Abstract
Oxidative stress and apoptosis are two key pathophysiological mechanisms underlying dopaminergic degeneration in Parkinson’s disease (PD). Recently, we identified that proteolytic activation of protein kinase C-delta (PKCδ), a member of the novel PKC family, contributes to oxidative stress-induced dopaminergic degeneration and that phosphorylation of tyrosine residue 311 (tyr311) on PKCδ is a key event preceding the PKCδ proteolytic activation during oxidative damage. Herein, we report that a non-receptor tyrosine kinase Fyn is significantly expressed in a dopaminergic neuronal N27 cell model. Exposure of N27 cells to the dopaminergic toxicant dieldrin (60 μM) rapidly activated Fyn kinase, PKCδ-tyr311 phosphorylation and proteolytic cleavage. Fyn kinase activation precedes the caspase-3-mediated proteolytic activation of PKCδ. Co-treatment with p60-tyrosine-specific kinase inhibitor (TSKI) almost completely attenuated dieldrin-induced phosphorylation of PKCδ-tyr311 and its proteolytic activation. Additionally, TSKI almost completely blocked dieldrin-induced apoptotic cell death. To further confirm Fyn’s role in the pro-apoptotic function of PKCδ, we adopted the RNAi approach. siRNA-mediated knockdown of Fyn kinase also effectively attenuated dieldrin-induced phosphorylation of PKCδ-tyr311, caspase-3-mediated PKCδ proteolytic cleavage, and DNA fragmentation, suggesting that Fyn kinase regulates the pro-apoptotic function of PKCδ. Collectively, these results demonstrate for the first time that Fyn kinase is a pro-apoptotic kinase that regulates upstream signaling of the PKCδ-mediated apoptotic cell death pathway in neurotoxicity models of pesticide exposure.
Keywords: pesticides, oxidative stress, kinases, apoptosis, neurodegeneration
1. Introduction
Parkinson’s disease (PD) is a widespread neurodegenerative movement disorder characterized by a preferential and progressive degeneration of dopaminergic neurons in the mesencephalic region of the brain, resulting in irreversible motor dysfunction characterized by bradykinesia, postural instability and muscular rigidity (Przedborski and Ischiropoulos, 2005; Przedborski and Vila, 2001; Schapira, 2009). This debilitating disorder affects about a million people in the United States with an estimated 50,000 new cases reported each year (Van Den Eeden et al., 2003). Intraneuronal proteinaceous inclusions termed as Lewy bodies mark the pathological significance of this disease (Roodveldt et al., 2008; Uversky et al., 2001). Despite research for several years, the etiopathogenesis of PD remains enigmatic. Well-documented disease etiology and epidemiology point to a multifactorial causation to this disease (Elbaz et al., 2007; Elbaz and Tranchant, 2007; Giasson and Lee, 2000; Paolini et al., 2004). Among the many etiological factors is environmental exposure to the organochloride pesticide dieldrin, which continues to pose a serious etiological threat in the vulnerability to PD even 30 years after the chemical was banned by the Environmental Protection Agency (EPA) in 1974 (Costello et al., 2009; Kitazawa et al., 2004; Kitazawa et al., 2001; Kitazawa et al., 2003, Anantharam, 2002; Paolini et al., 2004; Priyadarshi et al., 2000; Ritz and Yu, 2000; Tuchsen and Jensen, 2000). Prior to EPA ban, dieldrin was one of the most widely used insecticides in agriculture, and its long half-life has resulted in its bioaccumulation in the environment. As a result, humans continue to be exposed to dieldrin through the food chain (Chopra et al., 2010; Doong et al., 1999; Jorgenson, 2001; Mustafa et al., 2010; Phillips et al., 2010; Schafer and Kegley, 2002).
In addition to its long half-life, the lipophilicity of dieldrin also causes it to accumulate in the central nervous system and adipose tissue (Corrigan et al., 1996; Corrigan et al., 1998; Corrigan et al., 2000). Reports of higher levels of dieldrin in farm-raised salmon in comparison to the North Atlantic salmon, and higher serum levels of dieldrin among Iowa farmers reflect the environmental impact of dieldrin to the farming community (Corrigan et al., 1996; Corrigan et al., 1998; Fleming et al., 1994). In non-neuronal tissues, dieldrin toxicity has been linked to abnormalities in mammary gland development (Tarraf et al., 2003), increased risk to breast cancer (Cameron and Foster, 2008) and reproductive toxicity by affecting Leydig cell function (Fowler et al., 2007). Also, prenatal exposure to dieldrin in an animal model of PD exacerbated the toxic effects of the classical parkinsonian toxicant MPTP (Richardson et al., 2006). In vitro and in vivo studies indicate that the dopaminergic neuronal system is especially sensitive to dieldrin toxicity (Kitazawa et al., 2004; Kitazawa et al., 2001; Kitazawa et al., 2003; Sanchez-Ramos et al., 1998; Sharma et al., 2010). Dieldrin has also been shown to induce oxidative stress via elevation of reactive oxygen species in neuronal as well as non-neuronal cell types (Chun et al., 2001; Kannan et al., 2000). Despite the established association of dieldrin to PD epidemiology, the cellular mechanisms underlying dieldrin-induced dopaminergic degeneration is not completely known. A recent discovery of cooperative toxicity of dieldrin and lindane, another organochloride pesticide that also accumulates in the brain of PD patients (Corrigan et al., 2000; Fleming et al., 1994), further underlines the significance of a multitude of environmental factors contributing to the complex etiopathogenesis of this disorder (Sharma et al., 2010).
We have previously reported that caspase-3-dependent proteolytic activation of protein kinase C delta (PKCδ), plays an important role in the progression of dieldrin-induced apoptotic cascade in rat dopaminergic neurons (Kanthasamy et al., 2003; Kanthasamy et al., 2005; Kanthasamy et al., 2008; Kitazawa et al., 2001; Kitazawa et al., 2003). We recently demonstrated that dieldrin also impairs proteasomal activity resulting in the accumulation of proteins degraded by the ubiquitin-proteasome pathway (Sun et al., 2005). Additionally, we showed that phosphorylation of PKCδ at amino acid residue tyr311 occurs during oxidative stress in cellular models of PD (Kaul et al., 2005b). This study implicated the possibility that a Src family kinase (SFK) may lie upstream of PKCδ in the signaling cascade and the phosphorylation of tyr311 is a necessary step in the oxidative stress-induced proteolytic activation of PKCδ during dopaminergic neuronal apoptosis. In the present study, we sought to identify the member of SFK that lies upstream of PKCδ in the apoptotic signaling cascade. We show that Fyn kinase lies upstream in the apoptotic signaling cascade and activates PKCδ by tyr311 phosphorylation. Our functional studies show dieldrin-induced Fyn kinase activation contributes to apoptotic cell death in neurotoxic pesticide exposure.
2. Materials and Methods
2.1 Chemicals
Dieldrin was purchased from Sigma (St. Louis, MO). The primary antibodies used in this study - PKCδ (rabbit polyclonal), PKCδ-tyr311-phospho-specific (rabbit polyclonal), Fyn kinase (rabbit polyclonal) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), β-Actin antibody (mouse monoclonal, Sigma, St. Louis, MO) was purchased from Sigma. Secondary antibodies - IRDye 800-conjugated anti-rabbit (Rockland Immunochemicals, Gilbertsville, PA) and Alexa Fluor 680 conjugate anti-mouse (LICOR, Lincoln, NE) were used. Caspase substrate (Ac-DEVD-AFC) was obtained from Bachem Biosciences (King of Prussia, PA). {γ-32P}ATP was purchased from Perkin-Elmer Life Science Products (Boston, MA). The Bradford protein assay kit was purchased from Bio-Rad Laboratories (Hercules, CA). RPMI (Roswell Park Memorial Institute) 1640 medium, fetal bovine serum, L-glutamine, penicillin and streptomycin, Sytox green dye were purchased from Invitrogen (Carlsbad, CA). Src assay kit and Src kinase peptide was purchased from Millipore (Billerica, MA). Fyn kinase substrate was purchased from Enzo Life Sciences (Plymouth Meeting, PA). p60-Tyrosine Kinase Specific Inhibitor (TSKI) was synthesized at the Protein Facility, Office of Biotechnology, Iowa State University, Ames, IA.
2.2 Cell Culture
Rat mesencephalic dopaminergic cells (N27 cells) represent a homogenous population of tyrosine hydroxylase-positive neuronal cells. The cell line has been widely used as an in vitro model of PD (Afeseh Ngwa et al., 2009; Clarkson et al., 1999; Sharma et al., 2010; Song et al., 2010). N27 cells were cultured in RPMI 1640-medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 50 units of penicillin and 50 μg/ml of streptomycin. The N27 cells were maintained in a humidified atmosphere with 5% CO2 at 37°C as described previously (Yang et al., 2004; Zhang et al., 2007).
2.3 Treatment Paradigm
Dieldrin (60μM) was added to the cells for the duration of the experiment to evoke neurotoxic responses, and the concentration was deduced from previously published studies (Song et al., 2010; Sun et al., 2005). In the TSKI studies, cells with 70% confluency were pretreated with 5μM TSKI (Kaul et al., 2005a) for 30 min before proceeding with dieldrin treatment. At the end of the treatment, the cells were removed from the flask by using a cell scraper and centrifuged for 200 × g for 5 min, washed with ice cold phosphate buffered saline (PBS) twice, homogenized in radio-immunoprecipitation assay (RIPA) buffer and sonicated as described previously (Afeseh Ngwa et al., 2009). Cell lysates, collected by spinning down the cell fragments at 20,000 × g for 45 min at 4°C, were used to determine PKCδ-tyr311 phosphorylation and PKCδ proteolytic cleavage. Untreated cells were maintained in serum-free medium containing the DMSO (dimethyl sulfoxide, 0.1-0.2%) for the duration of the treatment and used as control samples.
