Abstract
The formation of the vascular system is one of the earliest and most important events during organogenesis in the developing embryo because the growing organism needs a transportation system to supply oxygen and nutrients and to remove waste products. Two distinct processes termed vasculogenesis and angiogenesis lead to a complex vasculature covering the entire body. Several cellular mechanisms including migration, proliferation, differentiation and maturation are involved in generating this hierarchical vascular tree. To achieve this aim, a multitude of signaling pathways need to be activated and coordinated in spatio-temporal patterns. Understanding embryonic molecular mechanism in angiogenesis further provides insight for therapeutic approaches in pathological conditions like cancer or ischemic diseases in the adult. In this review, we describe the current understanding of major signaling pathways that are necessary and active during vascular development.
Keywords: Vascular development, Embryogenesis, Angiogenic signaling, Endothelium, VEGF, Angiopoetins, TGF-beta, Review
2. INTRODUCTION
The vascular system is essential for all vertebrates because it provides tissues and organs with oxygen and nutrients. Due to a relatively short (0.1 to 0.2 mm) diffusion distance, the entire body is covered with an extensively branched network of blood vessels, which in conjunction with the heart and blood represents the circulatory system (1, 2). The connection between arteries and veins forming a closed circulation filled with blood was described for the first time in 1628 by William Harvey. Even though Harvey was not able to visualize the capillaries, he hypothesized their existence and accordingly they were specified in 1661 by Marcello Malpighi (3, 4).
Capillaries are the smallest vessels in the vasculature, and consist only of a single layer of endothelial cells (ECs), creating the inner lining of blood vessels (endothelium), and of occasional pericytes to maximize contact with the surrounding tissue. In larger vessels (arterioles and venules) the endothelium is accompanied by pericytes and connective tissue, whereas arteries and veins comprise of a thick layer of smooth muscle cells (SMC) and circumjacent connective tissue to stabilize these vessels. Oxygenated blood is pumped by the heart from the lungs with high pressure through arteries and arterioles into the capillaries, where material exchange with the tissue takes place. From there deoxygenated blood is transported with lower pressure and therefore flows much slower through venules and veins back to the lung. To withstand the high pressure, arteries require a stronger wall and have a thicker layer of SMCs compared to veins (5–7).
The circulatory vasculature serves not only to transport gases, nutrients, metabolic waste, macromolecules and liquids between tissues and organs, but also stabilizes the body temperature and pH to maintain homeostasis. Further important functions are to facilitate rapid communication between different organs mediated by hormones or cytokines, and finally to transport immune cells to sites of inflammation or infection (7–9). Consequentially, malformations or dysfunction of the vascular system have a huge impact on the whole organism and contribute to the pathogenesis of many diseases as, for instance, ischemic cardiovascular diseases (6). The importance of a complete and healthy vasculature is shown best during embryonic development because mice failing to establish functional blood vessels die early around day 10 (10).
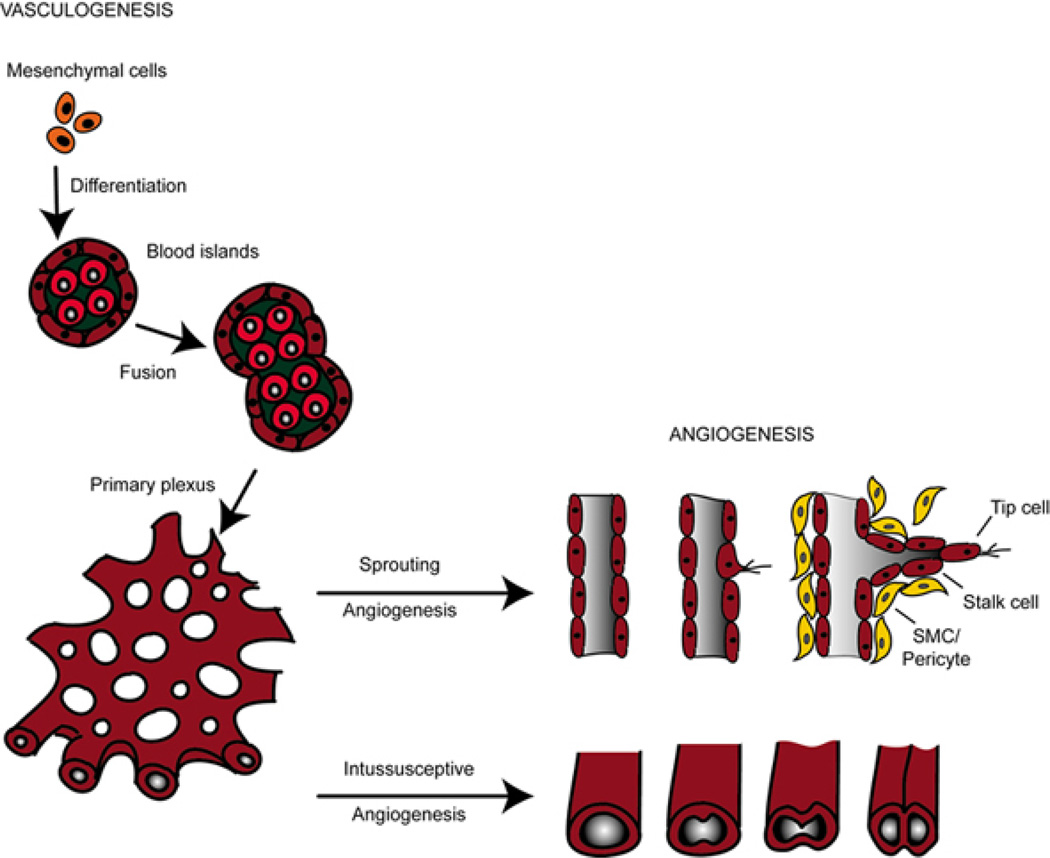
In the developing embryo the formation of the vascular system is one of the earliest events during organogenesis (11) (see Figure 1). Two distinct processes termed vasculogenesis and angiogenesis are involved in generating the vasculature of the growing organism (7).
Figure 1.
Schematic overview of vasculogenesis and angiogenesis. During development, mesodermal cells differentiate into haemangioblasts, which further develop into angioblast, the precursors of endothelial cells. Vasculogenesis involves the differentiation of endothelial cells (ECs) from these angioblast cells to form a primitive vascular plexus. Remodeling and growing processes of these pre-existing vessels is termed angiogenesis. Intussusceptive angiogenesis involves splitting by the formation of translumen pillars and growing of vessels. In sprouting angiogenesis, endothelial cells proliferate behind the tip cell of a growing branch in response to cytokines and lumens can form by vacuole fusion. Both forms of angiogenesis require the recruitment of smooth muscle cells (SMCs) or pericytes to stabilize the nascent vessels. Neighboring mesenchymal cells migrate towards the neovessel in response to platelet-derived growth factor (PDGF), which then differentiate into vascular SMCs (vSMCs) in response to transforming growth factor-beta (TGF-b) signaling.
During vasculogenesis mesodermal precursor cells migrate, differentiate and aggregate de novo to form a bipotential haemangioblast which gives rise to blood and ECs. Subsequently, the nascent blood vessels develop and generate a primitive vascular plexus. Further expansion and remodeling of this primary plexus of capillaries is denoted as angiogenesis and covers several steps including adhesion, proliferation, migration, differentiation and tube formation from already existing vessels as well as vessel maturation (7, 11). Over the past years, a plethora of signaling pathways has been shown to be involved in embryonic vascular development (Table 1). In particular, vascular endothelial growth factor (VEGF) and the angiopoetin/Tie receptor families, which are all nearly endothelial cell specific and crucial for the establishment of a functional vasculature, are the most extensively studied pathways. Other signaling pathways such as the Delta/Notch, ephrin/ephrine receptors, fibroblast growth factor (FGF), platelet derived growth factor (PDGF/PDGFR) and transforming growth factor beta (TGFb) family or pathways that are involved in axonal guidance such as the semaphorins, netrins and ROBO/Slits are not restricted to the endothelial lineage, but nevertheless play a specific and necessary role in vascular development (Table 1) (12). Although environmental factors such as hypoxia (13, 14) and hemodynamic forces (15, 16) modulate the establishment of the vasculature, the initial process of vasculogenesis seems to be genetically programmed (17–19).
Table 1.
