Abstract
Stem cell research plays an important role in orthopedic regenerative medicine today. Current literature provides us with promising results from animal research in the fields of bone, tendon, and cartilage repair. While early clinical results are already published for bone and cartilage repair, the data about tendon repair is limited to animal studies. The success of these techniques remains inconsistent in all three mentioned areas. This may be due to different application techniques varying from simple mesenchymal stem cell injection up to complex tissue engineering. However, the ideal carrier for the stem cells still remains controversial. This paper aims to provide a better understanding of current basic research and clinical data concerning stem cell research in bone, tendon, and cartilage repair. Furthermore, a focus is set on different stem cell application techniques in tendon reconstruction, cartilage repair, and filling of bone defects.
1. Introduction
Today great hope is set on regenerative medicine in all medical fields. Leland Kaiser introduced the term “Regenerative medicine” in 1992. He forecasted that “a new branch of medicine will develop that attempts to change the course of chronic diseases and in many instances will regenerate tired and failing organ systems” [1]. Since then, scientists all over the world try to develop cell-based approaches to regenerate damaged tissues, or even substitute whole organs [2].
Of course, regenerative medicine has developed to be of interest in orthopedics. There, great hope was set on regenerative medicine to develop alternative therapies for cartilage damage, arthritis, large bone defects, or atrophic tendon ruptures during the last decade. These are all indications, which are treatable only insufficiently with conventional implants and surgical procedures [3–10]. Therefore, they frequently result in decreased function of the musculoskeletal system or even loss of patients' mobility. In the worst case, the mentioned diseases even result in a loss of autonomy for the patient. In consequence, this implies immense costs for the health care systems all over the world.
In this review, we focus on application of stem cells in regenerative medicine for orthopedic indications. We present current approaches in stem cell-based therapy in orthopedics and review recent successes in basic science and clinical application of regenerative medicine approaches within the field.
2. Stem Cells
Stem cells are of particular interest in regenerative medicine. They inhere several unique characteristics that distinguish them from other cell types. Stem cells represent unspecialized cells, which have the ability to differentiate into different adult cell types. Here, it is important to distinguish embryonic stem cells, which are truly pluripotent from multipotent adult stem cells. Embryonic stem cells (ESCs) are only found in early developmental stages of the organism. They represent the only cell type, which has the ability to renew itself indefinitely and is truly pluripotent. As a unique precursor cell, it can differentiate into cells of all three germ layers [2]. In contrast, a variety of multipotent adult stem cells exists in assumedly all tissues of the organism. They are responsible for maintaining the integrity of the tissue they reside in. Usually, these adult stem cells show limited differentiation potential to tissues of one germ layer [2].
The use of human ESCs as a resource for cell therapeutic approaches is currently an intensively researched field [11–13]. From a legal and ethical point of view, research involving human embryonic cells is highly controversial and many countries are reviewing their legislation. Besides the ethical concerns, the use of embryonic stem cells is problematic, as the application of allogenic pluripotent cells inheres a distinct oncogenic potential that currently forbids the application in patients.
The work of Takahashi and Yamanaka in 2006 has opened new perspectives in regenerative medicine. His group was the first to demonstrate successful dedifferentiation of somatic cells into a pluripotent ESC-like status by transfection with four embryonic transcription factors [14]. The so-called induced pluripotent stem cells (iPS cells) provide the possibility of autologous therapy with pluripotent and easily accessible cells in the future. Beside the great potential this technique undoubtedly represents, it bears some essential safety problems which are currently far from being solved. As ESCs, these cells inhere a high oncogenic potential which currently forbids application in patients. If they are injected in an undifferentiated state, they cause teratomas, and mice generated from iPS cells show high rates of tumors. This oncogenicity may be due to the transcription factors used for dedifferentiation which are known to be oncogenes, due to the insufficient epigenetic remodeling or due to the oncogenic retroviruses used for transfection [15].
The use of adult stem cells raises less ethical concerns and has proved to be much safer than pluripotent stem cells. In addition, these cells have further advantages compared to ESCs, for example, a use for autologous cell therapies, using patients' own cells to reduce possible immune responses, is easier to realize. Nonetheless, the limited differentiation potential of adult stem cells narrows their applicability. Typically, adult stem cells can differentiate into the cell types of the tissue in which they reside. Mesenchymal stem cells have been found to be the most promising candidates, as they show good differentiation potential towards cartilage, tendon and bone cells. They can be isolated from a number of mesenchymal tissues as for example bone marrow, fat, synovial membrane, periosteum, and others [16]. Interestingly, these mesenchymal stem cells have been found to differ regarding their differentiation potential dependent on their tissue source [17].
As ethical and safety concerns currently forbid application of iPS cells and ESCs in patients [2, 18], we will focus on adult mesenchymal stem cells within the rest of the paper.
3. Application of Mesenchymal Stem Cells in Regenerative Medicine
Regenerative medicine mainly includes two different strategies of cell-based therapy. In the first approach, cells are applied to substitute damaged cells within a tissue to reconstitute its integrity and function. During this procedure called “cell therapy” a cell suspension is simply injected into the damaged tissue or into the blood circulation. The second approach called “tissue engineering” is more complex. Here, cells are combined with a three dimensional matrix to compose a tissue-like construct to substitute lost parts of the tissue, or even whole organs (Figure 1) [2].
Figure 1.
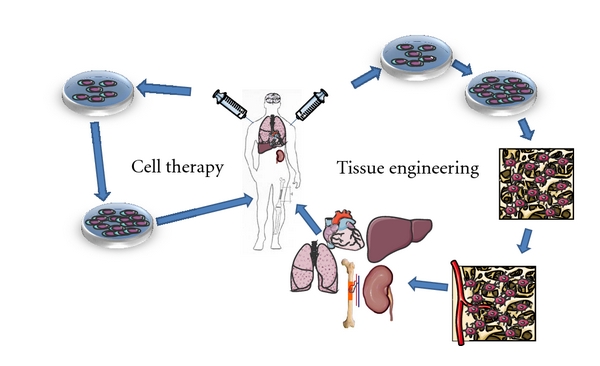
The two strategies of stem cell application in regenerative medicine. Stem cells are either isolated from the patient (autologous transplantation) or from other donors (allogenous transplantation). The cells are expanded in vitro and either applied directly to the patient to substitute lost cells (“cell therapy”), or seeded into 3 dimensional scaffolds (“Tissue engineering”) and differentiated into the demanded cell type. The composed artificial tissue construct is subsequently implanted into patients' tissue defect.
One of the most successful examples in “cell therapy” is the transplantation of hematopoietic stem cells. This procedure has now been practiced for decades to treat serious hematological diseases. For transplantation of bone marrow, hematopoietic stem cells are injected into the blood circulation of the recipient. Interestingly, they find their way to the bone marrow by a phenomenon termed “homing.” Chemokines were found to play a key role in homing of hematopoietic stem cells [19–21].
Several experiments have proven the ability of homing to injured tissue for several types of stem cells. In animal models of hepatic intoxication, partial hepatectomy, myocardial infarction, nephropathy, cerebral ischemia, lung injury, lung fibrosis, and local irradiation, stem cells enriched in injured tissue and partially differentiated into tissue-specific cell types after systemic injection [22–38]. Cell therapy with systemically injected mesenchymal stem cells was also performed in humans, showing beneficial effects in graft-versus-host disease or osteogenesis imperfecta [39, 40].
