Abstract
It was reported previously that isolated human islets from individuals with type 2 diabetes mellitus (T2DM) show reduced glucose-stimulated insulin release. To assess the possibility that impaired bioenergetics may contribute to this defect, glucose-stimulated respiration (V̇o2), glucose usage and oxidation, intracellular Ca2+, and insulin secretion (IS) were measured in pancreatic islets isolated from three healthy and three type 2 diabetic organ donors. Isolated mouse and rat islets were studied for comparison. Islets were exposed to a “staircase” glucose stimulus, whereas IR and V̇o2 were measured. V̇o2 of human islets from normals and diabetics increased sigmoidally from equal baselines of 0.25 nmol/100 islets/min as a function of glucose concentration. Maximal V̇o2 of normal islets at 24 mM glucose was 0.40 ± 0.02 nmol·min−1·100 islets−1, and the glucose S0.5 was 4.39 ± 0.10 mM. The glucose stimulation of respiration of islets from diabetics was lower, Vmax of 0.32 ± 0.01 nmol·min−1·100 islets−1, and the S0.5 shifted to 5.43 ± 0.13 mM. Glucose-stimulated IS and the rise of intracellular Ca2+ were also reduced in diabetic islets. A clinically effective glucokinase activator normalized the defective V̇o2, IR, and free calcium responses during glucose stimulation in islets from type 2 diabetics. The body of data shows that there is a clear relationship between the pancreatic islet energy (ATP) production rate and IS. This relationship was similar for normal human, mouse, and rat islets and the data for all species fitted a single sigmoidal curve. The shared threshold rate for IS was ∼13 pmol·min−1·islet−1. Exendin-4, a GLP-1 analog, shifted the ATP production-IS curve to the left and greatly potentiated IS with an ATP production rate threshold of ∼10 pmol·min−1·islet−1. Our data suggest that impaired β-cell bioenergetics resulting in greatly reduced ATP production is critical in the molecular pathogenesis of type 2 diabetes mellitus.
Keywords: glucokinase activators, pancreatic islets, type 2 diabetes, insulin secretion
impaired insulin secretion from pancreatic β-cells and insulin resistance of liver, muscle, and adipose tissue are the hallmarks of type 2 diabetes (T2DM). The disease has a complex genetic basis (21, 47, 56, 57, 66, 69), but its course of development is also critically influenced by lifestyle and is markedly accelerated by overnutrition and limited physical activity (68, 73). However, the causative molecular lesions of the affected tissues, particularly the defective pancreatic β-cells, have not been fully elucidated. Although it is still debated whether impaired insulin secretion as manifest in an abnormal glucose tolerance test is the result of reduced β-cell mass, of decreased function, or of a combination of these factors, strong evidence favors the view that compromised functional capacity of β-cells in T2DM makes a critical contribution in the pathogenesis of the disease (31, 33). Previous studies with islets of Langerhans isolated from the pancreas of patients with T2DM strongly support this assessment (3, 14, 15, 46).
Faulty bioenergetics as a potential cause of defective fuel-stimulated insulin release is a plausible biochemical explanation because its end product ATP, generated by metabolism of the physiological stimuli of glucose, amino acids, and fatty acids, serves as the preeminent obligatory factor in stimulus secretion coupling involving β-cell-specific mechanisms (3–5, 30, 46). The relative rates of ATP production and usage, manifest as the cellular P-potential (ATP/ADPxPi), determine the β-cell's membrane potential. The membrane is depolarized in a graded fashion as the net production rate of ATP increases during fuel stimulation until a threshold potential is reached for the opening of voltage-sensitive L-type Ca2+ channels (5, 23, 52). Consequently, global levels of intracellular free Ca2+, the essential second messenger for insulin secretion, rise sufficiently to trigger the process of hormone secretion. Electrical activity and free Ca2+ levels of β-cells are maintained as a direct function of the rate of fuel metabolism and ATP production, and they sustain corresponding rates of insulin secretion during prolonged fuel stimulation. Other second messengers are produced in the process of glucose stimulation, including cAMP, diacylglycerol, phosphoinositides, and inositol trisphosphate, affecting the levels or potentiating the action of Ca2+ (28, 60, 67). If these bioenergetic processes, metabolic coupling mechanisms, and the associated second-messenger enhancement were compromised in any way, a defect of insulin secretion would ensue, resulting in T2DM. Since β-cell fuel metabolism and stimulus secretion coupling involve numerous steps, it is difficult to identify those that might be critically impaired. The results of intensive genome-wide association studies, although they point in the direction of the β-cell, are at this state of knowledge of little help for conceptualizing a testable hypothesis that might explain the defect. The number of implicated genes is perplexingly large, and the impact of individual diabetes genes is small (21, 47, 56, 57, 66). However, the efficacy and mechanisms of action of approved (sulfonylureas, metformin, and thiazolidinediones) and experimental drugs [glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase IV (DPP IV) inhibitors, and glucokinase (GK) activators (GKAs)] suggest that fuel sensing, metabolic coupling, and their second-messenger augmentation might indeed be involved (2, 9, 36, 62). Therefore, it is important to study selected parameters that can provide a reliable quantitative assessment of β-cell bioenergetics and to relate them to the hormone-releasing function. On the basis of these considerations, we investigated human islets of Langerhans isolated from normal and diabetic pancreases and kept briefly in organ culture conditions to allow recovery from the stressful insult of the harvesting procedure. We measured oxygen consumption, glycolytic flux, and insulin secretion during simulated glucose tolerance tests. One of the newly discovered GKAs, piragliatin (26, 27, 51), was used in an attempt to remedy the defects of glucose metabolism or insulin secretion we observed. The rates of glycolysis and oxygen consumption provide a reliable measurement of the rate of ATP production of islet cells exposed to glucose, assuming that oxidative phosphorylation is well coupled. GKAs instead of sulfonylureas, GLP-1 receptor agonists, or other antidiabetic drugs were chosen for this purpose because the mechanism by which they augment energy production in β-cells is clearly defined. They directly stimulate GK enzymatic activity by allosteric activation and thereby augment glycolysis and glucose oxidation, leading to increased ATP production. This is not true for the other classes of drugs, which seem to be energetically neutral or are actually inhibitors of ATP generation (20, 36). GKAs increase the enzyme's affinity for glucose and also its maximal catalytic rate (26, 27, 51) and thereby stimulate insulin secretion (27, 51). They also enhance glucose induction of GK in isolated cultured pancreatic rat islets (51, 54), but with a considerable time delay so that enzyme induction is probably not a factor in the present studies. In vivo data indicate that these drugs lower blood sugar in normal laboratory animals and humans and in animal models of T2DM and humans with this disease (9, 26, 27, 51, 54).
The present data show that islets from type 2 diabetics have a significantly reduced respiratory and secretory response compared with controls when stimulated with a staircase glucose tolerance test. Both of these functional defects can be repaired largely by activation of the GK glucose sensor with the clinically effective specific allosteric activator piragliatin. This strongly suggests that decreased utilization of glucose with attendant decrease in glucose-stimulated insulin release (GSIR) plays a critical role in development of the disease.
METHODS
Human Islets
Human islets were received from the accredited Human Islet Resource Center at the University of Pennsylvania. The pancreas was procured and the isolation performed according to previously described protocols (15, 16). Assignment to the control or T2DM group was based on the pancreas donor's clinical case report and on Hb A1c results. We examined isolates from six separate donors; three were normal and three were diabetic. Normal donors were 45, 50, and 28 yr old; body mass indexes (BMIs) were 24.1, 28.5, and 24.7 and Hb A1c values 5.6, 5.8, and 5.7%, respectively. The type 2 diabetics were 58, 45, and 30 yr old with BMIs of 29.3, 34.1, and 25.9 and Hb A1c values of 9.3, 11.0, and 7.4%, respectively.
Animals
The research protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania (protocol no. 077603). Male B6D22F1 mice were used throughout. The animals were maintained on a 12:12-h light-dark cycle and were fed a standard rodent chow diet. Islets were always isolated from fed animals.
Islet Isolation and Culture
Rodent islets were isolated using collagenase (C9263; Sigma) digestion in Hanks' buffer, followed by separation of islets from exocrine tissue in a Ficoll (F-9378; Sigma) gradient. Isolated rodent islets were cultured at 37°C for 3–4 days in RPMI 1640 medium (Sigma) containing 10% fetal bovine serum, 10 ml/l penicillin-streptomycin-amphotericin B solution (Gibco-BRL, Grand Island, NY), and 10 mM glucose. Human islets were isolated from pancreatic tissue obtained through the local organ procurement organization. The pancreas was digested following the intraductal injection of collagenase (liberase; Roche, Nutley, NJ) at a concentration of 1.66 mg/ml in Hanks' balanced salt solution. Liberated islets were purified on continuous density gradients (Cellgro/Mediatech, Herndon, VA) using the COBE centrifuge. Islet fractions with >90% purity were cultured in CMRL-1066 medium (Cellgro/Mediatech) supplemented with 5 mM glucose, 10% endotoxin-free fetal calf serum (Cellgro/Mediatech), and antibiotic/antimycotic solution (Gibco-BRL) in a humidified 5% CO2 incubator at 25°C. For pilot studies, human islets were also cultured at higher temperatures and glucose levels as described for rodent islets.
Perifusion of Islets for Simultaneous Measurement of Oxygen Consumption and Insulin Release
As described previously (17), 700–800 islets were loaded into a 250-μl chamber using a P200 pipette and gel-loading tip and were allowed to settle for 1 min before the perfusate flow was started at 80 μl/min. The perifusate was a Krebs buffer (pH 7.4) containing 114 mmol/l NaCl, 5 mmol/l KCl, 24 mmol/l NaHCO3, 1 mmol/l MgCl2 6H2O, 2.2 mmol/l Ca2+, 1 mM Pi, 10 mmol/l HEPES (pH 7.4), 1% of BSA (fraction V, fatty acid free; Sigma-Aldrich). Glucose and other reagents were added to the perfusate reservoir at time points and concentrations that are recorded in the graphic material of the results. In the case of glucose as a stimulus, this approach resulted in a staircase profile of 3, 6, 12, and 24 mM steps.
