Abstract
The mechanical properties of healing myocardial infarcts are a critical determinant of pump function and the transition to heart failure. Recent reports suggest that modifying infarct mechanical properties can improve function and limit ventricular remodeling. However, little attempt has been made to identify the specific infarct material properties that would optimize left ventricular (LV) function. We utilized a finite-element model of a large anteroapical infarct in a dog heart to explore a wide range of infarct mechanical properties. Isotropic stiffening of the infarct reduced end-diastolic (EDV) and end-systolic (ESV) volumes, improved LV contractility, but had little effect on stroke volume. A highly anisotropic infarct, with high longitudinal stiffness but low circumferential stiffness coefficients, produced the best stroke volume by increasing diastolic filling, without affecting contractility or ESV. Simulated infarcts in two different locations displayed different transmural strain patterns. Our results suggest that there is a general trade-off between acutely reducing LV size and acutely improving LV pump function, that isotropically stiffening the infarct is not the only option of potential therapeutic interest, and that customizing therapies for different infarct locations may be important. Our model results should provide guidance for design and development of therapies to improve LV function by modifying infarct mechanical properties.
Keywords: anisotropy, finite-element model, infarct mechanics, ischemia, cardiac restraint device, polymer injection
Introduction
Each year, nearly 610,000 Americans experience a first myocardial infarction (MI); of these, the majority (82% of men and 77% of women) survive at least one year. In addition, each year 325,000 Americans experience a recurrent MI.[1] As a result, management of patients with a healing or healed MI is an increasingly important component of clinical cardiology practice.
Unlike many other tissues in the body, heart muscle cannot regenerate. Once myocardium dies during a heart attack, it is gradually replaced by scar tissue over the course of several weeks. Replacing healthy muscle with scar tissue reduces the pumping ability of the heart in the short term, and promotes the development of heart failure in the long term. Following myocardial infarction, standard therapies aim to: 1) reduce the amount of initial damage by opening occluded coronary arteries within the first few hours during a heart attack (fibrinolytic drugs, angioplasty, stents); and 2) reduce dilation and remodeling of the heart and delay the onset of heart failure (ACE inhibitors, beta-blockers). Despite current therapies, the risk of developing heart failure within 5 years after a first infarction increase with age (to >20% for patients over 70[1]) and infarct size. However, a number of exciting cellular, mechanical, and device therapies now promise to fundamentally change this landscape. These include myocardial regeneration, which aims to restore pump function and completely prevent heart failure by replacing scar tissue with functioning muscle,[2] and peri-infarct pacing, which aims to improve left ventricular (LV) function early after infarction by altering regional mechanics and energetics in the borderzone.[3,4]
Among novel therapies currently under development, direct modification of infarct mechanical properties has received less attention. The mechanical properties of healing myocardial infarcts are a critical determinant of both depression of pump function and the eventual transition to heart failure,[5] and reports that injecting polymers into the infarct can improve heart function[6-9] suggest that therapeutic modification of infarct mechanics might be possible. Because most plausible regenerative approaches will require weeks to fully implement, but much of the remodeling that predisposes to heart failure occurs in the first few days, we believe that early mechanical reinforcement has significant potential not only as a stand-alone therapy but also as an adjunct to other therapies.
Early models of the impact of infarct mechanics on left ventricular function predicted that stiffening an acute infarct would improve systolic function, but would also impair filling due to reduced diastolic ventricular compliance.[10,11] These models highlighted the importance of considering both systolic and diastolic function, but greatly oversimplified the mechanics by treating the infarct as an isotropic material with a single ‘stiffness’ value that completely described its mechanical properties. Normal myocardium deforms in a complex three-dimensional pattern with each heartbeat,[12-14] and healing myocardial infarcts can be highly anisotropic (having different mechanical properties in different directions); in fact, we proposed over a decade ago that scar anisotropy may preserve ventricular function.[15] When designing a therapy to modify infarct mechanics, selecting a target is therefore far from simple.
Computational models represent a useful way to explore a large parameter space. We utilized a finite-element model of the infarcted dog heart to explore a wide range of infarct mechanical properties and ask: What infarct properties provide the best LV function, and does the answer to this question depend on infarct size or location? Our model results should provide guidance for design and development of therapies to improve LV function by modifying infarct mechanical properties.
Methods
We utilized a freely available finite-element software package, Continuity® 6.3b, v4584 (www.continuity.ucsd.edu) to implement and adapt an existing three-dimensional model of the canine heart featuring accurate geometries of the left ventricle (LV) and right ventricle (RV) and detailed transmural organization of cardiac muscle fibers (Figure 1A).[16,17] An anisotropic exponential constitutive law for cardiac fibers governed passive filling,[16,18] while an active cardiac contraction model developed by Guccione et al. governed systolic function.[19,20] The model geometry consisted of 40 LV and 8 RV tricubic Hermite elements, with 1968 degrees of freedom. The non-linear FE model was solved with a modified Newton iteration algorithm. Integration was performed at 27 Gauss points (3×3×3) per element.[17]
Figure 1.

Finite element models of the infarcted canine heart. Each panel shows the end-systolic configuration at a left ventricular pressure of 100mmHg, including endocardial element boundaries (blue lines), the endocardial surface (red), the ischemic region (blue), and the muscle or infarct collagen fibers (white). A The baseline canine model contracts normally. B The acutely ischemic region (bff=18.5, bxx=3.58, bfx=1.63) bulges noticeably. C An anisotropic ischemic region with stiffer circumferential fibers (bff=50, bxx=bfx=1.63) stretches less in the circumferential direction but more longitudinally. D An anisotropic model with stiffer longitudinal fibers (bff=50, bxx=bfx=1.63) stretches less longitudinally, more circumferentially.
