Abstract
Cyclotides are a growing family of large plant-derived backbone-cyclized polypeptides (≈30 amino acids long) that share a disulfide-stabilized core characterized by an unusual knotted structure. Their unique circular backbone topology and knotted arrangement of three disulfide bonds makes them exceptionally stable to thermal, chemical, and enzymatic degradation compared to other peptides of similar size. Currently more than 100 sequences of different cyclotides have been characterized and the number is expected to increase dramatically in the coming years. Considering their stability, biological activities and ability to cross the cell membrane, cyclotides can be exploited to develop new peptide-based drugs with high potential for success. The cyclotide scaffold can be engineered or evolved using molecular evolution to inhibit protein-protein interactions implicated in cancer and other human diseases, or design new antimicrobial. The present review reports the biological diversity and therapeutic potential of natural and engineered cyclotides.
Keywords: Cyclic peptide, Cyclotides, Intein Kalata B1, MCoTI-I/II, Protein splicing, Protein engineering
Introduction
Head-to-tail or backbone-cyclized peptides are present throughout nature from bacteria to animals. Bacteria and fungi express numerous backbone-cyclized peptides that are currently in use as therapeutic agents [1]. For example, cyclosporin A is a fungal peptide with potent immunosuppressive properties and is used to treat organ transplant patients [2]. Daptomycin, is a 13-amino acid cyclic lipopeptide with a decanoyl side chain isolated from Streptomyces roseosporus that has recently been approved to treat infections against Gram-positive organisms, including multi-resistant strains [3]. In animals, the only known circular peptides are θ-defensins, which are expressed in blood leukocytes and bone marrow of Old World monkeys [4, 5]. θ-Defensins are antimicrobial peptides with broad-spectrum activities against bacteria, fungi, and viruses [6-8]. Backbone cyclized peptides have been also found in plants [9]. Sunflower trypsin inhibitor 1 (SFTI-1) for example is a bicyclic 14-residue long peptide found in sunflower seeds. SFTI-1 is the most potent known naturally occurring Bowman-Birk trypsin inhibitor [10]. Cyclotides, a novel family of small globular backbone-cyclized micro-proteins (≈ 30-residues long), are also naturally found in plants. Here, we review the properties of cyclotides and the latest developments in the use of the cyclotide scaffold to design novel peptide-based therapeutics.
Cyclotides, a novel ultrastable molecular scaffold
Cyclotides are small globular micro-proteins with a unique head-to-tail cyclized backbone, which is stabilized by three disulfide bonds (Fig. 1). Currently, over 140 sequences have been identified in the plant species Rubiaceae, Violaceae, and Cucurbitaceae [11]. Natural cyclotides have various activities including insecticidal [12, 13], uterotonic [14], anti-HIV [15], antimicrobial [16, 17], antitumor [18], antihelminthic [19, 20] and have been reported to cross cell membranes [21]. Their insecticidal and antihelminthic properties suggest that they may function as defense molecules in plants.
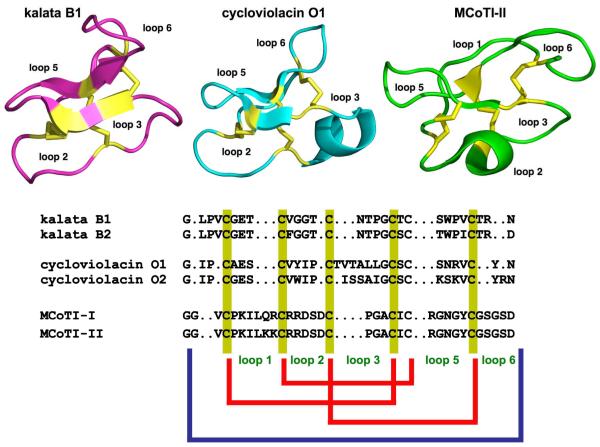
Figure 1.
Primary and tertiary structures of representative cyclotides from the bracelet (kalata B1; pdb ID: 1NB1 [108]), Möbius (cycloviolacin O1, pdb ID: 1NBJ [108]), and trypsin inhibitor (MCoTI-II, pdb ID: 1IB9 [109]) subfamilies. Conserved cysteine residues and disulfide bonds are shown in yellow. The blue line denotes the circular backbone.
Cyclotides share a unique head-to-tail circular knotted topology of three disulfide bridges, with one disulfide penetrating through a macrocycle formed by the two other disulfides and inter-connecting peptide backbones, forming what is called a cyclic cystine knot (CCK) motif (Fig. 1). The CCK topology is responsible for the high stability of cyclotides to enzymatic, thermal and chemical degradation [22]. The cyclotide family is divided into three structurally distinct families, Möbius, bracelet, and trypsin inhibitor subfamilies (Fig. 1). Möbius cyclotides are distinguished from bracelet cyclotides by the presence of a cis-Pro residue in loop 5. Trypsin inhibitor cyclotides have very different primary structures from Möbius and bracelet cyclotides, but retain the conserved cystine knot motif. Trypsin inhibitor cyclotides share a high sequence homology with related cystine-knot trypsin inhibitors found in squash such as EETI-II (Ecballium elaterium trypsin inhibitor II), and in fact can be considered cyclized homologs of these protease inhibitors. Thus, cyclotides can be considered natural combinatorial peptide libraries structurally constrained by the cystine-knot scaffold [23] and head-to-tail cyclization but are permissive of hypermutation of essentially all residues with the exception of the strictly conserved cysteines that comprise the knot [24-27].
Hence, cyclotides form a unique family of structurally-related peptides that possess remarkable stability due to the cystine knot, a small size making them readily accessible to chemical synthesis, and an excellent tolerance to sequence variations. Moreover, the first cyclotide to be discovered, kalata B1, is an orally effective uterotonic [14]. Intriguingly, the cyclotide MCoTI-II has also been shown to cross cell membranes through macropinocytosis [21]. We have also recently found that MCoTI-I has similar cellular-uptake properties to MCoTI-II (unpublished results). All of these features make cyclotides ideal tools for the development of a total novel class of peptide-based therapeutics.
Cyclotide biosynthesis
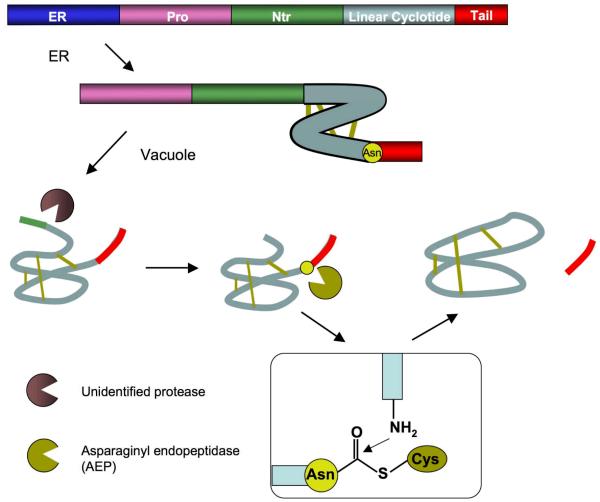
Cyclotides are ribosomally synthesized as precursor proteins, which consist of an endoplasmic reticulum (ER)-targeting sequence, a pro-region, a highly conserved N-terminal repeat (NTR) region, a mature cyclotide domain, and a C-terminal tail (Fig. 2). The combined NTR-cyclotide segment may contain one copy of the cyclotide sequence or there may be multiple copies of the same or different cyclotide sequences separated by additional NTR sequences. The precursor undergoes post-translational processing to generate a circular peptide by a mechanism that has not been completely elucidated yet [28, 29]. It has been hypothesized that a conserved Asn (or Asp) at the C-terminal cleavage site may be a recognition site by asparaginyl endoproteinase (AEP) for cyclization of the peptide in plants [28, 29]. AEP has been shown to be involved in the post-translational processing of concanavalin A from the jackbean [30], and therefore, it is possible this enzyme may be involved in the cyclization of other plant peptides. Studies using transgenic plants that express a cyclotide precursor have demonstrated the involvement of AEP and requirement for the asparagine residue in the cyclotide sequence [28, 29]. The authors showed that inhibition of AEP led to a decrease in the amount of cyclic product and an accumulation of linear peptides that were transiently expressed [29]. In addition, a complementary study showed that mutation of the asparagine residue or truncation of the conserved C-terminal tripeptide in transgenic plants resulted in no circular peptide production [28].