However, cell therapy alone is not sufficient to regenerate large tissue defects or even replace whole organs. Therefore, the approach of “tissue engineering” is the more promising strategy. In the process, tissue-specific cells are seeded on a scaffold imitating the architecture of the tissue-specific extracellular matrix. In the last decade, basic science has made great advantages in tissue engineering research, resulting in in vitro composition of multiple different functional tissue constructs [41]. Nonetheless, tissue engineering therapy has barely reached the patient [42]. The reason for the modest entering of tissue engineering methods into the clinic is the yet unsolved problem of vascularization [43]. Thus, an intact vascular network is a prerequisite to realize tissue constructs of more than 400 μm in diameter [44]. In the last decade many scientists in the field of tissue engineering have focused on solving the problem of vascularization. However, all efforts proving applicability for tissue engineering of large solid tissues or even whole organs in humans have failed so far (for paper see [43]). Nonetheless, tissue engineering was already successfully used in patients to substitute either hollow organs with limited wall diameter (trachea, bladder) or avascular tissues as cartilage [45–47]. In these cases, the diffusion trajectory is sufficient to maintain cell survival.
4. Participation of Mesenchymal Stem Cells in Tissue Regeneration
Mesenchymal stem cells have the ability to migrate chemotactically to tissues showing inflammation and injury in the organism [48]. Besides their unique ability to differentiate into different cell types, mesenchymal stem cells were found to secrete a variety of cytokines, showing anti-inflammatory activity and create an anabolic microenvironment [17]. Furthermore, direct cell-cell contact immunomodulation has also been shown. Thus, they participate in regeneration of injured tissues in different ways. On one hand, they directly differentiate into tissue-specific cells and thus substitute damaged or lost cells. On the other hand, they indirectly influence tissue regeneration by secretion of soluble factors. Thirdly, they are able to modulate the inflammatory response. Thus, they can promote vascularization, cell proliferation, differentiation and modulate an inflammatory process (Figure 2).
Figure 2.
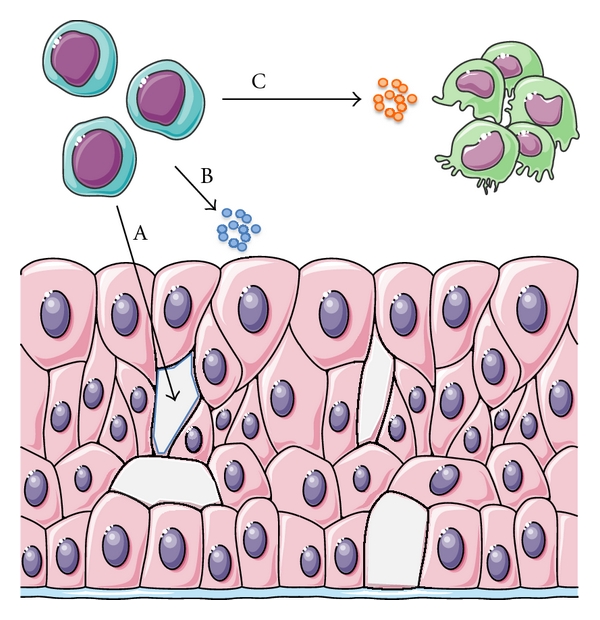
Stem cells participate in tissue regeneration in different ways. They directly differentiate into tissue-specific cells and thus substitute damaged or lost cells (A). They indirectly influence tissue regeneration by secretion of soluble factors. Here they promote vascularization, cell proliferation, differentiation within the tissue (B) and modulate inflammatory processes (C).
Indeed, there is evidence for all mentioned activities of MSCs in tissue regeneration from in vitro and in vivo experiments. The differentiation potential of MSCs was extensively studied in vitro. The cells were found to inhere the potential of multilineage differentiation towards possibly all kinds of mesenchymal cells such as cartilage, bone, tendon, and fat cells, and fibroblasts [69]. Excitingly, further studies revealed that differentiation capacity of MSCs seems not to be restricted to cells belonging to the mesenchymal lineage. They were shown to be able to differentiate towards cells from other germinal layers, as for example, neurons, glia cells, cardiomyocytes, endothelial cells and hepatocytes [70–73]. In vivo experiments and first clinical applications confirmed the ability of MSCs to engraft within a variety of injured tissues and differentiate into tissue-specific cells and thus substitute lost cellular function [17, 74].
In many studies, beneficial effects appeared without any detectable engraftment of the applied mesenchymal stem cells to the damaged tissue, however. Moreover, MSC protein extracts and conditioned medium from MSC cultures showed similar improvement of organ function in liver disorders or heart ischemia [75, 76]. Further investigation of MSCs revealed that they release paracrine factors for example IGF-1, HGF, VEGF, IGF-2, bFGF, or pre-microRNAs which protect's host cells, promote cell proliferation and enhance angiogenesis [77, 78]. These positive effects could partially be confirmed in vivo, where MSCs activated expression of some of the mentioned factors in the myocardium and promoted angiogenesis [79]. Furthermore, MSCs secrete paracrine factors which enhance lung function by regulating endothelial and epithelial permeability, decreasing inflammation, enhancing tissue repair, and inhibiting bacterial growth in acute lung injury and acute respiratory distress syndrome [80]. Beneficial effects of paracrine MSC signaling could also be confirmed in healing of cutaneous wounds [81]. The recently identified potential of paracrine MSC signaling on damaged tissue even caused some authors call MSCs an “injury drug store” [82].
Besides their mentioned differentiation potential and their ability to promote tissue regeneration by secretion of soluble factors, MSCs inhere extraordinary immunological properties. There is increasing evidence that the cells themselves are relatively nonimmunogenic and they can be readily transplanted between different individuals without initiating an immune response [83]. Furthermore, they proved to inhere anti-inflammatory and immunosuppressive capability in vitro and in vivo, where they can modulate immune responses on different targets. They inhibit maturation of immune cells, like helper T, cytotoxic T, dendritic, and B cells. Additionally, cells express a number of cytokines that can suppress inflammation, as for example TGF-beta1, NO, prostaglandin-E2, HLA-G, hepatocyte growth factor, and IL-10 [17]. The revealed anti-inflammatory effects of MSCs have opened a broad field of possible applications in transplantation and immune disorders. After confirming the anti-inflammatory effects in several animal models, first promising clinical applications have succeeded. In these applications, MSCs showed beneficial effects on graft versus host disease after hematopoietic stem cell transplantation and on Crohn's disease [84]. However, first results are promising and the in vivo application seems to be rather safe, as no serious side effects have been reported. Nonetheless, randomized trials have to follow to confirm these first results.
5. Application Techniques in Orthopedics
To differentiate between favorable application strategies the aim of treatment is one important factor. As mentioned before, MSCs have the potential to rebuild injured tissue but also to secrete growth factors for enhancing tissue regeneration. Depending on the underlying pathology, the treatment strategies differ considerably. In one patient a large tissue defect has to be filled by means of tissue engineering, whereas in another the substantial defect is bridged with residual tissue of low quality and only an improvement of healing environment is indicated.