Oxygen Consumption Measurements by Quenching Phosphorescence
Oxygen partial pressure was measured by the phosphorescence quenching method, using a new oxygen-sensitive phosphorescent, porphyrin-dendrimer Oxyphor G3 (palladium-tetrabenzoporphyrin, encapsulated inside gen 2 polyarylglycine dendrimer) (37). The inflow oxygen tension was measured in the absence of islets in the chamber before and after each experiment. Oxygen consumption by the islets was calculated from the difference in oxygen partial pressure between the influent and the effluent (oxygen extraction) and the rate at which medium flowed through the chamber.
Ramp Studies With “Micro” Perifusion System
To create a ramp-type stimulus profile, cultured islets (130 islets) were placed on a nylon filter in a plastic perifusion chamber (Millipore, Bedford, MA) and then perifused with a flow rate of 2 ml/min in an apparatus consisting of a computer-controlled, fast-performance HPLC system (Waters 625 LC System) that allowed programmable rates of flow and glucose concentration in the perfusate (18, 38, 39). The system also included a water bath (37°C) and a fraction collector (Waters Division of Millipore).
Glucose Usage
A radiometric method that is based on the production of tritiated water from [5-3H]glucose was used (78). A concentration range of [5-3H]glucose (12 μCi) was placed into vials containing buffer and 100 cultured islets after 1-h preincubation with different levels of glucose and a washing step. The radioactivity in all vials was counted on a Beckman 60001C liquid scintillation counter after addition of 10 ml of Cytoscint liquid scintillation fluid (ICN, Costa Mesa, CA). A blank control without islets was carried through the entire procedure, and 50 μl of tritiated water standard (Du Pont-New England Nuclear, Boston, MA) was treated in the same manner to allow the correction for incomplete transfer of tritium to the vial. Calculations were done according to Ashcroft et al. (6).
Glucose Oxidation
A radiometric method that is based on the production of 14CO2 from [U-14C]glucose was used. Cultured islets (100 islets) were placed in vials containing 0.5 ml of Krebs-Ringer buffer and preincubated for 1 h with different concentrations of glucose before 2 μCi [U-14C]glucose was injected into the vials (75). All vials were counted in a Beckman 60001C liquid scintillation counter. Background was determined by treating samples in the same way without adding islets, and a factor equaling the amount of radioactivity in the cup containing the hyamine hydroxide and the filter paper per amount of radioactivity in the buffer was measured. This factor was used to remove the background component from the amount of counts in the cup, yielding the amount of radioactive CO2 produced in 1 h. The rate of production of unlabeled CO2 from glucose was calculated by dividing the amount of radioactive CO2 produced by the specific activity of radiolabeled glucose, which yielded the production rate of unlabeled CO2 from glucose.
Computation of Total (i.e., Glycolytic Plus Oxidative Phosphorylation) ATP Generation Rates
From the rates of glycolysis (or glucose usage) and of respiration, it is possible to calculate the rate of ADP conversion to ATP based on the assumption that glycolysis generates two ATPs for glucose and that oxidative phosphorylation (Ox/Phos) operates with a P/O ratio of 2.5. It is also assumed that glycogen stores are negligible (53). Glycogenolysis could result in erroneous glycolysis data when the present radiometric assays are employed.
Ca2+ Measurement
Islets were loaded with fura-2 AM during a 40-min pretreatment at 37°C in 2 ml of Krebs-Ringer bicarbonate buffer supplemented with 5 μmol/l fura-2 acetoxymethylester (Molecular Probes, Eugene, OR). The loaded islets were transferred to the perifusion chamber and placed on the homeothermic platform of an inverted Zeiss microscope. Islets were perfused with Krebs-Ringer bicarbonate buffer at 37°C at a flow rate of 2 ml/min, whereas various treatments were applied to the islets (18, 19, 39). The intracellular Ca2+ was determined by the ratio of the excitation of fura at 334 and 380 nm. Emission was measured at 520 nm by a Zeiss charge-coupled device camera and calibrated using the Zeiss Ratio Vision Software.
Insulin Measurements
Insulin in the effluent was measured by double-antibody polyethylene glycol separation equilibrium radioimmunoassay (HI-14k and RI-13K for human and mouse insulin, respectively; Millipore).
Effect of Piragliatin on Enzyme Kinetics with Recombinant Human GK
The effect of the GKA piragliatin on the kinetics of pure recombinant human islet GK was studied as described previously (25, 71).
Statistical Analysis
Insulin data are presented the mean ± SE of experimental results from three to four islet isolates. Oxygen consumption and [Ca2+]i curves are presented as the mean of the three to four such experiments without SE due to the high frequency of sampling (10-s interval). In appropriate cases, significant differences between groups were determined by ANOVA with post hoc analysis using Dunnett's multiple comparison test. P ≤ 0.05 was considered significant.
RESULTS
Comparison of Piragliatin Effects on Glucose-Stimulated Insulin Release, Glycolysis, Glucose Oxidation, Respiration and Ca2+i of Normal Mouse, Rat, and Human Islets
Studies with control mouse and rat islets.
To assess the fundamental relationship between oxygen consumption, glycolysis, glucose oxidation, and insulin secretion in extensively studied animal model systems before embarking on studies with human islets, isolated mouse or rat islets were exposed to a “staircase” glucose stimulus from zero to 3, to 6, to 12, and then to 24 mM for 40 min each. Afterward, FCCP was added for 30 min to uncouple respiration and oxidative phosphorylation as test of mitochondrial normalcy. Finally, NaN3 was used at the end of each experiment to block the respiration. Insulin was measured in outflow samples and correlated with the O2 tension, also measured in the effluent.
The insulin secretory profile for mouse islets developed as expected (Fig. 1A); 3 mM glucose failed to stimulate insulin secretion but enhanced respiration by 37% (Fig. 1B), and 6 and 12 mM glucose stimulated insulin release (2- and 7-fold, respectively, compared with baseline at 0 mM glucose) and oxygen consumption rate (OCR; by 153 and 247%, respectively, compared with baseline at 0 mM glucose). Glucose at 24 mM increased insulin secretion but with no further enhancement of OCR, suggesting an increased relative contribution of glycolytic ATP to hormone release. Thus, both insulin release and oxygen consumption increased in a sigmoidal fashion as a function of stepwise increments of the glucose concentration in the perfusate (not graphically shown). However, the glucose dependency curve of oxygen consumption was left shifted compared with insulin release, suggesting that ATP and/or other mediators must first change to a critical level before initiating hormone release.
Fig. 1.
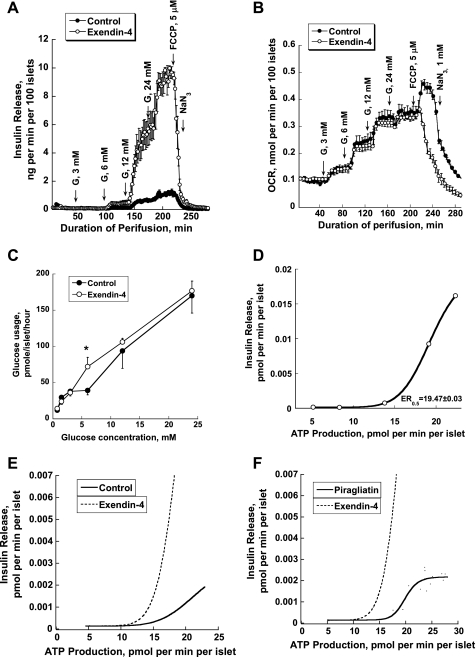
Effect of a glucokinase activator (GKA) on insulin release, respiration, glucose usage and oxidation, and intracellular calcium of isolated cultured mouse islets. A: insulin release patterns with glucose stimulation using stepwise increases of glucose from 0 to 3, 6, 12, and 24 mM in both the absence and presence of different concentrations of piragliatin (1, 2, 3, 10, and 30 μM). Insulin data are presented as the mean of experiments from 3 islet isolates. B: effects of piragliatin on islet respiration during stepwise increases of glucose concentration, followed by treatment with 5 μM of the uncoupler of oxidative phosphorylation (Ox/Phos) FCCP and 1 mM Na-azide. O2 consumption was determined with a method based on phosphorescence quenching of metalloporphyrins by oxygen (17). Oxygen consumption curves are presented as the means of the experiments from 3 isolates without SE. The high-frequency sampling at 10-s intervals resulted in smooth profiles that had a high interexperimental reproducibility, as also illustrated by the close overlap of the baselines and the decay curves after NaN3. C and D: glucose usage and oxidation in mouse islets as function of the glucose staircase. Each curve represents the mean ± SE of 3 experiments. E: relationship between energy generation and hormone secretion in form of an ATP production/insulin secretion diagram. ATP production was calculated based on oxygen consumption and glucose usage data, as described in methods. F: corresponding changes of intracellular Ca2+ of mouse islets due to stepwise increases of glucose from 0 to 1, 3, and 9 mM. The Fura-2 method was employed. Typical experiments are presented (n of the series = 3). *P ≤ 0.05. OCR, oxygen consumption rate.
Piragliatin (RO4389620, US patent 2007115968; Hoffmann-La Roche), an allosteric activator of GK that has been effective in clinical trials (9, 27), was used to stimulate glycolysis and oxidative glucose metabolism and to test whether an increase in substrate flow affects the relationship between glucose levels, metabolism, and insulin secretion. Piragliatin (1, 3, 10, and 30 μM) greatly left-shifted the glucose dependency curves of GSIR (Fig. 1A) and O2 use (Fig. 1B) in a clearly dose-dependent manner. At 1 μM, the GKA increased oxygen consumption of glucose at 3 and 6 mM. At 10 and 30 μM, maximal three- to fourfold stimulation of respiration was reached at the lowest glucose level of 3 mM, whereas at lower drug concentrations (1 and 3 μM) clear staircase profiles of glucose-induced respiration were observed. The maximal velocity (Vmax) of OCR was highest at 10 μM piragliatin, but further increasing the drug concentration to 30 μM, which is close to saturation of GK, actually lowered OCR by mechanisms that need to be explored. The maximally fourfold stimulation of respiration by glucose is very striking, suggesting that pancreatic mouse islets are substrate deficient after a 40-min equilibration period in the absence of exogenous fuel supply. This observation has its corollary in the “rundown” phenomenon that develops in isolated mouse and rat islets during substrate-free perifusion (22, 41). It is usually underappreciated that mouse pancreatic islets are energy deficient even at 3–5 mM glucose if other fuels are lacking (40).