Figure 2.
Schematic representation of the putative mechanism of protease-catalyzed cyclization for cyclotides [28, 29]. The prototypic linear precursor protein (top) comprises an endoplasmic reticulum (ER)-targeting sequence (dark blue), a pro-region (purple), an N-terminal repeat region (Ntr; green), a cyclotide domain (light grey) and a C-terminal tail (red). It also features a conserved asparagine (yellow) at the C-terminal cleavage point of the cyclotide domain. The precursor is processed in the ER and vacuole, disulfide bonds are formed (yellow), and a range of unidentified proteases (brown) trim the precursor. In the final stage, the active-site cysteine of an AEP (yellow) displaces the C-terminal tail to form an enzyme-acyl intermediate (boxed). This intermediate is then attacked by the cyclotide N-terminal glycine to form the mature cyclic peptide. Figure taken from reference [68].
Biological activities of naturally occurring cyclotides
The natural function of cyclotides appears to be in protection of plants against insects [12, 13], nematodes [20, 25], and mollusks [31]. Studies have demonstrated that cyclotides can suppress the growth and development of insect and nematode larvae. Various other studies have also shown cyclotides have antimicrobial, hemolytic, uterotonic, and anti-HIV activities. Much of these activities likely involve interaction of the cyclotide with membranes, although the mechanism of action is not totally well understood.
The first cyclotide discovered, kalata B1, was identified in the plant Oldenlandia affinis in central Africa in the 1960’s [32]. This plant was used by the natives to make a tea extract that was used to accelerate childbirth during labor [14, 33]. The main active ingredient in the tea extract was found to be a peptide that was named kalata B1, after the local name for the native medicine. The uterotonic properties of kalata B1 indicated that the peptide was orally bioavailable. The complete sequence, Cys-knotted arrangement, and cyclic nature of kalata B1 was determined 25 years after its original discovery [34]. Since the discovery of kalata B1, many more related cyclotides have been discovered and found to have various biological activities [35, 36].
Cyclotides were initially hypothesized to have antimicrobial activities based on the presence of hydrophilic and hydrophobic patches, which give an amphipathic character similar to classical antimicrobial peptides. The antimicrobial activities of cyclotides have been reported by two groups with conflicting results on the potency of kalata B1 against Escherichia coli and Staphylococcus aureus. In one study, kalata B1 was active against S. aureus, but not E. coli [16], and in the second study, the peptide had the reverse effect [17]. This is likely due to the technical differences in the experiments. Although kalata cyclotides are amphipathic, the overall charge is close to zero at neutral pH, making it unlikely that they interact with bacterial membranes electrostatically similar to classical cationic antimicrobial peptides [37]. Further studies are necessary to investigate the mechanisms of antimicrobial action given the growing occurrence of antibiotic resistance by microorganisms.
The anti-HIV properties of cyclotides have been extensively studied [15, 38-40]. They appear to mainly act by inhibiting viral entry into host cells as studies have shown a dose-dependent increase in cytoprotection [15]. This suggests the peptides may block binding or fusion of the virus, but the mechanism remains unclear. In general, cyclotide bioactivities appear to involve interactions with membranes and, therefore, this may be a mechanism for anti-HIV activities, by preventing fusion of the viral and host cell membranes. Studies have shown cyclotides can bind to model lipid membranes by surface plasmon resonance [41], and that binding occurs mainly through the peptide hydrophobic patches exposed on the surface [42-44]. This suggests membrane binding may be one mode for cyclotide activity against microorganisms.
Cyclotide interactions with membranes have also been suggested as their mechanism for cytotoxic activity. Studies have demonstrated antitumor activities of cyclotides, which were selective against cancer cell lines and solid tumors compared to normal mammalian cells [18, 45, 46]. Cancer cells differ from normal cells in the lipid and glycoprotein composition, which alters the overall net charge. The different potencies between cyclotide cytotoxicity are related to the three-dimensional structure as well as specific amino acid residues within the sequence [46, 47].
In addition to having antimicrobial and antitumor activities, some cyclotides have been found to cause extensive hemolysis of human and rat erythrocytes [16, 40, 48]. The cyclotide kalata B1 has strong hemolytic activity, although this can be eliminated by mutation to Ala of any one of eight residues located in the bioactive face of the molecule [25]. A more recent study has also shown that the hemolytic activity of kalata B1 could also be reduced or completely eliminated by mutation to Lys of any of the residues involved in either the bioactive or hydrophobic faces of kalata B1 [49]. The same study showed that the hemolytic, insecticidal and nematocidal activities of the different kalata B1 mutants were correlated, indicating there may be a common mechanism involving a cyclotide-membrane interaction [49]. The same authors have also shown recently the all D-analogue of kalata had similar nematicidal activity than the native peptide, thus corroborating that the biological activity is mediated by a cellular receptor [20]. In contrast, other cyclotides such as MCoTI-cyclotides have shown little or no hemolytic activity, thus demonstrating the diversity of cyclotide properties.
Another cyclotide with interesting biological activity is cyclopsychotride (Cpt) A. Cpt A is natural cyclotide obtained from the organic extract of the tropical plant Psychotropia longipes that has been reported to have neurotensin inhibition properties [50]. Cpt A was able to inhibit neurotensin binding to its receptor to HT-29 cell membranes with an IC50 ≈ 3 μM and increase intracellular Ca2+ levels in a concentration-dependent manner, which could not blocked by neurotensin antagonists [50]. Cpt A, however, showed a similar activity in two unrelated cell lines that did not express neurotensin receptors indicating that the mechanism of action is unlikely to be mediated through an interaction with the neurotensin receptor [50].
Chemical synthesis of cyclotides
Cyclotides are small peptides, approximately 30 amino acids long, and therefore can be readily synthesized by chemical methods using solid-phase peptide synthesis [51]. Chemical synthesis using a solid-phase approach has been utilized to generate native cyclotide structures as well as grafted analogues [52-56]. This method uses an intramolecular native chemical ligation [57], in which the peptide sequence contains an N-terminal cysteine and an α-thioester group at the C-terminus [58-60]. Both tert-butyloxyxarbonyl (Boc)- and 9-fluorenyloxycarbonyl (Fmoc)-based chemistries have been used to incorporate C-terminal thioesters during chain assembly (Boc) [61-63] or using a safety-catch based linkers (Fmoc) [60, 64-67]. Once the peptide is cleaved from the resin, both cyclization and folding are carried out in a single pot reaction.