2.4 Fyn kinase assay
At the end of dieldrin treatment, cells were washed with ice cold PBS and resuspended in PKC lysis buffer (Kaul et al., 2005a). 50μg of crude protein was incubated with 150 μM Fyn kinase substrate (Glu-Phe-Gly-Thr-Tyr-Gly-Thr-Leu-Ser-Lys-Lys-Lys) (Enzo Life Sciences, Plymouth Meeting, PA) or 150μM Src kinase substrate (Lys-Val-Glu-Lys-Ile-Gly-Glu-Gly-Thr-Tyr-Gly-Val-Val-Tyr-Lys), 100 μCi of {γ-32P}-ATP (Perkin Elmer), Src-Mn-ATP cocktail and Src reaction buffer (Upstate/Millipore) for 10 min at 30°C with agitation. 20μl of 40% tricholoroacetic acid was added to precipitate the Fyn kinase or Src kinase substrate peptide and 25μl of the mixture were spotted onto a P81 phosphocellulose square. Five min after spotting, the squares were washed 5 times in 0.75% phosphoric acid in PBS with a final wash step in acetone to fix the signals, according to the instructions of the manufacturer. The squares were transferred onto a scintillation vial and the counts per minute (CPM) counts were read in a liquid scintillation system after adding 5 ml of scintillation cocktail to each vial. The radioactivity counts were normalized to protein concentrations of the lysate.
2.5 siRNA transfection
The pre-validated Silencer® small interfering RNA (siRNA) specific to Fyn kinase mRNA (Catalog no. AM16708, siRNA ID no. 200364) or non-specific siRNA (no. 4611) were obtained from Ambion Inc. (Applied Biosystems/Ambion, Austin TX). The sequence for Fyn-siRNA is: sense, 5′-GCAUUACUCAGAGAGACCtt-3′; antisense, 5′-GGCUCUCUCUGAGUAAUGCtg-3′and for non-specific siRNA is sense, 5′AATTCTCACATTCGGAGAACCTGTCTC-3′; antisense, 5′-AAGTTCTCCGAAGTGTGAGAACCTGTCTC-3′. N27 cells were transiently transfected with the Fyn kinase specific and non-specific siRNA duplexes by the AMAXA Nucleofector Kit (AMAXA) Briefly, N27 cells were resuspended with transfection buffer provided with the kit to a final concentration of 4 to 5 × 106 cells/100μl and mixed with the siRNA duplexes. The final concentration of the siRNA was 5 nM. Electroporation was executed with an AMAXA Nucleofector instrument following the manufacturer’s protocol. The transfected neurons were either transferred to 6 well plate or T-175 flasks for 24 to 36 h before further treatments.
2.6 Enzymatic Assay for Caspase-3
After dieldrin exposure, cells were washed in ice-cold PBS (pH 7.4) and resuspended in caspase lysis buffer at 37°C for 20 min. Lysates were centrifuged at 14,000×g. Cell-free supernatants were incubated with Acetyl-DEVD-amino-4-methylcoumarin (50μM) - the fluorometric caspase-3 substrate used for the reaction as described previously (Afeseh Ngwa et al., 2009; Kitazawa et al., 2003). The formation of 7-amino-4-methylcoumarin (AFC), resulting from caspase-3 activity was measured fluorometrically, using a Gemini XS fluorescence microplate reader (Molecular Devices Inc.) at 400 nm excitation and 505 nm emission.
2.7 DNA Fragmentation Assay
DNA fragmentation was measured using Cell Death Detection ELISA Plus Assay Kit (Roche Diagnostics), as described in our recent publications (Kaul et al., 2005b; Song et al., 2010). This assay is a fast, highly sensitive and reliable assay for the detection of DNA fragmentation by the quantification of histone-associated low molecular weight DNA in the cytoplasm of cells. After centrifuging, the supernatants were dispensed into streptavidin-coated 96-well plates containing 80μl of HRP-conjugated antibody cocktail. After 2 h of incubation at ambient temperature, the absorbance of the ELISA reaction was measured at 490 nm and 405 nm using a microplate reader (SpectraMAX 190, Molecular Devices Inc.). The difference in the absorbance at OD 405 and OD 490 nm was used to measure the actual DNA fragmentation level.
2.8 Sytox cell death assay
Cell death was determined after exposing the N27 cells to dieldrin (Roth et al., 1997; Song et al., 2010) using the Sytox green cytotoxicity assay. The Sytox green cytotoxicity assay is based on the principle that Sytox green cannot enter cells with intact membranes (live cells), but permeates cells with compromised plasma membranes and intercalates with the DNA to produce green fluorescence (Roth et al., 1997). Briefly, N27 cells were knocked down for Fyn kinase using Fyn specific siRNA or non-specific siRNA transient transfection. Post-transfection, the cells were seeded at equal densities in 6 well plates. Briefly, cells were incubated with 60μM dieldrin under serum-free conditions and proceeded with the Sytox green assay, as described previously (Roth et al., 1997; Song et al., 2010). In the Sytox assay, dead cells can be viewed under a fluorescence microscope and the cell death could be quantified on a fluorescent microplate reader (SpectraMax Gemini XS, Molecular Devices Inc.) at an excitation at 485 nm and emission at 538 nm.
2.9 Western blot analysis
Western blotting for PKCδ-tyr311 phosphorylation, PKCδ proteolytic cleavage and Fyn kinase was performed as described previously (Afeseh Ngwa et al., 2009). Briefly, cell lysates containing equal amounts of protein were loaded in each lane and separated on a 10-12% SDS-PAGE gel. After separation, proteins were transferred to nitrocellulose membrane and non-specific binding sites were blocked by incubation for 1 h in Licor blocking buffer. The membranes were then treated with the appropriate antibodies, PKCδ polyclonal antibody (1:2000), Fyn polyclonal antibody (1:1000) and polyclonal PKCδ-tyr311 antibody (1:1000) followed by treatment with secondary anti-mouse or anti-rabbit antibodies, as appropriate. To confirm equal protein loading in each lane, the membranes were reprobed with monoclonal β-actin antibody (1:10000). Western blot was performed using IR-conjugated anti-rabbit dye, Alexa Flour 680 conjugated anti-mouse IgG secondary antibodies. Western blot images were captured and analyzed with an Odyssey IR Imaging System (LICOR) as per the manufacturer’s instructions after correcting integrated band intensity values for background as described previously (Afeseh Ngwa et al., 2009; Song et al., 2010).
2.10 Data analysis
Data analysis was performed using Prism 4.0 Software (GraphPad Prism, San Diego, CA). Data was first analyzed using Student t-test or one-way ANOVA, and then Tukey’s post-hoc test to compare all treatment groups. Differences with p<0.05 were considered significant.
3. Results
3.1 Fyn kinase but not Src kinase is activated in dieldrin-treated dopaminergic cells
We had previously shown that exposure of dopaminergic neuronal cells to dieldrin induces oxidative stress, mitochondrial dysfunction, proteasomal dysfunction, caspase-3 mediated proteolytic cleavage of PKCδ to mediate apoptotic cell death of dopaminergic neurons in a dose- and time-dependent manner (Kitazawa et al., 2001; Kitazawa et al., 2003; Song et al., 2010; Sun et al., 2005). Dieldrin-induced proteolytic cleavage of PKCδ can be blocked by co-treatment with caspase inhibitors (Kitazawa et al., 2003, Kanthasamy et al., 2008). Also our recent study demonstrated that pharmacological inhibition of non-receptor tyrosine kinases significantly prevented parkinsonian toxicant MPP+ induced PKCδ-tyr311 phosphorylation, proteolytic cleavage of PKCδ and attenuated apoptotic cell death of N27 dopaminergic neuronal cells (Song et al., 2010; Sun et al., 2005; Sun et al., 2007, Kaul, 2005b). Western blot analysis revealed that Src and Fyn are both expressed in N27 cells (Fig 1A). To determine which of the non-receptor tyrosine kinases, Src or Fyn are activated, we exposed N27 cells to 60 μM dieldrin for up to 60 min. Cell lysates were subject to in vitro kinase assays using Peptide-Lys-Val-Glu-Lys-Ile-Gly-Glu-Gly-Thr-Tyr-Gly-Val-Val-Tyr-Lys (Src kinase substrate) or Peptide-Glu-Phe-Gly-Thr-Tyr-Gly-Thr-Leu-Ser-Lys-Lys-Lys (Fyn kinase substrate) and {32P}-ATP as described in the methods section. Fyn and Src have almost similar affinities for their respective substrates, Src kinase peptide (Km 101.6μM) (Cheng et al., 1992) and Fyn kinase peptide (Km 70μM) (Cheng et al., 1992). As shown in Fig 1B, dieldrin-induced a two to three-fold increase in Fyn kinase activity in 15 minutes compared to vehicle-treated cell lysates. On the other hand, dieldrin failed to induce increases in Src-kinase activity even up to 60 min (Fig. 1C). Further, dieldrin did not inhibit basal Src kinase activity over a 60 min period in N27 cells suggesting that dieldrin does not inhibit Src kinase. These results suggest that Fyn kinase is preferentially activated in N27 dopaminergic cells in response to dieldrin exposure.
Fig. 1. Dieldrin exposure induces Fyn kinase activity and not Src kinase in dopaminergic neuronal cell model.
