Abstract
Our objective was to compare the effects of in vivo insulin on skeletal muscle glycogen synthase (GS) activity in normal (NGT) vs. impaired glucose-tolerant (IGT) obese postmenopausal women and to determine whether an increase in insulin activation of GS is associated with an improvement in insulin sensitivity (M) following calorie restriction (CR) and/or aerobic exercise plus calorie restriction (AEX + CR) in women with NGT and IGT. We did a longitudinal, clinical intervention study of CR compared with AEX + CR. Overweight and obese women, 49–76 yr old, completed 6 mo of CR (n = 46) or AEX + CR (n = 50) with V̇o2 max, body composition, and glucose tolerance testing. Hyperinsulinemic euglycemic (80 mU·m−2·min−1) clamps (n = 73) and skeletal muscle biopsies (before and during clamp) (n = 58) were performed before and after the interventions (n = 50). After 120 min of hyperinsulinemia during the clamp, GS fractional activity and insulin's effect to increase GS fractional activity (insulin − basal) were significantly lower in IGT vs. NGT (P < 0.01) at baseline. GS total activity increased during the clamp in NGT (P < 0.05), but not IGT, at baseline. CR and AEX + CR resulted in a significant 8% weight loss with reductions in total fat mass, visceral fat, subcutaneous fat, and intramuscular fat. Overall, M increased (P < 0.01), and the change in M (postintervention − preintervention) was associated with the change in insulin-stimulated GS fractional activity (partial r = 0.44, P < 0.005). In IGT, the change (postintervention − preintervention) in insulin-stimulated GS total activity was greater following AEX + CR than CR alone (P < 0.05). In IGT, insulin-stimulated GS-independent (P < 0.005) and fractional activity (P = 0.06) increased following AEX + CR. We conclude that the greatest benefits at the whole body and cellular level (insulin activation of GS) in older women at highest risk for diabetes are derived from a lifestyle intervention that includes exercise and diet.
Keywords: skeletal muscle, insulin action, aerobic exercise training, weight loss, aging
the diabetes prevention program research group demonstrated that weight loss and physical activity reduced the incidence of type 2 diabetes in individuals with impaired glucose tolerance (IGT) over an approximately 3-yr period (18). Although the lifestyle modification was more effective than metformin in preventing or delaying onset of type 2 diabetes (18), the cellular mechanisms behind the lifestyle changes were unaddressed in this large trial. Because current estimates indicate that more than 68% of middle-aged and older women are either overweight or obese in the US (23), this led us to examine potential mechanisms in postmenopausal women. Overweight and obesity are significantly associated with type 2 diabetes (22), with postmenopausal women at a higher risk for IGT and diabetes than younger women (6). Moreover, risk for IGT increases with obesity and age, and women with IGT are at a greater risk of developing diabetes (3).
We have demonstrated previously that a combined intervention of aerobic exercise plus weight loss improves whole body insulin sensitivity (M) during a 40 mU·m−2·min−1 hyperinsulinemic euglycemic clamp in a different group of overweight and obese postmenopausal women (26). Weight loss programs without exercise that reduce body weight ∼6% did not change insulin sensitivity (26), but larger losses of body weight (low-calorie dieting) increase M in obese men and premenopausal women (13, 25). In another example, insulin sensitivity by the minimal model technique increased comparably after 12% weight loss alone or combined with aerobic training in obese premenopausal women (28). Glycogen synthase (GS) is a key enzyme that is related to whole body insulin sensitivity across species and glucose tolerance (2, 7, 15, 24) with lower insulin activation of GS in IGT (12). An increased ability of insulin to activate GS may be involved in the improvement in insulin sensitivity following calorie restriction (CR) with or without exercise training (AEX); this has not been investigated previously in postmenopausal women. Therefore, we tested insulin activation of GS and M in postmenopausal women with normal glucose tolerance (NGT) and IGT before and after AEX + CR vs. CR. We hypothesized that an increase in insulin activation of GS would be associated with an increase in M after AEX + CR, and not after CR alone, regardless of glucose tolerance status.
MATERIALS AND METHODS
All subjects were healthy, overweight, and obese [body mass index (BMI) >25 kg/m2, range of 25–48 kg/m2] women between the ages of 49 and 76 yr. The women were postmenopausal and had not menstruated for ≥1 yr. Only women who were weight stable (<2.0 kg weight change in past year) and sedentary (<20 min of aerobic exercise twice/wk) were recruited. Subjects were screened by medical history questionnaire, physical examination, and fasting blood profile. Individuals with untreated hypertension or hyperlipidemia were referred to their doctor for therapy and entered the study if they were treated with an antihypertensive or lipid-lowering drug that did not affect glucose metabolism. All subjects were nonsmokers, showed no evidence of cancer, liver, renal, or hematological disease or other medical disorders, and underwent a Bruce graded treadmill test to exclude those with asymptomatic coronary artery disease.
One-hundred seventy-four women met study criteria and were assigned to CR (n = 86) or AEX + CR (n = 88). Sixty-three women dropped out of the program due to personal reasons, relocation, illness, and/or time constraints. Eight women completed the program but were not compliant to the weight loss or exercise guidelines described below. Eight women with unconfirmed diabetes or diagnosed with type 2 diabetes were excluded from the study. The baseline characteristics of those who dropped out did not differ from those who completed the study (data not shown). A total of 95 women completed the study (CR, n = 46; AEX + CR, n = 49). Because all 95 women had pre- and post-glucose tolerance testing, we divided them into NGT and IGT groups (1). Of the 95 women, 14 women (n = 5 in CR and n = 9 in AEX + CR) were using hormone replacement therapy prior to enrollment that did not change for the duration of the study, with the exception of one individual who came off of therapy during the intervention phase. The Institutional Review Board of the University of Maryland approved all methods and procedures. Each participant provided written, informed consent to participate in the study.