List of genes involved in vasculo-angiogenesis derived from knock-out mice
| Gene | Vascular Phenotype | Ref |
|---|---|---|
| Vegfa−/− | Heterozygous lethality after E9.5, abnormal blood island formation, reduction of embryonic vasculature | (55, 56) |
| Vegfa164/188−/− | 50% die shortly after birth with internal bleedings, other 50% die up to P14 due to ischemic cardiomyopathy | (186) |
| Vegfb−/− | Viable, but smaller heart and dysfunctional coronary vasculature | (187, 188) |
| Plgf−/− | Normal developmental vasculature, but impaired pathological angiogenesis | (189, 190) |
| Vegfr1−/− | Embryonic lethal between E8.5–E9.5, increased amount but disorganized ECs | (57) |
| Flk1 (Vegfr2)−/− | Embryonic lethal E8.5, failure of blood island formation | (28) |
| Vegfr3−/− | Embryonic lethal E9.5, disorganised and reduced ECs, defects in vascular remodelling in yolk sac and embryo | (58) |
| Ang1−/− | Phenotype resembles Tie2−/− | (73) |
| Ang2−/− | Lack of patterning and development of the lymphatic vessels, defects in the development of vascular bed of the retina | (75, 77) |
| Tie1−/− | Embryonic lethal around E13.5, exhibiting defects in EC sprouting and vessel integrity | (72, 74) |
| Tie2−/− | Embryonic lethal E9.5, impaired vessel branching and dilated vessels, haemorrhages | (71, 72) |
| Jag1−/− | Embryonic lethal around E9.5–E11.5, remodelling defects in yolk sac and embryo vasculature | (191) |
| Dll4+/− | Dependent on mouse strain, heterozygous lethality around E9.5–E10.5 due to defects in vascular remodelling in the yolk sac and disorganized vasculature in the embryo | (192, 193) |
| Notch1−/− | Embryonic lethal around E9.5, defects in vascular remodelling in the yolk sac and the embryo | (194) |
| Tie2-Cre Notch1−/− | Embryonic lethal around E9.5, defects in vascular remodelling in the yolk sac and the embryo | (195) |
| Notch1/4−/− | Embryonic lethal around E9.5, defects in vascular remodelling in the yolk sac and disorganized vasculature in the embryo | (194) |
| Hey1/2−/− | Embryonic lethal around E9.5, defects in vascular remodelling in the yolk sac and haemorrhages | (196, 197) |
| Fgf2−/− | Alive, decreased vascular tone and blood pressure | (198) |
| Fgfr1−/− | Embryonic lethal, reduction of paraxial mesoderm | (199) |
| Pdgfb−/− | Perinatal lethal, haematopoietic defects, microvascular haemorrhages, heart defects | (94, 95) |
| Pdgfrb−/− | Perinatal lethal, haematopoietic defects, microvascular haemorrhages | (93) |
| Tgfb1−/− | Embryonic lethal around E10.5, defects in haematopoiesis, yolk sac and embryonic vasculature, less vSMCs leading to enlarged and fragile vessels | (100) |
| Tgfb2−/− | Perinatal lethal, aortic arch and cardial septal defects | (200) |
| Bmp2−/− | Embryonic lethal around E8, mesoderm defects leading to disturbed amnion/chorion and cardiac development | (201) |
| Bmp4−/− | Embryonic lethal E7.5–E9.5, failure in mesoderm formation leading to loss of blood island formation | (106) |
| Tgfbr1 (Alk5)−/− | Phenotype like Tgfb1−/− | (202) |
| Tgfbr2−/− | Embryonic lethal around E10.5, defects in yolk sac haematopoiesis and vasculogenesis, fragile and dilated blood vessels | (203) |
| Actvrl1 (Alk1)−/− | Embryonic lethal around E11.5, hyperdilated vessels and remodelling defect, arteriovenous malformations | (101, 204) |
| Endoglin−/− | Embryonic lethal around E11.5, less vSMCs leading to enlarged and fragile vessels, similar to Tgfb1−/− | (205–208) |
| Bmpr1 (Alk3)−/− | Embryonic lethal between E6.5–E9.5, failure in mesoderm formation leading to loss of blood island formation | (107) |
| Bmpr2−/− | Embryonic lethal between E6.5–E9.5, failure in mesoderm formation leading to loss of blood island formation, defects in vascular remodelling and maturation | (109, 209, 210) |
| Smad1−/− | Embryonic lethal between E9.5–E10.5 caused by failure to remodel chorio allantoic circulation and to develop a placenta, some embryos show defects in yolk sac angiogenesis | (111) |
| Smad5−/− | Embryonic lethal between E9 –E11.5, defects in amnion, heart, allantois and yolk sac vascular remodelling | (112, 113) |
| Smad4−/− | Embryonic lethal around E6.5–E8.5, failure to initiate mesoderm formation | (211, 212) |
| Tie2-Cre Smad4−/− | Embryonic lethal around E10.5, cardiovascular defects, attenuated vessel sprouting and remodelling | (114) |
| Ihh−/− | 50% embryonic lethal at E10.5, failure in remodelling of yolk sac vasculature, fewer vessels | (213) |
| Shh−/− | Perinatal lethal, decreased branching morphogenesis and vasculature in lung | (214) |
| Ihh/Shh−/− | Embryonic lethal around E9.5, defects in vascular remodelling in the yolk sac and in the embryo | (215) |
| Ephrinb2−/− | Embryonic lethal around E11, defects in yolk sac and embryonic vasculature, myocardial trabeculation | (121, 216) |
| Tie2-Cre Ephrinb2−/− | Phenotype like Ephrinb2−/− | (217) |
| Ephb4−/− | Phenotype like Ephrinb2−/− | (122) |
| Neuropilin1−/− | Embryonic lethal around E12.5, defects in vascular remodelling in the yolk sac and in aortic arches | (218, 219) |
| Tie2-Cre Neuropilin1−/− | Perinatal lethal, defects in microvasculature | (220) |
| Neuropilin2−/− | Abnormal lymphatic vasculature, viable | (221) |
| Neuropilin1/2−/− | Embryonic lethal around E8.5, avascular yolk sac | (59) |
| Sema3a−/− | Mostly embryonic lethal, lack of anterior cardinal vein and intersomitic branching | (222, 223) |
| Sema5a−/− | Embryonic lethal around E11.5, decreased complexity of cranial cardinal vein and branches | (224) |
| Plexind1−/− | Perinatal lethal, defects in vascular and outflow tract separation, aberrant brachning of intersomitic vessels | (125, 225) |
| Unc5b−/− | Embryonic lethal around E12.5, abnormal excessive vessel branching | (226) |
| Tie2-Cre Unc5b−/− | Embryonic lethal between E12–E13.5, defects in placental vasculature | (227) |
| Scl (Tal1)−/− | Embryonic lethal around E9.5, failure of haematopoiesis | (228, 229) |
| Foxo1−/− | Embryonic lethal around E11, defects in branchial arches and impaired vascular development in yolk sac and embryo | (230) |
| Fli11−/− | Embryonic lethal around E12.5, haemorrhaging from dorsal aorta, neural tube and ventral brain | (231) |
| Hif1alpha−/− | Embryonic lethal around E11, malformations in heart, yolk sac and embryo vasculature | (232, 233) |
| Epas1 (/Hif2alpha)−/− | Embryonic lethal between E9.5 and E13.5 defective vascular remodelling in yolk sac and embryo | (234) |
| Arnt−/− | Embryonic lethal around E10, defective angiogenesis in yolk sac and branchial arch | (235) |
| Id1/3−/− | Embryonic lethal around E13.5, vascular malformations in the forbrain and absence of sprouting in neuroectoderm | (236) |
| c-myc−/− | Embryonic lethal around E10.5, defect of vasculogenesis and angiogenesis | (237) |
| Coup-tfII−/− | Embryonic lethal around E10.5, exhibiting defects in remodelling of primary plexus and intersomitic vessel growth, heart defects | (238) |
| Tie2-Cre Coup-tfII−/− | Embryonic lethal around E12, dilated and thin vessels, haemorrhages, loss of arteriovenous identity | (239) |
3. VASCULOGENESIS
Vasculogenesis is the process of de novo differentiation of endothelial cells from mesodermal precursor cells. Around E6–E6.5 during development of the mouse embryo, primitive endothelial cells begin to arise from mesodermal tissue located in the extra-embryonic yolk sac (20, 21) and afterwards in the embryo proper (22, 23) as well as in the allantois (24, 25) and placenta (26). One of the earliest markers for the endothelial progenitor cell lineage is fetal liver kinase (Flk1), also known as vascular endothelial growth factor receptor 2 (VEGFR2) (26), which is expressed together with the mesodermal marker Brachyury in mesodermal angioblast precursor cells located in the primitive streak (27). These progenitor cells migrate in response to endoderm-derived signals including FGF and bone morphogenetic protein 4 (BMP4) to sites of vascularization.– first into the extraembryonic visceral mesoderm of the yolk sac (11). There, mesodermal angioblast precursor cells proliferate, aggregate, differentiate and contribute to the formation of blood islands (28, 29).
In the early 20th century Sabin has observed a spatio-temporal proximity of endothelial and haematopoetic cell lineages in avian embryos and therefore postulated the existence of a common precursor, which was later called the haemangioblast by Murray (30–32). Indirect evidence for the existence of a bipotential progenitor came from Flk/VEGFR2 deficient mice that showed lack of blood islands and organized blood vessels in the yolk sac or embryo as well as only few haematopoetic progenitors (28, 33). Further insight for the haemangioblast theory was provided by a study using embryonic stem (ES) cells differentiating into blast colony-forming cells (BL-CFC). A single colony of these progenitor cells can give rise to an embryoid body containing both endothelial and haematopoetic lineages (34). This result was supported by Huber et al. using isolated haemangioblast cells from E7.5 mouse embryos that also could give rise to both lineages (27) as well as by a single cell fate map study in zebrafish (35).
However, another theory assumes that the haematopoetic lineage arises from an endothelial cell intermediate with haematopoetic potential, called haemogenic endothelium, and not directly from a mesodermal precursor (36–38).
Recently, two independent research groups provided additional evidence supporting this concept by the use of new imaging and cell tracking methods to analyze cell surface markers (39, 40). Mesodermal angioblast progenitors give rise to blood islands that consist of haematopoietic progenitor cells in the centre and a peripheral layer of angioblasts. Upon fusion processes of blood islands, a primitive capillary plexus arises (Figure 1). Thereafter, intraembryonic vasculogenesis is initiated along the paraaortic mesoderm (aorta-gonad-mesonephros (AGM) region) and subsequently capillary plexi are generated around the whole embryo (20, 41). The only exception for this process is the generation of the dorsal aorta and cardinal vein both of which are directly differentiated from mesodermal angioblast precursors (11, 42). Further pruning and maturation of the existing primary vascular plexus is described by the term angiogenesis (43, 44).
4. ANGIOGENESIS
Angiogenesis is defined as the growth of new blood vessels from pre-existing vessels. Two principal mechanisms - non-sprouting (intussusceptive) and sprouting angiogenesis - are distinguished in embryonic development (Figure 1). The major difference between both mechanisms is that sprouting angiogenesis is also invasive to unvascularized tissues like in the brain primordium, but the process is relatively slow and depends on cell proliferation (8). On the contrary, the process of intussusceptive angiogenesis allows formation of new blood vessels under flow and is quite fast since it appears within minutes to hours and does not primarily need proliferation of ECs (45).
Concurrent with sprouting angiogenesis intussusceptive angiogenesis occurs during lung, heart and yolk sac development (8). In lung development ECs, predominantly in venules or capillaries, invaginate into existing vessels and thereby create contact of opposing endothelium walls. In the following steps the endothelial bilayer becomes perforated and a transcapillary pillar is formed, which is further stabilized by invading pericytes producing collagen fibers. Finally, a longitudinal division of one vessel into two vessels is achieved (46–48).
For sprouting angiogenesis, ECs of a pre-existing vessel are activated by growth factors such as vascular endothelial growth factor (VEGF). Upon stimulation, ECs begin to release proteases such as members of the matrix metalloproteinase (MMP) family. The degradation of the extracellular matrix (ECM) surrounding the cells as well as of plasma proteins from the blood leads to a provisional scaffold facilitating EC migration (44, 49). Within the capillary vessel wall some ECs are selected for sprouting and the first cells of the growing sprout are denoted as tip cells. For example, during retina angiogenesis tip cells extend long filopodia to sense VEGF and thereupon migrate in a directed way along the VEGF concentration gradient. Interestingly, subsequent cells respond to VEGF by proliferation rather than by migration and are thus named stalk cells. Afterwards, they begin to form a lumen and give rise to a new vessel (7, 50).
Finally, maturation of this new blood vessel is achieved by recruitment of supporting cells, like pericytes or SMCs, and production of the basal lamina. Further growth of the organism requires remodeling of the vascular system induced by environmental stimuli like hypoxia, or changes in hemodynamic forces inducing multiple growth factors. In response to these stimuli the whole vascular tree is remodeled through branching and pruning (51).
5. MOLECULAR REGULATION OF VASCULAR DEVELOPMENT
Until today, a various number of angiogenic molecules and their corresponding signaling pathways have been identified to be involved in the complex processes of angiogenesis. Therefore growth factors, chemokines, receptors, adhesion molecules, transcription factors and intracellular signaling cascades need to be tightly orchestrated in a spatiotemporal manner to achieve the physiologic growth of the vasculature.
5.1. VEGF Signaling
Secreted vascular endothelial growth factor (VEGF) ligands and their cognate receptors and co-receptors play a pivotal role for all aspects of vascular development.– from the earliest event of endothelial cell lineage specification to vasculogenesis, angiogenesis and endothelial cell survival. VEGFRs are basically markers for commitment to the endothelial cell lineage. However, they can also be expressed in haematopoietic and neural stem cells, which is an exception to their endothelial cell specificity (52–54).
Gene deletion studies in mice revealed that a majority of genes that have a critical impact on the formation of the functional vessel network are related to the VEGF signaling pathway (23). Moreover, deletion of only a single allele of vegfa leads to embryonic lethality between day 11 and 12 due to severe defects in the vasculature (55, 56). Similar phenotypes are observed for homozygous deletions of any of the three major receptors VEGFR1/Flt1 (57), VEGFR2/Flk (28), VEGFR3/Flt4 (58) or of the two co-receptors Neuropilin 1 and 2 (59). Besides the ligand VEGFA there at least four more VEGF subtypes. Attenuation of any of the other VEGF subtypes (VEGFB -D and placenta growth factor (PLGF)) also has a great impact on the formation of a functional vasculature (see Table 1).
