin, administration of the Mc3/4r agonist, α-melanocyte-stimulating hormone (α-MSH) activates oxytocin neurons in the PVN (31, 47), Mc4r-expressing cells in the PVN are colocalized with oxytocin (36), and the anorexic effects of α-MSH are prevented by pretreatment with an oxytocin receptor antagonist (62). These findings collectively suggest that melanocortin signaling activates oxytocin neurons in the pPVN that in turn link input from leptin to hindbrain areas that process input from satiety signals such as CCK.
In DIO animals, attenuation of leptin-induced anorexia is associated with reduced activation by leptin of pSTAT3, a marker of leptin signaling, in the ARC, but not in other hypothalamic leptin-sensing neurons, suggesting that ARC neurons are a major site of leptin resistance (42). Combined with the finding that leptin fails to induce hypothalamic secretion of melanocortin peptides in DIO mice compared with chow-fed controls (17), we hypothesize that DIO is accompanied by an attenuation of leptin's ability to stimulate proopiomelanocortin neurons, thereby reducing the release of α-MSH on oxytocin neurons in the pPVN. This, in turn, reduces oxytocin secretion in the NTS, dampening the satiety response to CCK and favoring the consumption of larger meals, ultimately promoting weight gain over time. This model predicts that administration of oxytocin can overcome “leptin resistance” by activating oxytocin-sensitive neurons in hindbrain areas that promote the suppression of food intake.
Our findings that the effect of systemic oxytocin to activate cFLI in key hindbrain areas that contain oxytocin receptors (37) is not only maintained but enhanced in animals on a HFD relative to a LFD supports this model. One possible explanation for this effect is that HFD-induced obese animals have a reduced endogenous oxytocin tone, resulting in increased oxytocin sensitivity. Consistent with this model, lateral and fourth ventricular administration of an oxytocin receptor antagonist to block endogenous oxytocin in the hindbrain attenuates the satiety effects of CCK (6, 45) and stimulates food intake in rodents by increasing meal size (8, 9). However, although the satiety effect of CCK is not attenuated in oxytocin-deficient mice (38), reducing the expression of oxytocin-receptive cells in the NTS using a saporin toxin approach attenuates the satiety response to CCK (4).
Consistent with this hypothesis, a recent study reported that Syt4 is a regulator of exocytosis that is expressed in oxytocin neurons in the PVN and inhibits oxytocin release from these neurons (63). Support for a role of Syt4 in promoting DIO through inhibition of oxytocin release was provided by the finding that syt4-deficient (syt4−/−) mice are protected from DIO, whereas conversely, overexpression of Syt4 in oxytocin neurons increases food intake and body weight gain. Moreover, oxytocin release from the PVN was blunted in HFD relative to chow-fed control mice, whereas it was enhanced in chow-fed syt4−/− mice and was not attenuated by HFD feeding in these animals (63). Combined with evidence that chronic sucrose intake blunts activity of the anorexigenic oxytocin system (40), these support the hypothesis that oxytocin release is impaired in DIO (perhaps via a mechanism involving resistance to leptin signaling). Additional studies are warranted to test directly whether leptin-induced activation of oxytocin neurons in the pPVN, and the subsequent release of oxytocin in the NTS, is impaired in DIO animals.
Because oxytocin-induced weight loss could not be fully accounted for by reduced food intake alone, and since doses of oxytocin below those needed to reduce food intake nonetheless lowered body weight, it seems likely that oxytocin stimulates energy expenditure as well. While weight loss causes a decrease in energy expenditure in both rodents and humans (28, 33, 51), we found that in animals treated chronically with oxytocin, despite a persistent, sustained suppression of food intake and weight loss, energy expenditure levels were maintained in these animals similar to that of vehicle-treated controls. Thus, oxytocin administration blocked the effect of weight loss to reduce energy expenditure, an effect also observed in response to leptin administration (51). A role for oxytocin in the regulation of energy expenditure is further supported by evidence that central administration of oxytocin acutely stimulates energy expenditure in chow-fed rats (63), that pPVN oxytocin neurons project to the spinal cord (53), that a proportion of oxytocin neurons in the pPVN project postsynaptically to BAT (43) via the stellate ganglia (24), and that oxytocin-expressing neurons in the PVN are activated in mice following cold exposure (27). Moreover, oxytocin- and oxytocin receptor-deficient mice exhibit both an impaired thermogenic response to a cold challenge (27, 58) and reduced sympathetic tone (12), whereas central oxytocin administration induces hyperthermia in rabbits (35) and mice (39). These data suggest that pPVN oxytocin neurons participate in the regulation of thermoregulation as well as food intake, and both functions may participate in the control of energy reserves.
