Case Presentation
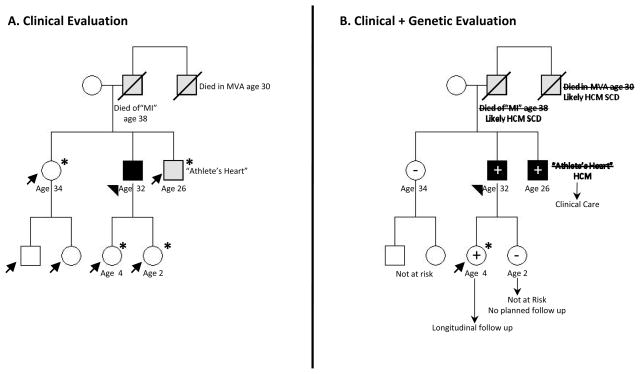
A healthy 32 year old male presents for evaluation of exertional dyspnea and syncope. A murmur was noted and echocardiography revealed marked septal hypertrophy with a resting left ventricular outflow tract (LVOT) gradient of 68 mmHg (Supplemental Video and Supplemental Figure 1). His father died of an “MI” at age 38, and his paternal uncle died as the driver in a single car accident at age 30. His younger brother is thought to have “athlete’s heart” (Figure 1A). After discussing his diagnosis of hypertrophic cardiomyopathy and implications for his family, he asks: “What will happen to my kids? Will I be able to feel well enough to exercise again?”
Figure 1. Family management in HCM.
A. Pedigree based on clinical evaluation: The proband (arrowhead) presented with symptoms, physical exam, and echo diagnostic for HCM. There are 6 other family members at risk for HCM (arrows). The diagnosis of “athlete’s heart” in his brother (a recreational athlete) is ambiguous. Guidelines recommend longitudinal clinical screening for his four 1st degree relatives (children and siblings (*)).1
B. Pedigree based on clinical and genetic evaluation: A pathogenic myosin heavy chain (MYH7) mutation was identified in the proband and predictive genetic testing performed in 1st degree relatives. Longitudinal clinical screening can now be focused on mutation carriers (+). Relatives who have not inherited the family’s pathogenic mutation (−) are not at risk for developing HCM or transmitting risk to their children. Only his daughter, the 1 mutation carrier currently without evidence of clinical HCM (arrow), is at risk and requires prospective serial follow up (*) to monitor for disease development. His brother’s diagnosis is clarified as HCM and appropriate care instituted. Defining the family’s genetic substrate also allows preimplantation genetic diagnosis (PGD) to attempt achieving a pregnancy that does not carry the mutation. PGD may be a particular consideration for families that experience consistently malignant disease.
Circles=females; squares=males; solid symbols=clinical diagnosis of HCM; Grey symbol= unclear clinical phenotype; Slash=deceased; +=mutation present; −=mutation absent
Background
Hypertrophic cardiomyopathy (HCM) is an intrinsic myocardial disorder characterized by unexplained left ventricular hypertrophy (LVH) that occurs in the absence of pressure overload or storage/infiltrative disease.1 Pathognomonic histological features are myocyte disarray and fibrosis (Supplemental Figure 2). The diagnosis is usually established by echocardiography, although cardiac magnetic resonance (CMR) imaging can provide additional information to characterize LV morphology and facilitate diagnosis. Late gadolinium enhancement (LGE) on CMR is thought to represent myocardial scar and is present in the majority of patients with HCM (Supplemental Figure 3). Although not universally adverse,2 LGE may be associated with worse composite outcomes including heart failure, hospitalization, and death.3, 4
The prevalence of unexplained LVH in the general population is ~1 in 500, predicting ~600,000 HCM patients in the United States. However, because cardiac hypertrophy is a relatively non-specific end result of multiple pathways, these estimates include patients with an assortment of different etiologies. This update will focus on primary HCM, most frequently caused by sarcomere mutations.
