Abstract
The maintenance of appropriate glucose levels is necessary for survival. Within the brain, specialized neurons detect glucose fluctuations and alter their electrical activity. These glucose-sensing cells include hypothalamic arcuate nucleus neurons expressing neuropeptide Y (NPY) and lateral hypothalamic area (LHA) neurons expressing orexin/hypocretins (ORX) or melanin-concentrating hormone (MCH). Within the LHA, a population of NPY-expressing cells exists; however, their ability to monitor energy status is unknown. We investigated whether NPY neurons located in the LHA, a classic hunger center, detect and respond to fluctuations in glucose availability and compared these responses with those of known LHA glucose sensors expressing ORX or MCH. Using mice expressing green fluorescent protein under the control of NPY regulatory elements, we identified LHA NPY cells and explored their anatomical distribution, neurochemical and electrical properties, in vivo responses to fasting and insulin-induced hypoglycemia, and in situ electrical responses to extracellular glucose. We report that NPY, ORX, and MCH are expressed in nonoverlapping populations within the LHA. Subpopulations of LHA NPY neurons were activated in vivo by both a 6-h fast and insulin-induced hypoglycemia. Likewise, increased extracellular glucose suppressed the electrical activity of approximately 70% of LHA NPY neurons in situ, eliciting hyperpolarization and activating background K+ currents. Furthermore, we report that the glucose sensitivity of LHA NPY neurons is significantly different from neighboring ORX and MCH neurons. These data suggest that NPY-expressing cells in the LHA are a novel population of glucose-sensing neurons that represent a new player in the brain circuitry integrating information about glucose homeostasis.
The maintenance of appropriate levels of glucose, as a primary fuel source, is required for survival. To protect health and viability, central and peripheral mechanisms are in place to detect low and high levels of glucose, which then trigger adaptive changes in hormone release, brain function, and feeding behavior. Brain glucose-sensing neurons were first reported approximately 50 yr ago and are categorized as glucose inhibited (GI; cells decrease their electrical activity in response to increased glucose concentration) or glucose excited (GE; cells increase their electrical activity in response to increased glucose concentration) (1–4). Many of the cells identified thus far to sense glucose also express neuropeptide(s) intimately involved in arousal and energy balance. Within the lateral hypothalamic area (LHA), neurons expressing the wake-promoting peptides orexins/hypocretins (ORX) are GI, whereas neurons expressing the sleep-promoting neuropeptide melanin-concentrating hormone (MCH) are GE (2, 5). Despite evidence that another distinct population of GI cells may exist in the LHA (6), the chemical identity and characterization of this population has not yet been described.
Neuropeptide Y (NPY) is a 36-amino acid peptide neurotransmitter in the pancreatic polypeptide family (7). Within the brain, NPY is expressed in multiple regions including the LHA and is implicated in numerous physiological processes including energy homeostasis (8). With relevance to energy balance, NPY is a potent orexigenic factor (9–11). In particular, NPY cells in the hypothalamic arcuate nucleus (ARC) project to the LHA (12, 13) and are crucial for feeding (14), and a subpopulation of ARC NPY cells are GI (15, 16). Also in the ARC is a key population of GE neurons that express proopiomelanocortin (4).
Recently a number of reports examining the contribution of non-ARC-derived hypothalamic NPY to energy balance have been published (17–19), highlighting the importance of NPY in the appropriate control of energy homeostasis. Although NPY cell bodies exist within the LHA, a brain region implicated in the control of energy balance (20), this specific population of cells has not been extensively studied, and their properties and regulation by body energy levels are unknown. Here we aimed to determine whether LHA NPY neurons are sensitive to energy status and fluctuations in glucose availability and whether these cells represent a novel population or comprise a subset of the previously described LHA glucose-sensing neurons.
Materials and Methods
Animals
Transgenic mice on a C57BL/6 background expressing tau sapphire green fluorescent protein (GFP) under the control of NPY regulatory elements (21) (NPY-GFP mice; generous gift from Dr. Jeffrey Friedman, Rockefeller University, New York, NY) and wild-type C57BL/6 mice were group housed and maintained on a 12-h light, 12-h dark cycle (lights on at 0600 h) with ad libitum access to chow diet and water unless otherwise stated. All experiments were in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986.
