SUMMARY
Gamma-frequency oscillations (GFOs, > 40 Hz) are a general network signature at seizure onset at all stages of development, with possible deleterious consequences in the immature brain. At early developmental stages, the simultaneous occurrence of GFOs in different brain regions suggests the existence of a long-ranging synchronizing mechanism at seizure onset. Here, we show that hippocampo-septal (HS) neurons, which are GABA long-range projection neurons, are necessary to entrain hippocampal interneurons into transient high frequency firing at the onset of epileptiform discharges in the intact, immature septo-hippocampal formation. The synchronized firing of interneurons in turn produces GFOs, which are abolished after the elimination of a small number of HS neurons. Since they provide the necessary fast conduit for pacing large neuronal populations and display intra- and extrahippocampal long-range projections, HS neurons appear to belong to the class of hub cells, which play a crucial role in the synchronization of developing networks.
INTRODUCTION
The immature brain shows a higher susceptibility to epileptic seizures compared to the mature one (Holmes, 1997). Although there is more resistance to acute seizure-induced cell loss than in the adult brain, both clinical (Baram, 2003; Lombroso, 2007) and experimental (Holmes et al., 1998) studies have confirmed that frequent or prolonged seizures lead to long-term impairments in brain development and functional abnormalities. Transient gamma-frequency oscillations (GFOs; > 40Hz) occurring at the onset of most seizures are a marker of a chronic epileptic condition (Worrell et al., 2004). GFOs have been proposed to participate to the induction of alterations of immature networks (Khalilov et al., 2005). These GFOs occur simultaneously in different brain regions, suggesting a wide network pacing system. Yet, the mechanisms underlying the emergence of GFOs and the control of their spatial synchronization are still unknown.
In adult networks, the mechanisms underlying GFOs genesis involve synaptic interactions between glutamatergic and GABA neurons as well as gap junctions (Bartos et al., 2007; Whittington and Traub, 2003). At early stages of postnatal development pyramidal cells are poorly developed and most function depends upon activation of GABA synapses (Ben Ari et al., 1997). In this context, GFOs mechanisms may differ from the adult situation, and reflect the particular anatomical and functional organization of immature networks (Khalilov et al., 2005). Hence, our goal was to identify the cellular and network mechanisms underlying the generation and synchronization of GFOs in various conditions during development. We used the intact in vitro septo-hippocampal preparation, in which various stimuli can be used to trigger epileptiform discharges characterized by GFOs at their onset (Khalilov et al., 2005; Quilichini et al., 2002), thus enabling the study of their underlying mechanisms.
RESULTS
GFOs occur simultaneously in the immature SHF at seizure onset
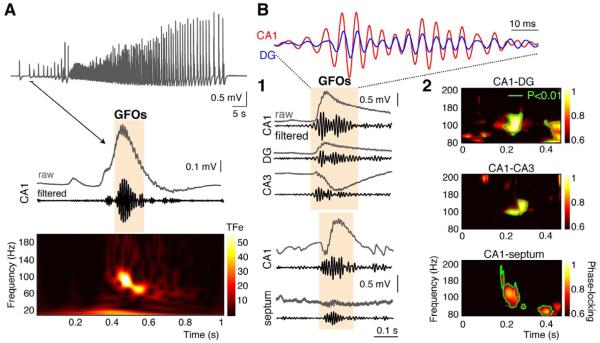
Field potential recordings in the intact septo-hippocampal formation (SHF), during the first postnatal week (P5-7) in low Mg2+ conditions (supplemental information), revealed the presence of transient GFOs (peak frequency: 88 ± 6 Hz, duration: 143 ± 19 ms, n=46 preparations) at the onset of regularly occurring ictal-like events (ILEs) (Quilichini et al., 2002) (Figures 1A-S1A). GFOs were coordinated throughout the preparation: they occurred simultaneously and were phase-locked in the different regions of the hippocampus and in the septum (φDG-φCA1 = 13.8 ± 17.8 °, φCA3-φCA1 = 85.4 ± 30.4 °, φseptum-φCA1 = 114.6 ± 31.5 °, n=3, Figure 1B). There is thus a mechanism in the septo-hippocampal region able to transiently synchronize networks in the gamma frequency range at the initiation of ILEs across quite large distances. Since this mechanism is at least present throughout CA1 (Figure S1B), it was further investigated in this region.
Figure 1. GFOs at seizure onset in the immature SHF.
(A) ILE recorded extracellularly in the CA1 area of a P7 SHF expresses GFOs at its onset as evidenced by 60-120 Hz band-pass filtering and by the time-frequency (TF) representation (TFe: TF energy, color coded in standard deviations above the mean power of an event-free baseline).
(B1) GFOs occurs simultaneously in the dentate gyrus (DG) and in the CA3 and CA1 regions, as well as between the septum and the CA1 region.
(B2) TF analysis of phase-locking between the different regions of the SHF reveals a significant enhancement of phase-synchrony around 100Hz (green contour line: p<0.01).