Besides the direct injection in the surrounding tissue, biomaterials are frequently used as carriers for drugs, bioactive molecules and cells. These materials have to fulfill some fundamental requirements. At first they have to be immune-compatible and nontoxic, whereas the degradation process must neither release toxic substances nor tissue-toxic concentrations of degradation products. For a later replacement with regenerated tissue, bio-degradable materials are important. The degradation velocity must be balanced as too fast and too slow are both detrimental. Beside these qualities, matrices formed from biomaterials must have distinct properties with regard to the desired kind of tissue. The prerequisite of mechanical strength, bioactivity, and kinetics of degradation and drug/cell release significantly varies between different repair tissues. Besides the used biomaterials themselves, the 3-dimensional structures of scaffolds have great influence on cell growth and differentiation. Scaffolds must be highly porous with interconnected pores of a diameter of at least 100 μm to allow ingrowth of cells and vessels [85]. Pore sizes between 100 and 400 μm are ideal.
Despite the tissue engineering of bone, for which various inorganic materials, such as hydroxyapatite, calcium phosphate, calcium carbonate, or glasses was tested, mainly organic biomaterials have been investigated for scaffold formation. These are either naturally derived, for example, collagen, fibrin, agarose, alginate, gelatin, silk or hyaluronic acid, or produced synthetically. Synthetically produced organic biomaterials are mainly polyhydroxyacids such as polyglycolides or polylactides. To control kinetics of degradation, recent studies were performed employing hydroxyl acid copolymers. Thus, it has been tried to adapt kinetics of degradation to those of tissue regeneration.
As these synthetic polymers often lack bioactivity, their surface was modified to alter cell adhesion, migration, differentiation, and proliferation in recent studies. Thus, they were coated or copolymerized with bioactive materials or functional groups were attached to the polymer chain before scaffold fabrication [86–88]. Apart from surface modifications with bioactive materials, scaffolds were coated directly with cytokines to control proliferation and differentiation of seeded cells [89]. Other authors describe the coating of scaffolds with genetic vectors to perform transfection of cells with different growth factors [90]. Biomaterials for tissue engineering can also carry drugs that prevent microbial colonization or control ingrowth of scaffolds into the surrounding tissue [91, 92].
5.1. Tendon Repair
Considering physiological properties of tendon tissue, an application technique via scaffolds with native extracellular matrix and the capability of cell seeding and adhesion would be ideal [93]. Based on this hypothesis, most of the current studies used scaffold application techniques. The few studies which favored direct application techniques injected the suspension of MSC into bone tunnels or on the bone surface before tendon refixation to improve tendon-to-bone healing [94, 95].
Scaffold application techniques for tendons can be divided into gel suspensions, 3D scaffolds of solid tissue, and hybrid techniques. Gel suspensions offer a perfect 3D filling of the defect, but the reduced stability in comparison to stable matrices may result in loss of gel at the repair site due to erosion. In a rabbit Achilles tendon model, Chong et al. [96] used a mixture of fibrin sealant and bone marrow-derived mesenchymal stem cells. The fibrin sealant was injected into the tendon and the repair site was additionally covered with the agent. Fibrin incorporates the advantages of a clinical use over years including FDA approval, bone marrow-derived mesenchymal stem cells remain viable in fibrin and published data indicate that fibrin itself has no effect on tendon healing [97]. In this study no differences between fibrin and fibrin with MSC could be shown histologically. In the early healing phase (3 weeks), significantly improved biomechanical properties were documented but not in subsequent time periods (6 and 12 weeks). In a rat rotator cuff model, Gulotta et al. [98] also used MSC in a fibrin sealant and placed it between tendon and bone before refixation of the tendon. In this acute tendon repair model they did not find any significant histological or biomechanical differences after 2 or 4 weeks, respectively. Noteworthy, the same group recently succeeded in enhancing tendon healing in the same rotator cuff model, applying transfected MSCs using the embryonic transcription factor MT1-MMP and the tendon transcription factor scleraxis [99, 100]. With a collagen gel, Awad et al. [101] presented a further gel-based application technique. They fixed a collagen gel with different concentrations of MSC to suture material and filled a defect in the rabbits' patellar tendon. After 12 and 26 weeks, significantly higher maximum stresses and moduli were documented compared to natural repair tissues. However, an adverse event was observed as there had been an increased number of intratendinous ossifications (28%). In comparison to the intact tendon only 25% of the ultimate load was reached with MSC. Regarding all groups, cell concentration had no significant influence on the outcome. This study group improved its application technique and presented a hybrid technique (MSC in a gel-collagen sponge composite) [102]. In the rabbit patellar tendon model, the biomechanical properties and cellular alignment were significantly improved in the MSC group after 12 weeks. A different matrix is presented by Omae et al. in in vitro and in vivo studies [103, 104]. Xenotendon slices with a thickness of 50 μm were decellularized and seeded with bone marrow stromal cells. The first results of the bundled construct in a patellar tendon rat model showed a survival of the stromal cells in all layers. In vivo results with MSC have not been published yet but the approach is promising.
In conclusion, the application of MSC in tendon repair shows promising but inhomogenous results in animal models. Current in vivo data favor the culture of MSC into a tissue-engineered construct, with the advantage of primary stability and allowing the cells to produce their own extracellular matrix. But there is no consensus about the ideal carrier construct. Clinical data are not yet available for MSC application in tendon repair.
5.2. Cartilage
Besides autograft transplantation and autologous chondrocyte transplantation, current therapeutic concepts of cartilage defects include the recruitment of MSC. Drilling, abrasion, or microfracturing of the subchondral bone aims at the recruitment of MSC from the subchondral bone to stimulate the formation of cartilage repair tissue. In experimental and clinical studies of these standard techniques, a nonhyaline cartilage with high proportions of fibrous elements and inferior functionality has been found [105].
For autologous cartilage repair various two- and three-dimensional constructs are available. Most of the matrices consist of natural polysaccharides and proteins, such as alginate and collagen. Furthermore, synthetic polymers are also available for example, polyethylene glycol (PEG) or polylactic acid (PLA). Successful outcome of a stem cell-based cartilage tissue engineering also depends on the design of extracellular matrix for a proper differentiation of MSCs into chondrocytes [106]. The most important property, namely, mechanical stability, to provide appropriate cell-matrix interactions to stimulate tissue growth and capability of functional tissue growth. The ideal matrix has sufficient strength to protect the cells from axial load and shear forces, is highly adhesive to remain stable in the repair site and possesses enough porosity to allow nutrient and differentiation factors to diffuse through it. Currently, a large number of in vitro studies focus on the optimal three-dimensional matrix.
Increasingly innovative matrices are tested in in vivo animal models. For example, Shafiee et al. [107] performed MSC-based cartilage repair in a rabbit model with full-thickness cartilage defects. They used poly(vinyl alcohol)/polycaprolactone (PVA/PCL) nanofibers as matrix which showed a support of MSC proliferation and chondrogenic differentiation in vitro. The animals treated with MSC showed an improved healing of the defects compared with the untreated control. Tay et al. [108] used alginate-embedded MSC for the repair of focal cartilage defects in a rabbit model. They compared the macroscopic and histological results of MSC versus autologous chondrocyte transplantation 6 months postoperatively. MSCs had a similar effectiveness as chondrocyte transplantation, MSC even showed a significantly better macroscopic score. Both treatments resulted in superior tissue regeneration compared with untreated control defects. These promising results from the laboratory resulted in the first clinical studies about cartilage repair with support of MSC. The earliest data are case series of Wakitani et al. [109, 110]. They performed a bone marrow aspiration from the iliac crest and the MSC were expanded in culture. Four weeks later, the MSC were implanted using a collagen gel and the defect was additionally covered with a periosteal flap. The authors describe satisfying clinical and macroscopic results, but the small number of patients, the retrospective study design and the missing control has to be taken into consideration. Nejadnik et al. [111] performed a matched pair analysis of 36 patients in each group who underwent autologous cartilage transplantation or implantation of MSC. The postoperative followup after 24 months showed no significant difference of different functional knee scores between the groups.
