Summary
Background
ALK gene rearrangement defines a new molecular subtype of non-small-cell lung cancer (NSCLC). In a recent phase 1 clinical trial, the ALK tyrosine-kinase inhibitor (TKI) crizotinib showed marked antitumour activity in patients with advanced, ALK-positive NSCLC. To assess whether crizotinib affects overall survival in these patients, we did a retrospective study comparing survival outcomes in crizotinib-treated patients in the trial and crizotinib-naive controls screened during the same time period.
Methods
We examined overall survival in patients with advanced, ALK-positive NSCLC who enrolled in the phase 1 clinical trial of crizotinib, focusing on the cohort of 82 patients who had enrolled through Feb 10, 2010. For comparators, we identified 36 ALK-positive patients from trial sites who were not given crizotinib (ALK-positive controls), 67 patients without ALK rearrangement but positive for EGFR mutation, and 253 wild-type patients lacking either ALK rearrangement or EGFR mutation. To assess differences in overall survival, we assessed subsets of clinically comparable ALK-positive and ALK-negative patients.
Findings
Among 82 ALK-positive patients who were given crizotinib, median overall survival from initiation of crizotinib has not been reached (95% CI 17 months to not reached); 1-year overall survival was 74% (95% CI 63–82), and 2-year overall survival was 54% (40–66). Overall survival did not differ based on age, sex, smoking history, or ethnic origin. Survival in 30 ALK-positive patients who were given crizotinib in the second-line or third-line setting was significantly longer than in 23 ALK-positive controls given any second-line therapy (median overall survival not reached [95% CI 14 months to not reached] vs 6 months [4–17], 1-year overall survival 70% [95% CI 50–83] vs 44% [23–64], and 2-year overall survival 55% [33–72] vs 12% [2–30]; hazard ratio 0·36, 95% CI 0·17–0·75; p=0·004). Survival in 56 crizotinib-treated, ALK-positive patients was similar to that in 63 ALK-negative, EGFR-positive patients given EGFR TKI therapy (median overall survival not reached [95% CI 17 months to not reached] vs 24 months [15–34], 1-year overall survival 71% [95% CI 58–81] vs 74% [61–83], and 2-year overall survival 57% [40–71] vs 52% [38–65]; p=0·786), whereas survival in 36 crizotinib-naive, ALK-positive controls was similar to that in 253 wild-type controls (median overall survival 20 months [95% CI 13–26] vs 15 months [13–17]; p=0·244).
Interpretation
In patients with advanced, ALK-positive NSCLC, crizotinib therapy is associated with improved survival compared with that of crizotinib-naive controls. ALK rearrangement is not a favourable prognostic factor in advanced NSCLC.
Introduction
Anaplastic lymphoma kinase (ALK) is one of the newest tyrosine-kinase targets in non-small-cell lung cancer (NSCLC). In about 4% of NSCLC tumours, ALK is aberrantly activated because of a chromosomal rearrangement, leading to expression of an oncogenic fusion kinase, such as EML4–ALK.1–5 Chromosomal rearrangements and other genetic alterations of ALK also occur in anaplastic large-cell lymphoma,6 in flam matory myofibroblastic tumour,7,8 and paediatric neuroblastoma.9–11 In NSCLC, ALK rearrangement is associated with distinct clinicopathological features, including young age of onset, absent or minimal smoking history, and adenocarcinoma histology.4,12–15 EML4–ALK and other oncogenic drivers such as mutant EGFR and oncogenic KRAS are generally mutually exclusive,12 consistent with the notion that ALK rearrangement defines a unique molecular subset of NSCLC.
Preclinical and clinical studies have shown that cancer cells harbouring EML4–ALK and other ALK abnormalities are exquisitely sensitive to ALK inhibition.3,16 In a recent phase 1 clinical trial, crizotinib (PF-02341066), the first clinically available tyrosine-kinase inhibitor (TKI) targeting ALK, showed marked antitumour activity in patients with advanced, ALK-positive NSCLC.17 Response and survival data from this trial were recently updated; among 119 evaluable patients, most of whom had received more than one previous line of therapy, the objective response rate was 61% and median progression-free survival (PFS) was 10 months.18 By comparison, standard single-agent chemotherapies for previously treated, unselected metastatic NSCLC are associated with an objective response rate of less than 10% and a median PFS of less than 3 months.19,20 In the phase 1 trial, crizotinib also showed substantial activity in one patient with ALK-rearranged inflammatory myofibroblastic tumour.21 Furthermore, the side-effects of crizotinib were generally mild and well tolerated.17 Based on these results and those from an ongoing phase 2 trial (NCT00932451), crizotinib has received accelerated approval in the USA.
The effect of crizotinib on overall survival in patients with ALK-positive NSCLC is not yet known. Overall survival has traditionally been regarded as the gold standard for evaluating clinical benefit in clinical trials. However, in the era of targeted therapies, prospective genotyping, and rapid drug development, assessing overall survival benefit poses unique challenges. For example, in the IPASS study,22 gefitinib was associated with a higher response rate and improved PFS compared with standard carboplatin plus paclitaxel chemotherapy in newly diagnosed, EGFR-mutation-positive patients. However, in the final analysis of IPASS, overall survival (a secondary endpoint) was similar between patients who received gefitinib and those who received carboplatin plus paclitaxel.23 Several factors might account for the similar survival outcomes. In particular, a large proportion of chemotherapy-treated patients received subsequent post-study treatments including gefitinib, effectively crossing over to the other study group. A similar issue might also confound evaluation of overall survival in the ongoing phase 3 registration trials of crizotinib, since ALK-positive patients randomised to receive chemotherapy who experience disease progression are eligible to cross over to receive crizotinib. With the recent approval of crizotinib in the USA, crizotinib might become a standard therapy for patients with ALK-positive NSCLC. As a result, assessment of overall survival benefit using a traditional randomised controlled trial with no crossover will not be possible.