Interneurons fire at high frequency during GFOs whereas HS cells fire before
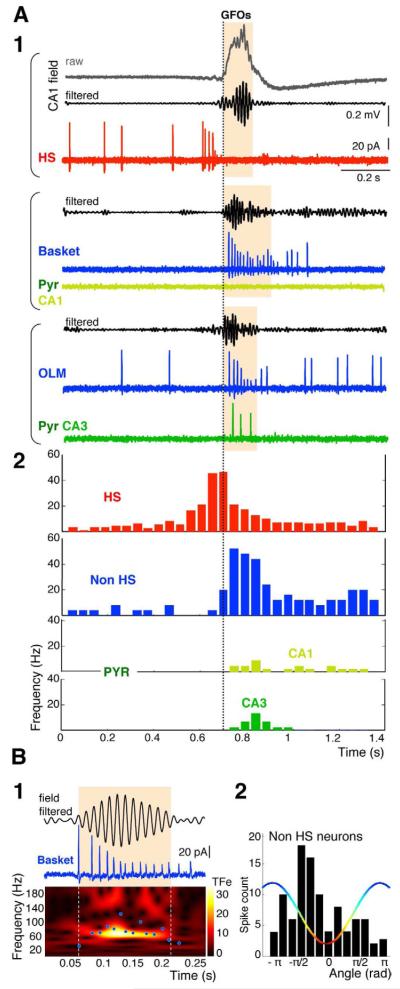
Large populations of neurons fire action potentials during GFOs, thus contributing to the field activity (Chrobak and Buzsaki, 1996). We thus determined the firing pattern of different neuronal classes during the GFOs. Using cell-attached recordings, we found that CA1 pyramidal cells (n=10) were either silent (n=8) or fired a single action potential (n=2) during GFOs, and CA3 pyramidal cells (n=10) fired at low rate (<25 Hz) (Figure 2A). Pyramidal cell always fired after GFOs initiation (Figure 2A2). In contrast, all the CA1 interneurons recorded (n=36; Figure S2), including basket cells (n=3), O-LM cells (n=7) and back-projecting cells (n=4), fired at high frequency exclusively during GFOs, reaching a maximum firing rate of 72 ± 10 Hz (range 40-100Hz), i.e. at nearly the same frequency as GFOs (Figure 2B1). Before GFOs occurrence, all GABA neurons, except Hippocampo-Septal (HS) cells described below, had a low firing rate (2 ± 2 Hz, range: 0-9 Hz, n=36) and the transition to high firing rate during GFOs was abrupt (Figure 2A2). Moreover, their action potentials were phase-locked to GFOs, arising preferentially at the descending phase of each cycle (−34.4 ± 80 °, R = 0.35, P < 0.001, Rayleigh test, Figure 2B2). These results are in agreement with the initial assumption that interneurons are the main contributors to GFOs generation. Accordingly, we found that GFOs depend upon GABAA but not AMPA receptor-activation (Figure S1C).
Figure 2. Interneurons fire during GFOs.
(A1) Firing patterns of simultaneously (bracket) recorded neurons (HS, basket, O-LM, CA1 and CA3 pyramidal cells, cell-attached recording, see Figure S2 for the morphology of the identified neurons) in three different experiments. The traces are aligned at the onset of each GFOs episode. After GFOs onset, interneurons fire at high frequency whilst pyramidal cells are either silent or fire at a lower frequency.
(A2) Mean firing activity of HS cells (n=23), interneurons (n=36), CA3 (n=10) and CA1 (n=10) pyramidal cells; bin size: 40 ms.
(B1) The instantaneous firing frequency of a basket cell (blue dots) is plotted against the GFOs TF plot. The neuron firing follows the GFOs frequency.
(B2) Interneurons (n=36) fire preferentially during the descending phase of the GFO cycle.
One type of GABA neuron, the HS cells, showed a different firing pattern. They always fired before GFOs onset, with a 10-300 ms time lag (69 ± 35 ms, n=23), reaching a maximal peak frequency of 96 ± 25 Hz (Figure 3A). These neurons belonged to the class of long-range projection GABA neurons (Figure 3A2) (Gulyas et al., 2003). Morphological analysis revealed that HS cells, although still in an early developmental stage, exhibited an extensive axon arborization in the different CA1 layers along the septo-temporal axis, as well as in the septum (Figure 3A2). During development, they are known to contact exclusively GABA neurons (Gulyas et al., 2003). Because of their early firing, we reasoned that HS cells might have a pivotal role in GFOs emergence by synchronizing their target interneurons (i.e. GABA neurons with exclusive local projections) into high frequency firing thus generating field GFOs. The firing patterns of the different cell types during GFOs are consistent with this hypothesis. If HS cells play a leading role in synchronizing their targets, we proposed the following scheme: 1) a progressive recruitment of HS cells, since transient network events may involve a progressive recruitment of leading cells (Menendez de la Prida et al., 2006); 2) a depolarizing action of GABA onto interneurons; 3) a recruitment of the interneurons (with exclusive local projections) by the HS cells. Finally, if a causal link exists between HS firing and GFOs emergence, preventing HS firing should abolish GFOs. We directly tested these proposals.
