Abstract
Hepatic stellate cells (HSCs) may play an important role in hepatic immune regulation by producing numerous cytokines/chemokines, and expressing Ag-presenting and T cell co-regulatory molecules. Due to disruption of the endothelial barrier during cold-ischemic storage and reperfusion of liver grafts, HSCs can interact directly with cells of the immune system. Endotoxin (LPS), levels of which increase in liver diseases and transplantation, stimulates the synthesis of many mediators by HSCs. We hypothesized that LPS-stimulated HSCs might promote hepatic tolerogenicity by influencing naturally-occurring immunosuppressive CD4+CD25+FoxP3+ regulatory T cells (Tregs). Following their portal venous infusion, allogeneic CD4+ T cells, including Tregs, were found closely associated with HSCs, and this association increased in LPS-treated livers. In vitro, both unstimulated and LPS-stimulated HSCs up-regulated Fas (CD95) expression on conventional CD4+ T cells and induced their apoptosis in a Fas/FasL-dependent manner. By contrast, HSCs induced Treg proliferation, which required cell-cell contact, and was MHC class II-dependent. This effect was augmented when HSCs were pretreated with LPS. LPS increased the expression of MHC class II, CD80 and CD86, and stimulated the production of IL-1α, IL-1β, IL-6, IL-10 and TNFα by HSCs. Interestingly, production of IL-1α, IL-1β, IL-6 and TNFα was strongly inhibited, but that of IL-10 enhanced, in LPS-pretreated HSC/Treg co-cultures. Adoptively transferred allogeneic HSCs migrated to the secondary lymphoid tissues and induced Treg expansion in lymph nodes. These data implicate endotoxins-stimulated HSCs as important immune regulators in liver transplantation by inducing selective expansion of tolerance-promoting Tregs, and reducing inflammation and allo-immunity.
Keywords: Hepatic stellate cells, lipopolysaccharide, liver, tolerance, Tregs
Introduction
The liver receives 75-80% of its blood supply from the portal vein that conveys food-derived and microbial products, Ags, endotoxins and xenobiotics, and plays a major defense role against potentially harmful factors on a continuous basis. This function is performed by the various hepatic cell populations in a coordinated manner. Despite its repertoire of immunologically active cell types (1, 2), the liver demonstrates a remarkable capacity to impart immune tolerance, as evidenced by viral persistence (e.g. hepatitis B and C), parasite infection (e.g., malaria parasite) and tumor metastasis. Liver tolerance is exemplified by the spontaneous acceptance of hepatic allografts across MHC barriers in animal models (3, 4) and human liver transplants (5, 6). Specific interactions between hepatic cell types and those of the immune system may determine the short- and long-term fate of liver allografts. Thus, donor liver-derived “passenger leukocytes” (microchimerism) can subvert anti-donor T cell responses (7-9). Other liver non-parenchymal cells (NPCs) can also exhibit tolerogenic properties (2). Thus, although hepatic dendritic cells (DCs), Kupffer cells (KCs) and liver sinusoidal endothelial cells (LSECs) have been studied extensively in this regard (10, 11), many unanswered questions remain concerning mechanisms underlying the tolerogenic properties of liver NPCs.
Hepatic stellate cells (HSCs), which are located in the perisinusoidal space (Space of Disse), have been investigated extensively for their role in liver fibrosis (12-14). Under physiological conditions, HSCs are quiescent and store most of the body’s retinoids (about 80%). During liver injury, they undergo activation, characterized by the loss of retinoids and transdifferentiation into highly proliferative and fibrogenic myofibroblast-like cells. The role of HSCs in hepatic immunobiology, and in particular transplant immunology, is understudied, despite their ability to produce an array of cytokines/chemokines (14-16) and their expression of Ag-presenting and co-regulatory molecules (17-21). Fully-activated, IFNγ-stimulated HSCs can inhibit allogeneic effector T cells (17), and promote IL-2-dependent expansion of regulatory T cells (Tregs) (19). Activated HSCs can also prevent DC-induced activation/proliferation of CD8+ T cells via a CD54-dependent mechanism (20). However, in the healthy donor liver, HSCs are quiescent, or may be activated transiently following graft reperfusion (22,23). Since the endothelial barrier is disrupted during cold ischemic preservation and reperfusion (I/R) of the graft (24, 25), there is direct contact between the cells in the hepatic sinusoids and perisinusoidal HSCs. Thus, the role of quiescent or transitionally-activated HSCs in immune regulation may be of importance in the control of alloimmunity following liver transplantation.
We have shown previously (26-28) that LPS stimulates the synthesis of pro-inflammatory mediators, such as IL-6 and TNF-α, by both quiescent and transitionally-activated HSCs. Circulating LPS levels are increased in liver disease and following liver transplantation, and remain elevated until graft function is fully restored (29). We hypothesized that LPS-stimulated quiescent or transitionally-activated HSCs might regulate the hepatic immune response by influencing allogeneic CD4+ (conventional and regulatory) T cells. We found that HSCs induced FasL-mediated death of allogeneic conventional CD4+ T cells, but caused MHC class II-dependent expansion of naturally-occurring allogeneic Tregs. The latter effect was augmented when HSCs were pre-exposed to LPS. Co-culture with Tregs resulted in augmentation of IL-10 secretion, and suppression of production of the pro-inflammatory cytokines IL-1α, IL-1β, IL-6, and TNF-α by LPS-pretreated HSCs. These data suggest that HSCs may play an important role in immune regulation early after liver transplantation.
Materials and Methods
In vivo exposure to LPS and cold/ischemic preservation of livers
Experimental protocols were approved by the University of Pittsburgh Animal Care and Use Committee, in accordance with NIH guidelines. Male C57BL/6 (H-2b) (B6) and BALB/c (H-2d) mice (6-8 wk old) were purchased from The Jackson Laboratory (Bar Harbor, Maine). Livers of B6 mice were perfused in situ with cold Belzer University of Wisconsin (UW) solution for 5 min via the portal vein, then harvested and stored in UW solution for 18h at 40C. The livers were then reperfused with 30 ml medium containing 16×106 purified bulk B6 or BALB/c CD4+ T cells via the portal vein (3 ml/min). The inferior vena cava was ligated below the liver and the perfusate collected from the suprahepatic vena cava. Retention of infused cells in the livers was determined by their enumeration in the outflow perfusate using a hemocytometer (30). In some experiments, B6 mice were treated with LPS (1 mg/kg; i.p.), 6h prior to perfusion. Control livers, following cold ischemic preservation, were reperfused with medium without cells. The liver lobes were cryopreserved for immunostaining.
HSC isolation
B6 mouse livers were perfused in situ through the inferior vena cava with 30-40 ml HBSS (without Ca2+), then digested with 30-40 ml HBSS (with Ca2+) containing collagenase type IV (0.25 mg/ml) (Worthington, Lakewood, NJ) and protease (0.50 mg/ml) (Sigma, St. Louis, MO) (26, 27). The cell suspension was filtered through 100 μm nylon mesh. Following removal of hepatocytes and cell debris by low speed centrifugation (50g; 2 min), HSCs were purified by Histodenz density gradient centrifugation, and suspended in DMEM containing 100 U/ml penicillin, 100 μg/ml streptomycin, 10% FBS and 10% horse serum. Cells were seeded in gelatin (0.1% in PBS)-coated plates at a density of 0.5×106/cm2 and 20 min later, loosely adherent HSCs were harvested and re-seeded in new 6 or 96-well flat-bottom plates. Cell purity, as assessed by vitamin A autofluorescence and desmin immunostaining, was >95%. Purity was further confirmed by FACS analysis (LSR Fortessa, BD Biosciences, San Jose, CA) using anti-CD68 (KCs), anti-CD11b (KCs and DCs), and anti-CD11c (DCs and NK cells) Abs (AbD Serotec, Oxford, UK or Biolegend, San Diego, CA) (supplemental Fig. 1).
Phenotypic characterization of HSCs
Cultured HSCs incubated in the absence or presence of 100 ng/ml LPS for 24h were harvested using trypsin/EDTA, suspended in DMEM, washed with PBS (2% BSA), and stained with the following Abs: MHC class I, MHC class II, CD40, CD80, CD86, ICAM-1, VCAM, B7-H1 and FasL (Biolegend). The cells were then fixed in 4% paraformaldehyde on ice for 20 min, permeabilized with 0.1% saponin for 30 min at room temperature, and stained with rabbit polyclonal anti-desmin Ab (Abcam, Cambridge, MA) at 1:200 dilution, followed by goat anti-rabbit IgG (H+L) secondary Ab (Invitrogen-Molecular Probes, Eugene, OR) at 1:400 dilution. In some experiments, expression of intracellular cytokines IL-6, IL-10 or TNFα was also determined. The stained cells were detected on a LSR Fortessa flow cytometer and analyzed using FlowJo 7.6.1 (Tree Star Inc., Ashland, OR).