Acute insulin-induced hypoglycemia
To prevent basal alterations in blood glucose between animals, mice were assessed in the light cycle when they are normally less active. Food was removed at 0800 h and adult male NPY-GFP mice were treated at 1400 h with an ip dose of insulin 1.5 U/kg to induce hypoglycemia (n = 6) or 0.9% saline (n = 6). Tail vein blood glucose was measured via a glucometer immediately before dosing and every 30 min thereafter for 60–90 min. After this, mice were deeply anesthetized, perfused with saline and then fixative, and brains extracted and prepared for immunohistochemical analysis as described below.
Acute food deprivation
Male (n = 8) and female (n = 8) adult NPY-GFP mice underwent a sex- and time-matched fed or fasted protocol. Fasted mice were food deprived for 6 h from 1600 h, encompassing 2 h before and 4 h after the onset of the dark cycle. Tail vein blood glucose was measured by glucometer at the start of the procedure at time 0 and 2, 3, 4, and 6 h later in ad libitum-fed and 6-h fasted mice.
A separate cohort of eight adult male NPY-GFP mice underwent the same ad libitum-fed or 6-h fasted protocol without blood sampling (n = 4 per group). Four hours into the dark phase (2200 h), mice were deeply anesthetized and brains were extracted, submerged in fixative, and prepared for immunohistochemical analysis as described below.
In situ hybridization histochemistry (ISHH) and immunohistochemistry (IHC)
Mice were anesthetized with ketamine (100 mg/kg, ip) and xylazine (20 mg/kg, ip), and transcardially perfused with diethylpyrocarbonate-treated 0.9% saline followed by phosphate-buffed 10% formalin. Brains were removed, postfixed for 4 h, and then submerged overnight in 30% sucrose in diethylpyrocarbonate-treated PBS. Brains were cut on a freezing sliding microtome at 25 μm (collected in 1:5 equal series).
NPY riboprobe generation and subsequent free-floating in situ hybridization was performed as previously described (22). After hybridization, tissue was processed for detection of GFP immunoreactivity (IR) by IHC.
Chromagenic and fluorescent IHC was performed as previously described (23). Primary antibodies used were rabbit anti-cFOS (1:10,000; Merck Chemicals, Nottingham, UK), rabbit anti-MCH and rabbit antiorexin-A (both 1:1,000; Phoenix Pharmaceuticals Europe, Karlsruhe, Germany), and goat anti-GFP (1:1,000; Abcam, Cambridge, UK). Secondary antibodies were either biotinylated (Jackson ImmunoResearch Europe, Newmarket, UK) or fluorophore conjugated (Molecular Probes, Eugene, OR). Chromagenic signal detection was achieved with an amplification step (Vectorstain Elite; Vector Laboratories, Peterborough, UK) and diaminobenzidine (Vector Laboratories).
LHA analysis was performed using a Zeiss Axioskop II (Carl Zeiss, Welwyn Garden City, UK) or Olympus BX61WI microscope (Olympus UK, Southend-on-Sea, UK) with attached charge-coupled device camera. Bregma levels were assigned using information from the publication, The Mouse Brain In Stereotaxic Coordinates (24) and digital images captured using Axiovision 4.3 software (Carl Zeiss) or Fluoview 2.1b (Olympus UK). Images were merged (where appropriate) using Adobe Photoshop CS3 (Adobe Systems Inc., San Jose, CA). Coexpression in dual-labeled cells was determined manually.
Electrophysiology
Standard current and voltage clamp whole-cell patch clamp electrophysiology was performed on male and female wild-type (14–25 d, n = 10) and NPY-GFP mice (12–30 d; n = 8) as previously described (25). Briefly, coronal brain slices (250 μm thick) containing the LHA were cut and maintained in oxygenated artificial cerebrospinal fluid containing 1 mM glucose except where indicated. GFP-expressing neurons in live slices were identified by epifluorescence using an Olympus BX50WI microscope equipped with filter block and mercury bulb light source. Recordings were made at 30–31 C with K-gluconate based pipette solutions as previously described (25). Junction potential error was estimated to be +10 mV and subtracted from voltage-clamp measurements. Input resistance was calculated from the slope of membrane current-voltage relationship between −80 and −60 mV. To examine whether the effects of glucose are mediated directly or via presynaptic input, one group of recordings was performed in the presence of tetrodotoxin. Some cells were also filled with biocytin to allow subsequent determination of cellular morphology or post-recording IHC for ORX or MCH as previously described (2).