Modeling Ischemia and Variable Anisotropy
We modeled ischemia due to ligation of the left anterior descending coronary artery (LAD) by setting intracellular calcium concentration to zero, eliminating contraction in a large anterior-apical region of the LV (22% of the endocardial surface, Figure 1B). The mechanics of the acutely ischemic region were therefore governed by the constitutive properties of normal, passive myocardium as expressed by a transversely isotropic exponential Fung-type strain energy function W:[21]
| (1) |
| (2) |
where Q is a function of components of the Lagrangian strain tensor E: fiber strain (Eff), cross-fiber strain (Ecc), radial strain (Ess), and shear strains (Efc, Efs, Ecs) and J is the determinant of the deformation gradient tensor F. Following Guccione et al.[21] and Costa et al.,[18] we represented normal passive canine myocardium with the coefficients C = 0.88 kPa, bff = 18.5, bxx = 3.58, and bfx = 1.63. Following Doll et al., we included the second term of Equation (1) and chose a large bulk modulus K = 100 kPa to represent the myocardium as nearly incompressible.[22]
In order to examine the effect of increasing circumferential stiffness in the infarct, we modified the transmural fiber distribution in the ischemic region, orienting fibers circumferentially at all depths (Figure 1C). We then held the coefficient C at 0.88 kPa, set the transverse and shear coefficients equal (bxx=bfx), and varied bff ≥ bxx=bfx over 2 orders of magnitude (1.63, 18.5, 50, and 100). Because the coefficient bxx modifies both cross-fiber and shear strains, we simulated increased longitudinal stiffness by orienting fibers longitudinally in the ischemic region (Figure 1D) and again exploring parameter combinations with bff≥bxx, rather than by considering cases where bff<bxx.
Because fiber orientations are represented in the model as smoothly varying fields, reorienting fibers in the ischemic region also affected fiber orientation somewhat in the border zone. We used the lateral distance from the ischemic region border at which transmural fiber angles returned to within 15° of their values in the baseline model as a measure of the width of the “structural” border zone we created by reorienting the fibers. This distance was 1.9 cm for the model with circumferentially oriented ischemic region fibers and 2.4 cm for the model with longitudinally oriented ischemic region fibers. To test whether altered fiber directions and mechanics in the border regions substantially impacted global function in the model, we compared results for isotropic infarct properties (bff=bxx=bfx) in the models with circumferential and longitudinal infarct fibers and confirmed that they were virtually identical (0.7±1.1% difference in simulated end-diastolic volume and 1.1±1.2% difference in simulated end-systolic volume across the four coefficient values).
Quantifying Model LV Function
We assessed LV pump function by constructing the end-diastolic (EDPVR) and end-systolic (ESPVR) pressure-volume relationships. Simulated inflation of the left ventricle to 2 kPa (15 mmHg) and the right ventricle to 0.8 kPa (6 mmHg) in 200 loading steps provided the exponential EDPVR directly. Cavity volume (EDV10) and strains recorded at a left ventricular pressure of 10 mmHg allowed detailed comparisons of end-diastolic behavior between models.
We constructed the ESPVR by simulating isovolumic contractions from several end-diastolic states and fitting the pressure-volume data with a straight line. The slope of the fitted ESPVR (Emax) provided an index of LV contractility. Simulated isovolumic contraction from an end-diastolic state yielding an end-systolic pressure of 100 mmHg for each model provided end-systolic cavity volume (ESV100), strains, and stresses for detailed comparisons between models. Finally, we computed the stroke volume each model would achieve at an end-diastolic pressure (EDP) of 10 mmHg and end-systolic pressure (ESP) of 100 mmHg in order to compare overall performance of the models, accounting for the balance between diastolic and systolic function.
Quantifying Regional Mechanics
All data for the regional deformation and stress analysis were exported from Continuity® at Gauss points of the anterior wall elements and rotated from fiber coordinates to cardiac coordinates. Each simulation (passive inflation to 2 kPa or inflation to lower pressures followed by isovolumic contraction) began at an unloaded configuration. Model Lagrangian strains at end diastole and end systole therefore reflected deformation relative to this unloaded reference state. While this provides a reasonable estimate of diastolic strains that might be measured in an experiment, systolic strains conventionally describe deformation from end diastole to end systole. We used the deformation gradient tensors describing deformation from the unloaded reference to an end-diastolic pressure of 10 mmHg (FED) and deformation from the same reference to an end-systolic pressure of 100 mmHg (FES) to compute strains reflecting deformation from end diastole to end systole:
| (3) |
We used a similar approach to compute a remodeling strain reflecting the difference between the end-diastolic states of the baseline and various infarct models:
| (4) |
Results
Global Function
Consistent with previous modeling studies that treated the infarct as an isotropic material, isotropically increasing infarct stiffness reduced diastolic and systolic volumes, improved LV contractility, and produced little net effect on stroke volume except for a drop in very soft infarcts (Figure 2, dashed diagonal line in each panel). The predicted impact of infarct anisotropy differed in systole and diastole. Systolic responses were nearly symmetric about the line of isotropy (Figure 2B, 2C); for example, coefficient values of bff=100 and bxx=bfx=1.63 produced nearly the same ESV100 and Emax, whether the stiffer fibers were oriented circumferentially or longitudinally. By contrast, diastolic filling was more sensitive to circumferential stiffness than to longitudinal stiffness (Figure 2A). Combining these two effects, a longitudinal stiffness coefficient of 50 and circumferential stiffness coefficient of 1.63 produced the highest predicted stroke volume of all combinations examined (Figure 2D).
Figure 2.