Study protocol.
Subjects received instruction in maintaining a weight-stable, Therapeutic Lifestyle Changes diet (19) by a registered dietitian 1 day/wk for 6–8 wk prior to baseline testing. Subjects were weight stable on the Therapeutic Lifestyle Changes diet prior to baseline testing and were instructed to maintain this dietary composition throughout the study.
Weight loss program.
All women in CR and AEX + CR attended weekly weight loss classes led by a registered dietitian for instruction in the Therapeutic Lifestyle Changes diet. Compliance was monitored by 7-day food records (or 24-h recalls) using the American Diabetes Association exchange list system. Women were instructed to reduce their caloric intake by 500 kcal/day. The average compliance for attendance to the weight loss classes was 86%.
Aerobic exercise program.
Women in the AEX + CR intervention exercised at the Baltimore Veterans Medical Center exercise facility three times/wk for 6 mo using treadmills and elliptical trainers. Each exercise session included a 5- to 10-min stretching and warmup phase and a 5- to 10-min cooldown phase. Women exercised at >85% heart rate reserve for 45 min. The average compliance for attendance to the exercise sessions was 87%.
Maximal aerobic uptake and body composition.
Maximal aerobic uptake (V̇o2max) was measured using a continuous treadmill test protocol (26). Height (cm) and weight (kg) were measured to calculate BMI, and waist and hip circumference were determined. Fat mass, lean tissue mass, and bone mineral content (fat-free mass = lean + bone) were determined by dual-energy X-ray absorptiometry (Prodigy; Lunar Radiation, Madison, WI). One individual refused her post-body composition scan. Fat-free mass was calculated for this individual using the mean decrease in fat-free mass for her group. A single computed tomography (Siemens Somatom Sensation 64 Scanner; Siemens, Fairfield, CT) scan at the L4–L5 region was used to determine visceral adipose tissue area and subcutaneous adipose tissue area and analyzed using Medical Image Processing, Analysis, and Visualization, version 7.0.0. A second scan at the midthigh was used to quantify muscle area, total fat area, and low-density lean tissue area by Hounsfield units (26); values of the right leg were used in the statistical analyses.
Metabolic testing.
All subjects were weight stabilized (± 2%) for ≥2 wk prior to metabolic testing before and after the interventions and were provided all meals as a eucaloric diet for 2 days before the clamp by a registered dietitian to control nutrient intake (26). All testing was performed in the morning after a 12-h overnight fast. At the end of 6 mo, the AEX + CR group was asked to continue the aerobic training 3 days/wk during the final testing period, and clamps were performed 36–48 h after the last bout of exercise.
Oral glucose tolerance test.
Blood samples were drawn before and at 30-min intervals for 2 h after ingestion of 75 g of glucose. Plasma glucose concentrations were measured using the glucose oxidase method (2300 STAT Plus; YSI, Yellow Springs, OH). The women were defined by glucose tolerance status (1).
Hyperinsulinemic euglycemic clamps with skeletal muscle biopsies and indirect calorimetry.
Whole body insulin sensitivity was measured using the hyperinsulinemic euglycemic clamp technique (8). Difficulty in obtaining venous access or a scheduling conflict occurred in 20 women at baseline (CR: n = 15; AEX + CR: n = 5) and five additional women after the interventions, so the clamp was not performed. Arterialized blood was obtained from a dorsal heated hand vein (21). Blood samples were obtained every 5 and 10 min for the determination of plasma glucose and insulin levels. A 10-min priming and continuous infusion of insulin (80 mU·m−2·min−1 Humulin; Eli Lilly, Indianapolis, IN) was performed for 180 min with a continuous infusion of 20% glucose solution starting at 10 min. Blood samples were collected in heparinized syringes, placed in prechilled test tubes containing 1.5 mg EDTA/ml of blood, and centrifuged at 4°C for plasma glucose and stored at −70°C until analysis for plasma insulin (27). Plasma glucose during each clamp period was not different before and after CR (NGT: 4.9 ± vs. 4.8 ± 0.07 mmol/l; IGT: 5.0 ± 0.13 vs. 5.0 ± 0.09 mmol/l) or AEX + CR (NGT: 5.0 ± 0.07 vs. 4.9 ± 0.07 mmol/l; IGT: 5.1 ± 0.08 vs. 5.0 ± 0.07 mmol/l). This was 97 ± 0.3% of the desired goal, with a coefficient of variation of 6.5 ± 0.2% in all clamps (n = 150). Plasma insulin concentrations during 120–180 min of the hyperinsulinemic euglycemic clamps was not different before or after the interventions (CR: NGT 1,113 ± 48 vs. 1,096 ± 55 pmol/l, IGT 1,181 ± 45 vs. 1,211 ± 77 pmol/l; AEX + CR: NGT 1,055 ± 25 vs. 1,043 ± 29 pmol/l, IGT 1,314 ± 67 vs. 1,267 ± 48 pmol/l). M was calculated from the amount of glucose infused after correction for glucose-equivalent space (glucose space correction). Twenty-four-hour urine collections were collected the day prior to each clamp. Continuous indirect calorimetry was performed prior to and during the last 30 min of the insulin infusion by the open-circuit dilution technique using a SensorMedics DeltaTrac cart (Yorba Linda, CA) to quantitate rates of glucose oxidation with correction for protein oxidation based on 24-h urinary urea nitrogen.
Skeletal muscle biopsies and analysis.
