Abstract
Oviducts respond to hormonal cues from ovaries with tissue proliferation and differentiation in preparation of transporting and fostering gametes. These responses produce oviducal microenvironments conducive to reproductive success. Here we investigated changes in circulating plasma sex steroid hormones concentrations and ovarian and oviducal mRNA expression to an in vivo gonadotropin (FSH) challenge in sexually immature, five-month-old alligators. Further, we investigated differences in these observed responses between alligators hatched from eggs collected at a heavily-polluted (Lake Apopka, FL) and minimally-polluted (Lake Woodruff, FL) site. In oviducts, we measured mRNA expression of estrogen, progesterone, and androgen receptors and also beta A and B subunits which homo- or heterodimerize to produce the transforming growth factor activin. In comparison, minimal inhibin alpha subunit mRNA expression suggests that these oviducts produce a primarily activin-dominated signaling milieu. Ovaries responded to a five-day FSH challenge with increased expression of steroidogenic enzyme mRNA which was concomitant with increased circulating sex steroid hormone concentrations. Oviducts in the FSH-challenged Lake Woodruff alligators increased mRNA expression of progesterone and androgen receptors, proliferating cell nuclear antigen, and the activin signaling antagonist follistatin. In contrast, Lake Apopka alligators displayed a diminished increase in ovarian CYP19A1 aromatase expression and no increase in oviducal AR expression, as compared to those observed in Lake Woodruff alligators. These results demonstrate that five-month-old female alligators display an endocrine-responsive ovarian-oviducal axis and environmental pollution exposure may alter these physiological responses.
Keywords: Alligator, Ovary, Oviduct, Sex Steroid Hormone Receptor, Activin, Follistatin
1. Introduction
The female reproductive tract exhibits alterations in structure and function developmentally as well as with seasonal reproductive activity. Oviducts, used here in the comparative sense meaning structures derived from the embryonic Müllarian duct, respond to endocrine signals through changes in gene expression, protein synthesis, and morphology with sexual maturation and reproductive activity. In adult tetrapods, oviducal microenvironments produced by these changes impact gamete maturation, fertilization, and early embryonic development [27, 28].
Sex steroid hormone receptors in the oviduct receive endocrine signals and regulate growth, differentiation, and protein secretion. Ligand binding of oviducal sex steroid hormone receptors results in a positive feedback that elevates expression levels of sex steroid hormone receptors, priming the tissue to receive further signals in general [15, 40]. Avian oviducts respond to estradiol treatments with proliferation of luminal epithelia and cytodifferentiation of tubular gland cells [4, 33]. Recently, neonatal exposure to the phytoestrogen genistein has been demonstrated to impact ovarian morphology and ovulation in mice [27]. This phytoestrogen treatment also impacted the adult reproductive oviducal environment resulting in decreased fertility. These data are similar to those that have been reported for decades in which perinatal diethylstilbestrol (DES) treatment, a pharmaceutical estrogen, alters the development and functioning of mouse and human reproductive tracts [48].
Estrogens exhibit ‘priming’ of the oviduct for later progesterone responsiveness through increased progesterone receptor (PR) expression levels [39, 59, 61]. Estrogenic treatment also increases androgen receptor (AR) mRNA expression in mouse fallopian tubes [55], suggesting androgen signaling plays a regulatory role in vertebrate oviducts. Further, plasma androgen concentrations are elevated at ovulation in various vertebrate species, such as in alligators and humans [7, 23]. A roll for AR signaling is further supported by the observations that AR-KO mice lack gonadotropin-induced endometrial growth [58] and AR mediated signaling plays an essential role in estradiol-induced proliferation of rat uterine epithelia [69]. Androgens and the AR expression also regulate oviducal function in non-mammalian tetrapods. In leopard gecko, shell gland differentiation positively correlates with circulating androgen level, whereas AR mRNA expression negatively correlates with the degree of shell gland differentiation [53]. In chicken oviducts, estrogen and progesterone induce the transcription of ovoalbumin; co-treatment with androgens enhance this induction [1]. These results support a role for androgen signaling in oviducal regulation across tetrapods.
Juvenile alligator oviducts respond to in vivo exogenous estrogenic and gonadotropin treatments. Treatment of 16-month-old female alligators with super-pharmacological doses of estrone led to pronounced oviducal hypertrophy with tall columnar epithelia surrounding enlarged lumens [14]. Further, treatment with pituitary extract hypertrophied the oviducts of 20-month-old alligators and increased convolutions and columnar epithelial height, but not oviducal size or diameter, in six-month-old animals [13]. Thus, similar to observations in other species [18], immature alligator oviducts are responsive to changes in reproductive signaling.
Estrogen and progesterone receptors have been identified and characterized in alligator oviducts [31, 32, 64] and environmental pollutants, such as DDT (1,1,1-trichloro-2,2-di(4-chlorophenyl)ethane) found in Lake Apopka, Florida, inhibit in vitro sex steroid hormone receptor binding kinetics [63]. Alligators from the polluted Lake Apopka have exhibited low reproductive success in association with elevated endocrine disrupting contaminant exposures for almost 30 years [22, 41, 43]. While the impact of these contaminants on alligator gonadal function has been a focus of considerable research [8, 9, 34, 41, 47], the impact of exposure to these contaminants on oviducal function has received little attention.
In mammals, activin signaling factors, like sex steroid hormones, play a role in regulating oviducal function. Activins and inhibins are transforming growth factor-β superfamily signaling ligands that are assembled through the dimerization of discrete subunits. Activin ligands act as agonists, work through membrane-bound activin receptor complexes, stimulate SMAD-mediated secondary messenger cascades, and ultimately modulate gene expression [11]. Homo- or heterodimerization of β subunits, βA (INHBA) or βB (INHBB), forms activin A (βA-βA), activin B (βB-βB), or activin AB (βA-βB), respectively. Inhibins or follistatin antagonizes activin signaling. Inhibins are heterodimers of a β subunit and an α subunit (INHA) forming either inhibin A (βA-α) or inhibin B (βB-α). Follistatin (FST) is a TGFβ superfamily ligand antagonist that binds and neutralizes secreted activins.
Maternally derived activins have been demonstrated in mammalian oviducts [3, 28] and critically influence pre-implantation embryogenesis [67]. For example, activins regulate developmental competence of in vitro-produced bovine embryos [67, 68]. Activin signaling is necessary for oviducal maintenance, where a loss of activin signaling is associated with endosalpingiosis, the formation of tubal cysts [5]. Activin receptor binding regulates intracellular SMAD signaling and ultimately modulation of gene expression through subsequent SMAD phosphorylation (pSMAD) and nuclear translocation. Mouse oviduct luminal epithelia express phospholylated SMAD proteins (pSMAD 2/3) and, therefore, are targets of activin signaling [60]. To date, the expression of oviducal activin signaling factors has received little-to-no investigation in non-mammalian vertebrates.