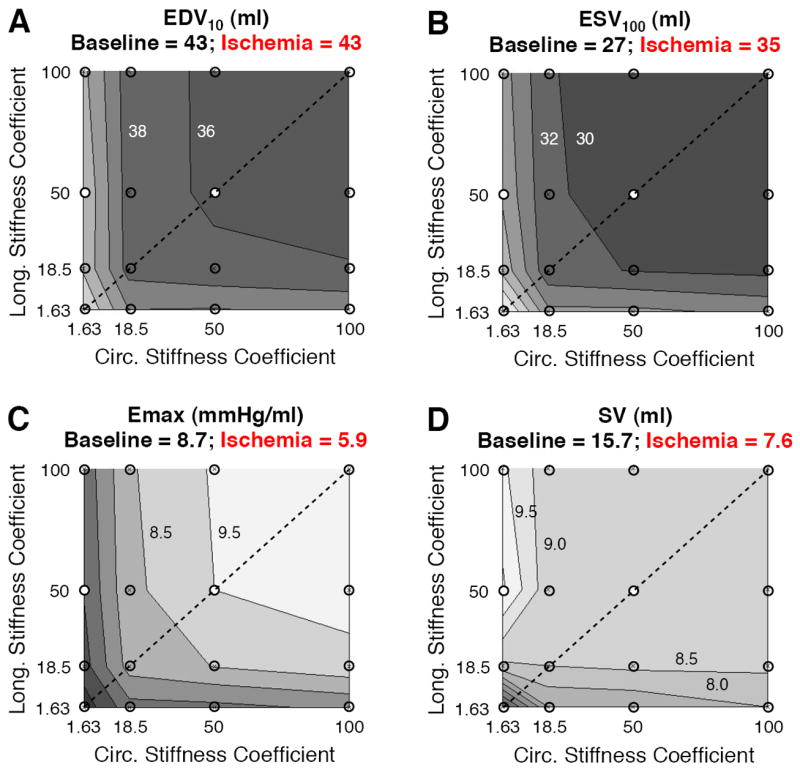
Impact of infarct mechanical properties on left ventricular function. Contour plots display variations in functional indices across the parameter space; titles in each panel provide values for the noninfarcted baseline model and acute ischemia model with normal fiber distribution and passive myocardial properties. A,B End-diastolic volume at a pressure of 10 mmHg and end-systolic volume at a pressure of 100 mmHg both decreased as infarct stiffness increased. C Isotropic stiffening provided the best systolic contractility as measured by the slope of the end-systolic pressure-volume relationship (Emax). D Anisotropic stiffening in the longitudinal direction produced the highest predicted stroke volume.
The results from this initial survey suggested that two possible strategies for mechanical reinforcement of anteroapical infarcts merit closer examination: isotropic stiffening and selective longitudinal stiffening. We therefore performed more detailed comparisons of pressure-volume relationships and regional mechanics among models reflecting the baseline state, acute ischemia, isotropic stiffening (bff=bxx=bfx=50), and longitudinal stiffening (bff=50, bxx=bfx=1.63, longitudinal fibers). Comparison of pressure-volume relationships showed that simulated acute ischemia reproduced the expected rightward shift of the ESPVR with no change in the EDPVR (Figure 3). Isotropic stiffening improved systolic function, shifting the ESPVR to the left, but also decreased the compliance of the LV, shifting the EDPVR to the left; the net improvement in stroke volume was modest. Longitudinal stiffening had little impact on systolic function compared to acute ischemia but shifted the EDPVR rightward, so that the predicted increase in stroke volume was due to increased filling.
Figure 3.

Impact of infarct mechanical properties on left ventricular function. End-systolic (ESPVR) and end-diastolic (EDPVR) pressure-volume relationships are displayed for 4 models, along with stroke volumes assuming a fixed end-diastolic pressure of 10 mmHg and end-systolic pressure of 100 mmHg: baseline (green), acutely ischemic (red), isotropic stiffening (bff=bxx=bfx=50, corresponds to white dot in center of each panel in Figure 2), and longitudinal stiffening (bff=50, bxx=bfx=1.63, white dot along left axis in Figure 2). Isotropic stiffening shifts the ESPVR and EDPVR leftward (blue) but has a modest net impact on predicted stroke volume. Longitudinal stiffening (black dotted lines) increases stroke volume due to increased filling rather than improved systolic function.
Regional Mechanics
Diastolic strains in the midwall dit not change during simulated ischemia, since the infarct material properties matched those of passive myocardium (Figure 4A). As expected, isotropic stiffening reduced diastolic strains in both directions. Longitudinal stiffening reduced longitudinal stretch during diastole but increased circumferential stretch. Remodeling strains reflected the differences in diastolic behavior: compared to baseline and acute ischemia, isotropic stiffening reduced circumferential and longitudinal dimensions of the infarct at end diastole, while longitudinal stiffening allowed circumferential expansion as it reduced longitudinal dimension (Figure 4B).
Figure 4.
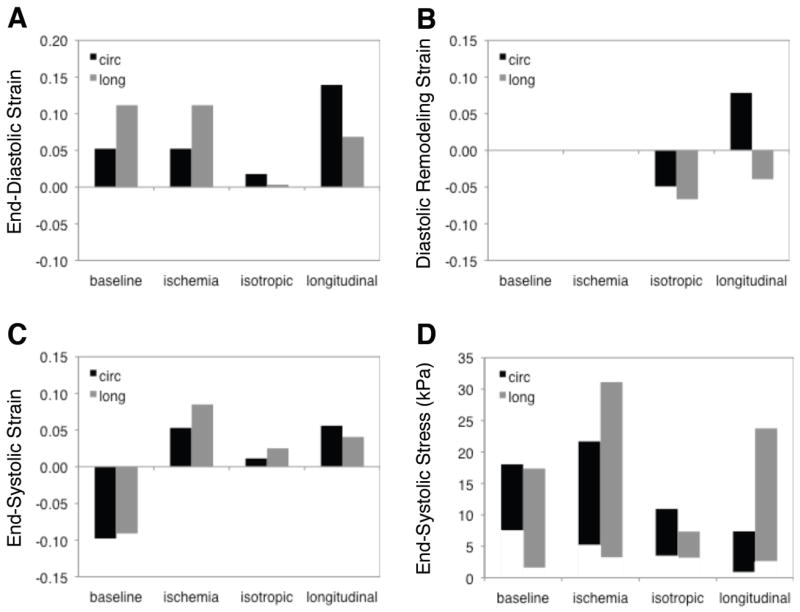
Impact of mechanical properties on midwall Lagrangian finite strains and transmural range of Cauchy stresses in the infarct at baseline, during simulated acute ischemia, following isotropic stiffening (bff=bxx=bfx=50, corresponds to white dot in center of each panel in Figure 2), and following longitudinal stiffening (bff=50, bxx=bfx=1.63, white dot along left axis in Figure 2). A,B Isotropic stiffening reduced diastolic stretch in both directions while longitudinal stiffening reduced longitudinal stretch and allowed increased circumferential stretch. C,D Isotropic stiffening reduced systolic stretching and stresses in both directions, while longitudinal stiffening reduced longitudinal strain and circumferential stress.