Biopsies were performed 36–48 h after the last bout of exercise. Prior to the start of the clamp, and 120-min during the glucose clamp, a vastus lateralis muscle biopsy was taken from each subject under local anesthesia for the measurement of GS activity. The muscle samples were lyophilized for 48 h and then dissected free of obvious connective tissue, fat, and blood. Microdissected samples were homogenized (0.67% wt/vol solution) in ice-cold buffer (pH 7.5) containing 0.1% 2-mercaptoethanol and (in mmol/l) 10 EDTA, 100 NaF, and 0.5 PMSF. The homogenate was centrifuged at 10,000 g for 2 min at 4°C. Total (10 mmol/l glucose 6-phosphate) and independent (0.1 mmol/l glucose 6-phosphate) GS activity were measured by adding 10 μl of supernatant to 60 μl of reaction mixture containing (in mmol/l) 50 Tris, 20 EDTA, 87.5 KF, 0.2 UDPG, 5,000 disintegrations·min−1·nmol−1 (UDP)-[U-14C]glucose, and 1% glycogen (24). The fractional activity of GS is the independent activity of GS divided by the total activity of GS and is expressed as a percent.
Statistics.
Baseline comparisons of the two intervention groups (CR vs. AEX + CR) and the two glucose tolerance groups (NGT vs. IGT) were performed using unpaired Student's t-tests.
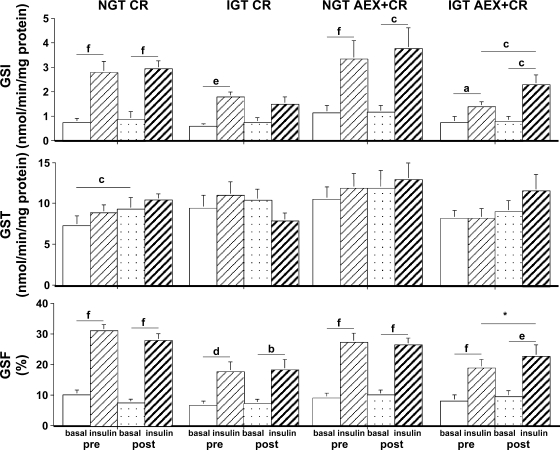
Paired Student t-tests were used to compare GS activity within each of the four combinations of intervention and glucose tolerance status (NGT CR, IGT CR, NGT AEX + CR, and IGT AEX + CR). The comparisons examined were 1) basal vs. insulin-stimulated GS preintervention, 2) basal vs. insulin-stimulated GS post intervention, 3) basal GS preintervention to basal GS postintervention, and 4) insulin-stimulated GS preintervention to insulin-stimulated GS postintervention (Fig. 1).
Fig. 1.
Basal and insulin-stimulated glycogen synthase (GS) activity by glucose tolerance status [normal (NGT) and impaired glucose tolerance (IGT)] within each intervention [calorie restriction (CR) alone, aerobic exercise training (AEX) + CR]. Top: GS-independent (GSI) activity. Middle: GS total (GST) activity. Bottom: GS fractional (GSF) activity. NGT CR, n = 13; IGT CR, n = 6; NGT AEX + CR, n = 20; IGT AEX + CR, n = 11. aP < 0.05; bP < 0.01; cP < 0.005; dP < 0.001; eP < 0.0005; fP < 0.0001. *P = 0.06 by paired Student's t-test. Pre, preintervention; post, postintervention.
The effect of the intervention (CR vs. AEX + CR) adjusted for glucose tolerance status (normal vs. impaired) was compared using ANOVA: change = baseline value + intervention group + glucose tolerance group + intervention × glucose tolerance group, followed by post hoc analyses (Tukey-Kramer when variances were equal; Scheffe otherwise) when the interaction was significant. When the intervention × glucose tolerance group interaction was not significant, it was dropped from the model, and the analysis was rerun. If neither term was significant, the overall effect was reported. Pearson correlations and partial correlations were used to assess relationships between key variables. Statistical significance was set at a two-tailed P < 0.05. Data were analyzed using SPSS (SPSS, Chicago, IL) and SAS (Cary, NC); results are expressed as means ± SE. Inferences were checked to make sure they were not the result of extreme values by identifying and deleting extreme values and rerunning the analyses or when cell sizes were small (<10) by nonparametric methods (Mann-Whitney U-test).
RESULTS
Baseline comparisons.
There were no significant differences between the AEX + CR and CR groups at baseline with respect to age, BMI, race, or NGT/IGT status. V̇o2max was higher at baseline in the AEX + CR than in the CR group (P < 0.05). There were no significant differences in measurements of glucose metabolism between AEX + CR and CR at baseline.
At baseline the NGT group was slightly younger (59 ± 1 vs. 64 ± 1 yr, P < 0.01) and had lower BMI than the IGT group (31.6 ± 0.6 vs. 33.7 ± 0.9 kg/m2, P < 0.05). As expected, fasting glucose (5.39 ± 0.09 vs. 5.18 ± 0.05 mmol/l, P < 0.05), G120 (8.82 ± 0.16 vs. 5.77 ± 0.13 mmol/l, P < 0.001), fasting insulin (104 ± 9 vs. 73 ± 3 pmol/l, P < 0.001), insulin120 (779 ± 105 vs. 393 ± 34 pmol/l, P < 0.001), and glucose AUC (1,039 ± 23 vs. 822 ± 15 mmol·l−1·120 min−1, P < 0.001) were significantly higher in IGT than in NGT. Insulin AUC (67,919 ± 7,642 vs. 53,475 ± 2,763 pmol·l−1·120 min−1) tended to be higher in IGT than in NGT (P < 0.06). M was lower in IGT than in NGT (55.2 ± 3.3 vs. 67.9 ± 2.2 μmol·kg fat-free mass−1·min−1, P < 0.01).
