Abstract
A subset of people with diabetes fail to mount defensive counterregulatory responses (CRR) to hypoglycemia. Although the mechanisms by which this occurs remain unclear, recurrent exposure to hypoglycemia may be an important etiological factor. We hypothesized that loss of CRR to recurrent exposure to hypoglycemia represents a type of stress desensitization, in which limbic brain circuitry involved in modulating stress responses might be implicated. Here, we compared activation of limbic brain regions associated with stress desensitization during acute hypoglycemia (AH) and recurrent hypoglycemia (RH). Healthy Sprague Dawley rats were exposed to either acute or recurrent 3-d hypoglycemia. We also examined whether changes in neuronal activation were caused directly by the CRR itself by infusing epinephrine, glucagon, and corticosterone without hypoglycemia. AH increased neuronal activity as quantified by c-fos immunoreactivity (FOS-IR) in the cingulate cortex and associated ectorhinal and perirhinal cortices but not in an adjacent control area (primary somatosensory cortex). FOS-IR was not observed after hormone infusion, suggesting that AH-associated activation was caused by hypoglycemia rather than by CRR. Importantly, AH FOS-IR activation was significantly blunted in rats exposed to RH. In conclusion, analogous with other models of stress habituation, activation in the cingulate cortex and associated brain areas is lost with exposure to RH. Our data support the hypothesis that limbic brain areas may be associated with the loss of CRR to RH in diabetes.
In clinical practice in diabetes, hypoglycemia, or even just fear of hypoglycemia, is the main factor limiting the extent to which average blood glucose levels can be lowered. This is a particular problem for a subgroup of patients who develop abnormalities in counterregulatory responses (CRR) that normally protect against hypoglycemia. Loss of CRR associated with decreased symptomatic awareness of hypoglycemia [termed hypoglycemia-associated autonomic failure (HAAF)] is a debilitating condition that significantly increases the risk of patients suffering from episodes of severe hypoglycemia.
To date, there has been a general assumption that HAAF is a direct consequence of alterations in the brain's specialized glucose-sensing apparatus, with research efforts focusing attention on identifying how hypoglycemia is detected, predominantly within the ventral hypothalamus (1, 2). Although progress has been made, the process underlying HAAF remains unclear. However, exposure to antecedent hypoglycemia itself is undoubtedly an important etiological factor (3).
Reduced defensive responses to recurrent, potentially damaging insults is also seen with some other physical or psychological stresses, where repeated or chronic exposure results in “stress habituation” with a reduction in responses when repeatedly challenged, perhaps as a defensive adaptation to limit expensive and potentially damaging stress responses (4). By analogy, HAAF may represent stress habituation to a recurrent homotypic stress, in this case hypoglycemia.
Current data suggest that key areas within the brain's limbic system may be involved in habituation to other stressors. For example, discrete areas within the medial prefrontal cortex have been implicated in habituation to recurrent restraint stress in rodents (5, 6). We hypothesized that discrete areas within the prefrontal cortex and/or connected limbic cortical areas might be involved in modulating responses to recurrent hypoglycemia (RH). Specifically, we anticipated that these brain areas would 1) be activated by acute hypoglycemia (AH) and 2) that this activation would be blunted after RH. We used immunohistochemical detection of the immediate early gene c-fos as an indicator of cellular activation associated with RH. c-fos is a commonly used marker of cellular activation, with expression in the nucleus induced within a few minutes of cell stimulation (7).
Research Design and Methods
Animals
Healthy male Sprague Dawley rats (300–325 g) were used throughout. Rats were group housed before survival surgery and individually housed after surgery on a 12-h light, 12-h dark cycle. In accordance with United Kingdom law, procedures were approved in advance after both a local University of Cambridge and a national (United Kingdom Home Office) review. All chemicals were from Sigma-Aldrich (Gillingham, United Kingdom) unless otherwise stated.
Surgical preparation
Under inhaled anesthesia, rats underwent survival surgery for implantation of vascular catheters to right jugular vein and/or left carotid artery as previously described (8).