T cell isolation and purification
Single cell suspension of splenocytes of BALB/c mice was treated with 0.84% NH4Cl to lyse RBCs, then T cells were enriched using sterile nylon wool fiber column according to the manufacturer’s protocol (Wako, Osaka, Japan). T lymphocytes were stained for CD3, CD4, CD8, CD25 (Biolegend), CD127 (eBioscience, San Diego, CA), and 7-AAD (BD PharMingen). Stained cells were acquired using a BD FACSAria. Dead cells were stained with 7-actinomycin D (7-AAD) for exclusion, and the viable CD3+CD8− cells with the following compositions were sorted: (a) bulk CD4+ T cells (CD4+CD25+/− including Tregs), (b) conventional CD4+ T cells (CD4+CD25− only), and (c) natural Tregs (CD4+CD25highCD127low/-) (31). Following sorting, individual cell populations were tested for their purity which was always >99%. Nearly 95% or more of the sorted Tregs were found to be positive for FoxP3 (data not shown).
Immuno-fluorescence microscopy
HSCs seeded on coverslips in co-culture with Tregs or liver sections (placed on glass slides) were stained with rabbit anti-desmin (Abcam), rat anti-FoxP3 (Alexis), rabbit anti-Ki67 (Abcam), rat anti-CD4 or rat anti-mouse CD31 Abs (Biolegend). Following primary staining, goat anti-rabbit Alexa-fluor-488, goat anti-rat Alexa-fluor-594 or donkey anti-rat Cy3 were used as secondary Abs, while DAPI was used as a nuclear stain. Coverslips or sections were mounted using gelvatol. The slides were placed at 40C overnight before microscopic examination (25).
Co-culture of HSCs with allogeneic CD4+ T cells
Overnight-cultured HSCs were treated with 270 μM gadolinium chloride (GdCl3) (Sigma-Aldrich) for 24h to block the activity of contaminating KCs, if any, then washed and stimulated with LPS (100 ng/ml) for 24h. The cells were then washed, and co-cultured in fresh medium with CFSE-labeled bulk CD4+ T cells, conventional CD4+ T cells or Tregs (at 1:10 ratio in 96-well flat-bottom plates). CFSE labeling was carried out as described previously (32). Polymyxin B (300 ng/ml) (Sigma) was added to the cultures to block the direct effect of any residual LPS on CD4+ T cells. In some experiments, HSCs were cultured in inserts (0.45 μm pore size) and separated from Tregs placed in the bottom portion of the wells (BD-Falcon) to determine whether their influence on T cells was due to soluble mediators or required cell-cell contact. Apoptosis was assessed by staining with 7-AAD and annexin-V, while cell proliferation was measured by CFSE dilution procedure (32). In some experiments, blocking Abs (anti-FasL, anti-I-A/I-E) or the co-stimulation blocking agent CTLA4-Ig were added at the start of cultures.
Quantification of cytokines
Cell-free supernatants were collected to measure concentrations of cytokines using a mouse 20-plex® Luminex™ beadset (BioSource International, San Diego, CA) and Luminex™ 100 IS apparatus (Luminex, Austin, TX).
Determination of the suppressive function of HSC-expanded Tregs
Untreated or LPS-pretreated B6 HSCs were co-cultured with purified BALB/c Tregs for 5 d in the presence of 200 U/ml IL-2, as described above. Tregs were recovered and, to increase their yield, re-incubated with fresh B6 HSCs + IL-2 for an additional 5 d. Tregs recovered from culture were characterized for their expression of glucocorticoid-induced TNFR-related protein (GITR), programmed death-1 (PD-1), lymphocyte-activation gene 3 protein (LAG-3), CD103, CCR7, CD62L, and intracellular CTLA4 and IL-10. To determine the suppressive potential of HSC-expanded Tregs, B6-Tregs (Ly5.2) were expanded with BALB/c HSCs, and then added at ratios of 1:10 and 1:20 in co-culture of CFSE-labeled conventional B6-CD4+ T cells (Ly5.2) and γ-irradiated BALB/c splenocytes (as stimulators) in 96-well plates for 96h (32). To some cultures, no Tregs or naïve or anti-CD3/CD28 Ab-activated conventional B6 CD4+ T cells (isolated from Ly5.1/Ly5.2 hybrid mice) were added to serve as negative controls. Suppression was determined by flow cytometry.
Adoptive transfer of Tregs
BALB/c HSC-expanded B6 Tregs were labeled with CFSE and adoptively transferred to B6 mice (1×106 Tregs/animal). One week later, the animals were euthanized, cells isolated from spleens and lymph nodes (axillary, inguinal, mesenteric, and renal), and the level of expression of FoxP3 in the CFSE-labeled cells assessed by FACS to determine their stability in vivo.
Adoptive transfer of HSCs
B6 HSCs (untreated or LPS-pretreated) were adoptively transferred to BALB/c mice through the lateral tail vein (5×106 cells/mouse). After 5 d, cells from blood, spleens, and lymph nodes were isolated and assessed for total number of the natural Tregs (CD4+FoxP3+Helios+). In some experiments, GFP+-B6 HSCs were adoptively transferred to BALB/c mice, and on d 2 and 5 after cell transfer, spleens and lymph nodes were collected, cryo-preserved and the migration of allogeneic HSCs and their interaction with Tregs analyzed by immunohistology.
Statistical analysis
Results are expressed as means ± SD. Statistical significance between groups was determined by Student’s ‘t’-test using Prism 5 Software package (GraphPad Software, GraphPad, San Diego, CA). A ‘p’ value <0.05 was considered statistically significant.
Results
LPS modulates the expression of Ag-presenting and T cell co-regulatory molecules on HSCs
HSCs expressed relatively high levels of surface MHC class I, but their expression of MHC class II, as well as T cell co-stimulatory molecules (CD40, CD80 and CD86) was very low (Fig. 1). LPS significantly increased expression of these molecules, except MHC class I. HSCs also expressed ICAM-1 and VCAM, programmed death ligand-1(PD-L1; B7-H1) and FasL (CD95L). LPS significantly increased surface expression of VCAM and FasL, as well as the intracellular expression of IL-10 in HSCs (Fig. 1).
FIGURE 1. Immunoregulatory molecules expressed by untreated and LPS-treated HSCs.
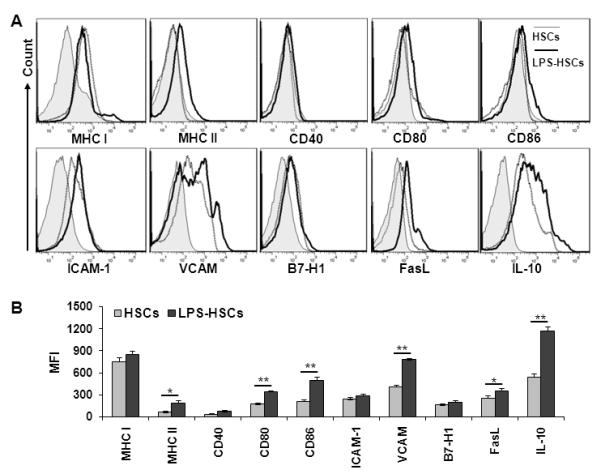
A, HSCs isolated from B6 mice were cultured overnight, treated with 270 μM GdCl3 for 24h, washed and stimulated with 100 ng/ml LPS for 24h. Cells were then stained for surface (MHC I, MHC II, CD40, CD80, CD86, ICAM-1, VCAM, B7-H1, and FasL) and intracellular (IL-10) molecules. Shaded histogram represents isotype control, while dotted and solid lines represent untreated and LPS-treated HSCs, respectively. B, Bar diagram shows mean fluorescence intensity (MFI) for each marker. The data are representative of at least three independent experiments. *p<0.05, **p < 0.01.
Retention of allogeneic CD4+ T cells by the liver
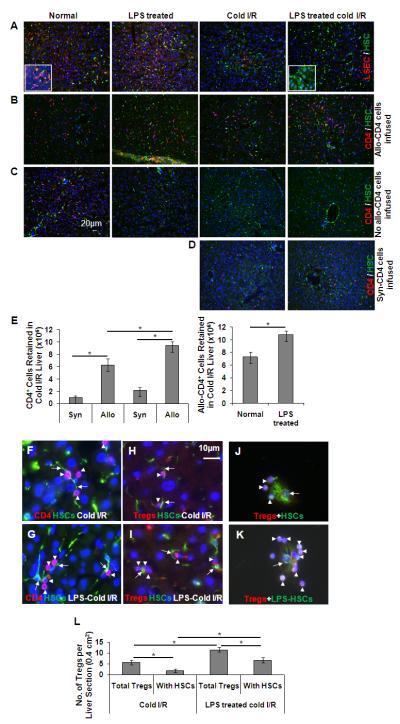
Despite their higher frequency in the liver (33), hepatic CD4+ T cells (30-40% of total intrahepatic lymphocytes in mice) have been understudied compared to CD8+ T cells (34). Here, we first determined whether HSCs might display enhanced interactions with allogeneic (BALB/c) CD4+ T cells following cold I/R of the B6 mouse liver. Consistent with previous observations (24, 25), LSEC-specific CD31 staining was markedly reduced in the cold I/R livers, indicating disruption of the endothelial barrier (Fig. 2A). Numerous infused allogeneic CD4+ T cells were retained in the liver, and I/R did not increase their retention (Fig. 2B). However, LPS pre-exposed livers (both un-preserved or normal and cold-preserved) retained significantly greater numbers of CD4+ T cells than the untreated livers (Fig. 2B, 2E); only a few CD4+ cells were observed in the control livers that were reperfused without allogeneic cells (Fig. 2C). In contrast to the allogeneic cells, significantly less retention of the infused syngeneic CD4+ T cells was observed in the cold I/R livers (Fig. 2D, 2E). These results demonstrate that the CD4+ T cells observed in the livers (Fig. 2B) were predominantly allogeneic. By high-power imaging, allogeneic CD4+ T cells were found to be retained mainly in the sinusoids of control livers (data not shown), and in close proximity to HSCs in cold-preserved livers (Fig. 2F, 2G). Interestingly, FoxP3+ Tregs were also found in close association with HSCs in cold I/R livers and this interaction was enhanced upon LPS-pretreatment (Fig. 2H, 2I, and 2L). In fact, about 50% of the retained Tregs were closely associated with HSCs. Immunostaining of HSC/Treg co-cultures also demonstrated the adherence of greater number of Tregs to LPS-pretreated (Fig. 2K) compared to untreated HSCs (Fig. 2J). These data indicate the ability of the liver to retain allogeneic CD4+ T cells regardless of cold preservation with augmentation of the effect upon LPS-pretreatment. The data further suggest that HSCs may play an important role in this phenomenon, especially when the endothelial barrier is disrupted due to cold I/R.