In the treatment of osteochondral lesions, the group of Buda et al. [66, 112] published clinical results of lesions in the femur condyle and the talus. In the talus group, MSC were taken from the iliac crest and incubated with a hyaluronic acid membrane (n = 25) or collagen powder (n = 23) before implantation in the defect in a single step procedure. 48 patients were examined clinically and radiologically after an average of 29 months postoperatively. The clinical scores revealed a significant improvement compared to postoperative scores whereas in the MRI and histology of second-look arthroscopies none showed complete hyaline cartilage. In the 20 patients with MSC therapy of the femur condyle satisfactory clinical results (IKDC 90.4 points) were also reported after an average of 29 months postoperatively. The MRI showed a satisfactory integration of the graft in 80% of the patients. Instead of direct defect coverage, some groups describe a simple intra-articular injection of MSC [113], with the intention of the ability of homing of the MSC. Centeno et al. report about an injection in a patient with early osteoarthritis of the knee. In the MRI followup after 6 months, they revealed an increased cartilage volume compared to point of time before injection.
In summary, all applications for clinical use are based on very small case series. The MSC application technique was adopted from the clinical experience of autologous chondrocyte transplantation (fibrin, collagen gel, periosteal flap). Before a clinical use can be recommended, basic research to optimize application techniques, cell preparation, and concentration are essential [114]. With improved knowledge from basic studies further evaluation of the clinical potential of MSC application has to be performed in larger randomized controlled trials.
5.3. Bone
In bone, the main focus of regenerative medicine approaches lies on atrophic non union and replacement of lost bone tissue. Large bone defects are usually caused by trauma, infection, or tumors, as atrophic nonunion are usually caused by insufficient blood supply, interposition of soft tissue or consequence after infection. Current treatment strategies include autologous bone grafts from the iliac crest, which is actually the gold standard—and as salvage procedures—autologous fibula graft transfer and allogenic bone graft transplantation. However, all mentioned techniques show limitations, as bone supply is limited, autologous bone harvesting is accompanied with high rates of morbidity and allogenic transplantation inheres the risk of transmission of diseases or rejection [115, 116].
In the last two decades, regenerative medicine approaches have been extensively studied to improve bone healing, or even generate functional bone tissue to substitute lost bone. Many in vitro studies were performed to investigate applicability of different stem cell types for bone regeneration. Here, promising capacity for differentiating towards bone cells, enhancing bone healing and vascularization could be proven for embryonic stem cells and different adult mesenchymal stem cells. However, due to the ethical and safety concerns mentioned above, only adult stem cells are presently taken into consideration for therapeutic applications [63]. Here, mesenchymal stem cells presently seem to be the most promising candidates for bone regeneration, due to their excellent osteogenic differentiation capacity [69].
In vitro trials found out that MSC strongly promote angiogenesis by paracrine factors after mechanical stimulation, as occurring during fracture healing [117], which makes MSC more interesting for bone regeneration. This paracrine enhancement of angiogenesis in bone regeneration could also be confirmed in animal models in vivo [118].
The capacity of mesenchymal stem cells for homing to injured tissues known from other fields was also demonstrated for fractures. Here, mesenchymal stem cells showed migration towards the fracture site after systemic application in a mouse model. The study further revealed that the cells enriched there and participated in fracture healing by paracrine induction of tissue healing, reduction of systemic and local inflammation and differentiating into bone cells [74]. However, the majority of the stem cells were trapped in the lungs after systemic application, thus making local application more practicable for bone regeneration [119].
Different groups achieved to compose small bone-like tissue constructs in vitro, by composing MSC with a variety of different biomaterials. Implanted into animals, several of these constructs survived in vivo [120]. However, researchers did not succeed in composing vital bone pieces in larger volumes, or even whole bones. This is due to the diffusion tract being larger than 200 μm. Beyond 200 μm, diffusion is not sufficient for providing cells with oxygen and nutrients. Therefore, functional vascularization is a prerequisite for survival of such solid tissues. Up to now, the problem of vascularization in tissue engineering is not yet solved, inhibiting the translation of tissue engineering methods into the clinic [43].
Nonetheless, regenerative medicine for bone healing has reached the patient in form of cell therapy approaches to treat localized bone defects or systemic diseases of the skeleton [39]. Here, autologous bone marrow or autologous mesenchymal stem cells was successfully implanted in a number of patients to enhance fracture/osteotomy healing, fill bone defects, treat pseudarthrosis, bone cysts, osteonecrosis, or enhance spinal fusion. Relevant clinical applications are summarized in Table 1.
Table 1.
Clinical applications of mesenchymal stem cells in bone regeneration.
| Author | Diagnosis | Application | n patients | Results |
|---|---|---|---|---|
| Treatment of nonunions | ||||
|
| ||||
| Connolly et al. 1991 [49] | Atrophic pseudarthrosis | Percutaneous autologous bone marrow injection | 20 | Healing capacity comparable to autologous cancellous bone grafting |
|
| ||||
| Garg et al. 1993 [50] | Nonunion in long bones | Percutaneous autologous bone marrow injection | 20 | 17 out of 20 cases united in 5 months |
|
| ||||
| Kettunen et al. 2002 [51] | Tibially delayed or non-union | Percutaneous autologous bone marrow injection | 41 | Appeared to be as effective as open techniques |
|
| ||||
| Hernigou et al. 2005 [52] | Atrophic pseudarthrosis | Percutaneous autologous bone marrow injection | 60 | Application is effective and safe Positive correlation between number of progenitor cells and callus volume |
|
| ||||
| Goel et al. 2005 [53] | Tibial non-union | Percutaneous autologous bone marrow injection | 20 | 15 out of 20 patients showed bone union |
|
| ||||
| Treatment of osteonecrosis | ||||
|
| ||||
| Hernigou and Beaujean 2002 [54] | Osteonecrosis femoral head | Injection of autologous bone marrow concentrate | 116 (189 hips) | Very good results in early stages Injection of greater number of progenitor cells transplanted had better outcomes |
|
| ||||
| Gangji et al. 2004 [55] | Osteonecrosis femoral head | Injection of autologous bone marrow concentrate | 13 (18 hips) | Significant reduction of pain, progression and improvement of function |
|
| ||||
| Hernigou et al. 2009 [56] | Osteonecrosis femoral head | Injection of autologous bone marrow concentrate | 342 (534 hips) | High amount of progenitor cells as predictor for successful outcome |
|
| ||||
| Enhancing spinal fusions | ||||
|
| ||||