In conclusion, our findings extend previous evidence and suggest that increased oxytocin signaling is capable of inducing both acute and chronic reductions of body weight in DIO rats both by suppressing food intake and by preventing the effect of weight loss to lower energy expenditure. Moreover, our data suggest that this effect of oxytocin to circumvent leptin resistance occurs through a mechanism in part involving activation of hindbrain neurons involved in satiety perception. Taken together, these data raise the possibility that pharmaceutical interventions that target this may be another potential avenue to treat obesity.
GRANTS
This work was supported by resources from the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, including the Department of Veterans Affairs Career Development Program, Merit Review Research Program, and the Career Scientist Program. D. G. Baskin is the recipient of a Department of Veterans Affairs Senior Research Career Scientist Award at the Veterans Affairs Puget Sound Health Care System. R. D. Reidelberger is the recipient of a Department of Veterans Affairs Research Career Scientist Award at the Department of Veterans Affairs Nebraska Western Iowa Health Care System. M. W. Schwartz is supported by NIH grants (DK-068304, DK-083042, DK-052989). G. J. Morton is supported by an NIH grant (DK-089056) and a Scientist Development Grant from the American Heart Association. This research was also supported by the Cellular and Molecular Imaging Core of the National Institutes of Health (NIH) Diabetes Endocrinology Research Center (DERC, P30 DK-17047), the NIH Nutrition Obesity Research Unit Animal Studies Physiology Core at the University of Washington (NORC, P30 DK-035816), and the NIH Mouse Metabolic Phenotyping Center Diabetes and Energy Balance Core (MMPC, U24 DK-076126).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.J.M., D.G.B., M.W.S., and J.E.B. conception and design of research; G.J.M., B.S.T., R.D.R., K.O., T.H.W.-H., and J.E.B. performed experiments; G.J.M., B.S.T., R.D.R., K.O., T.H.W.-H., and J.E.B. analyzed data; G.J.M., B.S.T., R.D.R., K.O., D.G.B., M.W.S., and J.E.B. interpreted results of experiments; G.J.M. and J.E.B. prepared figures; G.J.M. and J.E.B. drafted manuscript; G.J.M., B.S.T., R.D.R., K.O., T.H.W.-H., D.G.B., M.W.S., and J.E.B. edited and revised manuscript; G.J.M., B.S.T., R.D.R., K.O., T.H.W.-H., D.G.B., M.W.S., and J.E.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the excellent technical assistance provided by Jarrell Nelson for performing body composition measurements and Dr. Karl Kaiyala for performing multiple regression analysis for the normalization of energy expenditure as part of the University of Washington Nutrition Obesity Research Center Animal Studies Physiology Core. Serum oxytocin was measured by the University of Washington Mouse Metabolic Phenotyping Center Diabetes and Obesity Core.