To allow the quantitation of ATP production in pancreatic islets, the rates of glucose usage and oxidation were measured in addition to oxygen consumption using a batch incubation design, but otherwise conditions were comparable with the perifusion experiments. Both glucose usage and oxidation of mouse islets increased with the stepwise rise of glucose concentrations (Fig. 1, C and D). It is remarkable that glucose oxidation was only 20% of glucose usage at practically all glucose concentrations. Piragliatin at 1, 2, 3, and 20 μM shifted the glucose dependency curves of total usage and oxidation dose dependently to the left. The highest Vmax was reached at 3 μM. With 20 μM of the GKA, glucose usage and oxidation were maximally activated at low glucose levels, whereas at high glucose the activation of metabolism was less pronounced than with 3 μM of the drug, resulting in crossovers of the glucose concentration dependency curves of metabolism. Currently, there is no plausible explanation for the decreased GKA effectiveness at high glucose. The metabolic data were used to calculate how much ATP is produced in pancreatic islets stimulated with glucose. This calculation was based on the assumption that 2 ATPs/glucose are generated in glycolysis and that oxidative phosphorylation operates with an efficiency of a 2.5 P/O ratio. ATP production increased sigmoidally as a function glucose concentration (not shown). The relationship between ATP production and insulin release of mouse islets was fitted to a steep sigmoidal curve with a Hill coefficient of 11 (Fig. 1E), showing a threshold at ∼15 pmol·min−1·islet−1 and a half-maximal effective rate (ER0.5) of 19.88 ± 0.45 pmol·min−1·islet−1 of ATP production (r = 0.97).
Glucose- and piragliatin-induced changes in insulin secretion and oxygen consumption in mouse islets were correlated with the changes in free cytosolic calcium concentrations ([Ca2+]i) (Fig. 1F). One and 3 mM glucose did not change [Ca2+]i, and 9 mM glucose caused a two- to threefold increase in [Ca2+]i. As expected, piragliatin shifted the glucose dependency curves toward lower glucose concentrations.
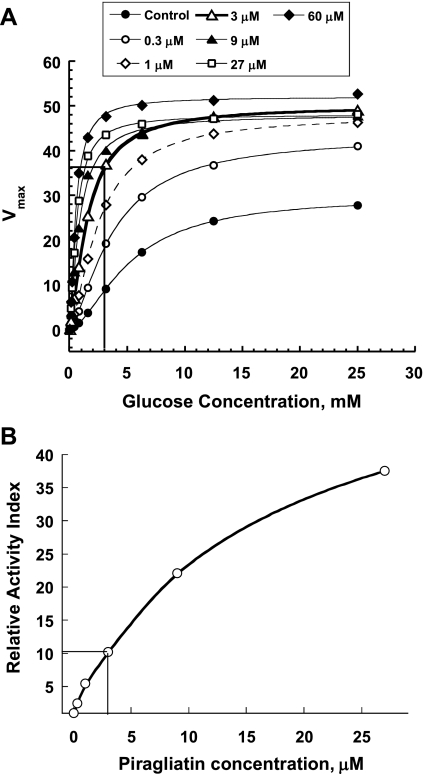
Rat islets were studied with protocols comparable with those employed for mouse islets. In this study, we tested only one concentration of piragliatin. The GKA concentration of 3 μM was chosen based on in vitro dose response curves with recombinant GK (Fig. 2) and on the basis of concentration dependency studies using mouse islets, as described above (Fig. 1). Piragliatin at 3 μM resulted in a three- to fourfold stimulation of GK at 3 mM glucose according to the drug titration of recombinant enzyme in solution (Fig. 2A), a concentration also observed to be optimal in the studies with mouse islets (Fig. 1). This drug concentration increased the relative activity index of GK ∼10-fold (Fig. 2B), which translates to a 50–75% threshold decrease for glucose-stimulated insulin secretion in man under control conditions (25, 51).
Fig. 2.
Effects of piragliatin on glucokinase (GK) activity. A: effect of the GK activator piragliatin on maximal velocity (Vmax) and glucose affinity of pure recombinant human islet GK. B: correlation between relative activity index (AI) of GK and threshold of glucose-stimulated insulin secretion. The AI is defined as follows: AI = (kcat/ S0.5h) × (2.5/2.5 + ATP km), and the relative AI at different GKA concentrations is obtained by normalizing to the AI under standard conditions (25).
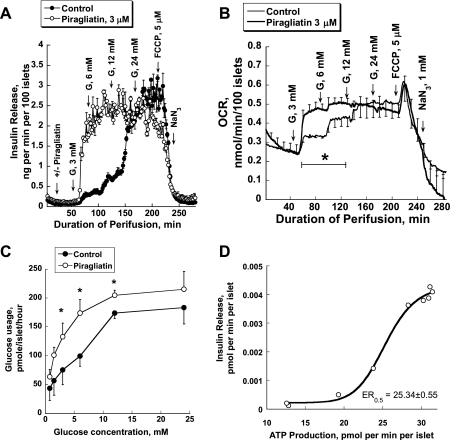
The insulin release and oxygen consumption profiles of rat islets were similar to those of mouse islets, except that maximal insulin release and the baseline of oxygen consumption were twofold higher in rat islets (Fig. 3, A and B). As in mouse islets, glucose usage in rat islets increased due to the stepwise rise of glucose concentrations (Fig. 3C). As expected, 3 μM piragliatin shifted the glucose dependency curves of insulin release, oxygen consumption, and glucose usage of rat islets to the left without increasing Vmax of the secretion or respiration rates. The relationship between ATP production and insulin release of rat islets was also fitted to a steep sigmoidal curve (Fig. 3D) with a threshold of ∼19 pmol·min−1·islet−1 and a ER0.5 of 25.34 ± 0.55 pmol·min−1·islet−1 of ATP production (r = 0.997).
Fig. 3.
Insulin release, respiration, glucose usage, and ATP production of isolated cultured rat islets. A: insulin release patterns in the absence and presence of 3 μM piragliatin. B: effects of piragliatin on islet respiration. C: glucose usage in rat islets as a function of the glucose levels. D: relationship between energy generation and hormone secretion in the form of an ATP production/insulin secretion diagram. Results are presented as means ± SE of experiments from 3 islet isolations.
Effect of islet organ culture conditions on insulin release in human islets.
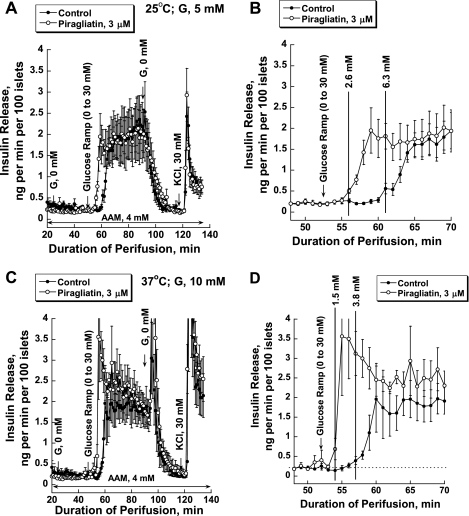
Rodent islets are routinely cultured for several days at 37°C and in the presence of 10 mM glucose. This is in contrast to human islets that were cultured for 3–5 days at 25°C and 5 mM glucose using a contractually mandated standard protocol of the Human Islet Resource Centers. We tested whether, and if so how much, lower glucose and temperature might affect the insulin secretory response. Insulin secretion was studied in islet perifusion experiments, using a glucose ramp of 0–30 mM within 40 min in the presence and absence of 3 μM piragliatin with islets cultured in routine fashion at low glucose and temperature. Glucose increased insulin release maximally by seven to nine times, with a threshold at 6.3 mM in the absence and 2.6 mM in the presence of the activator (Fig. 4, A and B). When human islets were kept in culture at 37°C and 10 mM glucose for 3–4 days and studied with the same protocol, small but significant functional differences were observed. Although the maximal rate of insulin secretion was little affected, the thresholds for glucose-stimulated insulin secretion were shifted to 3.8 mM in the absence and 1.5 mM in the presence of the activator (Fig. 4, C and D). Also, in the presence of the drug the first phase was more strongly accentuated. Since inclusion of an additional 3–4 days of culture at higher temperature lowers islet recovery significantly and since the functional impact of the difference in culture conditions on islet secretory function was relatively small, human islets for the present studies were used as prepared by the PENN Islet Procurement Center.
Fig. 4.
Effect of islet culturing condition on insulin release in human islets. A: results with islets cultured at 25°C and 5 mM glucose for ≥1 wk (n = 4–5). B: magnified view of selected section (48–70 min) of the experiment presented in A to pinpoint the threshold for insulin release and its drug-induced shift. C: islets cultured at 37°C and 10 mM glucose for an additional 3–4 days following the routine culture, as indicated above (n = 6). D: magnified view of a selected section (48–70 min) of the experiment presented in C to pinpoint the threshold for insulin release and its drug-induced shift.
Studies with control human islets.
Because of the limited availability of tissue, only one concentration of piragliatin (3 μM) was tested with human islets. Seven hundred to 800 islets comparable in size with that of rodent islets were hand-picked and transferred to the perifusion chamber. At 3 mM, glucose-stimulated insulin secretion was increased at a much lower glucose threshold than observed with mouse islets (Fig. 5, A and B). Glucose at 6 and 12 mM caused a biphasic and at 24 mM a monophasic increase in insulin secretion. The OCR increased by 48 and 58% at 6 and 12 mM glucose, respectively, compared with baseline at 0 mM glucose. As shown above for mouse and rat islets, 24 mM glucose had no effect on the OCR. Note that at high glucose concentrations (12 and 24 mM), changes in the OCR showed a transition response, an initial decrease followed by a new higher rate. For 12 mM glucose the initial decrease was followed by an overshoot, whereas for 24 mM glucose the return was almost to the previous rate, indicating temporary inhibition of mitochondrial metabolism by glycolysis. FCCP (5 μM) blocked insulin release and transiently increased the OCR, indicating coupling of islet respiration and oxidative phosphorylation. NaN3 blocked respiration. Thus, the OCR of healthy human islets increased sigmoidally as a function of the stepwise rise of glucose concentrations in a manner similar to that observed in mouse and rat islets (not shown). The maximal OCR was 0.40 ± 0.02 nmol·min−1·100 islets−1, and the glucose S0.5 was 4.39 ± 0.10 mM. Piragliatin (3 μM) did not change insulin release or the OCR when added during perifusion with 0 mM glucose; however, it greatly shifted the glucose dependency curve of insulin release and O2 use toward low glucose concentrations without significant changes in Vmax (Fig. 5, A and B), an indication of its specificity of action.