| Neen et al. 2006 [57] | Spinal fusions | Autologous bone marrow aspirate on hydroxyapatite-collagen I-composite | 50 | Similar healing capacity as autologous cancellous bone grafting in posterolateral fusion Inferior results in interbody fusions |
|
| ||||
| Gan et al. 2008 [58] | Spinal fusions | Bone marrow concentrate on tricalciumphosphate | 41 | After 34.5 months 95.1% cases showed good spinal fusion |
|
| ||||
| Filling bone cysts | ||||
|
| ||||
| Wright et al. 2008 [59] | Simple bone cysts | Intralesional injection of autologous bone marrow aspirate | 77 | Inferior results compared to injection of methylprednisolone |
|
| ||||
| Park et al. 2008 [60] | Simple bone cysts | Implantation of autologous bone marrow aspirate implanted in combination with either nonvital allogenic bone graft or injected with bone powder | 20 (23 cysts) | Injection of bone marrow-bone powder mix is effective alternative to open treatment |
|
| ||||
| Zamzam et al. 2009 [61] | Simple bone cysts | Percutaneous autologous bone marrow injection | 28 | Application is a safe and effective treatment |
|
| ||||
| Filling of bone defects | ||||
|
| ||||
| Salama and Weissman 1978 [62] | Different bone defects | Xenograft with bone marrow aspirate | 28 | Results have been “most satisfactory” |
|
| ||||
| Jäger et al. 2009 [63] | volumetric bone deficiencies | local autologous bone marrow/mesenchymal stem cell injection | 10 | May be a promising alternative to autogenous bone grafting |
|
| ||||
| Marcacci et al. 2007 [64] | Large bone diaphysis defect | autologous MSCs were expanded in vitro and seeded on hydroxyapatite scaffolds | 4 | Followup up to 7 years after surgery, good integration of implant, no secondary fractures |
|
| ||||
| Various applications | ||||
|
| ||||
| Hendrich et al. 2009 [65] | various bone healing disturbances | Bone marrow concentrate | 101 | Autogenous bone marrow concentrate application is safe |
|
| ||||
| Giannini et al. 2009 [66] | Osteochondral talus defects | arthroscopic-assisted injection of autologous bone marrow aspirate | 48 | Functional improvement |
|
| ||||
| Dallari et al. 2007 [67] | High tibial osteotomy | Lyophilized bone chips with platelets-enriched plasma with bone marrow aspirate | 33 | Lyophilized bone chips with platelets-enriched plasma with or without bone marrow aspirate enhance healing |
|
| ||||
| Kitoh et al. 2009 [68] | femoral and tibial lengthenings | Application of MSC expanded in vitro with PRP | 28 (51 osteotomies) | No enhancement of bone healing by MSC/PRP |
6. Conclusions
Current data provides a number of interesting approaches to treat musculoskeletal pathologies with the support of mesenchymal stem cells. But considering the limited, partially only preclinical data we believe that a standardized clinical application will take at least an additional 5 to 10 years. In order to realize the full therapeutic potential of stem cells, a number of open questions has to be to be answered. Besides the necessity of establishing further data about native stem cell function and pathways, basic research in the understanding of native tendon, bone, and cartilage regeneration also has to be continued. Especially signal pathways have to be understood because single-MSC application might be insufficient or only partially sufficient without the adequate signal for inducing tissue regeneration. The regenerated tissue also has to provide the appropriate 3-dimensional structure including production of extracellular matrix and biomechanical behavior according to native tissue. Therefore, tissue engineering will play an important role in the next years. In the near future, an interdisciplinary approach with biologists, bioengineers, and clinicians will be essential to achieve the clinical application of mesenchymal stem cells.
Acknowledgment
The authors thank Fritz Seidl, MA, for linguistically correcting the paper.
References
- 1.Kaiser LR. The future of multihospital systems. Topics in Health Care Financing. 1992;18(4):32–45. [PubMed] [Google Scholar]
- 2.Ehnert S, Glanemann M, Schmitt A, et al. The possible use of stem cells in regenerative medicine: dream or reality? Langenbeck's Archives of Surgery. 2009;394(6):985–997. doi: 10.1007/s00423-009-0546-0. [DOI] [PubMed] [Google Scholar]
- 3.Forriol F, Longo UG, Concejo C, Ripalda P, Maffulli N, Denaro V. Platelet-rich plasma, rhOP-1 (rhBMP-7) and frozen rib allograft for the reconstruction of bony mandibular defects in sheep. A pilot experimental study. Injury. 2009;40(supplement 3):S44–49. doi: 10.1016/S0020-1383(09)70011-7. [DOI] [PubMed] [Google Scholar]
- 4.Longo UG, Lamberti A, Khan WS, Maffulli N, Denaro V. Synthetic augmentation for massive rotator cuff tears. Sports Medicine and Arthroscopy Review. 2011;19(4):360–365. doi: 10.1097/JSA.0b013e318224e359. [DOI] [PubMed] [Google Scholar]
- 5.Kokkonen A, Ikävalko M, Tiihonen R, Kautiainen H, Belt EA. High rate of osteolytic lesions in medium-term followup after the AES total ankle replacement. Foot and Ankle International. 2011;32(2):168–175. doi: 10.3113/FAI.2011.0168. [DOI] [PubMed] [Google Scholar]
- 6.Pelissier P, Boireau P, Martin D, Baudet J. Bone reconstruction of the lower extremity: complications and outcomes. Plastic and Reconstructive Surgery. 2003;111(7):2223–2229. doi: 10.1097/01.PRS.0000060116.21049.53. [DOI] [PubMed] [Google Scholar]
- 7.Gladstone JN, Bishop JY, Lo IKY, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. American Journal of Sports Medicine. 2007;35(5):719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 8.Longo UG, Berton A, Alexander S, Maffulli N, Wallace AL, Denaro V. Biological resurfacing for early osteoarthritis of the shoulder. Sports Medicine and Arthroscopy Review. 2011;19(4):380–394. doi: 10.1097/JSA.0b013e318211c473. [DOI] [PubMed] [Google Scholar]
- 9.Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. American Journal of Sports Medicine. 2011;39(2):258–265. doi: 10.1177/0363546510390780. [DOI] [PubMed] [Google Scholar]
- 10.Maffulli N, Longo UG, Denaro V. Novel approaches for the management of tendinopathy. Journal of Bone and Joint Surgery. 2010;92(15):2604–2613. doi: 10.2106/JBJS.I.01744. [DOI] [PubMed] [Google Scholar]
- 11.Mummery C, Ward-van Oostwaard D, Doevendans P, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107(21):2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 12.Nir SG, David R, Zaruba M, Franz WM, Itskovitz-Eldor J. Human embryonic stem cells for cardiovascular repair. Cardiovascular Research. 2003;58(2):313–323. doi: 10.1016/s0008-6363(03)00264-5. [DOI] [PubMed] [Google Scholar]
- 13.Rubart M, Field LJ. Cardiac repair by embryonic stem-derived cells. Handbook of Experimental Pharmacology. 2006;(174):73–100. [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Rodolfa K, di Giorgio FP, Sullivan S. Defined reprogramming: a vehicle for changing the differentiated state. Differentiation. 2007;75(7):577–579. doi: 10.1111/j.1432-0436.2007.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mafi R, et al. Sources of adult mesenchymal stem cells applicable for musculoskeletal applications—a systematic review of the literature. Open Orthopaedics Journal. 2011;5(supplement 2):242–248. doi: 10.2174/1874325001105010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porada CD, Almeida-Porada G. Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Advanced Drug Delivery Reviews. 2010;62(12):1156–1166. doi: 10.1016/j.addr.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robbins RD, Prasain N, Maier BF, Yoder MC, Mirmira RG. Inducible pluripotent stem cells: not quite ready for prime time? Current Opinion in Organ Transplantation. 2010;15(1):61–67. doi: 10.1097/MOT.0b013e3283337196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lévesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by gcsf or cyclophosphamide. Journal of Clinical Investigation. 2003;111(2):187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lévesque JP, Hendy J, Winkler IG, Takamatsu Y, Simmons PJ. Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-KIT receptor (CD117) from the surface of hematopoietic progenitor cells. Experimental Hematology. 2003;31(2):109–117. doi: 10.1016/s0301-472x(02)01028-7. [DOI] [PubMed] [Google Scholar]