HCM demonstrates remarkable diversity in disease course, age of onset, pattern and extent of LVH, degree of obstruction, and risk for sudden cardiac death (SCD). Most patients do well, with normal life expectancy and manageable symptoms.5, 6 However, an important subset will experience severe sequelae, including sudden cardiac death, or progressive heart failure leading to death or cardiac transplantation. Indeed, HCM is a leading cause of non-violent sudden death in competitive athletes and young individuals in the United States.7
Genetics
Family studies in the 1980s led to the discovery of disease-causing mutations in genes encoding sarcomere proteins.8 Clinical genetic testing has been available since 2003 (see www.genetests.org for detailed information). Currently, a candidate-gene strategy is used, analyzing sarcomere genes and a limited number of genes associated with metabolic/storage and mitochondrial cardiomyopathies that may mimic HCM, but require different management.9
Sarcomere mutations are found in ~60–70% of adult and pediatric patients with a family history of HCM, and ~30–40% of apparently sporadic cases.8 Over 1000 distinct mutations have been identified, and most are private to a specific family. Mutations in myosin heavy chain (MYH7) and myosin binding protein C (MYBPC3) are most common, collectively accounting for ~80% of sarcomeric HCM. Due to the genetic and clinical heterogeneity seen in HCM, robust genotype-phenotype correlations have not yet emerged. As such, knowledge of the precise mutation usually does not alter management. However, adverse outcomes (cardiovascular death, stroke, progressive symptoms, systolic dysfunction) appear more prominent in HCM patients with sarcomere mutations compared to patients without an identifiable mutation.10 Additionally, patients with >1 mutation (~5% incidence) may have more severe disease, particularly in rare instances of triple mutations or homozygosity.11
The phenotypic expression and penetrance of mutations are variable and age-dependent. Clinical disease, as defined by the presence of LVH, often cannot be diagnosed before adolescence. Investigation is ongoing to characterize earlier phenotypes and improve understanding of the pathogenesis of HCM. For example, diastolic dysfunction,12 impaired myocardial energetics,13 and increased collagen synthesis14 are detectable in sarcomere mutation carriers even when LV wall thickness is normal.
The benefits and limitations of genetic testing in HCM are summarized in the Table. Although genetic testing can provide valuable information, accurate interpretation is complex. Unlike other laboratory testing, genetic testing results are probabilistic rather than binary or quantitative. Because of the profound natural variation in human genetic sequence and the diverse clinical manifestations of HCM, it may not be clear if a DNA variant identified in a patient is truly disease causing (pathogenic), disease modifying, or merely a clinically inconsequential polymorphism. Furthermore, results are dynamic. A variant’s classification as benign or pathogenic may change dramatically as new information emerges from sequencing both “normal” reference populations, as well as patients and families with HCM. The evolving nature of genetic testing results will require establishing novel systems to accommodate ongoing interaction between clinician and testing laboratory.
Table.
Benefits and Limitations of HCM Genetic Testing
BENEFITS
|
LIMITATIONS
|
Practically speaking, the benefit and impact of genetic testing is highest in larger families containing both affected and younger, apparently unaffected individuals. In this context, yield is higher, and relatives are available to both (a) participate in segregation analysis to help clarify the significance of ambiguous variants, and (b) benefit from definitive risk determination provided by genetic testing (Figure 1B).
Clinical Management
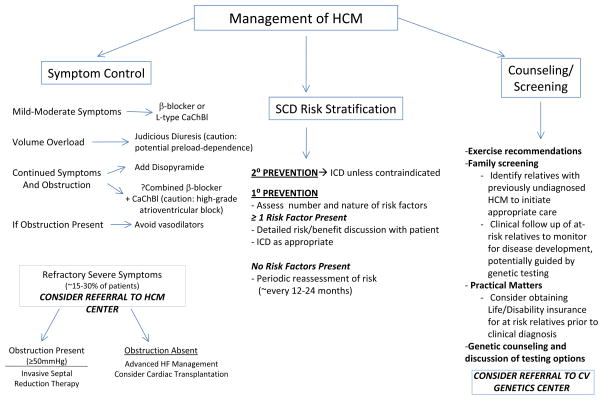
The clinical heterogeneity of HCM calls for individualized treatment. Three key components are considered: 1.) Symptom management; 2.) Risk stratification for SCD, and 3.) Counseling/Screening, including exercise and lifestyle recommendations, family screening, and genetic counseling (Figure 2).
