Abstract
We investigated the effects of frequency of hemodialysis on nutritional status by analyzing the data in the Frequent Hemodialysis Network Trial. We compared changes in albumin, body weight and composition among 245 patients randomized to 6- or 3-times per week in-center hemodialysis (Daily Trial) and 87 patients randomized to 6-times per week nocturnal or 3-times per week conventional hemodialysis, performed largely at home (Nocturnal Trial). In the Daily Trial, there were no significant differences between groups in changes in serum albumin or the equilibrated protein catabolic rate by 12 months. There was a significant relative decrease in pre-dialysis body weight of 1.5 ± 0.2 kg in the 6 times per week group at one month, but this significantly rebounded by 1.3 ± 0.5 kg over the remaining 11 months. Extracellular water decreased in the 6 times per week compared to the 3 per week hemodialysis group. There were no significant between-group differences in phase angle, intracellular water or body cell mass. In the Nocturnal Trial, there were no significant between-group differences in any study parameter. Any gain in “dry” body weight corresponded to increased adiposity rather than muscle mass but was not statistically significant. Thus, frequent in-center hemodialysis reduced extracellular water but did not increase serum albumin or body cell mass while frequent nocturnal hemodialysis yielded no net effect on parameters of nutritional status or body composition.
Introduction
Chronic kidney disease is often accompanied by reductions in serum albumin and prealbumin and progressive loss of muscle and adipose tissue, likely due to inadequate macronutrient intake, inflammation (1), metabolic acidosis (2,3), reduced physical activity (4), or a combination of these processes (5,6,7). Protein-energy wasting (PEW) generally tends to progress slowly once dialysis is initiated (8,9). Although several putative causal factors may be corrected by better control of uremia, the Mortality and Morbidity in Hemodialysis (HEMO) Study showed no associations between increased dialysis dose administered thrice-weekly and biochemical proxies of PEW assessed by caliper anthropometry (10). Frequent (“daily”) hemodialysis has been reported to preserve nutritional status (11;12;13). Previous studies of frequent hemodialysis were not randomized, typically had small sample sizes, and utilized anthropometric measures of body composition.
The Frequent Hemodialysis Network (FHN) Trials aimed to examine the effects of increased hemodialysis frequency on multiple intermediate outcome measures, including nutritional status and body composition. Frequent (6x per week) hemodialysis provided as in-center daily or nocturnal at-home hemodialysis was compared to conventional thrice weekly hemodialysis. The objectives and protocol summaries of both trials have been previously published (14). Limited by sample size, the FHN Trials were not designed to assess mortality or major health events.
We have previously reported that in-center and nocturnal frequent hemodialysis interventions failed to increase the 12-month serum albumin concentration, which we stipulated as the primary outcome for the nutritional status domain. In this manuscript, we present treatment effects on equilibrated protein catabolic rate (ePCR), a proxy for dietary protein intake, and body composition, as reflected by bioimpedance-measured resistance, reactance, phase angle and vector length and derived estimates of intracellular (ICW) and extracellular water (ECW) and body cell mass (BCM).
RESULTS
A total of 245 subjects were randomized in the Daily Trial and 87 subjects were randomized in the Nocturnal Trial. Baseline characteristics are summarized in Table 1. Subjects participating in both trials were diverse in terms of age, sex, race/ethnicity and other clinical characteristics. While the two trials were not formally compared, ESRD vintage was shorter and residual kidney function higher in the Nocturnal Trial. While we had planned to evaluate the effects of frequent hemodialysis on multiple aspects of nutritional status and body composition, our primary outcome within the nutrition domain was the change in serum albumin concentration from baseline to end-of-treatment (12 months). There were significant treatment differences in weekly standard Kt/Vurea, and per-session and weekly ultrafiltration volume, as previously reported (15,16).
Table 1. Subject Characteristics During Baseline.
| Variables | Daily Trial | Nocturnal Trial | ||||||
|---|---|---|---|---|---|---|---|---|
| N | All (N=245) | 3 times (N=120) | 6 times (N=125) | N | All (N=87) | 3 times (N=42) | 6 times (N=45) | |