At baseline, in response to insulin during the clamp, NGT women (n = 38) had a threefold increase in GS-independent activity (basal vs. insulin stimulated: 0.94 ± 0.16 vs. 3.02 ± 0.41 nmol·min−1·mg protein−1, P < 0.0001) and GS fractional activity (9.8 ± 1.0 vs. 28.9 ± 1.9%, P < 0.0001). IGT women (n = 20) had a 2.5-fold increase in GS-independent activity (0.75 ± 0.16 vs. 1.80 ± 0.26 nmol·min−1·mg protein−1, P < 0.001) and GS fractional activity (7.6 ± 1.2 vs. 18.4 ± 1.9%, P < 0.0001). The increases were significantly greater in NGT than in IGT (GS independent activity, P = 0.05; GS fractional activity, P < 0.001). Insulin-stimulated GS-independent activity (P = 0.01) and GS fractional activity (P < 0.001) were significantly lower in women with IGT compared with NGT. GS total activity increased significantly during the clamp in NGT (basal vs. insulin stimulated: 9.0 ± 0.9 vs. 10.4 ± 1.0 nmol·min−1·mg protein−1, P < 0.05) but not in IGT (9.8 ± 1.2 vs. 10.5 ± 1.3 nmol·min−1·mg protein−1).
Effects of the interventions on body composition, V̇o2max, and glucose metabolism.
There was no intervention (CR vs. AEX + CR) by glucose tolerance group (normal vs. IGT) interaction in any of the models described below (Table 1). Therefore, the interaction term was dropped from the model.
Table 1.
Physical and metabolic characteristics of the women by intervention
| CR |
AEX + CR |
P Value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NGT (n = 29) | IGT (n = 17) | NGT (n = 33) | IGT (n = 16) | ||||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Intervention | Group | Overall | |
| Age, yr | 60 ± 1 | 65 ± 2 | 59 ± 1 | 62 ± 2 | |||||||
| AA/C | 7/22 | 7/10 | 11/22 | 6/10 | |||||||
| Weight, kg (n = 95) | 88.3 ± 2.8 | 81.9 ± 2.9 | 84.4 ± 3.7 | 77.0 ± 3.3 | 81.4 ± 1.8 | 74.5 ± 1.8 | 91.4 ± 4.1 | 84.6 ± 3.6 | NS | NS | <0.001 |
| BMI, kg/m2 (n = 95) | 32.8 ± 0.9 | 30.4 ± 1.0 | 32.7 ± 1.2 | 29.8 ± 1.1 | 30.6 ± 0.6 | 28.0 ± 0.6 | 34.8 ± 1.4 | 32.3 ± 1.3 | NS | NS | <0.001 |
| Waist circumference, cm (n = 73) | 95.4 ± 1.9 | 89.5 ± 1.7 | 97.6 ± 3.2 | 93.0 ± 3.4 | 92.3 ± 1.6 | 86.8 ± 1.7 | 101.9 ± 5.1 | 99.3 ± 4.9 | NS | NS | <0.001 |
| %Body fat (n = 94) | 47.8 ± 0.8 | 45.5 ± 1.0 | 47.4 ± 1.0 | 44.0 ± 1.0 | 46.0 ± 0.9 | 41.9 ± 1.1 | 48.5 ± 1.3 | 45.6 ± 1.3 | NS | NS | <0.001 |
| Fat mass, kg (n = 94) | 43.0 ± 1.9 | 38.3 ± 2.0 | 40.9 ± 2.5 | 34.5 ± 2.2 | 38.0 ± 1.4 | 32.0 ± 1.5 | 45.1 ± 3.0 | 40.0 ± 2.7 | NS | NS | <0.001 |
| Fat-free mass, kg (n = 94) | 45.9 ± 1.1 | 44.5 ± 1.2 | 44.3 ± 1.4 | 42.4 ± 1.3 | 44.2 ± 0.8 | 43.2 ± 0.8 | 47.9 ± 2.2 | 46.9 ± 1.6 | NS | NS | <0.001 |
| V̇o2max, l/min (n = 82) | 1.54 ± 0.07 | 1.53 ± 0.07 | 1.38 ± 0.09 | 1.37 ± 0.08 | 1.80 ± 0.07 | 1.98 ± 0.08 | 1.72 ± 0.14 | 1.94 ± 0.17 | <0.001 | NS | |
| Visceral fat area, cm2 (n = 85) | 146.9 ± 12.6 | 127.1 ± 11.1 | 148.7 ± 11.6 | 126.5 ± 9.7 | 125.2 ± 9.7 | 102.1 ± 7.3 | 181.3 ± 27.6 | 162.1 ± 22.3 | NS | NS | <0.001 |
| Subcutaneous abdominal fat area, cm2 (n = 67) | 488.9 ± 26.1 | 435.2 ± 26.8 | 362.1 ± 37.1 | 315.5 ± 34.1 | 420.4 ± 20.9 | 360.1 ± 22.1 | 483.2 ± 43.1 | 425.4 ± 49.2 | NS | NS | <0.001 |
| Midthigh muscle area, cm2 (n = 86) | 70.0 ± 2.6 | 68.9 ± 2.3 | 73.6 ± 3.9 | 69.6 ± 4.3 | 71.7 ± 2.8 | 76.3 ± 3.0 | 78.0 ± 4.9 | 76.5 ± 4.7 | <0.01 | <0.05 | |
| Midthigh subcutaneous fat area, cm2 (n = 86) | 169.2 ± 11.2 | 148.3 ± 10.8 | 151.3 ± 11.4 | 128.2 ± 10.2 | 148.0 ± 7.4 | 127.4 ± 5.9 | 170.8 ± 17.2 | 147.1 ± 13.5 | NS | NS | <0.001 |
| Midthigh low-density lean tissue area, cm2 (n = 86) | 19.1 ± 1.3 | 17.9 ± 1.3 | 21.2 ± 2.1 | 20.8 ± 2.5 | 19.1 ± 0.9 | 17.8 ± 0.9 | 24.1 ± 3.1 | 22.9 ± 3.0 | NS | NS | <0.01 |
| Fasting plasma glucose, mmol/l (n = 94) | 5.30 ± 0.08 | 4.95 ± 0.07 | 5.36 ± 0.11 | 5.14 ± 0.12 | 5.09 ± 0.07 | 4.93 ± 0.07 | 5.42 ± 0.14 | 5.15 ± 0.12 | NS | NS | <0.001 |
