Abstract
The formation, maturation and dissolution of focal adhesions are basic prerequisites of cell migration and rely upon the recruitment, signalling and endocytosis of integrins. In many instances, extracellular matrix molecules are recognised by a number of integrins, and it is the sequential involvement of different integrins that allows establishment of cell polarity and migration toward a matrix stimulus. In this review we consider both the similarities and differences between two key fibronectin receptors: αvβ3 and α5β1 integrin. By considering the GTPase and kinase signalling and trafficking of two such closely-related receptors we begin to understand how cell migration is coordinated.
Keywords: Integrin, signalling, trafficking, endocytosis, focal adhesion, migration
1. Introduction
Intercellular communication in metazoa relies not only on autocrine, paracrine and exocrine signalling systems, but also on the structural and positional information encoded in extracellular matrices (ECM). Cell-ECM interactions act as sites of mechanotransduction, transmitting short-range tensile and elastic force across the plasma membrane, and interpreting long-range alterations in tissue flow. They also regulate chemical signalling by controlling the spatiotemporal assembly of enzymes and adaptors into dynamic complexes. At the cellular level, cell-ECM contact sites are elaborated as clusters of adhesion receptors, principally integrins, interacting extracellularly with ECM polymers and intracellularly with cytoskeletal and signalling components. The close integration of the external tissue structure with the internal signalling machinery enables exquisite environmental sensing. Fundamental cellular processes, including survival, division, differentiation and migration are reliant upon effective cell-ECM associations. For this reason, there has been much interest in defining the mechanisms of adhesion receptor-ligand binding and signalling. Understandably, for such an important protein assembly, the cell-ECM junction is complex, containing at least several hundred protein components. Currently, our insight into the molecular composition of adhesion complexes is limited; we understand which molecules and pathways are possible, but not the stoichiometry, turnover and dynamic relationships of molecules in real adhesion complexes. A number of recent reports have impacted significantly on these questions by opening up new avenues of research. We will draw together these diverse findings and predict directions that the field will take, with a particular view to understanding the spatial and temporal control of the mechanisms described and the molecular complexity associated with these processes.
The adhesion contacts of cultured cells have been broadly divided into three categories according to their molecular composition. Focal complexes (FX) are nascent adhesion plaques that are rich in phosphotyrosine, talin and αvβ3 integrin (1); focal adhesions (FA) develop from FX upon membrane retraction and contain α5β1 integrin and zyxin, in addition to the FX components (1,2); fibrillar adhesions (FB) develop as FA translocate centripetally across the ventral surface of the cell and have a limited molecular composition that includes α5β1 integrin and tensin, but lacks αvβ3 integrin, paxillin and phosphotyrosine (2,3). The sequential recruitment of adhesion contact components means that we must be careful not to over-interpret the partition into FX, FA and FB, and the cell-specific composition of the different contacts is still disputed, but the labels do reflect the molecular heterogeneity and dynamic relationship of integrin complexes. Integrin activation and the maturation of adhesion plaques is heavily influenced by the transmission of force through the actin cytoskeleton (4,5), which depends on both the rigidity of ECM and contraction of the cytoskeleton itself. Cytoskeletal reorganisation and contraction are driven by activation of the small GTPases Rac1 and RhoA (6), to the extent that activation of Rac1 drives FX formation, while suppression of RhoA blocks the transition to FA (7). Release of RhoA inhibition then drives FA formation and stress fibre contractility.
During biological processes such as cell migration and matrix fibrillogenesis, the heterodimer-specific functions of α5β1 and αvβ3 integrins are precisely co-ordinated. When considering the relative roles of α5β1 and αvβ3 integrins, there appears to be a dynamic interaction between the processes of FA formation/maturation, transduction of heterodimer-specific signalling and spatially restricted cell-surface expression of specific integrin heterodimers. In this review, we will consider these phenomena in turn and highlight mechanisms by which they influence one another. A recurring theme will be the concept of dynamic cross-regulation of these processes and how it is regulated by the extracellular environment. Specifically, how the spatiotemporal regulation of these processes, and their influence on one another, must be precisely co-ordinated to permit efficient cell migration.
Central to the ability of cells to transduce integrin heterodimer-specific intracellular signals in a spatially and temporally co-ordinated manner, are precisely regulated mechanisms to control cell surface localisation of receptors, including adhesive contact maturation and vesicular trafficking. The centripetal translocation of α5β1, but not αvβ3, is a defining characteristic of the maturation of adhesive contacts. A consequence of this is ligation of specific heterodimers in a spatially-restricted manner. Signalling as a consequence of ligand engagement of these different integrin heterodimers, particularly via the Rho family small GTPases and non-receptor tyrosine kinases, has a key role in regulating many of the cellular processes responsible for adhesion maturation and vesicular trafficking. Moreover, we will survey the present evidence that heterodimer-specific cytoplasmic interactions regulate integrin endocytosis and therefore cell surface availability/accessibility of receptors.