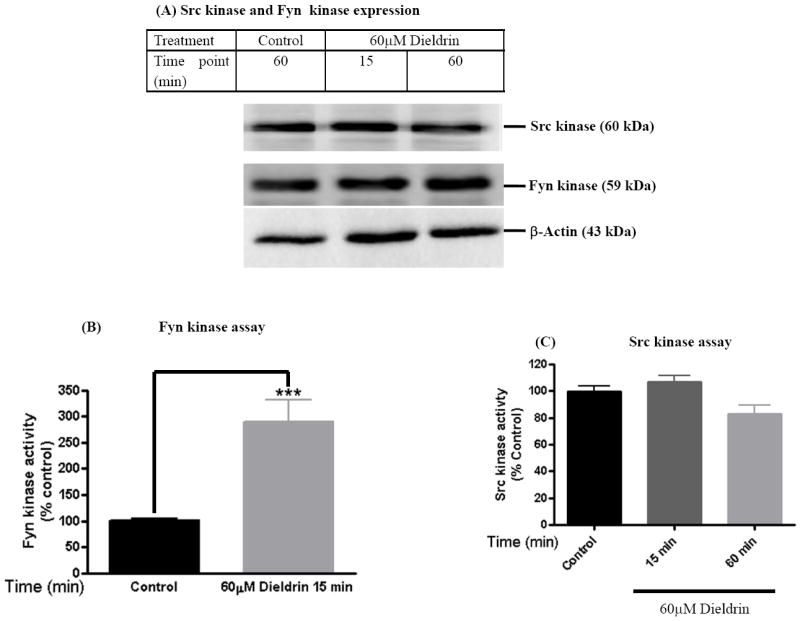
N27 dopaminergic cells treated with dieldrin (60μM) for a period of 15 min were lysed and ~50μg of protein was used for Western blot (A), Fyn kinase assay (B) and Src kinase assay (C) and as described in materials and methods. Dieldrin induced a statistically significant increase in 32P phosphorylation of the Fyn kinase substrate peptide (B) and not Src kinase substrate peptide (C) in dopaminergic neuronal cells. All the data represent mean ± S.E.M. for each of three independent experiments performed in triplicate or quadruplicate. ***p<0.001.
3.2 Dieldrin induces PKCδ-tyr311 phosphorylation
Several tyrosine kinases have been shown to phosphorylate tyr311 of PKCδ in response to several apoptotic stimuli with varied biological effects (Konishi et al., 2001; Murugappan et al., 2009; Song et al., 1998; Zhu et al., 2008). Since dieldrin induced increases in Fyn kinase activity, we performed Western blot to determine if dieldrin also induces tyrosine 311 phosphorylation of PKCδ. We used a phospho-specific antibody directed against tyr311 PKCδ protein that recognizes both full-length (72-74kDa) and cleaved (38kDa) phosphorylated PKCδ and an another antibody directed against native PKCδ that can recognize both phosphorylated and non-phosphorylated native (72-74) and cleaved PKCδ proteins (38-41kDa). As shown in Fig. 2A, Western blot analysis revealed that 60 μM dieldrin treatment induces PKCδ-tyr311 phosphorylation as early as 15 min using a phospho-specific antibody directed against phospho-tyr311 PKCδ (72-74 kDa). Tyr311 phosphorylation of PKCδ was quantified by densitometry of the phopho-tyr311 PKCδ to native PKCδ and was expressed as percentage of control. Densitometric analysis reveals that dieldrin induced a two- to three-fold increase in full-length PKCδ-tyr311 phosphorylation at 15 min (Fig. 2B). The time course study revealed that most of the native tyr311 phosphorylated PKCδ protein was cleaved within 60 min of incubation with dieldrin, as evidenced by a reduction in the intensity of the tyr311 phosphorylated full-length PKCδ and a concomitant increase in the tyr311 phosphorylated regulatory fragment (Fig. 2A). There was no increase in either native or cleaved tyr311 phosphorylated PKCδ levels in vehicle-treated cells.
Fig. 2. Dieldrin induces phosphorylation of PKCδ-tyr311 in dopaminergic neuronal cell model.
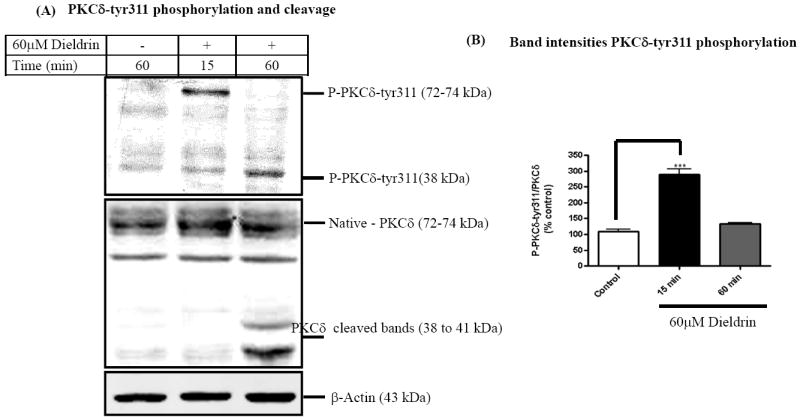
N27 dopaminergic cells treated with dieldrin (60μM) were lysed and ~50μg of protein was used to determine tyrosine phosphorylation of PKCδ by Western blot (A) Dieldrin-treated cells depicted increases in PKCδ-tyr311 phosphorylation as compared to the untreated cells and proteolytic cleavage of PKCδ native protein at 60 min. (B) Densitometric analysis of the immunoblots are expressed as relative band intensities of phospho-PKCδ-tyr311 to native-PKCδ (mean ± S.E.M. of three independent experiments). **p < 0.01.
Proteolytic cleavage of PKCδ observed in Fig. 2A was confirmed in experiments using antibody raised against native PKCδ. As shown in Fig. 2A, treatment of N27 cells with 60 μM dieldrin over a 60 min period resulted in native PKCδ (72-74 kDa) protein to be proteolytically cleaved to yield 38 kDa regulatory and 41 kDa catalytically active fragments. The time course study revealed significant levels of native PKCδ protein was cleaved within 1 h of incubation compared to vehicle-treated cells. Density of the 43 kDa β-actin band was identical in all lanes confirming equal protein loading. Our results are consistent with our previous study where 100-300 μM dieldrin induces an increase in cleaved PKCδ accompanied by a concomitant decrease in native full-length PKCδ over a 5 h period in a dose- and time-dependent manner (Kanthasamy et al., 2008; Kitazawa et al., 2003). These results suggest dieldrin-induced tyr311 phosphorylation of PKCδ is accompanied by its proteolytic cleavage.
3.3 Fyn kinase inhibitor attenuates PKCδ-tyr311 phosphorylation and PKCδ proteolytic activation
In order to determine the functional relevance of Fyn activation in dieldrin neurotoxicity, we examined whether non-receptor tyrosine kinase specific inhibitor (TSKI) alters the neurotoxic response of dieldrin. Our results show that pretreatment with 5μM TSKI effectively blocked dieldrin-induced Fyn kinase activity (Fig. 3A). Dieldrin induced a significant 75% increase in Fyn kinase activity compared to vehicle-treated cells. In the presence of TSKI and dieldrin, Fyn kinase activity was not significantly different from control. We also observed a significant reduction in basal Fyn kinase activity in TSKI-alone-treated cells. We also determined if pharmacological inhibition of Fyn kinase blocks tyr311 phosphorylation and proteolytic cleavage of PKCδ. As shown in Fig. 3B, pretreatment with tyrosine kinase-specific inhibitor (5μM TSKI) for 30 min significantly blocked 60 μM dieldrin-induced proteolytic cleavage of PKCδ. Similarly, pretreatment with TSKI also blocked dieldrin-induced PKCδ–tyr311 phosphorylation (Fig. 3C). Density of the 43 kDa β-actin band was identical in all lanes confirming equal protein loading. These results collectively suggest that Fyn kinase mediates PKCδ-tyr311 phosphorylation, proteolytic cleavage of PKCδ and that Fyn kinase possibly lies upstream of PKCδ in tyr311 phosphorylation-dependent activation during apoptotic signaling.
Fig. 3. Inhibition of Fyn kinase activity attenuates dieldrin-induced apoptosis in dopaminergic neuronal cell model.
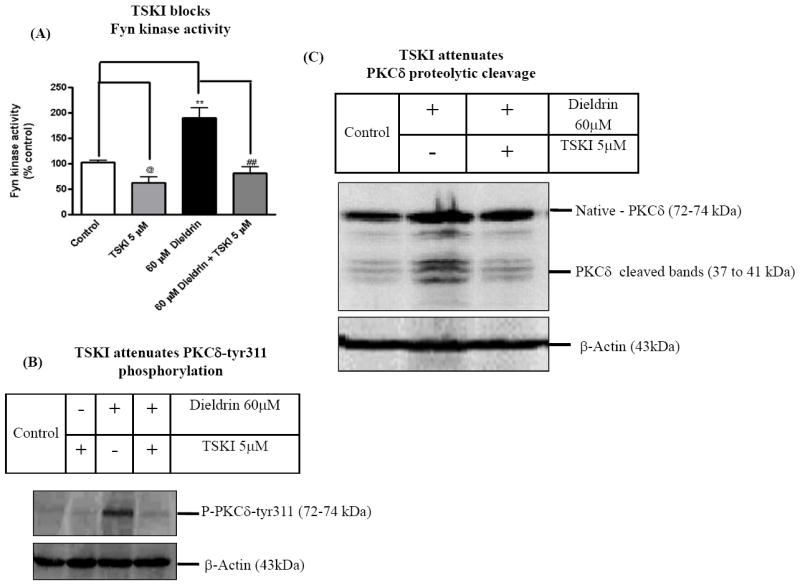
N27 dopaminergic cells treated with dieldrin (60μM) for 15 min and 60 min. At the end of treatments cells were lysed and cell lysates were analyzed for Fyn kinase activity, caspase-3 activity and DNA fragmentation. (A) Inhibition of dieldrin-induced Fyn kinase activation by TSKI. (B) and (C) Dieldrin-induced PKCδ-tyr311 phosphorylation and PKCδ proteolytic activation was assayed following TSKI pretreatment. The results are expressed as mean ± SEM of three independent experiments. @p <0.05, Control and 5 M TSKI, **p < 0.01 Control and 60μM Dieldrin, ## p < 0.01, 60μM Dieldrin and Dieldrin ± 5μM TSKI.