Recombinant expression of cyclotides
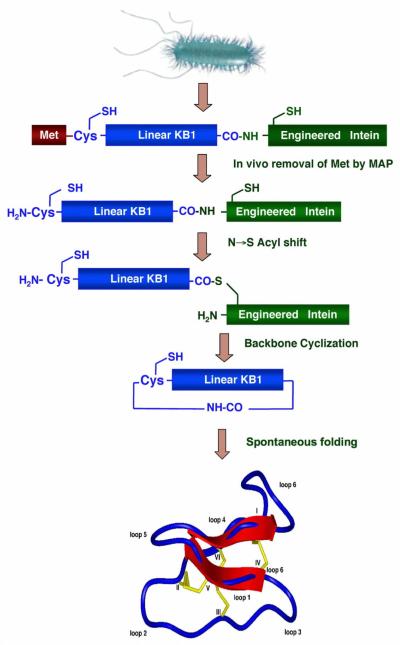
Cyclotides have also been produced recombinantly in bacteria through intramolecular native chemical ligation (see above) by using a modified protein splicing unit or intein (Fig. 3) (see reference [68] for a recent review). This method can generate folded cyclotides either in vivo or in vitro using standard bacterial expression systems [26, 69, 70]. Inteins are internal self-processing domains that undergo post-translational processing to splice together flanking external domains (exteins) [71]. The approach uses a modified intein fused to the C-terminus of the cyclotide sequence to allow the formation of an α-thioester at the C-terminus of recombinant polypeptides. To obtain the required N-terminal cysteine for cyclization, the peptide can be expressed with an N-terminal leading peptide signal, which can be cleaved either in vivo or in vitro by proteolysis or auto-proteolysis [68]. The simplest way to accomplish this is to introduce a Cys downstream of the initiating Met residue. Once the translation step is completed, the endogeneous methionyl aminopeptidases (MAP) removes the Met residue, thereby generating in vivo an N-terminal Cys residue [72-76]. The N-terminal Cys can then capture the reactive thioester in an intramolecular fashion to form a backbone-cyclized polypeptide (Fig. 3). Additional methods to generate an N-terminal cysteine have used exogenous proteases to cleave the leading signal after purification or in vivo by co-expressing the protease [77]. For example, the protease Factor Xa has been used to remove an N-terminal recognition sequence prior to a cysteine residue [59, 78]. Other proteases that have been used for this task include ubiquitin C-terminal hydrolase [79, 80], tobacco etch virus (TEV) protease [77], enterokinase [81] and thrombin [82]. The N-terminal pelB leader sequence has been used recently to direct newly synthesized fusion proteins to the E. coli periplasmic space where the corresponding endogenous leader peptidases [83, 84] can generate the desired N-terminal cysteine-containing protein fragment [85]. Besides proteases, protein splicing has also been used to produce recombinant N-terminal Cys-containing polypeptides. Some inteins can be modified in such a way that cleavage at the C-terminal splice junction can be accomplished in a pH- and temperature-dependent fashion [86-88].
Figure 3.
Intein-mediated backbone cyclization for the biosynthesis of cyclotides kalata B1 (kB1) and MCoTI-II in E. coli cells [69, 70]. The backbone cyclization of the linear cyclotide precursor is mediated by a modified protein splicing unit or intein. The cyclized product then folds spontaneously in the bacterial cytoplasm.
Intein-mediated backbone cyclization of polypeptides has also been recently used for the biosynthesis of the Bowman-Birk inhibitor SFTI-1 [89]. The biosynthesis of other cyclic peptides such as backbone-cyclized α-defensins and naturally occurring θ-defensins is currently underway in our laboratory.
Another approach to generate cyclic peptides in vivo is by protein trans-splicing. This approach utilizes a self-processing intein that is split into two fragments, an N-intein and a C-intein. This method has not been applied yet for the biosynthesis of cyclotides, but has been used to produce other natural cyclic peptides and genetically-encoded libraries of small cyclic peptides [90, 91]. It should be noted, however, that these systems require the presence of specific amino acid residues at both intein-extein junctions for efficient protein splicing to occur [90, 92, 93].
Designing cyclotides with novel biological activities
The unique properties associated with the cyclotide scaffold make them extremely valuable tools in drug discovery. There are several studies that have used the cyclotide molecular scaffold to graft peptide sequences and to generate libraries for the purpose of engineering cyclotides with novel biological functions (Fig. 4).
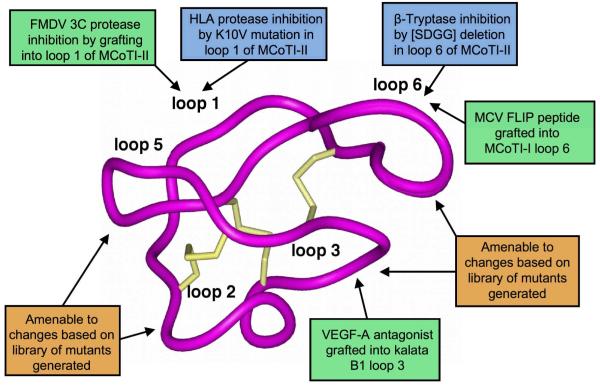
Figure 4.
Using the cyclotide molecular scaffold for drug design. Summary of the changes engineered into the cyclotide framework to introduce new biological functions. Peptide sequences have been successfully grafted into loop 3 of kalata B1 [94] and loops 1 and 6 of MCoTI-II [95] and MCoTI-I (unpublished results), respectively. Cyclotide based libraries have been generated using loops 2, 3, 5 and 6. The cyclotide structure used in the figure corresponds to MCoTI-II (pdb ID: 1IB9 [109]).
The plasticity of the cyclotide framework was first demonstrated by substituting hydrophobic residues in loop 5 of kalata B1 with polar and charged residues [24]. The mutated cyclotides retained the native fold of kalata B1, but were no longer hemolytic [24]. This showed that cyclotides were amendable to sequence changes, and interestingly, can be modified to change their biological functions.
The potential of grafted cyclotides was first demonstrated in a study aimed to develop novel anticancer peptide-based therapeutics [94]. In this work a peptide antagonist of angiogenesis was grafted into various loops of the kalata B1 scaffold [94]. The grafted cyclotide containing the vascular endothelial growth factor A (VEGF-A) antagonist sequence in loop 3 was found to adopt a CCK native fold and was biologically active at low micromolar concentrations. Additionally, the grafted cyclotide showed increased resistance to degradation in human serum. This study demonstrates the possibility of using the cyclotide scaffold to stabilize bioactive peptide epitopes, which may normally get degraded.
The utility of the cyclotide scaffold in drug design has also been recently shown by engineering non-native activities into the cyclotide MCoTI-II. MCoTI-II is a naturally occurring trypsin inhibitor (Ki ≈ 20 pM, which is the dissociation constant for inhibitor binding) found in the seeds of Momordica cochinchinensis, a tropical plant from the squash family. Mutation of the P1 residue in the active loop (loop 1, see Fig, 1) of the cyclotide produced several MCoTI-II analogs with different specificities towards different proteases [95]. Interestingly, several analogs showed activity against the foot-and-mouth-disease virus (FMDV) 3C protease, a Cys protease key for viral replication, in the low micromolar range [95]. This is the first reported peptide-based inhibitor for this protease and although the potency was relatively low, this study demonstrates the potential of using MCoTI-based cyclotides for designing novel protease inhibitors [95].
In a more recent study, the same authors also generated inhibitors of the serine proteases β-tryptase and human leukocyte elastase (HLE) using the backbone of MCoTI-II [96]. β-Tryptase is implicated in allergic and inflammatory disorders, and HLE has been associated with respiratory and pulmonary disorders. Replacing the P1 residue in loop 1 produced several MCoTI-II mutants (K6A and K6V) with activity against HLE with Ki values of 20-30 nM [96] and Ki values against trypsin above 1 μM. Removal of the SDGG peptide segment in loop 6 yielded a ß-tryptase inhibitor with a Ki ≈ 10 nM without significantly altering the three-dimensional structure as determined by NMR [96]. The authors hypothesized that deletion of the aspartic acid residue in MCoTI-II should improve activity by removal of repulsive electrostatic interactions with β-tryptase thus improving the inhibitory constant against trypsin 160-fold when compared to the wild-type MCoTI-II.
In addition to displaying biological activities, MCoTI-based peptides have also been shown to cross cell membranes in macrophage and breast cancer cell lines through macropinocytosis [21]. We have also found that grafting of a helix region from the molluscum contagiosum virus (MCV) FLICE-inhibitory protein (FLIP) into loop 6 of MCoTI-I yielded a folded cyclotide able to cross cell membranes and trigger apoptosis of virally infected cells (unpublished results), thus indicating that loop 6 may be used for grafting purposes without affecting cellular-uptake.
MCoTI cyclotides share a high sequence homology with related cystine-knot trypsin inhibitors found in squash, and could be considered cyclized homologs of these protease inhibitors. Squash cystine-knot trypsin inhibitors have also successfully been used to graft biological activities. For example, the RGD sequence was grafted into loop 1 of EETI-II yielding an EETI-II analog with platelet inhibitory activity [97]. The engineered proteins were much more potent in inhibiting platelet aggregation than the linear grafted peptides, thus highlighting the importance of grafting an epitope into a stable peptide-scaffold. These highly stable peptides could have clinical use for the treatment of patients with acute coronary syndrome, for example.