2. Alternative signals from fibronectin receptors
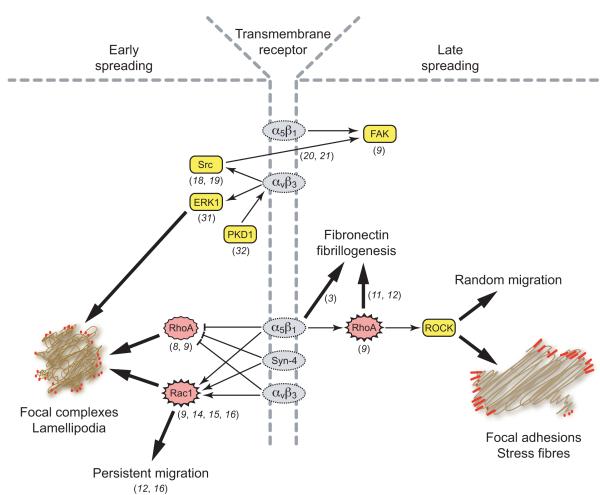
Heterogeneity in the integrin composition of adhesion plaques could feasibly lead to variation in the signalling cascades initiated in response to ECM engagement, and integrin-specific signalling pathways have been investigated extensively in both fibroblasts and epithelial cells. One prominent example is the regulation of Rho family GTPases. Engagement of either α5β1 or αvβ3 integrin is sufficient for the suppression of RhoA activity during the early stages of cell spreading on fibronectin (8,9), but while engagement of α5β1 integrin, found in FA and FB, is capable of reactivating RhoA, engagement of αvβ3 integrin, found in FX and FA, is not, reflecting the role of RhoA in the FX-FA transition (9) (Fig. 1). The morphologies of cells expressing specific integrins reflect these signalling differences as αvβ3-expressing cells have low RhoA activity and form broad lamellipodia, while cells expressing α5β1 integrin have high RhoA activity and form actin stress fibres. Pharmacological or dominant-negative inhibition of RhoA switches α5β1-expressing cells to an αvβ3-like morphology (10), demonstrating the key role of RhoA in mediating integrin-specific behaviour. The α5β1 integrin-RhoA link also explains some of the features of fibronectin fibrillogenesis, which requires both translocation of α5β1 integrin (3) and activation of RhoA (11). Cells expressing αvβ3, rather than α5β1, fail to organise fibronectin into fibrils but can be partially rescued by expression of dominant active RhoA (9,12). This is not to suggest that αvβ3 is simply an inert version of α5β1, as αvβ3 can mediate fibronectin fibrillogenesis, by an unknown alternative mechanism, in the developing embryo or when cells are cultured on laminin-coated surfaces (13). The alternative mechanisms of fibronectin fibrillogenesis are just one example of the molecular redundancy between integrins, and highlight the difficulties of drawing simple, linear connections between molecules that are frequently context-specific.
Fig. 1. α5β1 and αvβ3 integrins exert alternative influences over common signalling pathways.
A broad range of signalling molecules that include Rho-family GTPases and kinases are regulated in response to integrin-engagement by the ECM. The subtle differences between signals downstream of α5β1 and αvβ3 mean that changes in the integrin composition of adhesion complexes, as they mature, automatically trigger changes in the signals that regulate maturation itself. For example, the transition of αvβ3-rich FX into α5β1-rich FA causes a switch from Rac1-dominated to RhoA-dominated GTPase signalling that in turn causes an increase in cytoskeletal tension and adhesion complex maturation.
Unlike RhoA, Rac1 activation is influenced by both α5β1 and αvβ3 integrin. Clustering of cytoplasmic tails of either β1 or β3 integrin causes Rac1 activation (14) and steady-state activity levels are also similar between α5β1- or αvβ3-expressing cells (9). The regulation of Rac1 is complicated as engagement of α5β1 integrin causes membrane recruitment (15), while simultaneous engagement of the transmembrane proteoglycan, syndecan-4, causes GTP-loading (16). It is still not entirely clear whether αvβ3 integrin relies on cooperation with syndecans to the same extent as α5β1. Unlike α5β1, αvβ3-mediated adhesion appears to induce FA formation (9) and fibronectin fibrillogenesis (13) in the absence of syndecan engagement. However, other experiments have demonstrated that syndecan-1 plays an important role in the activation of αvβ3 integrin (17) and that reduction of syndecan-4 expression alters the morphology and migration of cells adhering through αvβ3 (12,16). As syndecans are understood to act as complementary receptors that fine-tune integrin function, it is logical that they should exert different influences over the different integrins, and resolving the subtle interplay between receptors will be an important step toward understanding how cells precisely regulate signalling cascades, both spatially and temporally.