| Age (years) | 245 | 50.4 ± 13.9 | 52.0 ± 14.1 | 48.9 ± 13.6 | 87 | 52.8 ± 13.6 | 54.0 ± 12.9 | 51.7 ± 14.4 |
| Male | 245 | 151 (61.6%) | 73 (60.8%) | 78 (62.4%) | 87 | 57 (65.5%) | 28 (66.7%) | 29 (64.4%) |
| Race/Ethnicity | 245 | 87 | ||||||
| White/Caucasion, non-Hispanic | 89 (36.3%) | 46 (38.3%) | 43 (34.4%) | 48 (55.2%) | 21 (50.0%) | 27 (60.0%) | ||
| Black/African-American/African | 102 (41.6%) | 53 (44.2%) | 49 (39.2%) | 23 (26.4%) | 11 (26.2%) | 12 (26.7%) | ||
| Hispanic & non-Black | 69 (28.2%) | 31 (25.8%) | 38 (30.4%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Asian | 16 (6.5%) | 5 (4.2%) | 11 (8.8%) | 12 (13.8%) | 7 (16.7%) | 5 (11.1%) | ||
| Other/Unknown | 22 (9.0%) | 8 (6.7%) | 14 (11.2%) | 1 (1.1%) | 1 (2.4%) | 0 (0%) | ||
| ESRD vintage (years) | 245 | 87 | ||||||
| < 1 year (%) | 40 (16.3%) | 20 (16.7%) | 20 (16.0%) | 45 (51.7%) | 25 (59.5%) | 20 (44.4%) | ||
| 1 to < 2 years (%) | 32 (13.15) | 15 (12.5%) | 17 (13.6%) | 13 (14.9%) | 5 (11.9%) | 8 (17.8%) | ||
| 2 – 5 years (%) | 76 (31.0%) | 42 (35.0%) | 34 (27.2%) | 13 (14.9%) | 5 (11.9%) | 8 (17.8%) | ||
| > 5 years (%) | 97 (39.6%) | 43 (35.8%) | 54 (43.2%) | 16 (18.4%) | 7 (16.7%) | 9 (20.0%) | ||
| Diabetes | 245 | 100 (40.8%) | 50 (41.7%) | 50 (40.0%) | 87 | 37 (42.5%) | 18 (42.9%) | 19 (42.2%) |
| Congestive heart failure | 245 | 49 (20.0%) | 24 (20.0%) | 25 (20.0%) | 87 | 12 (13.8%) | 7 (16.7%) | 5 (11.1%) |
| Peripheral arterial disease | 245 | 25 (10.2%) | 10 (8.33%) | 15 (12.0%) | 87 | 15 (17.2%) | 7 (16.7%) | 8 (17.8%) |
| Stroke | 245 | 18 (7.3%) | 9 (7.5%) | 9 (7.2%) | 87 | 2 (2.3%) | 1 (2.4%) | 1 (2.2%) |
| Average weekly enPCR (g/kg/d) | 245 | 1.02 ± 0.25 | 1.03 ± 0.25 | 1.02 ± 0.25 | 84 | 0.99 ± 0.25 | 0.99 ± 0.23 | 0.98 ± 0.28 |
| PCR | 245 | 63.4 ± 20.0 | 63.4 ± 19.1 | 63.5 ± 20.9 | 84 | 63.6 ± 21.3 | 64.4 ± 22.4 | 62.8 ± 20.3 |
| Residual kidney function (ml/min) | 245 | 87 | ||||||
| = 0 | 162 (66.1%) | 72 (60.0%) | 90 (72%) | 24 (27.6%) | 11 (26.2%) | 13 (28.9%) | ||
| 0 - 1 | 37 (15.1%) | 19 (15.8%) | 18 (14.4%) | 16 (18.4%) | 9 (21.4%) | 7 (15.6%) | ||
| 1 - 3 | 42 (17.1%) | 27 (22.5%) | 15 (12.0%) | 28 (32.2%) | 14 (33.3%) | 14 (31.1%) | ||
| > 3 | 4 (1.6%) | 2 (1.7%) | 2 (1.6%) | 19 (21.8%) | 8 (19.0%) | 11 (24.4%) | ||
| Creatinine (mg/dL) | 245 | 10.54 ± 2.72 | 10.32 ± 2.45 | 10.76 ± 2.95 | 87 | 8.74 ± 2.99 | 8.89 ± 3.06 | 8.59 ± 2.94 |
| Phosphate (mg/dL) | 245 | 5.78 ± 1.64 | 5.64 ± 1.53 | 5.91 ± 1.73 | 87 | 5.80 ± 1.61 | 5.77 ± 1.65 | 5.82 ± 1.59 |
| Hemoglobin (g/dL) | 244 | 11.9 ± 1.3 | 12.0 ± 1.2 | 11.9 ± 1.3 | 87 | 11.8 ± 1.1 | 11.9 ± 1.1 | 11.6 ± 1.1 |
| PTH (pg/mL = ng/L) | 244 | 312 (69, 901) | 287 (52, 873) | 334 (89, 984) | 87 | 322 (77, 635) | 340 (112, 618) | 296 (77, 640) |
| Albumin (g/dL) | 245 | 3.94 ± 0.42 | 3.94 ± 0.46 | 3.94 ± 0.37 | 87 | 3.91 ± 0.49 | 3.92 ± 0.51 | 3.90 ± 0.48 |
| Calcium (mg/dL) | 245 | 9.01 ± 0.92 | 9.04 ± 0.96 | 8.99 ± 0.89 | 87 | 8.83 ± 0.80 | 8.96 ± 0.79 | 8.71 ± 0.80 |
| Bicarbonate (mmol/L) | 245 | 23.7 ± 3.7 | 23.7 ± 4.0 | 23.7 ± 3.4 | 87 | 22.9 ± 3.8 | 22.8 ± 3.6 | 22.9 ± 4.0 |
| BMI (kg/m2) | 245 | 27.6 ± 6.7 | 27.6 ± 6.8 | 27.5 ± 6.6 | 87 | 29.1 ± 7.9 | 28.4 ± 7.6 | 29.8 ± 8.3 |
| Phase angle (degrees) | 234 | 5.43 ± 1.51 | 5.21 ± 1.21 | 5.65 ± 1.74 | 78 | 5.51 ± 1.48 | 5.54 ± 1.48 | 5.49 ± 1.51 |
| Intra-cellular water (L)/Weight (kg) | 234 | 0.267 ± 0.062 | 0.261 ± 0.061 | 0.273 ± 0.062 | 78 | 0.268 ± 0.066 | 0.273 ± 0.065 | 0.264 ± 0.068 |
| Postdialysis Weight (kg) | 245 | 78.2 ± 20.5 | 78.7 ± 20.5 | 77.7 ± 20.7 | 87 | 85.5 ± 25.4 | 83.3 ± 23.8 | 87.6 ± 27.0 |
| Adiposity (kg) | 234 | 36.9±13.7 | 37.6±13.7 | 36.3±13.8 | 80 | 39.4±16.2 | 37.9±14.6 | 40.9±17.7 |
| TBW | 234 | 44.2 ± 9.9 | 43.9± 10.2 | 44.5 ± 9.7 | 78 | 47.3 ± 11.8 | 46.7 ± 11.4 | 47.9 ± 12.2 |
| ECW | 234 | 22.8 ± 4.5 | 22.9 ± 4.7 | 22.7 ± 4.4 | 78 | 24.2 ± 5.9 | 23.8 ± 5.6 | 24.6 ± 6.2 |
| ECW/ICW | 234 | 1.13 ± 0.31 | 1.16 ± 0.30 | 1.12 ± 0.32 | 78 | 1.12 ± 0.32 | 1.11 ± 0.32 | 1.13 ± 0.33 |
| Resistance (ohms) | 234 | 474 ± 95 | 488 ± 99 | 460 ± 89 | 80 | 468 ± 100 | 470 ± 90 | 467 ± 111 |
| Reactance (ohms) | 234 | 48.5 ± 13.3 | 48.2 ± 13.6 | 49.0 ± 14.1 | 78 | 48.1 ± 13.5 | 48.7 ± 13.3 | 47.5 ± 13.8 |
| Phase angle (degrees) | 234 | 5.43 ± 1.51 | 5.21 ± 1.21 | 5.65 ± 1.74 | 78 | 5.51 ± 1.48 | 5.54 ± 1.48 | 5.49 ± 1.51 |
| Vector length () | 234 | 284.9 ± 62.9 | 291.8 ± 64.2 | 278.0 ± 61.1 | 78 | 273.4 ± 61.7 | 274.5 ± 57.3 | 272.3 ± 66.3 |
Results are shown as mean ± standard deviation, median and 10th & 90th percentiles range, or frequency (%), as appropriate
There were no significant differences between the treatment groups in each of the separate trials (Daily and Nocturnal) at baseline.