| Fasting plasma insulin, pmol/l (n = 93) | 78 ± 6 | 65 ± 4 | 100 ± 9 | 69 ± 6 | 67 ± 3 | 53 ± 3 | 109 ± 15 | 85 ± 9 | NS | NS | <0.001 |
| Glucose120, mmol/l (n = 94) | 5.67 ± 0.20 | 6.09 ± 0.29 | 8.93 ± 0.23 | 7.69 ± 0.37 | 5.83 ± 0.18 | 6.33 ± 0.21 | 8.69 ± 0.23 | 7.43 ± 0.34 | NS | NS | <0.001 |
| Insulin120, pmol/l (n = 92) | 417 ± 61 | 396 ± 43 | 830 ± 173 | 504 ± 65 | 361 ± 36 | 298 ± 27 | 716 ± 128 | 398 ± 53 | <0.05 | NS | |
| Glucose AUC, mmol•l−1•120 min−1 (n = 94) | 841 ± 20 | 827 ± 24 | 1036 ± 28 | 979 ± 34 | 806 ± 22 | 794 ± 17 | 1015 ± 27 | 925 ± 30 | NS | <0.05 | |
| Insulin AUC, pmol•l−1•120 min−1 (n = 88) | 56,618 ± 5,041 | 49,342 ± 3,795 | 71,208 ± 12,797 | 55,018 ± 7,976 | 46,682 ± 2,833 | 36,942 ± 2,165 | 58,037 ± 7,895 | 46,695 ± 6,079 | NS | NS | <0.001 |
| Glucose utilization, μmol•kg FFM−1•min−1 (n = 70) | 66.1 ± 3.6 | 69.5 ± 3.5 | 54.1 ± 4.0 | 58.7 ± 4.3 | 69.1 ± 2.9 | 77.4 ± 2.9 | 56.0 ± 5.1 | 62.2 ± 4.0 | NS | NS | <0.01 |
| Oxidative glucose, μmol•kg FFM−1•min−1 (n = 66) | 19.4 ± 1.1 | 18.0 ± 2.3 | 17.9 ± 2.7 | 19.2 ± 2.3 | 19.2 ± 2.0 | 23.0 ± 2.3 | 17.3 ± 2.6 | 15.8 ± 2.1 | NS | NS | NS |
| Nonoxidative glucose, μmol•kg FFM−1•min−1 (n = 66) | 46.7 ± 3.3 | 51.3 ± 3.8 | 33.7 ± 3.3 | 38.7 ± 4.5 | 50.0 ± 3.3 | 54.8 ± 3.2 | 37.6 ± 3.2 | 45.4 ± 2.4 | NS | NS | <0.01 |
Values are means ± SE.
CR, calorie restriction; AEX, aerobic exercise; NGT, normal glucose tolerance; IGT, impaired glucose tolerance; pre, preintervention; post, postintervention; AA, African-American; C, Caucasian; NS, nonsignificant; BMI, body mass index; AUC, area under the curve; FFM, fat-free mass.
The effect of the interventions in the 2 groups was compared using the following model: change = intervention group + glucose tolerance group + intervention × glucose tolerance group. There were no significant interaction effects.
There was an overall significant decrease in body weight (approximately −8%), percent body fat (−7%), total fat mass (−14%), visceral fat area (−13%), subcutaneous abdominal fat area (−12%), subcutaneous fat of the midthigh (−13%), and midthigh, low-density lean tissue (−4%). Intervention (independent of glucose tolerance group) was a significant predictor of change in V̇o2max and change in insulin120. V̇o2max increased (∼12%, P < 0.001) above baseline after AEX + CR and showed no significant change after CR. The changes were significantly different (P < 0.001). Insulin120 decreased after AEX + CR and CR (both P < 0.001); the changes were significantly different (P < 0.05).
Glucose tolerance group (independent of intervention) was a significant predictor of glucose AUC. Glucose area decreased in IGT (P < 0.01) and not after NGT; the changes were significantly different (P < 0.05).
Both intervention and glucose tolerance group were independent predictors of total thigh muscle area. Thigh muscle area decreased 3% after CR (P < 0.05) and increased 4% after AEX + CR (P < 0.06); the changes were significantly different (P < 0.005). Similarly, muscle area decreased 4% in subjects with IGT and increased 3% in subjects with NGT, with the difference in the changes in the two groups (P < 0.05).
Overall, fasting glucose (−4%), fasting insulin (−17%), and insulin AUC (−19%) decreased (all P < 0.001). Overall, M and nonoxidative glucose disposal significantly increased 14 and 24%, respectively (P < 0.01). Carbohydrate oxidation did not change.
Effects of the interventions on GS activity.
In the IGT AEX + CR group, insulin-stimulated GS-independent activity was significantly higher (P < 0.005), and insulin-stimulated GS fractional activity tended to be higher (P = 0.06) following intervention compared with baseline (Fig. 1).