In the absence of data from a randomised controlled trial, we addressed whether crizotinib improves survival by comparing overall survival in crizotinib-treated, ALK-positive patients with that in a control group consisting of patients who were retrospectively or prospectively shown to be ALK positive but never received crizotinib. Additionally, we examined overall surival in control groups of ALK-negative patients, including a TKI-sensitive, EGFR-mutant cohort and EGFR wild-type patients, to examine the survival effect of crizotinib in the context of other well defined subsets of NSCLC.
Methods
Study populations and procedures
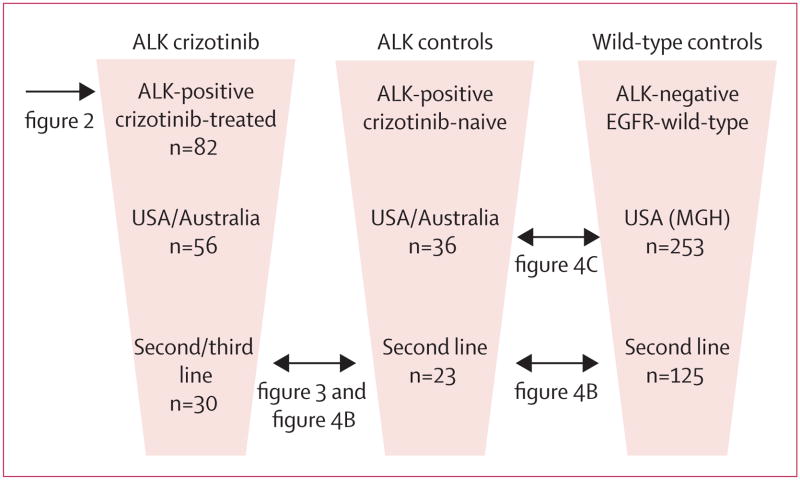
Three populations were used in these retrospective analyses (figure 1). The crizotinib group consisted of a subgroup of 82 patients with advanced ALK-positive NSCLC who received crizotinib in the phase 1 clinical trial (NCT00585195).17 These patients were enrolled at one of seven study sites between Dec 26, 2007, and Feb 10, 2010. For all patients, ALK positivity was confirmed by fluorescence in-situ hybridisation (FISH).12 ALK FISH was done before trial enrolment, using the initial diagnostic or surgical specimen, or a repeat biopsy specimen obtained for the purposes of genetic testing. The trial was designed to assess the safety, pharmacokinetic profile, and pharmacodynamic effects of crizotinib. Additionally, the trial design included expansion cohorts for molecularly defined patients, to identify early evidence of antitumour activity based on response rate. The trial was not designed to evaluate PFS or overall survival. Treatment histories, date of first administration of crizotinib, and survival data were obtained from the sponsor database, as of March 15, 2011. The protocol was approved by the institutional review board at each study centre.
Figure 1. Summary of study populations.
The three study populations and subsets analysed are shown. Double-headed arrows highlight comparisons between specific patient subsets shown in subsequent figures. MGH=Massachusetts General Hospital.
The ALK-positive control group for the present study included 36 patients with advanced, ALK-positive NSCLC who did not receive crizotinib. These crizotinib-naive patients were identified at four of the seven phase 1 study sites through retrospective and prospective screening efforts. Patients who were identified retrospectively had died before crizotinib was clinically available (between 1998 and 2008) or were screened solely for non-trial-related research purposes, or both. Patients identified prospectively were screened on or before Feb 10, 2010, with the intention of enrolment on a clinical trial of crizotinib. At the remaining three study sites, all ALK-positive patients had either already enrolled on the phase 1 trial of crizotinib, or were identified after Feb 10, 2010. Most of the ALK-positive controls (30 [83%] of 36 patients) were diagnosed with metastatic NSCLC between 2007 and 2010. For all control patients, ALK positivity was established based on FISH testing of diagnostic, surgical, or repeat biopsy specimens. All demographic, clinicopathological, treatment, and survival data were obtained from patient medical records via study investigators and were updated as of March 15, 2011.
ALK-negative controls in this study consisted of 320 patients with advanced NSCLC lacking ALK rearrangement. These patients underwent genetic testing at Massachusetts General Hospital (MGH; Boston, MA, USA) over the same time period as ALK-positive patients (up to Feb 10, 2010), and screened negative for ALK rearrangement by FISH. All patients were also screened for EGFR mutations by direct DNA sequencing of exons 18 to 21, or by a multiplex mutation-screening test (SNaPshot).24 There were no cases of coexisting ALK rearrangement and EGFR mutation. Of the 320 ALK-negative controls, 253 were negative for both ALK and EGFR mutations (wild-type controls). Data were obtained in the same manner as for ALK-positive patients, and updated as of March 15, 2011. The chart review of ALK-positive and ALK-negative patients was approved by the institutional review board at each of the participating centres.
Statistical analysis
Fisher’s exact test and Wilcoxon rank-sum test were used to assess the association of treatment group or genotype status with demographic and clinicopathological characteristics and treatment histories. The survival time of patients who were known to be alive at the time of the data update were censored at the date of last follow-up. Median follow-up time was calculated by the reverse Kaplan-Meier approach. Overall survival was estimated by the Kaplan-Meier method, and the log-rank test was used to compare the difference between patient or genotype groups. The hazard ratio (HR) between two groups was estimated by proportional hazards regression with a 95% Wald confidence interval (95% CI). Data analysis was done with SAS version 9.2, and all p values were two-sided.
Role of the funding source
Pfizer Oncology sponsored the phase 1 clinical trial of crizotinib. The sponsor provided funding and organisational support for the trial, and collected data for the 82 crizotinib-treated patients. The sponsor did not identify or collect data for the 36 crizotinib-naive patients. The sponsor did not design or provide funding for this study. The sponsor provided input to the authors on their analysis and interpretation of overall survival data. The sponsor was not involved in writing the report but reviewed the final version before submission. The corresponding author had full access to all the data in the study, and had final responsibility for the decision to submit for publication.