Model 1. RH, insulin or saline
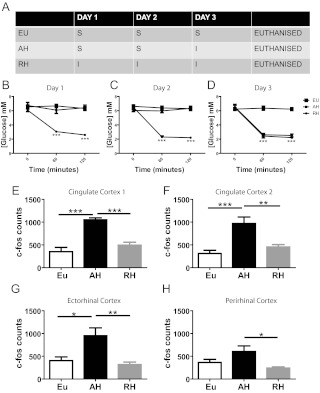
Three groups of rats were injected sc on three consecutive days as follows: 1) control/euglycemia (EU) had d 1–3 saline, 2) AH had d 1–2 saline and insulin (10 U/kg, Humulin S; Eli Lilly, Basingstoke, United Kingdom) on d 3, and 3) RH had d 1–3 insulin (Humulin S 10–6 U/kg) (Fig. 1A), a protocol which we have previously used to create impaired CRR (9). On each study day, blood glucose was measured at 0, 60, and 120 min using blood sampled from the tail vein; 180 min after injection on d 3, rats were transcardially perfused with saline, then fixative and brains were extracted and prepared for immunohistochemistry (IHC) as previously described (10).
Fig. 1.
Model 1. Insulin-induced hypoglycemia results in increased cingulate cortex, Ect, and PRh activation. Study design, showing saline (S) or insulin (I) treatment for each of the experimental groups on the three consecutive study days (A). Plasma glucose values for the three experimental groups of d 1 (B), d 2 (C), and d 3 (D). c-fos counts in cingulate cortex (E and F), Ect (G), and PRh (F) after 3 d of glycemic manipulation. Data are presented as mean ± sem, n = 8–10 in each group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Model 2. RH, matching exogenous insulin
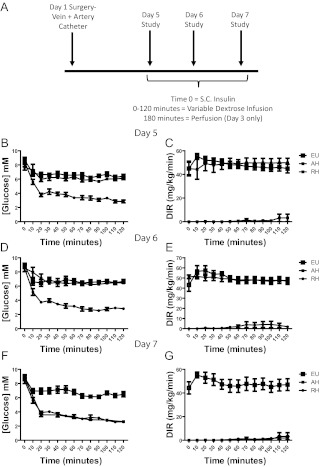
Although simple, a limitation of model 1, in which we used saline-injected controls, is that observed changes in brain activation could be attributable to insulin itself rather than hypoglycemia. To control for exogenous insulin, we therefore studied a further three groups of catheterized (jugular and carotid catheters) rats. In this model, on the three consecutive study days, sc Humulin S (10 U/kg) was given to all rats but with 20% dextrose being administered as an intravascular infusion as required to control plasma glucose. Food, but not water, was removed 1 h before insulin injections, and animals were adjusted to and maintained at glycemic targets for 120 min.
Using this protocol, 1) EU animals received 3 d of dextrose infusions to maintain plasma glucose above 6 mm, 2) AH animals received 2 d of EU followed by 1 d of hypoglycemia with plasma glucose lowered to 3 mm, and 3) RH received 3 d of hypoglycemia; 180 min after injection on d 3, rats were transcardially perfused with saline, then fixative, and brains removed for IHC processing.
Model 3. Recreating hormonal CRR independent of hypoglycemia
It is possible that any brain activation seen after AH may be a response to the CRR hormones that are elevated during hypoglycemia (and indeed lost with RH) and/or the physiological responses such as change in heart rate, etc., which these induce. To examine this, we studied a further group of chronically catheterized (jugular vein) rats which underwent 1 d studies with 120-min intravascular infusions of CRR hormones (corticosterone 3 μg/kg·min, adrenaline 3 μg/kg·min, and glucagon 5 ng/kg·min) to mimic the rise in CRR hormones seen during AH. Blood was sampled at 0 and 120 min for confirmation of plasma hormone concentrations; 180 min after the start of infusions, rats were transcardially perfused with saline, then fixative, and brains were extracted and prepared for IHC.