Serum Albumin Concentration
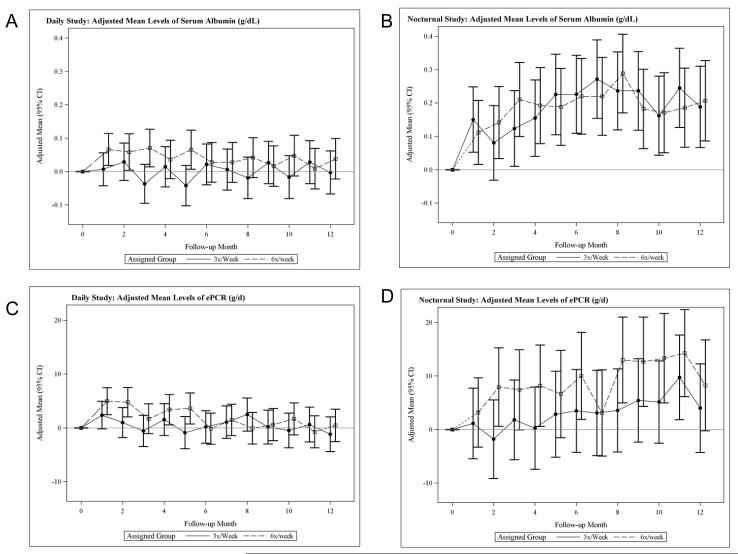
In the Daily Trial, predialysis serum albumin increased one month after randomization in the frequent compared to conventional (3x/week) group (relative difference 0.06 g/dL, 95% CI (−0.01 to +0.13 g/dL)). This difference was statistically significant over months 3-5 but was not sustained at 12 months. The change in albumin from baseline to 12 months did not differ significantly between treatment arms in either trial (Table 2, Figure 1, A and B). Based on a regression of serum albumin on the preceding interdialytic interval, we estimated that the shorter average interdialytic interval in the 6x versus the 3x per week group contributed 0.057 ± 0.013 g/dL to the treatment difference in serum albumin concentrations. Once this sampling bias is accounted for, the changes in serum albumin did not differ significantly between the treatment groups at any follow-up time. In the Nocturnal Trial, there were no significant between-group differences at any time, although in both groups combined, serum albumin increased by 0.19 ± 0.04 g/dL (p < 0.001) (Table 2, Figure 1B).
| Variable | Trial | Trt | Observed Data (Mean ± SD)1 | Adjusted Means and Treatment Effects2 (± SE or with 95% Confidence Intervals) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | F4 | F12 | Month 4 | Month 12 | |||||
| Change from Baseline |
Treatment Comparison (6x vs. 3x) |
Change from Baseline |
Treatment Comparison (6x vs. 3x) |
||||||
| Serum Albumin (g/dL)3 | Daily | 3x | 3.95 ± 0.44 | 3.94 ± 0.4 | 3.96 ± 0.4 | −0.02 ± 0.03 | 0.08 (0.01,0.14) P=0.016 |
0.00 ± 0.03 | 0.03 (−0.04,0.10) P=0.41 |
| 6x | 3.96 ± 0.36 | 4.0 ± 0.37 | 3.98 ± 0.36 | 0.06 ± 0.03 | 0.03 ± 0.03 | ||||
| Noct. | 3x | 3.93 ± 0.53 | 4.1 ± 0.45 | 4.12 ± 0.39 | 0.17 ± 0.05 | 0.03 (−0.10,0.16) P=0.65 |
0.20 ± 0.05 | −0.01 (−0.14,0.12) P=0.88 |
|
| 6x | 3.88 ± 0.49 | 4.1 ± 0.49 | 4.08 ± 0.53 | 0.2 ± 0.05 | 0.19 ± 0.05 | ||||
| ePCR (g/d)3 | Daily | 3x | 64.67 ± 17.86 | 65.09 ± 19.09 | 64.26 ± 20.02 | 0.03 ± 1.19 | 2.87 (−0.27,6.01) P=0.07 |
−0.35 ± 1.29 | 0.82 (−2.54,4.19) P=0.63 |
| 65.37 ± 21.23 | 67.83 ± 22.46 | 65.13 ± 22.53 | 2.90 ± 1.15 | 0.47 ± 1.21 | |||||
| Noct | 6x | 62.42 ± 21.6 | 63.28 ± 21.49 | 69.97 ± 24.23 | 1.65 ± 3.24 | 5.76 (−2.42,13.94) P=0.17 |
6.3 ± 3.33 | 5.65 (−2.98,14.27) P=0.20 |
|
| 62.86 ± 21.15 | 70.96 ± 22.05 | 74.55 ± 38.81 | 7.41 ± 3.24 | 11.94 ± 3.45 | |||||
| Predialysis Weight (kg)3 | Daily | 3x | 81.75 ± 20.26 | 81.89 ± 20.5 | 81.97 ± 20.37 | 0.25 ± 0.27 | −1.29 (−2.03,−0.56) P=0.001 |
0.15 ± 0.38 | −0.21 (−1.24,0.82) P=0.69 |
| 6x | 80.17 ± 21.26 | 79.25 ± 21.49 | 80.28 ± 21.51 | −1.05 ± 0.26 | −0.06 ± 0.36 | ||||
| Noct. | 3x | 85.83 ± 25.05 | 85.73 ± 25.71 | 86.57 ± 26.25 | −0.36 ± 0.49 | −0.44 (−1.79,0.91) P=0.52 |
0.51 ± 0.86 | 0.17 (−2.2,2.55) P=0.89 |
|
| 6x | 90.83 ± 28.95 | 89.69 ± 28.77 | 91.11 ± 28.87 | −0.8 ± 0.48 | 0.68 ± 0.85 | ||||
| Postdialysis Weight (kg)3 | Daily | 3x | 78.9 ± 19.76 | 79.1 ± 19.9 | 79.19 ± 19.86 | 0.37 ± 0.28 | −0.45 (−1.2,0.3) P=0.24 |
0.23 ± 0.45 | 0.62 (−0.59,1.83) P=0.32 |
| 6x | 77.01 ± 20.84 | 77.07 ± 21.18 | 78.15 ± 21.2 | −0.08 ± 0.27 | 0.85 ± 0.43 | ||||
| Noct. | 3x | 83.45 ± 24.08 | 83.3 ± 25.03 | 84.05 ± 25.64 | −0.44 ± 0.45 | −0.02 (−1.25,1.22) P=0.98 |
0.36 ± 0.79 | 0.51 (−1.66,2.69) P=0.64 |
|