FIGURE 2. (A-E) Disruption of liver sinusoidal endothelial cells (LSEC) and retention of allogeneic CD4+ T cells by the liver.
Livers of untreated or LPS (1 mg/kg; 6h)-treated B6 mice were flushed with cold UW solution, and either preserved at 40C for 18h or used immediately. The livers were reperfused with warm medium alone or medium containing FACS-purified BALB/c bulk CD4+ T cells (16×106). The livers were then fixed and cryopreserved for immunofluorescence microscopy. A, The sections show the presence or absence of endothelial cells in normal untreated, LPS-treated, cold-preserved (I/R), and LPS-pretreated and cold-preserved livers. LSEC (CD31; red stain) and HSCs (desmin; green stain). Magnification x20. Insets show images at higher (x100) magnification. B, The sections show the retention of allogeneic CD4+ T cells by the liver. CD4+ T cells (CD4; red stain) and HSCs (desmin; green stain). C, The sections show the host CD4+ T cells in indicated groups following reperfusion with medium only. D, The sections show the comparatively low retention of infused syngeneic CD4+ T cells. E, Bar graphs show the retention of CD4+ T cells in the various groups. (n=4 animals/group). *p<0.05. (F-L) Close association of allogeneic CD4+ T cells and Tregs with HSCs. F and G, Sections of cold I/R livers (untreated and LPS-pretreated), reperfused with allogeneic CD4+ T cells, showing the close interaction between HSCs (desmin; green stain-arrows) and CD4+ T cells (CD4; red stain-arrowheads). H and I, Sections of the cold I/R livers (untreated and LPS-pretreated), reperfused with allogeneic CD4+ T cells showing the close interaction between HSCs (desmin; green stain-arrows) and Tregs (FoxP3; red stain-arrowheads). Magnification x100. J and K, Co-culture of HSCs (untreated or LPS-pretreated) and purified allogeneic Tregs (on coverslips) showing the close interaction between HSCs (arrows) and Tregs (arrowheads). Magnification x100. L, Bar graph shows the total number of Tregs in the liver and Tregs closely associated with HSCs in liver sections of average area of 0.4 cm2 (n=4 animals/group). *p<0.05.
HSCs promote apoptosis of conventional CD4+ T cells via Fas-FasL interaction
Fas-FasL and B7-H1 (PD-L1)-PD1 interactions promote T cell death (36, 37). We next considered whether control or LPS-stimulated HSCs might induce conventional CD4+ T cell death via interactions of FasL and B7-H1 on HSCs with Fas and PD1 respectively on T cells. As shown in Figure 1, HSCs displayed low levels of FasL and B7-H1, and LPS treatment significantly increased FasL, but not B7-H1 expression. Interestingly, Fas expression increased strikingly on conventional CD4+ T cells co-cultured with HSCs, regardless of LPS pre-treatment (Fig. 3A). However, surface expression of PD1 on these T cells was not detected, without or with co-culture with HSCs ± LPS (Fig. 3B). Since PD1 is expressed on peripheral CD4+ and CD8+ T cells, NKT cells, B cells and monocytes and some DC subsets upon their activation by cytokines such as IL-2, IL-7, IL-15, IL-21 (38, 39), our data suggest that HSCs do not activate CD4+ T cells or produce cytokines that induce their expression of PD1. Furthermore, HSCs did not up-regulate the expression of the early activation markers CD69 or CD71 on conventional CD4+ T cells (data not shown). CFSE dilution assay also confirmed that HSCs did not induce proliferation of conventional CD4+ T cells (Fig. 3C). In co-culture with untreated or LPS-pretreated HSCs, apoptosis of conventional CD4+ T cells increased significantly and similarly, as indicated by 7-AAD and annexin V staining (Fig. 3D upper panel and bar graph). Partial reversal of HSCs ± LPS-induced apoptosis of CD4+CD25− T cells T cells occurred in the presence of anti-FasL blocking Ab (Fig. 3D, lower panel and bar graph). Apoptosis of conventional CD4+ T cells induced by co-culture with HSCs ± LPS was not altered by B7-H1 blocking Ab (data not shown). These results suggest that HSCs induce apoptosis of conventional CD4+ T cells primarily via Fas-FasL dependent mechanism.
FIGURE 3. Interaction of HSCs with allogeneic conventional CD4+ T cells.
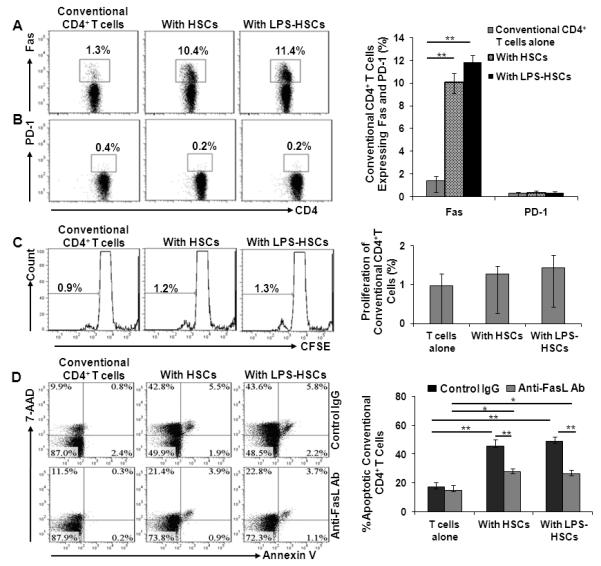
A and B, The expression of Fas (A) and PD-1 (B) on allogeneic CD4+ T cells cultured alone, with HSCs or with LPS-pretreated HSCs. Bar graph shows the percentage of conventional CD4+ T cells expressing Fas or PD-1 (n=3). C, Proliferation profile of the allogeneic CD4+ T cells. CFSE-labeled CD4+ T cells were cultured alone or with untreated or LPS (100 ng/ml)-pretreated HSCs. Bar graph shows the percentage of proliferating CD4+ T cells (mean + SD) (n=3). D, Apoptosis of allogeneic CD4+ T cells induced by HSCs. Apoptosis of CD4+ T cells was determined by flow analysis following 3 d co-culture with untreated or LPS (100 ng/ml)-pretreated HSCs, in the absence or presence of anti-FasL blocking Ab. The Ab was added to the HSC culture at a concentration of 10 μg/ml, 1h prior to the start of co-culture with CD4+ T cells. The bar graph shows the percentage of CD4+ T cells undergoing apoptosis (7-AAD+Annexin V−, 7-AAD+Annexin V+ and 7-AAD−Annexin V+ cells) in the absence or presence of anti-FasL Ab (n=3). *p <0.05, **p<0.01.
LPS-stimulated HSCs induce expansion of allogeneic FoxP3+ Tregs
In all Treg experiments, exogenous IL-2 (200 U/ml) was added since it is necessary for Treg survival, and our preliminary experiments showed no expression of IL-2 by unstimulated or LPS-stimulated HSCs, even when co-cultured with Tregs. Incubation of bulk allogeneic CD4+ T cells with untreated HSCs increased the numbers of the FoxP3+ Tregs significantly compared to controls; this effect was enhanced in co-culture with LPS-pretreated HSCs (Fig. 4A and bar graph). These Treg populations were positive for Helios, a member of Ikaros transcription factor family. Helios is preferentially expressed by naturally-occurring Tregs, but not by induced Tregs (40) (Fig. 4A). CFSE dilution assay showed proliferation of nearly 60% and 75% of Helios+ Tregs in the presence of control and LPS-pretreated HSCs, respectively (Fig. 4B). These data indicate that LPS-stimulated HSCs induce the expansion of Tregs from the bulk CD4+ T cell population. The HSC-induced Treg-expansion appeared to be allo-Ag specific since HSCs failed to expand syngeneic Tregs (data not shown).