Although the mechanisms by which integrins exert their influence on intracellular signals are still poorly resolved, heterodimer-specific associations with a number of protein kinases have been characterised. A range of tyrosine kinases including Hck, Lyn and c-Yes complex with the cytoplasmic domains of both β1 and β3 integrins, but the potential for alternative signalling pathways relies primarily on c-Src, which binds to, and is activated by, the clustered β3 cytoplasmic tail (18,19). Despite the role of Src in achieving full activation of another tyrosine kinase, focal adhesion kinase (FAK), the critical autophosphorylation of FAK on tyrosine-397 is delayed in αvβ3-expressing compared with α5β1-expressing cells (9). The recruitment of FAK to FA is accelerated in Src/Yes/Fyn-null cells (20), which suggests a model of Src recruitment to αvβ3-containing FX that precedes FAK recruitment to α5β1-containing FA. In support of this model, Src-independent autophosphorylation of FAK is blocked by a β1 fragment that inhibits cell spreading (21), FAK is enriched in FA compared to FX (1) and also enriched in α5β1-containing compared to αvβ3-containing pseudopodia (22). The reason for the enrichment of FAK in FA is unclear, as one of the recruitment pathways of FAK is by association with talin, which binds equally well to β1 and β3 cytoplasmic domains (23). The second method by which FAK might be recruited is through association with the adapter molecule paxillin. Like FAK, paxillin is enriched in FA over FX (1), but the paxillin that is found in FX is recruited before α5β1 is visibly clustered (24), which would argue that the differences in paxillin abundance are not entirely due to integrin specificity.
Heterodimer-specific relationships with serine/threonine kinases have also been characterised and often regulate integrin localisation, rather than mediating signals downstream of integrin engagement. The alpha and epsilon isoforms of protein kinase C (PKC) regulate trafficking of β1 integrin by binding to the cytoplasmic domain, either directly or in complex with the scaffolding protein RACK1, respectively (25-27). By contrast, β3-integrin interacts exclusively with PKCβ in a complex that again includes RACK1 and, like the β1-PKC complex, regulates cell migration (28). Although this study focused on integrin αIIbβ3 rather than αvβ3, it does suggest that β1 and β3 integrins are linked to similar, yet distinct pathways, though isoform-specific interactions. Of even greater interest is the potential for regulatory crosstalk between signals downstream of the different integrins. Ligation of α5β1 causes activation of calmodulin-dependent kinase II (CamKII) that is necessary for α5β1-mediated migration toward fibronectin (29). Not only is αvβ3-mediated migration independent of CamKII activity (30), but engagement of αvβ3 even inhibits CamKII activity to block α5β1-mediated migration (29). Interestingly, α5β1 does not exert a similarly antagonistic influence on αvβ3. Engagement of α5β1 and αvβ3 each make critical contributions toward suppression of PKA activity to the extent that engagement of α5β1 by low abundance ligand is necessary for efficient migration over the αvβ3-specifc ECM, vitronectin (30). Other integrin-specific interactions include the association of αvβ3 with ERK1 (31) and protein kinase D1 (PKD1) (32). ERK1 and PKD1 bind respectively to the central and membrane-distal NITY motifs of the β3 cytoplasmic domain, which are distinct from the talin-binding NPXY motif, and not conserved in β1. Disruption of the association of αvβ3 with either ERK1 or PKD1 blocks the initiation of FX formation and directionally persistent migration, by inhibiting Rab4-dependent recycling of αvβ3 as described below (Fig. 2).
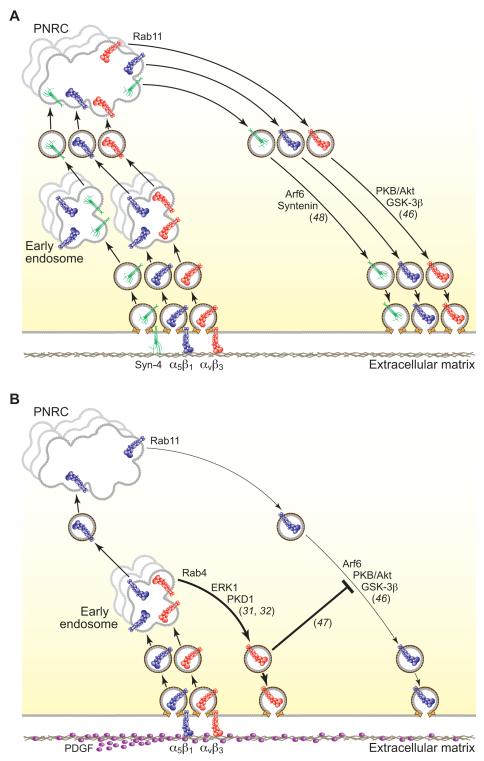
Fig. 2. Heterodimer-specific integrin recycling.