Immunohistochemistry
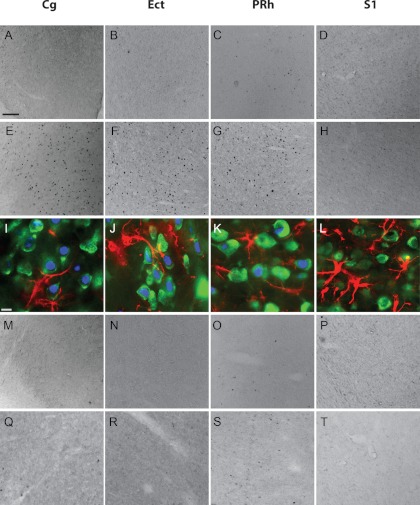
Hypoglycemia-induced activation patterns were examined using IHC as previously described (9, 11) using the after primary antibodies rabbit anti-c-fos (1:8000; Millipore, Billerica, MA), mouse antineuronal nuclei (NeuN) (1:1000; Millipore), and rabbit antiglial fibrillary acidic protein (GFAP) (1:1000; Millipore), and the after secondary antibodies biotinylated donkey antirabbit IgG (1:1000; Jackson ImmunoResearch, West Grove, PA), Alexa Fluor 488 donkey antimouse (1:1000; Invitrogen, Carlsbad, CA), and Alexa Fluor 568 donkey antirabbit (1:1000; Invitrogen), respectively (see further details in Supplemental data, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Based on initial screening of the pattern of distribution of c-fos immunoreactive (FOS-IR) cells in the brain, we identified four cortical areas of interest for a detailed comparison: cingulate cortex 1 (levels from bregma 1.80, 0.96, 0.36, −0.24, −0.84), cingulate cortex 2 (levels from bregma 1.80, 0.96, 0.36, −0.24, −0.84), perirhinal cortex (PRh) (levels from bregma −3.24, −3.84, −4.44, −5.04, −5.64), and ectorhinal cortex (Ect) (levels from bregma −3.24, −3.84, −4.44, −5.04, −5.64). We also examined the primary somatosensory cortex (S1) (levels from bregma 1.80, 0.96, 0.36, −0.24, −0.84) as a control cortical area adjacent to the cingulate cortex. AH-treated brains were then processed for triple IHC, for FOS-IR, the neuronal marker NeuN, and the glial marker GFAP, as previously described (10, 12).
Laboratory assays
Plasma glucose was measured using an Analox GM-9 analyzer (Analox Instruments, London, United Kingdom). Plasma corticosterone (MP Biomedicals, Orangeburg, NY) insulin and glucagon (Linco Research, St. Charles, MO) were measure by RIA in accord with manufacturer's instructions. Plasma epinephrine was measured using a 2-d-procedure ELISA kit (IBL, Hamburg, Germany).
Statistics
Data were analyzed by either 1) one-way ANOVA with Tukey's or Dunnett's post hoc comparisons, or 2) two-way ANOVA with post hoc Bonferroni test, as appropriate. Data are expressed as mean ± sem. For all analyses, statistical significance was assigned at P < 0.05.
Results
Model 1. Limbic cortical brain areas are activated by AH but not RH
The doses of insulin used resulted in 1 or 3 d of moderate hypoglycemia in AH and RH animals, respectively (Fig. 1, B–D). In AH, a significant increase in FOS-IR (suggesting increased cellular activation) was observed in the cingulate cortex, PRh, and Ect compared with EU treatment (Fig. 1, E–H). In contrast, FOS-IR in these regions in RH rats was not different from EU rats (Fig. 1, E–H). These data indicate that hypoglycemia-induced activation of key limbic brain areas is impaired after 2 d of previous exposure to the same hypoglycemic stimulus.