- 21.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nature Immunology. 2002;3(7):687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 22.Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108(7):863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 23.Fang B, Zheng C, Liao L, et al. Identification of human chronic myelogenous leukemia progenitor cells with hemangioblastic characteristics. Blood. 2005;105(7):2733–2740. doi: 10.1182/blood-2004-07-2514. [DOI] [PubMed] [Google Scholar]
- 24.François S, Mouiseddine M, Mathieu N, et al. Human mesenchymal stem cells favour healing of the cutaneous radiation syndrome in a xenogenic transplant model. Annals of Hematology. 2007;86(1):1–8. doi: 10.1007/s00277-006-0166-5. [DOI] [PubMed] [Google Scholar]
- 25.Glanemann M, Gaebelein G, Nussler N, et al. Transplantation of monocyte-derived hepatocyte-like cells (NeoHeps) improves survival in a model of acute liver failure. Annals of Surgery. 2009;249(1):149–154. doi: 10.1097/SLA.0b013e31818a1543. [DOI] [PubMed] [Google Scholar]
- 26.Harada K, Higaki S, Hashimoto K, et al. Study on the colonoscopic features of GVHD enteritis that developed after hematopoietic stem cell transplantation. Hepato-Gastroenterology. 2007;54(80):2221–2227. [PubMed] [Google Scholar]
- 27.Hauger O, Frost EE, van Heeswijk R, et al. MR evaluation of the glomerular homing of magnetically labeled mesenchymal stem cells in a rat model of nephropathy. Radiology. 2006;238(1):200–210. doi: 10.1148/radiol.2381041668. [DOI] [PubMed] [Google Scholar]
- 28.Inagaki Y, Higashiyama R, Okazaki I. Treatment strategy for liver fibrosis through recruitment and differentiation of bone marrow stem/progenitor cells. Hepatology Research. 2007;37(12):991–993. doi: 10.1111/j.1872-034X.2007.00267.x. [DOI] [PubMed] [Google Scholar]
- 29.Kawada H, Fujita J, Kinjo K, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104(12):3581–3587. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- 30.Mahmood A, Lu D, Qu C, Goussev A, Chopp M. Treatment of traumatic brain injury with a combination therapy of marrow stromal cells and atorvastatin in rats. Neurosurgery. 2007;60(3):546–553. doi: 10.1227/01.NEU.0000255346.25959.99. [DOI] [PubMed] [Google Scholar]
- 31.Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(14):8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oyagi S, Hirose M, Kojima M, et al. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. Journal of Hepatology. 2006;44(4):742–748. doi: 10.1016/j.jhep.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Rubart M, Field LJ. Cell-based approaches for cardiac repair. Annals of the New York Academy of Sciences. 2006;1080:34–48. doi: 10.1196/annals.1380.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubart M, Field LJ. Cardiac regeneration: repopulating the heart. Annual Review of Physiology. 2006;68:29–49. doi: 10.1146/annurev.physiol.68.040104.124530. [DOI] [PubMed] [Google Scholar]
- 35.Terai S, Yamamoto N, Omori K, Sakaida I, Okita K. A new cell therapy using bone marrow cells to repair damaged liver. Journal of Gastroenterology. 2002;37(supplement 14):162–163. doi: 10.1007/BF03326438. [DOI] [PubMed] [Google Scholar]
- 36.van Laake LW, van Hoof D, Mummery CL. Cardiomyocytes derived from stem cells. Annals of Medicine. 2005;37(7):499–512. doi: 10.1080/07853890500327843. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto N, Terai S, Ohata S, et al. A subpopulation of bone marrow cells depleted by a novel antibody, anti-Liv8, is useful for cell therapy to repair damaged liver. Biochemical and Biophysical Research Communications. 2004;313(4):1110–1118. doi: 10.1016/j.bbrc.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 38.Zhao DC, Lei JX, Chen R, et al. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World Journal of Gastroenterology. 2005;11(22):3431–3440. doi: 10.3748/wjg.v11.i22.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horwitz EM, Gordon PL, Koo WKK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(13):8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lazarus HM, Koc ON, Devine SM, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biology of Blood and Marrow Transplantation. 2005;11(5):389–398. doi: 10.1016/j.bbmt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Mikos AG, Herring SW, Ochareon P, et al. Engineering complex tissues. Tissue Engineering. 2006;12(12):3307–3339. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rustad KC, Sorkin M, Levi B, Longaker MT, Gurtner GC. Strategies for organ level tissue engineering. Organogenesis. 2010;6(3):151–157. doi: 10.4161/org.6.3.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends in Biotechnology. 2008;26(8):434–441. doi: 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 45.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367(9518):1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 46.Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. The Lancet. 2008;372(9655):2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 47.Yanaga H, Imai K, Fujimoto T, Yanaga K. Generating ears from cultured autologous auricular chondrocytes by using two-stage implantation in treatment of microtia. Plastic and Reconstructive Surgery. 2009;124(3):817–825. doi: 10.1097/PRS.0b013e3181b17c0e. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Li Y, Chen X, et al. MCP-1, MIP-1, IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture. Hematology. 2002;7(2):113–117. doi: 10.1080/10245330290028588. [DOI] [PubMed] [Google Scholar]
- 49.Connolly JF, Guse R, Tiedeman J, Dehne R. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clinical Orthopaedics and Related Research. 1991;(266):259–270. [PubMed] [Google Scholar]
- 50.Garg NK, Gaur S, Sharma S. Percutaneous autogenous bone marrow grafting in 20 cases of ununited fracture. Acta Orthopaedica Scandinavica. 1993;64(6):671–672. doi: 10.3109/17453679308994595. [DOI] [PubMed] [Google Scholar]
- 51.Kettunen J, Mäkelä EA, Turunen V, Suomalainen O, Partanen K. Percutaneous bone grafting in the treatment of the delayed union and non-union of tibial fractures. Injury. 2002;33(3):239–245. doi: 10.1016/s0020-1383(01)00075-4. [DOI] [PubMed] [Google Scholar]
- 52.Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions: influence of the number and concentration of progenitor cells. Journal of Bone and Joint Surgery: Series A. 2005;87(7):1430–1437. doi: 10.2106/JBJS.D.02215. [DOI] [PubMed] [Google Scholar]