Results
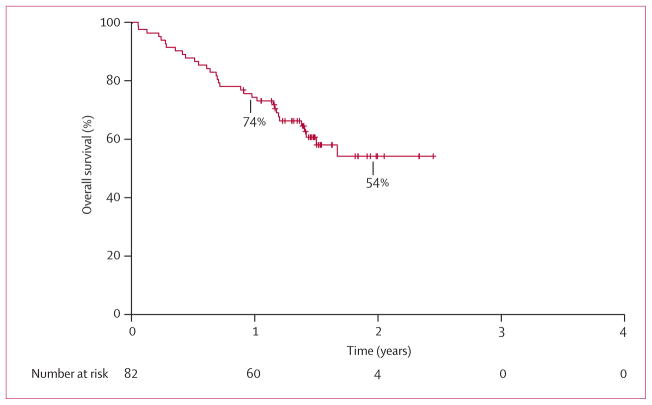
We assessed overall survival in the subgroup of 82 patients with advanced, ALK-positive NSCLC who had enrolled on the multicentre phase 1 clinical trial of crizotinib.17 These patients were mainly young (median age 51 years [range 25–78]), never smokers with adenocarcinoma histology, as previously reported.17 Among the 82 patients, 50 (61%) were enrolled at US study sites, 26 (32%) at the Korean site, and the remaining six (7%) in Australia. Since the protocol placed no restriction on the number of previous therapies, this number varied widely among patients, ranging from zero to seven previous lines (median two) of therapy for metastatic disease. 73 (89%) of the 82 patients had received at least one previous therapy for metastatic disease. As shown in figure 2, median overall survival from the date of first crizotinib dose has not been reached (95% CI 17 months to not reached), 1-year overall survival was 74% (95% CI 63–82), and 2-year overall survival was 54% (40–66). Overall survival did not differ based on age (≤50 vs >50 years, p=0·692), sex (p=0·975), smoking history (never vs any smoking, p=0·857), or ethnic origin (Asian vs non-Asian, p=0·857; webappendix p 2). Median follow-up for the 82 crizotinib-treated patients was 18 months (IQR 16–22).
Figure 2. Overall survival for ALK-positive, crizotinib-treated patients.
Overall survival is shown for the subset of 82 ALK-positive patients who enrolled on the international, multicentre phase 1 clinical trial of crizotinib.17 Overall survival was calculated from the date of first crizotinib dose.
Our control group of 36 ALK-positive patients who had not received crizotinib (figure 1) had been screened at four study sites (three in the USA, one in Australia) over the same time period as crizotinib-treated patients. Since no ALK-positive controls were identified at the Korean study site by the cutoff date of Feb 10, 2010, and since Asians might have different crizotinib pharmacokinetics than white people,25 we compared control, crizotinib-naive patients with the non-Korean cohort of crizotinib-treated patients (n=56). As shown in table 1, both demographic and clinicopathological characteristics were well balanced between the 36 control and 56 crizotinib-treated, ALK-positive patients. The groups were also well balanced in terms of presence of brain metastases at any time during the disease course. Whereas the number of previous therapies for metastatic disease ranged from zero to seven for crizotinib-treated patients, the number of therapies for ALK-positive control patients ranged from one to four. However, the median number of therapies was two for both groups (table 2). Additionally, control and crizotinib-treated patients had received similar types of standard therapies for advanced NSCLC, including platinum-based combination chemotherapy, pemetrexed or pemetrexed-containing regimens, and erlotinib or erlotinib-based regimens (table 2).
Table 1.
Demographic and clinicopathological characteristics of ALK-positive patients
| Crizotinib-treated, non-Korean (n=56) | ALK-positive controls (n=36) | p value | |
|---|---|---|---|
| Age in years, median (range) | 51 (28–78) | 51 (32–78) | 0·896 |
| Sex | |||
| Male | 30 (54%) | 16 (44%) | 0·522 |
| Female | 26 (46%) | 20 (56%) | |
| Ethnic origin | |||
| Asian | 3 (5%) | 3 (8%) | 0·732 |
| White | 48 (86%) | 31 (86%) | |
| Other | 5 (9%) | 2 (6%) | |
| Smoking history | |||
| Never | 46 (82%) | 24 (67%) | 0·255* |
| ≤10 pack-years | 7 (13%) | 7 (19%) | |
| >10 pack-years | 3 (5%) | 5 (14%) | |
| Pathology | |||
| Adenocarcinoma | 54 (96%) | 34 (94%) | 0·643† |
| Squamous-cell | 1 (2%) | 1 (3%) | |
| Large-cell | 1 (2%) | 1 (3%) | |
| Brain metastases | |||
| No | 27 (48%) | 19 (53%) | 0·831 |
| Yes | 29 (52%) | 17 (47%) | |
Data are n (%) unless otherwise stated.
Never or light smoker vs heavy smoker.
Adenocarcinoma vs other histologies.
Table 2.
Treatment histories of ALK-positive patients
| Crizotinib-treated, non-Korean (n=56) | ALK-positive controls (n=36) | p value | |
|---|---|---|---|
| Previous lines, median (range) | 2 (0–7) | 2 (1–4) | 0·678 |
| Platinum regimen | |||
| No | 12 (21%) | 6 (17%) | 0·788 |
| Yes | 44 (79%) | 30 (83%) | |
| Pemetrexed* | |||
| No | 26 (46%) | 12 (33%) | 0·279 |
| Yes | 30 (54%) | 24 (67%) | |
| Erlotinib† | |||
| No | 29 (52%) | 20 (56%) | 0·831 |
| Yes | 27 (48%) | 16 (44%) | |
Data are n (%) unless otherwise stated.
Includes pemetrexed or any pemetrexed-based regimen.
Includes erlotinib or any erlotinib-based regimen.