Uniform systolic contraction of the healthy myocardium converted to passive systolic stretch during acute ischemia, as expected (Figure 4C). At the midwall, the magnitude of the systolic stretch was greater in the longitudinal direction. As might be expected, isotropic stiffening reduced systolic stretching in both the circumferential and longitudinal directions, while longitudinal stiffening specifically reduced longitudinal systolic stretching.
The model predicted large transmural variations in stresses; we therefore computed stresses in the subepicardium (2.5% relative transmural depth), midwall (52%) and subendocardium (97.5%) and plotted the range of stresses across these locations (Figure 4D). Regional ischemia increased peak longitudinal stresses roughly two-fold and increased the transmural range of circumferential stresses. Isotropic reinforcement reduced both circumferential and longitudinal stresses in the infarct, while longitudinal reinforcement specifically reduced circumferential stresses. Although the mechanics of the border zone were not the primary focus of this paper, we did examine systolic stresses at the first row of Gauss points in the elements lateral to the infarct, approximately 0.4 cm from the infarct border. Changes in border zone stress followed those in the infarct: ischemia roughly doubled wall stresses; isotropic reinforcement reduced both circumferential and longitudinal stresses; and longitudinal reinforcement reduced circumferential but not longitudinal stresses.
Regional Deformation in Two Infarct Locations
Motivated by recent experimental results in our laboratory that showed that regional deformation in acute rat infarcts depends on infarct location,[23] we also briefly compared transmural strain distributions in simulated infarcts involving just the equatorial portion of the anterior wall or just the LV apex. These simulations predicted that strains vary transmurally in each location and differ between the two locations (Figure 5).
Figure 5.
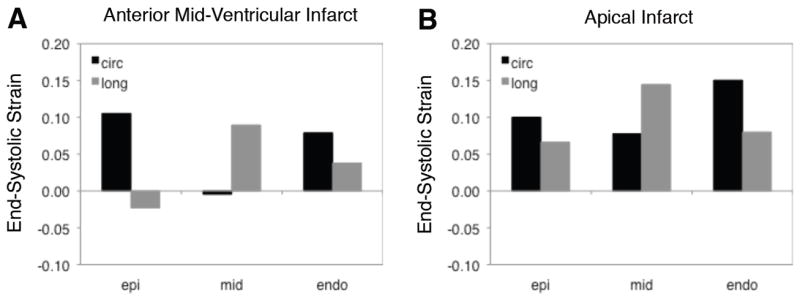
Impact of infarct location on transmural strain distributions. As expected, the balance of circumferential and longitudinal stretching varies transmurally during simulated acute ischemia. In addition, predicted strain patterns differ substantially between a mid-ventricular and apical location.
Discussion
It has been known for decades that the mechanical properties of healing myocardial infarcts are a critical determinant of pump function and left ventricular remodeling (reviewed by Holmes et al.[5]). It is therefore surprising that therapies designed to modify infarct mechanical properties are only now gaining traction. In part, reluctance to tamper with infarct properties may reflect the legacy of an ill-fated early clinical trial, in which patients were given post-infarction steroids based on animal data showing that steroids reduced infarct size. Several patients died and the trial was stopped;[24] later animal studies demonstrated that although steroids and other anti-inflammatory drugs reduce the size of the damaged region, they also impair healing and result in a thinner scar.[25-28]
The failure of post-infarction steroids illustrates why therapies to modify infarct properties must be rationally designed based on a deep understanding of infarct mechanics, heart function, and the relationship between the two. Because the mechanical coupling of the infarct to the surrounding myocardium is complex and three-dimensional, and infarct mechanics can affect systolic as well as diastolic function, computational models are essential to designing effective modifications of infarct mechanics. In the present study, we utilized a finite-element model of the infarcted dog heart to explore a wide range of infarct mechanical properties and ask what properties optimize function of the infarcted left ventricle. Our results suggest that different choices of infarct mechanical properties optimize different aspects of LV function, that isotropically stiffening the infarct is not the only option of potential therapeutic interest, and that customizing therapies for different infarct locations may be important.
Cardiac Restraint Devices
Cardiac restraint devices aim to physically arrest continuing ventricular dilation in patients with heart failure.[29-34] Over a decade ago, a group at the University of Pennsylvania (Penn) applied this same logic directly to the infarct – since early infarct expansion is the first step towards adverse ventricular remodeling and eventual heart failure, mechanically restraining the infarct early after infarction might limit remodeling. Their initial study showed that placing a piece of polypropylene mesh over the infarct region 10-14 days prior to infarction prevented adverse remodeling and the development of apical aneurysm following anteroapical infarction in sheep.[35] The Penn group later concluded that full cardiac restraint, rather than localized restraint of the infarct region, was required to significantly impact remodeling,[36] but other groups have reported improved remodeling and function with localized reinforcement applied as late as 8 weeks after infarction.[37]
The mechanics of full cardiac restraint early after infarction have been studied by MRI,[38,39] but the mechanics of local restraint have received less attention. Modeling and experimental studies of global restraint in failing hearts suggest a trade-off between preventing dilation and impairing ventricular function: increasing pressure applied to the epicardial surface gradually depresses cardiac output.[40,41] Devices such as the CorCap and Paracor likely affect stress and deformation similarly in the circumferential and longitudinal directions. By contrast, the design of the Myosplint suggests differential effects on the circumferential and longitudinal directions; it is therefore interesting that a computational study of the Myosplint predicted that a 25% reduction in end-diastolic LV diameter could be achieved without impairing cardiac output.[42] The study most relevant to local restraint was a model analysis of surgical anterior ventricular restoration by Dang et al.[43] The authors predicted that applying an isotropic synthetic patch to the endocardial surface of the LV after resecting an anteroapical infarct would depress stroke volume; stiffer patches reduced wall stress more than softer patches, but also depressed stroke volume more.