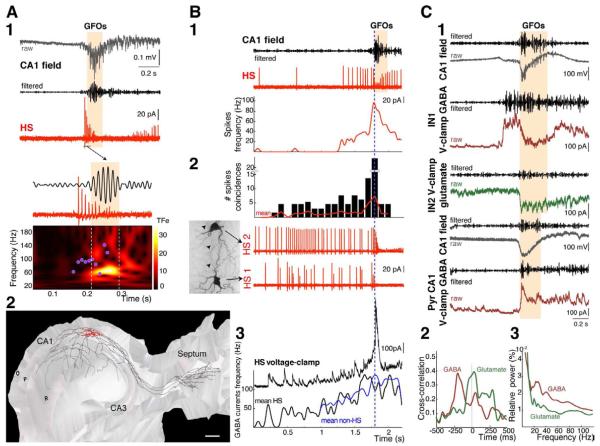
Figure 3. HS neurons firing precedes field GFOs.
(A1) The HS cell high frequency firing (cell-attached recording) precedes the GFOs. The decrease in spike amplitude during the burst is probably due to the inactivation of Na+ channels. Its instantaneous firing frequency (pink dots) is plotted against the GFOs TF plot.
(A2) Three-dimensional reconstruction of a HS cell. The soma (red) of the HS cell is located in stratum oriens and displays horizontally running dendrites (red). The axon (black) gives rise to many collaterals invading the septum as well as all layers of the CA1 hippocampal region along the septo-temporal axis.
O, stratum oriens; P, stratum pyramidale; R, stratum radiatum. Scale bar, 200 μm.
(B1) The progressive increase in the HS cell firing (top: cell-attached recording; bottom: instantaneous spike frequency) before GFOs onset suggests a build-up process.
(B2) Increased degree of spike coincidences (within 15ms, n=3) before GFOs in simultaneous recording of two connected HS cells (HS1 and HS2; cell-attached configuration). Morphological recovery of the pair of HS cells shows a putative synaptic contact (dotted circle) between the axon (arrowheads) of the HS-cell 1 and one dendrite of the HS-cell 2.
(B3) A progressive increase in the frequency of GABA postsynaptic currents in a HS cell can be seen 10-300 ms before GFOs as evidenced by mean instantaneous frequency of GABA currents received by HS cells (n=8) and interneurons (n=22).
(C1) Synaptic currents recorded in different O-LM interneurons (IN1 and IN2) and a CA1 pyramidal cell at holding potentials of +10 mV (GABA currents) and −60 mV (AMPA and NMDA receptor-mediated currents). Interneurons were recorded simultaneously during GFOs. The pyramidal cell was recorded later in the same preparation. The traces are aligned on GFOs onset. In interneurons, but not in pyramidal cells, GABA currents containing gamma-frequency components (40-120 Hz filtered) precede the GFOs by several hundreds of ms.
(C2) Mean cross-correlation between GABA currents or glutamatergic currents and the corresponding GFOs for several interneurons (n=17). Note the significant peaks around −200 ms in the GABA/field correlation.
(C3) High-frequency components (20-90 Hz) predominate in GABA currents over glutamatergic currents in interneurons (n=8) during GFOs.
HS cells orchestrate the synchronization of hippocampal interneurons during GFOs
We first investigated the possibility of a build-up mechanism among interconnected HS cells. We analyzed the firing behavior of eleven HS cell pairs (4 reciprocal and 7 unidirectional connections, Figure S3A-B). Before GFOs genesis, HS cell firing started to accelerate at a mean instantaneous frequency of 28 ± 11 Hz until an abrupt transition to high frequency firing occurred within 150 ± 65 ms before GFOs onset (Figure 3B). In keeping with a progressive recruitment of HS cells, there was a regular increase in GABAergic currents received by both HS cells and interneurons (Figure 3B3). Dual recordings revealed that HS cells were connected together and to other GABA neurons (4 unidirectional HS to O-LM connections, 3 HS to O-LM reciprocal connections, Figure S3A-B). During the build-up process, the frequency of GABA currents remained low, consistent with the low firing frequency of HS cells before their transition to high frequency firing (Figure 3B3). The switch to high frequency firing of HS cells correlated with the high frequency GABA currents received by the interneurons (Figure 3B3). Those results are thus in favor of a progressive recruitment of HS cells.
We then analyzed the nature of the neurotransmission between HS cells and their targets in normal ACSF (4 reciprocal and 7 unidirectional HS pairs, 4 unidirectional HS to O-LM connections, 3 HS to O-LM reciprocal connections, Figure S3A-B). The neurotransmission was extremely reliable at these synapses (1.0 release probability, n=25 connections). Intracellular chloride concentration increases in epileptic conditions in the immature brain, rendering GABA strongly excitatory (Dzhala et al., 2010). Using non-invasive measurements of the resting membrane potential and the reversal potential of chloride in different classes of interneurons, we found that ECl was 19.1 ± 1.9 mV (n= 6 HS cells, 3 O-LM cells, 1 Basket cell, 2 unidentified interneurons, Figure S3C-D) above resting membrane potential (−69.7 ± 2.8 mV, n=12), indicating a depolarizing action of GABA. We also found that high frequency stimulation of HS cells evoked action potentials in their target cells (n=5 SHFs, Figure S3E2b). These results show that HS cell firing can entrain their target neurons, probably via the depolarizing action of GABA.