REFERENCES
- 1. Amico JA, Vollmer RR, Karam JR, Lee PR, Li X, Koenig JI, McCarthy MM. Centrally administered oxytocin elicits exaggerated grooming in oxytocin null mice. Pharmacol Biochem Behav 78: 333–339, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Arletti R, Benelli A, Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides 10: 89–93, 1989 [DOI] [PubMed] [Google Scholar]
- 3. Arletti R, Benelli A, Bertolini A. Oxytocin inhibits food and fluid intake in rats. Physiol Behav 48: 825–830, 1990 [DOI] [PubMed] [Google Scholar]
- 4. Baskin DG, Kim F, Gelling RW, Russell BJ, Schwartz MW, Morton GJ, Simhan HN, Moralejo DH, Blevins JE. A new oxytocin-saporin cytotoxin for lesioning oxytocin-receptive neurons in the rat hindbrain. Endocrinology 151: 4207–4213, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benelli A, Bertolini A, Arletti R. Oxytocin-induced inhibition of feeding and drinking: no sexual dimorphism in rats. Neuropeptides 20: 57–62, 1991 [DOI] [PubMed] [Google Scholar]
- 6. Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG. Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res 993: 30–41, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Blevins JE, Morton GJ, Williams DL, Caldwell DW, Bastian LS, Wisse BE, Schwartz MW, Baskin DG. Forebrain melanocortin signaling enhances the hindbrain satiety response to CCK-8. Am J Physiol Regul Integr Comp Physiol 296: R476–R484, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brainstem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol 287: R87–R96, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Blouet C, Jo YH, Li X, Schwartz GJ. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci 29: 8302–8311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buijs RM. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res 192: 423–435, 1978 [DOI] [PubMed] [Google Scholar]
- 11. Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes 59: 323–329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Camerino C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity (Silver Spring) 17: 980–984, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Carson DS, Hunt GE, Guastella AJ, Barber L, Cornish JL, Arnold JC, Boucher AA, McGregor IS. Systemically administered oxytocin decreases methamphetamine activation of the subthalamic nucleus and accumbens core and stimulates oxytocinergic neurons in the hypothalamus. Addiction Biol 15: 448–463, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology 138: 4489–4492, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334: 292–295, 1996 [DOI] [PubMed] [Google Scholar]
- 16. El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 105: 1827–1832, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 5: 181–194, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet 25: 284–288, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81: 629–683, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282: 1568–1575, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Holder JL, Jr, Butte NF, Zinn AR. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum Mol Genet 9: 101–108, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Holder JL, Jr, Zhang L, Kublaoui BM, DiLeone RJ, Oz OK, Bair CH, Lee YH, Zinn AR. Sim1 gene dosage modulates the homeostatic feeding response to increased dietary fat in mice. Am J Physiol Endocrinol Metab 287: E105–E113, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Hung CC, Luan J, Sims M, Keogh JM, Hall C, Wareham NJ, O'Rahilly S, Farooqi IS. Studies of the SIM1 gene in relation to human obesity and obesity-related traits. Int J Obes (Lond) 31: 429–434, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Jansen AS, Wessendorf MW, Loewy AD. Transneuronal labeling of CNS neuropeptide and monoamine neurons after pseudorabies virus injections into the stellate ganglion. Brain Res 683: 1–24, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Kaiyala KJ, Morton GJ, Leroux BG, Ogimoto K, Wisse B, Schwartz MW. Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes 59: 1657–1666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaiyala KJ, Schwartz MW. Toward a more complete (and less controversial) understanding of energy expenditure and its role in obesity pathogenesis. Diabetes 60: 17–23, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kasahara Y, Takayanagi Y, Kawada T, Itoi K, Nishimori K. Impaired thermoregulatory ability of oxytocin-deficient mice during cold-exposure. Biosci Biotechnol Biochem 71: 3122–3126, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Keesey RE, Hirvonen MD. Body weight set-points: determination and adjustment. J Nutr 127: 1875S–1883S, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Kirchgessner AL, Sclafani A, Nilaver G. Histochemical identification of a PVN-hindbrain feeding pathway. Physiol Behav 42: 529–543, 1988 [DOI] [PubMed] [Google Scholar]
