Abstract
The syndecan transmembrane proteoglycans synergize with receptors for extracellular matrix molecules and growth factors to initiate cytoplasmic signals in response to a range of extracellular stimuli. Syndecans influence a wide range of physiological processes, but their contribution is most apparent during wound repair. Aspects of syndecan biology that have attracted research interest include extracellular matrix binding, outside-to-inside plasma membrane signal propagation, activation of cytoplasmic signals, and shedding of the syndecan extracellular domain, but the mechanisms by which syndecan cytoplasmic signals modulate extracellular function remain largely unresolved. Hayashida et al. have now discovered that association between an endocytic regulator, Rab5, and the syndecan-1 cytoplasmic domain controlled the shedding of the syndecan-1 extracellular domain. The work describes a mechanistic investigation into inside-to-outside syndecan signaling and highlights several gaps in our understanding of the relation between cell-surface receptors and proteases. In this Perspective, we summarize the current understanding of receptor interplay and identify the challenges that face investigators of adhesion- and growth factor–dependent signaling.
The syndecan transmembrane proteoglycans play critical regulatory roles in many biological processes, including wound healing, inflammation, neural patterning, and angiogenesis (1, 2). The mammalian syndecan family comprises four members, each with large heparan sulfate and chondroitin sulfate chains covalently attached to the extracellular domain (3) and short cytoplasmic tails that interact with a number of signaling adaptors and enzymes (1). Syndecan-1 is predominantly present on epithelial cells, whereas syndecan-4 is found ubiquitously but most notably on fibroblasts (3), and these two family members exhibit the closest functional similarities. Disruption of either of the genes that encode these proteins is nonlethal, but results in distinct wound-healing defects in mice (4, 5). In recent years, the study of the molecular mechanisms regulating syndecan function has gained momentum as the library of possible ligands and biological roles has expanded. Proteoglycans are not the primary receptors of extracellular matrix molecules, growth factors, or chemokines, but they cooperate with the prototypic receptors through simultaneous ligand engagement. Historically, the issues of ligand recognition and cytoplasmic signaling by syndecans have been addressed independently and, although there is strong evidence for signal transduction across the membrane (6), there is surprisingly little known about the mechanism by which intra- and extracellular domain functions are integrated.
Extracellular domain shedding is believed to play a key role in regulating the link between syndecan-ligand interactions and intracellular signaling. Proteolytic cleavage of the syndecan extracellular domain at a membrane-proximal site causes accumulation of shed ectodomains that compete with intact syndecans for extracellular ligands. The consequences of ectodomain competition are twofold, as both the signaling capabilities of the intact syndecan and the associated prototypic receptors are compromised (7, 8) (Fig. 1). Tissue fluids surrounding a wound contain an abundance of shed syndecan-1 and -4 ectodomains (9), which are thought to regulate inflammation and protect against tissue damage by modulating chemokine bioavailability. The syndecan ectodomains are cleaved by various secreted and membrane-associated matrix metalloproteinases (MMPs) that include MMP-7 (10), MMP-9 (11), and membrane type 1 (MT1)–MMP (MMP-14) (12). Syndecans are shed through both constitutive and inducible pathways, and shedding agonists include epidermal growth factor, thrombin, chemokines, and several bacterial virulence factors (9, 11, 13). The critical question, however, is whether ectodomain shedding is regulated indirectly by induced expression of metalloproteinases (11) or MMP inhibitors [e.g., tissue inhibitor of metalloproteinase 3 (TIMP-3) (14)], or whether the syndecan itself plays an active role in the process.
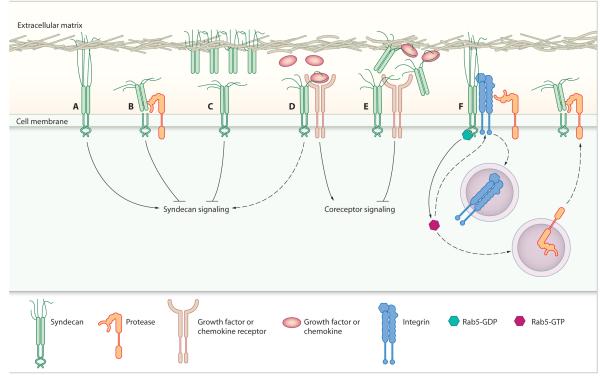
Fig. 1.