Figure 2. Key components in the clinical management of HCM.
CaChBl= Calcium Channel Blocker
Symptom Management
Exertional dyspnea and chest pain are the most common symptoms of HCM, presumably related to diastolic dysfunction, obstructive physiology, and ischemia from supply-demand mismatch or microvascular disease.15 Medical therapy is first line, using β-blockers or L-type calcium channel blockers (verapamil, diltiazem) to prolong diastolic filling and blunt dynamic intracavitary gradients. A small subset of patients may progress to “burned-out” or end stage HCM, marked by left ventricular systolic dysfunction (EF<50%), and occasionally LV wall thinning and chamber enlargement. End stage HCM is associated with worse outcomes.16 Management guidelines for heart failure apply. Atrial fibrillation (AF) is common in HCM, particularly as age and left atrial size increase. The risks of thromboembolism and recurrent AF are high. Anticoagulation is advised, even if only rare paroxysmal AF is documented and other traditional risk factors are not present.1, 17–19
Approximately two-thirds of HCM patients have obstructive physiology at rest or with physiologic provocation such as exercise, tachycardia, or volume depletion.20 Vasodilators (ACE-inhibitors, angiotensin receptor blockers, phosphodiesterase-5 inhibitors) may increase gradients and should be avoided in obstructive patients. Although gradients may be well-tolerated for long periods, the presence of obstruction (>30 mmHg) has been associated with progressive symptoms and HCM-related death.21 If obstructive physiology is prominent and limiting symptoms persist, long-acting disopyramide (150–300 mg bid) can be added.22 There are no data indicating that treating asymptomatic obstruction improves survival or natural history.
An important minority of patients may have medically refractory symptoms due to severe obstruction (≥50 mmHg at rest or with physiologic provocation) from systolic anterior motion of the mitral valve (SAM) and are candidates for invasive septal reduction therapy. 1 With surgical myectomy or alcohol septal ablation to induce a targeted myocardial infarction, septal thickness is reduced, altering hydrodynamic forces to decrease SAM and associated LV outflow tract obstruction and mitral regurgitation. In experienced hands, both procedures improve hemodynamics, symptoms, and exercise tolerance. Neither has been shown to prolong life or decrease sudden death risk. Myectomy has been considered first line due to greater experience (introduced ~mid 1960s), direct control over muscle resection, and the ability to address intrinsic mitral valve pathology if necessary. In experienced centers, long-term survival is excellent, and results are highly reliable and durable.23
Alcohol septal ablation was introduced in the mid 1990s24 and has primary advantages of shorter and easier recovery. Head-to-head comparisons of myectomy and septal ablation have not been performed. Observational studies and meta-analysis suggest similar morbidity and mortality (~1% mortality), although the need for permanent pacemaker and the likelihood of incomplete gradient reduction may be higher following ablation.25 No clear excess hazard has been associated with ablation, but concerns for potential long term problems, including proarrhythmia related to the infarct scar, have not been fully resolved, in part due to the relatively short follow up available for ablation and the stochastic nature of events. The choice of procedure must be individualized, based on age, comorbidities, cardiac morphology, suitability of coronary anatomy, intrinsic mitral valve disease, patient preference, and available expertise since operator experience is a key determinant of success.
Risk Stratification for Sudden Cardiac Death
Patients with HCM are at increased risk for sudden death. The estimated annualized rate of SCD is ~1% in the overall HCM population, but substantially higher in those at greatest risk. 1 SCD can occur at any age, with peak incidence spanning adolescence to young adulthood.26, 27 Pharmacologic therapy, including β-blockers and amiodarone, is not adequately protective. Implantable cardiovertor defibrillators (ICD) are effective26, 28 but have associated morbidity, particularly over time when implanted in young individuals. Patients are more likely to experience complications or inappropriate shocks than to receive an appropriate therapy.28, 29 The long term prognosis of patients who receive an appropriate ICD therapy is not necessarily adverse,30 suggesting distinct pathways mediating arrhythmias and other aspects of disease progression.