Model 2. Limbic cortical brain area activation is independent of insulin exposure
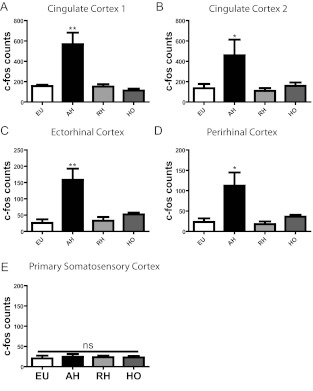
It is possible that the changes in FOS-IR seen in model 1 were caused by insulin rather than hypoglycemia per se. To examine this possibility, we studied animals that underwent three sequential days of studies but with identical exposure to insulin and infusion of exogenous dextrose as required to achieve glycemic targets (Fig. 2). In keeping with our hypothesis, significantly increased FOS-IR nuclei were observed in the cingulate cortex, Ect, and PRh after AH but not RH (see figure 4 below). This was not simply due to increased activation in all cortical brain areas, given that no changes were observed in an adjacent cortical control area (S1; for representative images see, Fig. 3). To determine whether FOS-IR expression represented neuronal or glial activation, we performed triple IHC for c-fos, neuronal marker NeuN, and glial marker GFAP in AH-treated rats. FOS-IR was predominantly neuronal, as demonstrated by colocalization of FOS-IR with NeuN, not GFAP (Fig. 3). These data suggest that hypoglycemia-induced changes in neuronal activation are independent of insulin exposure.
Fig. 2.
Model 2. Controlling for insulin exposure with 3 d hypoglycemia/EU. Schematic representation showing study design, with 3 d of insulin exposure, using a variable dextrose infusion (DIR) to maintain EU or allow hypoglycemia as desired (A). Glucose profiles and DIR for the three consecutive study days: d 5 (B and C), d 6 (D and E), and d 7 (E and F). Data are presented as mean ± sem, n = 8–10 in each group.
Fig. 4.
Model 2. Limbic cortical brain area activation is independent of insulin exposure. FOS-IR counts were quantified in; cingulate cortex 1 (A), cingulate cortex 2 (B), Ect (C), PRh (E), and S1 (E) after EU, AH, RH, and HO treatment. Data are mean ± sem, n = 8–10 in each group. *, P < 0.05; **, P < 0.01. ns, Not significant.
Fig. 3.
AH, but not RH, increases neuronal activity in the cingulate cortex (Cg), Ect, and PRh. FOS-IR in sections from representative treatment conditions, EU (A–D), AH (E–H), RH (M–P), and CRR HO (Q–T) treatment. I–L, Triple IHC for FOS-IR (blue), NeuN (green), and GFAP (red), carried out in AH-treated rats only. Scale bar in EU-treated Cg, 100 μm (applies to all except I—L). Scale bar in I, 20 μm (applies to I–L).
Cortical brain areas are not activated by hormonal CRR alone
To confirm that increased cortical brain activation after AH is not a response to the CRR hormones elevated during hypoglycemia, FOS-IR was assessed in rats treated with CRR hormones. Specifically, rats were infused with the CRR hormones resulting in levels of epinephrine (440 ± 72 to 5222 ± 695 pg/ml, P < 0.001), glucagon (38 ± 8 to 155 ± 31 pg/ml, P < 0.01), and corticosterone (258 ± 15 to 473 ± 51 ng/ml, P < 0.01), equivalent to previously reported concentrations during hypoglycemia (1, 2, 13). As expected, plasma glucose also rose in response to CRR infusion (8.7 ± 0.7 to 16.6 ± 2 mm, P < 0.01). Importantly, there was no increase in FOS-IR in the cortical brain areas assessed after hormone infusion only (HO) (Figs. 3 and 4), suggesting that changes in brain activation after AH are not an indirect consequence of the systemic CRR response.
Conclusions
The mechanisms by which some patients with diabetes develop impaired responses to hypoglycemia remain unclear. A better understanding of these changes and the brain areas involved might allow targeted therapies aimed at reversing these alterations, allowing patients to improve their glycemic control more safely. The data presented here demonstrate that AH activates the cingulate cortex and associated Ect and PRh but not other adjacent cortical areas. This activation is impaired when animals have been previously exposed to hypoglycemia. Importantly, data from model 2 show that these changes are not related to insulin but rather to hypoglycemia.