Additionally, the cyclotide scaffold can be engineered or evolved through molecular evolution techniches to selectively extracellular receptors such as binding G protein-coupled receptors to promote or block cell signaling [98]. These data demonstrate the versatility of the cyclotide scaffold and highlight the extraordinary pharmacological properties of MCoTI-cyclotides and related linear knottins, thus confirming the potential of Cys-knotted polypeptide scaffolds in peptide-based drug discovery [27].
Screening of cyclotide-based libraries
The ability to create cyclic polypeptides in vivo opens up the intriguing possibility of generating large libraries of cyclic polypeptides. Thus, libraries of genetically-encoded cyclic polypeptides containing billions of members can be readily generated using standard molecular recombinant tools. This tremendous molecular diversity allows one to perform selection strategies mimicking the evolutionary processes found in nature.
There are several examples where in vivo generated libraries of cyclic peptides have been used for rapid selection of biologically active peptides. For example protein trans-splicing has been used by several groups for the generation of libraries of small cyclic peptides (≤ 8 residues) in bacterial and mammalian cells [90, 92, 93, 99-101]. It should be pointed out, however, that the use of protein trans-splicing requires particular amino acids at the intein-extein junctions for efficient trans-splicing [93]. These requirements usually depend on the type of split-intein used, and seriously limit the diversity of the libraries that can be generated when using small cyclic peptide templates. Our group has recently demonstrated the expression in E. coli of libraries based on the cyclic peptide SFTI-1 (a backbone cyclized Bowman-Birk trypsin inhibitor) using an intein-mediated backbone cyclization approach (see above), which allows the biosynthesis of backbone-cyclized polypeptides without any sequence requirement limitation [89].
The use of small cyclic peptides, however, which technically can be considered a single closed loop (or 2 loops in the case of SFTI-1), could also limit the potency of the peptides selected, especially when targeting protein-protein interactions involving large binding surfaces. In these cases the use of peptide templates such as cyclotides with multiple variable loops could facilitate the selection of peptides with higher affinities.
The potential for generating cyclotide libraries was first explored by our group using the kalata B1 scaffold [69]. In this work wild-type and several mutants of kalata B1 were biosynthesized using an intramolecular native chemical ligation facilitated by a modified protein splicing unit. In this work, six different linear versions of kalata B1 were generated and expressed in E. coli as fusions to a modified version of the yeast vacuolar membrane ATPase (VMA) intein. Results demonstrated in vitro folding and cyclization of kalata B1 to varying degrees depending on which of the six native cysteine residues was at the N-terminus after cleavage of the initiation methionine by endogenous MAP. Cleavage and efficient cyclization of the different linear precursors did not occur equally, suggesting the amino acid residues near the intein as well as the predisposition to adopt a native fold of the corresponding linear precursor may determine the efficiency of the cleavage/cyclization step [69]. This information was used to express a small library based on the kalata B1 scaffold. This library was cyclized in vitro by incubation with a redox buffer containing reduced glutathione (GSH) as a thiol co-factor, thus mimicking the intracellular conditions, where GSH is the most abundant thiol co-factor. The use of GSH allows the cyclization and folding to happen in one step [69, 89]. Analysis of the cyclization/folding reaction by HPLC and mass spectrometry revealed that all the members of the kalata B1 based library were expressed and processed with similar yields to give the corresponding natively folded cyclotides [69].
More recently, we have also reported the biosynthesis of a genetically encoded library of MCoTI-I based cyclotides in E. coli cells [26]. The cyclization/folding of the library was performed either in vitro, by incubation with a redox buffer containing glutathione, or by in vivo self-processing of the corresponding precursor proteins. The bacterial gyrase A intein from Mycobacterium xenopus was used in this work [26]. This intein typically express at higher yields than the yeast VMA intein in E. coli expression systems [26]. The peptide libraries were purified and screened for activity using trypsin-immobilized sepharose beads, and then analyzed by HPLC and mass spectrometry. Out of 27 mutations studied, only two mutations, G27P and I22G, negatively affected the folding of the resulting cyclotides. All of the remaining cyclotides were able to fold with similar yields. The K6A mutant, as expected, was not able to bind trypsin. This residue is key for binding to the specificity pocket of trypsin, and can only be replaced by positively charged residues [102]. This mutant was found by NMR to adopt a native cyclotide structure, confirming that the lack of biological activity was due to the mutation and not to the ability to adopt a native fold. It is interesting to note that by modifying the nature of this residue, the specificity of the corresponding MCoTI-cyclotide can be changed to target other proteases [95, 96]. The rest of the MCoTI-based library members were able to bind trypsin, suggesting they were able to adopt a native cyclotide fold and retained biological activity. The affinity of each peptide was analyzed using a competitive trypsin-binding assay. The mutants had a wide range of affinity, some being greater than wild type MCoTI-I. The peptides with less affinity were mostly found in loop 1 and the C-terminal region of loop 6, both well conserved among other squash trypsin inhibitors. Overall, these data describe the structural requirements for correct formation of MCoTI-I and the residues that are key to modulating trypsin binding. To our knowledge, this is the first time that the biosynthesis of a genetically-encoded library of MCoTI-based cyclotides containing a complete suite of amino acid mutants is reported
The chemical synthesis of a complete suite of Ala mutants for kalata B1 has also been recently reported [25]. In this work all the mutants were fully characterized structurally and functionally. The results indicated that only two of the mutations explored (W23A and P24A, both located in loop 5, see Fig. 1) prevented folding [25]. The mutagenesis results obtained in our work with the cyclotide MCoTI-I show similar results highlighting the extreme robustness of the cyclotide scaffold to mutations. These studies show that cyclotides may provide an ideal scaffold for the biosynthesis of large combinatorial libraries inside living bacterial cells. These genetically-encoded libraries can then be screened in-cell for biological activity using high-throughput flow cytometry techniques for the rapid selection of novel biologically active cyclotides [69, 103, 104].
Summary and Concluding Remarks
In summary, cyclotides are a novel family of structurally related globular microproteins with a unique head-to-tail cyclized backbone, which is stabilized by three disulfide bonds [27, 105]. The number and positions of cysteine residues are conserved throughout the family, forming what is called cyclic cystine-knot (CCK) motif [35] that acts as a highly stable and versatile scaffold on which 5 hyper-variable loops are arranged (Fig 1). This CCK framework gives the cyclotides exceptional resistance to thermal and chemical denaturation, and enzymatic degradation. This is particularly important for the development of peptide-based therapeutics with oral bioavailability. In fact, the use of cyclotide-containing plants in indigenous medicine first highlighted the fact that the peptides are resistant to boiling and are apparently orally bioavailable [14, 32, 33]. Some cyclotides have also been shown to cross the cell membrane [21] thus allowing to target intracellular protein interactions, such as that mediated by viral FLIPs to prevent cell-death in virally infected cells (unpublished results). Cyclotides are also medium-sized polypeptides and therefore can be readily synthesized by standard solid-phase peptide-synthesis using either Boc-[54] or Fmoc-based [55] methodologies thus allowing the introduction of non-natural amino acids or other chemical modifications for lead optimization. They can also be encoded within standard cloning vectors and readily expressed in bacteria or animal cells [26, 70], thus making them ideal substrates for molecular evolution strategies to enable generation and selection of compounds with optimal binding and inhibitory characteristics using high throughput cell-based assays [106]. All of these characteristics make cyclotides appear as very promising leads or frameworks for development of peptide-based therapeutics and diagnostics [27, 68, 105, 107]
Acknowledgements
This work was supported by funding from the School of Pharmacy at the University of Southern California and by National Institute of Health award GM090323 to J.A.C.