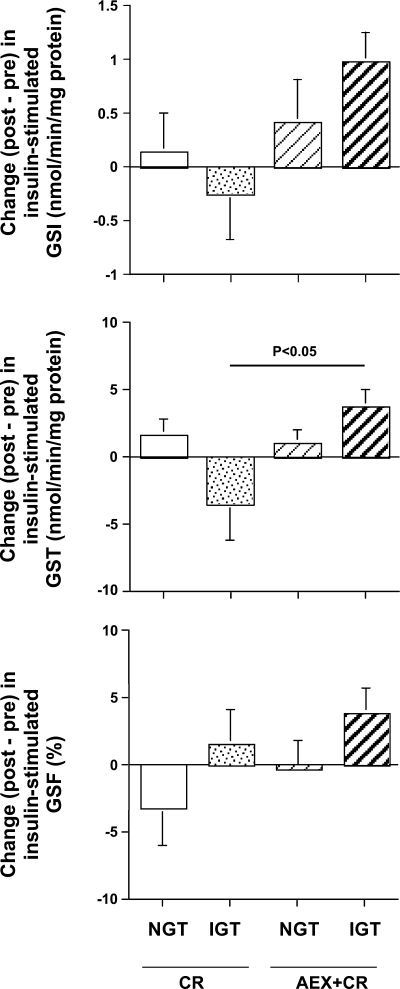
The ANOVA (n = 50) examining the change in insulin-stimulated GS total activity (postintervention − preintervention) revealed a significant intervention × glucose tolerance group interaction (P < 0.05). Post hoc analysis (Tukey-Kramer) demonstrated that, among women with IGT, insulin-stimulated GS total activity increased more after AEX + CR than after CR alone (P < 0.05; Fig. 2).
Fig. 2.
Effect of intervention (CR or AEX + CR) on insulin-stimulated GSI (top), GST (middle), and GSF (bottom) activity in women with NGT and IGT. Comparisons were made with ANOVA adjusted for baseline value, intervention, glucose tolerance status and, when appropriate, an intervention × glucose tolerance status, followed by Tukey-Kramer post hoc tests. Sample size as in Fig. 1.
The ANOVA (n = 50) examining the effect of insulin to increase GS total activity {[insulin-stimulated GS postintervention − basal GS postintervention] − [insulin-stimulated GS preintervention − basal GS preintervention] } demonstrated a significant interaction (P < 0.05). Post hoc analyses (Tukey-Kramer) revealed a tendency toward a larger increase in IGT women following AEX + CR than CR alone (P = 0.08).
Predictors of M before and after CR and AEX + CR.
Before (baseline) and after (post) the interventions, the change in GS fractional activity (insulin-stimulated − basal) was associated with M in the entire group (n = 57, partial r = 0.34, P < 0.01; and n = 49, partial r = 0.35, P < 0.05, respectively). The change in insulin-stimulated GS fractional activity (post − baseline) was associated with the change in M in the entire group (n = 48, partial r = 0.44, P < 0.005).
In the total group, the change in V̇o2max was a significant predictor of the change in M (n = 59, r = 0.27, P < 0.05); after controlling for intervention and glucose tolerance status, the value was r = 0.24, P < 0.07. The reduction in midthigh intramuscular fat predicted the change in M in the total group (n = 62, r = −0.26, P < 0.05, partialed for intervention and glucose tolerance status) and in the AEX + CR group (n = 36, r = −0.33, P = 0.05) partialed for glucose tolerance status.
DISCUSSION
This is the first investigation to study the effects of CR and AEX + CR on insulin sensitivity and skeletal muscle GS activity in a large sample of postmenopausal women with varying degrees of glucose tolerance. CR alone or combined with AEX results in similar and significant reductions in weight, total and abdominal fat, and thigh fat, which are accompanied by improvements in glucose homeostasis. We now demonstrate, for the first time, that AEX + CR increases insulin activation of GS in postmenopausal women with IGT.
In the current study, there was a remarkable 2.5- to threefold increase in GS fractional activity during steady-state plasma insulin levels of ∼1,100 pmol/l in women with IGT and NGT, respectively. This increase is surprisingly similar to that observed in obese premenopausal women at fivefold higher steady-state insulin levels (5,800 pmol/l) (9). At lower steady-state plasma insulin of 650 pmol/l, obese premenopausal women had a 1.6-fold increase in insulin activation of GS fractional activity relative to basal activity (9). In a study of older obese men by our group, the increase in GS fractional activity was twofold compared with basal activity at insulin concentrations of ∼1,200 pmol/l (16). In a study of middle-aged men and women, obese glucose-tolerant individuals had a significant 1.7-fold increase in GS fractional activity during the clamp (550 pmol/l insulin), whereas the individuals with IGT did not (1.4-fold; not significant) (12). We show that the effect of in vivo insulin to increase GS fractional activity was significantly lower in women with IGT than in women with NGT. In a recent study, insulin-stimulated incremental changes in skeletal muscle IRS, Akt, and GSK-3β were lower in subjects with IGT compared with NGT (17). These defects in insulin signaling and in insulin activation of GS may all contribute to lower M in IGT compared with NGT. Moreover, the increase in GS fractional activity in response to insulin in our obese postmenopausal women was greater (P < 0.05) than the increase in GS fractional activity in older obese men studied by our group (16), suggesting that higher insulin activation of GS fractional activity in the older obese women compared with the older obese men may explain the higher whole body insulin sensitivity in older women than in men (10).
GS total activity increased significantly during the clamp in women with NGT but not in women with IGT. Young healthy individuals have an increase in GS total activity during a euglycemic hyperinsulinemic clamp, but at steady-state plasma insulin concentrations of 3,800 pmol/l (20). This further supports the notion that postmenopausal women are very sensitive to the effects of in vivo insulin to increase GS activity.