VEGFA (VEGF subtype A) is the major mediator of blood vessel growth, survival and permeability when bound to VEGFR2, whereas, binding to VEGFR1 in a soluble form (sVEGFR1) seems to trap VEGFA and therefore may function as a decoy receptor or ligand reservoir (60–63). The precise function of VEGFR1 is still under debate, and seems to be dependent on its biological context. Mice deficient in VEGFR1 die of an excessive proliferation of angioblasts and malformation of blood vessels (57, 64) suggesting excessive VEGF signaling (28). On the other hand, VEGFA leads to weak phosphorylation of VEGFR1 (65), and additionally VEGFR1 is reported to crosstalk with VEGFR2, which may result in either inhibitory or stimulatory activity dependent on the biological context (reviewed in reference (66)).
VEGFA is expressed in multiple alternative splice isoforms with varying abilities to bind to VEGFRs and co-receptors such as neuropilins or heparin sulfate proteoglycans (HSPGs). VEGFRs belong to the superfamily of receptor tyrosine kinases (RTK) like PDGFRs or FGFRs that dimerize and become transphosphorylated upon activation. Binding of VEGF induces homodimerization of VEGFRs that leads to autophosphorylation and activation of different intracellular signaling cascades (66). For example, in a confluent endothelial cell layer Flk1 is associated with VE-cadherin through p120, beta-catenin and density enhanced phosphatase (DEP1). Altogether this complex promotes survival signals through phosphorylation of PI3K and Akt. The appearance of DEP1 in this case inhibits Erk signaling and subsequent proliferation of confluent cells (67–68).
VEGFB and PDGF can also mediate pro-angiogenic signals by binding to VEGFR1, but to a tenfold less extent compared to VEGFA binding to VEGFR2 (69). VEGFC and D bind to VEGFR3 and thereby stimulate lymphangiogenesis (70).
Taken together, activation of the VEGF pathway is essential for the generation of the EC lineage and the vasculature.
5.2. Ang/Tie2 Signaling
Other crucial members of the endothelial cell lineage specific RTKs are the tyrosine kinase with Ig and EGF homology (Tie1 and Tie2) receptors with their corresponding secreted ligands.– the angiopoetins (Ang1-2). These are also highly important regulators of angiogenic processes. Unlike VEGF deficient mice, gene deletion of Tie receptors reveal that formation of blood vessels proceeds normally but further maturation and expansion of the vasculature is disturbed resulting in embryonic lethality. Mice deficient in either the receptor Tie2 (71, 72) or its ligand Ang1 (73), which have the same phenotype, die at E9.5 to E12.5 because of multiple cardiovascular defects (Table 1). Therefore, Ang1 activity is suggested to be receptor-specific (73). Deletion of the other receptor Tie1 does not affect angiogenesis but displays a loss of vessel integrity and widespread edema resulting in lethality between E13 and birth (72, 74). In contrast, embryonic vascular development of Ang2 deficient mice seems to be normal (75), however, later on in adult mice vascular disorders have been found in various tissues i.e. kidney or retina (76, 77). Interestingly, in Ang2 overexpressing transgenic mice, blood vessel formation is disturbed and the phenotype resembles the Tie2 or Ang1 deficient phenotypes (78, 79). Taken together, loss and gain of function studies in mice suggest that Ang2 might function as an antagonist of Ang1 to modulate Tie2 receptor activity (79).
Binding of Ang1 to Tie2 receptors induces homodimerization. As the next step autophosporylation of the receptors and recruitment of intracellular adapter proteins that facilitate endothelial cell migration, survival, maturation and permeability can occur (80). As expected from the in vivo data, Ang2 can bind to Tie2 but does not activate it (81, 82). However, the role of Tie1 is still under debate. Full length Tie1 is either thought to form heterodimers with Tie2 (83) or to be an orphan receptor. Tie1 and VEGF signaling are functionally linked. Tie1 is cleaved by gamma-secretases following VEGF stimulation. The cleaved form, remaining in the cell membrane, interacts with Tie2 and is hypothesized to support Tie2 signaling (84, 85).
Taken together, stimulation of Tie2 by Ang1 induces remodeling and stabilization of blood vessels by recruiting pericytes and by formation of cell-cell and cell-matrix interactions and thus plays an important role in maintaining endothelial integrity and quiescence in the adult organism (86–89). On the contrary, Ang2 has a destabilizing effect on blood vessels, which may lead to vessel sprouting or regression dependent on tissue and biological context (i. e. availability of VEGFA) (90, 91).
5.3. PDGF and TGF-beta signaling
For stabilization of the newly formed vessels, recruitment of pericytes and smooth muscle cells is required and mainly involves PDGF, Ang1/Tie2 and TGF-beta signaling pathways. PDGFs are growth factors comprising a family of four (A- D) structurally related members binding to their appropriate RTKs.– PDGFR-alpha or.–beta, which may be present as homodimer or heterodimer (92).
During embryonic development of the vasculature, PDGF signaling has been shown to be indispensable for proliferation and migration of pericytes and SMCs. Deficiency of the PDGF-B chain or the PDGFR-beta in mice leads to severe vascular defects manifested in leakiness of vessels or in overproliferation of endothelial cells that is due to lack of pericytes (93–95). PDGF-B is secreted by endothelial cells, probably in response to VEGF stimulation, to recruit pericytes expressing PDGFR-beta. Additionally, pericytes themselves can secrete PDGF-B and thereby amplify the signal (96).
Another important growth factor released by ECs is TGF-beta. It induces attraction and differentiation of mesenchymal progenitor cells into pericytes and SMCs and synthesis of the extracellular matrix (97, 98). TGF-beta belongs to a large family of pleiotropic secreted growth factors, which also includes activins and bone morphogenetic proteins (BMPs). These ligands mediate their effect by binding to a complex of type I and type II serine/threonine kinase transmembrane receptors (99).
Interestingly, gene deletion studies of components related to the TGF-beta pathway (e.g. Tgfbr1 or 2) also revealed an abnormal formation of the primitive vascular plexus in mice, which suggests an important role of TGF-beta for endothelial cells besides decreased vessel wall integrity by impaired recruitment of SMCs (100, 101). Moreover, in vitro studies demonstrate that TGF-beta 1 has a context dependent biphasic effect on endothelial cells: at low concentrations extracellular TGF-beta 1 induces pro-angiogenic effects on endothelial cells. However, at high concentrations it leads to cytostasis, synthesis of extracellular matrix, vessel muscularization, and thereby to the status of quiescent endothelium (51). Furthermore, it is reported that TGF-beta family members additionally affect angiogenesis in a paracrine manner via stimulation of pro-angiogenic molecules like VEGF (102).
Early endothelial lineage specification is also controlled by members of the BMP family. In vitro cell culture models using murine embryonic stem (ES) cells to differentiate them towards the endothelial lineage revealed BMP4 to be indispensable for the generation of VEGFR positive embryonic endothelial progenitor cells (EPCs) (103). More recently, similar experiments using human ES cells came to the same conclusion. BMP4 was shown to signal downstream of Indian Hedgehog and to be essential for promoting endothelial cell differentiation from human ES cells (104). Even in a subgroup of EPCs derived from adult peripheral blood expression of BMP2/4 was observed and also implicated to be in conjunction with the capacity to promote neoangiogenesis (105).
More insights came from gene deletion studies in mice. Mice lacking BMP4 die around E7.5 with defects in mesoderm formation and patterning. Some embryos survive this developmental stage; however, they still die at E9.5 and show a vascular phenotype with a reduced number of blood islands (106). Accordingly, mice lacking either of the BMP type I receptors (Alk2 or Alk3) or BMPRII fail to complete gastrulation and die around the same time (E7.5.– E9.5) (107–109). Likewise, deletion of the intracellular effectors Smads display varying degrees of defects in vascular development. Targeted inactivation of Smad1 in mice results in defects in chorioallantoic fusion and embryos die between E9.5.– E10.5 (110, 111). Though overall vasculogenesis appears to be normal, some Smad1 deficient embryos show defects in yolk sac angiogenesis (111). Smad5 deficient and conditional endothelial specific Co-Smad4 deficient mice are embryonic lethal around midgestation due to vascular defects in the embryo proper and in the yolk sac (112–114). Interestingly, in these embryos vasculogenesis is effectively initiated in both the yolk sac and the embryo, but induction of angiogenesis fails (113, 114). Collectively, mice deficient for BMP ligands or receptors demonstrate the importance of BMP mediated signals in early embryonic development, but due to early embryonic lethality they do not yet allow full investigation of vasculogenesis or even angiogenesis. Of interest BMPER (BMP endothelial progenitor cell-derived regulator) is a BMP modulator that has been identified in a screen of VEGFR2 positive embryonal EPCs and therefore seems to be involved in endothelial cell lineage commitment and/or vasculogenesis (115). In addition, BMPER has been shown to enhance concentration-dependent angiogenic sprouting of endothelial cells (116), and loss of BMPER in the zebrafish model disturbed intersomitic vessel growth (117). These results point towards the necessity of a strict control and fine-tuning of the BMP signaling pathway to maintain proper blood vessel formation.
5.4. Vessel guidance molecules
An emerging vessel sprout needs to know in which direction it has to grow. Therefore vessel guidance is nessecary. Like the vascular system, the nervous system forms a complex network. Thus, it is not surprising that molecules which participate in axonal guidance are also found to be involved in EC sprouting (118, 119). Prominent protagonists of such proteins are the erythropoietin-producing human hepatocellular carcinoma (Eph) receptors, which are also members of the RTK family and their corresponding eph family receptor interacting proteins (ephrin ligands). Originally, Eph receptors and ephrin ligands were described as neuron-specific molecules that mediate attractive or repulsive guidance signals on growing neurons (120). However, EphrinB2 and EphB4 have been shown to be expressed in ECs and are key regulators of arteriovenous specification. Both EphrinB2 (121) or EphB4 (122) deficient mice die around embryonic day 10.5 displaying defects in vascular remodeling.
Other proteins involved in axonal guidance that are also important in vascular development are the Delta-like-4 (DLL4)/Notch system (123, 124), semaphorins/neuropilins/plexins (125, 126), Netrin/uncoordinated-5 (UNC5) (127) and Slit/round-about (ROBO) receptor (128, 129) (Table1).
Recent studies demonstrate that Notch receptors and their Delta-like-4 (DLL4) ligand coordinate tip/stalk cell behavior. DLL4 is prominently expressed in tip cells, whereas Notch1 is found in stalk cells. Disruption of the Notch signaling pathway leads to an increase in tip cells and massive endothelial cell sprouting and branching, indicating that Notch signaling is necessary for the stalk cell phenotype (124, 130). Notch signaling represses VEGFR2 expression in stalk cells and leads to proliferation of ECs in response to VEGFA stimulation instead of migration as it does in tip cells. Discrimination of tip versus stalk cell fate seems to be dependent on the amount of DLL4 expression induced by VEGFA. Cells with high DLL4 expression repress the tip cell phenotype of neighboring cells and are thus determined to be stalk cells (123, 124, 130, 131). Of interest, neuropilins (Nrp) also act as VEGFR co-receptors and enhance the affinity of VEGF to VEGFR2 and subsequent downstream signaling pathways. Furthermore, Nrp1 is specifically expressed on arteries and Nrp2 on veins (132–134).