Abbreviations
- AEP
asparaginyl endoproteinase
- Boc
tert-butyloxyxarbonyl
- EETI-II
Ecballium elaterium trypsin inhibitor II
- FMDV
foot-and-mouth-disease virus
- FLIP
FLICE-inhibitory protein
- Fmoc
9-fluorenyloxycarbonyl
- GSH
reduced glutathione
- HIV
human immunodeficiency virus
- HLE
human leukocyte elastase
- HPLC
high performance liquid chromatography
- MAP
methionyl aminopeptidase
- MCoTI
Momordica cochinchinensis trypsin inhibitor
- MCV
molluscum contagiosum virus
- NMR
nuclear magnetic resonance
- NTR
N-terminal repeat
- SFTI
sunflower trypsin inhibitor
- SPPS
solid-phase peptide synthesis
- VMA
vacuolar membrane ATPase
References
- [1].Mogi T, Kita K. Gramicidin S and polymyxins: the revival of cationic cyclic peptide antibiotics. Cell Mol Life Sci. 2009;66:3821–3826. doi: 10.1007/s00018-009-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Starzl TE, Klintmalm GB, Porter KA, Iwatsuki S, Schroter GP. Liver transplantation with use of cyclosporin a and prednisone. N Engl J Med. 1981;305:266–269. doi: 10.1056/NEJM198107303050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Enoch DA, Bygott JM, Daly ML, Karas JA. Daptomycin. J Infect. 2007;55:205–213. doi: 10.1016/j.jinf.2007.05.180. [DOI] [PubMed] [Google Scholar]
- [4].Tang YQ, Yuan J, Osapay G, Osapay K, Tran D, Miller CJ, Ouellette AJ, Selsted ME. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science. 1999;286:498–502. doi: 10.1126/science.286.5439.498. [DOI] [PubMed] [Google Scholar]
- [5].Garcia AE, Osapay G, Tran PA, Yuan J, Selsted ME. Isolation, synthesis, and antimicrobial activities of naturally occurring theta-defensin isoforms from baboon leukocytes. Infect Immun. 2008;76:5883–5891. doi: 10.1128/IAI.01100-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gallo SA, Wang W, Rawat SS, Jung G, Waring AJ, Cole AM, Lu H, Yan X, Daly NL, Craik DJ, Jiang S, Lehrer RI, Blumenthal R. Theta-defensins prevent HIV-1 Env-mediated fusion by binding gp41 and blocking 6-helix bundle formation. J Biol Chem. 2006;281:18787–18792. doi: 10.1074/jbc.M602422200. [DOI] [PubMed] [Google Scholar]
- [7].Wang W, Mulakala C, Ward SC, Jung G, Luong H, Pham D, Waring AJ, Kaznessis Y, Lu W, Bradley KA, Lehrer RI. Retrocyclins kill bacilli and germinating spores of Bacillus anthracis and inactivate anthrax lethal toxin. J Biol Chem. 2006;281:32755–32764. doi: 10.1074/jbc.M603614200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Seidel A, Ye Y, de Armas LR, Soto M, Yarosh W, Marcsisin RA, Tran D, Selsted ME, Camerini D. Cyclic and acyclic defensins inhibit human immunodeficiency virus type-1 replication by different mechanisms. PLoS One. 2010;5:e9737. doi: 10.1371/journal.pone.0009737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Craik DJ. Circling the enemy: cyclic proteins in plant defence. Trends Plant Sci. 2009;14:328–335. doi: 10.1016/j.tplants.2009.03.003. [DOI] [PubMed] [Google Scholar]
- [10].Korsinczky ML, Schirra HJ, Craik DJ. Sunflower trypsin inhibitor-1. Curr Protein Pept Sci. 2004;5:351–364. doi: 10.2174/1389203043379594. [DOI] [PubMed] [Google Scholar]
- [11].Wang CK, Kaas Q, Chiche L, Craik DJ. CyBase: a database of cyclic protein sequences and structures, with applications in protein discovery and engineering. Nucleic Acids Res. 2008;36:D206–210. doi: 10.1093/nar/gkm953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jennings C, West J, Waine C, Craik D, Anderson M. Biosynthesis and insecticidal properties of plant cyclotides: the cyclic knotted proteins from Oldenlandia affinis. Proc Natl Acad Sci U S A. 2001;98:10614–10619. doi: 10.1073/pnas.191366898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jennings CV, Rosengren KJ, Daly NL, Plan M, Stevens J, Scanlon MJ, Waine C, Norman DG, Anderson MA, Craik DJ. Isolation, solution structure, and insecticidal activity of kalata B2, a circular protein with a twist: do Mobius strips exist in nature? Biochemistry. 2005;44:851–860. doi: 10.1021/bi047837h. [DOI] [PubMed] [Google Scholar]
- [14].Gran L. Oxytocic principles of Oldenlandia affinis. Lloydia. 1973;36:174–178. [PubMed] [Google Scholar]
- [15].Gustafson KR, Sowder RC, Henderson LE, Parsons IC, Kashman Y, Cardellina JH, McMahon JB, Buckheit RW, Pannell LK, Boyd MR. Circulin-A and Circulin-B: Novel HIV-inhibitory macrocyclic peptides from the tropical tree Chassalia parvifolia. J Am Chem Soc. 1994;116:9337–9338. [Google Scholar]
- [16].Tam JP, Lu YA, Yang JL, Chiu KW. An unusual structural motif of antimicrobial peptides containing end-to-end macrocycle and cystine-knot disulfides. Proc Natl Acad Sci U S A. 1999;96:8913–8918. doi: 10.1073/pnas.96.16.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gran L, Sletten K, Skjeldal L. Cyclic peptides from Oldenlandia affinis DC. Molecular and biological properties. Chem Biodivers. 2008;5:2014–2022. doi: 10.1002/cbdv.200890184. [DOI] [PubMed] [Google Scholar]
- [18].Lindholm P, Goransson U, Johansson S, Claeson P, Gullbo J, Larsson R, Bohlin L, Backlund A. Cyclotides: a novel type of cytotoxic agents. Mol Cancer Ther. 2002;1:365–369. [PubMed] [Google Scholar]
- [19].Colgrave ML, Kotze AC, Ireland DC, Wang CK, Craik DJ. The anthelmintic activity of the cyclotides: natural variants with enhanced activity. Chembiochem. 2008;9:1939–1945. doi: 10.1002/cbic.200800174. [DOI] [PubMed] [Google Scholar]
- [20].Colgrave ML, Kotze AC, Huang YH, O’Grady J, Simonsen SM, Craik DJ. Cyclotides: natural, circular plant peptides that possess significant activity against gastrointestinal nematode parasites of sheep. Biochemistry. 2008;47:5581–5589. doi: 10.1021/bi800223y. [DOI] [PubMed] [Google Scholar]
- [21].Greenwood KP, Daly NL, Brown DL, Stow JL, Craik DJ. The cyclic cystine knot miniprotein MCoTI-II is internalized into cells by macropinocytosis. Int J Biochem Cell Biol. 2007;39:2252–2264. doi: 10.1016/j.biocel.2007.06.016. [DOI] [PubMed] [Google Scholar]
- [22].Colgrave ML, Craik DJ. Thermal, chemical, and enzymatic stability of the cyclotide kalata B1: the importance of the cyclic cystine knot. Biochemistry. 2004;43:5965–5975. doi: 10.1021/bi049711q. [DOI] [PubMed] [Google Scholar]
- [23].Craik DJ, Cemazar M, Wang CK, Daly NL. The cyclotide family of circular miniproteins: nature’s combinatorial peptide template. Biopolymers. 2006;84:250–266. doi: 10.1002/bip.20451. [DOI] [PubMed] [Google Scholar]
- [24].Clark RJ, Daly NL, Craik DJ. Structural plasticity of the cyclic-cystine-knot framework: implications for biological activity and drug design. Biochem J. 2006;394:85–93. doi: 10.1042/BJ20051691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Simonsen SM, Sando L, Rosengren KJ, Wang CK, Colgrave ML, Daly NL, Craik DJ. Alanine scanning mutagenesis of the prototypic cyclotide reveals a cluster of residues essential for bioactivity. J Biol Chem. 2008;283:9805–9813. doi: 10.1074/jbc.M709303200. [DOI] [PubMed] [Google Scholar]