We specifically investigated the tube region of five-month-old alligator oviducts, homologous to the magnum in birds, which is anterior to the utero-tubular junction and functions to secrete albumen proteins in reproductive adult animals [6, 50]. We examined the effects of an in vivo FSH challenge on ovarian steroidogenic enzyme mRNA expression levels, circulating sex steroid hormone concentrations, and oviducal sex steroid hormone receptors and activin-inhibin-follistatin signaling factor mRNA expression levels. In previous research, alligator oviducal responses to pituitary extract treatments has been hypothesized to be indirectly mediated via alteration of ovarian sex steroid synthesis by gonadotrophin treatment [13], thus we investigated if alligator oviducts express follicle stimulating hormone receptors (FSHR) and, therefore, could be directly responsive to a gonadotrophin challenge. We measured proliferating cell nuclear antigen (PCNA) mRNA expression levels to infer a proliferative oviducal response to treatment. Finally, we compared basal expression levels and challenge responsiveness observed in alligators hatched from eggs collected from two Florida lakes; a reference site (Lake Woodruff National Wildlife Refuge, FL) to those collected from a polluted environment (Lake Apopka, FL) where alligators have a history of decreased reproductive success and altered reproductive function [43].
2. Materials and Methods
We collected American alligator (Alligator mississippiensis, Daudin, 1801) eggs from nests at Lake Woodruff National Wildlife Refuge and Lake Apopka on June 27 and 28, respectively, 2005 (Permit #WX01310) prior to the period of temperature dependent sex determination [12]. Eggs were candled to assess viability at the University of Florida and eggs from six Lake Woodruff and five Lake Apopka clutches were used for this study. A subset of viable eggs from these clutches were systematically intermixed, placed into trays of damp sphagnum moss, and incubated at a female producing temperature of 30°C. Daily rotation of trays minimized regional temperature effects within incubators.
Animal procedures conformed to an IACUC approved protocol. Hatching animals were web tagged with numbered Monel tags and co-housed in a temperature-controlled animal room in tanks (~20 neonates/0.7 m3), and experienced a 16:8-hour photoperiod with heat lamps for basking and daily water changes. Ambient room temperatures ranged from 27°C to 31°C. Hatching occurred from August 31 through September 15. Hatch order systematically assigned animals to treatment groups within an FSH challenge study administered at approximately five months after hatching (average age = 141 days old; range = 131–153 days old). Challenge study animals received a daily IM injection of either 0.8% sterile saline vehicle (isotonic to alligator blood) or an FSH dose (50 ng/gram BM) to the base of the tail. Injections volumes were ~90 μl and were administered between 11:00 and 12:00 h. Animals either received one injection with necropsy on the following day (Day 2 animals) or one injection per day for four consecutive days with necropsy on the following day (Day 5 animals). Because reptile FSH preparations are not commercially available, we treated with ovine FSH (Sigma-Aldrich #F8174). Previous experimentation has shown robust hormonal and/or gonadal responsiveness to treatment in alligators [9, 37].
Necropsies commenced at 12:00 h on appointed days. Body mass (BM), total length (TL) and snout-vent lengths (SVL) were recorded. No differences in BM, SVL, or TL were observed between alligators within the two and five day FSH challenge comparing treatment group and lake of origin. Overall morphometric means and standard errors of animal body measures were: BM = 321 ± 8.6 g, SVL = 22.8 ± 0.2 cm, and TL = 46.8 ± 0.5 cm. Immediately prior to euthanasia, 1 ml of blood was collected from the supravertebral blood vessel, followed by a lethal dose (0.06 mg/g BM) of sodium pentobarbital (Sigma). Blood collected in a heparinized Vacutainer (BD Diagnostics) was kept on ice until centrifugation at 1,500 g for 20 min at 4°C. Plasma was stored at −80°C until radioimmunoassay (RIA). Plasma E2 and T concentrations were analyzed with a 96-well FlashPlate PLUS system (Perkin Elmer, Shelton, CT) as previously described [25]. From each animal, one ovary and the tube region of the oviduct (from alternating sides) were fixed in RNAlater (Ambion) according to manufacturer’s instructions and then stored at −80°C until RNA extraction. Standard paraffin histology of the contra-lateral ovary confirmed sex.
Our standard total RNA isolation and reverse transcription (RT) procedures have been previously reported in detail [41]. Animals with oviducts yielding less that 20 ng/μl total RNA were excluded from the study. Quantitative real-time PCR (Q-PCR) has been used to measure mRNA expression in American alligator tissues [24, 34, 44, 46, 47]. Table 1 reports Q-PCR primer sequence information, annealing temperatures, and accession numbers. The MyiQ single color detection system (BioRad, Hercules, CA) performed Q-PCR following manufactures protocol using iQ SYBR Green Supermix (BioRad) in triplicate reaction volumes of 15 μl with 0.6 μl of RT product (from a 20ul RT reaction containing 160 ng of total RNA by iScript cDNA synthesis kit, BioRad) and specific primer pairs. Q-PCR expression levels were calculated using gene specific, absolute standard curves, which contain the target cDNA at known concentrations. The use of absolute standard curves allows statistical comparisons of mRNA expression levels of different genes within and among samples. Sample means were normalized using ribosomal protein 18S mRNA expression.
Table 1.
| Gene | Forward Primer (5′ - 3′) | Anneal (°C) | Product (bp) | Accession |
|---|---|---|---|---|
| Reverse Primer (5′ - 3′) | ||||
| CYP11A1 | TCTGGAGTCAGTGTGCCATGTC | 60.0 | 101 | DQ007995 |
| TCATGCTCACCGCATCGAT | ||||
| CYP17A1 | CCAGAAAAAGTTCACCGAGCAC | 60.0 | 108 | DQ007997 |
| CGGCTGTTGTTGTTCTCCATG | ||||
| CYP19A1 | CAGCCAGTTGTGGACTTGATCA | 63.8 | 79 | AY029233 |
| TTGTCCCCTTTTTCACAGGATAG | ||||
| ESR1 | AAGCTGCCCCTTCAACTTTTTA | 66.5 | 72 | AB115909 |
| TGGACATCCTCTCCCTGCC | ||||
| ESR2 | AAGACCAGGCGCAAAAGCT | 66.0 | 72 | AB115910 |
| GCCACATTTCATCATTCCCAC | ||||
| AR | TGTGTTCAGGCCATGACAACA | 67.5 | 103 | AB186356 |
| GCCCATTTCACCACATGCA | ||||
| PR | AAATCCGTAGGAAGAACTGTCCAG | 67.5 | 71 | AB186354 |
| GACCTCCAAGGACCATTCCA | ||||
| FSHR | GAAATTACCAAACGAGGTTTTTCAA | 60.0 | 81 | DQ010157 |
| GGGCAGGAAACTGATTCTTGTC | ||||
| INHBA | ACCCACAGGTTACCGTGCTAA | 63.8 | 67 | DQ010152 |
| GCCAGAGGTGCCCGCTATA | ||||
| INHBB | GGGTCAGCTTCCTCCTTTCAC | 64.7 | 70 | DQ010153 |
| CGGTGCCCGGGTTCA | ||||
| INHA | ACAATCCACTTGTCCCAGCC | 70.0 | 68 | DQ010151 |
| CAACTGCCACCGCGC | ||||
| FST | CGAGTGTGCCCTCCTCAAA | 66.5 | 65 | DQ010156 |
| TGCCCTGATACTGGACTTCAAGT | ||||
| PCNA | AGCAGAAGACAATGCAGACAC | 62.0 | 199 | FJ824113 |
| TGCATCTCCAATATGGCTTAG |
JMP for windows version 7.0.2 (SAS Institute, Cary, NC) performed all statistical analyses. Morphometric data were log transformed and gene expression ratios were arcsin transformed to achieve homogeneous variances, as needed. Matched pairs t-tests compared gene expression levels within vehicle treatment groups to establish baseline dimorphic expressions. Two-way ANOVA followed by least square means Tukey-Kramer post-tests, when appropriate, compared body measurements, steroid hormone concentrations, and mRNA expression levels between control and FSH treatment groups (ANOVA factors: treatment and lake of origin) for animals in the two-day old and five-day old FSH challenge studies. Linear regression analysis was performed between selected mRNA expressions. Significance was set at P < 0.05.