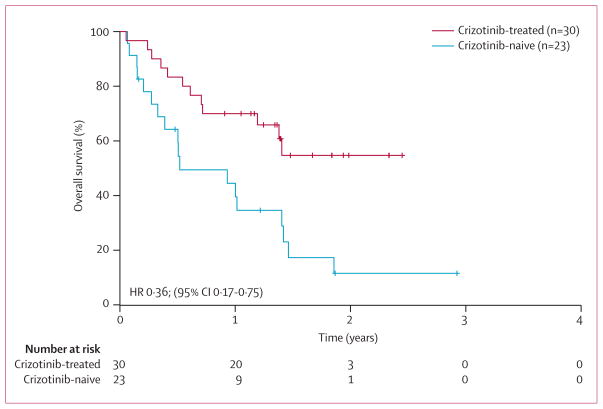
Overall survival in the 56 crizotinib-treated patients, calculated from the date of first crizotinib dose, was similar to that for the entire cohort of 82 patients: median overall survival has not been reached (95% CI 17 months to not reached); 1-year overall survival was 71% (95% CI 58–81), and 2-year overall survival was 57% (40–71). Median overall survival for the 36 crizotinib-naive, ALK-positive control patients, measured from the time of metastatic diagnosis, was 20 months (95% CI 13–26); 1-year overall survival was 72% (95% CI 54–84), and 2-year overall survival was 36% (19–54). However, the comparison of crizotinib-treated and crizotinib-naive patients is complicated by the fact that patients received crizotinib at different lines, from first-line through eighth-line (table 2). For both groups, assessing overall survival from the time of metastatic diagnosis is complicated by the fact that 20 (36%) of 56 crizotinib-treated patients received between three and seven previous lines of therapy. Thus, this approach could overestimate the survival benefit associated with crizotinib. We therefore examined survival outcomes among less heavily pretreated patients. Since only six (11%) of the 56 patients within the non-Korean cohort received crizotinib first-line, we could not compare survival outcomes in the first-line setting. Instead, we focused on the subset of patients who received crizotinib as second-line or third-line therapy and the subset of ALK-positive controls who received any second-line therapy. The clinicopathological features of these two subsets were comparable (webappendix p 3). Among 30 non-Korean patients given crizotinib in the second-line or third-line setting, median overall survival from crizotinib dosing has not been reached (95% CI 14 months to not reached); by contrast, median overall survival of 23 crizotinib-naive controls treated with any second-line therapy was 6 months (4–17; figure 3). 1-year overall survival was 70% (95% CI 50–83) for the crizotinib-treated subset versus 44% (23–64) for the crizotinib-naive subset; 2-year overall survival was 55% (33–72) versus 12% (2–30), respectively (HR 0·36, 95% CI 0·17–0·75; p=0·004). Similar results were obtained even when patients at the Korean study site treated with second-line or third-line crizotinib were included in this subset analysis (webappendix p 1). These results suggest that crizotinib might substantially improve survival outcomes in patients with advanced ALK-positive NSCLC.
Figure 3. Overall survival for crizotinib-treated versus crizotinib-naive, ALK-positive patients.
Overall survival was calculated from second-line or third-line crizotinib therapy in 30 ALK-positive patients, and from any second-line therapy in 23 ALK-positive controls.
To address whether the ALK-positive, crizotinib-naive controls were a valid comparator group for this survival analysis, we examined the screening and selection of control patients. Among the 36 controls, 12 (33%) were identified retrospectively and were not screened for the purposes of trial enrolment. These retrospective controls should accurately represent the natural history of ALK-positive NSCLC. The remaining 24 controls (67%) were identified prospectively with the intention of enrolling on the phase 1 trial. Ten (42%) of the 24 prospective controls remain trial eligible and could receive crizotinib on disease progression. The remaining 14 (58%) of the prospective controls did not receive crizotinib for various reasons, including 11 with unanticipated clinical deterioration (often while waiting for evidence of disease progression) or protocol eligibility issues, or both, and three who were lost to follow-up and never referred to a trial site. To assess whether the prospective controls might represent a subset of patients with a particularly poor prognosis, we compared overall survival for prospective and retrospective controls from the time of metastatic diagnosis. Compared with retrospective controls, prospective controls did not show a significant difference in overall survival (median 26 months [95% CI 13–48] vs 18 months [6–22], p=0·195; webappendix p 4). The lack of a significant difference might be a reflection of the small sample size of each group. However, these findings do support the use of both retrospectively and prospectively identified patients within the ALK-positive control cohort, since they could represent similar prognostic groups.
To evaluate the survival outcomes of ALK-positive patients in the context of the general NSCLC population, we identified an ALK-negative control group of patients with advanced NSCLC. This ALK-negative group was identified at the same time as the ALK-positive group through large-scale screening efforts at one US study centre (MGH). All patients were screened for ALK rearrangement and EGFR mutation. Among the 320 ALK-negative patients, we identified 67 with known EGFR sensitising mutations, referred to as EGFR-positive, and 253 without EGFR mutations (figure 1). Patients with atypical EGFR mutations such as exon 20 insertion were not included in this analysis. Patients who were both ALK-negative and EGFR-negative are referred to as wild-type. Table 3 summarises the demographic and clinicopathological features of ALK-positive, EGFR-positive, and wild-type patients. The cohort of 92 ALK-positive patients includes the 56 non-Korean, crizotinib-treated patients plus the 36 ALK-positive controls. Consistent with published studies,4,12,13,15 ALK-positive patients were significantly younger than either EGFR-positive or wild-type patients (p<0·0001; table 3). Other significant differences between ALK-positive and wild-type patients included never or light smoking history (91% vs 38%, p<0·0001) and adenocarcinoma histology (96% vs 88%, p=0·041). By contrast, ALK-positive and EGFR-positive patients were very similar with respect to smoking history and histological subtype (table 3). For all three groups, the proportion of patients with brain metastases at any time during their disease course was similar, and the median number of treatments received by each group was two (table 3).
Table 3.