A) In the absence of serum, both α5β1 and αvβ3 are trafficked, via the PNRC, along the long-loop recycling pathway. Syndecan-4 is also recycled via the long-loop pathway in a syntenin-dependent manner. B) Stimulation with PDGF triggers membrane delivery of αvβ3 via the short-loop pathway and suppresses long-loop recycling of α5β1; this allows spatial and functional compartmentalisation of the different heterodimers.
In summary, the pair-wise analysis of molecular interactions reveals a number of differences between α5β1 and αvβ3 integrins (Fig. 1). However, these studies are united by one common feature; namely that interfering with integrin-specific interactions consistently results in compromised cell migration rather than complete inhibition of cell adhesion. The subtlety of this phenotype emphasises the importance of the nucleation and maturation of adhesion plaques in coordinating cell behaviour, and as we go on to consider the consequences of the spatial localisation and appropriate trafficking of the different integrins this becomes increasingly apparent.
3. Receptor trafficking determines integrin function
Adhesion receptor trafficking is a precisely controlled mechanism that regulates the subcellular localisation of signalling cascades and cell-matrix traction. By targeting specific integrin heterodimers to discrete regions of membrane, the cell controls integrin accessibility and co-ordinates heterodimer-specific signalling. Additionally, integrins can be co-trafficked with other adhesion and growth factor receptors, with the result that the molecular processes that coordinate recycling of integrins may also regulate cell-surface delivery of receptors capable of modulating integrin function. In this section we will consider the role of adhesion receptor recycling as a means of differentially regulating the intracellular signalling of α5β1 and αvβ3 integrins (Fig. 2), and discuss the role of this regulation in adhesion plaque formation and directional cell migration.
Adhesion receptor internalisation occurs through both clathrin-dependent and clathrin-independent endocytosis and internalisation of these endocytic vesicles can occur in a dynamin-dependent or independent manner (33-35). Following endocytosis, receptors are trafficked to early endosomes, after which they face three possible fates: they can be recycled back to the membrane via the short-loop pathway, shuttled to the perinuclear recycling compartment (PNRC) before being returned to the membrane via the long-loop pathway, or alternatively, the receptors can be transported to late endosomes for degradation.
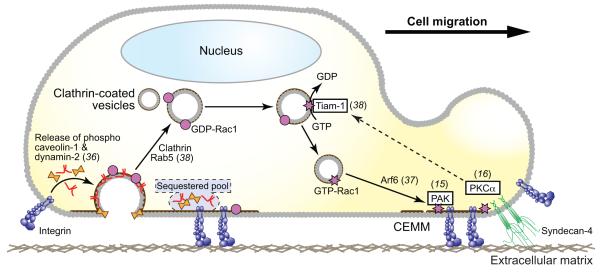
Cholesterol-enriched membrane microdomains (CEMMs), which include caveolae, are ordered regions of membrane that, as a result of their lipid composition, spatially restrict the distribution of, and signalling from, adhesion receptors (Fig. 3). Loss of integrin engagement results in internalisation of CEMMs due to the release of phosphorylated caveolin-1 and dynamin-2 from integrin clusters (36). Conversely, clustering of β1 integrin leads to the membrane localisation of CEMM markers and Rac1 (15), while direct clustering of the CEMM component, GM1, results in the membrane-recruitment of Rac1 and activation of its downstream effector, PAK (15). Anchorage-dependent regulation of CEMM membrane-targeting and Rac1 activation is mediated by microtubules and the vesicular trafficking-associated small GTPase, Arf6 (37). Interestingly, recent work by Palamidessi et al (2008) suggests that Rab5- and clathrin-dependent endocytosis is required for GTP-loading of Rac1 (38). Consistent with the role of Arf6 in CEMM recycling, Arf6 regulates membrane localisation of endocytically-regulated active Rac1 (37,38) (Fig. 3).
Fig. 3. Adhesion-dependent trafficking of Rac1.
At the trailing edge of a cell, disengagement of integrins from the ECM causes the release of sequestered phosphorylated caveolin-1 and dynamin-2. Liberation of these endocytic regulators causes internalisation of CEMMs and associated Rac1. In response to syndecan-4 engagement, Rac1 is reloaded with GTP and then recycled to the leading edge where matrix engagement causes Arf6-dependent recruitment of CEMMs.