Very recently two independent research groups reported Ephb2 to be not only involved in arteriovenous specification but also in angiogenic sprouting. Ephb2 has been shown to act as a positive regulator of VEGFR endocytosis, which then activates the downstream signaling that is required for VEGF-induced tip cell filopodia (135, 136).
5.5. Transcription factors
Whereas interaction of numerous growth factors, receptors and signaling pathways have been identified to be crucial for the process of vasculogenesis and angiogenesis, the transcriptional regulation of angiogenic molecules is less explored. The same is true regarding the impact of cytokines on regulation of transcription factors (TFs) in endothelial cells (137). A plethora of TFs seem to have important roles for the differentiation of endothelial cells and for the formation of the vasculature; members of the ETS family have a central role in this process. The other important families are the hypoxia-inducible factor (HIF), the forkhead box (FOX), the GATA, the sry-related HMG box (SOX), the homeobox (HOX) and finally the kruepple-like factor (KLF) transcription factors (138–142).
So far, ETS transcription factors have appeared to be central regulators of endothelial gene expression because ETS motifs are present in virtually all analyzed endothelial promoters and enhancers including VEGFR1, VEGFR2, Tie1 and Tie2 (137, 143). In addition, expression of ETS family member Fli-1 is detected in very early precursor cells of the haematopoietic and endothelial lineages and seems to be upstream of the TAL1/SCL and GATA2, which are known to be required for haemangioblast formation and VEGFR2 gene expression (144–146). To achieve endothelial-specific gene expression - as for example for VEGFR2 - different transcription factors have to act together in a complex manner. Besides ETS, Tal1 and GATA2, others like FOXH1 (147), HIF2-alpha (148), KLF2 (149) and HOXB5 (150) are known to participate in the regulation of promoter and enhancer regions of the vegfr2 gene.
Members of the HIF family are activated by the potent stimulus of hypoxia, which is known to be an important regulator of angiogenesis. Activated HIFs stimulate the expression of several angiogenic molecules, the most important being VEGF (151).
Endothelial cells express several members of the Fox transcription factor family including FOXC1/2, FOXF1, FOXH1 and FOXO1/3/4 and deletion studies in mice demonstrated severe defects in vascular development for all of them (reviewed in (140)). In addition to early functions in endothelial development, FoxC1 and FoxC2 also play an important role in arteriovenosus differentiation.
Interestingly, FOXO1-deficient mice die during embryogenesis and display malformations in major vessels of the embryo and in the yolk sac, whereas FOXO3 and 4 are viable and are grossly indistinguishable from wild type littermates (152). Furthermore, several studies investigated the role of FOXOs on ECs in the postnatal vasculature where overexpression of FOXO1 and FOXO3 in endothelial cells inhibited angiogenesis and silencing of FOXOs resulted in enhanced angiogenic processes (153). Moreover, mice lacking all three FOXOs developed haemangiomas and angiosarcomas, which demonstrated not only the importance of FOXOs for endothelial homeostasis and but also the tumor suppressor potential of FOXOs (154).
Hox proteins interact with numerous regulatory pathways like the FGF or BMP pathway, which are also known to be involved in vascularization (11). Multiple HOX transcription factors are reported to regulate expression of different angiogenesis related molecules: HOXA9 is a direct transactivator of ephb4 and enos (155, 156), HOXB3 stimulates expression of ephrina1 and HOXD3 coordinates expression of integrin alpha5, integrin beta3 and upa (urokinase-type plasminogen activator) and thus activate migration and sprouting of endothelial cells (157, 158). Along the same line of evidence, HOXB5 binds and transactivates the vegfr2 promoter and thereby increases the number of mature ECs (150). Additionally, HOXB5 has an activating effect on EC sprouting and coordinated vascular growth conferred by Ang2 (159). Another HOX-relative is prospero-related homeobox1 (PROX-1), which is a major regulator for lymphatic endothelial cell development (160). This is based on the fact that deletion of PROX-1 in mice results in loss of lymphatic-marker expression as well as the whole lymphatic vasculature (161).
Smad transcription factors were originally identified as a class of proteins that are related to the drosophila mothers against decapentaplegic protein (Mad) and the sma proteins from Caenorhabditis elegans. They are components of the TGF-beta and BMP signaling pathway and mediate signals upon activation through phosphorylation from the receptor into the nucleus. Regarding angiogenesis, Smad5 seems to be indispensable due to the fact that Smad-null embryos reveal distinct defects of the vasculature (112, 113). Likewise, Smad3 is reported to mediate pro-angiogenic effects by stimulating VEGFA expression (162). In contrast, Smad4 and Smad2 have been shown to inhibit angiogenesis by promoting expression of anti-angiogenic factors such as thrombospondin-1. Taken together, Smads display distinct roles in regulating angiogenesis (163).
The inhibitors of DNA binding (ID) 1–3 lack a DNA binding domain and consequently form heterodimers with other transcription factors to regulate gene expression. IDs are expressed during development in endothelial cells but are mostly absent in postnatal vasculature. Also, they are found to be highly expressed in blood vessels of tumors and control the expression of integrin alpha5, integrin beta3 and / or mmp2 (164).
Members of the KLF family are reported to be inducible in response to shear stress or insufficient vascular injury. Klf2 is induced by shear stress and KLF2 deficient mice die at E14.5 due to haemorrhage caused by incorrect vessel stabilization (165). Moreover, KLF2 is reported to possess anti-angiogenic effects by reducing endothelial cell proliferation and migration (149). But in contrast, very recently KLF2 is reported to be associated with the ETS transcription factors ERG and to synergistically activate the transcription of vegfr2 (166).
Other transcription factors are essential for controlling specification of endothelial cells into arterial, venous or lymphatic subtypes. This includes members of the SOX family, the hairy and enhancer of split-related (HEY) transcription factors, and chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII).
5.6. microRNAs
Besides transcriptional control of signaling pathways by transcription factors, the translational control of genes by microRNAs (miRNA) gained broad attention recently. MiRNAs are small non-coding RNAs, which bind imperfectly to the 3’- untranslated regions of different mRNAs and act to fine tune gene expression (167). The key enzymes to process miRNAs, Drosha and Dicer, have been targeted in endothelial cells by RNA interference resulting in decreased angiogenesis in vitro (168, 169). Accordingly, mice carrying a homozygous deletion of the first 2 exons of the Dicer gene die between E12.5–E14.5 displaying defective blood vessels in the yolk sac and in the embryo proper (170).
Of interest, some miRNAs are highly expressed in endothelial cells, namely miR-221/222, miR-21, the let-7 family, the miR-17~92 cluster, the miR-23~24 cluster and the endothelial cell specific miR-126 (168, 169). MiR-126 has been shown to be enriched in VEGFR-2 positive embryonal vascular progenitors as well as in mature endothelial cells (171). Deletion of miR-126 in mice resulted in severe vascular abnormalities: systemic edema and multifocal hemorrhages (172). In vitro or ex vivo experiments revealed miR-126 to be involved in endothelial cell sprouting, migration, proliferation and cytoskeleton organization. Mechanistically the effects of miR-126 in VEGF signaling are due to translational inhibition of negative regulators of the Akt and MAPK signaling pathways PIK3R2 and SPRED1, respectively (171, 172).
Recently, a connection between changes in haemodynamics and miR-126 expression was reported in the zebrafish model. Due to changes in blood flow, expression of KLF2a is increased, which leads to enhanced expression of miR-126. This miRNA is known to decrease expression of SPRED1, an inhibitor of VEGF signaling. Thus, miR-126 facilitates enhanced VEGF signaling due to changes in haemodynamics (173).
6. ARTERIOVENOUS DIFFERENTIATION
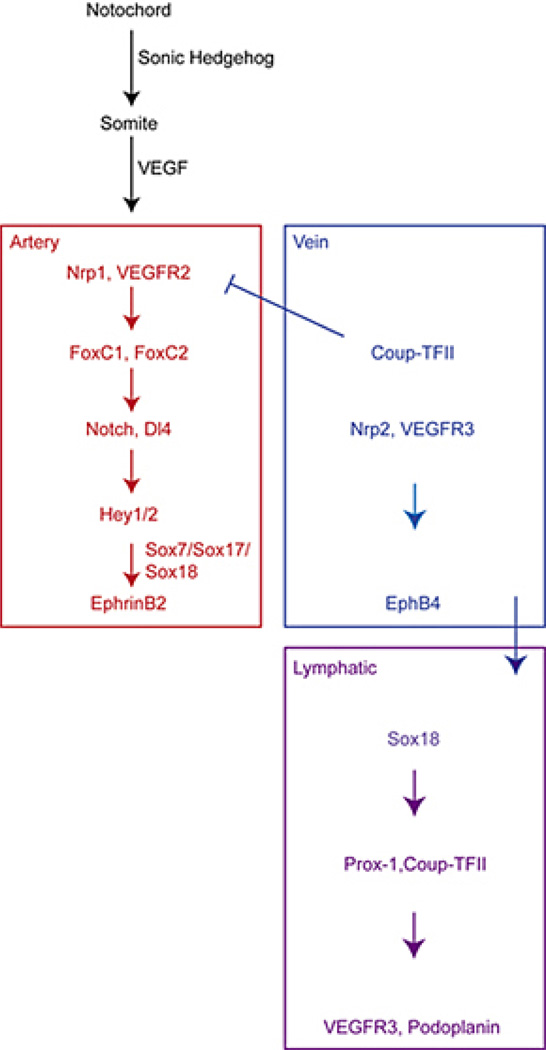
Arteries and veins are formed during remodeling processes of the primary capillary plexus in the early embryo. EphrinB2 is uniquely expressed by arteries and EphB4 by veins (121). In the past, the differentiation into arteries and veins was assumed to be induced by hemodynamic forces, but studies in zebrafish and mouse model systems have uncovered genetically predetermined events before the onset of blood flow (see Figure 2) (174). However, as known from coronary artery bypass grafting where veins are grafted into the arterial circulation and acquire arterial-like properties, there is plasticity of the vascular wall to changes of microenvironment and of blood pressure and flow (5).
Figure 2.
Arterial-venous and lymphatic specification in vertebrate embryo. Due to secretion of sonic hedgehog from the notochord VEGF expression is induced in adjacent somites. In nearby ECs VEGF leads to FoxC1 and FoxC2 mediated upregulation of components of the Notch signalling pathway (Notch-1 and DLL4). Thereupon Hey1/2 are expressed which, in addition to Sox7, Sox17 and Sox18, lead to arterial specification and EphrinB2 marker expression. COUP-TFII is responsible for suppression of Nrp1 expression and inhibits Notch signalling which leads to venous differentiation with the marker expression of Nrp2 and EphB4. Later on some venous ECs of the anterior cardinal vein begin to express Sox18 that precedes expression of Prox-1 and COUP-TFII. Prox-1 induces expression of lymphatic markers like podoplanin and is the major mediator for LECs specification. Modified from (175).