Our third proposal is a recruitment of interneurons by the HS cells. If HS firing entrains these interneurons, two requirements should be met: first, high frequency GABA currents should occur in interneurons before GFOs, since HS cells, which contact interneurons, always fire before GFOs at high frequency. Second, high frequency GABA currents should occur in turn after GFOs onset in pyramidal cells, since interneurons, which contact pyramidal cells, fire at high frequency during GFOs. Whole-cell recordings of interneurons revealed that large synaptic GABA currents with a high frequency component always preceded field GFOs (mean: 183 ms, range: 40-450 ms, n=17, Figure 3C). Since pyramidal cell-projecting interneurons fire at high frequency during GFOs (Figure 2A), pyramidal cells received a high frequency barrage of GABAergic inputs during GFOs (Figure 3C). The frequency of GABAergic inputs in pyramidal cells (88 ± 17 Hz, n=9) was similar to the firing frequency of interneurons and to the field GFOs. In contrast, the fast oscillatory component within the glutamatergic drive occurred after the initiation of GFOs in all recorded interneurons (n=17) and pyramidal cells (n=9), and had a lower magnitude than GABA currents (Figure 3C2-3). The analysis of synaptic activity thus reveals a sequential recruitment of the different actors in the network: at first a population of GABA neurons, which contact interneurons but not pyramidal cells (interneuron-specific GABA neurons), starts to fire before GFOs. This is consistent with the HS cells specific targeting and firing pattern. This suggests that GABAergic currents provide the main synaptic drive onto interneurons. Then, pyramidal cell-projecting interneurons fire during GFOs, as well as the pyramidal cells, although to a lesser extent.
HS cells are mandatory for GFOs emergence but not to seizure
If HS cells play a leading role and are necessary for GFOs emergence, preventing their firing should abolish GFOs. To test causality, we used GIN (GFP-expressing Inhibitory Neurons) mice in which green fluorescent protein (GFP) is expressed, via the GAD67 promoter, only in somatostatin-containing neurons including HS cells (Oliva et al., 2000). At P6, most GFP-containing neurons recorded in CA1 stratum oriens were identified post hoc as HS cells (72%, n=18/25). The 28% remaining ones were all identified as O-LM cells (n=7/25). Recordings of GFP-negative interneurons revealed the presence of O-LM cells, but not HS cells (n=14 O-LM cells and 14 other types of interneurons, not shown). This suggests that the vast majority of GFP-containing neurons in CA1 stratum oriens at P6 are HS cells (Figure S4A) and that many O-LM cells do not express GFP at this age. GFOs, cell firing patterns and synaptic inputs in GIN mice had properties similar to those found in rats (Figures 4A-S4B). We also used this preparation to test other models of acute seizures: high K+, kainic acid and 4-AP. In all three models, GFOs (96 ± 7 Hz, range: 40-180 Hz, n=22) were present at seizure onset, HS cells (115 ± 14 Hz, range: 40-208 Hz, n=13) started to fire before the GFOs (mean time lag: −105 ± 19 ms), and the interneurons generated spikes only during the GFOs (81 ± 11 Hz, range: 34-117 Hz, n=9; including 8 OLM cells, 1 backprojecting cell, Figure S4C-D-E). Whole-cell recordings of interneurons also showed large GABA synaptic currents preceding GFOs (102 ±31 ms, range: 35-210 ms, n=5, not shown). These features are similar to the ones obtained in low Mg2+ conditions, demonstrating the generality of the mechanism involved in triggering GFOs at ILEs onset at this stage of development.
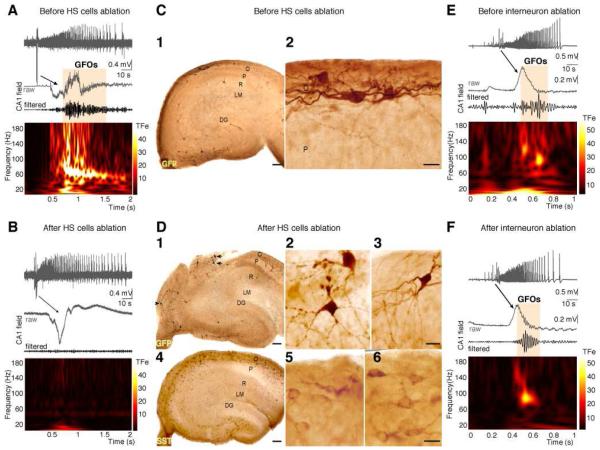
Figure 4. GFOs are abolished by the elimination of HS cells.