When considering the endocytosis of integrins themselves, it is clear that the coordination of a number of protein kinases is necessary for the trafficking of different integrin heterodimers. Using high-throughput screening, Pelkmans et al (2005) identified large numbers of kinases involved in different types and stages of endocytic recycling, and demonstrated that specific subsets of kinases have opposing effects on different modes of endocytosis (39). The processes that regulate endocytosis of integrins in a heterodimer-specific manner are still poorly understood but, as many of the kinases associated with internalisation of integrins can be differentially regulated by engagement of specific integrin heterodimers or their co-receptors, it is possible to postulate a number of heterodimer-specific recycling mechanisms. For example, caveolin-1 is phosphorylated on Y14 by Src (40), and phosphorylation of this residue is required for caveolin-mediated internalisation of CEMMs (36) and p190RhoGAP-dependent suppression of Rho activity (41). The differential regulation of Src by α5β1 and αvβ3 may provide a means by which integrins can influence endocytosis in a heterodimer-specific manner. Interestingly, in studies using cytoplasmic mutants of αIIbβ3 integrin, the distal NITY motif, which is unique to the β3 cytoplasmic domain, allows internalisation of β3. Substitution of this domain or mutation of tyrosine-759, which is a substrate for Src family kinases, suppresses endonexin-mediated uptake of β3 (42,43). Likewise, the isoform-specific interactions of PKCs, described above, make a major contribution to integrin trafficking. Stimulation of PKCα increases dynamin-dependent endocytosis and recycling of β1 integrin and, as a consequence, increases migration of carcinoma cells (26). In fibroblasts, PKCε, which binds to the cytoplasmic domain of β1 integrin, in complex with RACK1, phosphorylates the intermediate filament protein, vimentin, on the surface of integrin recyling endosomes (44). Phosphorylation of vimentin mediates the dissociation of PKCε from β1 integrin, and is essential for trafficking of β1 integrin back to the surface.
Microtubule targeting to adhesive contacts mediates FA disassembly, but it is not yet clear what role integrin endocytosis may play in this process. However, microtubule-dependent FA dissociation is dynamin-dependent (45) and microtubules regulate both internalisation and subsequent recycling of CEMMs (37), so it is likely that adhesion receptor endocytosis will play a significant role in microtubule-dependent FA disassembly.
As a result of recent advances in our understanding of vesicular trafficking, the processes regulating heterodimer-specific membrane-delivery of α5β1 and αvβ3 integrins are far better understood than those regulating heterodimer-specific endocytosis (Fig. 2). In serum-free conditions, fibroblasts and ovarian carcinoma cells recycle both α5β1 and αvβ3 integrins are recycled through the Rab11- and Arf6-dependent long-loop pathway, under the regulation of PKB/Akt and GSK-3β (32,46,47) (Fig. 2a). However upon platelet-derived growth factor (PDGF) stimulation, αvβ3 integrin, but not α5β1, is trafficked back to the membrane from early endosomes via the Rab4-mediated short-loop pathway (Fig. 2b). Short-loop recycling of αvβ3 depends upon direct association of the αvβ3 cytodomain with PKD1 (PKCμ) to the extent that substitution of the NITY motif, expression of a catalytically inactive mutant of PKD1, substitution of the autophosphorylated serine-916 of PKD1, or suppression of endogenous PKD1 expression, inhibits PDGF-stimulated short-loop recycling of αvβ3 (32,48). Significantly, disruption of the PKD1-αvβ3 interaction not only compromises short-loop recycling of αvβ3, but also increases long-loop recycling of α5β1, as does suppression of αvβ3 expression (48). The increase in α5β1 recycling, in response to inhibition of αvβ3, depended upon association of α5β1 with Rab-coupling protein (RCP), which in turn binds to Rab11 (46). These experiments provide a powerful example of direct antagonism between integrin heterodimers.
Integrins colocalise with other ECM and growth factor receptors in recycling endosomes. Perturbation of syndecan recycling by mutation of the PIP2-binding site of the scaffolding protein, syntenin, or expression of a dominant negative mutant of Arf6, leads to accumulation of syndecans, β1 integrin, and FGF receptor in long-loop recycling vesicles (49). Furthermore, clustering of syndecan-4, or FGF2 stimulation, regulates redistribution of syndecan-4 to CEMMs and promotes internalisation (35,50), raising the intriguing possibility that integrin co-receptors might influence heterodimer-specific recycling of integrins.
Throughout this review we have discussed the signalling consequences and trafficking mechanisms of α5β1 integrin, in comparison with αvβ3 integrin, and identified striking differences between the two. In this final section we consider the biological consequences of differential integrin signalling. Integrin endocytosis and exocytosis exerts a significant influence over cell migration, consistent with the role in regulating FX formation, stability, turnover, and spatial localisation. Phosphorylated caveolin-1 has a polarised distribution in migrating endothelial cells (51) and Src-family kinase-mediated caveolin-1 phosphorylation regulates directionally-persistent migration and cell polarisation. Moreover, caveolin-deficient fibroblasts, which are incapable of internalising CEMMs upon loss of integrin engagement, exhibit reduced directional persistence (36,41). Endocytosis mediated by the association of β1 integrin and PKCα regulates carcinoma cell migration (26,27) and recently, it has been demonstrated that direct association of the β1 cytoplasmic domain with the epithelial-specific Rab11 homologue, Rab25, restricts delivery of α5β1 to the leading edge and promotes tumour cell invasion of 3D matrices (52). Cell type-specific expression of molecules that regulate integrin trafficking is an area of research that requires further investigation and could eventually provide a strategy for targeting metastatic tumour cells without affecting the surrounding tissue. It is now established that activation of RhoA and its downstream effector, ROCK, promotes random, amoeboid migration (53). In the same way, suppression of α5β1-recycling, triggered by PDGF-stimulated αvβ3 short-loop recycling, promotes directionally persistent migration as a result of reduced ROCK and cofilin activity (48). This is consistent with the role of α5β1, but not αvβ3, in activating Rho upon ligand engagement (9) and the fact that cells over-expressing αvβ3, but not α5β1, migrate in a directionally-persistent manner (10). Danen et al. also showed that expression of αvβ3, rather than α5β1, reduced FA translocation and the turnover of FA components (10). In order for a cell to migrate efficiently it is necessary for FA stability to be actively regulated, so that FA stabilisation occurs only at points requiring static cell-ECM interactions to provide traction, and that FA formation, sliding and disassembly occur in other regions to allow cellular translocation. As such, it is arguable that precisely regulated migration can only be achieved when heterodimer-specific integrin signalling and recycling, and FA maturation, stabilisation and disassembly are completely integrated.