Data analysis
Data were analyzed with a t test, one-way ANOVA, or repeated-measures ANOVA, followed by Tukey’s post hoc tests, where appropriate. For all analyses, significance was assigned at the P ≤ 0.05 level. All data are presented as mean ± SEM.
Results
LHA NPY GFP-labeled neurons express NPY mRNA
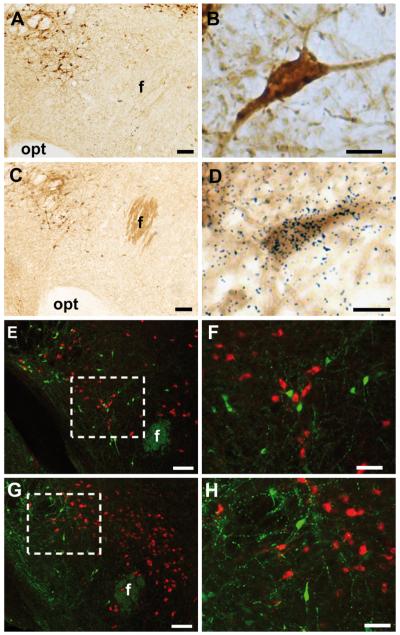
To investigate whether LHA NPY neurons respond to glucose, we used a NPY-GFP mouse line to facilitate the identification of NPY mRNA-expressing cells. Consistent with previous reports in rats (8), we detected endogenous NPY mRNA in the LHA. This endogenous NPY mRNA expression pattern was comparable with the distribution of GFP-IR in NPY-GFP mice (Fig. 1, A–D). To confirm exclusive expression of GFP in NPY mRNA-containing neurons within the LHA, dual-label ISHH/IHC was performed. We determined that, within the LHA, approximately half of NPY mRNA-expressing cells expressed GFP, whereas more than 95% of GFP-labeled cells coexpressed NPY mRNA (Fig. 1, C and D). These data indicate that GFP expression is eutopic in the LHA and confirm the validity of the NPY-GFP reporter line for assessing LHA NPY cell function.
FIG. 1.
Validation and neurochemical characterization of NPY-GFP mice. Single-label GFP-IR within the LHA of NPY-GFP mice at low (A) and high (B) magnification using chromagenic IHC. Dual ISHH and chromagenic IHC for NPY mRNA and GFP-IR at low (C) and high (D) magnification. Dense black granular staining directly overlaying brown GFP-IR cell body indicates a double-labeled cell. GFP-IR (green) (E and F) does not colocalize with MCH-IR [at low (E) and high (F) magnification] or ORX-A-IR (G and H) [at low (G) and high (H) magnification] (red). Boxed areas indicate the regions shown in higher magnification. Opt, Optic tract; f, fornix. Scale bars (A, C, E, and G), 100 μm; (B and D), 5 μm; (F and H), 50 μm.
LHA NPY cells are distinct from classic LHA glucosensors expressing ORX and MCH
The LHA is known to contain two distinct, nonoverlapping populations of glucose-sensing cells expressing ORX or MCH (2, 12, 13). To investigate whether LHA NPY is coexpressed with ORX or MCH, dual-label IHC was performed in NPY-GFP mice (n = 3). GFP was not coexpressed with either MCH (Fig. 1, E and F) or ORX (Fig. 1, G and H). These findings suggest that NPY, ORX, and MCH are expressed in distinct populations of LHA neurons.