There are few studies examining the effects of weight loss and/or aerobic exercise training on GS activity before and during a hyperinsulinemic euglycemic clamp in overweight or obese individuals and none in postmenopausal women. Insulin-stimulated GS activity did not change following a very low-calorie diet (11% weight reduction) in obese nondiabetic individuals (basal biopsies were not obtained) (4). A weight loss intervention of 4% in overweight individuals with and without diabetes also did not affect GS activity (basal or insulin stimulated) (14). In the current study, insulin activation of GS was not significantly changed by 8% CR in either NGT or IGT postmenopausal women. The results of all of these studies support the hypothesis that weight loss alone does not improve insulin activation of skeletal muscle GS.
In older obese men, a 6-mo AEX intervention significantly increased insulin activation of GS fractional activity compared with resistance training, with both interventions improving M (11). In younger obese men and women with and without type 2 diabetes, an 8-wk exercise-training regimen without weight loss increased M, basal GS-independent activity, and basal and insulin-stimulated GS total activity but did not affect basal or insulin-stimulated GS fractional activity (5). Although insulin-stimulated GS-independent activity increased in the nondiabetic subjects following AEX, it did not increase significantly in the subjects with type 2 diabetes following AEX (5). In the current study, M increased following the interventions. However, only the women with IGT showed a significant effect of in vivo insulin to increase GS-independent activity and a tendency to increase GS fractional activity following AEX + CR, whereas there was no effect of insulin activation on GS in women with NGT following AEX + CR. In addition, among women with IGT, insulin-stimulated GS total activity increased more after AEX + CR than after CR alone. One explanation for the lack of effect of AEX + CR on insulin activation of GS in women with NGT could be that these postmenopausal women were extremely sensitive to the effects of in vivo insulin to increase GS activity at baseline, as discussed above.
AEX + CR increases M in NGT obese postmenopausal women (26). However, in the current study, GS activity was not changed in women with NGT, suggesting that the improvement in M is independent of any change in skeletal muscle GS activation by insulin in these NGT women. These results are similar to the findings of Christ-Roberts et al. (5), where M increased following AEX in obese NGT men and women, yet insulin activation of GS did not improve. Therefore, mechanisms other than an increase in insulin activation of GS are responsible for the improvement in M following AEX in NGT. These mechanisms could include changes in GLUT4 protein expression. Basal GLUT4 protein expression increased, whereas insulin activation of insulin receptor substrate-1-associated phosphatidylinositol 3-kinase was not affected following AEX in obese NGT men and women (5). In our study of IGT obese postmenopausal women, insulin-stimulated GS-independent activity improved, suggesting that the increase in insulin-stimulated GS activity contributes to the improvement in insulin sensitivity in IGT after AEX + CR. AEX + CR also improved glucose metabolism, as evidenced by reductions in glucose AUC in the IGT obese postmenopausal women. These improvements were greater than that observed after AEX + CR in NGT, illustrating the importance of a lifestyle modification of exercise plus caloric restriction in individuals at greatest risk to develop type 2 diabetes.
The benefits of CR alone or combined with AEX in overweight and obese postmenopausal NGT and IGT women include robust changes in body composition and in whole body insulin sensitivity. The added benefits of AEX include improvements in physical fitness. In IGT, there is enhancement in insulin-stimulated GS activity following AEX + CR, and the effect of insulin to increase GS total activity is greater after AEX + CR compared with CR. Therefore, adding aerobic exercise training to caloric restriction will result in the greatest metabolic benefits for overweight and obese postmenopausal women with IGT. In accord with the results of the Diabetes Prevention Program, we demonstrate improvements in insulin sensitivity in older women with IGT and an increase in insulin activation of skeletal muscle GS.
GRANTS
This study was supported by funds from the Baltimore Veterans Affairs Medical Research Service, a Veterans Affairs Research Career Scientist Award, the Department of Veterans Affairs and Veterans Affairs Medical Center GRECC, National Institute on Aging Grants RO1-AG-19310 and R01-AG-20116, Claude D. Pepper Older Americans Independence Center Grant P30-AG-028747, the National Institute of Diabetes and Digestive and Kidney Diseases Mid-Atlantic Nutrition Obesity Research Center (NIH P30-DK-072488), and the General Clinical Research Center of the University of Maryland, Baltimore, Maryland (MO1-RR-016500).
DISCLOSURES
The authors have no conflict of interest.
AUTHOR CONTRIBUTIONS
A.S.R. and H.K.O. did the conception and design of the research; A.S.R. and H.K.O. performed the experiments; A.S.R., H.K.O., and J.D.S. analyzed the data; A.S.R. and H.K.O. interpreted the results of the experiments; A.S.R. and H.K.O. prepared the figures; A.S.R. and H.K.O. drafted the manuscript; A.S.R., H.K.O., and J.D.S. edited and revised the manuscript; A.S.R., H.K.O., and J.D.S. approved the final version of the manuscript.
ACKNOWLEDGMENTS
Our appreciation is extended to the women who participated in this study. We are indebted to Dr. Andrew P. Goldberg for advice and support. We are grateful to the medical team (Drs. Jacob Blumenthal and Ron Prigeon, Linda Hatler, Peter Normandt, and Lynn Stars-Zorn), the Geriatric Research, Education, and Clinical Center (GRECC) nurses, the laboratory technicians (Jonelle George, Melissa Gray, and Carol St. Clair), exercise physiologists (especially Lynda Robey and Gretchen Zietowski), and dieticians (especially Kelly Ort) of the Division of Gerontology and GRECC, and Dr. Dariush Elahi for their assistance with this project.