Infusion of CRR hormones did not activate these cortical areas, suggesting that the changes seen with hypoglycemia are not a consequence of CRR hormones. As anticipated, CRR infusion resulted in hyperglycemia, and although unlikely that this would have masked any effect of CRR hormones, this could be tested further by combining hormone infusions with an EU clamp to prevent the rise in glucose. It is also important to note that we have not examined directly whether CRR hormone rises during AH are related to HAAF.
Although most previous studies have focused on the role of the hypothalamus, some reports also implicate limbic activation in areas such as insular cortex, amygdala, and thalamus associated with AH (14–16). One group reported that thalamic activation was blunted with RH (15). Here, we report that acute activation of cingulate cortex, Ect, or PRh in rodents with hypoglycemia is blunted with repeat exposure to hypoglycemia. Another important difference is that none of these previous studies assessed neuronal activation independent of exogenous insulin exposure as we have done, despite the fact that numerous studies have reported an effect of circulating insulin on responses to hypoglycemia (e.g. Refs. 17, 18). Because FOS-IR is an indirect marker of activity, it is not clear whether our observations of increased cingulate cortex, Ect, or PRh activation represents a first order site or forms a part of the circuitry generating or facilitating autonomic responses to hypoglycemia and/or involved in the symptomatic awareness of hypoglycemia.
The cingulate cortex has been implicated in a number of biological processes, including monitoring of the internal milieu and control of autonomic function. It has also been reported to be activated in situations when errors in cognition are likely to happen (19) and is linked to hypothalamic and brain stem autonomic nuclei via descending neurological pathways (20). In keeping with actions to modulate the autonomic system, patients with cingulate damage fail to mount autonomic responses (20, 21). Anatomically, the cingulate projects to the ectorhinal and perirhinal areas in the parahippocampal gyrus of the temporal lobe via the cingulum but may also receive afferents back from these areas (22, 23). Our study does not allow us to examine whether the changes in ectorhinal/perirhinal activation are secondary to changes in cingulate activity. Both the Ect and PRh have been implicated in sympathetic activity, with reciprocal connections between both the ectorhinal, perirhinal, and common sympathetic output regions, such as the adrenal gland, stellate ganglion, and celiac ganglion (24). Therefore, it is possible that the cingulate cortex, Ect, and PRh collectively exert a controlling influence over autonomic outflow from the brain.
In keeping with our findings, noninvasive human imaging studies report activation by AH in a number of limbic brain areas implicated in facilitating/modifying responses to other stressors, including the cingulate/medial prefrontal cortex and thalamus (25–27). A role for the cingulate cortex, Ect, and/or PRh in HAAF has not previously been reported, although data suggest a hypoglycemia-mediating role for other limbic areas. For example, changes in thalamic activation have been reported in healthy humans after RH (28), and a direct modulatory role for the amygdala on CRR has been reported in rodents (29).
It is important to acknowledge that we show an association between RH and blunted cortical activation but do not prove that changes in these areas contribute to the etiology of HAAF. However, mechanistic studies suggest that efferent pathways from areas within the medial prefrontal cortex adjacent to the cingulate may be intrinsically involved in down-regulating responses to recurrent restraint stress, supporting a role for prefrontal cortical areas in the genesis of stress habituation (5).
In summary, our data show that limbic cortical brain areas, including the cingulate cortex, Ect, and PRh, are active when blood glucose falls and that this activity is blunted after RH. We speculate that these cortical areas may contribute to the development of HAAF. To date, most mechanistic work using rodent models to examine HAAF has concentrated on the hypothalamus. The significance of our work is that it suggests that broader brain areas may contribute to the development of HAAF and may thus represent new areas for targeted therapy aimed at minimizing problematic hypoglycemia in diabetes.