Insulin-induced hypoglycemia activates LHA NPY cells in vivo
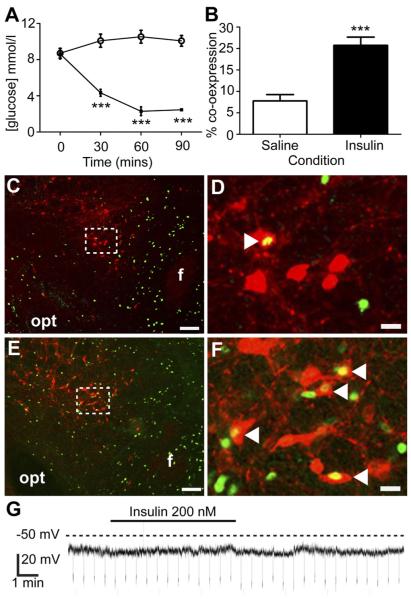
We next investigated whether LHA NPY neurons respond to changes in endogenous glucose levels. Hypoglycemia was elicited with a bolus of insulin (1.5 U/kg) and compared with saline vehicle treatment in NPY-GFP mice. Basal blood glucose levels between treatment groups were similar (Fig. 2A; vehicle 8.7 ± 0.5 mM; insulin 8.3 ± 0.6 mM; P > 0.05, n = 6 per group). In the insulin-treated group, hypoglycemia (<3mM glucose) was detected after 60 min and persisted until the termination of the procedure (Fig. 2A; vehicle 10.1 ± 0.6 mM; insulin 2.0 ± 0.3 mM; P ≤ 0.001). Within the LHA, insulin-induced hypoglycemia significantly increased FOS-IR in GFP-IR cells (Fig. 2B; P ≤ 0.001), indicating that a subpopulation of NPY neurons are activated by insulin-induced hypoglycemia.
FIG. 2.
Insulin-induced hypoglycemia increases FOS-IR in LHA NPY neurons. Insulin (1.5 U/kg, ip). Significantly reduced blood glucose (open circles, saline treated; black squares, insulin treated) (A) and significantly increased FOS-IR within GFP-IR neurons of the LHA compared with saline (B–F). Representative images (C–F) showing FOS-IR (green) and GFP-IR (red) within the LHA of saline (C, low magnification; D, high magnification) or insulin (E, low magnification; F, high magnification) treated mice. Boxed areas indicate the regions shown at high magnification. White arrowheads in D and F indicate double-labeled neurons. G, Representative whole-cell patch-clamp electrophysiological recording from an NPY-GFP mouse brain slice illustrating that, in the presence of 1 μM tetrodotoxin, insulin (200 nM) application had no significant effect on the resting membrane potential of LHA GFP-expressing cells (n = 9). Opt, Optic tract; f, fornix. Scale bars (C and E), 100 μm; (D and F), 15 μm. ***, P ≤ 0.001.
To determine whether the observed responses to insulin-induced hypoglycemia in vivo could be explained by direct action of insulin on LHA NPY neurons, we recorded, in situ, the electrical responses of LHA NPY neurons to bath-applied insulin (200 nM) in the presence of 1 μM tetrodotoxin using whole-cell patch clamp electrophysiology. There was no significant effect of insulin on membrane potential (Fig. 2G; 0.21 ± 0.51 mV depolarization; P > 0.05, n = 9), indicating that LHA NPY neurons do not directly respond to insulin.
Acute food deprivation lowers blood glucose and activates LHA NPY cells in vivo
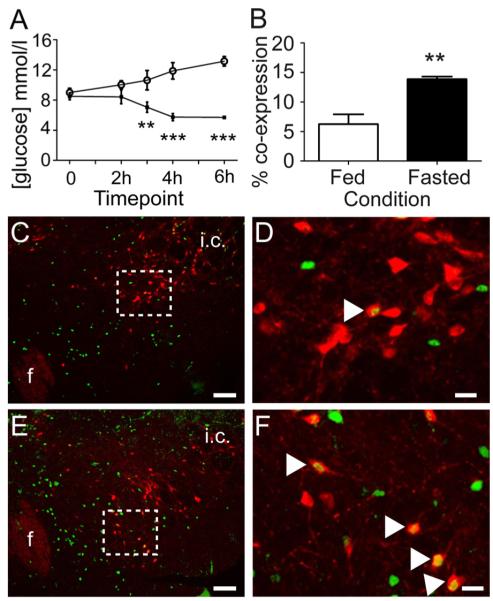
To determine whether LHA NPY cells are sensitive to physiological hypoglycemia, we first confirmed that acute 6 h food deprivation at the end of the light phase (2 h) and beginning of the dark phase (4 h) lowered blood glucose. We observed that food-deprived mice demonstrated significantly lower blood glucose compared with ad libitum-fed controls (Fig. 3A; 6 h fasted 5.71 ± 0.18 mM; fed postprandial blood glucose 13.15 ± 0.62 mM; P ≤ 0.001, n = 8 per group).
FIG. 3.