3. Results
3.1. Circulating Sex Steroid Hormone Concentrations
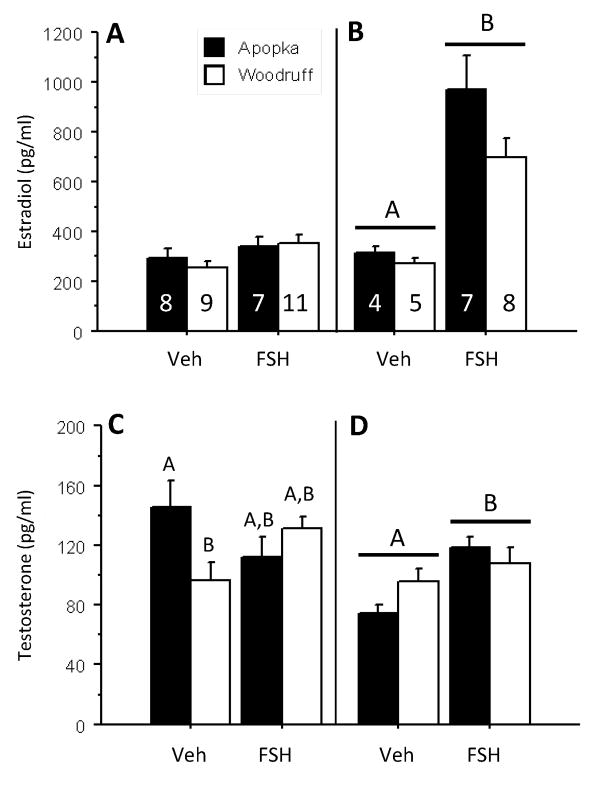
The two-day FSH challenge did not alter circulating estradiol-17β (E2) concentrations (Fig. 1A), or circulating testosterone (T) concentrations (Fig. 1C). Testosterone concentrations of Lake Apopka vehicle-treated animals were greater than those of vehicle-treated Lake Woodruff animals, but levels in two-day FSH challenged animals, from both lakes, did not differ from vehicle control levels (Fig. 1C).
Fig. 1.
Circulating plasma concentrations of 17β-estradiol (A,B) and testosterone (C,D) in five-month-old female alligators following FSH challenges (50 ng/gram BM daily) of two (A,C) and five (B,D) days duration: vehicle control treatments (Veh) or follicle stimulating hormone treatments (FSH). Bars report mean ± SEM and shading denotes animals hatched from eggs collected from: closed bars = Lake Apopka and open bars = Lake Woodruff. Horizontal lines over bars denote indicate statistical significance by treatment. Differing letter above bars indicate lake of origin by treatment statistical significances.
The five-day FSH challenge increased both circulating E2 concentrations (Fig. 1B; p < 0.001) and T concentrations (Fig. 1D; p = 0.041) with no differences by lake of origin. However, mean circulating E2 concentrations increased 2.7 times, compared to only 1.3 times for T concentrations.
3.2. Ovarian Steroidogenic Enzyme mRNA Expression Levels
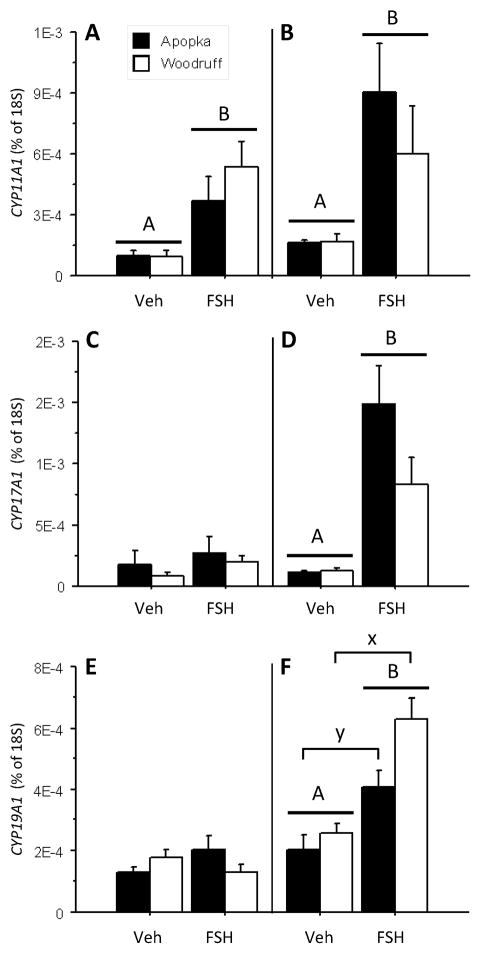
The two-day FSH challenge increased ovarian CYP11A1 mRNA expression (Fig. 2A; treatment effect p = 0.001), however it did not alter ovarian CYP17A1 (Fig. 2C) and CYP19A1 aromatase mRNA expression levels (Fig. 2E). Ovarian 18S mRNA expression levels were not different between treatment groups (data not shown).
Fig. 2.
Ovarian mRNA expression levels of steroidogenic enzymes CYP11A1 (A,B), CYP17A1 (C,D), and CYP19A1 (E,F) in five-month-old female alligators following FSH challenges (50 ng/gram BM daily) of two (A,C,E) and five (B,D,F) days duration: vehicle control treatments (Veh) or follicle stimulating hormone treatments (FSH). Bars report mean ± SEM and shading denotes animals hatched from eggs collected from: closed bars = Lake Apopka and open bars = Lake Woodruff. All mRNA expression sample means are normalized using 18S ribosomal expression levels. Horizontal lines with capitol letters over bars denote indicate statistical significance by treatment. Brackets with lower case letters above bars indicate lake of origin statistical significances.
The five-day FSH challenge significantly increased ovarian CYP11A1, CYP17A1, and CYP19A1 mRNA expression along with circulating E2 and T concentrations. The FSH challenge resulted in a treatment effect which increased ovarian CYP11A1 side chain cleavage (Fig. 2B; p = 0.014) and CYP17A1 17-alpha-hydroxylase (Fig. 2D; p < 0.001) mRNA expression levels. Further, the challenge resulted in a treatment and a lake of origin effect for ovarian CYP19A1 aromatase mRNA levels, with FSH treatment increasing expression (Fig. 2F; p < 0.001) so that Lake Woodruff animals exhibited greater expression levels than females from Lake Apopka (p = 0.025). Ovarian 18S mRNA expression levels were not different (data not shown).
3.3. Oviducal mRNA expression levels
The two-day FSH challenge did not alter expression levels of any oviducal mRNA examined (data not shown) and 18S mRNA expression levels showed no differences.