FIGURE 4. LPS-stimulated HSCs increase the incidence of FoxP3+CD4+ cells in bulk allogeneic CD4+ T cell cultures.
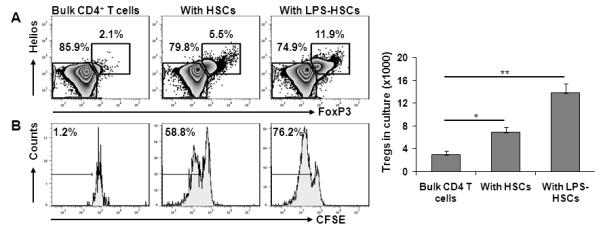
Purified bulk BALB/c CD4+ T cells were cultured for 5 d in flat-bottom 96-well plates alone, or with B6 HSCs preincubated without or with 100 ng/ml LPS for 24h at a HSC:Treg ratio of 1:10 for 5 d. T cells were then recovered for examination by flow cytometry. A. HSCs increase the incidence of CD4+FoxP3+Helios+ Tregs. B. Proliferation of FoxP3+Helios+ cells, as assessed by CFSE dilution assay. Bar graph shows the absolute numbers of Tregs in the cultures (n=3). *p <0.05, **p<0.01.
We next examined the influence of HSCs on purified Tregs. CFSE-labeled allogeneic Tregs were cultured alone or with HSCs (untreated or LPS-pretreated). Significant expansion of purified Tregs occurred in co-culture with untreated HSCs, and this effect was augmented when HSCs were pretreated with LPS (Fig. 5A). In a parallel experiment, separation of HSCs from Tregs in transwell cultures prevented Treg expansion (Fig. 5A and bar graph), indicating the need for cell-cell contact.
FIGURE 5. Proliferation of purified Tregs by HSCs requires cell contact.
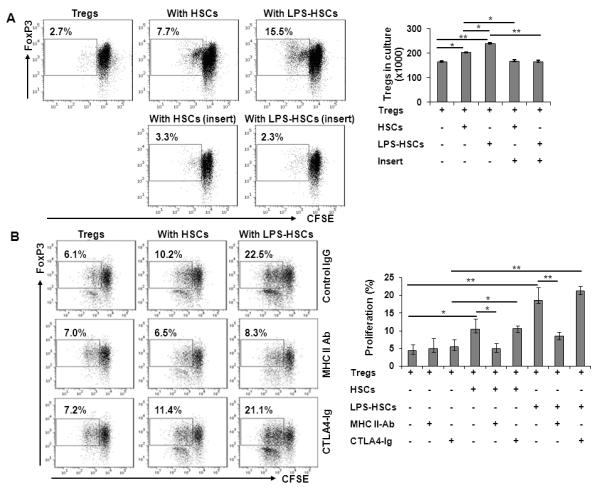
A, Tregs were cultured alone or with HSCs (untreated or LPS [100 ng/ml]-pretreated) at a HSC:Treg ratio of 1:10 for 5 d in the presence of 200 U/ml IL-2. CFSE dilution assay was performed to determine the proliferation of FoxP3+ cells. Proliferation of Tregs was blocked when the cells were separated from HSCs using trans-well culture inserts. Bar graph shows the absolute numbers of Treg at the end of culture period (n=3). B, HSC-induced Treg expansion is mediated by MHC class II on HSCs. HSCs, pre-incubated with 100 ng/ml LPS for 24h, were treated with 5 μg/ml anti-MHC class II (purified anti-mouse I-A/I-E) Ab, 5 μg/ml of the co-stimulation blocking agent CTLA4-Ig or control IgG for 30 min. The medium was aspirated and CFSE-labeled Tregs were added to the culture wells in fresh RPMI medium. At the end of incubation (d 5), proliferation of Tregs was measured by CFSE dilution assay. Bar diagram represents percent Treg proliferation (mean + SD) (n=3). *p<0.05, **p<0.01.
HSC-induced proliferation of Tregs is dependent on MHC class II
Even though HSCs produce a number of mediators, including TGF-β and retinoic acid that might affect the activation/function of Tregs (41), the data shown in Figure 5A demonstrated that HSC-induced Treg expansion was contact-dependent. Furthermore, incubation of Tregs in medium conditioned by HSCs (± LPS) did not stimulate their expansion (data not shown). Key cell surface-expressed molecules, such as MHC class II and CD80/CD86, have been implicated in Treg proliferation (42-44). LPS increased the expression of MHC class II, CD80 and CD86 on HSCs significantly (Fig. 1). Upon examining the role of these up-regulated molecules, blockade of MHC class II, but not the co-stimulatory molecules, significantly prevented HSC-induced Treg expansion (Fig. 5B and bar graph), suggesting a predominant role of TCR signaling in this effect.
Bi-directional interaction between HSCs and Tregs in the production of cytokines
Cytokines produced in the hepatic microenvironment following transplantation influence the course of allograft acceptance or rejection. Therefore, we ascertained the influence of HSC-Treg interaction on the production of anti-inflammatory (IL-10) and pro-inflammatory (IL-1α, IL-1β, IL-6, and TNF-α) cytokines. HSCs produced these cytokines spontaneously, and LPS pretreatment increased their production by HSCs significantly (Fig. 6A). Basal production of these cytokines by Tregs was very low/negligible, and in co-culture, their basal release by HSCs was abrogated. Interestingly, production of IL-10 by LPS-pretreated HSCs increased, and that of IL-1α, IL-1β, IL-6, and TNF-α was reduced significantly in co-culture with Tregs (Fig. 6A). Intracellular staining further confirmed production of these cytokines by HSCs (Fig. 6B). Although, we did not observe IL-6 and TNFα expression by Tregs cultured alone or with HSCs (±LPS) (data not shown), their IL-10 expression increased in co-culture with HSCs (Fig. 7A). These data indicate that bi-directional interaction between HSCs and Tregs can markedly affect the balance between pro- and anti-inflammatory cytokines production.
FIGURE 6. Production of cytokines by LPS-stimulated HSCs and in HSC/Treg co-cultures.
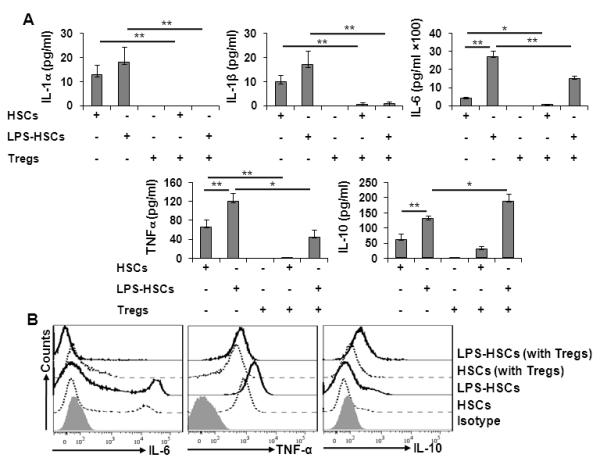
A, The supernatants from untreated or LPS (100 ng/ml)-pretreated HSCs, or from their co-cultures with Tregs, were aspirated after 5 d incubation, and the accumulated cytokines measured via Luminex assay (n=3). *p<0.05, **p<0.01. B, Intracellular IL-6, TNFα and IL-10 were stained in unstimulated or LPS (100 ng/ml)-prestimulated HSCs cultured alone or with Tregs for 5 d. The experiment was performed twice with essentially similar results.
FIGURE 7. HSCs favor retention of a regulatory phenotype and suppressive activity of proliferating Tregs.
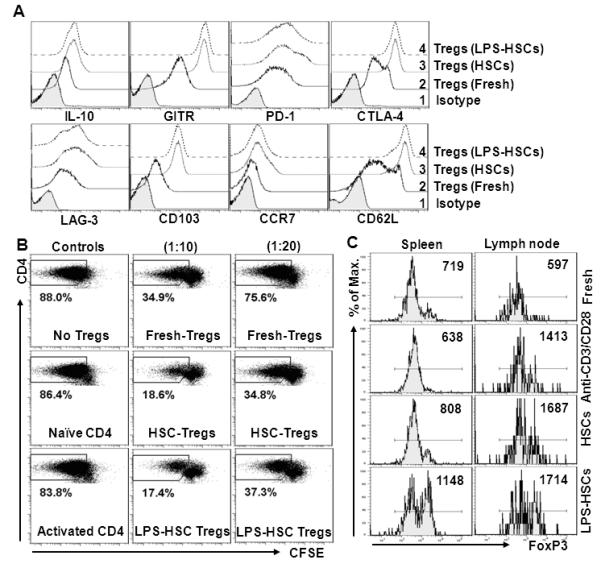
A, Purified Tregs co-cultured with HSCs (un-stimulated or LPS (100 ng/ml)-stimulated) were recovered, stained, and analyzed for the expression of the indicated molecules by flow cytometry. Shaded histogram, isotype control; solid lines, freshly-isolated Tregs; dotted lines, HSC-expanded Tregs; dashed lines, LPS-pretreated HSC-expanded Tregs. B, HSC-expanded allo-Tregs potently suppress the proliferation of syngeneic conventional CD4+ T cells. Purified naïve conventional B6 CD4+ T cells (Ly5.2; pre-stained with CFSE) were co-cultured with γ-irradiated BALB/c splenocytes (at a ratio of 10:1). Freshly-isolated B6 Tregs or Tregs recovered from co-cultures with untreated or LPS-pre-treated BALB/c HSCs were added at the indicated ratios to the conventional CD4+ T cell/splenocyte co-culture. As negative controls, no Tregs or naïve or anti-CD3/CD28Ab-activated conventional B6 CD4+ T cells (co-expressing Ly5.1 and 5.2) were added at 1:10 ratio. Following incubation for 96h, proliferation was measured in FoxP3− as well as CD45.1 and CD45.2 double-positive cells. The numbers in the panels indicate the incidence of proliferating CD4+ T cells as a percentage of total CD4+FoxP3− cells (n=2). C, In vivo stability of the HSC-expanded Tregs following their adoptive transfer (measured on the basis of their FoxP3 expression). Freshly-isolated, BALB/c HSC (untreated or LPS pre-treated)-expanded, and anti-CD3/CD28-expanded B6 Tregs were labeled with CFSE, and adoptively transferred to syngeneic B6 mice (1×106/animal). At 1 wk, expression of FoxP3 in labeled cells was determined in spleen and lymph nodes using FACS. Numbers in panels denote MFI. (n=2).