(A) GFOs recorded in the CA1 region of the SHF of GIN mice at P6 before targeted illumination.
(B) Specific elimination of GFP-positive GABA neurons by focused fluorescence illumination of the stratum oriens (SO) abolishes GFOs without affecting the occurrence of network discharges. ILEs recorded before and after illumination are similar, with tonic-clonic and clonic phases.
(C1) Distribution of GFP-positive GABA neurons in a hippocampal section of the GIN mice SHF used for electrophysiological recordings after sham fluorescence illumination.
(C2) Note the presence of healthy appearing putative HS cells.
(D) Two consecutive sections show the distribution of (1-2-3) GFP- and (4-5-6) somatostatin-containing neurons in GIN mice SHFs after focused SO fluorescence illumination and electrophysiological recordings.
(D1) In the close vicinity of the illuminated regions, GFP-containing neurons (arrows) display typical features of suffering neurons including beaded dendrites (D2).
(D3) In contrast, GFP-containing neurons (arrowhead in D1) located well outside (>500 μm) the illuminated area show healthy-like dendritic and axonal arborizations. Both in the directly illuminated and non-illuminated regions (D4-5-6), somatostatin-containing neurons can be clearly identified. This suggests that GFP-positive neurons were destroyed in the fluorescence-illuminated region, whilst GFP-negative neurons in the same illuminated area were preserved.
(D4) Outside this area, the cyto-architecture is mostly preserved.
Scales bars: C1 and D1-4: 100 μm; C2 and D2-3-5-6: 25 μm.
(E) GFOs recorded in the CA1 region of the SHF of GIN mice at P6 before GFP-negative neurons ablation.
(F) Elimination of 50 GFP-negative interneurons did not modify the expression of GFOs or the occurrence of ILEs.
Since the disappearance of a critical number of long-range projection neurons disrupts long-range synchrony (Dyhrfjeld-Johnsen et al., 2007), we eliminated stratum oriens GFP-positive cells successively along the septo-temporal axis using focused fluorescence illumination (Figure 4D, n=8 SHFs). The elimination of between 10 and 20 GFP-positive cells (n=8 SHFs) was sufficient to abolish GFOs without affecting the occurrence of ILEs (Figure 4A-B). The network structure and function did not appear damaged by this procedure: GFP-negative cells within the illuminated area and GFP-positive cells outside the illuminated area did not display apparent morphological damage (Figure 4D) and ILEs were still present (Figure 4B). The disappearance of GFOs could result from the loss of the trigger (HS cells) and/or the generator (interneurons). Since only a fraction of O-LM cells (GFP-positive) were removed from the circuitry and all the other generators (including GFP-negative O-LM and basket cells) were not affected, GFOs disappearance most likely results from the loss of a critical mass of HS cells (Figure 4D). Accordingly, the elimination of up to 50 GFP-negative interneurons (n=4 SHFs) did not affect the occurrence of GFOs at ILEs onset (Figure 5E-F). Finally, while recording from pairs of HS cells, we generated simultaneous trains of action potentials at 100 Hz in each pair. This was not sufficient to entrain the system to produce field GFOs, in keeping with the proposal that a critical mass of HS cells needs to be recruited.
DISCUSSION
In this study, we have shown in the immature SHF that: i) field GFOs are present at ILEs onset; ii) long-range projection HS cells start to fire at high frequency before field GFOs; iii) all the interneuron types recorded fire in turn high frequency action potentials arising preferentially at the descending phase of the GFOs; iv) GFOs are abolished after the elimination of a small number of HS cells. Taken together, we propose that HS cells, via their target-selective excitatory GABA synapses and their extensive axonal projections, provide the necessary fast conduit for synchronizing the hippocampal interneurons into transient high frequency firing, leading to GFOs emergence at the onset of epileptiform discharges.
The mechanisms that underlie GFOs in epileptogenic conditions, at early stages of development, contrast with those arising in physiological conditions. Several observations suggest that spontaneous GFOs are not present in developing networks. In rat pups, high-frequency (120-180 Hz) oscillations are observed in vivo in the hippocampus after the end of the second postnatal week (Buhl and Buzsaki, 2005). Moreover, various in vitro GFOs-generating procedures or agents, such as bath application of the ACh receptor agonist carbachol of intact cortex of newborn rats (Kilb and Luhmann, 2003) or high-frequency stimulation of CA1 afferents in rat hippocampal slices (Ruusuvuori et al., 2004), failed to generate GFOs during the first postnatal week. Because physiological GFOs are largely driven by glutamate in mature networks (Bartos et al., 2007; Fisahn et al., 1998; Traub et al., 1998; Whittington and Traub, 2003), these observations are consistent with the delayed maturation of glutamatergic synapses shown in a wide range of brain structures (Gozlan and Ben Ari, 2003). As suggested previously (Traub et al., 1998), developing networks would lack the critical density of functional glutamatergic synapses required for these oscillatory activities. However, GFOs can emerge in epileptogenic conditions, signaling a pathological state. We showed that AMPA receptor-activation is not necessary for GFOs expression and the glutamatergic drive always follows the GABAergic one in all neuron types. This is also consistent with other findings showing that synchronization of GABA neurons can occur in the absence of fast glutamatergic signaling via depolarizing GABA (Avoli and Perreault, 1987; Michelson and Wong, 1991). Furthermore, this is in agreement too with the tetanic model of GFOs that displays comparable GABA mechanisms to the low Mg2+ model (Fujiwara-Tsukamoto et al., 2006) since it also requires a depolarizing GABA action (Kohling et al., 2000), which is due to intracellular chloride accumulation during recurrent seizures (Dzhala et al., 2010).