Demographic and clinicopathological characteristics of ALK-positive and ALK-negative patients
| ALK-positive (n=92) | ALK-negative (n=320)
|
p value
|
|||
|---|---|---|---|---|---|
| EGFR-positive (n=67) | Wild-type* (n=253) | ALK-positive vs EGFR-positive | ALK-positive vs wild-type | ||
| Age in years, median (range) | 51 (28–78) | 63 (36–90) | 64 (27–87) | <0·0001 | <0·0001 |
| Number of treatments, median (range) | 2 (0–7) | 2 (0–10) | 2 (0–10) | 0·045 | 00·985 |
| Sex | |||||
| Male | 46 (50%) | 23 (34%) | 108 (43%) | 0·054 | 00·270 |
| Female | 46 (50%) | 44 (66%) | 145 (57%) | ||
| Ethnic origin | |||||
| Asian | 6 (7%) | 9 (13%) | 15 (6%)¶ | 0·356 | 00·088 |
| Caucasian | 79 (86%) | 54 (81%) | 231 (92%)¶ | ||
| Other | 7 (8%) | 4 (6%) | 6 (2%)¶ | ||
| Smoking history | |||||
| Never | 70 (76%) | 42 (63%) | 63 (25%) | 1·000† | <0·0001† |
| ≤10 pack-years | 14 (15%) | 20 (30%) | 32 (13%) | ||
| >10 pack-years | 8 (9%) | 5 (7%) | 158 (62%) | ||
| Pathology | |||||
| Adenocarcinoma | 88 (96%) | 66 (99%) | 223 (88%) | 0·398‡ | 00·041‡ |
| NOS | 0 (0%) | 0 (0%) | 15 (6%) | ||
| Squamous-cell | 2 (2%) | 1 (1%) | 10 (4%) | ||
| Large-cell | 2 (2%) | 0 (0%) | 5 (2%) | ||
| Brain metastases§ | |||||
| No | 46 (50%) | 29 (43%) | 147 (58%)¶ | 0·425 | 0·179 |
| Yes | 46 (50%) | 38 (57%) | 105 (42%)¶ | ||
Data are n (%) unless otherwise stated. NOS=not otherwise specified.
ALK-negative and EGFR-negative.
Never or light smoker vs heavy smoker.
Adenocarcinoma vs other histologies.
At any time during the disease course.
Missing information for one patient in these categories, so numbers total 252.
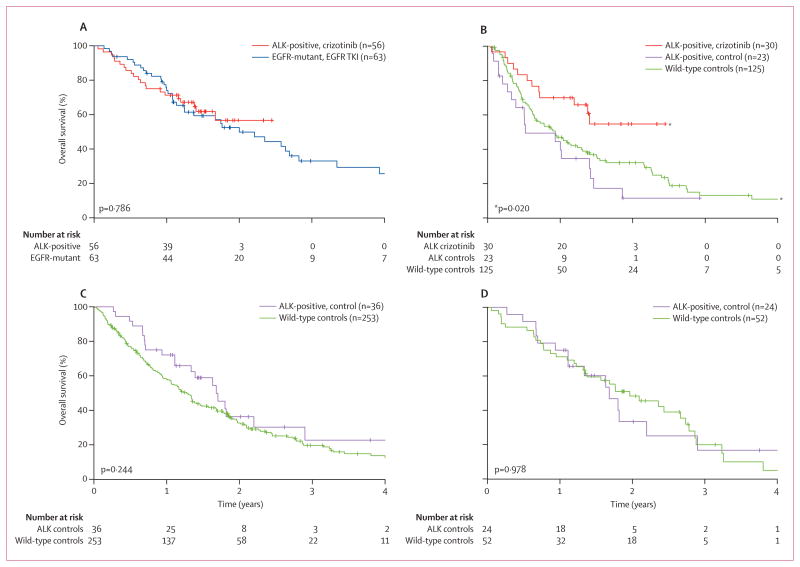
Patients who are EGFR-positive are a paradigm for successful targeting of a genetically defined subset of NSCLC. ALK positivity defines a new and distinct subset with similarities to EGFR-positive patients, in terms of clinicopathological features and responsive ness to TKIs. Therefore, we compared survival in ALK-positive versus ALK-negative, EGFR-positive patients, since the latter represent a well established, TKI-sensitive comparator population. First, we compared survival in crizotinib-treated, ALK-positive patients with that in EGFR-positive patients who received either gefitinib or erlotinib. As shown in figure 4A, from the time of starting TKI therapy, survival was similar for crizotinib-treated, ALK-positive patients (median overall survival not reached [95% CI 17 months to not reached], 1-year overall survival 71% [95% CI 58–81], 2-year overall survival 57% [40–71]) compared with EGFR TKI-treated, EGFR-positive patients (median overall survival 24 months [95% CI 15–34], 1-year overall survival 74% [61–83], 2-year overall survival 52% [38–65]; p=0·786). Since only six (11%) of 56 ALK-positive patients received crizotinib first-line, whereas most EGFR-positive patients (44 [70%] of 63) received first-line EGFR TKI therapy, we also compared survival by line of TKI therapy. Among the subsets of 30 ALK-positive and 19 EGFR-positive patients who received TKI therapy as their second-line or third-line agent, overall survival from the time of TKI therapy was also similar (median overall survival not reached [95% CI 14 months to not reached] vs 15 months [12–32]; 1-year overall survival 70% [95% CI 50–83] vs 72% [46–87], 2-year overall survival 55% [33–72] vs 43% [20–64], respectively; p=0·578). Thus, ALK-positive patients given crizotinib have a similar overall survival to EGFR-positive patients given an EGFR TKI.
Figure 4. Overall survival for ALK-positive versus ALK-negative patients.
(A) Overall survival for 56 crizotinib-treated, ALK-positive patients compared with 63 ALK-negative, EGFR-positive patients who received erlotinib or gefitinib. Overall survival was calculated from the start of crizotinib or EGFR TKI therapy. (B) Overall survival comparison for ALK-positive vs ALK-negative, EGFR-negative (wild-type) patients. Overall survival was calculated from second-line or third-line crizotinib in 30 ALK-positive patients and from any second-line therapy in 23 ALK-positive controls and 125 wild-type controls. p value is for the difference in overall survival between crizotinib-treated, ALK-positive patients and wild-type controls. (C) Overall survival comparison for 36 ALK-positive, crizotinib-naive patients vs 253 wild-type controls. Overall survival was calculated from metastatic diagnosis. (D) Overall survival analysis in younger (≤60 years) never or light-smoking patients in the ALK-positive, crizotinib-naive vs wild-type groups. Overall survival was calculated from metastatic diagnosis.