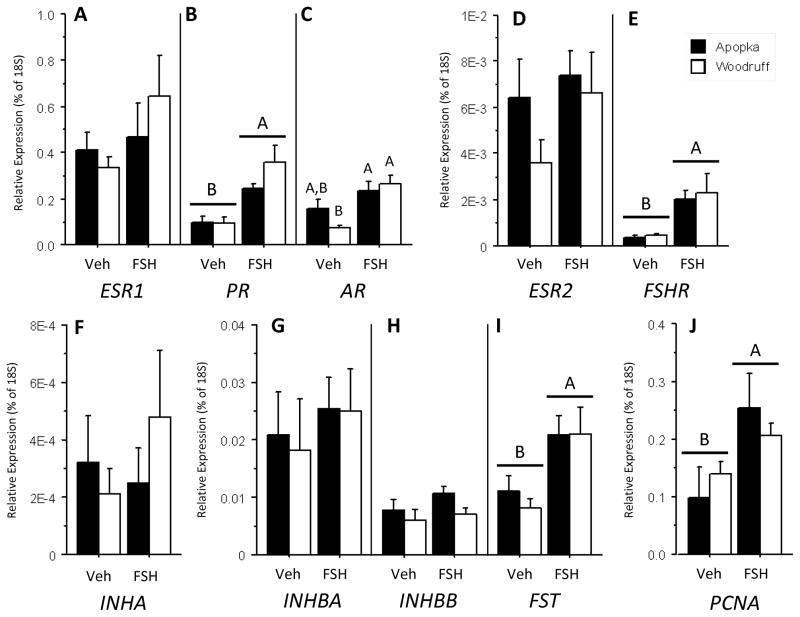
In the five-day FSH challenge, expression levels of ESR1 in vehicle treated oviducts (Fig. 3A) were greater than PR and AR expression levels (p < 0.001 for both, Figs. 3B and 3C respectively) and two orders of magnitude greater than those of ESR2 (Fig. 3D). We measured low levels of follicle stimulating hormone receptor (FSHR) in vehicle treated oviducts (Fig. 3E), as compared to sex steroid hormone receptor expression levels. Comparing activin-inhibin subunit mRNA expression levels in alligator oviducts, INHA mRNA levels (Fig. 2F) were two orders of magnitude less that INHBA and INHBB levels (Fig 3. G,H, respectively). Further, vehicle treated oviducts expressed greater levels of INHBA than INHBB (p = 0.046)
Fig. 3.
Oviducal mRNA expression levels of ESR1 (A), PR (B), AR (C), ESR2 (D), FSHR (E), INHA (F), INHBA (G), INHBB (H), FST (I), and PCNA (J) in five-month-old female alligators following FSH challenges (50 ng/gram BM daily) of five days duration: vehicle control treatments (Veh) or follicle stimulating hormone treatments (FSH). Bars report mean ± SEM and shading denotes animals hatched from eggs collected from: closed bars = Lake Apopka and open bars = Lake Woodruff. All mRNA expression sample means are normalized using 18S ribosomal expression levels. Horizontal lines with capitol letters over bars denote indicate statistical significance by treatment. Differing letter above bars indicate lake of origin by treatment statistical significances.
In contrast to the two-day challenge, the five-day FSH challenge resulted in some increased mRNA expression levels. Lake Woodruff animals exhibited increases in PR mRNA (treatment effect; p < 0.001) and AR mRNA expression levels (treatment by lake effect; p = 0.044), while only PR mRNA levels increased in Lake Apopka animals (Figs. 3B,C, respectively). Oviducal expression levels of PR and AR mRNA from challenged Lake Woodruff animals were linearly correlated (R2 = 0.53, p = 0.039), unlike those from Lake Apopka alligators (R2 = 0.09, p = 0.52). FSHR expression levels increased with FSH treatment (treatment effect; p < 0.001). No changes were observed in expression levels of activin-inhibin subunits with FSH treatment as compared to vehicle treated levels (Figs. 3F-H); though FSH stimulated INHBA mRNA expression levels were still greater than INHBB (p < 0.001). However, FST expression levels (Fig. 3I) increased in challenged animals (treatment effect; p = 0.004). We observed increased PCNA mRNA expression levels (Fig. 3J) with FSH challenge (treatment effect; p = 0.001).
4. Discussion
4.1. Basal expression levels and FSH challenge responses
Alligator ovaries shows dynamic morphological development and concomitant changes in mRNA expression levels during the first months post-hatching [44, 47]. Under laboratory grow out conditions, we have observed formation of ovarian follicles with enlarged, diplotene-arrested oocytes and a complement of granulosa within a complete basement membrane and associated thin thecal compartments between three- and five-months post-hatching. Here we demonstrate that during this developmental period, alligator reproductive tracts are responsive to an in vivo gonadotrophin stimulation. This would be induced by way of increased expression of steroidogenic enzymes, elevated circulating sex steroid hormone concentrations, and alteration of oviducal mRNA expression levels of sex steroid hormone receptors, activin signaling factors, and cell proliferation markers. Gonads of immature animals are often described as being quiescent (defined as inactive, quiet, at rest) and are assumed unresponsive until the initiation of brain-pituitary-gonad axis maturation at puberty. We have shown that the post-hatching alligator ovary is developmentally dynamic. Further, it is “listening” for endocrine signals and the reproductive tract, both gonad and oviduct, is competent to respond to received stimulation.
CYP11A1 side chain cleavage is the first enzyme of the steroidogenic cascade and synthesizes pregnenolone, the precursor to all other steroid hormones, from cholesterol substrate. In immature chicken ovaries, exogenous FSH induces steroidogenesis by increasing CYP11A1 mRNA expression and subsequent progesterone synthesis [26]. In five-month-old alligator ovaries, we observed increased CYP11A1 mRNA expression with a single FSH injection. This treatment, however, did not alter circulating E2 or T concentrations.
In the five-day FSH challenge, we observed treatment induced increases in CYP17A1 and CYP19A1 mRNA expression levels, the steroidogenic enzymes responsible for androgen synthesis and the aromatization of androgens to estrogens, respectively. Concomitantly, E2 and T concentrations increased. In light of a greater increase in E2 concentrations compared to T in FSH challenged animals, increased androgen synthesis related to increased CYP17A1 mRNA expression most likely resulted in increased aromatization of resulting androgens into estrogens, and thus only the observed minor elevation of T concentrations.
We observed dimorphic mRNA expression levels of oviducal sex steroid hormone receptors in oviducts of saline treated alligator in which ESR1 >PR/AR > ESR2. These results are parsimonious with observations in other vertebrates. Greater mRNA expression levels of ESR1 than ESR2 has been measured in rat [49, 65] and bovine oviducts [62]. This dimorphism in rat oviducts is in agreement with localization of expression; ESR1 has been detected in all cell types in rat and mouse oviducts, whereas ESR2 expression appears to be restricted to the epithelial cell compartment [49, 54]. Further, expression levels of PR and AR mRNA levels in rat oviducts were intermediate to greater ESR1 and lower ESR2 mRNA levels [49]. With FSH treatment, we observed no change in ESR1 or ESR2 but increased expression of PR mRNA in oviducts of all animals and increased AR mRNA in oviducts of Lake Woodruff animals. In light of these results, we posit that ESR1 signaling plays a greater signaling role in alligator oviducts than ESR2 and also PR and AR regulate oviducal physiology.