Acute food deprivation decreases blood glucose and increases FOS-IR in LHA NPY neurons. A, Mice that underwent a 6-h fast from 1600 to 2200 h demonstrated significantly lower blood glucose compared with ad libitum-fed controls (open circles, ad libitum fed; black squares, fasted). B, A separate group of mice that underwent a 6-h fast from 1600 to 2200 h demonstrated significantly more FOS-IR within GFP-IR neurons of the LHA compared with ad libitum-fed control animals. Representative images (C–F) showing FOS-IR (green) and GFP-IR (red) within the LHA of ad libitum-fed (C, low magnification; D, high magnification) or 6-h fasted (E, low magnification; F, high magnification) mice. Boxed areas indicate the regions shown at high magnification. White arrowheads in D and F indicate double-labeled neurons. F, Fornix; i.c., internal capsule. Scale bars (C and E), 100 μm; (D and F), 20 μm. **, P ≤ 0.01; ***, P ≤ 0.001.
In a separate group of NPY-GFP mice, we next investigated whether physiological fasting-induced hypoglycemia influenced the activity of LHA NPY neurons. Compared with ad libitum fed controls, 6 h food deprivation significantly increased FOS-IR in LHA GFP-IR cells (Fig. 3, B–F; P ≤ 0.01). These data indicate that a subset of LHA NPY neurons is responsive to physiologically relevant fluctuations in energy state.
Membrane properties of LHA NPY neurons in situ
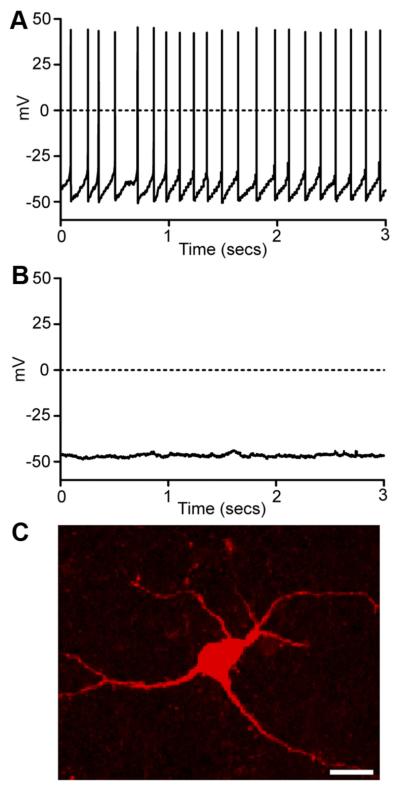
To explore functional properties and glucose responses of individual LHA NPY neurons, we recorded their electrical activity using whole-cell patch clamp electrophysiology in acute mouse brain slices, combined with biocytin filling to visualize cell morphology. Glucose concentrations used in situ (1–5 mM) were consistent with those found in blood in vivo after 6 h food restriction (blood glucose 5.71 ± 0.18 mM) and in time-matched ad libitum-fed controls (blood glucose 13.15 ± 0.62 mM) (Fig. 3A). Moreover, concentrations were also consistent with those reported in the LHA in vivo (26) and in other recent studies of hypothalamic glucosensing in vitro (2, 4, 5, 25). Under baseline conditions (1 mM extracellular glucose), approximately 60% (10 of 17 cells) of NPY cells fired spontaneous actions potentials with a frequency of 4.8 ± 2.0 Hz (Fig. 4A). In turn, approximately 40% (seven of 17 cells) were electrically silent with resting membrane potentials of −48.5 ± 3.0 mV (Fig. 4B). A switch between 1 and 5 mM extracellular glucose hyperpolarized approximately 70% (14 of 21) of LHA NPY cells (8.3 ± 1.0 mV hyperpolarization; P ≤ 0.001, n = 14). Seven of 10 spontaneously active cells were hyperpolarized by 5 mM glucose, demonstrating a significant reduction in spike frequency (Fig. 5A; 70.0 ± 16.0% reduction in firing rate; P ≤ 0.01, n = 7). The input resistance of LHA NPY neurons was 551 ± 71 MΩ (n = 14). Morphologically, NPY cells were multipolar (Fig. 4C) and generally resembled MCH and ORX neurons (2).
FIG. 4.
Intrinsic properties of LHA NPY neurons. A, Current-clamp recording (zero holding current) showing spontaneous action potential discharge (similar data were obtained in 10 of 17 cells). B, Current-clamp recording (zero holding current) showing an electrically silent cell (similar data were obtained in seven of 17 cells). C, A typical image of soma and proximal dendrites of a NPY-GFP cell filled with biocytin. Scale bar, 10 μm.