Myocyte disarray, fibrosis, and ischemia have been postulated as potential triggers, but the true mechanisms governing sudden death are unknown. Observational studies have identified five risk factors for SCD, including: family history of sudden death, unexplained syncope, non-sustained ventricular tachycardia on ambulatory monitoring, abnormal hypotensive blood pressure response to exercise (patients <50 years old), and severe LVH (>30 mm). However, these criteria are far from perfect. The positive predictive value of each individual risk factor is only ~20%. The absence of risk factors has been associated with a high negative predictive value and low risk, but risk factors are not identified in ~3% of SCD victims.31 Although the presence of multiple risk factors is associated with higher SCD rates,31 an ICD registry study did not identify an association between appropriate ICD therapies (which may overestimate true SCD events) and number of risk factors.28 Late gadolinium enhancement on CMR has been suggested as a potential tie-breaker in ICD decision making. Since the majority of HCM patients have LGE and statistical power has been limited by low SCD event rates, the incremental information provided by LGE is not yet clear. There is no role for routine invasive electrophysiological study.
Because highly reliable, patient-specific predictors of increased SCD risk are lacking, selection of appropriate candidates for primary prevention ICD therapy can be challenging. More precise means to stratify risk are needed. In the meantime, decision making is individualized, based on age, the number and nature of risk factors, clinical judgment, and active input from fully informed patients. ICDs are recommended for all patients with prior arrest/sustained VT (Class I recommendation), strongly considered for patients with ≥ 2 risk factors, and reasonable for those with 1 risk factor perceived to be at increased risk (e.g., a high proportion of affected family members suffering SCD; concerning syncope suggestive of malignant arrhythmia; Class II a recommendation). If an ICD is not implanted, periodic re-evaluation of risk (~every 12–24 months) is appropriate.1
Counseling
HCM has lifelong implications for patients and families and often impacts young, otherwise healthy individuals. Therefore lifestyle considerations (particularly regarding physical activity), family screening, and genetic counseling are important facets of clinical management.
Exercise and Lifestyle
Because HCM has been associated with sudden death in US competitive athletes,7 patients are advised to avoid intense competitive sports (systematic training involving extreme exertion to achieve athletic excellence). However, it is important to encourage patients to safely enjoy the numerous benefits of exercise. Moderate recreational exercise at a conversational pace is typically well-tolerated in asymptomatic and well compensated patients.1, 32 Patients should be instructed to maintain adequate hydration, avoid situations that may trigger excessive vasodilation, and avoid burst exertion particularly if they have obstructive physiology. Aggressive management of risk factors for atherosclerotic cardiac disease is also appropriate as concomitant coronary artery disease and HCM is associated with worse survival.33
Family Screening
The goal of family screening is to identify relatives with unrecognized HCM and to follow those at-risk for disease in order to minimize complications and assess SCD risk as appropriate. HCM follows autosomal dominant inheritance, therefore each 1st degree relative of an affected patient has a 50% chance of carrying the mutation and potentially developing HCM. Because both diagnosis and SCD risk are linked to the presence of LVH, and because the penetrance of LVH is age-dependent, serial clinical evaluation is appropriate. Screening is most frequent (annually) during adolescence-early adulthood (age 12–21 years) when phenotypic emergence of LVH is most common. Early childhood screening is appropriate if there is a family history of early onset disease or other concerns. During adulthood, screening is recommended every ~5 years, or in response to clinical change, as LVH can develop late in life.1, 18
If a pathogenic sarcomere mutation is identified in the family, predictive genetic testing provides a cost effective and definitive means of family screening because longitudinal evaluation can be focused on mutation carriers (Figure 1B). Other than serial clinical screening to assess for the emergence of clinically overt disease, optimal management of such preclinical (LVH-) mutation carriers has not yet been established. SCD risk is not thought to be increased. Formal exercise restrictions are not advocated by US consensus guidelines, although European Society of Cardiology recommendations are more limiting.34 Family history and individual factors are important considerations. Further assessment with exercise testing or Holter monitoring may be considered.