Isotropic Stiffening of Healing Infarcts
Several groups are exploring methods to stiffen infarcts, and some of these groups are using computational models to help design the interventions. Injecting biopolymers directly into a healing infarct reduces infarct thinning, infarct expansion, and ventricular remodeling and preserves ventricular function in several animal models.[6-9] While addition of a stiff material could obviously stiffen an infarct, even injections of soft materials may structurally reinforce an infarct by increasing wall thickness. Guccione and colleagues developed a finite-element model of the infarcted heart subjected to polymer injection[44] and used their model to identify the optimum distribution of injected material.[45] In an interesting result reminiscent of our Figure 2, they found that the most effective way to reduce wall stress was to inject as much material as possible. However, this strategy did not maximize stroke volume; when stroke volume was included as an objective, a very different optimum injection pattern resulted.[45]
Isotropic stiffening of the large anteroapical infarct in our model (diagonal line in each panel of Figure 2) improved systolic contractility (Emax), reduced end-systolic volume (ESV) and end-diastolic volume (EDV), and produced little change in stroke volume (SV). This is exactly what we expected based on published models.[10,11] Yet, our results appear to conflict with the studies of polymer injection reviewed above, some of which reported improved LV function following injection. In fact, the modeling and experimental studies generally agree, and apparent differences primarily reflect the use of different indices of LV function. For example, Ryan et al. measured regional mechanics and global function immediately before and 15 minutes after injection of a dermal filler compound into anteroapical infarcts in sheep.[9] In the dermal filler group, injection decreased EDV by an average of 6.2 ml and ESV by an average of 6.5 ml, suggesting very little change in stroke volume; because EDV and ESV decreased equally, ejection fraction (EF) increased. Data in their Table 1 also show that end-diastolic pressures were reduced by injection and that heart rate may have changed (cardiac output increased even though stroke volume apparently did not).
In interpreting their data, Ryan et al. focused on the effect of their therapy on chamber volumes and remodeling, but others have based claims of improved function on similar improvements in EF that may not reflect improved stroke volume or cardiac output. In a setting where diastolic compliance, end-diastolic pressure, and contractility are all changing, we agree with Wall et al.[44] that ventricular function curves (stroke volume or cardiac output plotted against end-diastolic pressure) acquired at a fixed heart rate are the best basis for comparing pump function. We reported stroke volumes rather than ventricular function curves in the present study only because we held end-diastolic pressure, end-systolic pressure, and heart rate constant in our simulations.
Anisotropic Stiffening of Healing Infarcts
We previously reported that healing myocardial scar in a pig model of myocardial infarction is structurally and mechanically anisotropic, and speculated that scar anisotropy might preserve LV function.[15] It was therefore intriguing that highly anisotropic infarct material properties produced the optimum stroke volume in the current study (Figure 2D). This result raises two interesting questions: could we achieve the infarct properties that optimized stroke volume in practice; and would we want to?
The optimal stroke volume occurred at a very high longitudinal stiffness coefficient but a very low circumferential stiffness coefficient – lower in fact than the cross-fiber coefficient values reported for passive canine myocardium. It seems unlikely that anyone would be willing to take the risk of intentionally softening an acute infarct in one direction, even while reinforcing it in another, but the contour plots suggest that most of the benefit could be achieved simply by selectively stiffening the longitudinal direction without altering the circumferential direction.
The more interesting question involves the trade-off between acute pump function and ventricular size. This same trade-off recurs as a theme throughout the discussion above, and highlights the essential question we cannot yet answer. Which strategy will produce the best long-term outcome following an acute myocardial infarct, as the heart grows and remodels and the neurohormonal environment responds – a therapy that initially limits dilation but impairs filling (isotropic stiffening), or a therapy that initially improves pump function without reducing LV size (longitudinal stiffening)? At present, our approach is to use computational modeling to select specific therapeutic options that appear to have beneficial acute effects, validate those predictions experimentally, then assess the long-term consequences in animal experiments. Computational models that represent cardiovascular remodeling accurately enough to predict how the heart will remodel after an intervention would be an important tool in designing new therapies, and a number of groups are developing such models (see for example Kroon et al. [46])
Timing of Interventions
We modeled the impact of modifying the mechanics of an acute infarct, before the local expansion and thinning and global dilation that typically occur with large anteroapical infarcts. Some of the studies reviewed above also considered the effects of altering the mechanics of an acute infarct (e.g. [36,35]), but most considered interventions in heart failure patients, long after the original myocardial infarction that may have triggered adverse remodeling and ultimately heart failure. Modifying infarct mechanics may have completely different effects early after infarction and later in the course of remodeling. Acting early, before too much adverse remodeling occurs, offers the promise of a bigger potential impact, specifically the possibility of delaying or even preventing the onset of heart failure. Early modification of infarct properties may also prove a useful temporary measure to protect against adverse remodeling while slower-acting regenerative therapies are deployed. Yet balancing the potential risks and benefits of aggressive therapies is much tougher at an earlier time point than in end-stage heart failure patients who have few other options.
Designing Therapies for Different Infarct Locations
Our finding that regional mechanics differ for simulated infarcts in two locations on the left ventricle suggests the possibility that mechanical therapies could be tailored for specific infarct locations. Conversely, it also suggests that some therapies may work better in some locations than others, so that any therapy should be tested for each specific infarct size and location in which it may be used. Transmural differences at both of the locations we simulated also suggest that even isotropic stiffening through polymer injection could be used to achieve net anisotropic reinforcement of an infarct by targeting the injection specifically to one transmural layer.