Fig. 5.
Effect of a GKA on secretion and metabolic profiles of isolated cultured human control islets. A: insulin release patterns with glucose stimulation using stepwise increases of glucose concentrations in the absence and presence of 3 μM piragliatin (n = 3). B: effect of piragliatin on islet respiration dynamics during the glucose staircase, followed by treatment with 5 μM of the uncoupler of Ox/Phos FCCP and 1 mM Na-azide (n = 3). C and D: effect of piragliatin on glucose usage and oxidation of human islets as a function of glucose concentrations (n = 3–4). E: ATP production/insulin secretion diagram. F: changes in intracellular Ca2+ of human islets due to stepwise increases of glucose (n = 3). Hb A1c levels for the 3 controls were 5.6, 5.7, and 5.7%. See legend to Fig. 1 for more information.
Both glucose usage and oxidation increased with rising glucose concentrations (Fig. 5, C and D). As was true for mouse islets, the oxidative catabolism of glucose was only a small fraction of glycolytic flux. Piragliatin, used at 3 μM and chosen for the perifusion studies because this concentration had proven to be optimal in the mouse and effective in rat, increased glucose usage and oxidation markedly at all glucose concentrations. However, at 10 μM the drug increased glucose oxidation only at low glucose concentrations, showing a crossover of concentration dependency curves of metabolism at 3–5 mM glucose that was, however, not seen for glucose usage. Figure 5E shows a clear relationship between insulin release and ATP production (calculated from OCR and glucose usage data) that was fitted to a sigmoidal curve similar to that for mouse and rat islets. Remarkably, the threshold and half-maximally effective rates of ATP production of human islets were very similar to those for mouse and rat islets, i.e., about 16 and 22.16 ± 0.38 pmol·min−1·islet−1 (r = 0.998), respectively.
Piragliatin-induced changes in insulin secretion, oxygen consumption, and glucose metabolism showed their corollaries in temporal [Ca2+]i concentration profiles (Fig. 5F). In contrast to mouse islets, 3 mM glucose transiently increased [Ca2+]i in human islets. High glucose (9 mM) caused a more than twofold increase in [Ca2+]i. As expected, in all cases piragliatin shifted the glucose dependency curve toward low glucose concentrations. However, surprisingly, for high glucose (9 mM) the [Ca2+]i in human islets was decreased at all drug dosages. An “off-spike” in [Ca2+]i was observed at all piragliatin concentrations during the washout period. This effect was specific for human islets and was not reproduced in mouse islets, even with glucose concentration as high as 16.7 mM and piragliatin concentrations ≥3 μM speaking against “off-target” action (data not shown). Thus, in human islets from normal organ donors, piragliatin greatly left-shifted the glucose dependency curve of OCR, GSIR, glucose usage, and oxidation and [Ca2+]i concentrations.
Comparison of the energy production/insulin secretion curves of normal mouse, rat, and human islets.
The threshold of insulin release as a function of glucose for normal human and rat islets was significantly left-shifted compared with that of normal mouse islets, and the maximal rate of hormone was higher in rat islets compared with human and mouse islets, confirming observations reported by others (29) (Fig. 6A). This resulted in differences of the glucose S0.5, which were 5.23 ± 0.13 (r = 0.999), 11.68 ± 0.13 mM (r = 0.999), and 8.42 ± 0.79 (r = 0.998) mM in human, mouse, and rat islets, respectively. Despite these differences in the glucose dependency curves of secretion profiles, the relationship between insulin secretion and ATP production was reliably fitted to one single sigmoidal curve, as can be seen in a scatter plot of combined data from mouse, rat, and human islets (Fig. 6B). This curve has a threshold of ∼13 pmol·min−1·islet−1. A similar fusion of two data sets into one single function was possible in studies with β-cell lines studied earlier that had characteristically diverging glucose dependency curves of insulin secretion, as illustrated in Fig. 6, C and D (44, 52). It is indeed remarkable that the energy production/insulin secretion curves of different tumorous β-cells and three different species agree so well qualitatively and also quantitatively (within a factor of ∼2). These results and their interpretation (Fig. 5) provide a new framework for β-cell exploration in which the rate of ATP production is the critical common denominator for GSIR.
Fig. 6.
Fusion of mouse, rat, and human islet ATP proction/insulin secretion diagrams and comparison with corresponding published data obtained with 2 functionally diverging β-cell lines (44, 52). A: secretion profiles of mouse, rat, and human islets as a function of the glucose concentration. B: combined energy production/insulin secretion curve with data on ATP production and insulin release from all 3 types of islets projected on 1 single continuous sigmoidal curve (note that results with 20–30 μM piragliatin in Fig. 1 were not included because respiration and glucose metabolism were inhibited at these high levels). C and D: analogous sets of data from 2 tumorous β-cell lines (44).
Potentiation of GSIR Influences the ATP Production/Insulin Secretion Diagram: Effect of Exendin-4 on the Energy Production/Insulin Secretion Diagram of Mouse Islets
Based on mouse, rat, and human data with glucose as fuel, it is proposed above that the relationship between energy production and insulin secretion can be represented by an ATP production/insulin secretion diagram that fits all three species (and also tumorous β-cells). However, it can be expected that large deviations from this curve can be caused by the neuroendocrine system, positive for GLP-1, glucagon, gastric inhibitory polypeptide, and acetylcholine but negative for somatostatin and catecholamines mediated primarily by altering receptor-dependent second-messenger signaling. To test this hypothesis, the theoretically and clinically highly relevant GLP-1 analog exendin-4 was used to stimulate the rate of insulin release maximally and assess the effect of this potentiation of glucose-dependent secretion on the metabolic rate. Exendin-4 at 50 nM increased insulin secretion fivefold at a glucose concentration ≥6 mM (Fig. 7A). This marked potentiation of glucose-dependent insulin release had no effect on the OCR (Fig. 7B). Note that the FCCP effect in exendin-4-treated islets was characteristically different from that seen in controls. FCCP caused an instant inhibition of respiration lacking the transient activation that usually results from uncoupling of oxidative phosphorylation. Currently, there is no compelling explanation for this striking exeption of FCCP action in the presence of exendin-4. Glucose usage at a wide range of glucose levels was barely affected by exendin-4, showing a significant increase only at 6 mM glucose (Fig. 7C). Based on these respiration and glucose usage data, the ATP production/insulin secretion diagram was generated and found to be sigmoidal (Fig. 7D), albeit with the threshold for insulin secretion shifted to the left (to ∼10 pmol·min−1·islet−1 of ATP produced) compared with glucose alone (Fig. 7E) or glucose plus piragliatin (Fig. 7F). The ER0.5 for exendin-4-treated islets was equal to 19.47 ± 0.03 pmol·min−1·islet−1 of ATP production (r = 1).
Fig. 7.
Effect of exendin-4 on secretion and metabolic profiles of isolated cultured mouse islets. A: insulin release patterns with glucose stimulation using stepwise increases in glucose concentrations in the absence and presence of 50 nM exendin-4 (n = 3). B: effect of exendin-4 on islet respiration (n = 3). C: effect of exendin-4 on glucose usage of mouse islets as a function of glucose concentrations (n = 3–4). D: ATP production/insulin secretion diagram. E: physiologically relevant comparison of ATP production/insulin secretion diagram of exendin-4-treated islets and controls. F: physiologically relevant comparison of ATP production/insulin secretion diagram in the presence of exendin-4 or piragliatin.
GK Activation Repairs Defects of Insulin Secretion, Oxygen Consumption, and Intracellular Ca2+ Responses in Islets from Type 2 Diabetics
Studies with human islets from type 2 diabetics compared with controls.