Genetic Counseling
Genetic counseling addresses the medical, psychological, and family aspects of HCM, including testing options and implications (who will benefit from the information; what reactions may be triggered by different results, particularly since the natural history of disease cannot currently be modified). The Genetic Information Nondiscrimination Act (GINA), signed into law in 2008, provides protection against health insurance and employment discrimination based on genetic testing or family history. However, this protection does not extend to life or disability insurance. At-risk relatives should consider obtaining such insurance before a clinical diagnosis is established.
Future Directions
Remarkable achievements have been made in characterizing the genetic basis of HCM. However, key advances are needed to transform clinical care; advances that include defining the molecular mechanisms that lead from mutation to disease, identifying pathways to target to interrupt phenotypic progression, and identifying more precise risk predictors for sudden death and heart failure. With this knowledge, we will be able to realize the ultimate goal: devising treatment to fundamentally change disease biology, to prevent development of HCM, and to limit life-threatening consequences.
CASE CONCLUSION
Symptoms improved substantially with long acting metoprolol. An ICD was implanted given recent exertional syncope and presumed HCM-related SCD in father and paternal uncle. Genetic testing identified a pathogenic mutation in MYH7, also present in his brother and 1 of his children (Figure 1B). Serial follow up is planned for his G+ daughter who currently has no evidence of HCM. No specific follow up is planned for his G- daughter, sister or her children.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by the National Institutes of Health (K23 HL078901 and 1P20HL101408)
Footnotes
Conflict of Interest Disclosures
None
References
- 1.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW. ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011 doi: 10.1016/j.jacc.2011.10.825. [DOI] [PubMed] [Google Scholar]
- 2.Maron MS, Appelbaum E, Harrigan CJ, Buros JL, Gibson CM, Hanna CA, Lesser JR, Udelson JE, Manning WJ, Maron BJ. Clinical Profile and Significance of Delayed Enhancement in Hypertrophic Cardiomyopathy. Circ Heart Fail. 2008;1:184–191. doi: 10.1161/CIRCHEARTFAILURE.108.768119. [DOI] [PubMed] [Google Scholar]
- 3.Bruder O, Wagner A, Jensen CJ, Schneider S, Ong P, Kispert EM, Nassenstein K, Schlosser T, Sabin GV, Sechtem U, Mahrholdt H. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:875–887. doi: 10.1016/j.jacc.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 4.O’Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, Webb J, Kulkarni M, Dawson D, Sulaibeekh L, Chandrasekaran B, Bucciarelli-Ducci C, Pasquale F, Cowie MR, McKenna WJ, Sheppard MN, Elliott PM, Pennell DJ, Prasad SK. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867–874. doi: 10.1016/j.jacc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Kofflard MJ, Ten Cate FJ, van der Lee C, van Domburg RT. Hypertrophic cardiomyopathy in a large community-based population: clinical outcome and identification of risk factors for sudden cardiac death and clinical deterioration. J Am Coll Cardiol. 2003;41:987–993. doi: 10.1016/s0735-1097(02)03004-8. [DOI] [PubMed] [Google Scholar]
- 6.Maron BJ, Casey SA, Hauser RG, Aeppli DM. Clinical course of hypertrophic cardiomyopathy with survival to advanced age. J Am Coll Cardiol. 2003;42:882–888. doi: 10.1016/s0735-1097(03)00855-6. [DOI] [PubMed] [Google Scholar]