The proposed functions of syndecan extracellular domain shedding. Extracellular matrix engagement of syndecans (A) elicits syndecan-specific intracellular signals, which are (B) terminated by proteolytic cleavage of the syndecan extracellular domain. (C) Cleaved syndecan ectodomains further disrupt syndecan signaling by competing with intact syndecan for matrix engagement. (D) The extracellular domain of membrane-bound syndecan promotes prototypic growth factor and chemokine receptor signaling by facilitating association with their ligands in a heparan sulfate–dependent manner, and (E) it is believed that shed syndecan ectodomains disrupt this signaling by sequestering the ligands away from their primary receptors. (F) Syndecan-1 proteolytic cleavage is regulated by a cytoplasmic domain interaction with Rab5-GDP. It was proposed that release of Rab5 from syndecan-1 permits Rab5 activation and modulates trafficking of integrins, which could serve to shield syndecans from proteolysis (15). Alternatively, regulation of Rab5 activity could mediate shedding by coordinating protease recycling.
Hayashida et al. have now provided the first report of syndecan-regulated syndecan shedding. The authors identified an association between the syndecan-1 cytoplasmic domain and the endocytic regulator, Rab5, which influenced ectodomain shedding, but not surface expression, of syndecan-1 (15). Syndecan-1 bound exclusively to the inactive, guanosine diphosphate (GDP)–bound form of Rab5, and it was postulated that the release of sequestered Rab5 might allow Rab5 activation (Fig. 1). It was further speculated that Rab5-dependent endocytosis of transmembrane receptors that shield syndecan-1 would expose the syndecan-1 extracellular domain to MMPs and would explain the phenomenon of regulated shedding. Alternatively, Rab5 could regulate the trafficking of membrane-associated MMPs or their inhibitors, and thus could control their ability to cleave syndecan-1 at the cell surface. Both of these potential mechanisms, although requiring further investigation, are intriguing and not necessarily mutually exclusive. In relation to the shielding concept, integrins would be ideal candidates for the associated receptors. Integrins and syndecans work synergistically during extracellular matrix engagement, with syndecan-1 cooperating with αvβ3 integrin (16), and syndecan-4 cooperating with α5β1 integrin (17). Endocytosis of various integrins is regulated by Rab5 (18, 19), and syndecans and integrins accumulate in the same endocytic vesicles (20). Furthermore, the protein cores of syndecan extracellular domains can act as integrin ligands, such that syndecan ectodomains can support integrin-mediated cell spreading (16, 21). The potential for direct interactions between the extracellular domains of these two families of adhesion receptors has major implications for the regulation of both integrin activity and shedding of the syndecan ectodomain. There is strong evidence linking integrin function with the expression, distribution, and activity of MMPs (both membrane-bound and soluble) and TIMPs (22-24), and many MMPs are regulated by endocytosis (25-27). Collectively, these reports of the mutual interdependence of syndecans, integrins, and MMPs for endocytosis indicate a robust, cyclic feedback loop that would hold in balance the abundance of cooperating receptors on the cell surface.
Apart from its role in the regulation of inflammatory responses, little is known about the biological significance of syndecan shedding. It is clear that extracellular cleavage of syndecan-1 by MT1-MMP promotes cell migration (28) and that the trafficking and cell surface distribution of MT1-MMP is polarized in migrating cells (29). Although this is thought to be associated primarily with spatially restricted extracellular matrix degradation, it would be interesting to know whether, in a migrating cell, syndecan-1 shedding occurs in a polarized manner. The spatial regulation of syndecan-extracellular matrix engagement through shedding is a concept worthy of attention. There is a precedent for syndecan-mediated regulation of directional cell migration, as extracellular engagement of syndecan-4 promotes directional persistence in a protein kinase C (PKC) α– and Rac1-dependent manner (6, 30), and the syndecan-1 cytoplasmic tail regulates migration toward laminin (31).