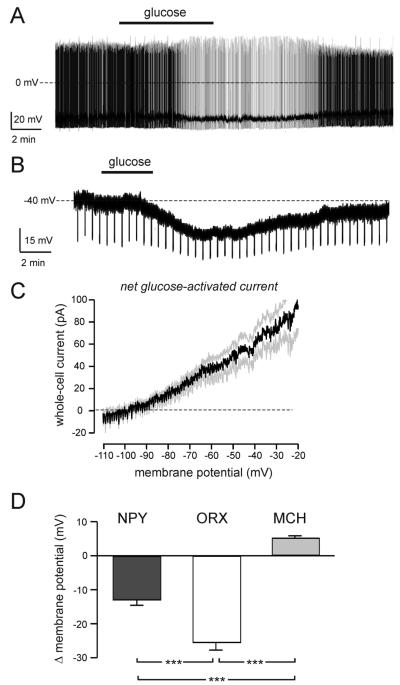
FIG. 5.
Glucose sensing by LHA NPY neurons in situ. A, Typical effect of an increase in extracellular glucose (switch from 1 to 5 mM glucose marked with black bar) in a spontaneously firing LHA NPY-GFP cell. Similar responses were obtained in seven cells. B, Effect of glucose (same concentration switch as in A) on the membrane potential of an LHA NPY-GFP cell in the presence of 1 μM tetrodotoxin. Downward deflections are responses to fixed-amplitude, 1-sec-long injections of hyperpolarizing current (note that elevated glucose decreases their amplitude, implying an increase in membrane conductance). Similar responses were obtained in six cells. C, The current-voltage relationship of the net glucose-activated current. Black line represents mean, gray lines represent SEM (means ± SEM of six cells). D, Effect of increased extracellular glucose (1 to 5 mM switch) on the membrane potential of LHA NPY (n = 6), MCH (n = 14), and ORX (n = 14) cells, recorded in the presence of 1 μM tetrodotoxin. ***, P ≤ 0.001.
Glucose responses of LHA NPY neurons in situ
In the presence of 1 μM tetrodotoxin, glucose still hyperpolarized 75% (six of eight cells) of LHA NPY cells, suggesting a postsynaptic action (Fig. 5B; 13.0 ± 1.6 mV hyperpolarization; P ≤ 0.001, n = 6). In these cells, the net glucose-activated current [measured using voltage-clamp ramps as in our previous studies (25)] had a reversal potential of −95.1 ± 1.8 mV (Fig. 5C; n = 6), suggesting a high selectivity for K+ ions (EK = −101 mV with our solutions). To compare the glucose sensitivity of LHA NPY neurons to that of known LHA glucosensors, namely ORX and MCH cells, we compared glucose-induced changes in membrane potential measured in the presence of 1 μM tetrodotoxin (Fig. 5D). In these experiments, NPY cells (n = 6) were identified by GFP expression before recordings, whereas ORX (n = 14) and MCH (n = 14) cells were identified by postrecording immunostaining. This revealed that LHA NPY neurons show significantly different glucose sensitivity from ORX or MCH neurons (Fig. 5D; P ≤ 0.001).
Discussion
The data presented here advance the understanding of the neurochemical and physiological identity of LHA glucose-sensing neurons. Although such cells were first described several decades ago (1, 3), their identities began to be clarified only very recently, with reports of ORX expression in GI cells, and MCH expression in GE cells (2, 5, 27). Moreover, an intriguing report identified a subpopulation of LHA GI neurons that are ORX negative (6), but their chemical identity and their mechanism of inhibition by glucose remained to be clarified.