- 7.Maron BJ. Sudden death in young athletes. N Engl J Med. 2003;349:1064–1075. doi: 10.1056/NEJMra022783. [DOI] [PubMed] [Google Scholar]
- 8.Konno T, Chang S, Seidman JG, Seidman CE. Genetics of hypertrophic cardiomyopathy. Curr Opin Cardiol. 2010 doi: 10.1097/HCO.0b013e3283375698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arad M, Maron BJ, Gorham JM, Johnson WH, Jr, Saul JP, Perez-Atayde AR, Spirito P, Wright GB, Kanter RJ, Seidman CE, Seidman JG. Glycogen storage diseases presenting as hypertrophic cardiomyopathy. N Engl J Med. 2005;352:362–372. doi: 10.1056/NEJMoa033349. [DOI] [PubMed] [Google Scholar]
- 10.Olivotto I, Girolami F, Ackerman MJ, Nistri S, Bos JM, Zachara E, Ommen SR, Theis JL, Vaubel RA, Re F, Armentano C, Poggesi C, Torricelli F, Cecchi F. Myofilament protein gene mutation screening and outcome of patients with hypertrophic cardiomyopathy. Mayo Clin Proc. 2008;83:630–638. doi: 10.4065/83.6.630. [DOI] [PubMed] [Google Scholar]
- 11.Girolami F, Ho CY, Semsarian C, Baldi M, Will ML, Baldini K, Torricelli F, Yeates L, Cecchi F, Ackerman MJ, Olivotto I. Clinical features and outcome of hypertrophic cardiomyopathy associated with triple sarcomere protein gene mutations. J Am Coll Cardiol. 55:1444–1453. doi: 10.1016/j.jacc.2009.11.062. [DOI] [PubMed] [Google Scholar]
- 12.Ho CY, Sweitzer NK, McDonough B, Maron BJ, Casey SA, Seidman JG, Seidman CE, Solomon SD. Assessment of diastolic function with Doppler tissue imaging to predict genotype in preclinical hypertrophic cardiomyopathy. Circulation. 2002;105:2992–2997. doi: 10.1161/01.cir.0000019070.70491.6d. [DOI] [PubMed] [Google Scholar]
- 13.Crilley JG, Boehm EA, Blair E, Rajagopalan B, Blamire AM, Styles P, McKenna WJ, Ostman-Smith I, Clarke K, Watkins H. Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of hypertrophy. J Am Coll Cardiol. 2003;41:1776–1782. doi: 10.1016/s0735-1097(02)03009-7. [DOI] [PubMed] [Google Scholar]
- 14.Ho CY, Lopez B, Coelho-Filho OR, Lakdawala NK, Cirino AL, Jarolim P, Kwong R, Gonzalez A, Colan SD, Seidman JG, Diez J, Seidman CE. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med. 2010;363:552–563. doi: 10.1056/NEJMoa1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olivotto I, Cecchi F, Gistri R, Lorenzoni R, Chiriatti G, Girolami F, Torricelli F, Camici PG. Relevance of coronary microvascular flow impairment to long-term remodeling and systolic dysfunction in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2006;47:1043–1048. doi: 10.1016/j.jacc.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 16.Harris KM, Spirito P, Maron MS, Zenovich AG, Formisano F, Lesser JR, Mackey-Bojack S, Manning WJ, Udelson JE, Maron BJ. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation. 2006;114:216–225. doi: 10.1161/CIRCULATIONAHA.105.583500. [DOI] [PubMed] [Google Scholar]
- 17.Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation. 2001;104:2517–2524. doi: 10.1161/hc4601.097997. [DOI] [PubMed] [Google Scholar]
- 18.Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, Shah PM, Spencer WH, 3rd, Spirito P, Ten Cate FJ, Wigle ED. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 2003;42:1687–1713. doi: 10.1016/s0735-1097(03)00941-0. [DOI] [PubMed] [Google Scholar]
- 19.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Zamorano JL. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) J Am Coll Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT, Nistri S, Cecchi F, Udelson JE, Maron BJ. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232–2239. doi: 10.1161/CIRCULATIONAHA.106.644682. [DOI] [PubMed] [Google Scholar]