3.4 Pharmacological inhibition of Fyn kinase protects against dieldrin induced apoptotic cell death
We examined whether attenuation of Fyn kinase activation has any effect on dieldrin-induced apoptotic events. Since the pharmacological inhibitor specific to Fyn kinase was not available, we used TSKI which inhibits Src kinase and other tyrosine kinases at IC50 >7.5μM (Sato et al., 1990). To determine, if TSKI also protects against dieldrin-induced apoptotic cell death, we used caspase-3 activity and DNA fragmentation as markers of apoptotic cell death in N27 dopaminergic cells. As shown in Fig. 4A, pretreatment with 5μM TSKI significantly blocked 60 μM dieldrin-induced increases in caspase-3 activity. Quantitative analysis revealed an increase in caspase-3 activity by 200 to 250% in dieldrin treated cells, whereas TSKI treatment almost completed blocked dieldrin-induced caspase-3 activation. To further confirm whether TSKI protects against dieldrin-induced apoptotic cell death, we performed a quantitative DNA fragmentation assay as described in our previous publications (Afeseh Ngwa et al., 2009; Kaul et al., 2005b). Fig. 4B shows that the dieldrin-induced DNA fragmentation in N27 cells was significantly attenuated in cells pretreated with Fyn kinase inhibitor TSKI (5μM). Quantitative analysis revealed an increase in DNA fragmentation by 100 to 125% in dieldrin treated cells, and that TSKI treatment brought back the dieldrin-induced DNA fragmentation to basal level. Together these data indicate the pro-apoptotic role of Fyn kinase in environmental toxicant dieldrin-induced apoptosis of dopaminergic neuronal cells.
Fig. 4. Phosphorylation at tyr311 on PKCδ is essential for dieldrin- induced apoptosis.
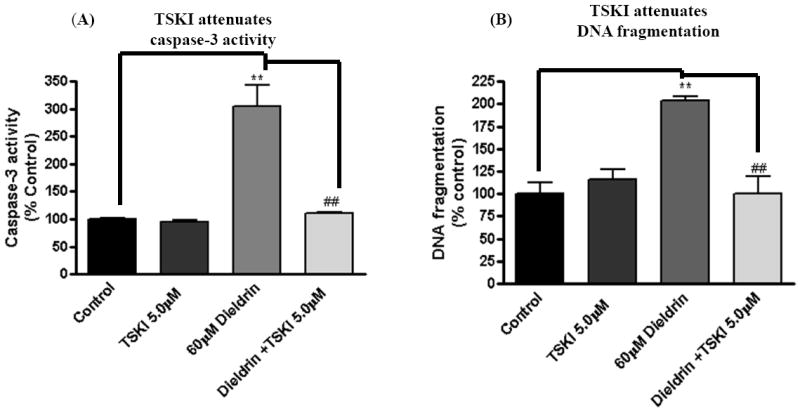
N27 dopaminergic cells treated with dieldrin (60μM) for 15 min and 60 min. At the end of treatments cells were lysed and cell lysates were analyzed for tyr311 phosphorylation and PKCδ proteolytic activation by immunoblotting. (A) TSKI inhibited dieldrin induced caspase-3 activation. (B) TSKI attenuated dieldrin induced DNA fragmentation. The data in (A) and (B) are representative blots from three independent experiments. The results are expressed as mean ± SEM of three independent experiments. **p < 0.01, ## p < 0.01.
3.5. Attenuation of dieldrin-induced PKCδ-tyr311 phosphorylation and proteolytic activation of PKCδ by siRNA-Fyn
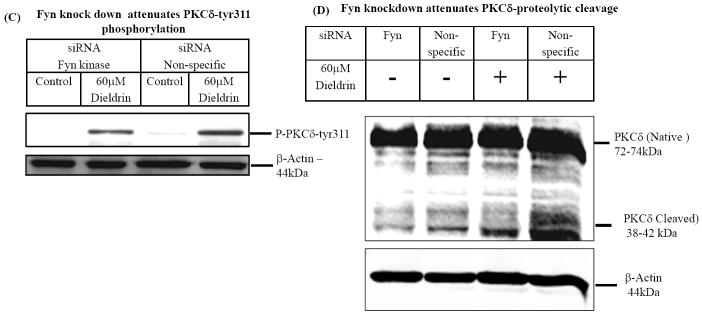
To further confirm the role of Fyn in PKCδ kinase activation during dieldrin neurotoxicity, we used RNAi approaches. N27 cells were transfected siRNA-Fyn or siRNA-Non-Specific, cell lysates were extracted at 24 h post transfection and the levels of Fyn kinase expression were determined by Western blotting. As shown in Fig. 5A, Fyn-siRNA significantly suppressed Fyn expression. Non-specific siRNA (siRNA-NS) had no silencing effect on Fyn expression at 24 h post-transfection (Fig. 5A). Densitometric analysis data to determine the efficiency of Fyn knock-down indicates a significant reduction of Fyn expression in Fyn siRNA treated compared to non-specific RNA treated cells (Fig. 5B). Next we determined if siRNA-Fyn blocks dieldrin-induced PKCδ-tyr311 phosphorylation and proteolytic cleavage of PKCδ in siRNA-Fyn and siRNA-NS transfected N27 cells during dieldrin treatment. As shown in Fig. 5, we detected dieldrin induced tyr311 phosphorylation of PKCδ at 15 min (Fig. 5C) and proteolytic cleavage of PKCδ in 60 min (Fig. 5D) in N27 cells transfected with non-specific siRNA (siRNA-NS). On the contrary, dieldrin failed to induce PKCδ-tyr311 phosphorylation at 15 min (Fig. 5C) and proteolytic cleavage of PKCδ in 60 min (Fig. 5D) in N27 cells transfected with Fyn specific siRNA (siRNA-Fyn). These results demonstrate that siRNA-Fyn effectively suppressed dieldrin-induced PKCδ-tyr311 phosphorylation and proteolytic cleavage.
Fig. 5. Fyn kinase phosphorylates PKCδ on tyr311 to modulate its dieldrin-induced proteolytic activation.
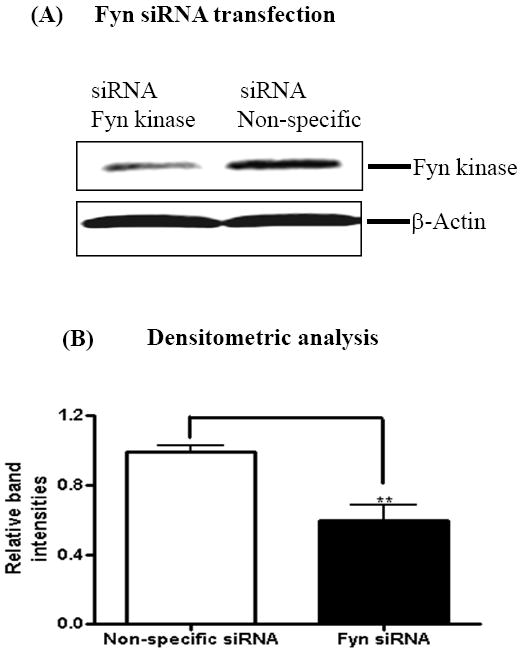
N27 dopaminergic cells were transiently transfected with Fyn kinase specific siRNA and non-specific siRNA and treated with 60μM Dieldrin for a duration of 15 min and 60 min. At the end of treatments, cell lysates were immunoblotted for Fyn kinase, PKCδ and PKCδ-tyr311 phosphorylation. (A) Western blotting for Fyn kinase shows knockdown of Fyn kinase. (B) Densitometric analysis of 59 kDa Fyn kinase band intensities. (C) and (D) Immunoblotting shows that Fyn-specific siRNA inhibited dieldrin-induced PKCδ proteolytic activation and PKCδ-tyr311 phosphorylation.
3.6. RNAi-mediated knockdown of Fyn kinase rescues dopaminergic neuronal cells from dieldrin -induced cytotoxic and apoptotic cell death
To further substantiate the pro-apoptotic function of Fyn in dieldrin-induced apoptotic cell death, we examined the effect of Fyn siRNA on dieldrin-induced increases in caspase-3 enzyme activity, cytotoxic and apoptotic cell death. We performed Sytox green cytotoxicity assays to determine cell viability (Afeseh Ngwa et al., 2009; Kaul et al., 2005b). Microscopic analysis revealed that exposure to 60 μM dieldrin for 60 min induced a significant increase in the number of Sytox green positive cells in non-specific siRNA transfected N27 cells compared with untreated controls (Fig. 6A). On the other hand, microscopic analysis clearly displayed the protective effect of Fyn knockdown, as evident by the reduced number of Sytox-positive green cells in siRNA-Fyn transfected N27 cells (Fig. 6A). The neuroprotective effect of siRNA-Fyn on dieldrin-induced cell death was further confirmed by the quantification of Sytox green fluorescence using a multiplate reader. Cytotoxic cell death was significantly increased by 400% in cells exposed to 60 μM dieldrin for 60 min in siRNA-NS transfected N27 cells as compared to untreated control cells (Fig. 6B). Dieldrin-induced cell death was dramatically attenuated in siRNA-Fyn transfected cells (Fig. 6B).