- [26].Austin J, Wang W, Puttamadappa S, Shekhtman A, Camarero JA. Biosynthesis and biological screening of a genetically encoded library based on the cyclotide MCoTI-I. Chembiochem. 2009;10:2663–2670. doi: 10.1002/cbic.200900534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jagadish K, Camarero JA. Cyclotides, a promising molecular scaffold for peptide-based therapeutics. Biopolymers. 2010 doi: 10.1002/bip.21433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gillon AD, Saska I, Jennings CV, Guarino RF, Craik DJ, Anderson MA. Biosynthesis of circular proteins in plants. Plant J. 2008;53:505–515. doi: 10.1111/j.1365-313X.2007.03357.x. [DOI] [PubMed] [Google Scholar]
- [29].Saska I, Gillon AD, Hatsugai N, Dietzgen RG, Hara-Nishimura I, Anderson MA, Craik DJ. An asparaginyl endopeptidase mediates in vivo protein backbone cyclization. J Biol Chem. 2007;282:29721–29728. doi: 10.1074/jbc.M705185200. [DOI] [PubMed] [Google Scholar]
- [30].Sheldon PS, Keen JN, Bowles DJ. Post-translational peptide bond formation during concanavalin A processing in vitro. Biochem J. 1996;320(Pt 3):865–870. doi: 10.1042/bj3200865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Plan MR, Saska I, Cagauan AG, Craik DJ. Backbone cyclised peptides from plants show molluscicidal activity against the rice pest Pomacea canaliculata (golden apple snail) J Agric Food Chem. 2008;56:5237–5241. doi: 10.1021/jf800302f. [DOI] [PubMed] [Google Scholar]
- [32].Gran L. On the effect of a polypeptide isolated from “Kalata-Kalata” (Oldenlandia affinis DC) on the oestrogen dominated uterus. Acta Pharmacol Toxicol (Copenh) 1973;33:400–408. doi: 10.1111/j.1600-0773.1973.tb01541.x. [DOI] [PubMed] [Google Scholar]
- [33].Gran L, Sandberg F, Sletten K. Oldenlandia affinis (R&S) DC. A plant containing uteroactive peptides used in African traditional medicine. J Ethnopharmacol. 2000;70:197–203. doi: 10.1016/s0378-8741(99)00175-0. [DOI] [PubMed] [Google Scholar]
- [34].Saether O, Craik DJ, Campbell ID, Sletten K, Juul J, Norman DG. Elucidation of the primary and three-dimensional structure of the uterotonic polypeptide kalata B1. Biochemistry. 1995;34:4147–4158. doi: 10.1021/bi00013a002. [DOI] [PubMed] [Google Scholar]
- [35].Craik DJ, Daly NL, Bond T, Waine C. Plant cyclotides: A unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J Mol Biol. 1999;294:1327–1336. doi: 10.1006/jmbi.1999.3383. [DOI] [PubMed] [Google Scholar]
- [36].Goransson U, Luijendijk T, Johansson S, Bohlin L, Claeson P. Seven novel macrocyclic polypeptides from Viola arvensis. J Nat Prod. 1999;62:283–286. doi: 10.1021/np9803878. [DOI] [PubMed] [Google Scholar]
- [37].Henriques ST, Craik DJ. Cyclotides as templates in drug design. Drug Discov Today. 15:57–64. doi: 10.1016/j.drudis.2009.10.007. [DOI] [PubMed] [Google Scholar]
- [38].Daly NL, Gustafson KR, Craik DJ. The role of the cyclic peptide backbone in the anti-HIV activity of the cyclotide kalata B1. FEBS Lett. 2004;574:69–72. doi: 10.1016/j.febslet.2004.08.007. [DOI] [PubMed] [Google Scholar]
- [39].Chen B, Colgrave ML, Daly NL, Rosengren KJ, Gustafson KR, Craik DJ. Isolation and characterization of novel cyclotides from Viola hederaceae: solution structure and anti-HIV activity of vhl-1, a leaf-specific expressed cyclotide. J Biol Chem. 2005;280:22395–22405. doi: 10.1074/jbc.M501737200. [DOI] [PubMed] [Google Scholar]
- [40].Wang CK, Colgrave ML, Gustafson KR, Ireland DC, Goransson U, Craik DJ. Anti-HIV cyclotides from the Chinese medicinal herb Viola yedoensis. J Nat Prod. 2008;71:47–52. doi: 10.1021/np070393g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kamimori H, Hall K, Craik DJ, Aguilar MI. Studies on the membrane interactions of the cyclotides kalata B1 and kalata B6 on model membrane systems by surface plasmon resonance. Anal Biochem. 2005;337:149–153. doi: 10.1016/j.ab.2004.10.028. [DOI] [PubMed] [Google Scholar]
- [42].Shenkarev ZO, Nadezhdin KD, Sobol VA, Sobol AG, Skjeldal L, Arseniev AS. Conformation and mode of membrane interaction in cyclotides. Spatial structure of kalata B1 bound to a dodecylphosphocholine micelle. FEBS J. 2006;273:2658–2672. doi: 10.1111/j.1742-4658.2006.05282.x. [DOI] [PubMed] [Google Scholar]
- [43].Shenkarev ZO, Nadezhdin KD, Lyukmanova EN, Sobol VA, Skjeldal L, Arseniev AS. Divalent cation coordination and mode of membrane interaction in cyclotides: NMR spatial structure of ternary complex Kalata B7/Mn2+/DPC micelle. J Inorg Biochem. 2008;102:1246–1256. doi: 10.1016/j.jinorgbio.2008.01.018. [DOI] [PubMed] [Google Scholar]
- [44].Wang CK, Hu SH, Martin JL, Sjogren T, Hajdu J, Bohlin L, Claeson P, Goransson U, Rosengren KJ, Tang J, Tan NH, Craik DJ. Combined X-ray and NMR analysis of the stability of the cyclotide cystine knot fold that underpins its insecticidal activity and potential use as a drug scaffold. J Biol Chem. 2009;284:10672–10683. doi: 10.1074/jbc.M900021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Svangard E, Goransson U, Hocaoglu Z, Gullbo J, Larsson R, Claeson P, Bohlin L. Cytotoxic cyclotides from Viola tricolor. J Nat Prod. 2004;67:144–147. doi: 10.1021/np030101l. [DOI] [PubMed] [Google Scholar]
- [46].Herrmann A, Burman R, Mylne JS, Karlsson G, Gullbo J, Craik DJ, Clark RJ, Goransson U. The alpine violet, Viola biflora, is a rich source of cyclotides with potent cytotoxicity. Phytochemistry. 2008;69:939–952. doi: 10.1016/j.phytochem.2007.10.023. [DOI] [PubMed] [Google Scholar]
- [47].Herrmann A, Svangard E, Claeson P, Gullbo J, Bohlin L, Goransson U. Key role of glutamic acid for the cytotoxic activity of the cyclotide cycloviolacin O2. Cell Mol Life Sci. 2006;63:235–245. doi: 10.1007/s00018-005-5486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chen B, Colgrave ML, Wang C, Craik DJ. Cycloviolacin H4, a hydrophobic cyclotide from Viola hederaceae. J Nat Prod. 2006;69:23–28. doi: 10.1021/np050317i. [DOI] [PubMed] [Google Scholar]
- [49].Huang YH, Colgrave ML, Clark RJ, Kotze AC, Craik DJ. Lysine-scanning mutagenesis reveals an amendable face of the cyclotide kalata B1 for the optimization of nematocidal activity. J Biol Chem. 2010;285:10797–10805. doi: 10.1074/jbc.M109.089854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Witherup KM, Bogusky MJ, Anderson PS, Ramjit H, Ransom RW, Wood T, Sardana M. Cyclopsychotride A, a biologically active, 31-residue cyclic peptide isolated from Psychotria longipes. J Nat Prod. 1994;57:1619–1625. doi: 10.1021/np50114a002. [DOI] [PubMed] [Google Scholar]
- [51].Marglin A, Merrifield RB. Chemical synthesis of peptides and proteins. Annu Rev Biochem. 1970;39:841–866. doi: 10.1146/annurev.bi.39.070170.004205. [DOI] [PubMed] [Google Scholar]
- [52].Tam JP, Lu Y-A. Synthesis of Large Cyclic Cystine-Knot Peptide by Orthogonal Coupling Strategy Using Unprotected Peptide Precursor. Tetrathedron Lett. 1997;38:5599–5602. [Google Scholar]