HSC-expanded Tregs retain regulatory properties and exhibit potent suppressive function
The principal function of Tregs is maintenance of homeostasis by the suppression of exaggerated immune responses. Therefore, we examined Tregs for expression of molecules that are responsible for suppressive function. About 80-90% of HSC-expanded Tregs maintained FoxP3 expression (data not shown), and were uniformly positive for GITR (Fig. 7A), a potent T cell co-stimulatory receptor and regulator of Treg function, deficiency of which abrogates the suppressive function of Tregs and increases T cell proliferation (45). HSC-expanded Tregs expressed high levels of PD1 (surface) and CTLA-4 (intracellular), which are implicated in induction of allograft tolerance by suppressing the activation, proliferation and survival of CD4+ and CD8+ T cells (37, 46). The proliferating Tregs were positive for LAG3, a protein expressed by activated natural Tregs and an augmenter of their regulatory function (47), CD103 (integrin αEβ7), a marker for memory/activated Tregs, and CCR7 and CD62L, the molecules implicated in their migration in non-lymphoid tissues (48).
We then tested the suppressive function of Tregs co-cultured with HSCs (unstimulated and LPS-stimulated). Tregs that proliferated in co-culture with untreated or LPS-pretreated HSCs caused greater suppression of alloreactive CD4+ T cell proliferation than freshly-isolated Tregs. Only approximately 20% alloreactive CD4+ T cells proliferated when cultured with Tregs harvested from HSC co-cultures (untreated and LPS-pretreated, respectively) in contrast to approximately 35% that proliferated with freshly-isolated Tregs at the same suppressor-effector cell ratio (Fig. 7B). In contrast, the addition of naïve or activated conventional B6 CD4+ T cells (co-expressing Ly5.1 and Ly5.2) (negative controls) instead of Tregs (at 1:10 ratio) demonstrated very mild inhibition of proliferation (Fig. 7B). These data suggest that, in vitro HSC-expanded Tregs retain a suppressive phenotype and function. Upon examining their stability in vivo following adoptive transfer, HSC-expanded Tregs located in the spleen and lymph nodes were found to express a high level of FoxP3 even after a week in comparison to the anti-CD3/CD28 Ab-expanded Tregs (Fig. 7C). These data indicate superior suppressive potential and in vivo stability of HSC-expanded Tregs.
HSCs preferentially increase Tregs in lymph nodes in vivo
To ascertain the in vivo relevance of HSC-induced Treg expansion, we adoptively transferred B6 HSCs (untreated or LPS-pretreated) into BALB/c mice. After 5 d, naturally-occurring Tregs (CD4+FoxP3+Helios+) were assessed in blood, spleen and lymph nodes. The number of theseTregs in the blood decreased in mice that received HSCs (Fig. 8A, 8B). Although the proportion of Tregs in total CD4+ T cells increased (Fig. 8A), their absolute numbers decreased significantly in the spleens of mice that received HSCs (Fig. 8B). Interestingly, Tregs increased significantly in lymph nodes of mice that received HSCs, the effect being greater when LPS-pretreated HSCs were transferred (Fig. 8A and 8B). Immunostaining for Ki67 showed proliferation of FoxP3+ Tregs in the lymph nodes (Fig. 8C), but not spleens (data not shown). Tregs were found in close proximity of adoptively transferred GFP+ HSCs in the lymph nodes and spleen on d 2 (Fig. 8D, 8E), and a small number could be observed in lymph nodes even on d 5 (data not shown). These data indicate the potential of HSCs to recruit and expand Tregs in vivo.
FIGURE 8. In vivo migration of HSCs and proliferation of Tregs (CD4+FoxP3+Helios+).
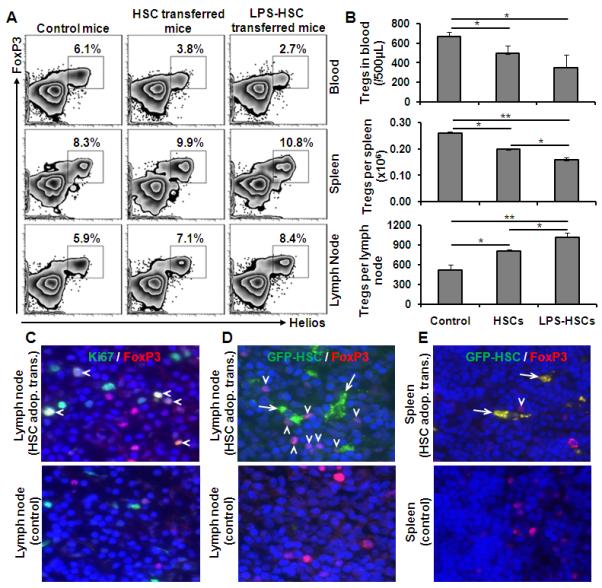
Untreated or LPS-pretreated B6 HSCs were adoptively transferred to BALB/c mice (5×106 HSCs/mouse). After 5 d, blood, spleen and lymph nodes were collected for Treg enumeration. A, Percent incidence of Tregs in various compartments (gated on total CD4). B, Bar diagram shows the absolute numbers of Tregs (n=3 animals in each group). *p<0.05, **p <0.01. C, Immunohistology (d 5) of lymph nodes of BALB/c mice that received B6 HSCs by adoptive transfer shows proliferating Tregs [FoxP3 cells (red), Ki67 (green) and nuclear stain DAPI (blue)] indicated with arrow heads. Controls did not receive adoptively transferred HSCs. D and E, Immunohistology of lymph nodes and spleens of BALB/c mice that received GFP+ B6 HSCs by adoptive transfer (d 2) and control showing the migration of HSCs (green; arrow) closely-associated with FoxP3+ cells (red; arrow head) (n=4 animals/group).
Discussion
CD4+CD25+FoxP3+ Tregs suppress the activation/proliferation of autoreactive CD4+ and CD8+ T cells and control allograft rejection, infection-induced immune responses, and inflammatory diseases (49). Expansion of Tregs in a murine model of spontaneous liver allograft acceptance and their potential role in hepatic immune tolerance (50) have been reported. Thus, the differential effects of perisinusoidal HSCs observed in this study (apoptosis of naïve conventional CD4+ T cells and expansion of Tregs) indicate that HSCs may play a critical role in liver allograft acceptance/tolerance, especially since the endothelial barrier is disrupted due to cold-ischemic storage and reperfusion of the graft (24, 25). However, even when the sinusoidal endothelium is intact, HSCs can interact with cells in the sinusoids via their cytoplasmic processes penetrating through the SEC fenestrations (13). In this regard, hepatocytes that are located beneath HSCs have been shown to interact with T cells through fenestrations in LSECs (51). Indeed, we observed close association of CD4+ T cells with HSCs in vivo and in vitro, which may be due to the expression of ICAM-1 and VCAM by HSCs (Fig. 1) (21, 52, 53). HSCs are also a principal source of fibronectin (54) that can recruit lymphocytes in the reperfused liver graft by interacting with α4β1 (55). Moreover, retinoic acid, stored mainly by HSCs, enhances the expression of integrin α4β7 on naïve CD4+ T cells which causes their homing towards non-lymphoid tissues (56). Furthermore, HSCs produce CXCL9 (MIG) and CXCL10 (IP-10) (data not shown) that can recruit CXCR3+CD4+ T cells following cold liver I/R injury (57). Together, these observations suggest that HSCs may constitute a major hepatic cell type in regulating immune responses. Indeed, the role of HSCs in immune regulation is exemplified by their ability to protect co-transplanted islet allografts from rejection (58) and to enhance hepatocyte engraftment (59).