- 53.Goel A, Sangwan SS, Siwach RC, Ali AM. Percutaneous bone marrow grafting for the treatment of tibial non-union. Injury. 2005;36(1):203–206. doi: 10.1016/j.injury.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clinical Orthopaedics and Related Research. 2002;(405):14–23. doi: 10.1097/00003086-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Gangji V, Hauzeur JP, Matos C, de Maertelaer V, Toungouz M, Lambermont M. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. Journal of Bone and Joint Surgery: Series A. 2004;86(6):1153–1160. doi: 10.2106/00004623-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Hernigou P, Poignard A, Zilber S, Rouard H. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian Journal of Orthopaedics. 2009;43(1):40–45. doi: 10.4103/0019-5413.45322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neen D, Noyes D, Shaw M, Gwilym S, Fairlie N, Birch N. Healos and bone marrow aspirate used for lumbar spine fusion: a case controlled study comparing healos with autograft. Spine. 2006;31(18):E636–E640. doi: 10.1097/01.brs.0000232028.97590.12. [DOI] [PubMed] [Google Scholar]
- 58.Gan Y, Dai K, Zhang P, Tang T, Zhu Z, Lu J. The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials. 2008;29(29):3973–3982. doi: 10.1016/j.biomaterials.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 59.Wright JG, Yandow S, Donaldson S, Marley L. A randomized clinical trial comparing intralesional bone marrow and steroid injections for simple bone cysts. Journal of Bone and Joint Surgery: Series A. 2008;90(4):722–730. doi: 10.2106/JBJS.G.00620. [DOI] [PubMed] [Google Scholar]
- 60.Park IH, Micic ID, Jeon IH. A study of 23 unicameral bone cysts of the calcaneus: open chip allogeneic bone graft versus percutaneous injection of bone powder with autogenous bone marrow. Foot and Ankle International. 2008;29(2):164–170. doi: 10.3113/FAI.2008.0164. [DOI] [PubMed] [Google Scholar]
- 61.Zamzam MM, Abak AA, Bakarman KA, Al-Jassir FF, Khoshhal KI, Zamzami MM. Efficacy of aspiration and autogenous bone marrow injection in the treatment of simple bone cysts. International Orthopaedics. 2009;33(5):1353–1358. doi: 10.1007/s00264-008-0619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salama R, Weissman SL. The clinical use of combined xenografts of bone and autologous red marrow. A preliminary report. Journal of Bone and Joint Surgery: Series B. 1978;60(1):111–115. doi: 10.1302/0301-620X.60B1.342531. [DOI] [PubMed] [Google Scholar]
- 63.Jäger M, Jelinek EM, Wess KM, et al. Bone marrow concentrate: a novel strategy for bone defect treatment. Current Stem Cell Research and Therapy. 2009;4(1):34–43. doi: 10.2174/157488809787169039. [DOI] [PubMed] [Google Scholar]
- 64.Marcacci M, Kon E, Moukhachev V, et al. Stem cells associated with macroporous bioceramics for long bone repair: 6- To 7-year outcome of a pilot clinical study. Tissue Engineering. 2007;13(5):947–955. doi: 10.1089/ten.2006.0271. [DOI] [PubMed] [Google Scholar]
- 65.Hendrich C, et al. Safety of autologous bone marrow aspiration concentrate transplantation: initial experiences in 101 patients. Orthopedic Reviews. 2009;1(1, article e32) doi: 10.4081/or.2009.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giannini S, Buda R, Vannini F, Cavallo M, Grigolo B. One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clinical Orthopaedics and Related Research. 2009;467(12):3307–3320. doi: 10.1007/s11999-009-0885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dallari D, Savarino L, Stagni C, et al. Enhanced tibial osteotomy healing with use of bone grafts supplemented with platelet gel or platelet gel and bone marrow stromal cells. Journal of Bone and Joint Surgery: Series A. 2007;89(11):2413–2420. doi: 10.2106/JBJS.F.01026. [DOI] [PubMed] [Google Scholar]
- 68.Kitoh H, Kawasumi M, Kaneko H, Ishiguro N. Differential effects of culture-expanded bone marrow cells on the regeneration of bone between the femoral and the tibial lengthenings. Journal of Pediatric Orthopaedics. 2009;29(6):643–649. doi: 10.1097/BPO.0b013e3181b2afb2. [DOI] [PubMed] [Google Scholar]
- 69.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 70.Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochemical and Biophysical Research Communications. 2005;332(2):370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 71.Bossolasco P, Cova L, Calzarossa C, et al. Neuro-glial differentiation of human bone marrow stem cells in vitro. Experimental Neurology. 2005;193(2):312–325. doi: 10.1016/j.expneurol.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 72.Fukuda K. Reprogramming of bone marrow mesenchymal stem cells into cardiomyocytes. Comptes Rendus: Biologies. 2002;325(10):1027–1038. doi: 10.1016/s1631-0691(02)01524-x. [DOI] [PubMed] [Google Scholar]
- 73.Schwartz RE, Reyes M, Koodie L, et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. Journal of Clinical Investigation. 2002;109(10):1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Granero-Molto F, Weis JA, Miga MI, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27(8):1887–1898. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dai W, Hale SL, Kloner RA. Role of a paracrine action of mesenchymal stem cells in the improvement of left ventricular function after coronary artery occlusion in rats. Regenerative Medicine. 2007;2(1):63–68. doi: 10.2217/17460751.2.1.63. [DOI] [PubMed] [Google Scholar]
- 76.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nature Medicine. 2005;11(4):367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 77.Yu XY, Geng YJ, Li XH, et al. The effects of mesenchymal stem cells on c-kit up-regulation and cell-cycle re-entry of neonatal cardiomyocytes are mediated by activation of insulin-like growth factor 1 receptor. Molecular and Cellular Biochemistry. 2009;332(1-2):25–32. doi: 10.1007/s11010-009-0170-x. [DOI] [PubMed] [Google Scholar]
- 78.Chen TS, Lai RC, Lee MM, Choo ABH, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Research. 2009;38(1):215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. American Journal of Physiology: Heart and Circulatory Physiology. 2009;296(6):H1888–H1897. doi: 10.1152/ajpheart.00186.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review: mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29(6):913–919. doi: 10.1002/stem.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Experimental Cell Research. 2010;316(14):2213–2219. doi: 10.1016/j.yexcr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9(1):11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Devine SM, Bartholomew AM, Mahmud N, et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Experimental Hematology. 2001;29(2):244–255. doi: 10.1016/s0301-472x(00)00635-4. [DOI] [PubMed] [Google Scholar]
- 84.Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Annals of the New York Academy of Sciences. 2009;1176:101–117. doi: 10.1111/j.1749-6632.2009.04607.x. [DOI] [PubMed] [Google Scholar]
- 85.Ma PX. Biomimetic materials for tissue engineering. Advanced Drug Delivery Reviews. 2008;60(2):184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bosetti M, Santin M, Lloyd AW, Denyer SP, Sabbatini M, Cannas M. Cell behaviour on phospholipids-coated surfaces. Journal of Materials Science: Materials in Medicine. 2007;18(4):611–617. doi: 10.1007/s10856-007-2309-1. [DOI] [PubMed] [Google Scholar]
- 87.Zhang H, Lin CY, Hollister SJ. The interaction between bone marrow stromal cells and RGD-modified three-dimensional porous polycaprolactone scaffolds. Biomaterials. 2009;30(25):4063–4069. doi: 10.1016/j.biomaterials.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu X, Won Y, Ma PX. Surface modification of interconnected porous scaffolds. Journal of Biomedical Materials Research: Part A. 2005;74(1):84–91. doi: 10.1002/jbm.a.30367. [DOI] [PubMed] [Google Scholar]
- 89.Tachibana A, Nishikawa Y, Nishino M, Kaneko S, Tanabe T, Yamauchi K. Modified keratin sponge: binding of bone morphogenetic protein-2 and osteoblast differentiation. Journal of Bioscience and Bioengineering. 2006;102(5):425–429. doi: 10.1263/jbb.102.425. [DOI] [PubMed] [Google Scholar]