Fig. 6. Fyn siRNA protects dopaminergic neurons from dieldrin-induced apoptosis.
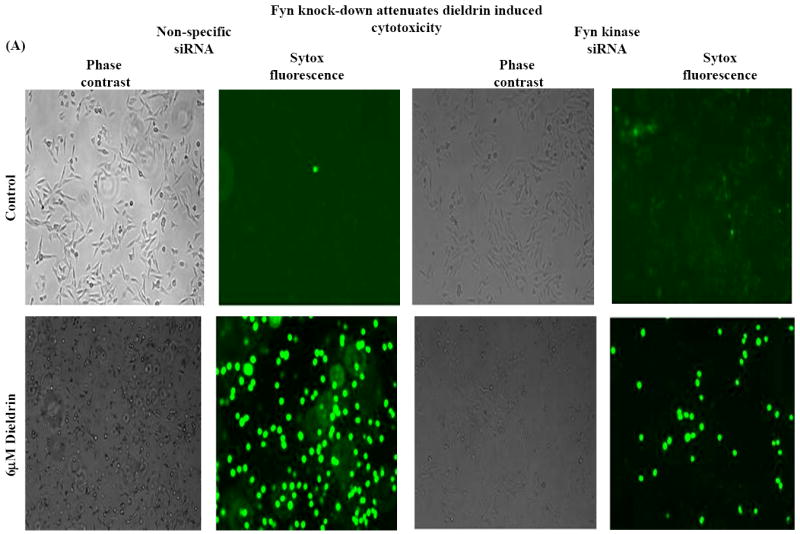
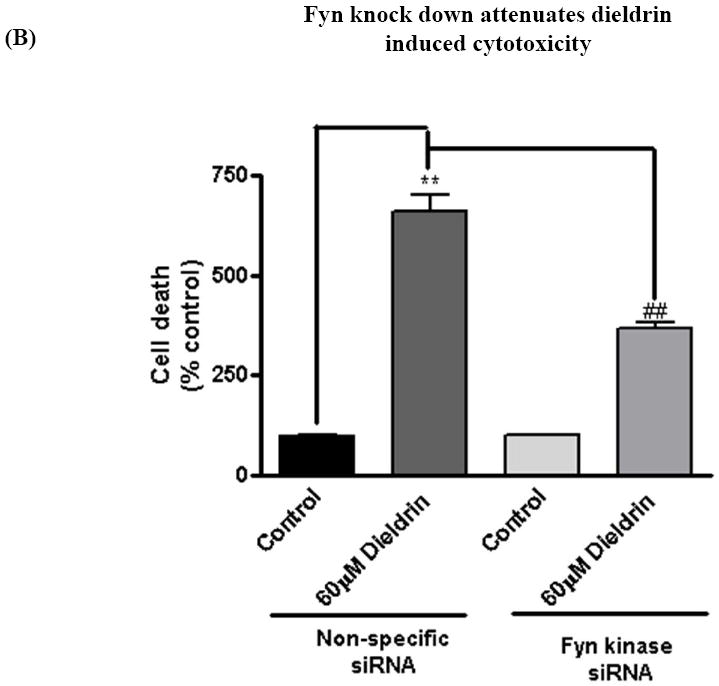
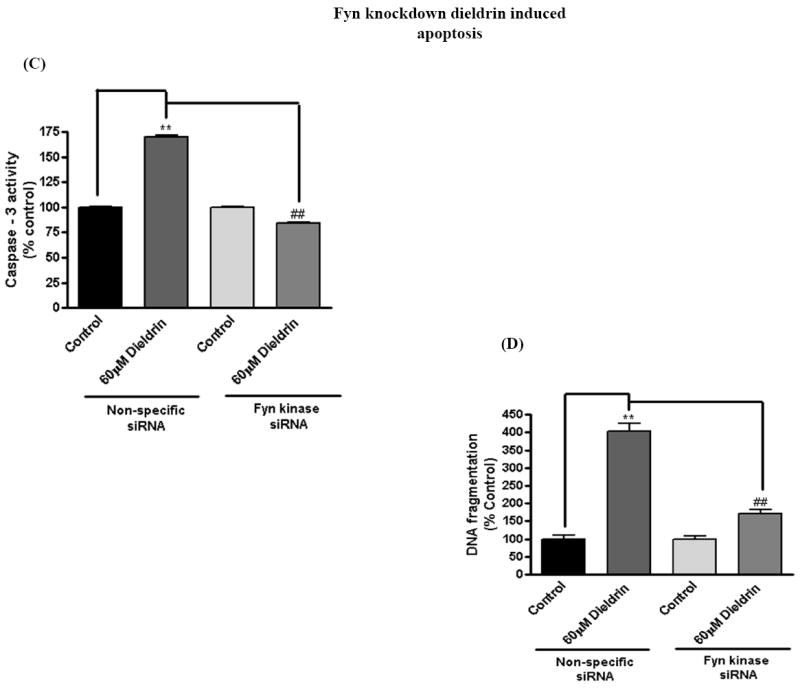
N27 dopaminergic neuronal cells were transiently transfected with Fyn kinase specific siRNA and non-specific siRNA. Cells were treated with 60μM Dieldrin for 60 min and assayed for Sytox green cytotoxicity assay, caspase-3 assay and DNA fragmentation. (A) Phase-contrast and fluorescence microscopic images of Sytox fluorescence staining in the non-specific and Fyn siRNA-transfected dopaminergic neurons. (B) Quantification of the Sytox staining in a fluorescence plate reader. (C) and (D) Caspase-3 activation and DNA fragmentation in siRNA-Fyn and siRNA-non-specific transfected N27 dopaminergic cells. The results are expressed as mean ± SEM of two independent experiments in triplicate or quadruplicate. **p < 0.01,## p < 0.01.
To determine if siRNA-Fyn also blocks dieldrin-induced caspase-3 activity and DNA fragmentation, we performed caspase-3 assays and DNA fragmentation ELISA sandwich assays in siRNA-Fyn and siRNA-NS transfected cells after dieldrin exposure. Caspase-3 activity was significantly increased in cells exposed to dieldrin (60 μM) for 60 min in siRNA-NS transfected N27 cells as compared to untreated control cells (Fig. 6C). Dieldrin-induced caspase-3 activation was almost completely suppressed in siRNA-Fyn transfected cells. To further characterize the functional role of Fyn activation in apoptotic cell death, we examined the effect of siRNA-Fyn on dieldrin-induced DNA fragmentation. DNA fragmentation was increased 2.5-fold in siRNA-NS transfected N27 cells following dieldrin treatment and was almost completely blocked in siRNA-Fyn transfected cells (Fig. 6D). Together, these results demonstrate that siRNA-Fyn effectively suppressed caspase-3 activation and thereby attenuated dieldrin-induced DNA fragmentation, demonstrating a key proapoptotic function of Fyn kinase in dieldrin-induced dopaminergic cell death.
4. Discussion
Previously we have shown that dieldrin induces caspase-3-mediated proteolytic activation of PKCδ by proteolysis in which the native kinase (72-74kDa) is cleaved resulting in regulatory (38kDa) and catalytic (41kDa) fragments (Kanthasamy et al., 2003; Kitazawa et al., 2003). This event persistently activates the kinase to mediate apoptosis of dopaminergic neuronal cells (Kitazawa et al., 2003). In the current study, we show that the environmental neurotoxicant dieldrin induces Fyn kinase activity in dopaminergic neuronal cells to modulate tyr311 phosphorylation-dependent activation of PKCδ and apoptosis. The important findings of the present study are: 1) dieldrin rapidly induces Fyn kinase activation and PKCδ-tyr311 phosphorylation as early as 15 min following dieldrin exposure in dopaminergic neuronal cells, 2) the pharmacological inhibitor TSKI protects against dieldrin-induced phosphorylation of PKCδ-tyr311, caspase-3-mediated PKCδ proteolytic activation and apoptotic cell death of dopaminergic cells, and 3) Both TSKI and Fyn siRNA both protected against dieldrin-induced neurotoxicity. Fig.7 explains the signaling schematic of how Fyn mediates the PKCδ-tyr311 phosphorylation-dependent activation during dopaminergic apoptosis. These findings suggest that Fyn is an important proapoptotic molecule and Fyn activation is an early signaling event in the execution of apoptosis following exposure of dopaminergic cells to the environmental neurotoxicant dieldrin. To our knowledge, this is the first report of Fyn kinase activation during the apoptotic cell death of nigral dopaminergic neurons following exposure to an environmental neurotoxicant.
Fig. 7. Schematic representation of dieldrin-induced apoptotic signaling cascade.
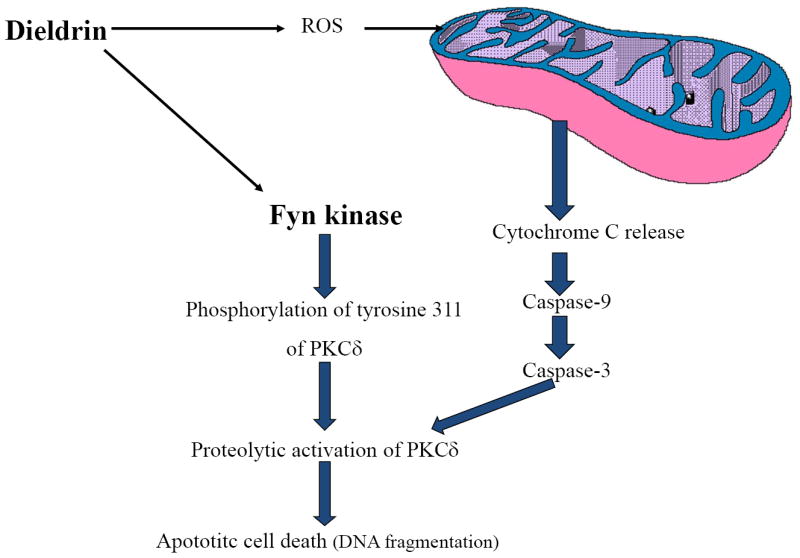
Exposure of dopaminergic cells to environmental neurotoxicant dieldrin induces an early signaling event of activating Fyn kinase. Fyn kinase phosphorylates PKCδ-tyr311, which is at close proximity to the caspase-3 recognition site on PKCδ. Upon phosphorylation of this tyrosine residue, the caspase-3 site becomes available for proteolysis of PKCδ. As shown in our previous publication (Kaul et al., 2005b), mitochondrial-dependent proteolytic activation of PKCδ results in apoptotic cell death of dopaminergic neurons.