The data reported here indicate that NPY-expressing LHA cells, which are distinct from ORX and MCH cells, act as sensors of body energy levels in vivo and are inhibited by glucose in situ. Interestingly, under baseline in situ conditions (1 mM glucose), we detected a greater proportion of spontaneously active LHA NPY cells (defined as generating spontaneous action potentials) than we observed in vivo (defined as expressing FOS-IR) following either insulin-induced hypoglycemia or a 6-h fast. A plausible explanation for the differences in percent of NPY-GFP neuron activation in vitro and in vivo is methodological. The electrophysiological experiments investigated the effect of glucose on individual neurons, whereas in vivo experiments were a snapshot of all LHA NPY-GFP neuron activity at a particular time point using immunohistochemistry to detect the indirect marker of neuronal activity, the immediate early gene c-fos. Moreover, the in vivo experiments investigated the effect of fluctuations of glucose within an intact system. It is therefore possible that inhibitory presynaptic signaling pathways influenced the activity of NPY-GFP neurons in vivo, inputs that were compromised in vitro. Alternatively, the in vivo increase in FOS-IR in NPY-GFP neurons may be an indirect effect caused by glucose and/or insulin activity at neurons providing synaptic first- or other-order interactions with LHA NPY neurons.
Although it is likely that LHA NPY neurons may also be modulated indirectly, our data suggest that glucose is capable of directly inhibiting LHA NPY neurons through a mechanism involving membrane hyperpolarization and opening of background K+ channels in the cell membrane. A similar glucose-sensing mechanism has been recently reported in ORX cells (25) and ventromedial hypothalamic neurons (28). Although the molecular steps involved remain to be determined (discussed in Refs. 25 and 29), our results support the idea that glucose-stimulated K+ channels play a wider role in brain function than originally anticipated (25).
The quantitative comparison of direct glucose responses of the three neurochemically distinct classes of LHA neurons (Fig. 5D) indicate that NPY cells display a glucose sensitivity that is significantly different from ORX and MCH cells (i.e. larger than MCH cells, smaller than ORX cells). This may suggest that LHA NPY, ORX, and MCH cells could be involved in different aspects of glucose homeostasis.
Recent data demonstrating a role for dorsomedial hypothalamic nucleus NPY neurons in glucose homeostasis and energy balance (18, 19) highlight the increasing understanding of the importance of non-ARC NPY neurons in energy homeostasis. Given the paucity of data related to NPY LHA neurons in the literature, it has not yet been clarified where these neurons project or the inputs they receive. However, it has been reported that NPY action within the LHA is orexigenic (10), and overexpression of NPY within the LHA promotes hyperphagia, obesity, decreased dark cycle core temperature, and decreased locomotor activity (30).
In summary, the data presented here characterize a population of NPY neurons that have not been well described and reveal a novel central neurocircuit that integrates information about glucose homeostasis in a way that is neurochemically and electrophysiologically distinct from known LHA glucose sensors.
Acknowledgments
This work was supported by the Sir Jules Thorne Trust (to P.H.), Juvenile Diabetes Research Foundation Grants 1-2003-78 and 1-2006-29 (to M.L.E.); Diabetes United Kingdom Grant RD05/003059 (to M.L.E.); the European Research Council Grant FP7 StG (to D.I.B.); the Wellcome Trust Grant WT081713 (to L.K.H.); and the Cambridge Medical Research Council Centre for the Study of Obesity and Related Disorders (to all authors).
Abbreviations
- ARC
Arcuate nucleus
- GE
glucose excited
- GFP
green fluorescent protein
- GI
glucose inhibited
- IHC
immunohistochemistry
- IR
immunoreactivity
- ISHH
in situ hybridization histochemistry
- LHA
lateral hypothalamic area
- MCH
melanin-concentrating hormone
- NPY
neuropeptide Y
- ORX
orexins/hypocretins
Footnotes
Disclosure Summary: The authors have nothing to disclose.