We also assessed survival in ALK-positive patients versus wild-type controls, for whom there are generally no effective TKIs. Analyses of overall survival measured from the time of metastatic diagnosis or from the time of second-line therapy yielded similar results. As shown in figure 4B, median overall survival from second-line therapy among ALK-positive patients who did not receive crizotinib (n=23) was 6 months (95% CI 4–17), whereas that of wild-type controls (n=125) was 11 months [8–15]; however, this difference was not significant (p=0·175), suggesting that ALK-positive controls might have similar survival to wild-type controls. By comparison, ALK-positive patients given second-line or third-line crizotinib had significantly better survival than wild-type controls (HR 0·49, 95% CI 0·27–0·91; p=0·020; figure 4B), suggesting that crizotinib-treated, ALK-positive patients have better survival than the general population of NSCLC patients. From time of metastatic diagnosis, the survival of 36 crizotinib-naive, ALK-positive controls did not differ significantly from that of the entire group of 253 wild-type controls, with a median overall survival of 20 months (95% CI 13–26) versus 15 months (13–17; HR 0·77, 95% CI 0·50–1·19; p=0·244; figure 4C).
Comparing the survival of ALK-positive controls versus wild-type controls is potentially complicated by the fact that ALK-positive patients had distinct clinicopathological features (table 3). The most important feature was never or light smoking status, a well-established prognostic factor. Therefore, we compared ALK-positive controls, most of whom were never or light smokers, with the subset of wild-type controls who were never or light smokers. Among never or light smoking patients younger than 60 years, overall survival from the time of metastatic diagnosis was similar between 24 ALK-positive controls and 52 wild-type controls; median overall survival was 20 months (95% CI 13–26) versus 24 months (16–33; HR 1·01, 95% CI 0·55–1·85; p=0·978; figure 4D). Similar results were obtained from analysis of the subset of never or light smoking patients with adenocarcinoma histology (data not shown). Taken together, these results suggest that in the absence of crizotinib therapy, the survival outcome of ALK-positive patients is similar to that of clinically comparable NSCLC patients who are ALK-negative and EGFR-negative.
Discussion
Our data suggest that overall survival for patients with advanced ALK-positive NSCLC was significantly longer in the subset of patients given second-line or third-line crizotinib than in clinically comparable, crizotinib-naive controls. Thus, crizotinib might prolong overall survival in ALK-positive NSCLC. Additionally, crizotinib-naive, ALK-positive patients had a generally poor outcome, similar to that of the general population of NSCLC patients. Thus, ALK rearrangement is not a favourable prognostic factor in advanced NSCLC.
Crizotinib has already shown impressive single-agent activity in ALK-positive NSCLC, as assessed by response rate and PFS;17,18 however, the effect of crizotinib on overall survival is important to establish as an indication of ultimate clinical benefit. Overall survival has traditionally been regarded as the most reliable endpoint in evaluating an experimental cancer therapy, with improvement in overall survival serving as an important cornerstone for regulatory approval. Overall survival is typically assessed in a randomised controlled study, to enable the most direct and unbiased comparison of survival outcomes. With the advent of highly effective, targeted therapies for select molecularly defined patients, there is increasing uncertainty whether overall survival as assessed in randomised controlled studies is a practical or meaningful primary endpoint. Assessment of overall survival is confounded by subsequent post-study treatments, particularly salvage therapy with the experimental drug for control patients. This type of crossover might diminish overall survival differences between the control and experimental groups. In both the first-line and second-line registration trials of crizotinib, patients in the chemotherapy group are permitted to crossover and receive crizotinib at the time of progression. Thus, it might be that neither of these randomised trials will show a definitive result with regard to the overall survival benefit of crizotinib.
To assess the potential effect of crizotinib on overall survival, we compared survival in a subset of ALK-positive patients who were given the drug in the phase 1 clinical trial, with that of clinically comparable, ALK-positive controls. This survival analysis has several limitations, the most important of which is that it was a retrospective, non-randomised study with unmatched and potentially unbalanced study populations. Survival differences between treated patients and historical or historical-like controls can be difficult to interpret because of confounding by differences in patient selection, and differences in standard and supportive-care treatments. These differences would be minimised in a randomised controlled study. In our analysis, the potential for patient selection bias is particularly important to address. ALK-positive patients who received crizotinib were all participants in a clinical trial and had to meet specific eligibility requirements for enrolment. More than a third of the crizotinib group had received three or more prior lines of therapy,17 suggesting a potentially more indolent disease course. By contrast, ALK-positive controls did not participate in a trial of crizotinib and represent a more heterogeneous population. Nearly a third of the control patients (11 of 36) were screened for ALK with the intention of enrolling on a trial, but were subsequently deemed ineligible or inappropriate for the trial. Patient selection bias could therefore have contributed to a systematic imbalance favouring improved survival in the crizotinib group and worse survival in the control group. A second limitation with our analysis is that we did not have detailed information on post-crizotinib therapies. As of last follow-up, 25 (30%) of the 82 patients were known to have received additional chemotherapies after relapsing on crizotinib, and these could have contributed to an imbalance favouring the crizotinib group. Finally, with respect to the prognostic importance of ALK, a limitation in this analysis is that the wild-type comparator group was probably heterogeneous at the molecular level, with some patients potentially harbouring other oncogenic drivers such as mutant KRAS and HER2. The molecular heterogeneity of the wild-type patients might or might not be an accurate reflection of that in the general NSCLC population.