Supplementary Material
Acknowledgements
This work was supported by Juvenile Diabetes Research Foundation Grants 1-2003-78 and 1-2006-29 and the Diabetes United Kingdom Grant RD05/003059 (to M.L.E.), by the Wellcome Trust Grant WT081713 (to L.K.H.), by the Sir Jules Thorn Charitable Trust (P.H.), and by the Cambridge Medical Research Council Centre for Study of Obesity and Related Disorders to all authors.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AH
- Acute hypoglycemia
- CRR
- counterregulatory response
- Ect
- ectorhinal cortex
- EU
- euglycemia
- FOS-IR
- c-fos immunoreactive
- GFAP
- glial fibrillary acidic protein
- HAAF
- hypoglycemia-associated autonomic failure
- HO
- hormone infusion only
- IHC
- immunohistochemistry
- NeuN
- neuronal nuclei
- PRh
- perirhinal cortex
- RH
- recurrent hypoglycemia
- S1
- primary somatosensory cortex.
References
- 1. McCrimmon RJ, Evans ML, Fan X, McNay EC, Chan O, Ding Y, Zhu W, Gram DX, Sherwin RS. 2005. Activation of ATP-sensitive K+ channels in the ventromedial hypothalamus amplifies counterregulatory hormone responses to hypoglycemia in normal and recurrently hypoglycemic rats. Diabetes 54:3169–3174 [DOI] [PubMed] [Google Scholar]
- 2. Evans ML, McCrimmon RJ, Flanagan DE, Keshavarz T, Fan X, McNay EC, Jacob RJ, Sherwin RS. 2004. Hypothalamic ATP-sensitive K+ channels play a key role in sensing hypoglycemia and triggering counterregulatory epinephrine and glucagon responses. Diabetes 53:2542–2551 [DOI] [PubMed] [Google Scholar]
- 3. Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA. 1994. Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Lancet 344:283–287 [DOI] [PubMed] [Google Scholar]
- 4. Grissom N, Bhatnagar S. 2009. Habituation to repeated stress: get used to it. Neurobiol Learn Mem 92:215–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weinberg MS, Johnson DC, Bhatt AP, Spencer RL. 2010. Medial prefrontal cortex activity can disrupt the expression of stress response habituation. Neuroscience 168:744–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diorio D, Viau V, Meaney MJ. 1993. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci 13:3839–3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Curran T, Miller AD, Zokas L, Verma IM. 1984. Viral and cellular fos proteins: a comparative analysis. Cell 36:259–268 [DOI] [PubMed] [Google Scholar]
- 8. Flanagan DE, Keshavarz T, Evans ML, Flanagan S, Fan X, Jacob RJ, Sherwin RS. 2003. Role of corticotrophin-releasing hormone in the impairment of counterregulatory responses to hypoglycemia. Diabetes 52:605–613 [DOI] [PubMed] [Google Scholar]
- 9. Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Marcus JN, Holstege H, Lee CE, Cone RD, Elmquist JK. 2002. Activation of central melanocortin pathways by fenfluramine. Science 297:609–611 [DOI] [PubMed] [Google Scholar]
- 10. Lam DD, Zhou L, Vegge A, Xiu PY, Christensen BT, Osundiji MA, Yueh CY, Evans ML, Heisler LK. 2009. Distribution and neurochemical characterization of neurons within the nucleus of the solitary tract responsive to serotonin agonist-induced hypophagia. Behav Brain Res 196:139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Osundiji MA, Zhou L, Shaw J, Moore SP, Yueh CY, Sherwin R, Heisler LK, Evans ML. 2010. Brain glucosamine boosts protective glucoprivic feeding. Endocrinology 151:1499–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guillod-Maximin E, Lorsignol A, Alquier T, Pénicaud L. 2004. Acute intracarotid glucose injection towards the brain induces specific c-fos activation in hypothalamic nuclei: involvement of astrocytes in cerebral glucose-sensing in rats. J Neuroendocrinol 16:464–471 [DOI] [PubMed] [Google Scholar]