Limitations and Sources of Error
The primary limitation of this study is that the predictions for infarcts with anisotropic material properties were not validated experimentally. The original model of LAD ischemia in the dog that formed the starting point for our studies was validated extensively against regional strains by its developers at the University of California, San Diego.[47,48] We later showed that it captured the mechanics well enough to make other predictions for which it was not specifically developed. For example, we simulated acutely ischemic regions of varying sizes and found that the relationship between endocardial wall motion and ischemic region size matched experiments closely.[49] Our current predictions for the effects of acute ischemia on global hemodynamics match published experimental data,[50] and our predictions for isotropic stiffening match data on the acute impact of polymer injection,[9] providing general confidence in the model framework. Specific validation of the predicted impact of longitudinal stiffening is the next step, and will require new experimental methods for altering infarct properties.
Conclusions
Direct mechanical reinforcement of acute myocardial infarcts has significant potential to improve function and limit remodeling in the critical first few days following infarction. Because the mechanical coupling of the infarct to the surrounding myocardium is complex and three-dimensional, and infarct mechanics can affect systolic as well as diastolic function, computational models are essential to designing effective modifications of infarct mechanics. We utilized a finite-element model of the infarcted dog heart to explore a wide range of infarct mechanical properties and ask what properties optimize function of the infarcted left ventricle. Our results suggest that there is a general trade-off between acutely reducing LV size and acutely improving LV pump function, that isotropically stiffening the infarct is not the only option of potential therapeutic interest, and that customizing therapies for different infarct locations may be important. Future work should focus on impact of different reinforcement strategies on long-term remodeling and progression to heart failure.
Acknowledgments
This work was funded by a Coulter Foundation Translational Research Grant (JWH, GA) and by NIH R01 HL-075639 (JWH). The authors wish to thank Dr. Roy Kerckhoffs at the University of California, San Diego, for his advice and assistance.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: A report from the american heart association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667.CIRCULATIONAHA.109.192667 [DOI] [PubMed] [Google Scholar]
- 2.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451(7181):937–942. doi: 10.1038/nature06800.nature06800 [DOI] [PubMed] [Google Scholar]
- 3.Chung ES, Menon SG, Weiss R, Schloss EJ, Chow T, Kereiakes DJ, Mazur W, Salo RW, Galle E, Pastore JM. Feasibility of biventricular pacing in patients with recent myocardial infarction: Impact on ventricular remodeling. Congest Heart Fail. 2007;13(1):9–15. doi: 10.1111/j.1527-5299.2007.05868.x. [DOI] [PubMed] [Google Scholar]
- 4.Shuros AC, Salo RW, Florea VG, Pastore J, Kuskowski MA, Chandrashekhar Y, Anand IS. Ventricular preexcitation modulates strain and attenuates cardiac remodeling in a swine model of myocardial infarction. Circulation. 2007;116(10):1162–1169. doi: 10.1161/CIRCULATIONAHA.107.696294.CIRCULATIONAHA.107.696294 [DOI] [PubMed] [Google Scholar]
- 5.Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng. 2005;7:223–253. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- 6.Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10(3-4):403–409. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 7.Dai W, Wold LE, Dow JS, Kloner RA. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: A novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 2005;46(4):714–719. doi: 10.1016/j.jacc.2005.04.056.S0735-1097(05)01201-5 [DOI] [PubMed] [Google Scholar]
- 8.Mukherjee R, Zavadzkas JA, Saunders SM, McLean JE, Jeffords LB, Beck C, Stroud RE, Leone AM, Koval CN, Rivers WT, Basu S, Sheehy A, Michal G, Spinale FG. Targeted myocardial microinjections of a biocomposite material reduces infarct expansion in pigs. Ann Thorac Surg. 2008;86(4):1268–1276. doi: 10.1016/j.athoracsur.2008.04.107.S0003-4975(08)00955-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan LP, Matsuzaki K, Noma M, Jackson BM, Eperjesi TJ, Plappert TJ, St John-Sutton MG, Gorman JH, 3rd, Gorman RC. Dermal filler injection: A novel approach for limiting infarct expansion. Ann Thorac Surg. 2009;87(1):148–155. doi: 10.1016/j.athoracsur.2008.09.028.S0003-4975(08)02024-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janz RF, Waldron RJ. Predicted effect of chronic apical aneurysms on the passive stiffness of the human left ventricle. Circulation Research. 1978;42(2):255–263. doi: 10.1161/01.res.42.2.255. [DOI] [PubMed] [Google Scholar]
- 11.Bogen DK, Rabinowitz SA, Needleman A, McMahon TA, Abelmann WH. An analysis of the mechanical disadvantage of myocardial infarction in the canine left ventricle. Circulation Research. 1980;47:728–741. doi: 10.1161/01.res.47.5.728. [DOI] [PubMed] [Google Scholar]
- 12.Costa KD, Takayama Y, McCulloch AD, Covell JW. Laminar fiber architecture and three-dimensional systolic mechanics in canine ventricular myocardium. Am J Physiol. 1999;276(2 Pt 2):H595–607. doi: 10.1152/ajpheart.1999.276.2.H595. [DOI] [PubMed] [Google Scholar]