Human exposure to organochlorine pesticides is widespread owing to large scale usage of these pesticides starting in the 1940s, their resistance to biodegradation and the resultant bioaccumulation in the food chain. Significant levels of residual organochloride pesticides including dieldrin continue to be detected in the soil, sediment, serum and breast milk of the general population throughout the world (Kanthasamy et al., 2005; Miller and Milne, 2008; Mueller et al., 2008; Mustafa et al.; Sun et al., 2006a; Sun et al., 2006b; Sun et al., 2006c). Epidemiological and case-control studies continue to associate the increased incidence of PD with elevated exposure to dieldrin (Corrigan et al., 1996; Corrigan et al., 1998; Corrigan et al., 2000; Elbaz et al., 2009; Elbaz and Tranchant, 2007; Fleming et al., 1994; Hancock et al., 2008; Kamel et al., 2007; Li et al., 2005). The increased susceptibility of dopaminergic neurons to the toxic effects of dieldrin in comparison to other neuronal cell types is well established (Hatcher et al., 2007; Kitazawa et al., 2004; Kitazawa et al., 2001; Sanchez-Ramos et al., 1998; Sharma et al., 2010).
Non-receptor tyrosine kinases are involved in multiple proliferative and cell death signaling pathways. Previously it has been shown that toxicants such as methylmercury, lead, and herbicides, including paraquat and members of the pyrethroid insecticide group, activate Fyn kinase in the central nervous system (CNS) progenitor cells. Activation of Fyn by this diverse group of chemicals was implicated as a factor in pro-oxidant-mediated disruption of normal CNS development (Li et al., 2007). Fyn has also been shown to be a substrate of caspase-3 in T cells undergoing physiological apoptosis (Ricci et al., 2001; Ricci et al., 1999). Here we show that acute dieldrin treatment induces activation of Fyn kinase but not Src kinase in dopaminergic neuronal cells during the early stages of apoptosis. Dieldrin increased superoxide production via protein kinase C and tyrosine kinases in human neutrophil (Pelletier and Girard, 2002; Pelletier et al., 2001), but the isoforms involved in the dieldrin exposure were not identified. Recently we and others demonstrated that phosphorylation of PKCδ tyr311 by non-receptor tyrosine kinase and caspase-3-dependant cleavage are the key determinants of pro-apoptotic function of PKCδ (Kaul et al., 2005b; Lu et al., 2007). Non-receptor tyrosine kinases that have been known to phosphorylate PKCδ are Src, Fyn, Lyn, Lck, and Syk. Generally, phosphorylation of tyrosine residues on PKCδ is associated with increases in kinase activity, altered sub-cellular localization and change in its substrate preferences (Gschwendt et al., 1994; Haleem-Smith et al., 1995; Li et al., 1994). The tyrosine residues on PKCδ that can be phosphorylated during oxidative stress include tyr-52 and tyr-187 on the N terminus of the protein, tyr-512 and tyr-523 on the C terminus, and tyr-311 at the intermediate hinge region (Li et al., 1996; Li et al., 1994). Konishi et al. have demonstrated that H2O2 treatment induces the phosphorylation of PKCδ at various tyrosine residues including tyr-311, tyr-332, and tyr-512 in COS cells, but phosphorylation at tyr-311 is critical for initiation of PKCδ catalytic activity (Konishi et al., 1997; Konishi et al., 2001). PKCδ tyrosine phosphorylation has also been shown to regulate pro-oxidant etoposide-induced apoptotic cell death in C-6 glial cells (Blass et al., 2002). Ceramide-induced phosphorylation of tyrosines - tyr311 and tyr332 of PKCδ in the golgi complex was associated with increased kinase activity and apoptosis (Kajimoto et al., 2001; Kajimoto et al., 2004). Recently, Lu et al. showed phosphorylation of tyr332 was necessary for the caspase-3-dependant proteolytic cleavage of PKCδ during TRAIL-induced apoptosis in HeLa cells (Lu et al., 2007). However, none of these studies demonstrated that phosphorylation of PKCδ-tyr311 is required for caspase-3-mediated proteolytic activation of PKCδ. We have previously shown that over-expression of PKCδY311F mutant in dopaminergic cells attenuated pro-oxidant-induced proteolytic activation of PKCδ and apoptosis (Kaul et al., 2005b).
In the present study, we demonstrate that pharmacological inhibition of Fyn kinase with TSKI significantly inhibited dieldrin-induced PKCδ-tyr311 phosphorylation, caspase-3-mediated PKCδ proteolytic cleavage and DNA fragmentation. Since TSKI is not just a specific inhibitor of Fyn but widely used as inhibitor of Src family kinases (Kaul et al., 2005b), we further employed siRNA targeted against Fyn to specifically inhibit Fyn function. Here we show that siRNA targeted against Fyn kinase rescues dopaminergic cells from dieldrin-induced PKCδ-tyr311 phosphorylation, caspase-3-mediated PKCδ proteolytic cleavage and DNA fragmentation cells clearly establishes a proapoptotic function for Fyn kinase in dopaminergic cell death. Fyn siRNA suppressed dieldrin induced cytotoxic cell death to lesser extent than caspase-3 activation and DNA fragmentation at 1 h and we attribute this to caspase-3 activation and DNA fragmentation preceding cytotoxic cell death. We believe a complete protection would be observed over a 3 h exposure period. Although TSKI and Fyn siRNA both attenuated dieldrin-induced dopaminergic cell death, results obtained with siRNA-mediated suppression of Fyn kinase activity unequivocally confirm the role of Fyn kinase compared to TSKI, which is not a selective inhibitor of Fyn kinase. On the other hand, one also cannot rule out the role of caspase-independent cell death pathways that may contribute to dieldrin-induced effects on cell death.
In conclusion, we demonstrate that Fyn kinase-mediated tyr311 phosphorylation and caspase-3-dependent proteolytic activation of PKCδ facilitates dopaminergic cell death in cell culture models of PD. Selective targeting of the proapoptotic Fyn kinase by siRNA could rescue dopaminergic neurons. The results obtained from the N27 rat dopaminergic neuronal cell line also further necessitate examination of similar changes in human cell lines. Currently, we are using SH-SY5Y, a human neuroblastoma cell line to study neurotoxic mechanisms. Preliminary studies indicate that neurotoxicants induce proteolytic activation of PKCδ in SH-SY5Y cells. We are currently investigating the role of Fyn in this human cell model. Further evaluation of changes to Fyn activity in lower and environmentally relevant concentrations of dieldrin is also being pursued. The proapoptotic function of Fyn kinase in dopaminergic degeneration may have therapeutic implications in environmentally linked Parkinson’s disease.
Acknowledgments
This work was supported in part by National Institutes of Health Grants NS 45133, ES10586, NS38644, NS65167 and NS074443-01. We thank Mary Ann deVries for assistance in the preparation of this manuscript.