Binding of EphrinB2 to its receptor EphB4 requires cell-cell contact because both are membrane bound molecules. Interestingly, Ephrin ligand and Eph receptors are able to signal bidirectional (forward: EphrinB - EphB and reverse: EphB - EphrinB). This interaction is essential to establish arterial-venous boundaries and proper vascular development. To support this notion gene deletion studies in mice revealed embryonic death at E9.5 due to failure in vascular remodeling (175). Several genes act upstream of EphrinB2 and EphB4 to establish arteriovenous differentiation (see also Figure 2). Sonic hedgehog is secreted by the notochord and induces arterial differentiation via expression of VEGF in nearby somites (176). ECs cultured in the presence of VEGF showed an upregulation of components of the Notch signaling pathway and subsequently of Hey1/2 (177). Hey 1/2 together with Sox7, Sox17 and Sox18 lead to arterial marker expression (EphrinB2 and neuropilin1) and accordingly arterial specification. Loss of any component of the Notch pathway in zebrafish and mice results in arteriovenous malformations indicating the importance of Notch signaling in arterial specification (178).
For venous specification, expression of Coup-TFII is indispensable because it inhibits Notch signaling and expression of Nrp1 and therefore arterial specification. Furthermore, it induces expression of venous markers (EphB4 and Nrp2) and thus venous specification (175).
The development of coronary arteries is still under debate. Early on observations of anatomical studies in humans suggested that they emerge from the aorta (179). However, chimeric chick-quail experiments had come to the conclusion that endothelial cells of the coronary arteries arise through differentiation of the proepicardium (180, 181). In contrast, lineage-tracing experiments in mice found only very few ECs that differentiate from the proepicardium (182, 183). Very recently Red-Horse et al. showed by histological and clonal analysis in mice that coronary arteries develop from differentiated venous ECs in the sinus venosus and from the endocardium (184). Interestingly, quite similar observations have been made for the development of the lymphatic vasculature. Subsequent to arterial-venous differentiation, lymphatic specification begins with the expression of SOX18 preceding PROX-1 in some ECs of the anterior cardinal veins. Overexpression of SOX18 in cultured ECs results in increased expression of lymphatic markers including PROX-1 (185). PROX-1 itself is essential for lymphatic development because inactivation in mice results in loss of lymphatic markers and lack of lymphatic vasculature (Figure 2) (161).
Besides arterial-venous differentiation further pruning and remodeling of the vascular tree involves vessel regression and tissue specialization to build a full functional circulatory vasculature. ECs are a quite heterogeneous cell population that must adapt to the underlying tissue and thus display organ specificity (4, 5).
7. CONCLUDING REMARKS
For a long time the VEGF signaling pathway inherited the key position for vascular development. However, as we discussed above many other pathways regulate or are controlled by the VEGF pathway. Accordingly, an interacting network of signal transduction governs the outcome for further blood vessel development, homeostasis or even regression of existing blood vessels. Furthermore, the discovery of microRNAs as regulators of gene expression adds an additional element to fine tune signals from different pathways. In the future, it will be interesting to see how the orchestra of vascular signaling pathways assemble, and also how they will play together during embryogenesis.
ACKNOWLEDGEMENTS
We thank Sheena Kinniry for critical reading the manuscript. This work was supported by DFG Mo973/5-1 and DFG Mo973/6-1 to M.M.
Abbreviations
- AGM
aorta-gonad-mesonephros
- Alk
activin receptor-like Kinase
- Ang
angiopoetins
- BMP
bone morphogenetic protein
- BMPER
BMP endothelial cell precursor-derived regulator
- BMPRIa/b
BMP-type Ia/b receptor
- co-Smad
common mediator Smad
- COUP-TFII
chicken ovalbumin upstream promoter-transcription factor II
- DLL4
delta-like-4
- EC
endothelial cells
- ECM
extracellular matrix
- EPC
endothelial progenitor cell
- Eph
erythropoietin-producing human hepatocellular carcinoma receptor
- Erk
extracellular-signal regulated kinase
- ESC
embryonic stem cell
- FGF/R
fibroblast growth factor/ receptor
- FOX
forkhead box
- HEY
hairy and enhancer of split-related
- HIF
hypoxia-inducible factor
- HOX
homeobox
- HSPG
heparan sulphate proteoglycans
- ID
inhibitors of DNA binding
- I-Smad
inhibitory Smads
- KLF
kruepple-like factor
- MAPK
mitogen-activated protein kinases
- miRNA
micro Ribonucleic acid
- MMP
matrix metalloproteinase
- Nrp
neuropilin
- PDGF/R
platelet-derived growth factor/ receptor
- PI3K
phosphatidylinositol-3-kinase
- PLGF
placental growth factor
- PROX-1
prospero-related homeobox1
- ROBO
round-about receptor
- R-Smad
receptor-regulated Smad
- RTK
receptor tyrosine kinase
- Shh
sonic hedgehog
- Smad
sma proteins from Caenorhabditis elegans and drosophila mothers against decapentaplegic protein (MAD)
- SMC
smooth muscle cell
- SOX
sry related HMG box
- TF
transcription factor
- TGF-beta/R
transforming growth factor-beta/ receptor
- Tie
tyrosine kinase with Ig and EGF homology
- UNC5
uncoordinated-5
- VE-cadherin
vascular endothelial-cadherin
- VEGF/R
vascular endothelial growth factor/ receptor
REFERENCES
- 1.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 2.Fraisl P, Mazzone M, Schmidt T, Carmeliet P. Regulation of angiogenesis by oxygen and metabolism. Dev Cell. 2009;16:167–179. doi: 10.1016/j.devcel.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Brown A, Barnes J. William Harvey (1578–1657) and Marcello Malpighi (1628–1694): linked in blood, paralleled in life. Adler Mus Bull. 1994;20:14–23. [PubMed] [Google Scholar]
- 4.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 5.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 7.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 8.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 9.Aird WC. Mechanisms of endothelial cell heterogeneity in health and disease. Circ Res. 2006;98:159–162. doi: 10.1161/01.RES.0000204553.32549.a7. [DOI] [PubMed] [Google Scholar]
- 10.Semenza GL. Vasculogenesis, angiogenesis, and arteriogenesis: mechanisms of blood vessel formation and remodeling. J Cell Biochem. 2007;102:840–847. doi: 10.1002/jcb.21523. [DOI] [PubMed] [Google Scholar]
- 11.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 12.Rossant J, Howard L. Signaling pathways in vascular development. Annu Rev Cell Dev Biol. 2002;18:541–573. doi: 10.1146/annurev.cellbio.18.012502.105825. [DOI] [PubMed] [Google Scholar]
- 13.Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramirez-Bergeron DL, Runge A, Dahl KD, Fehling HJ, Keller G, Simon MC. Hypoxia affects mesoderm and enhances hemangioblast specification during early development. Development. 2004;131:4623–4634. doi: 10.1242/dev.01310. [DOI] [PubMed] [Google Scholar]
- 15.Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones EA, Baron MH, Fraser SE, Dickinson ME. Measuring hemodynamic changes during mammalian development. Am J Physiol Heart Circ Physiol. 2004;287:H1561–H1569. doi: 10.1152/ajpheart.00081.2004. [DOI] [PubMed] [Google Scholar]
- 17.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 18.Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 19.Isogai S, Lawson ND, Torrealday S, Horiguchi M, Weinstein BM. Angiogenic network formation in the developing vertebrate trunk. Development. 2003;130:5281–5290. doi: 10.1242/dev.00733. [DOI] [PubMed] [Google Scholar]
- 20.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 21.Ferkowicz MJ, Yoder MC. Blood island formation: longstanding observations and modern interpretations. Exp Hematol. 2005;33:1041–1047. doi: 10.1016/j.exphem.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Drake CJ, Fleming PA. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood. 2000;95:1671–1679. [PubMed] [Google Scholar]
- 23.Argraves WS, Drake CJ. Genes critical to vasculogenesis as defined by systematic analysis of vascular defects in knockout mice. Anat Rec A Discov Mol Cell Evol Biol. 2005;286:875–884. doi: 10.1002/ar.a.20232. [DOI] [PubMed] [Google Scholar]
- 24.Downs KM, Gifford S, Blahnik M, Gardner RL. Vascularization in the murine allantois occurs by vasculogenesis without accompanying erythropoiesis. Development. 1998;125:4507–4520. doi: 10.1242/dev.125.22.4507. [DOI] [PubMed] [Google Scholar]
- 25.Caprioli A, Jaffredo T, Gautier R, Dubourg C, Dieterlen-Lievre F. Blood-borne seeding by hematopoietic and endothelial precursors from the allantois. Proc Natl Acad Sci U S A. 1998;95:1641–1646. doi: 10.1073/pnas.95.4.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1993;118:489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- 27.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 28.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 29.Risau W, Sariola H, Zerwes HG, Sasse J, Ekblom P, Kemler R, Doetschman T. Vasculogenesis and angiogenesis in embryonic-stem-cell-derived embryoid bodies. Development. 1988;102:471–478. doi: 10.1242/dev.102.3.471. [DOI] [PubMed] [Google Scholar]
- 30.Sabin FR. Preliminary note on the differentiation of angioblasts and the method by which they produce blood-vessels, blood plasma, and red blood-cells as seen in the living chick. Anat Rec. 1917;13:19–204. doi: 10.1089/152581602753448496. [DOI] [PubMed] [Google Scholar]
- 31.Murray PDF. The Development in vitro of the Blood of the Early Chick Embryo. Proceedings of the Royal Society of London. Series B, Biological Science. 1932;111:497–521. [Google Scholar]
- 32.Jin SW, Patterson C. The Opening Act. Vasculogenesis and the Origins of Circulation. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.107.161539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shalaby F, Ho J, Stanford WL, Fischer KD, Schuh AC, Schwartz L, Bernstein A, Rossant J. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 34.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 35.Vogeli KM, Jin SW, Martin GR, Stainier DY. A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature. 2006;443:337–339. doi: 10.1038/nature05045. [DOI] [PubMed] [Google Scholar]
- 36.Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lievre F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998;125:4575–4583. doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- 37.Nishikawa SI, Nishikawa S, Kawamoto H, Yoshida H, Kizumoto M, Kataoka H, Katsura Y. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity. 1998;8:761–769. doi: 10.1016/s1074-7613(00)80581-6. [DOI] [PubMed] [Google Scholar]
- 38.North TE, de Bruijn MF, Stacy T, Talebian L, Lind E, Robin C, Binder M, Dzierzak E, Speck NA. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- 39.Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009 doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- 41.Sadlon TJ, Lewis ID, D'Andrea RJ. BMP4: its role in development of the hematopoietic system and potential as a hematopoietic growth factor. Stem Cells. 2004;22:457–474. doi: 10.1634/stemcells.22-4-457. [DOI] [PubMed] [Google Scholar]
- 42.Ishii Y, Langberg J, Rosborough K, Mikawa T. Endothelial cell lineages of the heart. Cell Tissue Res. 2009;335:67–73. doi: 10.1007/s00441-008-0663-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 44.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 45.Burri PH, Hlushchuk R, Djonov V. Intussusceptive angiogenesis: its emergence, its characteristics, and its significance. Dev Dyn. 2004;231:474–488. doi: 10.1002/dvdy.20184. [DOI] [PubMed] [Google Scholar]