In ovo FSH treatment results in oviducal responsiveness in chickens. Treated embryos display enlarged oviducal size, increased maturation state, increased mitotic index of epithelial cells, and increased PR immunoreactivity [18, 19]. This responsiveness was hypothesized to be an indirect effect of FSH stimulation by way of elevated ovarian sex steroid hormone production. However, studies have reported FSHR expression in human fallopian tubes and these tissues could be a direct target of gonadotropin signaling, with endometrial expression fluctuating with menstrual cycle [36, 70]. Further, FSHR mRNA is present in the bovine cervix (homologous to the alligator uterotubular junction) and these levels positively correlate with circulating FSH concentrations [56, 57]. Therefore, we cannot rule out a direct effect of FSH treatment on alligator oviduct physiology. We measured FSHR expression in alligator oviducts and these levels were increased with FSH treatment. These results suggest a direct gonadotropin signaling effect on the oviduct. However, in light of low expression levels (on par with those of ESR2 mRNA) this signaling pathway may not be a dominant factor in oviducal regulation when compared to sex steroid hormone receptors.
We are the first to report mRNA expression of activin-related signaling factors in a non-mammalian oviduct. Expression levels of INHBA, INHBB, and INHA were dimorphic and similar to expression levels observed in the reproductive tracts of mammals, including humans [3, 16, 28, 52]. In mouse oviduct, INHBA and INHBB are abundantly expressed when compared to weak INHA expression [28]. Therefore, the elevated expression of INHBA and INHBB subunits in alligator oviduct supports a hypothesis that an activin-enriched (i.e. predominantly beta subunit expression) oviducal milieu could be common across vertebrates.
Like activins, FST is believed to play a beneficial role in oocyte competence and early embryogenesis [38]. Follistatin is expressed in human endometrial cells and this expression fluctuates with reproductive state [29, 30]. It is hypothesized that epithelial-derived FST regulates the levels of bioavailable activin in the uterine lumen. Our demonstration of FST mRNA expression level responsiveness to FSH challenge suggests the presence of an activin-follistatin regulatory system in alligator oviducts. Further, this regulation is present and competent in the oviducts of a five-month old alligator.
To infer a proliferative oviducal response to the FSH challenge, we measured mRNA expression levels of PCNA, a sliding ring clamp that interacts with DNA polymerase during DNA replication and expression is maximal around the S phase of the cell cycle [35]. We measured increased expression with FSH challenge. This elevation of mitotic activity is most likely a result of oviducal stimulation by way of elevated circulating sex steroid hormones. However, an alternative hypothesis of a direct influence of FSH on oviducal proliferation exists. In cultured rat granulosa cells, FSH stimulation in the presence of activin A results in a synergistic increase in DNA synthesis, marked by increased PCNA mRNA and protein [10]. We have observed the expression of FSHR and INHBA in alligator oviduct and, therefore, a putative pathway to FSH induced elevation of PCNA expression.
4.2. Environmental contaminant exposure
Alligators used in this study were hatched from eggs collected from two Florida lakes; a reference site (Lake Woodruff National Wildlife Refuge) and a polluted environment (Lake Apopka). Lake Apopka alligators have a history of decreased reproductive success and altered reproductive function [43] concomitant with persistently high body burden levels of organochloride pesticide contamination, including DDT and deriviatives such as p,p′-DDE (1,1-dichloro-2,2-bis ethylene) [17]. The DDT metabolite p-p′-DDE can modulate aromatase gene expression [2], possesses anti-androgenic activity through competatatively binding the androgen receptor without activiating AR-dependent pathways [20, 21], and has been linked to the reproductive and endocrine alterations in alligators [22, 42]. Organochloride pesticide contaminants are conveyed in alligators from mother to eggs and result in poor clutch viability [51].
When offspring from these two lakes are compared, we observed a lake of origin difference in ovarian CYP19A1 mRNA expression and a lake by treatment difference in oviducal AR mRNA expression levels. In previous studies from our laboratory, we observed no differences in basal ovarian CYP19A mRNA expression levels between juveniles from Lakes Woodruff and Apopka [34, 41, 47]. However, here we demonstrated a lake of origin, ovarian aromatase expression difference in FSH-challenged alligators with Lake Apopka alligators expressing lower levels of aromatase mRNA when compared to Lake Woodruff alligators[46]. Alligator ovaries undergo substantial morphological alteration during the months post-hatching, including follicle formation and granulosa and thecal cell development [44, 45]. We hypothesize that during this period of dynamic ovarian development, an FSH challenge, as performed in this study, reveals physiological differences in endocrine responsively between Lake Woodruff and Lake Apopka female alligators not readily observed when basal expression levels are examined alone. In support of this hypothesis, we have recently observed a lack of response, to an FSH challenge, in ovarian CYP19A1 mRNA expression levels in five-day-old alligator from Lake Apopka [46].
As with aromatase expression, FSH challenge did not increase oviducal AR mRNA expression in five-month-old Lake Apopka animals, as compared to Lake Woodruff females, but the pattern for this gene was different. That is, oviducal tissues from Lake Apopka females exhibited higher expression levels of AR mRNA prior to treatment and when stimulated showed a slight increase in expression. In contrast, the oviducal tissues obtained from Lake Woodruff females had low levels of AR mRNA and showed a significant response to FSH challenge with increased expression. We hypothesize, that this difference in Lake Apopka alligator oviducal AR mRNA basal levels and responsiveness could be the result of increased AR signaling during development. AR signaling during oviduct development is important, as demonstrated in the mouse, and that exposure in ovo and post-hatching to maternally-contributed, anti-androgenic contaminants, such as p-p′-DDE in yolk could influence signaling. That is, even though p,p′-DDE has been demonstrated to be anti-androgenic – an antagonist of the receptor - in many species, it also has shown some affinity for the ER and has been shown to influence sex determination in an estrogenic fashion in at least one study of alligators and a freshwater turtle [66]. Given the presumed background of low androgens during the development of a female, we hypothesize that any altered androgenic or estrogenic signaling during development could alter future responsiveness to stimulation. In light of the growing understanding of the role androgen signaling plays in oviducal regulation, this physiological difference could forecast pathologies in the reproductive competence of mature Lake Apopka alligators that need to be examined in the future.
5. Conclusions
In this study, we have shown that five-month-old female alligators display a responsive reproductive ovarian-oviducal axis. In vivo gonadotropin treatments increased circulating estradiol concentrations, ovarian steroidogenic enzyme mRNA expression levels, and oviducal mRNA expression levels of sex steroid hormone receptors, activin-binding proteins, and mitotic factors. Taken together, these results show that the reproductive tracts of female alligators during this period of dynamic development are competent to receive and respond to endogenous endocrine signals. Additionally, we have shown differences in some observed physiological responses between alligators collected from areas of differing levels of pesticide contamination highlighting the potential impact of variable environmental quality on alligator sexual development and function.
Highlights.
Five-month-old female alligators display an endocrine-responsive ovarian-oviducal axis.
Relative oviducal mRNA expression levels of sex steroid hormone receptors and activin signaling factors are similar to those observed in other vertebrates.
In vivo FSH treatments increased circulating estradiol and testosterone and some ovarian and oviducal mRNA expressions.
Differing levels of pesticide contamination exposure correlated to differences in CYP19A1 and AR mRNA expression levels.
Acknowledgments
We thank Allan Woodward and other colleagues from the Florida FWC, for continuing assistance with fieldwork and permitting. This work was supported in part by grant funding from the NIEHS (R21 ES014053-01) and HHMI Professor’s Program, to LJG.