- 30. Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature 435: 673–676, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol 22: 1723–1734, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kublaoui BM, Holder JL, Jr, Gemelli T, Zinn AR. Sim1 haploinsufficiency impairs melanocortin-mediated anorexia and activation of paraventricular nucleus neurons. Mol Endocrinol 20: 2483–2492, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 332: 621–628, 1995 [DOI] [PubMed] [Google Scholar]
- 34. Lighton J. Flow through respirometry: the equations. In: Measuring Metabolic Rate: A Manual for Scientists. New York: Oxford Univ. Press, 2008 [Google Scholar]
- 35. Lipton JM, Glyn JR. Central administration of peptides alters thermoregulation in the rabbit. Peptides 1: 15–18, 1980 [DOI] [PubMed] [Google Scholar]
- 36. Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci 23: 7143–7154, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Loup F, Tribollet E, Dubois-Dauphin M, Pizzolato G, Dreifuss JJ. Localization of oxytocin binding sites in the human brainstem and upper spinal cord: an autoradiographic study. Brain Res 500: 223–230, 1989 [DOI] [PubMed] [Google Scholar]
- 38. Mantella RC, Rinaman L, Vollmer RR, Amico JA. Cholecystokinin and d-fenfluramine inhibit food intake in oxytocin-deficient mice. Am J Physiol Regul Integr Comp Physiol 285: R1037–R1045, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Mason GA, Caldwell JD, Stanley DA, Hatley OL, Prange AJ, Jr, Pedersen CA. Interactive effects of intracisternal oxytocin and other centrally active substances on colonic temperatures of mice. Regul Pept 14: 253–260, 1986 [DOI] [PubMed] [Google Scholar]
- 40. Mitra A, Gosnell BA, Schioth HB, Grace MK, Klockars A, Olszewski PK, Levine AS. Chronic sugar intake dampens feeding-related activity of neurons synthesizing a satiety mediator, oxytocin. Peptides 31: 1346–1352, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, Schwartz MW. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest 115: 703–710, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145: 4880–4889, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience 110: 515–526, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides 12: 113–118, 1991 [DOI] [PubMed] [Google Scholar]
- 45. Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology 129: 785–791, 1991 [DOI] [PubMed] [Google Scholar]
- 46. Olson BR, Freilino M, Hoffman GE, Stricker EM, Sved AF, Verbalis JG. c-Fos expression in rat brain and brainstem nuclei in response to treatments that alter food intake and gastric motility. Mol Cell Neurosci 4: 93–106, 1993 [DOI] [PubMed] [Google Scholar]
- 47. Olszewski PK, Wirth MM, Shaw TJ, Grace MK, Billington CJ, Giraudo SQ, Levine AS. Role of α-MSH in the regulation of consummatory behavior: immunohistochemical evidence. Am J Physiol Regul Integr Comp Physiol 281: R673–R680, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Rinaman L. Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats. J Comp Neurol 399: 101–109, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Rinaman L, Rothe EE. GLP-1 receptor signaling contributes to anorexigenic effect of centrally administered oxytocin in rats. Am J Physiol Regul Integr Comp Physiol 283: R99–R106, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 115: 3579–3586, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab 87: 2391–2394, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol 205: 260–272, 1982 [DOI] [PubMed] [Google Scholar]
- 54. Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes 46: 2119–2123, 1997 [DOI] [PubMed] [Google Scholar]
- 55. Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, van Dijk G, Baskin DG, Schwartz MW. Melanocortin receptors in leptin effects. Nature 390: 349, 1997 [DOI] [PubMed] [Google Scholar]
- 56. Stanley BG, Lanthier D, Leibowitz SF. Multiple brain sites sensitive to feeding stimulation by opioid agonists: a cannula-mapping study. Pharmacol Biochem Behav 31: 825–832, 1988 [DOI] [PubMed] [Google Scholar]
- 57. Swaab DF, Purba JS, Hofman MA. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: a study of five cases. J Clin Endocrinol Metab 80: 573–579, 1995 [DOI] [PubMed] [Google Scholar]
- 58. Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport 19: 951–955, 2008 [DOI] [PubMed] [Google Scholar]
- 59. Tolson KP, Gemelli T, Gautron L, Elmquist JK, Zinn AR, Kublaoui BM. Postnatal Sim1 deficiency causes hyperphagic obesity and reduced Mc4r and oxytocin expression. J Neurosci 30: 3803–3812, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis HR., Jr Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest 99: 385–390, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yosten GL, Samson WK. The anorexigenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin receptor antagonist. Am J Physiol Regul Integr Comp Physiol 298: R1642–R1647, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang G, Bai H, Zhang H, Dean C, Wu Q, Li J, Guariglia S, Meng Q, Cai D. Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron 69: 523–535, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zittel TT, De Giorgio R, Sternini C, Raybould HE. Fos protein expression in the nucleus of the solitary tract in response to intestinal nutrients in awake rats. Brain Res 663: 266–270, 1994 [DOI] [PubMed] [Google Scholar]