4. Conclusions
Although progress towards an understanding of cell adhesion complex assembly and turnover has been rapid, a number of major gaps remain. Defining the proteomes of receptor-specific adhesion complexes using mass spectrometry and sophisticated imaging techniques will provide insights into the extent that complexes vary. Systems analysis of signalling modules and networks will shed light on the molecular mechanisms responsible for controlling assembly and maturation of adhesion complexes. Such approaches will also delineate the cross-regulation between different integrin heterodimers, and explain how adhesion complexes initiate long-range effects on cell differentiation. In this context, analyses of the convergence of signalling by other receptor families, such as growth factor, chemokine and cytokine receptors, will lead to an explanation of the cell’s sensory functions that are modulated by adhesion to the ECM.
Acknowledgements
The authors were supported by Wellcome Trust grant 074941 (MRM, MDB and MJH) and by a BBSRC CASE PhD studentship, sponsored by GlaxoSmithKline (AB).
References
- 1.Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci. 2003;116:4605–4613. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- 2.Zamir E, Katz M, Posen Y, Erez N, Yamada KM, Katz BZ, Lin S, Lin DC, Bershadsky A, Kam Z, Geiger B. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat Cell Biol. 2000;2:191–196. doi: 10.1038/35008607. [DOI] [PubMed] [Google Scholar]
- 3.Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, Lin S, Hahn C, Yamada KM. Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J Cell Biol. 2000;148:1075–1090. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katsumi A, Naoe T, Matsushita T, Kaibuchi K, Schwartz MA. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J Biol Chem. 2005;280:16546–16549. doi: 10.1074/jbc.C400455200. [DOI] [PubMed] [Google Scholar]
- 6.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 7.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 8.Bass MD, Morgan MR, Roach KA, Settleman J, Goryachev AB, Humphries MJ. p190RhoGAP is the convergence point of adhesion signals from alpha 5 beta 1 integrin and syndecan-4. J Cell Biol. 2008;181:1013–1026. doi: 10.1083/jcb.200711129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danen EH, Sonneveld P, Brakebusch C, Fassler R, Sonnenberg A. The fibronectin-binding integrins alpha5beta1 and alphavbeta3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J Cell Biol. 2002;159:1071–1086. doi: 10.1083/jcb.200205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danen EH, van Rheenen J, Franken W, Huveneers S, Sonneveld P, Jalink K, Sonnenberg A. Integrins control motile strategy through a Rho-cofilin pathway. J Cell Biol. 2005;169:515–526. doi: 10.1083/jcb.200412081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huveneers S, Truong H, Fassler R, Sonnenberg A, Danen EH. Binding of soluble fibronectin to integrin {alpha}5{beta}1 - link to focal adhesion redistribution and contractile shape. J Cell Sci. 2008;121:2452–2462. doi: 10.1242/jcs.033001. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi S, Leiss M, Moser M, Ohashi T, Kitao T, Heckmann D, Pfeifer A, Kessler H, Takagi J, Erickson HP, Fassler R. The RGD motif in fibronectin is essential for development but dispensable for fibril assembly. J Cell Biol. 2007;178:167–178. doi: 10.1083/jcb.200703021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berrier AL, Martinez R, Bokoch GM, LaFlamme SE. The integrin beta tail is required and sufficient to regulate adhesion signaling to Rac1. J Cell Sci. 2002;115:4285–4291. doi: 10.1242/jcs.00109. [DOI] [PubMed] [Google Scholar]
- 15.Del Pozo MA, Alderson NB, Kiosses WB, Chiang HH, Anderson RG, Schwartz MA. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- 16.Bass MD, Roach KA, Morgan MR, Mostafavi-Pour Z, Schoen T, Muramatsu T, Mayer U, Ballestrem C, Spatz JP, Humphries MJ. Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J Cell Biol. 2007;177:527–538. doi: 10.1083/jcb.200610076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beauvais DM, Burbach BJ, Rapraeger AC. The syndecan-1 ectodomain regulates alphavbeta3 integrin activity in human mammary carcinoma cells. J Cell Biol. 2004;167:171–181. doi: 10.1083/jcb.200404171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci U S A. 2003;100:13298–13302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huveneers S, van den Bout I, Sonneveld P, Sancho A, Sonnenberg A, Danen EH. Integrin alpha v beta 3 controls activity and oncogenic potential of primed c-Src. Cancer Res. 2007;67:2693–2700. doi: 10.1158/0008-5472.CAN-06-3654. [DOI] [PubMed] [Google Scholar]