Footnotes
Conflict of interest There are no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afeseh Ngwa H, Kanthasamy A, Anantharam V, Song C, Witte T, Houk R, et al. Vanadium induces dopaminergic neurotoxicity via protein kinase Cdelta dependent oxidative signaling mechanisms: relevance to etiopathogenesis of Parkinson’s disease. Toxicol Appl Pharmacol. 2009;240:273–85. doi: 10.1016/j.taap.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blass M, Kronfeld I, Kazimirsky G, Blumberg PM, Brodie C. Tyrosine phosphorylation of protein kinase Cdelta is essential for its apoptotic effect in response to etoposide. Mol Cell Biol. 2002;22:182–95. doi: 10.1128/MCB.22.1.182-195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HL, Foster WG. Dieldrin promotes resistance to anoikis in breast cancer cells in vitro. Reprod Toxicol. 2008;25:256–62. doi: 10.1016/j.reprotox.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Cheng HC, Nishio H, Hatase O, Ralph S, Wang JH. A synthetic peptide derived from p34cdc2 is a specific and efficient substrate of src-family tyrosine kinases. J Biol Chem. 1992;267:9248–56. [PubMed] [Google Scholar]
- Chopra AK, Sharma MK, Chamoli S. Bioaccumulation of organochlorine pesticides in aquatic system-an overview. Environ Monit Assess. 2010;173:905–916. doi: 10.1007/s10661-010-1433-4. [DOI] [PubMed] [Google Scholar]
- Chun HS, Gibson GE, DeGiorgio LA, Zhang H, Kidd VJ, Son JH. Dopaminergic cell death induced by MPP(+), oxidant and specific neurotoxicants shares the common molecular mechanism. J Neurochem. 2001;76:1010–21. doi: 10.1046/j.1471-4159.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- Clarkson ED, Edwards-Prasad J, Freed CR, Prasad KN. Immortalized dopamine neurons: A model to study neurotoxicity and neuroprotection. Proc Soc Exp Biol Med. 1999;222:157–63. doi: 10.1046/j.1525-1373.1999.d01-126.x. [DOI] [PubMed] [Google Scholar]
- Corrigan FM, French M, Murray L. Organochlorine compounds in human brain. Hum Exp Toxicol. 1996;15:262–4. doi: 10.1177/096032719601500314. [DOI] [PubMed] [Google Scholar]
- Corrigan FM, Murray L, Wyatt CL, Shore RF. Diorthosubstituted polychlorinated biphenyls in caudate nucleus in Parkinson’s disease. Exp Neurol. 1998;150:339–42. doi: 10.1006/exnr.1998.6776. [DOI] [PubMed] [Google Scholar]
- Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D. Organochlorine insecticides in substantia nigra in Parkinson’s disease. J Toxicol Environ Health A. 2000;59:229–34. doi: 10.1080/009841000156907. [DOI] [PubMed] [Google Scholar]
- Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol. 2009;169:919–26. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dente L, Vetriani C, Zucconi A, Pelicci G, Lanfrancone L, Pelicci PG, et al. Modified phage peptide libraries as a tool to study specificity of phosphorylation and recognition of tyrosine containing peptides. J Mol Biol. 1997;269:694–703. doi: 10.1006/jmbi.1997.1073. [DOI] [PubMed] [Google Scholar]
- Doong RA, Lee CY, Sun YC. Dietary intake and residues of organochlorine pesticides in foods from Hsinchu, Taiwan. J AOAC Int. 1999;82:677–82. [PubMed] [Google Scholar]
- Elbaz A, Clavel J, Rathouz PJ, Moisan F, Galanaud JP, Delemotte B, et al. Professional exposure to pesticides and Parkinson disease. Ann Neurol. 2009;66:494–504. doi: 10.1002/ana.21717. [DOI] [PubMed] [Google Scholar]
- Elbaz A, Dufouil C, Alperovitch A. Interaction between genes and environment in neurodegenerative diseases. C R Biol. 2007;330:318–28. doi: 10.1016/j.crvi.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Elbaz A, Tranchant C. Epidemiologic studies of environmental exposures in Parkinson’s disease. J Neurol Sci. 2007;262:37–44. doi: 10.1016/j.jns.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Fleming L, Mann JB, Bean J, Briggle T, Sanchez-Ramos JR. Parkinson’s disease and brain levels of organochlorine pesticides. Ann Neurol. 1994;36:100–3. doi: 10.1002/ana.410360119. [DOI] [PubMed] [Google Scholar]
- Fowler PA, Abramovich DR, Haites NE, Cash P, Groome NP, Al-Qahtani A, et al. Human fetal testis Leydig cell disruption by exposure to the pesticide dieldrin at low concentrations. Hum Reprod. 2007;22:2919–27. doi: 10.1093/humrep/dem256. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Lee VM. A new link between pesticides and Parkinson’s disease. Nat Neurosci. 2000;3:1227–8. doi: 10.1038/81737. [DOI] [PubMed] [Google Scholar]
- Gschwendt M, Kielbassa K, Kittstein W, Marks F. Tyrosine phosphorylation and stimulation of protein kinase C delta from porcine spleen by src in vitro. Dependence on the activated state of protein kinase C delta. FEBS Lett. 1994;347:85–9. doi: 10.1016/0014-5793(94)00514-1. [DOI] [PubMed] [Google Scholar]
- Haleem-Smith H, Chang EY, Szallasi Z, Blumberg PM, Rivera J. Tyrosine phosphorylation of protein kinase C-delta in response to the activation of the high-affinity receptor for immunoglobulin E modifies its substrate recognition. Proc Natl Acad Sci U S A. 1995;92:9112–6. doi: 10.1073/pnas.92.20.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock DB, Martin ER, Mayhew GM, Stajich JM, Jewett R, Stacy MA, et al. Pesticide exposure and risk of Parkinson’s disease: a family-based case-control study. BMC Neurol. 2008;8:6. doi: 10.1186/1471-2377-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher JM, Richardson JR, Guillot TS, McCormack AL, Di Monte DA, Jones DP, et al. Dieldrin exposure induces oxidative damage in the mouse nigrostriatal dopamine system. Exp Neurol. 2007;204:619–30. doi: 10.1016/j.expneurol.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgenson JL. Aldrin and dieldrin: a review of research on their production, environmental deposition and fate, bioaccumulation, toxicology, and epidemiology in the United States. Environ Health Perspect. 2001;109(Suppl 1):113–39. doi: 10.1289/ehp.01109s1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimoto T, Ohmori S, Shirai Y, Sakai N, Saito N. Subtype-specific translocation of the delta subtype of protein kinase C and its activation by tyrosine phosphorylation induced by ceramide in HeLa cells. Mol Cell Biol. 2001;21:1769–83. doi: 10.1128/MCB.21.5.1769-1783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimoto T, Shirai Y, Sakai N, Yamamoto T, Matsuzaki H, Kikkawa U, et al. Ceramide-induced apoptosis by translocation, phosphorylation, and activation of protein kinase Cdelta in the Golgi complex. J Biol Chem. 2004;279:12668–76. doi: 10.1074/jbc.M312350200. [DOI] [PubMed] [Google Scholar]
- Kamel F, Engel LS, Gladen BC, Hoppin JA, Alavanja MC, Sandler DP. Neurologic symptoms in licensed pesticide applicators in the Agricultural Health Study. Hum Exp Toxicol. 2007;26:243–50. doi: 10.1177/0960327107070582. [DOI] [PubMed] [Google Scholar]
- Kannan K, Holcombe RF, Jain SK, Alvarez-Hernandez X, Chervenak R, Wolf RE, et al. Evidence for the induction of apoptosis by endosulfan in a human T-cell leukemic line. Mol Cell Biochem. 2000;205:53–66. doi: 10.1023/a:1007080910396. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V. Role of proteolytic activation of protein kinase Cdelta in oxidative stress-induced apoptosis. Antioxid Redox Signal. 2003;5:609–20. doi: 10.1089/152308603770310275. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V. Dieldrin-induced neurotoxicity: relevance to Parkinson’s disease pathogenesis. Neurotoxicology. 2005;26:701–19. doi: 10.1016/j.neuro.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Yang Y, Anantharam V, Kanthasamy A. Environmental neurotoxin dieldrin induces apoptosis via caspase-3-dependent proteolytic activation of protein kinase C delta (PKCdelta): Implications for neurodegeneration in Parkinson’s disease. Mol Brain. 2008;1:12. doi: 10.1186/1756-6606-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul S, Anantharam V, Kanthasamy A, Kanthasamy AG. Wild-type alpha-synuclein interacts with pro-apoptotic proteins PKCdelta and BAD to protect dopaminergic neuronal cells against MPP+-induced apoptotic cell death. Brain Res Mol Brain Res. 2005a;139:137–52. doi: 10.1016/j.molbrainres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Kaul S, Anantharam V, Yang Y, Choi CJ, Kanthasamy A, Kanthasamy AG. Tyrosine phosphorylation regulates the proteolytic activation of protein kinase Cdelta in dopaminergic neuronal cells. J Biol Chem. 2005b;280:28721–30. doi: 10.1074/jbc.M501092200. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy A, Kanthasamy AG. Dieldrin promotes proteolytic cleavage of poly(ADP-ribose) polymerase and apoptosis in dopaminergic cells: protective effect of mitochondrial anti-apoptotic protein Bcl-2. Neurotoxicology. 2004;25:589–98. doi: 10.1016/j.neuro.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells. Free Radic Biol Med. 2001;31:1473–85. doi: 10.1016/s0891-5849(01)00726-2. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin induces apoptosis by promoting caspase-3-dependent proteolytic cleavage of protein kinase Cdelta in dopaminergic cells: relevance to oxidative stress and dopaminergic degeneration. Neuroscience. 2003;119:945–64. doi: 10.1016/s0306-4522(03)00226-4. [DOI] [PubMed] [Google Scholar]
- Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, et al. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci U S A. 1997;94:11233–7. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi H, Yamauchi E, Taniguchi H, Yamamoto T, Matsuzaki H, Takemura Y, et al. Phosphorylation sites of protein kinase C delta in H2O2-treated cells and its activation by tyrosine kinase in vitro. Proc Natl Acad Sci U S A. 2001;98:6587–92. doi: 10.1073/pnas.111158798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AA, Mink PJ, McIntosh LJ, Teta MJ, Finley B. Evaluation of epidemiologic and animal data associating pesticides with Parkinson’s disease. J Occup Environ Med. 2005;47:1059–87. doi: 10.1097/01.jom.0000174294.58575.3e. [DOI] [PubMed] [Google Scholar]