Three lines of evidence support the validity of our conclusions and address the specific issue of patient selection bias. First, the ALK-positive cohorts were well balanced in terms of demographic features, including age, sex, ethnic origin, and smoking history (table 1), which are important factors when assessing survival outcomes.26–28 Ethnic origin was important to control for since Asian patients have a higher response rate to crizotinib,18 which might be partly due to pharmacokinetic differences and higher effective crizotinib concentrations.25 We were not able to compare performance status, a well established prognostic factor in advanced NSCLC, since this was not consistently assessed in control patients at the start of each line of therapy. However, the cohorts were balanced with regard to another potentially important prognostic indicator—presence of brain metastases.29 Second, although patients who received crizotinib did have a greater range in number of previous therapies than controls (0–7 vs 1–4), which could have contributed to their survival advantage, the median number of prior therapies was two in both groups (table 2). Exposure to standard platinum-based combination chemotherapy was similar between the two groups, as was exposure to therapies that might be particularly active or inactive in ALK-positive NSCLC, such as pemetrexed30,31 and erlotinib,12 respectively. Finally, survival differences between the crizotinib and control groups were seen in subsets of patients with similar numbers of previous therapies; we compared overall survival in patients after second-line or third-line crizotinib with that of controls after any second-line therapy. Although the numbers of patients in this subset analysis were small, there was a significant survival difference favouring the crizotinib group. This subset analysis should minimise patient selection bias, and the skewing of survival outcomes by control patients with rapidly progressive disease who never received second-line therapy, and by crizotinib-treated patients with more indolent disease who were able to have several lines of therapy before enrolling on a trial. We would also expect the inclusion of patients given crizotinib in either the second-line or third-line setting, as opposed to a pure second-line population, to potentially bias against the crizotinib group; however, crizotinib therapy was associated with longer survival than standard non-crizotinib therapies.
In this study, the control group represents the natural history of advanced ALK-positive NSCLC in the absence of effective targeted therapy. Until now, the natural history of the disease has been poorly understood, with only a few case series reporting data on survival. For example, in an unadjusted analysis of a small cohort of 103 surgically resected patients, 12 of whom were ALK-positive based on rapid amplification of cDNA ends (RACE)-coupled PCR sequencing, there was a small, non-significant improvement in survival in ALK-positive compared with ALK-negative patients.15 By contrast, within a cohort of never-smokers with lung adenocarcinoma screened at the Mayo Clinic (Rochester, MN, USA), there was significantly worse stage-adjusted, 5-year PFS or recurrence-free survival among ALK-positive compared with ALK-negative patients.32 A worse outcome was also recently reported in a case-matched retrospective study of Korean patients with ALK-positive or ALK-negative NSCLC.33 Our results show that in the absence of crizotinib, ALK-positive patients have similar survival to the general population of wild-type patients lacking either ALK or EGFR. The same result was noted even after accounting for prognostic factors such as age and smoking history. Taken together, these findings suggest that in the absence of crizotinib therapy, ALK rearrangement is not a favourable prognostic factor for survival.
In conclusion, the prolonged survival associated with crizotinib therapy in this study, along with the documented response rate and median PFS, all support the notion that crizotinib can fundamentally alter the natural history of ALK-positive NSCLC (panel). The true effect of crizotinib on overall survival as assessed in a randomised controlled study without crossover might never be established. However, based on the data presented here, other estimates of clinical benefit such as objective response rate and PFS, and anticipated improvements in quality of life, crizotinib will probably become a standard of care for patients with advanced, ALK-positive NSCLC.
Supplementary Material
Systematic review
We did a systematic review of the literature before initiating this survival analysis. We searched PubMed using the search terms “ALK” and “lung cancer”. In 2007, ALK rearrangement was first reported as a novel oncogenic driver in NSCLC.1 At that time, crizotinib was already being tested in a phase 1 clinical trial. Crizotinib was found to be highly active in patients with advanced, ALK-positive NSCLC.17 Responses were often substantial and durable; however, it was unknown whether these responses would translate into a clinically meaningful improvement in overall survival. Additionally, although there were many case series reporting on the clinicopathological features associated with ALK rearrangement, there was no published data on the natural history of ALK-positive NSCLC. Therefore, it was unknown whether ALK-positive patients might have an intrinsically better prognosis than the general population of patients with NSCLC, and derive comparable benefit from standard therapies. To address these questions, crizotinib is currently being assessed in two randomised phase 3 studies, both of which are still accruing patients. The overall survival benefit of crizotinib might be difficult to establish in these trials because of crossover, so we assessed survival benefit with a retrospective analysis of survival outcomes among ALK-positive patients given crizotinib on the single-arm phase 1 trial, versus ALK-positive control patients given standard therapies for advanced NSCLC.
Interpretation
Our results provide evidence that crizotinib might improve overall survival in patients with advanced, ALK-positive NSCLC. In the absence of crizotinib, patients with ALK-positive NSCLC have a similar prognosis to the general population of NSCLC patients. We recommend that clinicians consider ALK testing for patients with advanced lung adenocarcinoma, particularly those who are known to be negative for EGFR and KRAS mutations. In the USA, clinicians may prescribe crizotinib for patients with advanced, ALK-positive NSCLC. Outside the USA, clinicians should direct these patients to clinical trials of crizotinib.
Acknowledgments
Funding: Pfizer Inc, V Foundation for Cancer Research.
We thank our patients and their families. We also thank members of the phase 1 study teams for their hard work, dedication, and support. The phase 1 trial of crizotinib was sponsored by Pfizer Inc. This study was supported by internal funds from the MGH Cancer Center and MGH Pathology Department. ATS is supported in part by the V Foundation for Cancer Research and by the Sig Adler Cancer Research Fund. BYY and DBC are supported by an NCI Lung Cancer Spore (P50-CA090578). DBC also receives funding from the American Cancer Society. Heather Saxton (Acumed; New York, NY, USA) assisted in collecting author statement forms, with funding from Pfizer.
Footnotes
Contributors
ATS and BYY designed the study and analysed the data. ATS, BJS, GJR, JG, GIS, DBC, S-HIO, MB, RS, RGM, MV-G, RCD, Y-JB, ELK, JWC, AJI, and DRC collected data. ATS, BYY, BJS, GJR, JAE, KK, PS, YT, KDW, and DRC interpreted the data. ATS wrote the report. All authors read and provided comments on the report.