- 21.Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, Cecchi F, Maron BJ. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348:295–303. doi: 10.1056/NEJMoa021332. [DOI] [PubMed] [Google Scholar]
- 22.Sherrid MV, Barac I, McKenna WJ, Elliott PM, Dickie S, Chojnowska L, Casey S, Maron BJ. Multicenter study of the efficacy and safety of disopyramide in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;45:1251–1258. doi: 10.1016/j.jacc.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S, Gersh BJ, Ackerman MJ, McCully RB, Dearani JA, Schaff HV, Danielson GK, Tajik AJ, Nishimura RA. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:470–476. doi: 10.1016/j.jacc.2005.02.090. [DOI] [PubMed] [Google Scholar]
- 24.Sigwart U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet. 1995;346:211–214. doi: 10.1016/s0140-6736(95)91267-3. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura RA, Ommen SR. Septal reduction therapy for obstructive hypertrophic cardiomyopathy and sudden death: what statistics cannot tell you. Circ Cardiovasc Interv. 3:91–93. doi: 10.1161/CIRCINTERVENTIONS.110.952085. [DOI] [PubMed] [Google Scholar]
- 26.Maron BJ, Shen WK, Link MS, Epstein AE, Almquist AK, Daubert JP, Bardy GH, Favale S, Rea RF, Boriani G, Estes NA, 3rd, Spirito P. Efficacy of implantable cardioverter-defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med. 2000;342:365–373. doi: 10.1056/NEJM200002103420601. [DOI] [PubMed] [Google Scholar]
- 27.Elliott P, Spirito P. Prevention of hypertrophic cardiomyopathy-related deaths: theory and practice. Heart. 2008;94:1269–1275. doi: 10.1136/hrt.2008.154385. [DOI] [PubMed] [Google Scholar]
- 28.Maron BJ, Spirito P, Shen WK, Haas TS, Formisano F, Link MS, Epstein AE, Almquist AK, Daubert JP, Lawrenz T, Boriani G, Estes NA, 3rd, Favale S, Piccininno M, Winters SL, Santini M, Betocchi S, Arribas F, Sherrid MV, Buja G, Semsarian C, Bruzzi P. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. Jama. 2007;298:405–412. doi: 10.1001/jama.298.4.405. [DOI] [PubMed] [Google Scholar]
- 29.Lin G, Nishimura RA, Gersh BJ, Phil D, Ommen SR, Ackerman MJ, Brady PA. Device complications and inappropriate implantable cardioverter defibrillator shocks in patients with hypertrophic cardiomyopathy. Heart. 2009;95:709–714. doi: 10.1136/hrt.2008.150656. [DOI] [PubMed] [Google Scholar]
- 30.Maron BJ, Haas TS, Shannon KM, Almquist AK, Hodges JS. Long-term survival after cardiac arrest in hypertrophic cardiomyopathy. Heart Rhythm. 2009;6:993–997. doi: 10.1016/j.hrthm.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Elliott PM, Gimeno Blanes JR, Mahon NG, Poloniecki JD, McKenna WJ. Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet. 2001;357:420–424. doi: 10.1016/S0140-6736(00)04005-8. [DOI] [PubMed] [Google Scholar]
- 32.Maron BJ, Zipes DP. Introduction: eligibility recommendations for competitive athletes with cardiovascular abnormalities-general considerations. J Am Coll Cardiol. 2005;45:1318–1321. doi: 10.1016/j.jacc.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Sorajja P, Ommen SR, Nishimura RA, Gersh BJ, Berger PB, Tajik AJ. Adverse prognosis of patients with hypertrophic cardiomyopathy who have epicardial coronary artery disease. Circulation. 2003;108:2342–2348. doi: 10.1161/01.CIR.0000097110.55312.BF. [DOI] [PubMed] [Google Scholar]
- 34.Pelliccia A, Zipes DP, Maron BJ. Bethesda Conference #36 and the European Society of Cardiology Consensus Recommendations revisited a comparison of U.S. and European criteria for eligibility and disqualification of competitive athletes with cardiovascular abnormalities. J Am Coll Cardiol. 2008;52:1990–1996. doi: 10.1016/j.jacc.2008.08.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.