Although very high frequency oscillations (HFOs in the ripples 140–200 Hz or fast ripples 200–500 Hz range) can be recorded in adult epileptic networks in vitro (Khosravani et al., 2005; Traub et al., 2001) and in vivo (Jirsch et al., 2006), the GFOs recorded in our conditions never reached such frequencies during the first post-natal week, probably reflecting the immature stage of development (Buhl and Buzsaki, 2005). It has been suggested that recurrent glutamatergic synaptic transmission (Dzhala and Staley, 2004) and pyramidal axo-axonic gap junctions (Traub et al., 2002) are instrumental in the generation of HFOs in the adult brain. This implies that these additional mechanisms are not yet fully functional. Nevertheless, the immature hippocampus has already reached a sufficient level of organization to generate GFOs (40-100 Hz) under epileptogenic conditions.
Collectively, our results provide strong support for the concept that, in different epileptogenic conditions at early stages of development, long-range projection neurons can entrain interneurons, with exclusive local connectivity, into transient high frequency firing leading to GFOs. Although most of HS cells continued to fire at high frequency during GFOs, thus contributing to their expression, their main impact appears to be coordinating the activity of their targets. In the adult brain, long-range projection neurons, which can contact both pyramidal cells and interneurons (Jinno et al., 2007; Takacs et al., 2008), may fulfill the role of synchronizing elements (Tort et al., 2007). In our conditions, HS cells do not appear to functionally contact pyramidal cells, as GABAergic currents should have occurred simultaneously in pyramidal cells and interneurons. Whether this discrepancy reflects a maturation process of these neurons and/or the existence of different classes of long-range projection neurons (Jinno et al., 2007) remains to be determined.
Interestingly, GFP expression in GIN mice is driven via the GAD67 promoter. GAD67 expression is developmentally regulated and is lower at the end of the first postnatal week compared to adults (Jiang et al., 2001). Hence at P6, GFP-negative neurons might include immature somatostatin-containing neurons (in which GAD67 expression is still low and would increase later in development) in addition to SST-negative neurons. This also suggests that HS cells, which form the vast majority of GFP-positive neurons, already display at P6 features of mature neurons compared to others futures somatostatin-containing interneurons. We show here that these long-range projection neurons play a key role in triggering network synchronization and GFOs expression. Interestingly, some “connector hub neurons” described in immature mouse hippocampal slices also show an extended axonal arborization (within the hippocampus), a similar co-activation (built-up of synchronization) before the onset of network activity (giant depolarizing potentials), and orchestration of spontaneous network synchronization (Bonifazi et al., 2009). Besides, early-generated GABA hub neurons express preferentially somatostatin and were recently proposed to develop into GABA projection neurons (Picardo et al., 2011). It has also been suggested that GABA neurons displaying long-range axonal arborization extending the outside the hippocampus would carry such a hub function and would support the emergence of network oscillations (Buzsaki et al., 2004). Thus, HS cells are likely to belong to this class of hub neurons and to form such a hub network critical for broad network synchronization during development. In keeping with such an assumption, the elimination of some HS cells abolished GFOs, but not the ILEs themselves.
Altogether, our results have two major implications: i) In contrast to adult networks (Jirsch et al., 2006; Steriade and Demetrescu, 1966) and with the caveat that we used an in vitro acute model of epilepsy, we propose that GFOs may not be causally linked to seizure genesis at early stages of development. They would sign the activity of a network before its transition to the ictal discharge; ii) Few hub-like GABA neurons, i.e. HS cells, are able to synchronize wide reaching large neuronal populations, enabling GFOs to emerge, a phenomenon that may also be valid in physiological conditions.
EXPERIMENTAL PROCEDURES
Intact septo-hippocampal formations (SHFs) were prepared from 5-7 day old rats and GIN mice. Extracellular recordings, cell attached and voltage-clamp whole-cell recordings were performed at 33°C from hippocampal CA1 pyramidal cells and interneurons. GFOs were quantified using wavelet time-frequency analysis. GABAergic and glutamatergic synaptic currents were measured at +10 mV and −60 mV, respectively. Resting membrane potential and the reversal potential of GABAergic currents were measured using single NMDA and GABAA receptor channel recordings in the cell attached configuration. Synaptic connections between cells were determined by making one cell fire an action potential and by detecting the presence of a postsynaptic GABA current in the second cell. Ablation of GFP-containing neurons was obtained following 5 min long high power fluorescence focused through a 60× objective. All recorded cells were filled with biocytin for post hoc morphological identification. They were reconstructed using the Neurolucida system. Immunohistochemical labelings for GFP- and somatostatin-containing neurons were performed using polyclonal antisera directed against GFP and somatostatin, respectively.