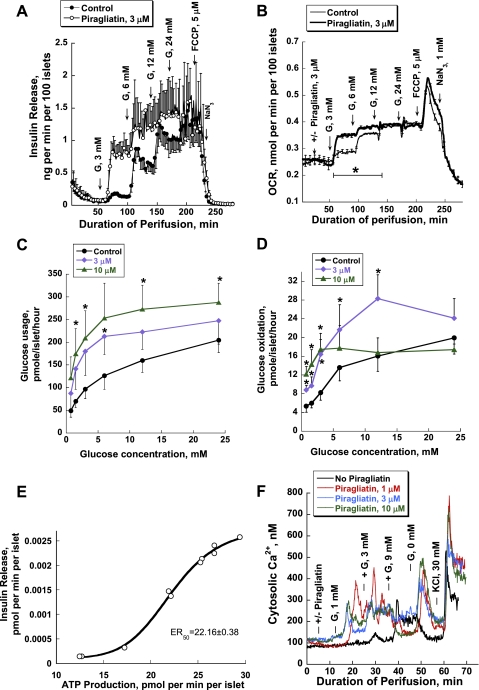
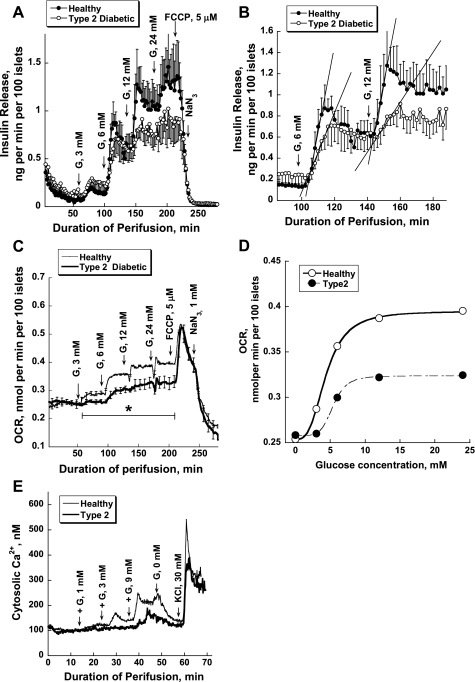
High-quality human islets from patients with T2DM are very scarce. Therefore, we opted to restrict our measurements to perifusion studies, providing OCR and insulin secretion in the absence and presence of 3 μM GKA, complemented by calcium measurements (Fig. 8). We did not have sufficient material to measure glycolysis and glucose oxidation in this series of studies. The glucose dependency curves of insulin secretion and respiration of diabetic islets showed decreased maximal rates and a right shift for the OCR compared with those of the control islets (Fig. 8, A, C, and D). It is worthy of note that the baselines for both parameters are comparable. In the insulin secretion profiles, the difference is most pronounced at the 6- and 12-mM glucose steps showing decreased rates of rise, a decreased extent, and loss of biphasicity (Fig. 8B). The glucose dependency of the OCR of the diabetic islets is relatively flat and is reduced by 50% at 24 mM glucose (Fig. 8C). FCCP (5 μM) blocked insulin release instantly and transiently increased OCR in control and diabetic islets to the same level, indicating that strong coupling exists between islet respiration and oxidative phosphorylation in both types of islets, making a defect in Ox/Phos as cause of β-cell dysfunction unlikely (Fig. 8C). V̇o2 of healthy and diseased human islets increased sigmoidally as a function of the stepwise rise of glucose concentrations but showed a clear right shift of the concentration dependency curve of islets from diabetics (Fig. 8D). In islets from type 2 diabetics, the maximal stimulation of respiration (Vmax) by glucose was reduced from 0.40 ± 0.02 nmol·min−1·100 islets−1 in control to 0.32 ± 0.01 nmol·min−1·100 islets−1, and the S0.5 rose from 4.39 ± 0.01 mM in control to 5.43 ± 0.13 mM (Fig. 8D). Figure 8E presents changes in intracellular [Ca2+]i of human islets. Three millimolars glucose caused a transient and 9 mM glucose a biphasic sustained increase in [Ca2+]i of control islets. Diabetic islets responded only to 9 mM, and this response was delayed and significantly lower than 0 in controls.
Fig. 8.
Impaired insulin release (A and B), oxygen consumption (C and D), and intracellular calcium (E) of isolated islets from control and type 2 diabetic organ donors; see legends to Figs. 1 and 4 for more details and comparison. Six separate donors were examined; 3 were normal and 3 were diabetic. Normal donors were 45, 50, and 28 yr old; body mass indexes (BMI) were 24.1, 28.5, and 24.7 and Hb A1c values 5.6, 5.8, and 5.7%, respectively. The type 2 diabetics were 58, 45, and 30 yr old with BMI of 29.3, 34.1, and 25.9 and Hb A1c values of 9.3, 11.0, and 7.4%, respectively. Results are presented as means ± SE (SE when applicable) of 3 experiments.
Piragliatin fully repairs the functional defect of islets from type 2 diabetics.
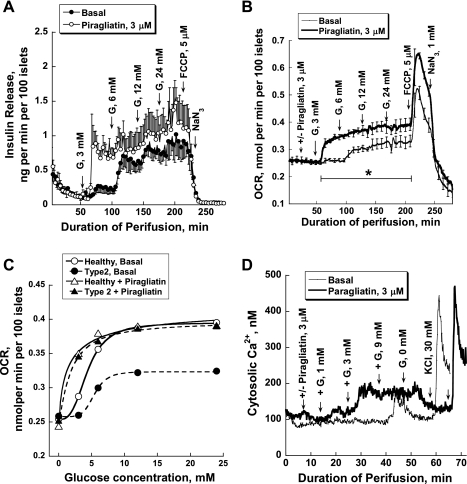
In the next series of experiments, we tested whether piragliatin might improve insulin secretion, oxygen consumption, and calcium responses to glucose in human islets from type 2 diabetic organ donors. Both insulin release and oxygen consumption curves were left-shifted by 3 μM of the drug (Fig. 9, A and B). The insulin release and OCR profiles for control and T2DM islets in response to the staircase stimulus of glucose were practically indistinguishable when the activator was present, indicating full repair of the functional lesion (Fig. 9C). Figure 9D shows the [Ca2+]i data of type 2 diabetic human islets. The typical example illustrates that piragliatin greatly improved the calcium response.
Fig. 9.
Piragliatin repairs defective insulin release, oxygen consumption, and calcium responses to glucose of islets isolated from type 2 diabetic organ donors. A: insulin release (n = 3). B: oxygen consumption for the same experiments presented in A (n = 3). C: comparison of OCR glucose dependencies of healthy islets and those of diabetics and of the effect of piragliatin on these 2 sets of islets. D: GKA effect on intracellular calcium concentrations.
DISCUSSION
Three aspects of this study require discussion: 1) the rationale and the strategy of the study, 2) the direct demonstration of defects in oxidative glucose metabolism as cause of impaired insulin secretion of islets from individuals with T2DM and of repair of the defects by a pharmacological GKA, and 3) the demonstration that GSIR of normal mouse, rat, and human pancreatic islets is characterized by a single steeply sigmoidal “energy production/insulin secretion” diagram implicating the rate of ATP production as the predominant coupling process in GSIR.
Rationale and Strategy of the Study
The molecular pathology of T2DM and its usually slow development with aging remains a mystery, although extensive clinical investigations of the pathophysiology have provided an exhaustive phenomenological description, and genome-wide association studies have amassed compelling evidence for linkage of numerous diabetes genes implicating involvement of the β-cell (21, 47, 56, 57, 66, 69). A less explored approach is to identify the underlying defects “ex iuvantibus” using pharmacology. As the study was being designed we considered four clinically highly effective medications: 1) sulfonylurea compounds (62), 2) metformin and thiazolidinediones (11), 3) GLP-1 receptor agonists or DDP IV inhibitors (2), and 4) GKAs (26, 51). Common to all four is that their mechanisms of action are largely known. Sulfonylureas and equivalent drugs depolarize β-cells by inhibiting the KATP channel and sensitizing the cells to amino acids, stimulatory neurotransmitters, and endocrine modifiers (17, 18, 38, 72). However, there is evidence that they also inhibit oxidative glucose metabolism and reduce glucose responsiveness (20, 32, 55, 70). In this context, it is of interest that GKAs are effective in sulfonylurea-desensitized rats (64). Metformin and thiazolidinediones are insulin sensitizers targeting AMP kinase or the transcription factor PPARγ (10, 11, 77) but have been shown recently to inhibit glucose-stimulated insulin release by interfering with ATP production (36). GLP-1 receptor activation, directly by pharmacological agonists or indirectly by inhibiting the degradation of endogenous GLP-1, results in the glucose-dependent potentiation of β-cell function via cAMP signaling (2, 34). GKAs stimulate glycolysis, glucose oxidation, and ATP production, thereby facilitating all glucose-dependent processes of β-cells (26, 27, 51, 54). Because glucose stimulation of β-cells is compromised in T2DM, we chose to assess the efficacy of a GKA in correcting the observed functional defects of isolated human islets. Insulin release, glycolysis, glucose oxidation, respiration, and intracellular Ca2+ were monitored in the presence and absence of piragliatin. It was expected that this would reveal whether a defect in glucose metabolism in T2DM islets, should it exist, was responsible for the altered insulin release and abnormally low Ca2+ levels. In order for this strategy to work, it was important to carry out detailed parallel studies with islets from other species, in this case normal mouse and rat, to relate our findings to those for well-studied biological model systems.
GKA Repairs a Defect of Bioenergetics in Isolated Islets of Langerhans of Type 2 Diabetics
Although an insulin secretion defect is known to exist in human T2DM (3, 14, 15, 59), a convincing explanation remains far from established. The marked reduction of glucose-stimulated oxygen consumption, a clear manifestation of limited ATP production observed here, provides a good causal explanation for the impaired secretory function because ATP and the P-potential play an essential role in fuel-stimulated secretion coupling. Several pertinent publications also support the present view that impaired energetics are central to the understanding of the pancreatic β-cell defect in T2DM. Anello et al. (3) and Del Guerra et al. (14) have shown that reduced insulin secretion in response to glucose in human type 2 diabetic islets is associated with lower ATP levels, a lower ATP/ADP ratio, and impaired hyperpolarization of the mitochondrial membrane. These authors also reported the increased protein expression of uncoupling protein 2, complex I and complex V of the respiratory chain, and a higher level of nitrotyrosine, an indicator of oxidative stress. MacDonald et al. (48) have shown that the activities of the mitochondrial enzymes, glycerol phosphate dehydrogenase, pyruvate carboxylase, and succinyl-CoA:3-ketoacid-CoA transferase were decreased significantly in islets isolated from type diabetic donors. Using MKR mice (a model of type 2 diabetes), Lu et al. (46) have shown that decreased mitochondrial function and abnormal morphology occur even before the onset of hyperglycemia and play a role in β-cell failure and type 2 diabetes in these mice. The authors found reduced hyperpolarization of ΔΨm, impaired Ca2+ signaling, reduced ATP/ADP ratios during glucose stimulation, and a decrease in proteins of the mitochondrial inner membrane, including rate-limiting enzymes of the TCA cycle and multiple components involved in OxPhos. These data were associated with a decreased respiration by mitochondria. They proposed that the inability of glucose to hyperpolarize ΔΨm is likely a key defect resulting in a reduced ATP/ADP ratio and glucose-stimulated insulin secretion. Collectively, these data suggest that alterations in mitochondria may be a culprit in the pathogenetic processes culminating in type 2 diabetes (59).