- [53].Tam JP, Lu YA. A biomimetic strategy in the synthesis and fragmentation of cyclic protein. Protein Sci. 1998;7:1583–1592. doi: 10.1002/pro.5560070712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Daly NL, Love S, Alewood PF, Craik DJ. Chemical synthesis and folding pathways of large cyclic polypeptides: studies of the cystine knot polypeptide kalata B1. Biochemistry. 1999;38:10606–10614. doi: 10.1021/bi990605b. [DOI] [PubMed] [Google Scholar]
- [55].Thongyoo P, Tate EW, Leatherbarrow RJ. Total synthesis of the macrocyclic cysteine knot microprotein MCoTI-II. Chem Commun (Camb) 2006:2848–2850. doi: 10.1039/b607324g. [DOI] [PubMed] [Google Scholar]
- [56].Aboye T. Leta, Clark RJ, Craik DJ, Goransson U. Ultra-stable peptide scaffolds for protein engineering-synthesis and folding of the circular cystine knotted cyclotide cycloviolacin O2. Chembiochem. 2008;9:103–113. doi: 10.1002/cbic.200700357. [DOI] [PubMed] [Google Scholar]
- [57].Dawson PE, Muir TW, Clark-Lewis I, Kent SBH. Synthesis of Proteins by Native Chemical Ligation. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- [58].Camarero JA, Muir TW. Chemoselective backbone cyclization of unprotected peptides. J. Chem. Soc., Chem. Comm. 1997;1997:1369–1370. [Google Scholar]
- [59].Camarero JA, Muir TW. Biosynthesis of a Head-to-Tail Cyclized Protein with Improved Biological Activity. J. Am. Chem. Soc. 1999;121:5597–5598. [Google Scholar]
- [60].Camarero JA, Mitchell AR. Synthesis of proteins by native chemical ligation using Fmoc-based chemistry. Protein Pept Lett. 2005;12:723–728. doi: 10.2174/0929866054864166. [DOI] [PubMed] [Google Scholar]
- [61].Camarero JA, Cotton GJ, Adeva A, Muir TW. Chemical ligation of unprotected peptides directly from a solid support. J Pept Res. 1998;51:303–316. doi: 10.1111/j.1399-3011.1998.tb00428.x. [DOI] [PubMed] [Google Scholar]
- [62].Beligere GS, Dawson PE. Conformationally assisted ligation using C-terminal thioester peptides. J. Am. Chem. Soc. 1999;121:6332–6333. [Google Scholar]
- [63].Camarero JA, Adeva A, Muir TW. 3-Thiopropionic acid as a highly versatile multidetachable thioester resin linker. Lett. Pept. Sci. 2000;7:17–21. [Google Scholar]
- [64].Ingenito R, Bianchi E, Fattori D, Pessi A. Solid phase synthesis of peptide C-terminal thioesters by Fmoc/t-Bu chemistry Source. J. Am. Chem. Soc. 1999;121:11369–11374. [Google Scholar]
- [65].Shin Y, Winans KA, Backes BJ, Kent SBH, Ellman JA, Bertozzi CR. Fmoc-Based Synthesis of Peptide-aThioesters: Application to the Total Chemical Synthesis of a Glycoprotein by Native Chemical Ligation. J. Am. Chem. Soc. 1999;121:11684–11689. [Google Scholar]
- [66].Camarero JA, Hackel BJ, de Yoreo JJ, Mitchell AR. Fmoc-based synthesis of peptide alpha-thioesters using an aryl hydrazine support. J Org Chem. 2004;69:4145–4151. doi: 10.1021/jo040140h. [DOI] [PubMed] [Google Scholar]
- [67].Blanco-Canosa JB, Dawson PE. An efficient Fmoc-SPPS approach for the generation of thioester peptide precursors for use in native chemical ligation. Angew Chem Int Ed Engl. 2008;47:6851–6855. doi: 10.1002/anie.200705471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sancheti H, Camarero JA. “Splicing up” drug discovery. Cell-based expression and screening of genetically-encoded libraries of backbone-cyclized polypeptides. Adv Drug Deliv Rev. 2009;61:908–917. doi: 10.1016/j.addr.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kimura RH, Tran AT, Camarero JA. Biosynthesis of the cyclotide kalata B1 by using protein splicing. Angewandte Chemie (International ed. 2006;45:973–976. doi: 10.1002/anie.200503882. [DOI] [PubMed] [Google Scholar]
- [70].Camarero JA, Kimura RH, Woo YH, Shekhtman A, Cantor J. Biosynthesis of a fully functional cyclotide inside living bacterial cells. Chembiochem. 2007;8:1363–1366. doi: 10.1002/cbic.200700183. [DOI] [PubMed] [Google Scholar]
- [71].Perler FB, Adam E. Protein splicing and its applications. Curr. Opin. Biotechnol. 2000:377–383. doi: 10.1016/s0958-1669(00)00113-0. [DOI] [PubMed] [Google Scholar]
- [72].Hirel PH, Schmitter MJ, Dessen P, Fayat G, Blanquet S. Extent of N-terminal methionine excision from Escherichia coli proteins is governed by the side-chain length of the penultimate amino acid. Proc. Natl. Acad. Sci. U S A. 1989;86:8247–8251. doi: 10.1073/pnas.86.21.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Dwyer MA, Lu W, Dwyer JJ, Kossiakoff AA. Biosynthetic phage display: a novel protein engineering tool combining chemical and genetic diversity. Chem. Biol. 2000;7:263–274. doi: 10.1016/s1074-5521(00)00102-2. [DOI] [PubMed] [Google Scholar]
- [74].Iwai H, Pluckthum A. Circular b-lactamase: stability enhancement by cyclizing the backbone. FEBS Lett. 1999:166–172. doi: 10.1016/s0014-5793(99)01220-x. [DOI] [PubMed] [Google Scholar]
- [75].Camarero JA, Fushman D, Cowburn D, Muir TW. Peptide chemical ligation inside living cells: in vivo generation of a circular protein domain. Bioorg Med Chem. 2001;9:2479–2484. doi: 10.1016/s0968-0896(01)00217-6. [DOI] [PubMed] [Google Scholar]
- [76].Cotton GJ, Ayers B, Xu R, Muir TW. Insertion of a Synthetic Peptide into a Recombinant Protein Framework; A Protein Biosensor. J. Am. Chem. Soc. 1999;121:1100–1101. [Google Scholar]
- [77].Tolbert TJ, Wong C-H. New methods for proteomic research: preparation of proteins with N-terminal cysteines for labeling and conjugation. Angew. Chem. Int. Ed. 2002;41:2171–2174. doi: 10.1002/1521-3773(20020617)41:12<2171::aid-anie2171>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- [78].Erlanson DA, Chytil M, Verdine GL. The leucine zipper domain controls the orientation of AP-1 in the NFAT.AP-1.DNA complex. Chem Biol. 1996;3:981–991. doi: 10.1016/s1074-5521(96)90165-9. [DOI] [PubMed] [Google Scholar]
- [79].Baker RT, Smith SA, Marano R, McKee J, Board PG. Protein expression using cotranslational fusion and cleavage of ubiquitin. Mutagenesis of the glutathione-binding site of human Pi class glutathione S-transferase. J Biol Chem. 1994;269:25381–25386. [PubMed] [Google Scholar]
- [80].Varshavsky A. Ubiquitin fusion technique and related methods. Methods Enzymol. 2005;399:777–799. doi: 10.1016/S0076-6879(05)99051-4. [DOI] [PubMed] [Google Scholar]
- [81].Dykes CW, Bookless AB, Coomber BA, Noble SA, Humber DC, Hobden AN. Expression of atrial natriuretic factor as a cleavable fusion protein with chloramphenicol acetyltransferase in Escherichia coli. Eur J Biochem. 1988;174:411–416. doi: 10.1111/j.1432-1033.1988.tb14113.x. [DOI] [PubMed] [Google Scholar]