Abbreviations
- AR
androgen receptor
- CYP11A1
cytochrome P450, family 11, subfamily A, polypeptide 1 (side chain cleavage)
- CYP17A1
cytochrome P450, family 17, subfamily A, polypeptide 1 (17-alpha-hydroxylase)
- CYP19A1
cytochrome P450, family 19, subfamily A, polypeptide 1 (aromatase)
- DDT
1,1,1-trichloro-2,2-di(4-chlorophenyl)ethane
- DES
diethylstilbestrol
- ESR1
estrogen receptor 1 or alpha
- ESR2
estrogen receptor 2 or beta
- E2
estradiol-17β
- FSHR
follicle stimulating hormone receptor
- FST
follistatin
- INHA
inhibin alpha subunit
- INHBA
inhibin beta A subunit
- INHBB
inhibin beta B subunit
- PCNA
proliferating cell nuclear antigen
- p,p′-DDE
1,1-dichloro-2,2-bis ethylene
- PR
progesterone receptor
- SVL
snout-vent length
- T
testosterone
- TL
total length
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arao Y, Miyatake N, Yamamoto E, Usiku H, Matsuura A, Ninomiya Y, Masushige S, Hasegawa T, Kato S. Steroid Hormones Differentially Induce Transcription of the Chicken Ovalbumin Gene, but Stabilize the mRNA with the Same Half-Life. Journal of Biochemistry. 1996;120:710–715. doi: 10.1093/oxfordjournals.jbchem.a021469. [DOI] [PubMed] [Google Scholar]
- 2.Arukwe A. Organ-specific patterns of P450arom gene isoforms are modulated by p,p′-DDE in adult male European common frog, Rana temporaria. Mar Environ Res. 2006;62:S215–S218. doi: 10.1016/j.marenvres.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 3.Bahathiq AO, Stewart RL, Wells M, Moore HD, Pacey AA, Ledger WL. Production of activins by the human endosalpinx. Journal of Clinical Endocrinology & Metabolism. 2002;87:5283–5289. doi: 10.1210/jc.2001-011884. [DOI] [PubMed] [Google Scholar]
- 4.Berg C, Holm L, Brandt I, Brunstrom B. Anatomical and histological changes in the oviducts of Japanese quail, Coturnix japonica, after embryonic exposure to ethynyloestradiol. Reproduction. 2001;121:155–165. doi: 10.1530/rep.0.1210155. [DOI] [PubMed] [Google Scholar]
- 5.Bristol-Gould SK, Hutten CG, Sturgis C, Kilen SM, Mayo KE, Woodruff TK. The development of a mouse model of ovarian endosalpingiosis. Endocrinology. 2005;146:5228–5236. doi: 10.1210/en.2005-0697. [DOI] [PubMed] [Google Scholar]
- 6.Buhi WC, Alvarez IM, Binelli M, Walworth ES, Guillette LJ. Identification and characterization of proteins synthesized de novo and secreted by the reproductive tract of the American alligator, Alligator mississippiensis. Journal of Reproduction and Fertility. 1999;115:201–213. doi: 10.1530/jrf.0.1150201. [DOI] [PubMed] [Google Scholar]
- 7.Castracane VD, Stewart DR, Gimpel T, Overstreet JW, Lasley BL. Maternal serum androgens in human pregnancy: early increases within the cycle of conception. Human Reproduction. 1998;13:460–464. doi: 10.1093/humrep/13.2.460. [DOI] [PubMed] [Google Scholar]
- 8.Crain DA, Spiteri ID, Guillette LJ. The functional and structural observations of the neonatal reproductive system of alligators exposed in ovo to atrazine, 2,4-D, or estradiol. Toxicology and Industrial Health. 1999;15:180–185. doi: 10.1191/074823399678846565. [DOI] [PubMed] [Google Scholar]
- 9.Edwards TM, Gunderson MP, Milnes MR, Guillette LJ. Gonadotropin-induced testosterone response in peripubertal male alligators. General and Comparative Endocrinology. 2004;135:372–380. doi: 10.1016/j.ygcen.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 10.El-Hefnawy T, Zeleznik AJ. Synergism between FSH and activin in the regulation of proliferating cell nuclear antigen (PCNA) and cyclin D2 expression in rat granulosa cells. Endocrinology. 2001;142:4357–4362. doi: 10.1210/endo.142.10.8438. [DOI] [PubMed] [Google Scholar]
- 11.Ethier JF, Findlay JK. Roles of activin and its signal transduction mechanisms in reproductive tissues. Reproduction. 2001;121:667–675. doi: 10.1530/rep.0.1210667. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson MWJ, Joanen T. Temperature-dependent sex determination in Alligator mississippiensis. Journal of Zoology. 1983;200:143–177. [Google Scholar]
- 13.Forbes TR. Studies on the reproductive system of the alligator I. The effects of prolonged injections of pituitary whole gland extract in the immature alligator. Anatomical Record. 1937;70:113–137. [Google Scholar]
- 14.Forbes TR. Studies on the reproductive system of the alligator III. The action of testosteroine on the accessory sex structures of recently hatched female alligators. Anatomical Record. 1938;72:87–95. [Google Scholar]
- 15.Freifeld M, Feil P, Bardin C. The in vivo regulation of the progesterone “receptor” in guinea pig uterus: dependence on estrogen and progesterone. Steroids. 1974;23:93–103. doi: 10.1016/0039-128x(74)90143-3. [DOI] [PubMed] [Google Scholar]
- 16.Gandolfi F, Modina S, Brevini TAL, Passoni L, Artini P, Petraglia F, Lauria A. Activin beta(A) subunit is expressed in bovine oviduct. Molecular Reproduction and Development. 1995;40:286–291. doi: 10.1002/mrd.1080400304. [DOI] [PubMed] [Google Scholar]
- 17.Garrison AW, Guillette LJ, Wiese TE, Avants JK. Persistent organochlorine pesticides and their metabolites in alligator livers from Lakes Apopka and Woodruff, Florida, USA. Int J Environ Anal Chem. 2010;90:159–170. [Google Scholar]
- 18.Gonzalez-Moran G. Effect of follicle-stimulating hormone on different cell sub-populations in the ovary of newly hatched chicks treated during embryonic development. British Poultry Science. 1998;39:128–132. doi: 10.1080/00071669889501. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Moran G, Camacho-Arroyo I. Changes in the presence of progesterone receptor isoforms in the oviduct magnum of newly-hatched chicks after gonadotropins treatment. Life Sciences. 2003;73:871–882. doi: 10.1016/s0024-3205(03)00353-9. [DOI] [PubMed] [Google Scholar]
- 20.Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Veeramachaneni DN, Wilson V, Price M, Hotchkiss A, Orlando E, Guillette L. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update. 2001;7:248–264. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- 21.Gray LE, Wolf C, Lambright C, Mann P, Price M, Cooper RL, Ostby J. Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p,p ′-DDE, and ketoconazole) and toxic substances (dibutyl- and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicology and Industrial Health. 1999;15:94–118. doi: 10.1177/074823379901500109. [DOI] [PubMed] [Google Scholar]
- 22.Guillette LJ, Crain DA, Gunderson MP, Kools SAE, Milnes MR, Orlando EF, Rooney AA, Woodward AR. Alligators and endocrine disrupting contaminants: A current perspective. American Zoologist. 2000;40:438–452. [Google Scholar]
- 23.Guillette LJ, Woodward AR, Crain DA, Masson GR, Palmer BD, Cox MC, Qui YX, Orlando EF. The reproductive cycle of the female American alligator (Alligator mississippiensis) General and Comparative Endocrinology. 1997;108:87–101. doi: 10.1006/gcen.1997.6953. [DOI] [PubMed] [Google Scholar]