Supplementary Material
Highlights.
Long-range projection cells are important for synchronizing γ-frequency oscillations
Hippocampo-Septal cells show a built-up in their synchronization before field GFOs
HS cells entrain the interneurons firing, which in turn produces transient GFOs
Elimination of a small number of hub HS neurons abolishes the expression of GFOs
ACKNOWLEDGEMENTS
This work was supported by INSERM, Fondation pour la Recherche sur le Cerveau, Fédération Française de la Recherche sur l’Epilepsie, Fondation pour la Recherche Médicale (PPQ), Ligue Française Contre l’Epilepsie (PPQ), NIH (F33NS062617 to DAT and CB), The Philippe Foundation (DAT) and the Letten Foundation (CB). Initial experiments were performed in Y. Ben-Ari’s lab (INMED-INSERM U29). We thank G. Buzsàki and D. Johnston for helpful comments on the manuscript, and M. Fontes for hosting AC, ME and CB in his laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental information includes four figures, full experimental procedures and associated references.
REFERENCES
- Avoli M, Perreault P. A GABAergic depolarizing potential in the hippocampus disclosed by the convulsant 4-aminopyridine. Brain Res. 1987;400:191–195. doi: 10.1016/0006-8993(87)90671-8. [DOI] [PubMed] [Google Scholar]
- Baram TZ. Long-term neuroplasticity and functional consequences of single versus recurrent early-life seizures. Ann Neurol. 2003;54:701–705. doi: 10.1002/ana.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Ben Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated ‘menage a trois’. Trends Neurosci. 1997;20:523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- Bonifazi P, Goldin M, Picardo MA, Jorquera I, Cattani A, Bianconi G, Represa A, Ben-Ari Y, Cossart R. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science. 2009;326:1419–1424. doi: 10.1126/science.1175509. [DOI] [PubMed] [Google Scholar]
- Buhl DL, Buzsaki G. Developmental emergence of hippocampal fast-field “ripple” oscillations in the behaving rat pups. Neuroscience. 2005;134:1423–1430. doi: 10.1016/j.neuroscience.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Geisler C, Henze DA, Wang XJ. Interneuron Diversity series: Circuit complexity and axon wiring economy of cortical interneurons. Trends Neurosci. 2004;27:186–193. doi: 10.1016/j.tins.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsaki G. High-frequency oscillations in the output networks of the hippocampal-entorhinal axis of the freely behaving rat. J.Neurosci. 1996;16:3056–3066. doi: 10.1523/JNEUROSCI.16-09-03056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyhrfjeld-Johnsen J, Santhakumar V, Morgan RJ, Huerta R, Tsimring L, Soltesz I. Topological determinants of epileptogenesis in large-scale structural and functional models of the dentate gyrus derived from experimental data. J Neurophysiol. 2007;97:1566–1587. doi: 10.1152/jn.00950.2006. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Kuchibhotla KV, Glykys JC, Kahle KT, Swiercz WB, Feng G, Kuner T, Augustine GJ, Bacskai BJ, Staley KJ. Progressive NKCC1-dependent neuronal chloride accumulation during neonatal seizures. J Neurosci. 2010;30:11745–11761. doi: 10.1523/JNEUROSCI.1769-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhala VI, Staley KJ. Mechanisms of fast ripples in the hippocampus. J Neurosci. 2004;24:8896–8906. doi: 10.1523/JNEUROSCI.3112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Fujiwara-Tsukamoto Y, Isomura Y, Takada M. Comparable GABAergic mechanisms of hippocampal seizurelike activity in posttetanic and low-Mg2+ conditions. J Neurophysiol. 2006;95:2013–2019. doi: 10.1152/jn.00238.2005. [DOI] [PubMed] [Google Scholar]
- Gozlan H, Ben Ari Y. Interneurons are the source and the targets of the first synapses formed in the rat developing hippocampal circuit. Cerebral Cortex. 2003;13:684–692. doi: 10.1093/cercor/13.6.684. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Hajos N, Katona I, Freund TF. Interneurons are the local targets of hippocampal inhibitory cells which project to the medial septum. Eur J Neurosci. 2003;17:1861–1872. doi: 10.1046/j.1460-9568.2003.02630.x. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Gairsa JL, Chevassus-Au-Louis N, Ben Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann.Neurol. 1998;44:845–857. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- Jiang M, Oliva AA, Jr., Lam T, Swann JW. GABAergic neurons that pioneer hippocampal area CA1 of the mouse: morphologic features and multiple fates. J Comp Neurol. 2001;439:176–192. doi: 10.1002/cne.1341. [DOI] [PubMed] [Google Scholar]