An important consideration regarding the role of HSCs in hepatic immune regulation is their strong reactivity to LPS, levels of which are elevated pre- and post-transplantation (29). In this study, both untreated and LPS-pretreated HSCs induced apoptosis of conventional allogeneic CD4+ T cells. This property of HSCs contrasts with that of KCs, SECs and hepatocytes that induce the proliferation of CD4+CD25− T cells (60). Although, it is unclear why up-regulation of Fas expression on naïve conventional allogeneic CD4+ T cells occurred without their activation/proliferation, our data are consistent with inhibition of activation/proliferation, together with apoptosis, of naïve conventional CD4+ T cells due to FasL-Fas cross-linking in the presence of TCR ligation (36, 61). The B7-H1-PD1 pathway has also been reported to be important for the inhibition of T cell proliferation and their apoptosis (37). Fully-activated HSCs have been shown to induce apoptosis of DC-activated CD4+ T cells, in part via a B7-H1/PD1-dependent mechanism, as a result of enhanced B7-H1 expression on HSCs and that of PD1 on DC-stimulated allogeneic CD4+CD25− cells (17). However, we did not observe such an effect of HSCs on conventional CD4+ T cells. It appears from our studies that quiescent HSCs do not induce PD-1 expression on naïve conventional CD4+ T cells (Fig. 3C). Together with the low expression of B7-H1 on quiescent HSCs, this may be the reason why the PD-1-B7-H1 pathway did not play a role in quiescent HSC-induced apoptosis of naïve conventional allogeneic CD4+ T cells. Thus, our results indicate that Fas/FasL interaction may be a key mechanism underlying quiescent HSC-induced elimination of conventional allogeneic CD4+CD25− T cells. Our data also support the concept (62) that auto- or alloreactive T cells in target organs are activated mainly by ‘non-professional’ APCs and deleted by apoptosis through the Fas/FasL pathway, in the absence of adequate co-stimulation.
It has been reported previously (19) that culture (7-10 d)-activated mouse HSCs (i.e. cells that are retinoid-deficient and fibrogenic), once they have been stimulated with IFN-γ for an additional 72h, cause expansion of Tregs in an IL-2-dependent manner. By contrast, the activation state of HSCs examined in the present study was very low, and likely achieved soon after liver transplantation due to the release of free radicals and inflammatory cytokines (14-16). Furthermore, no IFN-γ could be detected in the supernatants of HSC cultures (control or LPS-stimulated) or HSC/Treg co-cultures (data not shown). Thus, our data indicate that HSCs can cause Treg expansion both with or without LPS stimulation, and in an IFN-γ-independent manner. Considering the distinct effects of LPS and IFN-γ on cytokine production by HSCs, it is reasonable to speculate that the effects of LPS and IFN-γ in causing Treg expansion and promotion of tolerance may be additive or synergistic.
Wiegard et al. (60) reported that KCs, but not SECs or hepatocytes, caused Treg expansion. Interestingly, however, KC-expanded Tregs were less potent than freshly-isolated Tregs in suppressing CD4+ T cell proliferation. In contrast, we found that Tregs expanded by HSCs were much more potent in suppressing CD4+ T cell proliferation than freshly-isolated Tregs. This can be explained by the retention of a suppressive phenotype and enhanced FoxP3 expression by HSC-expanded Tregs (Fig. 7). Additionally, the suppressive activity of Tregs was reversed when KCs were stimulated with LPS (66), while the magnitude of suppressor activity of Tregs expanded by untreated and LPS-stimulated HSCs in this study was similar. This observation is interesting since FoxP3 expression is directly linked to Treg function (63), and Tregs from genetically-engineered mice that express low levels of FoxP3 lose suppressive activity and gain effector cell functions (IL-2, IL-4 and IFN-γ production) simultaneously (64). The mechanisms underlying this interesting paradox are the topics of future investigation.
IL-10 inhibits the synthesis of many pro-inflammatory cytokines and chemokines, including TNF-α and IL-6 (65), and reduces Ag-specific CD4+ and CD8+ T cell activation and responses (66, 67). We observed constitutive and LPS-induced production of IL-10 by HSCs that was enhanced in co-culture with Tregs. Previous work (65, 68) has shown that liver DCs and KCs, as well as LSECs, produce IL-10, and that LPS stimulates IL-10 synthesis by human KCs and SECs. It has been postulated that a mechanism underlying the inhibition of Treg suppressor activity by DCs or KCs is due to IL-6 released by the latter cell types (60, 69). Here, we found that LPS-pretreated HSCs produced comparatively large amounts of IL-6. However, this effect was abrogated when Tregs were present in the co-cultures. Interestingly, the release of other pro-inflammatory cytokines (IL-1α, IL-1β and TNF-α) was also reduced significantly in co-cultures of LPS-pretreated HSCs and Tregs, and IFN-γ and IL-4 were not detected. In contrast, secretion of IL-10 increased significantly in the same co-cultures. These data, and the increased hepatic expression of IL-10, concurrent with decreased IFN-γ, IL-6 and TNF-α expression in ConA-tolerant mice (70), suggest that a reciprocal relationship between proinflammatory cytokines and anti-inflammatory IL-10 may be important in hepatic immune tolerance. The mechanism(s) underlying increased IL-10 and decreased pro-inflammatory cytokine expression in Treg/LPS-pretreated HSC cocultures is unclear, but increased IL-10 has been shown to suppress IL-6 production by SECs and KCs (71). Thus, a similar regulatory effect of IL-10 on pro-inflammatory cytokine production observed in the present study cannot be ruled out. Together, the bidirectional interaction between LPS-stimulated HSCs and Tregs may be important in promoting tolerance and reducing inflammatory responses.
Our study demonstrates an absolute requirement for cell-cell contact and MHC class II-dependence for Treg expansion by HSCs. Even though constitutive expression of MHC class II is low on HSCs, it increases significantly upon LPS stimulation, which is consistent with enhanced Treg expansion by LPS-pretreated HSCs. Irla et al. reported recently (72) that MHC class II-dependent, myelin Ag-specific interaction of plasmacytoid DCs with naturally-occurring Tregs in lymph nodes caused their expansion during autoimmune encephalomyelitis. The role of Tregs in this latter model was indicated by exacerbated pathology in MHC class II-deficient mice. Mast cells have also been shown to stimulate proliferation of naturally-occurring Tregs in an MHC class II-dependent manner (73). Experimental evidence also suggests the role of co-regulatory molecules (for instance CD80/CD86 and B7-H1) in Treg expansion (74, 75). However, almost complete inhibition of HSC-induced Treg expansion by anti-MHC class II Ab suggests predominance of TCR ligation in this phenomenon. Moreover, mast cell-induced Treg expansion is also unaffected by anti-B7-H1 Ab (73). Although our data demonstrate that MHC class II-dependent expansion of Tregs by HSCs may be an important mechanism of hepatic immune regulation, we note that KCs also express MHC class II constitutively (11). Thus, a similar MHC class II-dependent effect of KCs on Treg expansion cannot be ruled out. However, sinusoidal endothelium is disrupted for a considerable time, and KC as well as DC numbers decrease after reperfusion (76), emphasizing the potential significance of HSCs in liver allograft tolerance, especially during the initial period following graft reperfusion. The homing of Tregs has been shown to be critical for the development of peripheral tolerance and acceptance of heart allografts (77). HSC-expanded Tregs expressed CCR7 and especially CD62L, molecules implicated in homing of Tregs to secondary lymphoid tissues, at a high level (Fig. 7A). These findings are consistent with our in vivo observations in which adoptively transferred HSCs enhanced homing/recruitment as well as the expansion of Tregs in the lymph nodes (Fig. 8).
In conclusion, expansion of Tregs and concomitant apoptosis of naïve conventional CD4+ T cells by HSCs could favor immunological tolerance after liver transplantation, and also be of value for ex vivo expansion of Tregs for potential therapeutic applications.
Supplementary Material
Acknowledgments
We thank Ms. Rachel Stewart, Mr. Jesse Ennis and Mr. Mark Ross for excellent technical assistance. We thank Dr. David M. Rothstein and Dr. Geetha Chalasni for providing GFP-B6 and CD45.1/CD45.2-B6 mice, respectively.
This work was supported by National Institutes of Health Grant PO1A1081678. G.R. is supported by a Junior Faculty Grant from the American Diabetes Association, by a Joseph A. Patrick Research Fellowship in Transplantation, and by a CTSI-PEIR grant (funded through grant number UL1 RR024153 from the National Center for Research Resources). T.L.S. was the recipient of American Society of Transplantation Basic Science Fellowship. S.K. is supported by NIH grant DK071753.