- 46.Patan S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J Neurooncol. 2000;50:1–15. doi: 10.1023/a:1006493130855. [DOI] [PubMed] [Google Scholar]
- 47.Djonov V, Baum O, Burri PH. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 2003;314:107–117. doi: 10.1007/s00441-003-0784-3. [DOI] [PubMed] [Google Scholar]
- 48.Kurz H, Burri PH, Djonov VG. Angiogenesis and vascular remodeling by intussusception: from form to function. News Physiol Sci. 2003;18:65–70. doi: 10.1152/nips.01417.2002. [DOI] [PubMed] [Google Scholar]
- 49.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 50.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.ten Dijke P, Arthur HM. Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007;8:857–869. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- 52.Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 53.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tam BY, Wei K, Rudge JS, Hoffman J, Holash J, Park SK, Yuan J, Hefner C, Chartier C, Lee JS, Jiang S, Nayak NR, Kuypers FA, Ma L, Sundram U, Wu G, Garcia JA, Schrier SL, Maher JJ, Johnson RS, Yancopoulos GD, Mulligan RC, Kuo CJ. VEGF modulates erythropoiesis through regulation of adult hepatic erythropoietin synthesis. Nat Med. 2006;12:793–800. doi: 10.1038/nm1428. [DOI] [PubMed] [Google Scholar]
- 55.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 56.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 57.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 58.Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- 59.Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Tashiro F, Niwa H, Miyazaki Ji J, Hirota S, Kitamura Y, Kitsukawa T, Fujisawa H, Klagsbrun M, Hori M. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci U S A. 2002;99:3657–3662. doi: 10.1073/pnas.022017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kearney JB, Ambler CA, Monaco KA, Johnson N, Rapoport RG, Bautch VL. Vascular endothelial growth factor receptor Flt-1 negatively regulates developmental blood vessel formation by modulating endothelial cell division. Blood. 2002;99:2397–2407. doi: 10.1182/blood.v99.7.2397. [DOI] [PubMed] [Google Scholar]
- 62.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 63.Hiratsuka S, Nakao K, Nakamura K, Katsuki M, Maru Y, Shibuya M. Membrane fixation of vascular endothelial growth factor receptor 1 ligand-binding domain is important for vasculogenesis and angiogenesis in mice. Mol Cell Biol. 2005;25:346–354. doi: 10.1128/MCB.25.1.346-354.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fong GH, Zhang L, Bryce DM, Peng J. Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development. 1999;126:3015–3025. doi: 10.1242/dev.126.13.3015. [DOI] [PubMed] [Google Scholar]
- 65.Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, Kroll J, Plaisance S, De Mol M, Bono F, Kliche S, Fellbrich G, Ballmer-Hofer K, Maglione D, Mayr-Beyrle U, Dewerchin M, Dombrowski S, Stanimirovic D, Van Hummelen P, Dehio C, Hicklin DJ, Persico G, Herbert JM, Communi D, Shibuya M, Collen D, Conway EM, Carmeliet P. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003;9:936–943. doi: 10.1038/nm884. [DOI] [PubMed] [Google Scholar]
- 66.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 67.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 68.Grazia Lampugnani M, Zanetti A, Corada M, Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A, Kemler R, Daniel TO, Dejana E. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol. 2003;161:793–804. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shibuya M. Vascular endothelial growth factor-dependent and -independent regulation of angiogenesis. BMB Rep. 2008;41:278–286. doi: 10.5483/bmbrep.2008.41.4.278. [DOI] [PubMed] [Google Scholar]
- 70.Karkkainen MJ, Makinen T, Alitalo K. Lymphatic endothelium: a new frontier of metastasis research. Nat Cell Biol. 2002;4:E2–E5. doi: 10.1038/ncb0102-e2. [DOI] [PubMed] [Google Scholar]
- 71.Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- 72.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 73.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 74.Puri MC, Rossant J, Alitalo K, Bernstein A, Partanen J. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. Embo J. 1995;14:5884–5891. doi: 10.1002/j.1460-2075.1995.tb00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 76.Pitera JE, Woolf AS, Gale NW, Yancopoulos GD, Yuan HT. Dysmorphogenesis of kidney cortical peritubular capillaries in angiopoietin-2-deficient mice. Am J Pathol. 2004;165:1895–1906. doi: 10.1016/S0002-9440(10)63242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hackett SF, Ozaki H, Strauss RW, Wahlin K, Suri C, Maisonpierre P, Yancopoulos G, Campochiaro PA. Angiopoietin 2 expression in the retina: upregulation during physiologic and pathologic neovascularization. J Cell Physiol. 2000;184:275–284. doi: 10.1002/1097-4652(200009)184:3<275::AID-JCP1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 78.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 79.Reiss Y, Droste J, Heil M, Tribulova S, Schmidt MH, Schaper W, Dumont DJ, Plate KH. Angiopoietin-2 impairs revascularization after limb ischemia. Circ Res. 2007;101:88–96. doi: 10.1161/CIRCRESAHA.106.143594. [DOI] [PubMed] [Google Scholar]
- 80.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 81.Fiedler U, Krissl T, Koidl S, Weiss C, Koblizek T, Deutsch U, Martiny-Baron G, Marme D, Augustin HG. Angiopoietin-1 and angiopoietin-2 share the same binding domains in the Tie-2 receptor involving the first Ig-like loop and the epidermal growth factor-like repeats. J Biol Chem. 2003;278:1721–1727. doi: 10.1074/jbc.M208550200. [DOI] [PubMed] [Google Scholar]
- 82.Macdonald PR, Progias P, Ciani B, Patel S, Mayer U, Steinmetz MO, Kammerer RA. Structure of the extracellular domain of Tie receptor tyrosine kinases and localization of the angiopoietin-binding epitope. J Biol Chem. 2006;281:28408–28414. doi: 10.1074/jbc.M605219200. [DOI] [PubMed] [Google Scholar]
- 83.Marron MB, Hughes DP, Edge MD, Forder CL, Brindle NP. Evidence for heterotypic interaction between the receptor tyrosine kinases TIE-1 and TIE-2. J Biol Chem. 2000;275:39741–39746. doi: 10.1074/jbc.M007189200. [DOI] [PubMed] [Google Scholar]
- 84.Tsiamis AC, Morris PN, Marron MB, Brindle NP. Vascular endothelial growth factor modulates the Tie-2:Tie-1 receptor complex. Microvasc Res. 2002;63:149–158. doi: 10.1006/mvre.2001.2377. [DOI] [PubMed] [Google Scholar]
- 85.Marron MB, Singh H, Tahir TA, Kavumkal J, Kim HZ, Koh GY, Brindle NP. Regulated proteolytic processing of Tie1 modulates ligand responsiveness of the receptor-tyrosine kinase Tie2. J Biol Chem. 2007;282:30509–30517. doi: 10.1074/jbc.M702535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jones N, Iljin K, Dumont DJ, Alitalo K. Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat Rev Mol Cell Biol. 2001;2:257–267. doi: 10.1038/35067005. [DOI] [PubMed] [Google Scholar]
- 87.Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12:235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 88.Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D, Deutsch U, Koh GY, Olsen BR, Alitalo K. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol. 2008;10:527–537. doi: 10.1038/ncb1715. [DOI] [PubMed] [Google Scholar]
- 89.Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, Shibuya M, Takakura N, Koh GY, Mochizuki N. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008;10:513–526. doi: 10.1038/ncb1714. [DOI] [PubMed] [Google Scholar]
- 90.Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005;118:771–780. doi: 10.1242/jcs.01653. [DOI] [PubMed] [Google Scholar]
- 91.Eklund L, Olsen BR. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp Cell Res. 2006;312:630–641. doi: 10.1016/j.yexcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 92.Cao Y, Cao R, Hedlund EM. R Regulation of tumor angiogenesis and metastasis by FGF and PDGF signaling pathways. J Mol Med. 2008;86:785–789. doi: 10.1007/s00109-008-0337-z. [DOI] [PubMed] [Google Scholar]
- 93.Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- 94.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- 95.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 96.Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen S, Lechleider RJ. Transforming growth factor-beta-induced differentiation of smooth muscle from a neural crest stem cell line. Circ Res. 2004;94:1195–1202. doi: 10.1161/01.RES.0000126897.41658.81. [DOI] [PubMed] [Google Scholar]
- 98.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 99.Goumans MJ, Liu Z, ten Dijke P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–127. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- 100.Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development. 1995;121:1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- 101.Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, Li E. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A. 2000;97:2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deckers MM, van Bezooijen RL, van der Horst G, Hoogendam J, van Der Bent C, Papapoulos SE, Lowik CW. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002;143:1545–1553. doi: 10.1210/endo.143.4.8719. [DOI] [PubMed] [Google Scholar]
- 103.Park C, Afrikanova I, Chung YS, Zhang WJ, Arentson E, Fong Gh G, Rosendahl A, Choi K. A hierarchical order of factors in the generation of FLK1- and SCL-expressing hematopoietic and endothelial progenitors from embryonic stem cells. Development. 2004;131:2749–2762. doi: 10.1242/dev.01130. [DOI] [PubMed] [Google Scholar]
- 104.Little SC, Mullins MC. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat Cell Biol. 2009 doi: 10.1038/ncb1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smadja DM, Bieche I, Silvestre JS, Germain S, Cornet A, Laurendeau I, Duong-Van-Huyen JP, Emmerich J, Vidaud M, Aiach M, Gaussem P. Bone morphogenetic proteins 2 and 4 are selectively expressed by late outgrowth endothelial progenitor cells and promote neoangiogenesis. Arterioscler Thromb Vasc Biol. 2008;28:2137–2143. doi: 10.1161/ATVBAHA.108.168815. [DOI] [PubMed] [Google Scholar]
- 106.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 107.Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- 108.Gu Z, Reynolds EM, Song J, Lei H, Feijen A, Yu L, He W, MacLaughlin DT, van den Eijnden-van Raaij J, Donahoe PK, Li E. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development. 1999;126:2551–2561. doi: 10.1242/dev.126.11.2551. [DOI] [PubMed] [Google Scholar]
- 109.Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol. 2000;221:249–258. doi: 10.1006/dbio.2000.9670. [DOI] [PubMed] [Google Scholar]
- 110.Tremblay KD, Dunn NR, Robertson EJ. Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development. 2001;128:3609–3621. doi: 10.1242/dev.128.18.3609. [DOI] [PubMed] [Google Scholar]
- 111.Lechleider RJ, Ryan JL, Garrett L, Eng C, Deng C, Wynshaw-Boris A, Roberts AB. Targeted mutagenesis of Smad1 reveals an essential role in chorioallantoic fusion. Dev Biol. 2001;240:157–167. doi: 10.1006/dbio.2001.0469. [DOI] [PubMed] [Google Scholar]
- 112.Chang H, Huylebroeck D, Verschueren K, Guo Q, Matzuk MM, Zwijsen A. Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development. 1999;126:1631–1642. doi: 10.1242/dev.126.8.1631. [DOI] [PubMed] [Google Scholar]
- 113.Yang X, Castilla LH, Xu X, Li C, Gotay J, Weinstein M, Liu PP, Deng CX. Angiogenesis defects and mesenchymal apoptosis in mice lacking SMAD5. Development. 1999;126:1571–1580. doi: 10.1242/dev.126.8.1571. [DOI] [PubMed] [Google Scholar]