| 6x | 88.55 ± 28.19 | 87.8 ± 28.56 | 89.07 ± 28.56 | −0.45 ± 0.44 | 0.88 ± 0.78 | ||||
| Reactance (ohms) | Daily | 3x | 48.2 ± 12.6 | 47.8 ± 15.1 | 47.4 ± 15.2 | −0.6 ± 1.2 | 5.7 (2.5, 8.9) P<0.001 |
−0.9 ± 1.5 | 5.2 (1.3, 9.2) P=0.010 |
| 6x | 48.9 ± 14.1 | 54.5 ± 14.3 | 53.3 ± 15.3 | 5.1 ± 1.2 | +4.3 ± 1.4 | ||||
| Noct. | 3x | 48.7 ± 13.3 | 51.4 ± 15.1 | 53.9 ± 13.9 | 2.6 ± 2.6 | 3.4 (−3.4, 10.2) P=0.32 |
+4.5 ± 2.5 | 1.4 (−5.1, 7.9) P=0.67 |
|
| 6x | 47.5 ± 13.8 | 54.3 ± 16.9 | 53.9 ± 16.2 | 6.0 ± 2.6 | +5.8 ± 2.5 | ||||
| Resistance (ohms ) | Daily | 3x | 488 ± 99 | 471 ± 92 | 466 ± 91 | −11.0 ± 5.8 | +36.9 (21.8, 52.1) P<0.001 |
−11.7 ± 7.4 | 30.4 (11.1, 49.6) P=0.002 |
| 6x | 460 ± 89 | 494 ± 96 | 492 ± 93 | 26.0 ± 5.4 | +18.7 ± 7.0 | ||||
| Noct. | 3x | 470 ± 90 | 471 ± 98 | 483 ± 108 | 3.4 ± 9.3 | +30.8 (4.7, 57.0) P=0.021 |
+6.2 ± 12.2 | 17.9 (−16.5, 52.2) P=0.30 |
|
| 6x | 467 ± 111 | 493 ± 117 | 481 ± 94 | 34.3 ± 9.3 | +24.1 ± 12.5 | ||||
| Phase Angle (degrees) | Daily | 3x | 5.21 ± 1.21 | 5.32 ± 1.44 | 5.34 ± 1.58 | 0.01 ± 0.13 | +0.30 (−0.03, 0.63) P=0.075 |
−0.02 ± 0.15 | 0.28 (−0.11, 0.67) P=0.16 |
| 6x | 5.65 ± 1.74 | 5.91 ± 1.85 | 5.78 ± 1.96 | 0.31 ± 0.12 | +0.25 ± 0.14 | ||||
| Noct. | 3x | 5.54 ± 1.48 | 5.76 ± 1.48 | 5.98 ± 1.66 | 0.16 ± 0.24 | +0.09 (−0.53, 0.72) P=0.77 |
+0.37 ± 0.22 | −0.05 (−0.66, 0.56) P=0.87 |
|
| 6x | 5.49 ± 1.51 | 5.81 ± 1.69 | 5.79 ± 1.67 | 0.25 ± 0.24 | +0.32 ± 0.23 | ||||
| Vector Length (ohms/m) | Daily | 3x | 291.8 ± 64.2 | 281.7 ± 59.3 | 278.1 ± 58.2 | −6.9 ± 3.5 | +23.4*** (14.1, 32.7) P<0.001 |
−7.5 ± 4.6 | 19.6** (7.6, 31.6) P=0.0015 |
| 6x | 278.0 ± 61.1 | 298.8 ± 66.8 | 297.4 ± 65.2 | 16.5 ± 3.3 | +12.1 ± 4.3 | ||||
| Noct. | 3x | 274.5 ± 57.3 | 279.9 ± 67.3 | 289.4 ± 75.1 | 4.1 ± 5.7 | 17.5* (1.4, 33.5) P=0.033 |
+6.0 ± 7.5 | 9.4 (−11.6, 30.5) P=0.38 |
|
| 6x | 460.5 ± 95.4 | 496.7 ± 117.5 | 484.2 ± 93.5 | 35.6 ± 9.7 | +25.2 ± 12.7 | ||||
| Total Body Water (L) | Daily | 3x | 43.9 ± 10.2 | 44.1 ± 9.7 | 44.9 ± 9.6 | 0.5 ± 0.3 | −1.7 (−2.4, −1.0) P<0.001 |
+0.6 ± 0.3 | −1.3 (−2.1, −0.4) P=0.004 |
| 6x | 44.5 ± 9.7 | 43.0 ± 9.9 | 43.1 ± 10.2 | −1.2 ± 0.2 | −0.7 ± 0.3 | ||||
| Noct. | 3x | 46.7 ± 11.4 | 45.9 ± 11.4 | 44.7 ± 11.3 | −0.3 ± 0.4 | −1.1 (−2.3, 0.1) P=0.065 |
−0.1 ± 0.6 | −0.4 (−2.2, 1.3) P=0.63 |
|
| 6x | 47.9 ± 12.2 | 47.1 ± 12.2 | 48.1 ± 11.9 | −1.4 ± 0.4 | −0.5 ± 0.6 | ||||
| Kinetic Volume (L)3 | Daily | 3x | 36.2 ± 8.6 | 37.2 ± 9.3 | 37.1 ± 8.5 | 0.62 ± 0.45 | −1.96 (−3.14,−0.78) P=0.001 |
0.31 ± 0.48 | −1.55 (−2.8,−0.29) P=0.02 |
| 6x | 36.8 ± 9.5 | 35.6 ± 8.5 | 35.6 ± 9.1 | −1.34 ± 0.43 | −1.24 ± 0.45 | ||||
| Noct. | 3x | 38.2 ± 12.1 | 39.6 ± 13.6 | 38.7 ± 13.5 | 1.32 ± 1.44 | −2.53 (−6.21,1.14) P=0.18 |
0.97 ± 1.47 | −0.56 (−4.38,3.26) P=0.77 |
|
| 6x | 37.6 ± 9.3 | 36.5 ± 11.0 | 41.2 ± 20.5 | −1.22 ± 1.43 | 0.42 ± 1.51 | ||||
| Extra-cellular Water (L) | Daily | 3x | 22.9 ± 4.7 | 23.1 ± 4.7 | 23.4 ± 4.9 | 0.26 ± 0.23 | −1.26 (−1.87, −0.65) P<0.001 |
+0.44 ± 0.26 | −1.12 (−1.83, −0.41) P=0.002 |
| 6x | 22.7 ± 4.4 | 21.4 ± 4.3 | 21.6 ± 4.4 | −1.00 ± 0.22 | −0.68 ± 0.25 | ||||
| Noct. | 3x | 23.8 ± 5.6 | 23.2 ± 5.9 | 22.3 ± 5.4 | −0.38 ± 0.48 | −0.76 (−2.09, 0.58) P=0.26 |
−0.68 ± 0.45 | 0.02 (−1.23, 1.27) P=0.98 |
|
| 6x | 24.6 ± 6.2 | 23.7 ± 6.3 | 24.3 ± 5.9 | −1.13 ± 0.48 | −0.66 ± 0.46 | ||||