- 90.Ueblacker P, Wagner B, Vogt S, et al. In vivo analysis of retroviral gene transfer to chondrocytes within collagen scaffolds for the treatment of osteochondral defects. Biomaterials. 2007;28(30):4480–4487. doi: 10.1016/j.biomaterials.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 91.Lucke M, Wildemann B, Sadoni S, et al. Systemic versus local application of gentamicin in prophylaxis of implant-related osteomyelitis in a rat model. Bone. 2005;36(5):770–778. doi: 10.1016/j.bone.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 92.Schmidmaier G, Wildemann B, Bail H, et al. Local application of growth factors (insulin-like growth factor-1 and transforming growth factor-β1) from a biodegradable poly(D,L-lactide) coating of osteosynthetic implants accelerates fracture healing in rats. Bone. 2001;28(4):341–350. doi: 10.1016/s8756-3282(00)00456-7. [DOI] [PubMed] [Google Scholar]
- 93.Longo UG, Lamberti A, Maffulli N, Denaro V. Tissue engineered biological augmentation for tendon healing: a systematic review. British Medical Bulletin. 2011;98(1):31–59. doi: 10.1093/bmb/ldq030. [DOI] [PubMed] [Google Scholar]
- 94.Ju YJ, Muneta T, Yoshimura H, Koga H, Sekiya I. Synovial mesenchymal stem cells accelerate early remodeling of tendon-bone healing. Cell and Tissue Research. 2008;332(3):469–478. doi: 10.1007/s00441-008-0610-z. [DOI] [PubMed] [Google Scholar]
- 95.Nourissat G, Diop A, Maurel N, et al. Mesenchymal stem cell therapy regenerates the native bone-tendon junction after surgical repair in a degenerative rat model. PLoS ONE. 2010;5(8) doi: 10.1371/journal.pone.0012248. Article ID e12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chong AK, Ang AD, Goh JCH, et al. Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit Achilles tendon model. Journal of Bone and Joint Surgery. 2007;89(1):74–81. doi: 10.2106/JBJS.E.01396. [DOI] [PubMed] [Google Scholar]
- 97.Lusardi DA, Cain JE., Jr. The effect of fibrin sealant on the strength of tendon repair of full thickness tendon lacerations in the rabbit Achilles tendon. Journal of Foot and Ankle Surgery. 1994;33(5):443–447. [PubMed] [Google Scholar]
- 98.Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a Rotator cuff repair model. American Journal of Sports Medicine. 2009;37(11):2126–2133. doi: 10.1177/0363546509339582. [DOI] [PubMed] [Google Scholar]
- 99.Gulotta LV, Kovacevic D, Montgomery S, Ehteshami JR, Packer JD, Rodeo SA. Stem cells genetically modified with the developmental gene MT1-MMP improve regeneration of the supraspinatus tendon-to-bone insertion site. American Journal of Sports Medicine. 2010;38(7):1429–1437. doi: 10.1177/0363546510361235. [DOI] [PubMed] [Google Scholar]
- 100.Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow-derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. American Journal of Sports Medicine. 2011;39(6):1282–1289. doi: 10.1177/0363546510395485. [DOI] [PubMed] [Google Scholar]
- 101.Awad HA, Boivin GP, Dressler MR, Smith FNL, Young RG, Butler DL. Repair of patellar tendon injuries using a cell-collagen composite. Journal of Orthopaedic Research. 2003;21(3):420–431. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- 102.Juncosa-Melvin N, Boivin GP, Gooch C, et al. The effect of autologous mesenchymal stem cells on the biomechanics and histology of gel-collagen sponge constructs used for rabbit patellar tendon repair. Tissue Engineering. 2006;12(2):369–379. doi: 10.1089/ten.2006.12.369. [DOI] [PubMed] [Google Scholar]
- 103.Omae H, et al. Engineered tendon with decellularized xenotendon slices and bone marrow stromal cells: an in vivo animal study. doi: 10.1002/term.423. Journal of Tissue Engineering and Regenerative Medicine. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Omae H, Zhao C, Yu LS, An KN, Amadio PC. Multilayer tendon slices seeded with bone marrow stromal cells: a novel composite for tendon engineering. Journal of Orthopaedic Research. 2009;27(7):937–942. doi: 10.1002/jor.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kalson NS, Gikas PD, Briggs TW. Current strategies for knee cartilage repair. International Journal of Clinical Practice. 2010;64(10):1444–1452. doi: 10.1111/j.1742-1241.2010.02420.x. [DOI] [PubMed] [Google Scholar]
- 106.Seo S, Na K. Mesenchymal stem cell-based tissue engineering for chondrogenesis. Journal of Biomedicine and Biotechnology. 2011;2011:8 pages. doi: 10.1155/2011/806891. Article ID 806891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shafiee A, Soleimani M, Chamheidari GA, et al. Electrospun nanofiber-based regeneration of cartilage enhanced by mesenchymal stem cells. Journal of Biomedical Materials Research: Part A. 2011;99(3):467–478. doi: 10.1002/jbm.a.33206. [DOI] [PubMed] [Google Scholar]
- 108.Tay LX, et al. Treatment outcomes of alginate-embedded allogenic mesenchymal stem cells versus autologous chondrocytes for the repair of focal articular cartilage defects in a rabbit model. The American Journal of Sports Medicine. 2012;40(1):83–90. doi: 10.1177/0363546511420819. [DOI] [PubMed] [Google Scholar]
- 109.Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. Journal of Tissue Engineering and Regenerative Medicine. 2007;1(1):74–79. doi: 10.1002/term.8. [DOI] [PubMed] [Google Scholar]
- 110.Kuroda R, Ishida K, Matsumoto T, et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis and Cartilage. 2007;15(2):226–231. doi: 10.1016/j.joca.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 111.Nejadnik H, Hui JH, Choong EPF, Tai BC, Eng Hin Lee Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. American Journal of Sports Medicine. 2010;38(6):1110–1116. doi: 10.1177/0363546509359067. [DOI] [PubMed] [Google Scholar]
- 112.Buda R, Vannini F, Cavallo M, Grigolo B, Cenacchi A, Giannini S. Osteochondral lesions of the knee: a new one-step repair technique with bone-marrow-derived cells. Journal of Bone and Joint Surgery. 2010;92(supplement 2):2–11. doi: 10.2106/JBJS.J.00813. [DOI] [PubMed] [Google Scholar]
- 113.Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11(3):343–353. [PubMed] [Google Scholar]
- 114.Khan WS, Longo UG. ACI and MACI procedures for cartilage repair utilise mesenchymal stem cells rather than chondrocytes. Medical Hypotheses. 2011;77(2):p. 309. doi: 10.1016/j.mehy.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 115.Cypher TJ, Grossman JP. Biological principles of bone graft healing. Journal of Foot and Ankle Surgery. 1996;35(5):413–417. doi: 10.1016/s1067-2516(96)80061-5. [DOI] [PubMed] [Google Scholar]
- 116.Finkemeier CG. Bone-grafting and bone-graft substitutes. Journal of Bone and Joint Surgery: Series A. 2002;84(3):454–464. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 117.Kasper G, Dankert N, Tuischer J, et al. Mesenchymal stem cells regulate angiogenesis according to their mechanical environment. Stem Cells. 2007;25(4):903–910. doi: 10.1634/stemcells.2006-0432. [DOI] [PubMed] [Google Scholar]
- 118.Schumann P, Tavassol F, Lindhorst D, et al. Consequences of seeded cell type on vascularization of tissue engineering constructs in vivo. Microvascular Research. 2009;78(2):180–190. doi: 10.1016/j.mvr.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 119.Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology. 2008;47(2):126–131. doi: 10.1093/rheumatology/kem206. [DOI] [PubMed] [Google Scholar]
- 120.Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Engineering. 2005;11(7-8):1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]