Abbreviations
- I/R
ischemic preservation and reperfusion
- DCs
dendritic cells
- HSCs
hepatic stellate cells
- KCs
Kupffer cells
- LSECs
liver sinusoidal endothelial cells
- Tregs
regulatory T cells
References
- 1.Crispe IN. The liver as a lymphoid organ. Annu. Rev. Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 2.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat. Rev. Immunol. 2010;10:753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 3.Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, Binns RM, Devies DA. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 4.Sun J, Sheil AG, Wang C, Wang L, Rokahr K, Sharland A, Jung SE, Li L, McCaughan GW, Bishop GA. Tolerance to rat liver allografts: IV. Acceptance depends on the quantity of donor tissue and on donor leukocytes. Transplantation. 1996;62:1725–1730. doi: 10.1097/00007890-199612270-00005. [DOI] [PubMed] [Google Scholar]
- 5.Pons JA, Revilla-Nuin B, Baroja-Mazo A, Ramírez P, Martínez-Alarcón L, Sánchez-Bueno F, Robles R, Rios A, Aparicio P, Parrilla P. FoxP3 in peripheral blood is associated with operational tolerance in liver transplant patients during immunosuppression withdrawal. Transplantation. 2008;86:1370–1378. doi: 10.1097/TP.0b013e318188d3e6. [DOI] [PubMed] [Google Scholar]
- 6.Castellaneta A, Thomson AW, Nayyar N, de Vera M, Mazariegos GV. Monitoring the operationally tolerant liver allograft recipient. Curr. Opin. Organ. Transplant. 2010;15:28–34. doi: 10.1097/MOT.0b013e328334269a. [DOI] [PubMed] [Google Scholar]
- 7.Kreisel D, Petrowsky H, Krasinskas AM, Krupnick AS, Szeto WY, McLean AD, Popma SH, Gelman AE, Traum MK, Furth EE, et al. The role of passenger leukocyte genotype in rejection and acceptance of rat liver allografts. Transplantation. 2002;73:1501–1507. doi: 10.1097/00007890-200205150-00022. [DOI] [PubMed] [Google Scholar]
- 8.Bishop GA, Wang C, Sharland AF, McCaughan G. Spontaneous acceptance of liver transplants in rodents: evidence that liver leucocytes induce recipient T-cell death by neglect. Immunol. Cell Biol. 2002;80:93–100. doi: 10.1046/j.1440-1711.2002.01049.x. [DOI] [PubMed] [Google Scholar]
- 9.Araújo MB, Leonardi LS, Leonardi MI, Boin IF, Magna LA, Donadi EA, Kraemer MH. Prospective analysis between the therapy of immunosuppressive medication and allogeneic microchimerism after liver transplantation. Transpl. Immunol. 2009;20:195–198. doi: 10.1016/j.trim.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Knechtle SJ, Kwun J. Unique aspects of rejection and tolerance in liver transplantation. Semin. Liver Dis. 2009;29:91–101. doi: 10.1055/s-0029-1192058. [DOI] [PubMed] [Google Scholar]
- 11.Tiegs G, Lohse AW. Immune tolerance: what is unique about the liver. J. Autoimmun. 2010;34:1–6. doi: 10.1016/j.jaut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 13.Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin. Liver Dis. 2001;21:311–335. doi: 10.1055/s-2001-17550. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi CR. Stellate Cells. In Molecular Pathology of Liver Diseases. In: Monga SP, editor. Molecular Pathology Library, Part 1. Springer; 2010. pp. 53–80. [Google Scholar]
- 15.Marra F. Hepatic stellate cells and the regulation of liver inflammation. J. Hepatol. 1999;31:1120–1130. doi: 10.1016/s0168-8278(99)80327-4. [DOI] [PubMed] [Google Scholar]
- 16.Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Sem. Liver Dis. 2001;21:397–416. doi: 10.1055/s-2001-17554. [DOI] [PubMed] [Google Scholar]
- 17.Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, Fung JJ, Lu L, Qian S. Inhibition of T cell responses by hepatic stellate cells via B7-H1 mediated T cell apoptosis. Hepatology. 2004;40:1312–1321. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 18.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, Modlin RL, Liblau RS, Gressner AM, Kaufmann SH. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. SH. [DOI] [PubMed] [Google Scholar]
- 19.Jiang G, Yang HR, Wang L, Wildey GM, Fung JJ, Qian S, Lu L. Hepatic stellate cells preferentially expand allogeneic CD4+CD25+FoxP3+ regulatory T cells in an IL-2-dependent manner. Transplantation. 2008;86:1492–1502. doi: 10.1097/TP.0b013e31818bfd13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schildberg FA, Kurts C, Knolle PA. Prominent regulatory but weak antigen-presenting cell function of hepatic stellate cells. Hepatology. 2011;54:1108. doi: 10.1002/hep.24565. [DOI] [PubMed] [Google Scholar]
- 21.Schildberg FA, Wojtalla A, Siegmund SV, Endl E, Diehl L, Abdullah Z, Kurts C, Knolle PA. Murine hepatic stellate cells veto CD8 T cell activation by a CD54-dependent mechanism. Hepatology. 2011;54:262–272. doi: 10.1002/hep.24352. [DOI] [PubMed] [Google Scholar]
- 22.Cheng F, Li Y, Feng L, Li S. Hepatic stellate cell activation and hepatic fibrosis induced by ischemia/reperfusion injury. Transplant Proc. 2008;40:2167–2170. doi: 10.1016/j.transproceed.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Q, Ng KT, Fan ST, Lim ZX, Guo DY, Liu XB, Liu Y, Poon RT, Lo CM CM, Man K. Distinct mechanism of small-for-size fatty liver graft injury-- Wnt4 signaling activates hepatic stellate cells. Am. J. Transplant. 2010;10:1178–1188. doi: 10.1111/j.1600-6143.2010.03102.x. [DOI] [PubMed] [Google Scholar]
- 24.Gao W, Bentley RC, Madden JF, Clavien PA. Apoptosis of sinusoidal endothelial cells is a critical mechanism of preservation injury in rat liver transplantation. Hepatology. 1998;27:1652–1660. doi: 10.1002/hep.510270626. [DOI] [PubMed] [Google Scholar]
- 25.Stolz DB, Ross MA, Ikeda A, Tomiyama K, Kaizu T, Geller DA, Murase N. Sinusoidal endothelial cell repopulation following ischemia/reperfusion injury in rat liver transplantation. Hepatology. 2007;46:1464–1475. doi: 10.1002/hep.21887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uemura T, Gandhi CR. Inhibition of DNA synthesis in cultured hepatocytes by endotoxin-conditioned medium of activated stellate cells is transforming growth factor-β- and nitric oxide-independent. Br. J. Pharmacol. 2001;133:1125–1133. doi: 10.1038/sj.bjp.0704151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thirunavukkarasu C, Uemura T, Wang LF, Watkins SC, Gandhi CR. Normal rat hepatic stellate cells respond to endotoxin in LBP-independent manner to produce inhibitor(s) of DNA synthesis in hepatocytes. J. Cell Physiol. 2005;204:654–665. doi: 10.1002/jcp.20366. [DOI] [PubMed] [Google Scholar]
- 28.Thirunavukkarasu C, Watkins SC, Gandhi CR. Mechanisms of endotoxin-induced NO, IL-6 and TNF-alpha production in activated rat hepatic stellate cells: role of p38MAPK. Hepatology. 2006;44:389–398. doi: 10.1002/hep.21254. [DOI] [PubMed] [Google Scholar]
- 29.Miyata T, Yokoyama I, Todo S, Tzakis A, Selby R, Starzl TE. Endotoxaemia, pulmonary complications, and thrombocytopenia in liver transplantation. Lancet. 1989;2:189–191. doi: 10.1016/s0140-6736(89)90373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehal WZ, Juedes AE, Crispe IN. Selective retention of activated CD8+ T cells by the normal liver. J. Immunol. 1999;163:3202–3210. [PubMed] [Google Scholar]
- 31.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ Treg cells. J. Exp. Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raimondi G, Sumpter TL, Matta BM, Pillai M, Corbitt N, Vodovotz Y, Wang Z, Thomson AW. Mammalian target of rapamycin inhibition and alloantigen-specific regulatory T cells synergize to promote long-term graft survival in immunocompetent recipients. J. Immunol. 2010;184:624–636. doi: 10.4049/jimmunol.0900936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klugewitz K, Adams DH, Emoto M, Eulenburg K, Hamann A. The composition of intrahepatic lymphocytes: shaped by selective recruitment? Trends Immunol. 2004;25:590–594. doi: 10.1016/j.it.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Bowen DG, McCaughan GW, Bertolino P. Intrahepatic immunity: a tale of two sites? Trends Immunol. 2005;26:512–517. doi: 10.1016/j.it.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 35.McAleer JP, Rossi RJ, Vella AT. Lipopolysaccharide potentiates effector T cell accumulation into nonlymphoid tissues through TRIF. J. Immunol. 2009;182:5322–5330. doi: 10.4049/jimmunol.0803616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maksimow M, Söderström TS, Jalkanen S, Eriksson JE, Hänninen A. Fas costimulation of naive CD4 T cells is controlled by NF-kappaB signaling and caspase activity. J. Leukoc. Biol. 2006;79:369–377. doi: 10.1189/jlb.0505238. [DOI] [PubMed] [Google Scholar]