REFERENCES
- 1. American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 32, Suppl 1: S62–S67, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bogardus C, Lillioja S, Stone K, Mott D. Correlation between muscle glycogen synthase activity and in vivo insulin action in man. J Clin Invest 73: 1185–1190, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bogardus C, Tataranni PA. Reduced early insulin secretion in the etiology of type 2 diabetes mellitus in Pima Indians. Diabetes 51, Suppl 1: S262–S264, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Bryson JM, King SE, Burns CM, Baur LA, Swaraj S, Caterson ID. Changes in glucose and lipid metabolism following weight loss produced by a very low calorie diet in obese subjects. Int J Obes Relat Metab Disord 20: 338–345, 1996 [PubMed] [Google Scholar]
- 5. Christ-Roberts CY, Pratipanawatr T, Pratipanawatr W, Berria R, Belfort R, Kashyap S, Mandarino LJ. Exercise training increases glycogen synthase activity and GLUT4 expression but not insulin signaling in overweight nondiabetic and type 2 diabetic subjects. Metabolism 53: 1233–1242, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, Saydah SH, Williams DE, Geiss LS, Gregg EW. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002. Diabetes Care 29: 1263–1268, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Damsbo P, Hermann LS, Vaag A, Hother-Nielsen O, Beck-Nielsen H. Irreversibility of the defect in glycogen synthase activity in skeletal muscle from obese patients with NIDDM treated with diet and metformin. Diabetes Care 21: 1489–1494, 1998 [DOI] [PubMed] [Google Scholar]
- 8. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E214–E223, 1979 [DOI] [PubMed] [Google Scholar]
- 9. Evans DJ, Murray R, Kissebah AH. Relationship between skeletal muscle insulin resistance, insulin-mediated glucose disposal, and insulin binding. Effects of obesity and body fat topography. J Clin Invest 74: 1515–1525, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferrara CM, Goldberg AP, Nicklas BJ, Sorkin JD, Ryan AS. Gender disparities in insulin action and body fat distribution in obese, older men and women. Appl Physiol Nutr Metab 33: 784–790, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferrara CM, Goldberg AP, Ortmeyer HK, Ryan AS. Effects of aerobic and resistive exercise training on glucose disposal and skeletal muscle metabolism in older men. J Gerontol A Biol Sci Med Sci 61: 480–487, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Golay A, Munger R, Assimacopoulos-Jeannet F, Bobbioni-Harsch E, Habicht F, Felber JP. Progressive defect of insulin action on glycogen synthase in obesity and diabetes. Metabolism 51: 549–553, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes 48: 839–847, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Johnson AB, Argyraki M, Thow JC, Broughton D, Jones IR, Taylor R. Effects of intensive dietary treatment on insulin-stimulated skeletal muscle glycogen synthase activation and insulin secretion in newly presenting type 2 diabetic patients. Diabet Med 7: 420–428, 1990 [DOI] [PubMed] [Google Scholar]
- 15. Johnson AB, Argyraki M, Thow JC, Jones IR, Broughton D, Miller M, Taylor R. Impaired activation of skeletal muscle glycogen synthase in non-insulin-dependent diabetes mellitus is unrelated to the degree of obesity. Metabolism 40: 252–260, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Joseph LJ, Ortmeyer HK, McLenithan JC, Prior SJ, Blumenthal JB, Katzel LI, Goldberg AP, Ryan AS. Exercise and weight loss improve glucose utilization and glycogen synthase fractional activity in obese older men (Abstract). Diabetes 57: A302, 2008. [Google Scholar]
- 17.Kim do M, Jang HJ, Han SJ, Ha ES, Kim YK, Park JW, Song KE, Jung SH, Ahn SM, Choi SE, Kim HJ, Kim DJ, Lee HC, Lee KW. Classical PKC is not associated with defective insulin signaling in patients with impaired glucose tolerance. Diabetes Res Clin Pract 83: 334–340, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393–403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J. Summary of American Heart Association Diet and Lifestyle Recommendations revision 2006. Arterioscler Thromb Vasc Biol 26: 2186–2191, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Mandarino LJ, Wright KS, Verity LS, Nichols J, Bell JM, Kolterman OG, Beck-Nielsen H. Effects of insulin infusion on human skeletal muscle pyruvate dehydrogenase, phosphofructokinase, and glycogen synthase. Evidence for their role in oxidative and nonoxidative glucose metabolism. J Clin Invest 80: 655–663, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGuire EA, Helderman JH, Tobin JD, Andres R, Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol 41: 565–573, 1976 [DOI] [PubMed] [Google Scholar]
- 22. Mokdad AH, Stroup DF, Giles WH; Behavioral Risk Factor Surveillance Team Public health surveillance for behavioral risk factors in a changing environment. Recommendations from the Behavioral Risk Factor Surveillance Team. MMWR Recomm Rep 52: 1–12, 2003 [PubMed] [Google Scholar]
- 23. Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295: 1549–1555, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Ortmeyer HK, Bodkin NL, Hansen BC. Insulin-mediated glycogen synthase activity in muscle of spontaneously insulin-resistant and diabetic rhesus monkeys. Am J Physiol Regul Integr Comp Physiol 265: R552–R558, 1993 [DOI] [PubMed] [Google Scholar]
- 25. Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med 133: 92–103, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Ryan AS, Nicklas BJ, Berman DM. Aerobic exercise is necessary to improve glucose utilization with moderate weight loss in women. Obesity (Silver Spring) 14: 1064–1072, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Ryan AS, Nicklas BJ, Berman DM. Racial differences in insulin resistance and mid-thigh fat deposition in postmenopausal women. Obes Res 10: 336–344, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Schenk S, Harber MP, Shrivastava CR, Burant CF, Horowitz JF. Improved insulin sensitivity after weight loss and exercise training is mediated by a reduction in plasma fatty acid mobilization, not enhanced oxidative capacity. J Physiol 587: 4949–4961, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]