In summary, it is now becoming clear that syndecans fulfill dual roles: First, syndecans function as coreceptors, facilitating interactions between extracellular ligands and receptors; second, syndecans have signaling capabilities and propagate intracellular signals in response to extracellular matrix engagement. Therefore, the most important role of syndecans is in the integration of these two apparently distinct processes. Some remaining questions are as follows: What are the signaling consequences of syndecan ectodomain shedding? What role does syndecan shedding play in directional cell migration? Do syndecans regulate recycling of other cell surface receptors? If so, can this function be regulated by syndecan shedding or syndecan-dependent regulation of Rab5? And, precisely what effect does syndecan shedding have on growth factor and chemokine receptor signaling? As we move toward a greater understanding of the complexity of signaling networks, through proteomic analyses and systems biology combined with precise molecular manipulation of syndecan function both in vitro and in vivo, it is likely that many of these issues will be resolved.
References
- 1.Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol. 2007;8:957–969. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexopoulou AN, Multhaupt HA, Couchman JR. Syndecans in wound healing, inflammation and vascular biology. Int. J. Biochem. Cell Biol. 2007;39:505–528. doi: 10.1016/j.biocel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 4.Stepp MA, Gibson HE, Gala PH, Iglesia DD, Pajoohesh-Ganji A, Pal-Ghosh S, Brown M, Aquino C, Schwartz AM, Goldberger O, Hinkes MT, Bernfield M. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. J. Cell Sci. 2002;115:4517–4531. doi: 10.1242/jcs.00128. [DOI] [PubMed] [Google Scholar]
- 5.Echtermeyer F, Streit M, Wilcox-Adelman S, Saoncella S, Denhez F, Detmar M, Goetinck P. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J. Clin. Invest. 2001;107:R9–R14. doi: 10.1172/JCI10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bass MD, Roach KA, Morgan MR, Mostafavi-Pour Z, Schoen T, Muramatsu T, Mayer U, Ballestrem C, Spatz JP, Humphries MJ. Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J. Cell Biol. 2007;177:527–538. doi: 10.1083/jcb.200610076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanderson RD, Yang Y, Kelly T, MacLeod V, Dai Y, Theus A. Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: Growth regulation and the prospect of new cancer therapies. J. Cell. Biochem. 2005;96:897–905. doi: 10.1002/jcb.20602. [DOI] [PubMed] [Google Scholar]
- 8.Buczek-Thomas JA, Nugent MA. Elastase-mediated release of heparan sulfate proteoglycans from pulmonary fibroblast cultures: A mechanism for basic fibroblast growth factor (bFGF) release and attenuation of bFGF binding following elastase-induced injury. J. Biol. Chem. 1999;274:25167–25172. doi: 10.1074/jbc.274.35.25167. [DOI] [PubMed] [Google Scholar]
- 9.Subramanian SV, Fitzgerald ML, Bernfield M. Regulated shedding of syndecan-1 and -4 ectodomains by thrombin and growth factor receptor activation. J. Biol. Chem. 1997;272:14713–14720. doi: 10.1074/jbc.272.23.14713. [DOI] [PubMed] [Google Scholar]
- 10.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 11.Brule S, Charnaux N, Sutton A, Ledoux D, Chaigneau T, Saffar L, Gattegno L. The shedding of syndecan-4 and syndecan-1 from HeLa cells and human primary macrophages is accelerated by SDF-1/CXCL12 and mediated by the matrix metalloproteinase-9. Glycobiology. 2006;16:488–501. doi: 10.1093/glycob/cwj098. [DOI] [PubMed] [Google Scholar]
- 12.Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, Furukawa M, Sato H. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J. Biol. Chem. 2003;278:40764–40770. doi: 10.1074/jbc.M306736200. [DOI] [PubMed] [Google Scholar]