It needs to be appreciated that in normal islet tissue the oxidative component of glucose metabolism generates about 70–80% of the total ATP and that glycolysis contributes the other 20–30% to energy production. Lack of islet tissue availability prohibited us from measuring glycolysis in islets from diabetics at this stage of the project. However, it is highly unlikely that accelerated glycolysis of diabetic islets could compensate for the shortfall of mitochondrial ATP, thus weakening the present interpretation because of the demonstrated 30% reduction of GK expression (14, 43). Respiration is a global parameter of energy metabolism, and it remains to be established where exactly in the complex pathway the defect lies. The results of FCCP uncoupling of Ox/Phos are inconsistent with a major mitochondrial coupling defect. The results of GKA activation suggest that the overall capacities for glucose metabolism and thus of glucokinase activity are not significantly reduced. Therefore, it is likely that regulation of metabolism is at fault, as indicated by several observations. We have shown, for example, that pyruvate cycling is dysregulated with greatly enhanced rates at low glucose, that flux through the GABA shunt is reduced, and that diabetic islets have a distorted amino acid profile (Ref. 43 and Li C, Liu C, Nissim I, Chen J, Chen P, Doliba N, Nissim I, Daikhin Y, Stokes D, Yudkoff M, Bennett MJ, Stanley CA, Matschinsky FM, and Naji A, unpublished observations), suggesting dysregulation of anaplerosis and cataplerosis. The remedial action of GKA at 3 mM glucose is particularly intriguing in light of the fact that 24 mM glucose, approximately equivalent to 3 mM in the presence of a GKA, is unable to elicit a comparable functional response. One can only speculate about possible explanations for this observation. Glucose transport is not rate limiting in normal β-cells, but a marked reduction in diabetes could become a factor (52). This possibility cannot be fully excluded because activation of glucose phosphorylation could compensate for such a defect. However, the evidence for impaired GK function of T2DM β-cells is compelling. Most importantly, GK is reduced significantly in islets isolated from type 2 diabetics (14, 43). Furthermore, since β-cell GK is normally found in functionally significant association with mitochondria (12), in insulin granules (58), and in complex with the bifunctional enzyme phosphofructo-2-kinase/fructose-2,6-bisphosphatase (7), it is conceivable that any or all of these associations are disrupted in T2DM islets impairing catalysis and that GKAs restore enzyme activity. The efficacy of a GKA in fully restituting the pathologically low glucose-induced respiration and insulin release and greatly improving the Ca2+ response is indeed striking. This result offers a plausible explanation for the efficacy of piragliatin in a clinical trial (9, 51) and illustrates the potential of this new class of antidiabetic agents. Evidence is increasing that glucose stimulates replication of normal β-cells, allowing their adaptative hyperplasia in insulin resistance states, for example, in pregnancy and obesity (74, 76). If this proliferative glucose action depends on glucose metabolism, as current knowledge implies, it is predictable from the present data that it might be curbed in the diabetic state and that GKAs may repair this refractoriness. Realizing that the action of GLP-1, which is thought to stimulate β-cell replication (2), depends absolutely on intact glucose metabolism, experimental trials that would use a therapy of GLP-1 receptor activation by exanitide or DPP IV inhibitors combined with metabolic potentiation by GKAs are recommended.
It has been and is repeatedly questioned, explicitly (63) or implicitly (36), whether antidiabetic pharmacotherapies that are based on the stimulation of the β-cells are conceptually defendable. The argument is that “overworking” the already compromised β-cell would lead to “exhaustion” associated with impaired function and in the long run cell death. The issue will perhaps be settled only by long-term human trials because negative and positive theoretical and experimental arguments can be made on both sides of the controversial issue. In any case, the present results show 1) that human islet tissue from type 2 diabetics has a defect in glucose-dependent energy production and insulin release persisting for days ex vivo and 2) that this defect appears to be in part due to dysregulation of the GK glucose sensor because it can be overcome by a relatively low dose of a GKA. This drug activates the enzyme allosterically and is effective, whereas 24 mM hyperglycemia, which acts through “substrate site activation” of the enzyme, is not. Since GKAs are also insulin sensitizers by their powerful hepatic action (1, 9, 27, 51, 54), their medical potential should not be underestimated and should be explored further. Phosphorylated peptide mimetics of the BH3 domain of BAD (SAHBs or stapled α-helix BCl2 domains) have been reported to activate GK by a mechanism that is fundamentally different from that of GKAs and to repair a β-cell glucose-sensing defect of BAD−/− mice (13). It is thought that SAHBs facilitate the multicomplex association of the enzyme with the mitochondrial membrane that is apparently essential for its operation in the β-cell. It will be highly informative to test whether these agents repair the defect in β-cell bioenergetics in T2DM islets in a manner similar to GKA, particularly in view of a report that the small isoform of BAD is markedly reduced in mitochondria of T2DM pancreatic islets (45).
Heuristic Significance of an Energy Production/Insulin Secretion Diagram for Understanding Normal and Impaired Stimulus-Secretion Coupling
The present study illustrates that even a limited exploration of scarce human islet material is useful when it derives additional strength from the application of new technology and extensive parallel studies with rodent islets. The optical determination of oxygen pressure in the pancreatic islet perifusion experiment made it possible to measure islet respiration stably for several hours. As a result, the OCR could be related to glycolysis, glucose oxidation, and insulin secretion and allow for calculation of the ATP production rate as a function of the glucose concentration. Making the reasonable assumption that islet glycogen stores are negligible (53) and that coupling of Ox/Phos is intact as shown here, we were able to establish for the first time an islet “ATP Production/Insulin Secretion” diagram. For mouse, rat, and man the curve is the same (surely in its physiologically relevant part), is steeply sigmoidal, and has a threshold for GSIR at ∼15 pmol·islet−1·min−1, a Hill coefficient of ∼11, which is an indication of highly cooperative coupling mechanisms, and a half-maximal effective rate (ER50) of ∼25 pmol·islet−1·min−1. These values imply that ATP turns over about twice/min at the threshold and about four times at maximal glucose stimulation but that ATP turnover is changed relatively little (perhaps not more than 30%) in the physiologically important segment of the curve. These ATP production rates extrapolate to a remarkable fact. At the threshold for insulin release, pancreatic islets generate ATP at a rate of about six times their weight in 24 h and at a rate of about 13 times their weight in 24 h when stimulated maximally by glucose, placing them among the energetically most active tissues. It is speculated that the ATP Production/Insulin Secretion diagram that is a manifestation of this fact has fundamental and medical significance comparable with that of the classical “Frank/Starling” curve of the heart. In the heart it is the venous return and the ensuing muscle fiber stretch that determines cardiac output, maintaining the match between the pump rate and body oxygen requirements. In β-cells, it is the fuel load and the ensuing ATP turnover that determines insulin output and maintains glucose homeostasis. Available knowledge suggests that this curve is modified in a major way by the neuroendocrine system (positively by the vagus and GLP-1 but negatively by sympathetic input, adrenaline, and somatostatin) (2, 17, 18). These neuroendocrine modifications are caused not by alteration of ATP production (17) and associated metabolic coupling but instead by second-messenger signaling (2). Indeed, our data show that exendin-4, a GLP-1 analog, which greatly potentiates insulin secretion, changes the diagram markedly; the threshold for insulin release is shifted from an ATP production rate of ∼15 to one of ∼10 pmol·islet−1·min−1. This effect of exendin-4 is most likely due to generation of second messengers and activation of PKA. The present data also show that, energetically speaking, exendin-4 is more effective than the GKA piragliatin, as demonstrated by the threshold shift in the diagram. It will be important to investigate how other physiological and experimental fuel stimulants or pharmacological agents other than GKAs and GLP-1 affect this basic relationship in normal islets and also in islets from type 2 diabetics. Scarcity of well-preserved tissue has thus far prevented us from quantitating glycolysis of islets from type 2 diabetics, precluding the construction of an ATP production/insulin secretion diagram as influenced by the disease and by GKAs. However, we predict that glycolysis is reduced similarly to glucose oxidation and that the diagram of the diabetics will exhibit a characteristic pattern showing an incomplete curve when untreated (perhaps not exceeding the ER50 mark) but with a capacity for full activation in the presence of GKAs or possibly other drugs.
A recent comparative biochemical study on patterns of enzymes thought to be critically involved in fuel stimulation of pancreatic β-cells and showing striking quantitative differences between human and rat pancreatic islets deserves comment in this context (49). It was reported that pyruvate carboxylase and citrate lyase were substantially lower in humans compared with rat islet, which also manifested itself in greatly reduced pyruvate cycling. This observation raised significant questions as to the unique and general importance attributed to this pathway in stimulus-secretion coupling for GSIR. It was also found that human islets have a relatively high capacity (compared with rat) for generating acetoacetate in response to high glucose. This is attributed to operation of a metabolic pathway that leads from glucose-derived mitochondrial acetyl-CoA via acetoacetyl-CoA to acetoacetate. According to this proposal, acetoacetate then exits to the cytosol, where it serves as a precursor for acetyl-CoA, malonyl-CoA, hydroxylmethylglutaryl-CoA, and long-chain acyl-CoA, and these function as metabolic coupling factors. An alternative hypothesis would be that the main physiological direction of flux for this pathway is catabolic rather than anabolic, being involved in the generation of ATP when ketones are abundant, maintaining basal insulin release in the fasting state as proposed many years ago (8, 50, 65).
The outstanding unresolved question in this context is the nature of the ATP-using processes in the β-cell. Since exocytosis per se has an extremely low energy requirement, as demonstrated by the fact that manifold potentiation of GSIR has little, if any, effect on the OCR (Ref. 17 and the present study), other ATP sinks have to be identified (24). They include ion pumping of calcium and sodium, protein biosynthesis, protein phosphorylations and dephosphorylations, and various forms of futile cycling and metabolic shunts, the latter processes perhaps quantitatively highly significant. The following futile cycles have been described: pyruvate cycling (30), glucose cycling (35), fatty acid/triglyceride cycling (61), and the GABA shunt (42). It is hypothesized here that these energy-consuming cycling processes serve an important regulatory function, facilitating the precise integration of metabolic input from multiple metabolic pathways involved in fuel sensing and stimulation of insulin release and biosynthesis. It is anticipated that analysis of stimulus-secretion coupling within the framework of the energy production/insulin secretion relationship will help settle important, unresolved questions about the nature and importance of putative metabolic coupling factors and second messengers compared with the unquestionably essential role that is played by the rate of ATP production.