- 37.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, Fauci AS. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21induce the expression of programmed death-1 and its ligands. J. Immunol. 2008;181:6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 40.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 42.Lau AW, Biester S, Cornall RJ, Forrester JV. Lipopolysaccharide-activated IL-10-secreting dendritic cells suppress experimental autoimmune uveoretinitis by MHCII-dependent activation of CD62L-expressing regulatory T cells. J. Immunol. 2008;180:3889–3899. doi: 10.4049/jimmunol.180.6.3889. [DOI] [PubMed] [Google Scholar]
- 43.Darrasse-Jéze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao KH, Masilamani RF, Dustin ML, Rudensky A, Liu K, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J. Exp. Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou T, Caton AJ, Koretzky GA, Kambayashi T. Dendritic cells induce regulatory T cell proliferation through antigen-dependent and -independent interactions. J. Immunol. 2010;185:2790–2799. doi: 10.4049/jimmunol.0903740. [DOI] [PubMed] [Google Scholar]
- 45.Liao G, Nayak S, Regueiro JR, Berger SB, Detre C, Romero X, de Waal Malefyt R, Chatila TA, Herzog RW, Terhorst C. GITR engagement preferentially enhances proliferation of functionally competent CD4+CD25+FoxP3+ regulatory T cells. Int. Immunol. 2010;22:259–270. doi: 10.1093/intimm/dxq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, Sharpe AH, Powrie F. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J. Immunol. 2006;177:4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowaslky J, Levitsky HI, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 48.Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J. Exp. Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rudensky AY. Regulatory T cells and Foxp3. Immunol. Rev. 2011;241:260–268. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li W, Kuhr CS, Zheng XX, Carper K, Thomson AW, Reyes JD, Perkins JD. New insights into mechanisms of spontaneous liver transplant tolerance: the role of Foxp3-expressing CD25+CD4+ regulatory T cells. Am. J. Transplant. 2008;8:1639–1651. doi: 10.1111/j.1600-6143.2008.02300.x. [DOI] [PubMed] [Google Scholar]
- 51.Bertolino P, Trescol-Biémont MC, Thomas J, Fazekas de St Groth B, Pihlgren M, Marvel J, Rabourdin-Combe C. Death by neglect as a deletional mechanism of peripheral tolerance. Int. Immunol. 1999;11:1225–1238. doi: 10.1093/intimm/11.8.1225. [DOI] [PubMed] [Google Scholar]
- 52.Hellerbrand S, Wang C, Tsukamoto H, Brenner DA, Rippe RA. Expression of intracellular adhesion molecule 1 by activated hepatic stellate cells. Hepatology. 1996;24:670–676. doi: 10.1002/hep.510240333. [DOI] [PubMed] [Google Scholar]
- 53.Knittel T, Dinter C, Kobold D, Neubauer K, Mehde M, Eichhorst S, Ramadori G. Expression and regulation of cell adhesion molecules by hepatic stellate cells (HSC) of rat liver: involvement of HSC in recruitment of inflammatory cells during hepatic tissue repair. Am. J. Pathol. 1999;154:153–167. doi: 10.1016/s0002-9440(10)65262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aziz-Seible RS, Casey CA. Fibronectin: Functional character and role in alcoholic liver disease. World J. Gastroenterol. 2011;17:2482–2499. doi: 10.3748/wjg.v17.i20.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore C, Shen XD, Fondevila C, Gao F, Coito AJ. Blockade of fibronectin-alpha4beta1 adhesive interactions down-regulates cyclooxygenase-2 inducible nitric oxide synthase and prolongs recipient survival in a 24-hour model of cold hepatic ischemia-reperfusion injury. Transplant. Proc. 2005;37:1682–1683. doi: 10.1016/j.transproceed.2005.03.146. [DOI] [PubMed] [Google Scholar]
- 56.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhai Y, Shen XD, Hancock WW, Gao F, Qiao B, Lassman C, Belperio JA, Strieter RM, Busuttil RW, Kupiec-Weglinski JW. CXCR3+CD4+ T cells mediate innate immune function in the pathophysiology of liver ischemia/reperfusion injury. J. Immunol. 2006;176:6313–6322. doi: 10.4049/jimmunol.176.10.6313. [DOI] [PubMed] [Google Scholar]
- 58.Chen CH, Kuo LM, Chang Y, Wu W, Goldbach C, Ross MA, Stolz DB, Chen L, Fung JJ, Lu L, et al. In vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology. 2006;44:1171–1181. doi: 10.1002/hep.21379. [DOI] [PubMed] [Google Scholar]
- 59.Benten D, Kumaran V, Joseph B, Schattenberg J, Popov Y, Schuppan D, Gupta S. Hepatocyte transplantation activates hepatic stellate cells with beneficial modulation of cell engraftment in the rat. Hepatology. 2005;42:1072–1081. doi: 10.1002/hep.20889. [DOI] [PubMed] [Google Scholar]
- 60.Wiegard C, Frenzel C, Herkel J, Kallen KJ, Schmitt E, Lohse AW. Murine liver antigen presenting cells control suppressor activity of CD4+CD25+ regulatory T cells. Hepatology. 2005;42:193–199. doi: 10.1002/hep.20756. [DOI] [PubMed] [Google Scholar]
- 61.Paulsen M, Mathew B, Qian J, Lettau M, Kabelitz D, Janssen O. FasL cross-linking inhibits activation of human peripheral T cells. Int. Immunol. 2009;21:587–598. doi: 10.1093/intimm/dxp028. [DOI] [PubMed] [Google Scholar]
- 62.Pender MP. Activation-induced apoptosis of autoreactive and alloreactive T lymphocytes in the target organ as a major mechanism of tolerance. Immunol. Cell Biol. 1999;77:216–223. doi: 10.1046/j.1440-1711.1999.00818.x. [DOI] [PubMed] [Google Scholar]
- 63.Egawa T, Tillman RE, Naoe Y, Taniuchi I, Littman DR. The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. J. Exp. Med. 2007;204:1945–1957. doi: 10.1084/jem.20070133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 65.Knolle P, Schlaak J, Uhrig A, Kempf P, zum Buschenfelde KHM, Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J. Hepatol. 1995;22:226–229. doi: 10.1016/0168-8278(95)80433-1. [DOI] [PubMed] [Google Scholar]
- 66.Groux H, Bigler M, de Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4 T cells. J. Exp. Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knolle PA, Germann T, Treichel U, Uhrig A, Schmitt E, Hegenbarth S, Lohse AW, Gerken G. Endotoxin down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells. J. Immunol. 1999;162:1401–1407. [PubMed] [Google Scholar]
- 68.Bamboat ZM, Stableford JA, Plitas G, Burt BM, Nguyen HM, Welles AP, Gonen M, Young JW, DeMatteo RP. Human liver dendritic cells promote T cell hyporesponsiveness. J. Immunol. 2009;182:1901–1911. doi: 10.4049/jimmunol.0803404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 70.Erhardt A, Biburger M, Papadopoulos T, Tiegs G. IL-10, regulatory T cells, and Kupffer cells mediate tolerance in concanavalin A-induced liver injury in mice. Hepatology. 2007;45:475–485. doi: 10.1002/hep.21498. [DOI] [PubMed] [Google Scholar]
- 71.Knolle PA, Loser E, Protzer U, Duchmann R, Schmitt E, zum Buschenfelde KHM, Rose-John S, Gerken G. Regulation of endotoxin-induced IL-6 production in liver entothelial cells and Kupffer cells by IL-10. Clin. Exp. Immunol. 1997;107:555–561. doi: 10.1046/j.1365-2249.1997.d01-959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Irla M, Küpfer N, Suter T, Lissilaa R, Benkhoucha M, Skupsky J, Lalive PH, Fontana A, Reith W, Hugues S. MHC class II-restricted antigen presentation by plasmacytoid dendritic cells inhibits T cell-mediated autoimmunity. J. Exp. Med. 2010;207:1891–1905. doi: 10.1084/jem.20092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kambayashi T, Allenspach EJ, Chang JT, Zou T, Shoag JE, Reiner SL, Katon AJ, Koretzky GA. Inducible MHC class II expression by mast cells supports effector and regulatory T cell activation. J. Immunol. 2009;182:4686–4695. doi: 10.4049/jimmunol.0803180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hombach AA, Kofler D, Hombach A, Rappl G, Abken H. Effective proliferation of human regulatory T cells requires a strong costimulatory CD28 signal that cannot be substituted by IL-2. J. Immunol. 2007;179:7924–7931. doi: 10.4049/jimmunol.179.11.7924. [DOI] [PubMed] [Google Scholar]
- 75.Chen X, Jensen PE. Cutting edge: primary B lymphocytes preferentially expand allogeneic FoxP3+ CD4 T cells. J. Immunol. 2007;179:2046–2050. doi: 10.4049/jimmunol.179.4.2046. [DOI] [PubMed] [Google Scholar]
- 76.Bamboat ZM, Ocuin LM, Balachandran VP, Obaid H, Plitas G, DeMatteo RP. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. J. Clin. Invest. 2010;120:559–569. doi: 10.1172/JCI40008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ochando JC, Yopp AC, Yang Y, Garin A, Li Y, Boros P, Liodra J, Ding Y, Lira SA, Krieger NR, et al. Lymph node occupancy is required for the peripheral development of alloantigen-specific Foxp3+ regulatory T cells. J. Immunol. 2005;174:6993–7005. doi: 10.4049/jimmunol.174.11.6993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.