- 13.Popova TG, Millis B, Bradburne C, Nazarenko S, Bailey C, Chandhoke V, Popov SG. Acceleration of epithelial cell syndecan-1 shedding by anthrax hemolytic virulence factors. BMC Microbiol. 2006;6:8. doi: 10.1186/1471-2180-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzgerald ML, Wang Z, Park PW, Murphy G, Bernfield M. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J. Cell Biol. 2000;148:811–824. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashida K, Stahl PD, Park PW. Syndecan-1 ectodomain shedding is regulated by the small GTPase Rab5. J. Biol. Chem. 2008;283:35435–35444. doi: 10.1074/jbc.M804172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beauvais DM, Burbach BJ, Rapraeger AC. The syndecan-1 ectodomain regulates αvβ3 integrin activity in human mammary carcinoma cells. J. Cell Biol. 2004;167:171–181. doi: 10.1083/jcb.200404171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mostafavi-Pour Z, Askari JA, Parkinson SJ, Parker PJ, Ng TT, Humphries MJ. Integrin-specific signaling pathways controlling focal adhesion formation and cell migration. J. Cell Biol. 2003;161:155–167. doi: 10.1083/jcb.200210176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pellinen T, Arjonen A, Vuoriluoto K, Kallio K, Fransen JA, Ivaska J. Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of β1-integrins. J. Cell Biol. 2006;173:767–780. doi: 10.1083/jcb.200509019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valdembri D, Caswell PT, Anderson KI, Schwarz JP, Konig I, Astanina E, Caccavari F, Norman JC, Humphries MJ, Bussolino F, Serini G. Neuropilin-1/GIPC1 signaling regulates α5β1 integrin traffic and function in endothelial cells. PLoS Biol. 2009;7:e25. doi: 10.1371/journal.pbio.1000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmermann P, Zhang Z, Degeest G, Mortier E, Leenaerts I, Coomans C, Schulz J, N’Kuli F, Courtoy PJ, David G. Syndecan recycling [corrected] is controlled by syntenin-PIP2 interaction and Arf6. Dev. Cell. 2005;9:377–388. doi: 10.1016/j.devcel.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Whiteford JR, Couchman JR. A conserved NXIP motif is required for cell adhesion properties of the syndecan-4 ectodomain. J. Biol. Chem. 2006;281:32156–32163. doi: 10.1074/jbc.M605553200. [DOI] [PubMed] [Google Scholar]
- 22.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Hinsbergh VW, Engelse MA, Quax PH. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler. Thromb. Vasc. Biol. 2006;26:716–728. doi: 10.1161/01.ATV.0000209518.58252.17. [DOI] [PubMed] [Google Scholar]
- 24.Chirco R, Liu XW, Jung KK, Kim HR. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev. 2006;25:99–113. doi: 10.1007/s10555-006-7893-x. [DOI] [PubMed] [Google Scholar]
- 25.Remacle AG, Rozanov DV, Baciu PC, Chekanov AV, Golubkov VS, Strongin AY. The transmembrane domain is essential for the microtubular trafficking of membrane type-1 matrix metalloproteinase (MT1-MMP) J. Cell Sci. 2005;118:4975–4984. doi: 10.1242/jcs.02610. [DOI] [PubMed] [Google Scholar]
- 26.Van den Steen PE, Van Aelst I, Hvidberg V, Piccard H, Fiten P, Jacobsen C, Moestrup SK, Fry S, Royle L, Wormald MR, Wallis R, Rudd PM, Dwek RA, Opdenakker G. The hemopexin and O-glycosylated domains tune gelatinase B/MMP-9 bioavailability via inhibition and binding to cargo receptors. J. Biol. Chem. 2006;281:18626–18637. doi: 10.1074/jbc.M512308200. [DOI] [PubMed] [Google Scholar]
- 27.Yang Z, Strickland DK, Bornstein P. Extracellular matrix metalloproteinase 2 levels are regulated by the low density lipoprotein-related scavenger receptor and thrombospondin 2. J. Biol. Chem. 2001;276:8403–8408. doi: 10.1074/jbc.M008925200. [DOI] [PubMed] [Google Scholar]
- 28.Su G, Blaine SA, Qiao D, Friedl A. Membrane type 1 matrix metalloproteinase-mediated stromal syndecan-1 shedding stimulates breast carcinoma cell proliferation. Cancer Res. 2008;68:9558–9565. doi: 10.1158/0008-5472.CAN-08-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bravo-Cordero JJ, Marrero-Diaz R, Megias D, Genis L, Garcia-Grande A, Garcia MA, Arroyo AG, Montoya MC. MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. EMBO J. 2007;26:1499–1510. doi: 10.1038/sj.emboj.7601606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthews HK, Marchant L, Carmona-Fontaine C, Kuriyama S, Larrain J, Holt MR, Parsons M, Mayor R. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development. 2008;135:1771–1780. doi: 10.1242/dev.017350. [DOI] [PubMed] [Google Scholar]
- 31.Chakravarti R, Sapountzi V, Adams JC. Functional role of syndecan-1 cytoplasmic V region in lamellipodial spreading, actin bundling, and cell migration. Mol. Biol. Cell. 2005;16:3678–3691. doi: 10.1091/mbc.E04-10-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]