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants DK-22122 (to F. M. Matschinsky) and DK-053012 (to C. A. Stanley) and by the Institute for Diabetes, Obesity, and Metabolism (via NIDDK Grant DK-19525).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.M.D., C.A.S., D.F.W., J.G., R.S., A.N., and F.M.M. did the conception and design of the research; N.M.D., W.Q., H.N., C. Liu, C.W.B., J.S., H.W.-C., and C. Li performed the experiments; N.M.D. analyzed the data; N.M.D., C. Li, C.A.S., D.F.W., J.G., R.S., A.N., and F.M.M. interpreted the results of the experiments; N.M.D. prepared the figures; N.M.D. and F.M.M. drafted the manuscript; N.M.D. and F.M.M. edited and revised the manuscript; N.M.D. and F.M.M. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the the critical contribution to this study by the team of the Human Islet Resource Center at University of Pennsylvania, especially to Zaw Min, Yanping Luo, Seyed Ziaie, Yanjing Li, and Kumar Vivek. We thank Inderroop Singh for technical assistance and Markiyan Doliba for assistance in the statistical analysis of data.
REFERENCES
- 1. Agius L. Glucokinase and molecular aspects of liver glycogen metabolism. Biochem J 414: 1–18, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Ahren B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov 8: 369–385, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Anello M, Lupi R, Spampinato D, Piro S, Masini M, Boggi U, Del Prato S, Rabuazzo AM, Purrello F, Marchetti P. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia 48: 282–289, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature 312: 446–448, 1984 [DOI] [PubMed] [Google Scholar]
- 5. Ashcroft FM, Harrison DE, Ashcroft SJ. A potassium channel modulated by glucose metabolism in rat pancreatic beta-cells. Adv Exp Med Biol 211: 53–62, 1986 [DOI] [PubMed] [Google Scholar]
- 6. Ashcroft SJ, Weerasinghe LC, Bassett JM, Randle PJ. The pentose cycle and insulin release in mouse pancreatic islets. Biochem J 126: 525–532, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baltrusch SW, Okar DA, Tiedge M, Lange AJ. Interaction of GK with the bifunctional enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (6PF2K/F26P2ase). In: Frontiers in Diabetes Glucokinase and Glycemic Disease: From Basic to Novel Therapeutics, edited by Matschinsky FM. Basel, Switzerland: Karger, 2004, p. 360–378 [Google Scholar]
- 8. Boden G, Chen X. Effects of fatty acids and ketone bodies on basal insulin secretion in type 2 diabetes. Diabetes 48: 577–583, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Bonadonna RC, Heise T, Arbet-Engels C, Kapitza C, Avogaro A, Grimsby J, Zhi J, Grippo JF, Balena R. Piragliatin (RO4389620), a novel glucokinase activator, lowers plasma glucose both in the postabsorptive state and after a glucose challenge in patients with type 2 diabetes mellitus: a mechanistic study. J Clin Endocrinol Metab 95: 5028–5036, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, Roden M, Gnaiger E, Nohl H, Waldhausl W, Furnsinn C. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes 53: 1052–1059, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Campbell IW, Mariz S. Beta-cell preservation with thiazolidinediones. Diabetes Res Clin Pract 76: 163–176, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB, Gygi SP, Korsmeyer SJ. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature 424: 952–956, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Danial NN, Walensky LD, Zhang CY, Choi CS, Fisher JK, Molina AJ, Datta SR, Pitter KL, Bird GH, Wikstrom JD, Deeney JT, Robertson K, Morash J, Kulkarni A, Neschen S, Kim S, Greenberg ME, Corkey BE, Shirihai OS, Shulman GI, Lowell BB, Korsmeyer SJ. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med 14: 144–153, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Del Guerra S, Lupi R, Marselli L, Masini M, Bugliani M, Sbrana S, Torri S, Pollera M, Boggi U, Mosca F, Del Prato S, Marchetti P. Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes 54: 727–735, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Deng S, Vatamaniuk M, Huang X, Doliba N, Lian MM, Frank A, Velidedeoglu E, Desai NM, Koeberlein B, Wolf B, Barker CF, Naji A, Matschinsky FM, Markmann JF. Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes 53: 624–632, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Deng S, Vatamaniuk M, Lian MM, Doliba N, Wang J, Bell E, Wolf B, Raper S, Matschinsky FM, Markmann JF. Insulin gene transfer enhances the function of human islet grafts. Diabetologia 46: 386–393, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Doliba NM, Qin W, Vatamaniuk MZ, Buettger CW, Collins HW, Magnuson MA, Kaestner KH, Wilson DF, Carr RD, Matschinsky FM. Cholinergic regulation of fuel-induced hormone secretion and respiration of SUR1−/− mouse islets. Am J Physiol Endocrinol Metab 291: E525–E535, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Doliba NM, Qin W, Vatamaniuk MZ, Li C, Zelent D, Najafi H, Buettger CW, Collins HW, Carr RD, Magnuson MA, Matschinsky FM. Restitution of defective glucose-stimulated insulin release of sulfonylurea type 1 receptor knockout mice by acetylcholine. Am J Physiol Endocrinol Metab 286: E834–E843, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Doliba NM, Qin W, Vinogradov SA, Wilson DF, Matschinsky FM. Palmitic acid acutely inhibits acetylcholine- but not GLP-1-stimulated insulin secretion in mouse pancreatic islets. Am J Physiol Endocrinol Metab 299: E475–E485, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doliba NM, Vatamaniuk MZ, Buettger CW, Qin W, Collins HW, Wehrli SL, Carr RD, Matschinsky FM. Differential effects of glucose and glyburide on energetics and Na+ levels of betaHC9 cells: nuclear magnetic resonance spectroscopy and respirometry studies. Diabetes 52: 394–402, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Doria A, Patti ME, Kahn CR. The emerging genetic architecture of type 2 diabetes. Cell Metab 8: 186–200, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gao ZY, Li G, Najafi H, Wolf BA, Matschinsky FM. Glucose regulation of glutaminolysis and its role in insulin secretion. Diabetes 48: 1535–1542, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Ghosh A, Ronner P, Cheong E, Khalid P, Matschinsky FM. The role of ATP and free ADP in metabolic coupling during fuel-stimulated insulin release from islet beta-cells in the isolated perfused rat pancreas. J Biol Chem 266: 22887–22892, 1991 [PubMed] [Google Scholar]
- 24. Gilbert M, Jung SR, Reed BJ, Sweet IR. Islet oxygen consumption and insulin secretion tightly coupled to calcium derived from L-type calcium channels but not from the endoplasmic reticulum. J Biol Chem 283: 24334–24342, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gloyn AL, Odili S, Buettger C, Njolstad PR, Shiota C, Magnuson MA, Matschinsky FM. A mathematical model predicts the threshold for glucose stimulated insulin release for GCK gene mutations that cause hyper- and hypoglycemia. In: Glucokinase and Glycemic Disease: From Basics to Novel Therapeutics, edited by Matschinsky FM, Magnuson MA. New York: Karger, 2004, p. 92–109 [Google Scholar]
- 26. Grimsby J, Berthel SJ, Sarabu R. Glucokinase activators for the potential treatment of type 2 diabetes. Curr Top Med Chem 8: 1524–1532, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Grimsby J, Sarabu R, Corbett WL, Haynes NE, Bizzarro FT, Coffey JW, Guertin KR, Hilliard DW, Kester RF, Mahaney PE, Marcus L, Qi L, Spence CL, Tengi J, Magnuson MA, Chu CA, Dvorozniak MT, Matschinsky FM, Grippo JF. Allosteric activators of glucokinase: potential role in diabetes therapy. Science 301: 370–373, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 49: 1751–1760, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Henquin JC, Dufrane D, Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes 55: 3470–3477, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Jitrapakdee S, Wutthisathapornchai A, Wallace JC, MacDonald MJ. Regulation of insulin secretion: role of mitochondrial signalling. Diabetologia 53: 1019–1032, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 46: 3–19, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355: 2427–2443, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Kahn SE, Zraika S, Utzschneider KM, Hull RL. The beta cell lesion in type 2 diabetes: there has to be a primary functional abnormality. Diabetologia 52: 1003–1012, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kang G, Chepurny OG, Holz GG. cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic beta-cells. J Physiol 536: 375–385, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khan A, Ostenson CG, Berggren PO, Efendic S. Glucocorticoid increases glucose cycling and inhibits insulin release in pancreatic islets of ob/ob mice. Am J Physiol Endocrinol Metab 263: E663–E666, 1992 [DOI] [PubMed] [Google Scholar]