| Intra-cellular Water (L) | Daily | 3x | 21.0 ± 6.5 | 21.1 ± 6.4 | 21.6 ± 6.1 | 0.27 ± 0.20 | −0.46 (−0.99, 0.08) P=0.094 |
+0.13 ± 0.23 | −0.19 (−0.81, 0.44) P=0.562 |
| 6x | 21.9 ± 6.9 | 21.6 ± 7.0 | 21.5 ± 7.4 | −0.19 ± 0.19 | −0.05 ± 0.22 | ||||
| Noct. | 3x | 22.8 ± 7.3 | 22.8 ± 6.8 | 22.4 ± 7.5 | −0.02 ± 0.33 | −0.12 (−1.04, 0.80) P=0.80 |
+0.53 ± 0.43 | −0.22 (−1.45, 1.01) P=0.73 |
|
| 6x | 23.3 ± 7.6 | 23.4 ± 7.4 | 23.8 ± 7.5 | −0.14 ± 0.33 | +0.31 ± 0.44 | ||||
| Body Cell Mass (kg) | Daily | 3x | 26.6 ± 8.2 | 26.7 ± 8.0 | 27.3 ± 7.7 | 0.34 ± 0.25 | −0.58 (−1.26, 0.10) P=0.094 |
+0.17 ± 0.30 | −0.23 (−1.03, 0.56) P=0.56 |
| 6x | 27.7 ± 8.8 | 27.3 ± 8.8 | 27.3 ± 9.3 | −0.24 ± 0.24 | −0.07 ± 0.28 | ||||
| Noct. | 3x | 28.9 ± 9.2 | 28.8 ± 8.6 | 28.4 ± 9.5 | −0.02 ± 0.41 | −0.15 (−1.31, 1.01) P=0.80 |
+0.67 ± 0.55 | −0.28 (−1.84, 1.28) P=0.73 |
|
| 6x | 29.5 ± 9.6 | 29.7 ± 9.4 | 30.2 ± 9.5 | −0.17 ± 0.41 | +0.39 ± 0.56 | ||||
| Lean Body Mass (kg) | Daily | 3x | 44.0 ± 10.2 | 44.2 ± 9.7 | 45.0 ± 9.6 | 0.53 ± 0.26 | −1.68 (−2.37, −0.99) P<0.001 |
+0.58 ± 0.32 | −1.26 (−2.12, −0.41) P=0.004 |
| 6x | 44.6 ± 9.8 | 43.1 ± 9.9 | 43.2 ± 10.3 | −1.15 ± 0.24 | −0.68 ± 0.30 | ||||
| Noct. | 3x | 46.3 ± 11.7 | 46.1 ± 11.5 | 44.8 ± 11.4 | −0.23 ± 0.41 | −1.11 (−2.25, 0.04) P=0.057 |
−0.04 ± 0.61 | −0.45 (−2.18, 1.28) P=0.61 |
|
| 6x | 47.4 ± 12.5 | 47.2 ± 12.2 | 48.2 ± 12.0 | −1.34 ± 0.41 | −0.49 ± 0.63 | ||||
| % Adiposity | Daily | 3x | 37.6 ± 13.7 | 36.8 ± 13.4 | 37.3 ± 12.8 | −0.23 ± 0.29 | +0.40 (−0.38, 1.18) P=0.31 |
−0.09 ± 0.41 | 0.76 (−0.34, 1.85) P=0.17 |
| 6x | 36.3 ± 13.8 | 36.2 ± 13.5 | 35.5 ± 12.9 | 0.18 ± 0.27 | +0.67 ± 0.38 | ||||
| Noct. | 3x | 37.9 ± 14.6 | 37.7 ± 14.5 | 37.5 ± 15.1 | −0.53 ± 0.57 | +0.78 (−0.78, 2.35) P=0.32 |
−0.17 ± 0.81 | 1.90 (−0.36, 4.17) P=0.10 |
|
| 6x | 40.9 ± 17.7 | 42.5 ± 18.1 | 44.4 ± 18.5 | 0.25 ± 0.56 | +1.73 ± 0.82 | ||||
Means and standard deviations are provided for constant cohorts with nonmissing values at each of the baseline, month 4 and month 12 visits. Sample sizes ranges from 77 to 116 (3x per week) and 89 to 123 (6x per week) in the Daily Trial, and from 35 to 42 (3x per week) and 33 to 44 (6x per week) in the Nocturnal Trial.
Adjusted means and treatment effects were estimated under mixed effects models with adjustment for the baseline level of the outcome and clinical center in the Daily Trial, and the baseline level of the outcome in the Nocturnal Trial.
Month 4 and Month 12 designate averages over Months 3-5 and Months 10-12, respectively, for outcomes measured a monthly kinetic modeling sessions.
Figure 1.
Adjusted mean changes in predialysis albumin in the daily trial (A) and the nocturnal trial (B) and mean changes in equilibrated protein catabolic rate (ePCR) in the daily trial (C) and in the nocturnal trial (D).
Equilibrated Protein Catabolic Rate (ePCR)
For both the Daily and Nocturnal and Trials, there were no significant differences in ePCR between the treatment groups at 1, 4 or 12 months (Figure 1, panels C and D). In the Nocturnal Trial, mean ePCR increased by 9.1 ± 2.6 g/day from baseline to 12 months in both treatment groups combined (Figure 1D). The increases in serum albumin and ePCR persisted and remained statistically significant compared to baseline when the Nocturnal Trial analysis was restricted to patients with baseline GFR < 1.70 ml/min, the median baseline GFR.