- 13. Leon-Quinto T, Adnot P, Portha B. 1997. Alteration of the counterregulatory hormones in the conscious rat after protein-energy restriction. Diabetologia 40:1028–1034 [DOI] [PubMed] [Google Scholar]
- 14. Evans SB, Wilkinson CW, Bentson K, Gronbeck P, Zavosh A, Figlewicz DP. 2001. PVN activation is suppressed by repeated hypoglycemia but not antecedent corticosterone in the rat. Am J Physiol Regul Integr Comp Physiol 281:R1426–R1436 [DOI] [PubMed] [Google Scholar]
- 15. Paranjape SA, Briski KP. 2005. Recurrent insulin induced hypoglycemia causes site specific patterns of habituation or amplification of CNS neuronal genomic activation. Neuroscience 130:957–970 [DOI] [PubMed] [Google Scholar]
- 16. Al-Noori S, Sanders N, Taborsky G, Wilkinson C, Zavosh A, West C, Sanders C, Figlewicz D. 2008. Recurrent hypoglycemia alters hypothalamic expression of the regulatory proteins Fos B and synaptophysin. Am J Physiol Regul Integr Comp Physiol 295 R1446–R1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davis SN, Goldstein RE, Price L, Jacobs J, Cherrington AD. 1993. The effects of insulin on the counterregulatory response to equivalent hypoglycemia in patients with insulin-dependent diabetes mellitus. J Clin Endocrinol Metab 77:1300–1307 [DOI] [PubMed] [Google Scholar]
- 18. Davis SN, Dunham B, Walmsley K, Shavers C, Neal D, Williams P, Cherrington AD. 1997. Brain of the conscious dog is sensitive to physiological changes in circulating insulin. Am J Physiol Endocrinol Metab 272:E567–E575 [DOI] [PubMed] [Google Scholar]
- 19. Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. 1998. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280:747–749 [DOI] [PubMed] [Google Scholar]
- 20. Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. 2003. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci 4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. 2003. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain 126:2139–2152 [DOI] [PubMed] [Google Scholar]
- 22. Jones BF, Witter MP. 2007. Cingulate cortex projections to the parahippocampal region and hippocampal formation in the rat. Hippocampus 17:957–976 [DOI] [PubMed] [Google Scholar]
- 23. Deacon TW, Eichenbaum H, Rosenberg P, Eckmann KW. 1983. Afferent connections of the perirhinal cortex in the rat. J Comp Neurol 220:168–190 [DOI] [PubMed] [Google Scholar]
- 24. Westerhaus MJ, Loewy AD. 2001. Central representation of the sympathetic nervous system in the cerebral cortex. Brain Res 903:117–127 [DOI] [PubMed] [Google Scholar]
- 25. Teh MM, Dunn JT, Choudhary P, Samarasinghe Y, Macdonald I, O'Doherty M, Marsden P, Reed LJ, Amiel SA. 2010. Evolution and resolution of human brain perfusion responses to the stress of induced hypoglycemia. NeuroImage 53:584–592 [DOI] [PubMed] [Google Scholar]
- 26. Dunn JT, Cranston I, Marsden PK, Amiel SA, Reed LJ. 2007. Attenuation of amydgala and frontal cortical responses to low blood glucose concentration in asymptomatic hypoglycemia in type 1 diabetes. Diabetes 56:2766–2773 [DOI] [PubMed] [Google Scholar]
- 27. Teves D, Videen TO, Cryer PE, Powers WJ. 2004. Activation of human medial prefrontal cortex during autonomic responses to hypoglycemia. Proc Natl Acad Sci USA 101:6217–6221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arbelaez AM, Powers WJ, Videen TO, Price JL, Cryer PE. 2008. Attenuation of counterregulatory responses to recurrent hypoglycemia by active thalamic inhibition. Diabetes 57:470–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou L, Podolsky N, Sang Z, Ding Y, Fan X, Tong Q, Levin BE, McCrimmon RJ. 2010. The medial amygdalar nucleus: a novel glucose-sensing region that modulates the counterregulatory response to hypoglycemia. Diabetes 59:2646–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.