- Jinno S, Klausberger T, Marton LF, Dalezios Y, Roberts JD, Fuentealba P, Bushong EA, Henze D, Buzsaki G, Somogyi P. Neuronal diversity in GABAergic long-range projections from the hippocampus. J Neurosci. 2007;27:8790–8804. doi: 10.1523/JNEUROSCI.1847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–1608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- Khalilov I, Le Van Quyen M, Gozlan H, Ben-Ari Y. Epileptogenic actions of GABA and fast oscillations in the developing hippocampus. Neuron. 2005;48:787–796. doi: 10.1016/j.neuron.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Khosravani H, Pinnegar CR, Mitchell JR, Bardakjian BL, Federico P, Carlen PL. Increased high-frequency oscillations precede in vitro low-Mg seizures. Epilepsia. 2005;46:1188–1197. doi: 10.1111/j.1528-1167.2005.65604.x. [DOI] [PubMed] [Google Scholar]
- Kilb W, Luhmann HJ. Carbachol-induced network oscillations in the intact cerebral cortex of the newborn rat. Cereb Cortex. 2003;13:409–421. doi: 10.1093/cercor/13.4.409. [DOI] [PubMed] [Google Scholar]
- Kohling R, Vreugdenhil M, Bracci E, Jefferys JG. Ictal epileptiform activity is facilitated by hippocampal GABAA receptor-mediated oscillations. J.Neurosci. 2000;20:6820–6829. doi: 10.1523/JNEUROSCI.20-18-06820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombroso CT. Neonatal seizures: gaps between the laboratory and the clinic. Epilepsia. 2007;48(Suppl 2):83–106. doi: 10.1111/j.1528-1167.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- Menendez de la Prida L, Huberfeld G, Cohen I, Miles R. Threshold behavior in the initiation of hippocampal population bursts. Neuron. 2006;49:131–142. doi: 10.1016/j.neuron.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Michelson HB, Wong RK. Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science. 1991;253:1420–1423. doi: 10.1126/science.1654594. [DOI] [PubMed] [Google Scholar]
- Oliva AA, Jr., Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci. 2000;20:3354–3368. doi: 10.1523/JNEUROSCI.20-09-03354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picardo MA, Guigue P, Bonifazi P, Batista-Brito R, Allene C, Ribas A, Fishell G, Baude A, Cossart R. Pioneer GABA cells comprise a subpopulation of hub neurons in the developing hippocampus. Neuron. 2011;71:695–709. doi: 10.1016/j.neuron.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilichini PP, Diabira D, Chiron C, Ben Ari Y, Gozlan H. Persistent epileptiform activity induced by low Mg2+ in intact immature brain structures. Eur.J Neurosci. 2002;16:850–860. doi: 10.1046/j.1460-9568.2002.02143.x. [DOI] [PubMed] [Google Scholar]
- Ruusuvuori E, Li H, Huttu K, Palva JM, Smirnov S, Rivera C, Kaila K, Voipio J. Carbonic anhydrase isoform VII acts as a molecular switch in the development of synchronous gamma-frequency firing of hippocampal CA1 pyramidal cells. J Neurosci. 2004;24:2699–2707. doi: 10.1523/JNEUROSCI.5176-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Demetrescu M. Post-primary cortical responses to flashes and their specific potentiation by steady light. Electroencephalogr Clin Neurophysiol. 1966;20:576–590. doi: 10.1016/0013-4694(66)90022-8. [DOI] [PubMed] [Google Scholar]
- Takacs VT, Freund TF, Gulyas AI. Types and synaptic connections of hippocampal inhibitory neurons reciprocally connected with the medial septum. Eur J Neurosci. 2008;28:148–164. doi: 10.1111/j.1460-9568.2008.06319.x. [DOI] [PubMed] [Google Scholar]
- Tort AB, Rotstein HG, Dugladze T, Gloveli T, Kopell NJ. On the formation of gamma-coherent cell assemblies by oriens lacunosum-moleculare interneurons in the hippocampus. Proc Natl Acad Sci U S A. 2007;104:13490–13495. doi: 10.1073/pnas.0705708104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Draguhn A, Whittington MA, Baldeweg T, Bibbig A, Buhl EH, Schmitz D. Axonal gap junctions between principal neurons: a novel source of network oscillations, and perhaps epileptogenesis. Rev.Neurosci. 2002;13:1–30. doi: 10.1515/revneuro.2002.13.1.1. [DOI] [PubMed] [Google Scholar]
- Traub RD, Kopell N, Bibbig A, Buhl EH, LeBeau FE, Whittington MA. Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. J Neurosci. 2001;21:9478–9486. doi: 10.1523/JNEUROSCI.21-23-09478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Spruston N, Soltesz I, Konnerth A, Whittington MA, Jefferys GR. Gamma-frequency oscillations: a neuronal population phenomenon, regulated by synaptic and intrinsic cellular processes, and inducing synaptic plasticity. Prog.Neurobiol. 1998;55:563–575. doi: 10.1016/s0301-0082(98)00020-3. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–682. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004;127:1496–1506. doi: 10.1093/brain/awh149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.