- 114.Lan Y, Liu B, Yao H, Li F, Weng T, Yang G, Li W, Cheng X, Mao N, Yang X. Essential role of endothelial Smad4 in vascular remodeling and integrity. Mol Cell Biol. 2007;27:7683–7692. doi: 10.1128/MCB.00577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moser M, Binder O, Wu Y, Aitsebaomo J, Ren R, Bode C, Bautch VL, Conlon FL, Patterson C. BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Mol Cell Biol. 2003;23:5664–5679. doi: 10.1128/MCB.23.16.5664-5679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heinke J, Wehofsits L, Zhou Q, Zoeller C, Baar KM, Helbing T, Laib A, Augustin H, Bode C, Patterson C, Moser M. BMPER Is an Endothelial Cell Regulator and Controls Bone Morphogenetic Protein-4-Dependent Angiogenesis. Circ Res. 2008 doi: 10.1161/CIRCRESAHA.108.178434. [DOI] [PubMed] [Google Scholar]
- 117.Moser M, Yu Q, Bode C, Xiong JW, Patterson C. BMPER is a conserved regulator of hematopoietic and vascular development in zebrafish. J Mol Cell Cardiol. 2007;43:243–253. doi: 10.1016/j.yjmcc.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 119.Eichmann A, Makinen T, Alitalo K. Neural guidance molecules regulate vascular remodeling and vessel navigation. Genes Dev. 2005;19:1013–1021. doi: 10.1101/gad.1305405. [DOI] [PubMed] [Google Scholar]
- 120.Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu Rev Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- 121.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 122.Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell. 1999;4:403–414. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- 123.Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci U S A. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. DLL4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 125.Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev Cell. 2004;7:107–116. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 126.Torres-Vazquez J, Gitler AD, Fraser SD, Berk JD, Van NP, Fishman MC, Childs S, Epstein JA, Weinstein BM. Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev Cell. 2004;7:117–123. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 127.Wilson BD, Ii M, Park KW, Suli A, Sorensen LK, Larrieu-Lahargue F, Urness LD, Suh W, Asai J, Kock GA, Thorne T, Silver M, Thomas KR, Chien CB, Losordo DW, Li DY. Netrins promote developmental and therapeutic angiogenesis. Science. 2006;313:640–644. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Park KW, Morrison CM, Sorensen LK, Jones CA, Rao Y, Chien CB, Wu JY, Urness LD, Li DY. Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev Biol. 2003;261:251–267. doi: 10.1016/s0012-1606(03)00258-6. [DOI] [PubMed] [Google Scholar]
- 129.Bedell VM, Yeo SY, Park KW, Chung J, Seth P, Shivalingappa V, Zhao J, Obara T, Sukhatme VP, Drummond IA, Li DY, Ramchandran R. roundabout4 is essential for angiogenesis in vivo. Proc Natl Acad Sci U S A. 2005;102:6373–6378. doi: 10.1073/pnas.0408318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sainson RC, Aoto J, Nakatsu MN, Holderfield M, Conn E, Koller E, Hughes CC. Cell-autonomous notch signaling regulates endothelial cell branching and proliferation during vascular tubulogenesis. Faseb J. 2005;19:1027–1029. doi: 10.1096/fj.04-3172fje. [DOI] [PubMed] [Google Scholar]
- 131.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (DLL4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 133.Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G. Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165 (corrected) J Biol Chem. 2000;275:18040–18045. doi: 10.1074/jbc.M909259199. [DOI] [PubMed] [Google Scholar]
- 134.Kawamura H, Li X, Goishi K, van Meeteren LA, Jakobsson L, Cebe-Suarez S, Shimizu A, Edholm D, Ballmer-Hofer K, Kjellen L, Klagsbrun M, Claesson-Welsh L. Neuropilin-1 in regulation of VEGF-induced activation of p38MAPK and endothelial cell organization. Blood. 2008;112:3638–3649. doi: 10.1182/blood-2007-12-125856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, Acker-Palmer A. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 2010;465:487–491. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- 136.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, Barberis A, Benjamin LE, Makinen T, Nobes CD, Adams RH. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 137.De Val S, Black BL. Transcriptional control of endothelial cell development. Dev Cell. 2009;16:180–195. doi: 10.1016/j.devcel.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.De Val S, Chi NC, Meadows SM, Minovitsky S, Anderson JP, Harris IS, Ehlers ML, Agarwal P, Visel A, Xu SM, Pennacchio LA, Dubchak I, Krieg PA, Stainier DY, Black BL. Combinatorial regulation of endothelial gene expression by ets and forkhead transcription factors. Cell. 2008;135:1053–1064. doi: 10.1016/j.cell.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Oettgen P. Transcriptional regulation of vascular development. Circ Res. 2001;89:380–388. doi: 10.1161/hh1701.095958. [DOI] [PubMed] [Google Scholar]
- 140.Papanicolaou KN, Izumiya Y, Walsh K. Forkhead transcription factors and cardiovascular biology. Circ Res. 2008;102:16–31. doi: 10.1161/CIRCRESAHA.107.164186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gorski DH, Walsh K. The role of homeobox genes in vascular remodeling and angiogenesis. Circ Res. 2000;87:865–872. doi: 10.1161/01.res.87.10.865. [DOI] [PubMed] [Google Scholar]
- 142.Atkins GB, Jain MK. Role of Kruppel-like transcription factors in endothelial biology. Circ Res. 2007;100:1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- 143.Bernat JA, Crawford GE, Ogurtsov AY, Collins FS, Ginsburg D, Kondrashov AS. Distant conserved sequences flanking endothelial-specific promoters contain tissue-specific DNase-hypersensitive sites and over-represented motifs. Hum Mol Genet. 2006;15:2098–2105. doi: 10.1093/hmg/ddl133. [DOI] [PubMed] [Google Scholar]
- 144.Bloor AJ, Sanchez MJ, Green AR, Gottgens B. The role of the stem cell leukemia (SCL) gene in hematopoietic and endothelial lineage specification. J Hematother Stem Cell Res. 2002;11:195–206. doi: 10.1089/152581602753658402. [DOI] [PubMed] [Google Scholar]
- 145.Lugus JJ, Chung YS, Mills JC, Kim SI, Grass J, Kyba M, Doherty JM, Bresnick EH, Choi K. GATA2 functions at multiple steps in hemangioblast development and differentiation. Development. 2007;134:393–405. doi: 10.1242/dev.02731. [DOI] [PubMed] [Google Scholar]
- 146.Kappel A, Schlaeger TM, Flamme I, Orkin SH, Risau W, Breier G. Role of SCL/Tal-1, GATA, and ets transcription factor binding sites for the regulation of flk-1 expression during murine vascular development. Blood. 2000;96:3078–3085. [PubMed] [Google Scholar]
- 147.Choi J, Dong L, Ahn J, Dao D, Hammerschmidt M, Chen JN. FoxH1 negatively modulates flk1 gene expression and vascular formation in zebrafish. Dev Biol. 2007;304:735–744. doi: 10.1016/j.ydbio.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kappel A, Ronicke V, Damert A, Flamme I, Risau W, Breier G. Identification of vascular endothelial growth factor (VEGF) receptor-2 (Flk-1) promoter/enhancer sequences sufficient for angioblast and endothelial cell-specific transcription in transgenic mice. Blood. 1999;93:4284–4292. [PubMed] [Google Scholar]
- 149.Bhattacharya R, Senbanerjee S, Lin Z, Mir S, Hamik A, Wang P, Mukherjee P, Mukhopadhyay D, Jain MK. Inhibition of vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis by the Kruppel-like factor KLF2. J Biol Chem. 2005;280:28848–28851. doi: 10.1074/jbc.C500200200. [DOI] [PubMed] [Google Scholar]
- 150.Wu Y, Moser M, Bautch VL, Patterson C. HoxB5 is an upstream transcriptional switch for differentiation of the vascular endothelium from precursor cells. Mol Cell Biol. 2003;23:5680–5691. doi: 10.1128/MCB.23.16.5680-5691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 152.Hosaka T, Biggs WH, 3rd, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci U S A. 2004;101:2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115:2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bruhl T, Urbich C, Aicher D, Acker-Palmer A, Zeiher AM, Dimmeler S. Homeobox A9 transcriptionally regulates the EphB4 receptor to modulate endothelial cell migration and tube formation. Circ Res. 2004;94:743–751. doi: 10.1161/01.RES.0000120861.27064.09. [DOI] [PubMed] [Google Scholar]
- 156.Rossig L, Urbich C, Bruhl T, Dernbach E, Heeschen C, Chavakis E, Sasaki K, Aicher D, Diehl F, Seeger F, Potente M, Aicher A, Zanetta L, Dejana E, Zeiher AM, Dimmeler S. Histone deacetylase activity is essential for the expression of HoxA9 and for endothelial commitment of progenitor cells. J Exp Med. 2005;201:1825–1835. doi: 10.1084/jem.20042097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Myers C, Charboneau A, Boudreau N. Homeobox B3 promotes capillary morphogenesis and angiogenesis. J Cell Biol. 2000;148:343–351. doi: 10.1083/jcb.148.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Boudreau N, Andrews C, Srebrow A, Ravanpay A, Cheresh DA. Induction of the angiogenic phenotype by Hox D3. J Cell Biol. 1997;139:257–264. doi: 10.1083/jcb.139.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Winnik S, Klinkert M, Kurz H, Zoeller C, Heinke J, Wu Y, Bode C, Patterson C, Moser M. HoxB5 induces endothelial sprouting in vitro and modifies intussusceptive angiogenesis in vivo involving Angiopoietin-2. Cardiovasc Res. 2009 doi: 10.1093/cvr/cvp133. [DOI] [PubMed] [Google Scholar]
- 160.Petrova TV, Makinen T, Makela TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Yla-Herttuala S, Alitalo K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. Embo J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 162.Sanchez-Elsner T, Botella LM, Velasco B, Corbi A, Attisano L, Bernabeu C. Synergistic cooperation between hypoxia and transforming growth factor-beta pathways on human vascular endothelial growth factor gene expression. J Biol Chem. 2001;276:38527–38535. doi: 10.1074/jbc.M104536200. [DOI] [PubMed] [Google Scholar]
- 163.Hamik A, Wang B, Jain MK. Transcriptional regulators of angiogenesis. Arterioscler Thromb Vasc Biol. 2006;26:1936–1947. doi: 10.1161/01.ATV.0000232542.42968.e3. [DOI] [PubMed] [Google Scholar]
- 164.Benezra R, Rafii S, Lyden D. The Id proteins and angiogenesis. Oncogene. 2001;20:8334–8341. doi: 10.1038/sj.onc.1205160. [DOI] [PubMed] [Google Scholar]
- 165.Lee JS, Yu Q, Shin JT, Sebzda E, Bertozzi C, Chen M, Mericko P, Stadtfeld M, Zhou D, Cheng L, Graf T, MacRae CA, Lepore JJ, Lo CW, Kahn ML. Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev Cell. 2006;11:845–857. doi: 10.1016/j.devcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 166.Meadows SM, Salanga MC, Krieg PA. Kruppel-like factor 2 cooperates with the ETS family protein ERG to activate Flk1 expression during vascular development. Development. 2009 doi: 10.1242/dev.029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 168.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 169.Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 170.Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem. 2005;280:9330–9335. doi: 10.1074/jbc.M413394200. [DOI] [PubMed] [Google Scholar]
- 171.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nicoli S, Standley C, Walker P, Hurlstone A, Fogarty KE, Lawson ND. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 2010;464:1196–1200. doi: 10.1038/nature08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Torres-Vazquez J, Kamei M, Weinstein BM. Molecular distinction between arteries and veins. Cell Tissue Res. 2003;314:43–59. doi: 10.1007/s00441-003-0771-8. [DOI] [PubMed] [Google Scholar]