- 13.Arts T, Costa KD, Covell JW, McCulloch AD. Relating myocardial laminar architecture to shear strain and muscle fiber orientation. Am J Physiol Heart Circ Physiol. 2001;280(5):H2222–2229. doi: 10.1152/ajpheart.2001.280.5.H2222. [DOI] [PubMed] [Google Scholar]
- 14.Takayama Y, Costa KD, Covell JW. Contribution of laminar myofiber architecture to load-dependent changes in mechanics of lv myocardium. Am J Physiol Heart Circ Physiol. 2002;282(4):H1510–1520. doi: 10.1152/ajpheart.00261.2001. [DOI] [PubMed] [Google Scholar]
- 15.Holmes JW, Nunez JA, Covell JW. Functional implications of myocardial scar structure. Am J Physiol Heart Circ Physiol. 1997;272:H2123–2130. doi: 10.1152/ajpheart.1997.272.5.H2123. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen PMF, LeGrice IJ, Smaill BH, Hunter PJ. Mathematical model of geometry and fibrous structure of the heart. Am J Physiol Heart Circ Physiol. 1991;260:H1365–H1378. doi: 10.1152/ajpheart.1991.260.4.H1365. [DOI] [PubMed] [Google Scholar]
- 17.Kerckhoffs RC, Neal ML, Gu Q, Bassingthwaighte JB, Omens JH, McCulloch AD. Coupling of a 3d finite element model of cardiac ventricular mechanics to lumped systems models of the systemic and pulmonic circulation. Ann Biomed Eng. 2007;35(1):1–18. doi: 10.1007/s10439-006-9212-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costa KD, Hunter PJ, Wayne JS, Waldman LK, Guccione JM, McCulloch AD. A three-dimensional finite element method for large elastic deformations of ventricular myocardium: Ii--prolate spheroidal coordinates. J Biomech Eng. 1996;118(4):464–472. doi: 10.1115/1.2796032. [DOI] [PubMed] [Google Scholar]
- 19.Guccione JM, McCulloch AD. Mechanics of active contraction in cardiac muscle: Part i--constitutive relations for fiber stress that describe deactivation. J Biomech Eng. 1993;115(1):72–81. doi: 10.1115/1.2895473. [DOI] [PubMed] [Google Scholar]
- 20.Guccione JM, Costa KD, McCulloch AD. Finite element stress analysis of left ventricular mechanics in the beating dog heart. J Biomech. 1995;28(10):1167–1177. doi: 10.1016/0021-9290(94)00174-3.0021929094001743 [DOI] [PubMed] [Google Scholar]
- 21.Guccione JM, McCulloch AD, Waldman LK. Passive material properties of intact ventricular myocardium determined from a cylindrical model. J Biomech Eng. 1991;113(1):42–55. doi: 10.1115/1.2894084. [DOI] [PubMed] [Google Scholar]
- 22.Doll S, Schweizerhof K. On the development of volumetric strain energy functions. J Appl Mech. 2000;67:17–21. [Google Scholar]
- 23.Fomovsky GM, Holmes JW. Collagen fiber structure correlates with mechanical environment in healing myocardial infarcts. 2009 Summer Bioengineering Conference; Squaw Valley, CA. June 17-20, 2009. [Google Scholar]
- 24.Roberts R, DeMello V, Sobel BE. Deleterious effects of methyprednisolone in patients with myocardial infarction. Circulation. 1976;53(3 Suppl I):I-204–I-205. [PubMed] [Google Scholar]
- 25.Kloner RA, Fishbein MC, Lew H, Maroko PR, Braunwald E. Mummification of the infarcted myocardium by high dose corticosteroids. Circulation. 1978;57(1):56–63. doi: 10.1161/01.cir.57.1.56. [DOI] [PubMed] [Google Scholar]
- 26.Brown EJ, Kloner RA, Schoen FJ, Hammerman H, Hale S, Braunwald E. Scar thinning due to ibuprofen administration after experimental myocardial infarction. American Journal of Cardiology. 1983;51:877–883. doi: 10.1016/s0002-9149(83)80148-9. [DOI] [PubMed] [Google Scholar]
- 27.Hammerman H, Kloner RA, Hale S, Schoen FJ, Braunwald E. Dose-dependent effects of short-term methylprednisolone on myocardial infarct extent scar formation and ventricular function. Circulation. 1983;68(2):446–452. doi: 10.1161/01.cir.68.2.446. [DOI] [PubMed] [Google Scholar]
- 28.Hammerman H, Kloner RA, Schoen FJ, Brown EJ, Hale S, Braunwald E. Indomethacin-induced scar thinning after experimental myocardial infarction. Circulation. 1983;67(6):1290–1295. doi: 10.1161/01.cir.67.6.1290. [DOI] [PubMed] [Google Scholar]
- 29.Mann DL, Acker MA, Jessup M, Sabbah HN, Starling RC, Kubo SH. Clinical evaluation of the corcap cardiac support device in patients with dilated cardiomyopathy. Ann Thorac Surg. 2007;84(4):1226–1235. doi: 10.1016/j.athoracsur.2007.03.095.S0003-4975(07)00892-2 [DOI] [PubMed] [Google Scholar]
- 30.Starling RC, Jessup M, Oh JK, Sabbah HN, Acker MA, Mann DL, Kubo SH. Sustained benefits of the corcap cardiac support device on left ventricular remodeling: Three year follow-up results from the acorn clinical trial. Ann Thorac Surg. 2007;84(4):1236–1242. doi: 10.1016/j.athoracsur.2007.03.096.S0003-4975(07)00893-4 [DOI] [PubMed] [Google Scholar]
- 31.Ailawadi G, Kron IL. New strategies for surgical management of ischemic cardiomyopathy. Expert Rev Cardiovasc Ther. 2008;6(4):521–530. doi: 10.1586/14779072.6.4.521. [DOI] [PubMed] [Google Scholar]
- 32.Klodell CT, Jr, Aranda JM, Jr, McGiffin DC, Rayburn BK, Sun B, Abraham WT, Pae WE, Jr, Boehmer JP, Klein H, Huth C. Worldwide surgical experience with the paracor heartnet cardiac restraint device. J Thorac Cardiovasc Surg. 2008;135(1):188–195. doi: 10.1016/j.jtcvs.2007.09.034.S0022-5223(07)01640-6 [DOI] [PubMed] [Google Scholar]
- 33.Jugdutt BI. Current and novel cardiac support therapies. Curr Heart Fail Rep. 2009;6(1):19–27. doi: 10.1007/s11897-009-0005-9. [DOI] [PubMed] [Google Scholar]