- 24.Gunderson MP, Kohno S, Blumberg B, Iguchi T, Guillette LJ. Up-regulation of the alligator CYP3A77 gene by toxaphene and dexamethasone and its short term effect on plasma testosterone concentrations. Aquatic Toxicology. 2006;78:272–283. doi: 10.1016/j.aquatox.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Hamlin HJ, Lowers RH, Albergotti LC, McCoy MW, Mutz J, Guillette LJ. Environmental Influence on Yolk Steroids in American Alligators (Alligator mississippiensis) Biology of Reproduction. 2010;83:736–741. doi: 10.1095/biolreprod.110.085142. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez AG, Bahr JM. Role of FSH and epidermal growth factor (EGF) in the initiation of steroidogenesis in granulosa cells associated with follicular selection in chicken ovaries. Reproduction. 2003;125:683–691. [PubMed] [Google Scholar]
- 27.Jefferson WN, Padilla-Banks E, Goulding EH, Lao SPC, Newbold RR, Williams CJ. Neonatal exposure to genistein disrupts ability of female mouse reproductive tract to support preimplantation embryo development and implantation. Biology of Reproduction. 2009;80:425–431. doi: 10.1095/biolreprod.108.073171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones RL, Kaitu’u-Lino TJ, Nie G, Sanchez-Partida LG, Findlay JK, Salamonsen LA. Complex expression patterns support potential roles for maternally derived activins in the establishment of pregnancy in mouse. Reproduction. 2006;132:799–810. doi: 10.1530/REP-06-0034. [DOI] [PubMed] [Google Scholar]
- 29.Jones RL, Salamonsen LA, Findlay JK. Potential roles for endometrial inhibins, activins and follistatin during human embryo implantation and early pregnancy. Trends in Endocrinology and Metabolism. 2002;13:144–150. doi: 10.1016/s1043-2760(01)00559-8. [DOI] [PubMed] [Google Scholar]
- 30.Jones RL, Salamonsen LA, Zhao YC, Ethier JF, Drummond AE, Findlay JK. Expression of activin receptors, follistatin and betaglycan by human endometrial stromal cells; consistent with a role for activins during decidualization. Molecular Human Reproduction. 2002;8:363–374. doi: 10.1093/molehr/8.4.363. [DOI] [PubMed] [Google Scholar]
- 31.Katsu Y, Bermudez DS, Braun EL, Helbing C, Miyagawa S, Gunderson MP, Kohno S, Bryan TA, Guillette LJ, Iguchi T. Molecular cloning of the estrogen and progesterone receptors of the American alligator. General and Comparative Endocrinology. 2004;136:122–133. doi: 10.1016/j.ygcen.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Katsu Y, Matsubara K, Kohno S, Matsuda Y, Toriba M, Oka K, Guillette LJ, Jr, Ohta Y, Iguchi T. Molecular cloning, characterization, and chromosome mapping of reptilian estrogen receptors. Endocrinology. 2010;151:5710–5720. doi: 10.1210/en.2010-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohler P, Grimley P, O’Malley B. Estrogen-induced cytodifferentiation of the ovalbumin-secreting glands of the chick oviduct. J Cell Biol. 1969;40:8–27. doi: 10.1083/jcb.40.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohno S, Bermudez DS, Katsu Y, Iguchi T, Guillette LJ. Gene expression patterns in juvenile American alligators (Alligator mississippiensis) exposed to environmental contaminants. Aquatic Toxicology. 2008;88:95–101. doi: 10.1016/j.aquatox.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Krishna TSR, Kong XP, Gary S, Burgers PM, Kuriyan J. Crystal-structure of the eukaryotic DNA-polymerase processivity factor PCNA. Cell. 1994;79:1233–1243. doi: 10.1016/0092-8674(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 36.La Marca A, Artenisio AC, Stabile G, Rivasi F, Volpe A. Evidence for cycle-dependent expression of follicle-stimulating hormone receptor in human endometrium. Gynecological Endocrinology. 2005;21:303–306. doi: 10.1080/09513590500402756. [DOI] [PubMed] [Google Scholar]
- 37.Lance VA, Vliet KA. Effects of mammalian gonadotropins on testosterone secrestion in male alligators. Journal Of Experimental Zoology. 1987;241:91–94. doi: 10.1002/jez.1402410111. [DOI] [PubMed] [Google Scholar]
- 38.Lee KB, Bettegowda A, Wee G, Ireland JJ, Smith GW. Molecular determinants of oocyte competence: potential functional role for maternal (oocyte-Derived) follistatin in promoting bovine early embryogenesis. Endocrinology. 2009;150:2463–2471. doi: 10.1210/en.2008-1574. [DOI] [PubMed] [Google Scholar]
- 39.Mester J, Baulieu EE. Progesterone receptors in chick oviduct - determination of total concentration of binding-sites in cytosol and nuclear fraction and effect of progesterone on their distribution. European Journal of Biochemistry. 1977;72:405–414. doi: 10.1111/j.1432-1033.1977.tb11265.x. [DOI] [PubMed] [Google Scholar]
- 40.Milgrom E, Thi L, Atger M, Baulieu E. Mechanisms regulating the concentration and the conformation of progesterone receptor(s) in the uterus. J Biol Chem. 1973;248:6366–6374. [PubMed] [Google Scholar]
- 41.Milnes MR, Bryan TA, Katsu Y, Kohno S, Moore BC, Iguchi T, Guillette LJ. Increased posthatching mortality and loss of sexually dimorphic gene expression in alligators (Alligator mississippiensis) from a contaminated environment. Biology of Reproduction. 2008;78:932–938. doi: 10.1095/biolreprod.107.064915. [DOI] [PubMed] [Google Scholar]
- 42.Milnes MR, Bryan TA, Medina JG, Gunderson MP, Guillette LJ., Jr Developmental alterations as a result of in ovo exposure to the pesticide metabolite p,p′-DDE in Alligator mississippiensis, General and Comparative Endocrinology. 2005;144:257–263. doi: 10.1016/j.ygcen.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Milnes MR, Guillette LJ. Alligator Tales: New lessons about environmental contaminants from a sentinel species. Bioscience. 2008;58:1027–1036. [Google Scholar]
- 44.Moore BC, Hamlin HJ, Botteri NL, Guillette LJ. Gonadal mRNA expression levels of TGFβ signaling factors correspond with post hatching morphological development in American alligators. Sexual Development. 2010;4 doi: 10.1159/000277934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore BC, Hamlin HJ, Botteri NL, Lawler AN, Mathavan KK, Guillette LJ. Posthatching development of Alligator mississippiensis ovary and testis. Journal of Morphology. 2010;271:580–595. doi: 10.1002/jmor.10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore BC, Kohno S, Cook RW, Alvers AL, Hamlin HJ, Woodruff TK, Guillette LJ. Altered sex formone concentrations and gonadal mRNA expression levels of activin signaling factors in hatchling alligators from a contaminated Florida lake. Journal of Experimental Zoology Part a-Ecological Genetics and Physiology. 2010;313A:218–230. doi: 10.1002/jez.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore BC, Milnes MR, Kohno S, Katsu Y, Iguchi T, Guillette LJ. Influences of sex,iIncubation temperature, and environmental quality on gonadal estrogen and androgen receptor messenger RNA expression in juvenile American alligators (Alligator mississippiensis) Biology of Reproduction. 2010;82:194–201. doi: 10.1095/biolreprod.109.077305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newbold RR. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicology and Applied Pharmacology. 2004;199:142–150. doi: 10.1016/j.taap.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 49.Okada A, Ohta Y, Inoue S, Hiroi H, Muramatsu M, Iguchi T. Expression of estrogen, progesterone and androgen receptors in the oviduct of developing, cycling and pre-implantation rats. Journal of Molecular Endocrinology. 2003;30:301–315. doi: 10.1677/jme.0.0300301. [DOI] [PubMed] [Google Scholar]