- 175.Aitsebaomo J, Portbury AL, Schisler JC, Patterson C. Brothers and sisters: molecular insights into arterial-venous heterogeneity. Circ Res. 2008;103:929–939. doi: 10.1161/CIRCRESAHA.108.184937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, Shapiro R, Taylor FR, Baker DP, Asahara T, Isner JM. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 177.Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–939. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hofmann JJ, Iruela-Arispe ML. Notch signaling in blood vessels: who is talking to whom about what? Circ Res. 2007;100:1556–1568. doi: 10.1161/01.RES.0000266408.42939.e4. [DOI] [PubMed] [Google Scholar]
- 179.Hutchins GM, Kessler-Hanna A, Moore GW. Development of the coronary arteries in the embryonic human heart. Circulation. 1988;77:1250–1257. doi: 10.1161/01.cir.77.6.1250. [DOI] [PubMed] [Google Scholar]
- 180.Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 181.Manner J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat Rec. 1999;255:212–226. doi: 10.1002/(sici)1097-0185(19990601)255:2<212::aid-ar11>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 182.Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Red-Horse K, Ueno H, Weissman IL, Krasnow MA. Coronary arteries form by developmental reprogramming of venous cells. Nature. 2010;464:549–553. doi: 10.1038/nature08873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Francois M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M, Davidson T, Tutt D, Cheah KS, Stacker SA, Muscat GE, Achen MG, Dejana E, Koopman P. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643–647. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- 186.Carmeliet P, Ng YS, Nuyens D, Theilmeier G, Brusselmans K, Cornelissen I, Ehler E, Kakkar VV, Stalmans I, Mattot V, Perriard JC, Dewerchin M, Flameng W, Nagy A, Lupu F, Moons L, Collen D, D'Amore PA, Shima DT. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med. 1999;5:495–502. doi: 10.1038/8379. [DOI] [PubMed] [Google Scholar]
- 187.Bellomo D, Headrick JP, Silins GU, Paterson CA, Thomas PS, Gartside M, Mould A, Cahill MM, Tonks ID, Grimmond SM, Townson S, Wells C, Little M, Cummings MC, Hayward NK, Kay GF. Mice lacking the vascular endothelial growth factor-B gene (Vegfb) have smaller hearts, dysfunctional coronary vasculature, and impaired recovery from cardiac ischemia. Circ Res. 2000;86:E29–E35. doi: 10.1161/01.res.86.2.e29. [DOI] [PubMed] [Google Scholar]
- 188.Aase K, von Euler G, Li X, Ponten A, Thoren P, Cao R, Cao Y, Olofsson B, Gebre-Medhin S, Pekny M, Alitalo K, Betsholtz C, Eriksson U. Vascular endothelial growth factor-B-deficient mice display an atrial conduction defect. Circulation. 2001;104:358–364. doi: 10.1161/01.cir.104.3.358. [DOI] [PubMed] [Google Scholar]
- 189.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, Vandendriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 190.Luttun A, Brusselmans K, Fukao H, Tjwa M, Ueshima S, Herbert JM, Matsuo O, Collen D, Carmeliet P, Moons L. Loss of placental growth factor protects mice against vascular permeability in pathological conditions. Biochem Biophys Res Commun. 2002;295:428–434. doi: 10.1016/s0006-291x(02)00677-0. [DOI] [PubMed] [Google Scholar]
- 191.Xue Y, Gao X, Lindsell CE, Norton CR, Chang B, Hicks C, Gendron-Maguire M, Rand EB, Weinmaster G, Gridley T. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet. 1999;8:723–730. doi: 10.1093/hmg/8.5.723. [DOI] [PubMed] [Google Scholar]
- 192.Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci U S A. 2004;101:15949–15954. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18:2469–2473. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 195.Limbourg FP, Takeshita K, Radtke F, Bronson RT, Chin MT, Liao JK. Essential role of endothelial Notch1 in angiogenesis. Circulation. 2005;111:1826–1832. doi: 10.1161/01.CIR.0000160870.93058.DD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kokubo H, Miyagawa-Tomita S, Nakazawa M, Saga Y, Johnson RL. Mouse hesr1 and hesr2 genes are redundantly required to mediate Notch signaling in the developing cardiovascular system. Dev Biol. 2005;278:301–309. doi: 10.1016/j.ydbio.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 198.Zhou M, Sutliff RL, Paul RJ, Lorenz JN, Hoying JB, Haudenschild CC, Yin M, Coffin JD, Kong L, Kranias EG, Luo W, Boivin GP, Duffy JJ, Pawlowski SA, Doetschman T. Fibroblast growth factor 2 control of vascular tone. Nat Med. 1998;4:201–207. doi: 10.1038/nm0298-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Deng CX, Wynshaw-Boris A, Shen MM, Daugherty C, Ornitz DM, Leder P. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 1994;8:3045–3057. doi: 10.1101/gad.8.24.3045. [DOI] [PubMed] [Google Scholar]
- 200.Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- 202.Larsson J, Goumans MJ, Sjostrand LJ, van Rooijen MA, Ward D, Leveen P, Xu X, ten Dijke P, Mummery CL, Karlsson S. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. Embo J. 2001;20:1663–1673. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Oshima M, Oshima H, Taketo MM. TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol. 1996;179:297–302. doi: 10.1006/dbio.1996.0259. [DOI] [PubMed] [Google Scholar]
- 204.Urness LD, Sorensen LK, Li DY. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat Genet. 2000;26:328–331. doi: 10.1038/81634. [DOI] [PubMed] [Google Scholar]
- 205.Bourdeau A, Dumont DJ, Letarte M. A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest. 1999;104:1343–1351. doi: 10.1172/JCI8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 207.Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, Charlton R, Parums DV, Jowett T, Marchuk DA, Burn J, Diamond AG. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol. 2000;217:42–53. doi: 10.1006/dbio.1999.9534. [DOI] [PubMed] [Google Scholar]
- 208.Sorensen LK, Brooke BS, Li DY, Urness LD. Loss of distinct arterial and venous boundaries in mice lacking endoglin, a vascular-specific TGFbeta coreceptor. Dev Biol. 2003;261:235–250. doi: 10.1016/s0012-1606(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 209.Liu D, Wang J, Kinzel B, Mueller M, Mao X, Valdez R, Liu Y, Li E. Dosage-dependent requirement of BMP type II receptor for maintenance of vascular integrity. Blood. 2007;110:1502–1510. doi: 10.1182/blood-2006-11-058594. [DOI] [PubMed] [Google Scholar]
- 210.Beppu H, Ichinose F, Kawai N, Jones RC, Yu PB, Zapol WM, Miyazono K, Li E, Bloch KD. BMPR-II heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1241–L1247. doi: 10.1152/ajplung.00239.2004. [DOI] [PubMed] [Google Scholar]
- 211.Yang X, Li C, Xu X, Deng C. The tumor suppressor SMAD4/DPC4 is essential for epiblast proliferation and mesoderm induction in mice. Proc Natl Acad Sci U S A. 1998;95:3667–3672. doi: 10.1073/pnas.95.7.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sirard C, de la Pompa JL, Elia A, Itie A, Mirtsos C, Cheung A, Hahn S, Wakeham A, Schwartz L, Kern SE, Rossant J, Mak TW. The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev. 1998;12:107–119. doi: 10.1101/gad.12.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Byrd N, Becker S, Maye P, Narasimhaiah R, St-Jacques B, Zhang X, McMahon J, McMahon A, Grabel L. Hedgehog is required for murine yolk sac angiogenesis. Development. 2002;129:361–372. doi: 10.1242/dev.129.2.361. [DOI] [PubMed] [Google Scholar]
- 214.Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8:1083–1086. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- 215.Zhang XM, Ramalho-Santos M, McMahon AP. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R asymmetry by the mouse node. Cell. 2001;105:781–792. [PubMed] [Google Scholar]
- 216.Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gerety SS, Anderson DJ. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development. 2002;129:1397–1410. doi: 10.1242/dev.129.6.1397. [DOI] [PubMed] [Google Scholar]
- 218.Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126:4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- 219.Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn. 2004;231:503–509. doi: 10.1002/dvdy.20148. [DOI] [PubMed] [Google Scholar]
- 220.Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yuan L, Moyon D, Pardanaud L, Breant C, Karkkainen MJ, Alitalo K, Eichmann A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129:4797–4806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- 222.Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383:525–528. doi: 10.1038/383525a0. [DOI] [PubMed] [Google Scholar]
- 223.Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, Tessier-Lavigne M, Taniguchi M, Puschel AW, Bussolino F. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- 224.Fiore R, Rahim B, Christoffels VM, Moorman AF, Puschel AW. Inactivation of the Sema5a gene results in embryonic lethality and defective remodeling of the cranial vascular system. Mol Cell Biol. 2005;25:2310–2319. doi: 10.1128/MCB.25.6.2310-2319.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 226.Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, Sugiyama D, Breant C, Claes F, De Smet F, Thomas JL, Autiero M, Carmeliet P, Tessier-Lavigne M, Eichmann A. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–186. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- 227.Navankasattusas S, Whitehead KJ, Suli A, Sorensen LK, Lim AH, Zhao J, Park KW, Wythe JD, Thomas KR, Chien CB, Li DY. The netrin receptor UNC5B promotes angiogenesis in specific vascular beds. Development. 2008;135:659–667. doi: 10.1242/dev.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Robb L, Lyons I, Li R, Hartley L, Kontgen F, Harvey RP, Metcalf D, Begley CG. Absence of yolk sac hematopoiesis from mice with a targeted disruption of the scl gene. Proc Natl Acad Sci U S A. 1995;92:7075–7079. doi: 10.1073/pnas.92.15.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Shivdasani RA, Mayer EL, Orkin SH. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature. 1995;373:432–434. doi: 10.1038/373432a0. [DOI] [PubMed] [Google Scholar]
- 230.Furuyama T, Kitayama K, Shimoda Y, Ogawa M, Sone K, Yoshida-Araki K, Hisatsune H, Nishikawa S, Nakayama K, Nakayama K, Ikeda K, Motoyama N, Mori N. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J Biol Chem. 2004;279:34741–34749. doi: 10.1074/jbc.M314214200. [DOI] [PubMed] [Google Scholar]
- 231.Spyropoulos DD, Pharr PN, Lavenburg KR, Jackers P, Papas TS, Ogawa M, Watson DK. Hemorrhage, impaired hematopoiesis, and lethality in mouse embryos carrying a targeted disruption of the Fli1 transcription factor. Mol Cell Biol. 2000;20:5643–5652. doi: 10.1128/mcb.20.15.5643-5652.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. Embo J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Peng J, Zhang L, Drysdale L, Fong GH. The transcription factor EPAS-1/hypoxia-inducible factor 2alpha plays an important role in vascular remodeling. Proc Natl Acad Sci U S A. 2000;97:8386–8391. doi: 10.1073/pnas.140087397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- 236.Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O'Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, Benezra R. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401:670–677. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- 237.Baudino TA, McKay C, Pendeville-Samain H, Nilsson JA, Maclean KH, White EL, Davis AC, Ihle JN, Cleveland JL. c-Myc is essential for vasculogenesis and angiogenesis during development and tumor progression. Genes Dev. 2002;16:2530–2543. doi: 10.1101/gad.1024602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999;13:1037–1049. doi: 10.1101/gad.13.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]