References
- 1.Anand BK, Chhina GS, Sharma KN, Dua S, Singh B. Activity of single neurons in the hypothalamic feeding centers: effect of glucose. Am J Physiol. 1964;207:1146–1154. doi: 10.1152/ajplegacy.1964.207.5.1146. [DOI] [PubMed] [Google Scholar]
- 2.Burdakov D, Gerasimenko O, Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci. 2005;25:2429–2433. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and osmosensitive neurones of the rat hypothalamus. Nature. 1969;222:282–284. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- 4.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 5.Kong D, Vong L, Parton LE, Ye C, Tong Q, Hu X, Choi B, Brüning JC, Lowell BB. Glucose stimulation of hypothalamic MCH neurons involves K(ATP) channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell Metab. 2010;12:545–552. doi: 10.1016/j.cmet.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu XH, Morris R, Spiller D, White M, Williams G. Orexin a preferentially excites glucose-sensitive neurons in the lateral hypothalamus of the rat in vitro. Diabetes. 2001;50:2431–2437. doi: 10.2337/diabetes.50.11.2431. [DOI] [PubMed] [Google Scholar]
- 7.Tatemoto K. Neuropeptide Y: complete amino acid sequence of the brain peptide. Proc Natl Acad Sci USA. 1982;79:5485–5489. doi: 10.1073/pnas.79.18.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris BJ. Neuronal localisation of neuropeptide Y gene expression in rat brain. J Comp Neurol. 1989;290:358–368. doi: 10.1002/cne.902900305. [DOI] [PubMed] [Google Scholar]
- 9.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 10.Stanley BG, Chin AS, Leibowitz SF. Feeding and drinking elicited by central injection of neuropeptide Y: evidence for a hypothalamic site(s) of action. Brain Res Bull. 1985;14:521–524. doi: 10.1016/0361-9230(85)90100-5. [DOI] [PubMed] [Google Scholar]
- 11.Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- 12.Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M, Elmquist JK. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- 13.Broberger CL, De Lecea L, Sutcliffe JG, Hökfelt T. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J Comp Neurol. 1998;402:460–474. [PubMed] [Google Scholar]
- 14.Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, Barsh GS, Horvath TL, Brüning JC. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 15.Fioramonti X, Contié S, Song Z, Routh VH, Lorsignol A, Pénicaud L. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide Y and pro-opio melanocortin networks? Diabetes. 2007;56:1219–1227. doi: 10.2337/db06-0567. [DOI] [PubMed] [Google Scholar]
- 16.Muroya S, Yada T, Shioda S, Takigawa M. Glucose-sensitive neurons in the rat arcuate nucleus contain neuropeptide Y. Neurosci Lett. 1999;264:113–116. doi: 10.1016/s0304-3940(99)00185-8. [DOI] [PubMed] [Google Scholar]
- 17.Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1030–R1036. doi: 10.1152/ajpregu.00734.2002. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Scott KA, Hyun J, Tamashiro KL, Tray N, Moran TH, Bi S. Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance. J Neurosci. 2009;29:179–190. doi: 10.1523/JNEUROSCI.4379-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao PT, Yang L, Aja S, Moran TH, Bi S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metab. 2011;13:573–583. doi: 10.1016/j.cmet.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernardis LL, Bellinger LL. The lateral hypothalamic area revisited: ingestive behavior. Neurosci Biobehav Rev. 1996;20:189–287. doi: 10.1016/0149-7634(95)00015-1. [DOI] [PubMed] [Google Scholar]
- 21.Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 22.Stanley S, Pinto S, Segal J, Pérez CA, Viale A, DeFalco J, Cai X, Heisler LK, Friedman JM. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proc Natl Acad Sci USA. 2010;107:7024–7029. doi: 10.1073/pnas.1002790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam DD, Zhou L, Vegge A, Xiu PY, Christensen BT, Osundiji MA, Yueh CY, Evans ML, Heisler LK. Distribution and neurochemical characterization of neurons within the nucleus of the solitary tract responsive to serotonin agonist-induced hypophagia. Behav Brain Res. 2009;196:139–143. doi: 10.1016/j.bbr.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Academic Press; San Diego: 2001. [Google Scholar]
- 25.Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O’Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 26.Silver IA, Erecińska M. Glucose-induced intracellular ion changes in sugar-sensitive hypothalamic neurons. J Neurophysiol. 1998;79:1733–1745. doi: 10.1152/jn.1998.79.4.1733. [DOI] [PubMed] [Google Scholar]
- 27.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 28.Williams RH, Burdakov D. Silencing of ventromedial hypothalamic neurons by glucose-stimulated K(+) currents. Pflugers Arch. 2009;458:777–783. doi: 10.1007/s00424-009-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burdakov D, Lesage F. Glucose-induced inhibition: how many ionic mechanisms? Acta Physiol (Oxford, England) 2010;198:295–301. doi: 10.1111/j.1748-1716.2009.02005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiesjema B, Adan RA, Luijendijk MC, Kalsbeek A, la Fleur SE. Differential effects of recombinant adeno-associated virus-mediated neuropeptide Y overexpression in the hypothalamic paraventricular nucleus and lateral hypothalamus on feeding behavior. J Neurosci. 2007;27:14139–14146. doi: 10.1523/JNEUROSCI.3280-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]