- Li W, Chen XH, Kelley CA, Alimandi M, Zhang J, Chen Q, et al. Identification of tyrosine 187 as a protein kinase C-delta phosphorylation site. J Biol Chem. 1996;271:26404–9. doi: 10.1074/jbc.271.42.26404. [DOI] [PubMed] [Google Scholar]
- Li W, Mischak H, Yu JC, Wang LM, Mushinski JF, Heidaran MA, et al. Tyrosine phosphorylation of protein kinase C-delta in response to its activation. J Biol Chem. 1994;269:2349–52. [PubMed] [Google Scholar]
- Li Z, Dong T, Proschel C, Noble M. Chemically diverse toxicants converge on Fyn and c-Cbl to disrupt precursor cell function. PLoS Biol. 2007;5:e35. doi: 10.1371/journal.pbio.0050035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Lee HK, Xiang C, Finniss S, Brodie C. The phosphorylation of tyrosine 332 is necessary for the caspase 3-dependent cleavage of PKCdelta and the regulation of cell apoptosis. Cell Signal. 2007;19:2165–73. doi: 10.1016/j.cellsig.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Miller KD, Milne P. Determination of pesticide residues (> 0.5 microg/L) in soft drinks and sports drinks by gas chromatography with mass spectrometry: collaborative study. J AOAC Int. 2008;91:202–36. [PubMed] [Google Scholar]
- Mueller JF, Harden F, Toms LM, Symons R, Furst P. Persistent organochlorine pesticides in human milk samples from Australia. Chemosphere. 2008;70:712–20. doi: 10.1016/j.chemosphere.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Murugappan S, Chari R, Palli VM, Jin J, Kunapuli SP. Differential regulation of threonine and tyrosine phosphorylations on protein kinase Cdelta by G-protein-mediated pathways in platelets. Biochem J. 2009;417:113–20. doi: 10.1042/BJ20080235. [DOI] [PubMed] [Google Scholar]
- Mustafa M, Pathak R, Tripathi AK, Ahmed RS, Guleria K, Banerjee BD. Maternal and cord blood levels of Aldrin and Dieldrin in Delhi population. Environ Monit Assess. doi: 10.1007/s10661-010-1307-9. [DOI] [PubMed] [Google Scholar]
- Paolini M, Sapone A, Gonzalez FJ. Parkinson’s disease, pesticides and individual vulnerability. Trends Pharmacol Sci. 2004;25:124–9. doi: 10.1016/j.tips.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Pelletier M, Girard D. Dieldrin induces human neutrophil superoxide production via protein kinases C and tyrosine kinases. Hum Exp Toxicol. 2002;21:415–20. doi: 10.1191/0960327102ht272oa. [DOI] [PubMed] [Google Scholar]
- Pelletier M, Roberge CJ, Gauthier M, Vandal K, Tessier PA, Girard D. Activation of human neutrophils in vitro and dieldrin-induced neutrophilic inflammation in vivo. J Leukoc Biol. 2001;70:367–73. [PubMed] [Google Scholar]
- Phillips PJ, Nowell LH, Gilliom RJ, Nakagaki N, Murray KR, VanAlstyne C. Composition, distribution, and potential toxicity of organochlorine mixtures in bed sediments of streams. Sci Total Environ. 408:594–606. doi: 10.1016/j.scitotenv.2009.09.052. [DOI] [PubMed] [Google Scholar]
- Priyadarshi A, Khuder SA, Schaub EA, Shrivastava S. A meta-analysis of Parkinson’s disease and exposure to pesticides. Neurotoxicology. 2000;21:435–40. [PubMed] [Google Scholar]
- Przedborski S, Ischiropoulos H. Reactive oxygen and nitrogen species: weapons of neuronal destruction in models of Parkinson’s disease. Antioxid Redox Signal. 2005;7:685–93. doi: 10.1089/ars.2005.7.685. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Vila M. The last decade in Parkinson’s disease research. Basic sciences. Adv Neurol. 2001;86:177–86. [PubMed] [Google Scholar]
- Ricci JE, Lang V, Luciano F, Belhacene N, Giordanengo V, Michel F, et al. An absolute requirement for Fyn in T cell receptor-induced caspase activation and apoptosis. FASEB J. 2001;15:1777–9. doi: 10.1096/fj.00-0665fje. [DOI] [PubMed] [Google Scholar]
- Ricci JE, Maulon L, Luciano F, Guerin S, Livolsi A, Mari B, et al. Cleavage and relocation of the tyrosine kinase P59FYN during Fas-mediated apoptosis in T lymphocytes. Oncogene. 1999;18:3963–9. doi: 10.1038/sj.onc.1202782. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Wang M, Dean ED, Pennell KD, Miller GW. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson’s disease. Faseb J. 2006;20:1695–7. doi: 10.1096/fj.06-5864fje. [DOI] [PubMed] [Google Scholar]
- Ritz B, Yu F. Parkinson’s disease mortality and pesticide exposure in California 1984-1994. Int J Epidemiol. 2000;29:323–9. doi: 10.1093/ije/29.2.323. [DOI] [PubMed] [Google Scholar]
- Roodveldt C, Christodoulou J, Dobson CM. Immunological features of alpha-synuclein in Parkinson’s disease. J Cell Mol Med. 2008;12:1820–9. doi: 10.1111/j.1582-4934.2008.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Poot M, Yue ST, Millard PJ. Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. Appl Environ Microbiol. 1997;63:2421–31. doi: 10.1128/aem.63.6.2421-2431.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ramos J, Facca A, Basit A, Song S. Toxicity of dieldrin for dopaminergic neurons in mesencephalic cultures. Exp Neurol. 1998;150:263–71. doi: 10.1006/exnr.1997.6770. [DOI] [PubMed] [Google Scholar]
- Sato K, Miki S, Tachibana H, Hayashi F, Akiyama T, Fukami Y. A synthetic peptide corresponding to residues 137 to 157 of p60v-src inhibits tyrosine-specific protein kinases. Biochem Biophys Res Commun. 1990;171:1152–9. doi: 10.1016/0006-291x(90)90805-w. [DOI] [PubMed] [Google Scholar]
- Schafer KS, Kegley SE. Persistent toxic chemicals in the US food supply. J Epidemiol Community Health. 2002;56:813–7. doi: 10.1136/jech.56.11.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH. Etiology and pathogenesis of Parkinson disease. Neurol Clin. 2009;27:583–603. v. doi: 10.1016/j.ncl.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Sharma H, Zhang P, Barber DS, Liu B. Organochlorine pesticides dieldrin and lindane induce cooperative toxicity in dopaminergic neurons: role of oxidative stress. Neurotoxicology. 2010;31:215–22. doi: 10.1016/j.neuro.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Song C, Kanthasamy A, Anantharam V, Sun F, Kanthasamy AG. Environmental neurotoxic pesticide increases histone acetylation to promote apoptosis in dopaminergic neuronal cells: relevance to epigenetic mechanisms of neurodegeneration. Mol Pharmacol. 2010;77:621–32. doi: 10.1124/mol.109.062174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JS, Swann PG, Szallasi Z, Blank U, Blumberg PM, Rivera J. Tyrosine phosphorylation-dependent and -independent associations of protein kinase C-delta with Src family kinases in the RBL-2H3 mast cell line: regulation of Src family kinase activity by protein kinase C-delta. Oncogene. 1998;16:3357–68. doi: 10.1038/sj.onc.1201886. [DOI] [PubMed] [Google Scholar]
- Sun F, Anantharam V, Latchoumycandane C, Kanthasamy A, Kanthasamy AG. Dieldrin induces ubiquitin-proteasome dysfunction in alpha-synuclein overexpressing dopaminergic neuronal cells and enhances susceptibility to apoptotic cell death. J Pharmacol Exp Ther. 2005;315:69–79. doi: 10.1124/jpet.105.084632. [DOI] [PubMed] [Google Scholar]
- Sun F, Kanthasamy A, Anantharam V, Kanthasamy AG. Environmental neurotoxic chemicals-induced ubiquitin proteasome system dysfunction in the pathogenesis and progression of Parkinson’s disease. Pharmacol Ther. 2007;114:327–44. doi: 10.1016/j.pharmthera.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Sun F, Wong SS, Li GC, Chen SN. A preliminary assessment of consumer’s exposure to pesticide residues in fisheries products. Chemosphere. 2006a;62:674–80. doi: 10.1016/j.chemosphere.2005.04.112. [DOI] [PubMed] [Google Scholar]
- Sun P, Backus S, Blanchard P, Hites RA. Temporal and spatial trends of organochlorine pesticides in Great Lakes precipitation. Environ Sci Technol. 2006b;40:2135–41. doi: 10.1021/es051861g. [DOI] [PubMed] [Google Scholar]
- Sun P, Blatnchard P, Brice K, Hites RA. Atmospheric organochlorine pesticide concentrations near the Great Lakes: temporal and spatial trends. Environ Sci Technol. 2006c;40:6587–93. doi: 10.1021/es060858+. [DOI] [PubMed] [Google Scholar]
- Tarraf C, El-Sabban M, Bassam R, Beyrouthy M, Chamoun J, Talhouk R. Functional consequence of exposure to dieldrin on mammary development and function. Food Addit Contam. 2003;20:819–28. doi: 10.1080/0265203031000138231. [DOI] [PubMed] [Google Scholar]
- Tuchsen F, Jensen AA. Agricultural work and the risk of Parkinson’s disease in Denmark, 1981-1993. Scand J Work Environ Health. 2000;26:359–62. doi: 10.5271/sjweh.554. [DOI] [PubMed] [Google Scholar]
- Uversky VN, Li J, Fink AL. Pesticides directly accelerate the rate of alpha-synuclein fibril formation: a possible factor in Parkinson’s disease. FEBS Lett. 2001;500:105–8. doi: 10.1016/s0014-5793(01)02597-2. [DOI] [PubMed] [Google Scholar]
- Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, et al. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–22. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- Yang Y, Kaul S, Zhang D, Anantharam V, Kanthasamy AG. Suppression of caspase-3-dependent proteolytic activation of protein kinase C delta by small interfering RNA prevents MPP+-induced dopaminergic degeneration. Mol Cell Neurosci. 2004;25:406–21. doi: 10.1016/j.mcn.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Zhang D, Kanthasamy A, Yang Y, Anantharam V, Kanthasamy A. Protein kinase C delta negatively regulates tyrosine hydroxylase activity and dopamine synthesis by enhancing protein phosphatase-2A activity in dopaminergic neurons. J Neurosci. 2007;27:5349–62. doi: 10.1523/JNEUROSCI.4107-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Brodie C, Balasubramanian S, Eckert RL. Multiple PKCdelta tyrosine residues are required for PKCdelta-dependent activation of involucrin expression--a key role of PKCdelta-Y311. J Invest Dermatol. 2008;128:833–45. doi: 10.1038/sj.jid.5701103. [DOI] [PMC free article] [PubMed] [Google Scholar]