Body weight
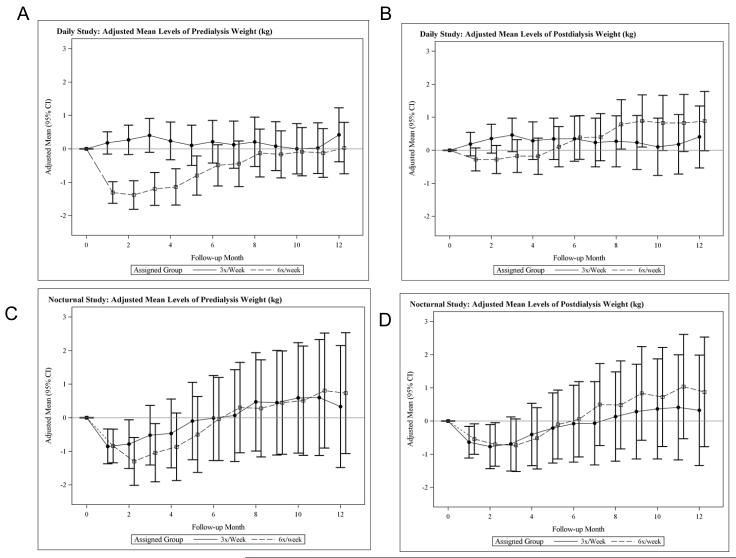
In the Daily Trial, there was a significant relative decrease (frequent versus conventional) in predialysis body weight evident within one month (Figure 2A). Between one and 12 months, the relative change in predialysis body weight was 1.3 ± 0.5 kg (p=0.007) higher in the 6x per week group. Post-dialysis body weight changed in parallel in both treatment groups (Figure 2B). Between one and 12 months, the relative change in post-dialysis body weight was 1.1 ± 0.5 kg (p = 0.04) higher in the 6x per week group.
Figure 2.
Adjusted mean changes in predialysis weight (A) and post-dialysis weight (B) in the daily trial and in predialysis weight (C) and post-dialysis weight (D) in the nocturnal trial.
In the Nocturnal Trial (Table 2, Figures 2 C and D), pre and post-dialysis weights in both treatment groups combined decreased by one month after baseline, reaching a nadir at two months, with a slow increase toward and then beyond baseline by 12 months, but with no significant difference between treatment groups.
Measured Parameters: Reactance, Resistance, Phase Angle and Vector Length
In the Daily Trial, there were statistically significant relative increases in measured reactance and resistance in the 6x per week group at months 4 and 12. The vector length was relatively lengthened in the 6x per week group, reflecting reduced tissue hydration (Table 2).
In the Nocturnal Trial, none of the between-group comparisons reached statistical significance, with the exception of vector length at four months, where the vector length was significantly lengthened in the 6x per week group (Table 2).
Derived Estimates of Body Composition
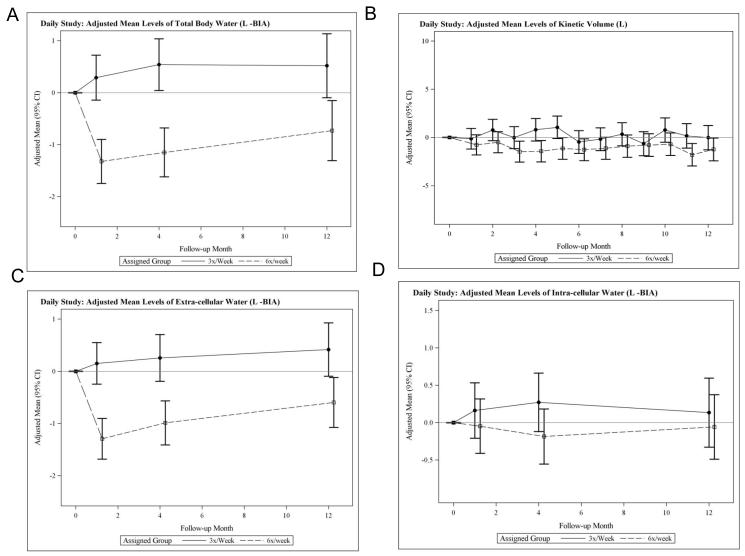
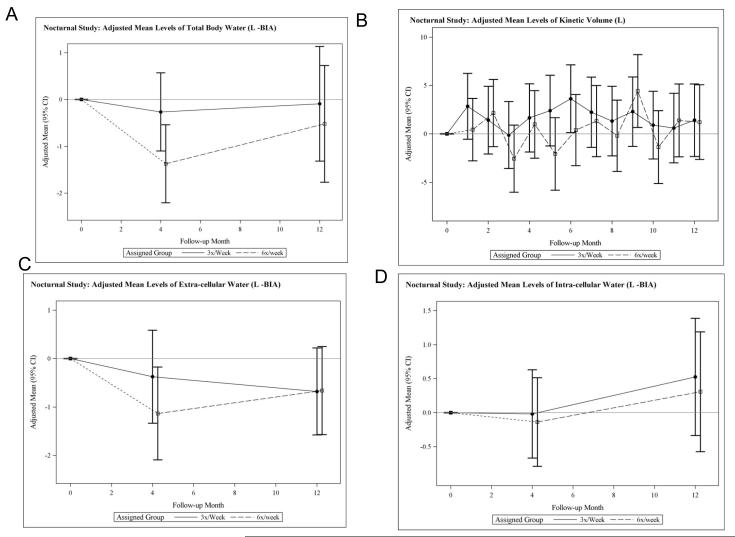
In the Daily Trial, there were large relative decreases (frequent versus conventional) in total body water (TBW) evident at one month, which remained statistically significant at four and 12 months (Table 2, Figure 3A). The relative reduction in TBW was confirmed using an independent determination of the urea distribution volume from monthly urea kinetic modeling (Table 2, Figure 3B). Changes in estimates of extracellular water (ECW) mirrored those of TBW (Figure 3C), suggesting that the relative decline in TBW was driven by a change in ECW, an observation consistent with the changes in vector length described above. There were no significant relative changes in intracellular water (ICW) (Table 2, Figure 3D). Relative changes in TBW, ECW, and ICW during the Nocturnal Trial were small in magnitude and not statistically significant (Table 2, Figure 4).