Conflicts of interest
ATS, BJS, DBC, RGM, Y-JB, ELK, and AJI received honoraria or consulting fees from Pfizer. RGM served as a paid expert witness for Pfizer. BJS, GJR, GIS, RGM, and Y-JB received research funding from Pfizer. KK, PS, YT, and KDW are employees and stockholders of Pfizer. AJI received honoraria or consulting fees from Abbott Laboratories. ATS, GJR, JAE, and JWC received honoraria or consulting fees from ARIAD. ATS and GJR received honoraria or consulting fees from Chugai. GJR received honoraria or consulting fees from Merck, Boehringer-Ingelheim, and Tragara. JAE received consulting fees and research funding from Novartis and AstraZeneca. All other authors declared no conflicts of interest.
References
- 1.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–66. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 2.Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 3.Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14:4275–83. doi: 10.1158/1078-0432.CCR-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115:1723–33. doi: 10.1002/cncr.24181. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi K, Choi YL, Soda M, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res. 2008;14:6618–24. doi: 10.1158/1078-0432.CCR-08-1018. [DOI] [PubMed] [Google Scholar]
- 6.Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–84. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 7.Griffin CA, Hawkins AL, Dvorak C, Henkle C, Ellingham T, Perlman EJ. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59:2776–80. [PubMed] [Google Scholar]
- 8.Coffin CM, Patel A, Perkins S, Elenitoba-Johnson KS, Perlman E, Griffin CA. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol. 2001;14:569–76. doi: 10.1038/modpathol.3880352. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Takita J, Choi YL, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455:971–74. doi: 10.1038/nature07399. [DOI] [PubMed] [Google Scholar]
- 10.George RE, Sanda T, Hanna M, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;455:975– 78. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janoueix-Lerosey I, Lequin D, Brugieres L, et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature. 2008;455:967–70. doi: 10.1038/nature07398. [DOI] [PubMed] [Google Scholar]
- 12.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247–53. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol. 2009;22:508–15. doi: 10.1038/modpathol.2009.2. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi T, Sonobe M, Kobayashi M, et al. Clinicopathologic features of non-small-cell lung cancer with EML4-ALK fusion gene. Ann Surg Oncol. 2010;17:889–97. doi: 10.1245/s10434-009-0808-7. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Zhang S, Yang X, et al. Fusion of EML4 and ALK is associated with development of lung adenocarcinomas lacking EGFR and KRAS mutations and is correlated with ALK expression. Mol Cancer. 2010;9:188. doi: 10.1186/1476-4598-9-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDermott U, Iafrate AJ, Gray NS, et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res. 2008;68:3389–95. doi: 10.1158/0008-5472.CAN-07-6186. [DOI] [PubMed] [Google Scholar]
- 17.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camidge DR, Bang Y-J, Kwak EL, et al. Progression-free survival (PFS) from a phase 1 study of crizotinib (PF-02341066) in patients with ALK-positive non-small cell lung cancer (NSCLC) Proc Am Soc Clin Oncol. 2011;29:2501 (abstr). [Google Scholar]
- 19.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 20.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 21.Butrynski JE, D’Adamo DR, Hornick JL, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363:1727–33. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 23.Yang C-H, Fukuoka M, Mok TS, et al. Final overall survival results from a phase III randomized, open-label, first-line study of gefitinib v carboplatin/paclitaxel in clinically selected patients with advanced non-small cell lung cancer in Asia. Ann Oncol. 2010;21(suppl 8):1–2. [Google Scholar]
- 24.Dias-Santagata D, Akhavanfard S, David SS, et al. Rapid targeted mutational analysis of human tumours: a clinical platform to guide personalized cancer medicine. EMBO Mol Med. 2010;2:146–58. doi: 10.1002/emmm.201000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ou SI, Salgia R, Clark JW. Comparison of crizotinib (PF-02341066) pharmacokinetics between Asian and non-Asian patients with advanced malignancies. J Thorac Oncol. 2010;5(suppl 5):382. [Google Scholar]
- 26.Kawaguchi T, Takada M, Kubo A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol. 2010;5:620–30. doi: 10.1097/JTO.0b013e3181d2dcd9. [DOI] [PubMed] [Google Scholar]
- 27.Kawaguchi T, Takada M, Kubo A, et al. Gender, histology, and time of diagnosis are important factors for prognosis: analysis of 1499 nevers-mokers with advanced non-small cell lung cancer in Japan. J Thorac Oncol. 2010;5:1011–17. doi: 10.1097/JTO.0b013e3181dc213e. [DOI] [PubMed] [Google Scholar]
- 28.Ou SH, Ziogas A, Zell JA. Asian ethnicity is a favorable prognostic factor for overall survival in non-small cell lung cancer (NSCLC) and is independent of smoking status. J Thorac Oncol. 2009;4:1083–93. doi: 10.1097/JTO.0b013e3181b27b15. [DOI] [PubMed] [Google Scholar]
- 29.Zabel A, Debus J. Treatment of brain metastases from non-small-cell lung cancer (NSCLC): radiotherapy. Lung Cancer. 2004;45(suppl 2):247–52. doi: 10.1016/j.lungcan.2004.07.968. [DOI] [PubMed] [Google Scholar]
- 30.Camidge DR, Kono SA, Lu X, et al. Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J Thorac Oncol. 2011;6:774–80. doi: 10.1097/JTO.0b013e31820cf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JO, Kim TM, Lee SH, et al. Anaplastic lymphoma kinase translocation: a predictive biomarker of pemetrexed in patients with non-small cell lung cancer. J Thorac Oncol. 2011 doi: 10.1097/JTO.0b013e3182208fc2. published online June 2. [DOI] [PubMed] [Google Scholar]
- 32.Yang P, Kulig K, Oliviera AM, et al. Anaplastic lymphoma kinase (ALK) status and clinical outcomes by IHC and FISH: a retrospective study of never-smoker, adenocarcinoma lung cancer cases (Abstract 47PD) Lung Cancer. 2011;71(suppl 2):26–28. [Google Scholar]
- 33.Kim D, Lee JK, Park HS, et al. Comparative analyses of overall survival of anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC) patients who did not receive ALK inhibitors. Proc Am Soc Clin Oncol. 2011;29:7515 (abstr). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.