- 50.Palmer BD, Guillette LJ. Alligators provide evidence for the evolution of an archosaurian mode of ovipary. Biology of Reproduction. 1992;46:39–47. doi: 10.1095/biolreprod46.1.39. [DOI] [PubMed] [Google Scholar]
- 51.Rauschenberger RH, Wiebe JJ, Sepulveda MS, Scarborough JE, Gross TS. Parental exposure to pesticides and poor clutch viability in American alligators. Environ Sci Technol. 2007;41:5559–5563. doi: 10.1021/es0628194. [DOI] [PubMed] [Google Scholar]
- 52.Refaat BA, Bahathiq AO, Sockanathan S, Stewart RL, Wells M, Ledger WL. Production and localization of activins and activin type IIA and IIB receptors by the human endosalpinx. Reproduction. 2004;128:249–255. doi: 10.1530/rep.1.00156. [DOI] [PubMed] [Google Scholar]
- 53.Rhen T, Sakata JT, Woolley S, Porter R, Crews D. Changes in androgen receptor mRNA expression in the forebrain and oviduct during the reproductive cycle of female leopard geckos, Eublepharis macularius. General and Comparative Endocrinology. 2003;132:133–141. doi: 10.1016/s0016-6480(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 54.Shao R, Egecioglu E, Weijdegard B, Kopchick JJ, Fernandez-Rodriguez J, Andersson N, Billig H. Dynamic regulation of estrogen receptor-alpha isoform expression in the mouse fallopian tube: mechanistic insight into estrogen-dependent production and secretion of insulin-like growth factors. American Journal of Physiology-Endocrinology and Metabolism. 2007;293:E1430–E1442. doi: 10.1152/ajpendo.00384.2007. [DOI] [PubMed] [Google Scholar]
- 55.Shao RJ, Ljungstrom K, Weijdegard B, Egecioglu E, Fernandez-Rodriguez J, Zhang FP, Thurin-Kjellberg A, Bergh C, Billig H. Estrogen-induced upregulation of AR expression and enhancement of AR nuclear translocation in mouse fallopian tubes in vivo. American Journal of Physiology-Endocrinology and Metabolism. 2007;292:E604–E614. doi: 10.1152/ajpendo.00350.2006. [DOI] [PubMed] [Google Scholar]
- 56.Shemesh M. Actions of gonadotrophins on the uterus. Reproduction. 2001;121:835–842. doi: 10.1530/rep.0.1210835. [DOI] [PubMed] [Google Scholar]
- 57.Shemesh M, Mizrachi D, Gurevich M, Stram Y, Shore LS, Fields MJ. Functional importance of bovine myometrial and vascular LH receptors and cervical FSH receptors. Seminars in Reproductive Medicine. 2001;19:87–96. doi: 10.1055/s-2001-13915. [DOI] [PubMed] [Google Scholar]
- 58.Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, Sakari M, Takada I, Nakamura T, Metzger D, Chambon P, Kanno J, Yoshikawa H, Kato S. Premature ovarian failure in androgen receptor-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:224–229. doi: 10.1073/pnas.0506736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Syvala H, Vienonen A, Ylikomi T, Blauer M, Zhuang YH, Tuohimaa P. Expression of the chicken progesterone receptor forms A and B is differentially regulated by estrogen in vivo. Biochemical and Biophysical Research Communications. 1997;231:573–576. doi: 10.1006/bbrc.1997.6149. [DOI] [PubMed] [Google Scholar]
- 60.Tian X, Halfhill AN, Diaz FJ. Localization of phosphorylated SMAD proteins in granulosa cells, oocytes and oviduct of female mice. Gene Expr Patterns. 2010;10:105–112. doi: 10.1016/j.gep.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Toft D, O’Malley B. Target tissue receptors for progesterone: the influence of estrogen treatment. Endocrinology. 1972;90:1041–1045. doi: 10.1210/endo-90-4-1041. [DOI] [PubMed] [Google Scholar]
- 62.Ulbrich SE, Kettler A, Einspanier R. Expression and localization of estrogen receptor alpha, estrogen receptor beta and progesterone receptor in the bovine oviduct in vivo and in vitro. Journal of Steroid Biochemistry and Molecular Biology. 2003;84:279–289. doi: 10.1016/s0960-0760(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 63.Vonier PM, Crain DA, McLachlan JA, Guillette LJ, Arnold SF. Interaction of environmental chemicals with the estrogen and progesterone receptors from the oviduct of the American alligator. Environmental Health Perspectives. 1996;104:1318–1322. doi: 10.1289/ehp.961041318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vonier PM, Guillette LJ, McLachlan JA, Arnold SF. Identification and characterization of estrogen and progesterone receptors from the oviduct of the American alligator (Alligator mississippiensis) Biochemical and Biophysical Research Communications. 1997;232:308–312. doi: 10.1006/bbrc.1997.6274. [DOI] [PubMed] [Google Scholar]
- 65.Wang H, Eriksson H, Sahlin L. Estrogen receptors alpha and beta in the female reproductive tract of the rat during the estrous cycle. Biology of Reproduction. 2000;63:1331–1340. doi: 10.1095/biolreprod63.5.1331. [DOI] [PubMed] [Google Scholar]
- 66.Willingham E, Crews D. Sex reversal effects of environmentally relevant xenobiotic concentrations on the red-eared slider turtle, a species with temperature-dependent sex determination. General and Comparative Endocrinology. 1999;113:429–435. doi: 10.1006/gcen.1998.7221. [DOI] [PubMed] [Google Scholar]
- 67.Yoshioka K, Suzuki C, Iwamura S. Activin A and follistatin regulate developmental competence of in vitro-produced bovine embryos. Biology of Reproduction. 1998;59:1017–1022. doi: 10.1095/biolreprod59.5.1017. [DOI] [PubMed] [Google Scholar]
- 68.Yoshioka K, Suzuki C, Iwamura S. Effects of activin A and follistatin on developmental kinetics of bovine embryos: cinematographic analysis in a chemically defined medium. Journal of Reproduction and Fertility. 2000;118:119–125. [PubMed] [Google Scholar]
- 69.Zhang WH, Ekman J, Almkvist A, Saji S, Wang L, Warner M, Gustafsson JA. Involvement of androgen receptor in 17 beta-estradiol-induced cell proliferation in rat uterus. Biology of Reproduction. 2002;67:616–623. doi: 10.1095/biolreprod67.2.616. [DOI] [PubMed] [Google Scholar]
- 70.Zheng WX, Magid MS, Kramer EE, Chen YT. Follicle-stimulating hormone receptor is expressed in human ovarian surface epithelium and fallopian tube. American Journal of Pathology. 1996;148:47–53. [PMC free article] [PubMed] [Google Scholar]