Figure 3.
Adjusted mean changes from baseline in total body water (TBW) measured by bioimpedance (A), in adjusted mean changes in the volume of distribution of urea measured by kinetic modeling (B), in adjusted mean changes from baseline in extracellular water (C) and in adjusted mean changes from baseline n intracellular water (D) in the daily trial
Figure 4.
Adjusted mean changes from baseline in total body water (TBW) measured by bioimpedance (A), in adjusted mean changes in the volume of distribution of urea measured by kinetic modeling (B), in adjusted mean changes from baseline in extracellular water (C) and in adjusted mean changes from baseline n intracellular water (D) in the nocturnal trial.
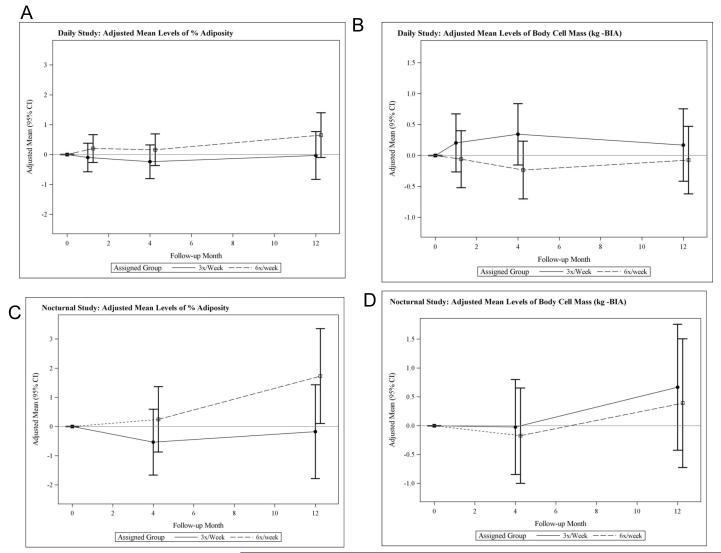
There was a relative increase in estimated adiposity in the 6x per week group although these changes did not reach statistical significance in either trial (Table 2, Figures 5, A and C). Similarly, there were no statistically significant relative changes in ICW or body cell mass (BCM), the metabolically active component of lean body mass (LBM) (Table 2, Figures 5, B and D). Indeed, the relative decrease in LBM could be explained virtually entirely by the relative decrease in ECW. There was no evidence of enhanced ICW or BCM to reflect either anabolism or preservation of BCM (Table 2, Figure 5, B and D).
Figure 5.
Adjusted mean changes from baseline in percent adiposity (A) and in body cell mass (BCM) (kg) estimated by BIA in the daily trial, and adjusted mean changes from baseline in percent adiposity (C) and in body cell mass (BCM) (kg) estimated by BIA (D) in the nocturnal trial.
DISCUSSION
Frequent hemodialysis has been reported to preserve nutritional status and prevent or attenuate the anticipated decline in BCM (17,18,19) and has been associated with improved appetite, increased protein and caloric intake, and incremental increases in dry weight, muscle mass, and serum albumin in various studies (17,18,19,20). In contrast to most previous studies, the FHN Daily and Nocturnal Trials were randomized and utilized monthly measures of serum albumin, ePCR, and serial bioimpedance-based rather than anthropometric measures of body composition (14,19). The HEMO trial, a 2 × 2 factorial randomized trial of >1800 subjects dialyzed 3x per week at standard and high per session eKt/Vurea and with high and low flux dialyzers, showed a progressive decline in serum albumin, enPCR, and body weight (10) unaffected by dialysis dose or flux. No significant effect on weight was observed during the first year of HEMO, but over time there were decreases in both estimated muscle and fat mass in all groups (21).
The FHN trials showed no statistically significant between-group differences in serum albumin from baseline to 12 months. While serum albumin concentrations have been reported to increase during the first year of dialysis (22), possibly related to a reduction in proteinuria or improved nutritional status, the observed increases in serum albumin in both arms of the Nocturnal Trial were not associated with residual kidney function or proximity to the initiation of dialysis therapy (data not shown).
Body weight is an imprecise nutritional marker in patients on dialysis, as weight gain may reflect increases in ECW, BCM (reported by an increase in ICW), and/or fat mass. A biphasic pattern of change in body weight has previously been described after switching from 3x to 6x per week dialysis (23). Presumably the initial drop is due to a reduction in ECW, and subsequent weight gain results from improved appetite and increased tissue weight. In the Daily Trial, the monthly predialysis body weights in subjects randomized to the 6x per week group followed such a pattern. BIA-derived data at one month confirmed a reduction in TBW and ECW with no significant change in ICW. At 12 months, the average predialysis body weight had returned to baseline levels, while the reduction in ECW persisted. There was no evidence of a gain in ICW and correspondingly, BCM. These results suggest that the body weight gain was in a non-hydrated body compartment, likely fat. Whether an increase in adiposity in patients on hemodialysis is beneficial or harmful is unknown; observational data suggest that higher body mass index is associated with enhanced survival (24).
We found no significant changes in ICW or phase angle with frequent as compared to conventional hemodialysis. In an adult population, changes in ICW and phase angle result predominantly from changes in muscle mass, since non-muscle organ mass should remain relatively constant over time. Acidosis (25,26), inflammation (27,28), and reduced physical activity, all common in the dialysis population, are associated with decline in muscle mass, while increased resistive training (29) or androgen replacement may be associated with increased muscle mass (3030,31). Thus, the expected effect of change in dialysis frequency might be one of protection from loss rather than an increase in muscle mass. Our observations suggest that factors responsible for the deterioration of nutritional status seen in other studies was attenuated or possibly prevented because of patient selection, “adequate” hemodialysis in the 3x per week group, or that the period of observation was too simply too short.
Our study is strengthened by data from two randomized clinical trials involving a relatively large sample of subjects reasonably representative of the North American hemodialysis population. We also included monthly measures of nutritional parameters, and used serial bioimpedance-based measures of body composition. This study also has limitations. We did not measure adiposity directly in this study. Our estimation of change in adiposity and difference in adiposity between the groups is based on subtraction of two relatively large values, body weight and TBW from one another. Each is accompanied by a measurement error, decreasing precision in our estimate of differences in body fat mass between treatment groups. We used single frequency BIA rather than isotope dilution methodology to determine body composition and calculated adiposity by assuming hydration of fat free mass (FFM) of 0.73. The relative expansion of ECW found in patients on hemodialysis might lead to an underestimation of the hydration of FFM. Nevertheless, ECW/ICW varies greatly in humans as a function of age, sex (32) and obesity (33) with no significant change in the hydration of FFM measured directly.