- 20.Volberg T, Romer L, Zamir E, Geiger B. pp60(c-src) and related tyrosine kinases: a role in the assembly and reorganization of matrix adhesions. J Cell Sci. 2001;114:2279–2289. doi: 10.1242/jcs.114.12.2279. [DOI] [PubMed] [Google Scholar]
- 21.Berrier AL, Jones CW, LaFlamme SE. Tac-beta1 inhibits FAK activation and Src signaling. Biochem Biophys Res Commun. 2008;368:62–67. doi: 10.1016/j.bbrc.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao Y, Schwarzbauer JE. Accessibility to the fibronectin synergy site in a 3D matrix regulates engagement of alpha5beta1 versus alphavbeta3 integrin receptors. Cell Commun Adhes. 2006;13:267–277. doi: 10.1080/15419060601072215. [DOI] [PubMed] [Google Scholar]
- 23.Calderwood DA, Fujioka Y, de Pereda JM, Garcia-Alvarez B, Nakamoto T, Margolis B, McGlade CJ, Liddington RC, Ginsberg MH. Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc Natl Acad Sci U S A. 2003;100:2272–2277. doi: 10.1073/pnas.262791999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiseman PW, Brown CM, Webb DJ, Hebert B, Johnson NL, Squier JA, Ellisman MH, Horwitz AF. Spatial mapping of integrin interactions and dynamics during cell migration by image correlation microscopy. J Cell Sci. 2004;117:5521–5534. doi: 10.1242/jcs.01416. [DOI] [PubMed] [Google Scholar]
- 25.Besson A, Wilson TL, Yong VW. The anchoring protein RACK1 links protein kinase Cepsilon to integrin beta chains. Requirements for adhesion and motility. J Biol Chem. 2002;277:22073–22084. doi: 10.1074/jbc.M111644200. [DOI] [PubMed] [Google Scholar]
- 26.Ng T, Shima D, Squire A, Bastiaens PI, Gschmeissner S, Humphries MJ, Parker PJ. PKCalpha regulates beta1 integrin-dependent cell motility through association and control of integrin traffic. Embo J. 1999;18:3909–3923. doi: 10.1093/emboj/18.14.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsons M, Keppler MD, Kline A, Messent A, Humphries MJ, Gilchrist R, Hart IR, Quittau-Prevostel C, Hughes WE, Parker PJ, Ng T. Site-directed perturbation of protein kinase C-integrin interaction blocks carcinoma cell chemotaxis. Mol Cell Biol. 2002;22:5897–5911. doi: 10.1128/MCB.22.16.5897-5911.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buensuceso CS, Obergfell A, Soriani A, Eto K, Kiosses WB, Arias-Salgado EG, Kawakami T, Shattil SJ. Regulation of outside-in signaling in platelets by integrin-associated protein kinase C beta. J Biol Chem. 2005;280:644–653. doi: 10.1074/jbc.M410229200. [DOI] [PubMed] [Google Scholar]
- 29.Blystone SD, Slater SE, Williams MP, Crow MT, Brown EJ. A molecular mechanism of integrin crosstalk: alphavbeta3 suppression of calcium/calmodulin-dependent protein kinase II regulates alpha5beta1 function. J Cell Biol. 1999;145:889–897. doi: 10.1083/jcb.145.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim S, Harris M, Varner JA. Regulation of integrin alpha vbeta 3-mediated endothelial cell migration and angiogenesis by integrin alpha5beta1 and protein kinase A. J Biol Chem. 2000;275:33920–33928. doi: 10.1074/jbc.M003668200. [DOI] [PubMed] [Google Scholar]
- 31.Roberts MS, Woods AJ, Shaw PE, Norman JC. ERK1 associates with alpha(v)beta 3 integrin and regulates cell spreading on vitronectin. J Biol Chem. 2003;278:1975–1985. doi: 10.1074/jbc.M208607200. [DOI] [PubMed] [Google Scholar]
- 32.Woods AJ, White DP, Caswell PT, Norman JC. PKD1/PKCmu promotes alphavbeta3 integrin recycling and delivery to nascent focal adhesions. Embo J. 2004;23:2531–2543. doi: 10.1038/sj.emboj.7600267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caswell PT, Norman JC. Integrin trafficking and the control of cell migration. Traffic. 2006;7:14–21. doi: 10.1111/j.1600-0854.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- 34.Jones MC, Caswell PT, Norman JC. Endocytic recycling pathways: emerging regulators of cell migration. Curr Opin Cell Biol. 2006;18:549–557. doi: 10.1016/j.ceb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Tkachenko E, Lutgens E, Stan RV, Simons M. Fibroblast growth factor 2 endocytosis in endothelial cells proceed via syndecan-4-dependent activation of Rac1 and a Cdc42-dependent macropinocytic pathway. J Cell Sci. 2004;117:3189–3199. doi: 10.1242/jcs.01190. [DOI] [PubMed] [Google Scholar]