- [82].Liu D, Xu R, Dutta K, Cowburn D. N-terminal cysteinyl proteins can be prepared using thrombin cleavage. FEBS Lett. 2008;582:1163–1167. doi: 10.1016/j.febslet.2008.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Dalbey RE, Lively MO, Bron S, van Dijl JM. The chemistry and enzymology of the type I signal peptidases. Protein Sci. 1997;6:1129–1138. doi: 10.1002/pro.5560060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Paetzel M, Dalbey RE, Strynadka NC. Crystal structure of a bacterial signal peptidase apoenzyme: implications for signal peptide binding and the Ser-Lys dyad mechanism. J Biol Chem. 2002;277:9512–9519. doi: 10.1074/jbc.M110983200. [DOI] [PubMed] [Google Scholar]
- [85].Hauser PS, Ryan RO. Expressed protein ligation using an N-terminal cysteine containing fragment generated in vivo from a pelB fusion protein. Protein Expr Purif. 2007;54:227–233. doi: 10.1016/j.pep.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Evans TC, Benner J, Xu M-Q. The in Vitro Ligation of Bacterially Expressed Proteins Using an Intein from Metanobacterium thermoautotrophicum. J. Biol. Chem. 1999;274:3923–3926. doi: 10.1074/jbc.274.7.3923. [DOI] [PubMed] [Google Scholar]
- [87].Southworth MW, Amaya K, Evans TC, Xu MQ, Perler FB. Purification of proteins fused to either the amino or carboxy terminus of the Mycobacterium xenopi gyrase A intein. Biotechniques. 1999;27116:110–114. 118–120. doi: 10.2144/99271st04. [DOI] [PubMed] [Google Scholar]
- [88].Mathys S, Evans TC, Chute IC, Wu H, Chong S, Benner J, Liu XQ, Xu MQ. Characterization of a self-splicing mini-intein and its conversion into autocatalytic N- and C-terminal cleavage elements: facile production of protein building blocks for protein ligation. Gene. 1999;231:1–13. doi: 10.1016/s0378-1119(99)00103-1. [DOI] [PubMed] [Google Scholar]
- [89].Austin J, Kimura RH, Woo YH, Camarero JA. In vivo biosynthesis of an Ala-scan library based on the cyclic peptide SFTI-1. Amino Acids. 2010;38:1313–1322. doi: 10.1007/s00726-009-0338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Abel-Santos E, Scott CP, Benkovic SJ. Use of inteins for the in vivo production of stable cyclic peptide libraries in E. coli. Methods Mol. Biol. 2003;205:281–294. doi: 10.1385/1-59259-301-1:281. [DOI] [PubMed] [Google Scholar]
- [91].Tavassoli A, Benkovic SJ. Split-intein mediated circular ligation used in the synthesis of cyclic peptide libraries in E. coli. Nat Protoc. 2007;2:1126–1133. doi: 10.1038/nprot.2007.152. [DOI] [PubMed] [Google Scholar]
- [92].Scott CP, Abel-Santos E, Wall M, Wahnon D, Benkovic SJ. Production of cyclic peptides and proteins in vivo. Proc. Natl. Acad. Sci. USA. 1999;96:13638–13643. doi: 10.1073/pnas.96.24.13638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Scott CP, Abel-Santos E, Jones AD, Benkovic SJ. Structural requirements for the biosynthesis of backbone cyclic peptide libraries. Chem. Biol. 2001;8:801–815. doi: 10.1016/s1074-5521(01)00052-7. [DOI] [PubMed] [Google Scholar]
- [94].Gunasekera S, Foley FM, Clark RJ, Sando L, Fabri LJ, Craik DJ, Daly NL. Engineering stabilized vascular endothelial growth factor-A antagonists: synthesis, structural characterization, and bioactivity of grafted analogues of cyclotides. J Med Chem. 2008;51:7697–7704. doi: 10.1021/jm800704e. [DOI] [PubMed] [Google Scholar]
- [95].Thongyoo P, Roque-Rosell N, Leatherbarrow RJ, Tate EW. Chemical and biomimetic total syntheses of natural and engineered MCoTI cyclotides. Org Biomol Chem. 2008;6:1462–1470. doi: 10.1039/b801667d. [DOI] [PubMed] [Google Scholar]
- [96].Thongyoo P, Bonomelli C, Leatherbarrow RJ, Tate EW. Potent inhibitors of beta-tryptase and human leukocyte elastase based on the MCoTI-II scaffold. J Med Chem. 2009;52:6197–6200. doi: 10.1021/jm901233u. [DOI] [PubMed] [Google Scholar]
- [97].Reiss S, Sieber M, Oberle V, Wentzel A, Spangenberg P, Claus R, Kolmar H, Losche W. Inhibition of platelet aggregation by grafting RGD and KGD sequences on the structural scaffold of small disulfide-rich proteins. Platelets. 2006;17:153–157. doi: 10.1080/09537100500436663. [DOI] [PubMed] [Google Scholar]
- [98].Gruber CW, Muttenthaler M, Freissmuth M. Ligand-Based Peptide Design and Combinatorial Peptide Libraries to Target G Protein-Coupled Receptors. Curr Pharm Des. 2010 doi: 10.2174/138161210793292474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Kinsella TM, Ohashi CT, Harder AG, Yam GC, Li W, Peelle B, Pali ES, Bennett MK, Molineaux SM, Anderson DA, Masuda ES, Payan DG. Retrovirally delivered random cyclic Peptide libraries yield inhibitors of interleukin-4 signaling in human B cells. J Biol Chem. 2002;277:37512–37518. doi: 10.1074/jbc.M206162200. [DOI] [PubMed] [Google Scholar]
- [100].Cheng L, Naumann TA, Horswill AR, Hong SJ, Venters BJ, Tomsho JW, Benkovic SJ, Keiler KC. Discovery of antibacterial cyclic peptides that inhibit the ClpXP protease. Protein Sci. 2007;16:1535–1542. doi: 10.1110/ps.072933007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Tavassoli A, Lu Q, Gam J, Pan H, Benkovic SJ, Cohen SN. Inhibition of HIV budding by a genetically selected cyclic peptide targeting the Gag-TSG101 interaction. ACS Chem Biol. 2008;3:757–764. doi: 10.1021/cb800193n. [DOI] [PubMed] [Google Scholar]
- [102].Hernandez JF, Gagnon J, Chiche L, Nguyen TM, Andrieu JP, Heitz A, Hong T. Trinh, Pham TT, Le Nguyen D. Squash trypsin inhibitors from Momordica cochinchinensis exhibit an atypical macrocyclic structure. Biochemistry. 2000;39:5722–5730. doi: 10.1021/bi9929756. [DOI] [PubMed] [Google Scholar]
- [103].Giepmans BN, Adams SR, Ellisman MH, Tsien RY. The fluorescent toolbox for assessing protein location and function. Science. 2006;312:217–224. doi: 10.1126/science.1124618. [DOI] [PubMed] [Google Scholar]
- [104].You X, Nguyen AW, Jabaiah A, Sheff MA, Thorn KS, Daugherty PS. Intracellular protein interaction mapping with FRET hybrids. Proc Natl Acad Sci U S A. 2006;103:18458–18463. doi: 10.1073/pnas.0605422103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Daly NL, Rosengren KJ, Craik DJ. Discovery, structure and biological activities of cyclotides. Adv Drug Deliv Rev. 2009;61:918–930. doi: 10.1016/j.addr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- [106].Kimura RH, Steenblock ER, Camarero JA. Development of a cell-based fluorescence resonance energy transfer reporter for Bacillus anthracis lethal factor protease. Anal Biochem. 2007;369:60–70. doi: 10.1016/j.ab.2007.05.014. [DOI] [PubMed] [Google Scholar]
- [107].Kolmar H. Biological diversity and therapeutic potential of natural and engineered cystine knot miniproteins. Curr Opin Pharmacol. 2009;9:608–614. doi: 10.1016/j.coph.2009.05.004. [DOI] [PubMed] [Google Scholar]
- [108].Rosengren KJ, Daly NL, Plan MR, Waine C, Craik DJ. Twists, knots, and rings in proteins. Structural definition of the cyclotide framework. J Biol Chem. 2003;278:8606–8616. doi: 10.1074/jbc.M211147200. [DOI] [PubMed] [Google Scholar]
- [109].Felizmenio-Quimio ME, Daly NL, Craik DJ. Circular proteins in plants: solution structure of a novel macrocyclic trypsin inhibitor from Momordica cochinchinensis. J Biol Chem. 2001;276:22875–22882. doi: 10.1074/jbc.M101666200. [DOI] [PubMed] [Google Scholar]