- 36. Lamontagne J, Pepin E, Peyot ML, Joly E, Ruderman NB, Poitout V, Madiraju SR, Nolan CJ, Prentki M. Pioglitazone acutely reduces insulin secretion and causes metabolic deceleration of the pancreatic beta-cell at submaximal glucose concentrations. Endocrinology 150: 3465–3474, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lebedev AY, Cheprakov AV, Sakadzić S, Boas DA, Wilson DF, Vinogradov SA. Dendritic phosphorescent probes for oxygen imaging in biological systems. ACS Appl Mater Interfaces 1: 1292–1304, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li C, Buettger C, Kwagh J, Matter A, Daikhin Y, Nissim IB, Collins HW, Yudkoff M, Stanley CA, Matschinsky FM. A signaling role of glutamine in insulin secretion. J Biol Chem 279: 13393–13401, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Li C, Chen P, Palladino A, Narayan S, Russell LK, Sayed S, Xiong G, Chen J, Stokes D, Butt YM, Jones PM, Collins HW, Cohen NA, Cohen AS, Nissim I, Smith TJ, Strauss AW, Matschinsky FM, Bennett MJ, Stanley CA. Mechanism of hyperinsulinism in short-chain 3-hydroxyacyl-CoA dehydrogenase deficiency involves activation of glutamate dehydrogenase. J Biol Chem 285: 31806–31818, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li C, Matter A, Kelly A, Petty TJ, Najafi H, MacMullen C, Daikhin Y, Nissim I, Lazarow A, Kwagh J, Collins HW, Hsu BY, Nissim I, Yudkoff M, Matschinsky FM, Stanley CA. Effects of a GTP-insensitive mutation of glutamate dehydrogenase on insulin secretion in transgenic mice. J Biol Chem 281: 15064–15072, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Li C, Najafi H, Daikhin Y, Nissim IB, Collins HW, Yudkoff M, Matschinsky FM, Stanley CA. Regulation of leucine-stimulated insulin secretion and glutamine metabolism in isolated rat islets. J Biol Chem 278: 2853–2858, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Li C, Nissim I, Chen P, Buettger C, Najafi H, Daikhin Y, Nissim I, Collins HW, Yudkoff M, Stanley CA, Matschinsky FM. Elimination of KATP channels in mouse islets results in elevated [U-13C]glucose metabolism, glutaminolysis, and pyruvate cycling but a decreased gamma-aminobutyric acid shunt. J Biol Chem 283: 17238–17249, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li CL, Nissim I, Chen P, Doliba N, Nissim I, Daikhin Y, Stokes D, Yudkoff M, Stanley CA, Matschinsky FM, Daikhin A. Islets of human type 2 diabetics have decreased GABA shunt and dysregulated glucose metabolism (Abstract). Diabetes 58, Suppl 1: A424, 2009. [Google Scholar]
- 44. Liang Y, Bai G, Doliba N, Buettger C, Wang L, Berner DK, Matschinsky FM. Glucose metabolism and insulin release in mouse beta HC9 cells, as model for wild-type pancreatic β-cells. Am J Physiol Endocrinol Metab 270: E846–E857, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Liu S, Okada T, Assmann A, Soto J, Liew CW, Bugger H, Shirihai OS, Abel ED, Kulkarni RN. Insulin signaling regulates mitochondrial function in pancreatic beta-cells. PLoS One 4: e7983, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lu H, Koshkin V, Allister EM, Gyulkhandanyan AV, Wheeler MB. Molecular and metabolic evidence for mitochondrial defects associated with beta-cell dysfunction in a mouse model of type 2 diabetes. Diabetes 59: 448–459, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lyssenko V, Jonsson A, Almgren P, Pulizzi N, Isomaa B, Tuomi T, Berglund G, Altshuler D, Nilsson P, Groop L. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med 359: 2220–2232, 2008 [DOI] [PubMed] [Google Scholar]
- 48. MacDonald MJ, Longacre MJ, Langberg EC, Tibell A, Kendrick MA, Fukao T, Ostenson CG. Decreased levels of metabolic enzymes in pancreatic islets of patients with type 2 diabetes. Diabetologia 52: 1087–1091, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. MacDonald MJ, Longacre MJ, Stoker SW, Kendrick M, Thonpho A, Brown LJ, Hasan NM, Jitrapakdee S, Fukao T, Hanson MS, Fernandez LA, Odorico J. Differences between human and rodent pancreatic islets: low pyruvate carboxylase, atp citrate lyase, and pyruvate carboxylation and high glucose-stimulated acetoacetate in human pancreatic islets. J Biol Chem 286: 18383–18396, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Madison LL, Mebane D, Unger RH, Lochner A. The hypoglycemic action of ketones. II. Evidence for a stimulatory feedback of ketones on the pancreatic beta cells. J Clin Invest 43: 408–415, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nat Rev Drug Discov 8: 399–416, 2009 [DOI] [PubMed] [Google Scholar]
- 52. Matschinsky FM. Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes 45: 223–241, 1996 [DOI] [PubMed] [Google Scholar]
- 53. Matschinsky FM, Ellerman JE, Landgraf , krzanowski J, Kotler-Brajtburg J, Fertel R. Quantitative histochemistry of glucose metabolism in the islets of Langerhans. Curr Probl Clin Biochem 3: 143–182, 1971 [PubMed] [Google Scholar]
- 54. Matschinsky FM, Zelent B, Doliba NM, Kaestner KH, Vanderkooi JM, Grimsby J, Berthel SJ, Sarabu R. Research and development of glucokinase activators. In: Diabetes − Perspectives in Drug Therapy, edited by Schwanstecher M. New York: Springer, 2011, p. 357–401 [DOI] [PubMed] [Google Scholar]
- 55. Matthews DR, Cull CA, Stratton IM, Holman RR, Turner RC. UKPDS 26: Sulphonylurea failure in non-insulin-dependent diabetic patients over six years. UK Prospective Diabetes Study (UKPDS) Group. Diabet Med 15: 297–303, 1998 [DOI] [PubMed] [Google Scholar]
- 56. McCarthy MI, Froguel P. Genetic approaches to the molecular understanding of type 2 diabetes. Am J Physiol Endocrinol Metab 283: E217–E225, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Meigs JB, Shrader P, Sullivan LM, McAteer JB, Fox CS, Dupuis J, Manning AK, Florez JC, Wilson PW, D'Agostino RB, Sr, Cupples LA. Genotype score in addition to common risk factors for prediction of type 2 diabetes. N Engl J Med 359: 2208–2219, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Miwa IT, Yoshie S. Glucokinase in beta-cell insulin-secretory granules. In: Glucokinase and Glycemic Disease: From Basics to Novel Therapeutics, edited by Matschinsky FM. New York: Karger, 2004, p. 350–359 [Google Scholar]
- 59. Mulder H, Ling C. Mitochondrial dysfunction in pancreatic beta-cells in Type 2 diabetes. Mol Cell Endocrinol 297: 34–40, 2009 [DOI] [PubMed] [Google Scholar]
- 60. Newgard CB, Matschinsky FM. Substrate control of insulin release. In: Handbook of Physiology, edited by Jefferson L. Oxford, CA: Oxford Univ. Press, 2001, p. 125–151 [Google Scholar]
- 61. Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes 55, Suppl 2: S16–S23, 2006 [DOI] [PubMed] [Google Scholar]
- 62. Nolte MS. Pancreatic Hormones and Antidiabetic Drugs. New York: McGraw Hill, 2007, p. 697–698 [Google Scholar]
- 63. O'Doherty RM, Lehman DL, Telemaque-Potts S, Newgard CB. Metabolic impact of glucokinase overexpression in liver: lowering of blood glucose in fed rats is accompanied by hyperlipidemia. Diabetes 48: 2022–2027, 1999 [DOI] [PubMed] [Google Scholar]
- 64. Ohyama S, Takano H, Iino T, Nishimura T, Zhou YP, Langdon RB, Zhang BB, Eiki J. A small-molecule glucokinase activator lowers blood glucose in the sulfonylurea-desensitized rat. Eur J Pharmacol 640: 250–256, 2010 [DOI] [PubMed] [Google Scholar]
- 65. Owen OE, Reichard GA, Jr, Markus H, Boden G, Mozzoli MA, Shuman CR. Rapid intravenous sodium acetoacetate infusion in man. Metabolic and kinetic responses. J Clin Invest 52: 2606–2616, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pearson ER. Translating TCF7L2: from gene to function. Diabetologia 52: 1227–1230, 2009 [DOI] [PubMed] [Google Scholar]
- 67. Prentki M, Matschinsky FM. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev 67: 1185–1248, 1987 [DOI] [PubMed] [Google Scholar]
- 68. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 37: 1595–1607, 1988 [DOI] [PubMed] [Google Scholar]
- 69. Ridderstrale M, Groop L. Genetic dissection of type 2 diabetes. Mol Cell Endocrinol 297: 10–17, 2009 [DOI] [PubMed] [Google Scholar]
- 70. Riedel AA, Heien H, Wogen J, Plauschinat CA. Loss of glycemic control in patients with type 2 diabetes mellitus who were receiving initial metformin, sulfonylurea, or thiazolidinedione monotherapy. Pharmacotherapy 27: 1102–1110, 2007 [DOI] [PubMed] [Google Scholar]
- 71. Sagen JV, Odili S, Bjørkhaug L, Zelent D, Buettger C, Kwagh J, Stanley C, Dahl-Jørgensen K, de Beaufort C, Bell GI, Han Y, Grimsby J, Taub R, Molven A, Søvik O, Njølstad PR, Matschinsky FM. From clinicogenetic studies of maturity-onset diabetes of the young to unraveling complex mechanisms of glucokinase regulation. Diabetes 55: 1713–1722, 2006 [DOI] [PubMed] [Google Scholar]
- 72. Shiota C, Larsson O, Shelton KD, Shiota M, Efanov AM, Hoy M, Lindner J, Kooptiwut S, Juntti-Berggren L, Gromada J, Berggren PO, Magnuson MA. Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. J Biol Chem 277: 37176–37183, 2002 [DOI] [PubMed] [Google Scholar]
- 73. Smith DO, LeRoith D. Insulin resistance syndrome, pre-diabetes, and the prevention of type 2 diabetes mellitus. Clin Cornerstone 6: 7–6, 2004 [DOI] [PubMed] [Google Scholar]
- 74. Sorenson RL, Stout LE, Brelje TC, Jetton TL, Matschinsky FM. Immunohistochemical evidence for the presence of glucokinase in the gonadotropes and thyrotropes of the anterior pituitary gland of rat and monkey. J Histochem Cytochem 55: 555–566, 2007 [DOI] [PubMed] [Google Scholar]
- 75. Sweet IR, Li G, Najafi H, Berner D, Matschinsky FM. Effect of a glucokinase inhibitor on energy production and insulin release in pancreatic islets. Am J Physiol Endocrinol Metab 271: E606–E625, 1996 [DOI] [PubMed] [Google Scholar]
- 76. Takamoto I, Terauchi Y, Kubota N, Ohsugi M, Ueki K, Kadowaki T. Crucial role of insulin receptor substrate-2 in compensatory beta-cell hyperplasia in response to high fat diet-induced insulin resistance. Diabetes Obes Metab 10, Suppl 4: 147–156, 2008 [DOI] [PubMed] [Google Scholar]
- 77. Wang X, Zhou L, Shao L, Qian L, Fu X, Li G, Luo T, Gu Y, Li F, Li J, Zheng S, Luo M. Troglitazone acutely activates AMP-activated protein kinase and inhibits insulin secretion from beta cells. Life Sci 81: 160–165, 2007 [DOI] [PubMed] [Google Scholar]
- 78. Zawalich WS, Dye ES, Matschinsky FM. Metabolism and insulin-releasing capabilities of glucosamine and N-acetylglucosamine in isolated rat islets. Biochem J 180: 145–152, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]