- 36.Del Pozo MA, Balasubramanian N, Alderson NB, Kiosses WB, Grande-Garcia A, Anderson RG, Schwartz MA. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–908. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balasubramanian N, Scott DW, Castle JD, Casanova JE, Schwartz MA. Arf6 and microtubules in adhesion-dependent trafficking of lipid rafts. Nat Cell Biol. 2007;9:1381–1391. doi: 10.1038/ncb1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palamidessi A, Frittoli E, Garre M, Faretta M, Mione M, Testa I, Diaspro A, Lanzetti L, Scita G, Di Fiore PP. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–147. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 39.Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, Krausz E, Zerial M. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature. 2005;436:78–86. doi: 10.1038/nature03571. [DOI] [PubMed] [Google Scholar]
- 40.Li S, Seitz R, Lisanti MP. Phosphorylation of caveolin by src tyrosine kinases. The alpha-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J Biol Chem. 1996;271:3863–3868. [PubMed] [Google Scholar]
- 41.Grande-Garcia A, Echarri A, de Rooij J, Alderson NB, Waterman-Storer CM, Valdivielso JM, del Pozo MA. Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases. J Cell Biol. 2007;177:683–694. doi: 10.1083/jcb.200701006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Datta A, Huber F, Boettiger D. Phosphorylation of beta3 integrin controls ligand binding strength. J Biol Chem. 2002;277:3943–3949. doi: 10.1074/jbc.M109536200. [DOI] [PubMed] [Google Scholar]
- 43.Gawaz M, Besta F, Ylanne J, Knorr T, Dierks H, Bohm T, Kolanus W. The NITY motif of the beta-chain cytoplasmic domain is involved in stimulated internalization of the beta3 integrin A isoform. J Cell Sci. 2001;114:1101–1113. doi: 10.1242/jcs.114.6.1101. [DOI] [PubMed] [Google Scholar]
- 44.Ivaska J, Vuoriluoto K, Huovinen T, Izawa I, Inagaki M, Parker PJ. PKCepsilon-mediated phosphorylation of vimentin controls integrin recycling and motility. Embo J. 2005;24:3834–3845. doi: 10.1038/sj.emboj.7600847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol. 2005;7:581–590. doi: 10.1038/ncb1262. [DOI] [PubMed] [Google Scholar]
- 46.Caswell PT, Chan M, Lindsay AJ, McCaffrey MW, Boettiger D, Norman JC. Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol. 2008;183:143–155. doi: 10.1083/jcb.200804140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts MS, Woods AJ, Dale TC, Van Der Sluijs P, Norman JC. Protein kinase B/Akt acts via glycogen synthase kinase 3 to regulate recycling of alpha v beta 3 and alpha 5 beta 1 integrins. Mol Cell Biol. 2004;24:1505–1515. doi: 10.1128/MCB.24.4.1505-1515.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White DP, Caswell PT, Norman JC. alpha v beta3 and alpha5beta1 integrin recycling pathways dictate downstream Rho kinase signaling to regulate persistent cell migration. J Cell Biol. 2007;177:515–525. doi: 10.1083/jcb.200609004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimmermann P, Zhang Z, Degeest G, Mortier E, Leenaerts I, Coomans C, Schulz J, N’Kuli F, Courtoy PJ, David G. Syndecan recycling is controlled by syntenin-PIP2 interaction and Arf6. Dev Cell. 2005;9:377–388. doi: 10.1016/j.devcel.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 50.Tkachenko E, Simons M. Clustering induces redistribution of syndecan-4 core protein into raft membrane domains. J Biol Chem. 2002;277:19946–19951. doi: 10.1074/jbc.M200841200. [DOI] [PubMed] [Google Scholar]
- 51.Beardsley A, Fang K, Mertz H, Castranova V, Friend S, Liu J. Loss of caveolin-1 polarity impedes endothelial cell polarization and directional movement. J Biol Chem. 2005;280:3541–3547. doi: 10.1074/jbc.M409040200. [DOI] [PubMed] [Google Scholar]
- 52.Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, Mills GB, Humphries MJ, Messent AJ, Anderson KI, McCaffrey MW, Ozanne BW, Norman JC. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 53.Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–719. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]