- 34.Topkara VK, Kondareddy S, Mann DL. Modulation of left ventricular dilation remodeling with epicardial restraint devices in postmyocardial infarction heart failure. Curr Heart Fail Rep. 2009;6(4):229–235. doi: 10.1007/s11897-009-0032-6. [DOI] [PubMed] [Google Scholar]
- 35.Kelley ST, Malekan R, Gorman JH, 3rd, Jackson BM, Gorman RC, Suzuki Y, Plappert T, Bogen DK, Sutton MG, Edmunds LH., Jr Restraining infarct expansion preserves left ventricular geometry and function after acute anteroapical infarction. Circulation. 1999;99(1):135–142. doi: 10.1161/01.cir.99.1.135. [DOI] [PubMed] [Google Scholar]
- 36.Enomoto Y, Gorman JH, 3rd, Moainie SL, Jackson BM, Parish LM, Plappert T, Zeeshan A, St John-Sutton MG, Gorman RC. Early ventricular restraint after myocardial infarction: Extent of the wrap determines the outcome of remodeling. Ann Thorac Surg. 2005;79(3):881–887. doi: 10.1016/j.athoracsur.2004.05.072. discussion 881-887.S0003-4975(04)01215-9 [DOI] [PubMed] [Google Scholar]
- 37.Liao SY, Siu CW, Liu Y, Zhang Y, Chan WS, Wu EX, Wu Y, Nicholls JM, Li RA, Benser ME, Rosenberg SP, Park E, Lau CP, Tse HF. Attenuation of left ventricular adverse remodeling with epicardial patching after myocardial infarction. J Card Fail. 2010;16(7):590–598. doi: 10.1016/j.cardfail.2010.02.007.S1071-9164(10)00070-9 [DOI] [PubMed] [Google Scholar]
- 38.Pilla JJ, Blom AS, Gorman JH, 3rd, Brockman DJ, Affuso J, Parish LM, Sakamoto H, Jackson BM, Acker MA, Gorman RC. Early postinfarction ventricular restraint improves borderzone wall thickening dynamics during remodeling. Ann Thorac Surg. 2005;80(6):2257–2262. doi: 10.1016/j.athoracsur.2005.05.089. [DOI] [PubMed] [Google Scholar]
- 39.Blom AS, Pilla JJ, Arkles J, Dougherty L, Ryan LP, Gorman JH, 3rd, Acker MA, Gorman RC. Ventricular restraint prevents infarct expansion and improves borderzone function after myocardial infarction: A study using magnetic resonance imaging, three-dimensional surface modeling, and myocardial tagging. Ann Thorac Surg. 2007;84(6):2004–2010. doi: 10.1016/j.athoracsur.2007.06.062.S0003-4975(07)01368-9 [DOI] [PubMed] [Google Scholar]
- 40.Ghanta RK, Rangaraj A, Umakanthan R, Lee L, Laurence RG, Fox JA, Bolman RM, 3rd, Cohn LH, Chen FY. Adjustable, physiological ventricular restraint improves left ventricular mechanics and reduces dilatation in an ovine model of chronic heart failure. Circulation. 2007;115(10):1201–1210. doi: 10.1161/CIRCULATIONAHA.106.671370.CIRCULATIONAHA.106.671370 [DOI] [PubMed] [Google Scholar]
- 41.Jhun CS, Wenk JF, Zhang Z, Wall ST, Sun K, Sabbah HN, Ratcliffe MB, Guccione JM. Effect of adjustable passive constraint on the failing left ventricle: A finite-element model study. Ann Thorac Surg. 2010;89(1):132–137. doi: 10.1016/j.athoracsur.2009.08.075.S0003-4975(09)01793-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guccione JM, Salahieh A, Moonly SM, Kortsmit J, Wallace AW, Ratcliffe MB. Myosplint decreases wall stress without depressing function in the failing heart: A finite element model study. Ann Thorac Surg. 2003;76(4):1171–1180. doi: 10.1016/s0003-4975(03)00731-8. discussion 1180.S0003497503007318 [DOI] [PubMed] [Google Scholar]
- 43.Dang AB, Guccione JM, Zhang P, Wallace AW, Gorman RC, Gorman JH, 3rd, Ratcliffe MB. Effect of ventricular size and patch stiffness in surgical anterior ventricular restoration: A finite element model study. Ann Thorac Surg. 2005;79(1):185–193. doi: 10.1016/j.athoracsur.2004.06.007.S0003-4975(04)01236-6 [DOI] [PubMed] [Google Scholar]
- 44.Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Theoretical impact of the injection of material into the myocardium: A finite element model simulation. Circulation. 2006;114(24):2627–2635. doi: 10.1161/CIRCULATIONAHA.106.657270.CIRCULATIONAHA.106.657270 [DOI] [PubMed] [Google Scholar]
- 45.Wenk JF, Wall ST, Peterson RC, Helgerson SL, Sabbah HN, Burger M, Stander N, Ratcliffe MB, Guccione JM. A method for automatically optimizing medical devices for treating heart failure: Designing polymeric injection patterns. J Biomech Eng. 2009;131(12):121011. doi: 10.1115/1.4000165. [DOI] [PubMed] [Google Scholar]
- 46.Kroon W, Delhaas T, Bovendeerd P, Arts T. Structure and torsion in the normal and situs inversus totalis cardiac left ventricle. Ii. Modeling cardiac adaptation to mechanical load. Am J Physiol Heart Circ Physiol. 2008;295(1):H202–210. doi: 10.1152/ajpheart.00877.2007.00877.2007 [DOI] [PubMed] [Google Scholar]
- 47.Mazhari R, McCulloch AD. Integrative models for understanding the structural basis of regional mechanical dysfunction in ischemic myocardium. Ann Biomed Eng. 2000;28(8):979–990. doi: 10.1114/1.1308502. [DOI] [PubMed] [Google Scholar]
- 48.Mazhari R, Omens JH, Covell JW, McCulloch AD. Structural basis of regional dysfunction in acutely ischemic myocardium. Cardiovascular Research. 2000;47(2):284–293. doi: 10.1016/s0008-6363(00)00089-4. [DOI] [PubMed] [Google Scholar]
- 49.Herz SL, Hasegawa T, Makaryus AN, Parker KM, Homma S, Wang J, Holmes JW. Quantitative three-dimensional wall motion analysis predicts ischemic region size and location. Ann Biomed Eng. 2010;38(4):1367–1376. doi: 10.1007/s10439-009-9880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sunagawa K, Maughan WL, Sagawa K. Effect of regional ischemia on the left ventricular end-systolic pressure-volume relationship of isolated canine hearts. Circulation Research. 1983;52(2):170–178. doi: 10.1161/01.res.52.2.170. [DOI] [PubMed] [Google Scholar]