In conclusion, frequent in-center hemodialysis significantly reduced ECW but failed to anabolize (i.e., no increase in serum albumin or BCM). Any gain in “dry” body weight corresponded to increased adiposity rather than muscle mass. Frequent nocturnal hemodialysis yielded no net effect on parameters of nutritional status or body composition.
Brief Methods
Study Design
The Frequent Hemodialysis Network (FHN) Daily Trial was a multicenter, prospective, randomized trial of frequent (6x per week), as compared with conventional (3x per week) in-center hemodialysis. The FHN Nocturnal Trial was a multicenter, prospective, randomized trial of frequent (6x per week) nocturnal at-home hemodialysis, as compared with conventional hemodialysis (3x per week), with the majority of conventional subjects receiving home-based therapy. The study designs including specific inclusion and exclusion criteria, and data collection procedures have been described previously (14).
Study Population
Subjects with ESRD requiring maintenance hemodialysis who achieved mean eKt/Vurea >1.0 for the last two baseline dialysis sessions and weighed > 30 kg were eligible for inclusion. Major exclusion criteria included age <13 (Daily) or <18 (Nocturnal) years, residual kidney function >3 mL/min/35L (Daily) or mean of creatinine and urea clearance >10 mL/min/1.73m2 (Nocturnal). Informed consent was obtained from each subject. The study was approved by the Institutional Review Board at each participating study site.
Intervention, control and adherence
In the Daily Trial, subjects who were assigned to hemodialysis 6x per week (n=125) had a target equilibrated Kt/Vn (where Vn=3.271×V2/3) of 0.9 provided that each session length was between 1.5 and 2.75 hours. Subjects who were assigned to 3x per week hemodialysis (n=120) continued their usual dialysis prescriptions, which included a minimum target equilibrated Kt/Vurea (the ratio of the equilibrated urea clearance during each dialysis session (Kt) to the patient’s volume of urea distribution (V)) of 1.1 and a session length of 2.5 to 4.0 hours.
In the Nocturnal Trial, subjects were assigned to dialyze either 3x per week (n=42) to a prescribed standardized Kt/Vurea of > 2.0/week and a session length of ≥ 2.5 hours or 6x per week (n=45) to a standardized Kt/Vurea of ≥ 4.0/week for ≥ 6 hours per session. For both Trials, we calculated adherence as the ratio of dialysis sessions attended to dialysis sessions prescribed, by month.
Outcome Measures
The pre-specified primary outcome for the “nutrition domain” was serum albumin concentration, measured monthly throughout the follow-up period. Additional outcomes with monthly measurements included pre- and postdialysis weight, urea kinetic volume (V), and protein catabolic rate (ePCR) calculated using the equilibrated post-dialysis BUN. We analyzed absolute ePCR in g/day without normalizing to V, to avoid confounding with projected changes in V.
Body composition was assessed by single frequency (50 kHz) (Quantum, RJL Systems, Inc.) bioelectrical impedance analysis (BIA) at baseline, 1 month, 4 months and 12 months in the Daily trial, and at baseline, 4 months, and 12 months in the Nocturnal trial. The protocol instructed clinical centers to implement the BIA procedure before a mid-week hemodialysis session for subjects with at least one intact leg and arm when feasible; however, a minority of BIA assessments were performed on other days or after dialysis. We measured reactance (Xc) and resistance (R) and calculated phase angle, the arc tangent of the Xc to R ratio. We multiplied the arc tangent of Xc/R by 180/π to convert from radians to degrees. We used reactance to estimate total body potassium (TBK) by the method of Kotler, et al. (34). We estimated body cell mass (BCM) using the equation:
(35).
ICW was then calculated as 0.73 × BCM (36).
We estimated adiposity (fat mass) by subtracting FFM (estimated as TBW/0.73) from total (post-dialysis) body weight.
Statistical Analysis
Continuous variables were summarized using means and standard deviations (SD), and categorical variables were summarized using frequencies and proportions. Descriptive summaries of changes in treatment-related variables are provided for the constant cohort with non-missing values at baseline and at months 4 and 12 after randomization.
The effects of randomized treatment assignment on outcomes with monthly measurements (predialysis serum albumin, ePCR, pre and post dialysis weight and kinetic volume) were estimated using mixed effects analyses, with covariate adjustment for the baseline level of the outcome and clinical center in the Daily Trial, and the baseline level of the outcome in the Nocturnal Trial. We used a combined compound-symmetry first order auto-regressive covariance matrix to account for correlations in measurements over time (37). A heteroscedastic extension of the covariance model was used for the pre- and post-dialysis weights in the Daily Trial to account for a greater variability in weight at baseline than during follow-up. The analytic approach accounted for non-missing early measurements in the analysis of changes to later time points in cases for subjects who died or dropped out of the study during the follow-up period. Treatment effects were assessed primarily by comparisons between randomized groups of adjusted mean changes from baseline to the average level during months 10-12. Additional comparisons between randomized groups were defined for the mean changes from baseline to the average level during months 3-5 and for the mean changes from months 3-5 to the average level during months 10-12 to separately assess effects of the treatment interventions on early and later changes. In accordance with the study design, primary emphasis was given to comparisons between treatment groups; however, further contrasts were defined to estimate mean changes over each of these time intervals (baseline to months 10-12, baseline to months 3-5, and months 3-5 to months 10-12) within each randomized group and for the average of the two groups combined. Finally, the same mixed effects models were used to provide adjusted mean changes from baseline to each follow-up month for plots representing the pattern of change over the full 1-year follow-up period.
Similar mixed effects models were used to estimate treatment effects on changes in BIA measurements; only in this case an unstructured covariance model was used to account for serial correlation in repeated measurements within the same patients (baseline and months 1, 4, and 12 in the Daily Trial; baseline and months 4 and 12 in the Nocturnal Trial). Analogous to monthly outcomes, we focused primarily on comparisons of changes to the end of follow-up (month 12), but also evaluated treatment effects on changes from baseline to month 4 and from month 4 to month 12. The mixed effects models for total body water and intracellular body water were extended in both trials by adding linear interaction terms to investigate if the treatment effects differed among subjects with lower and higher levels of the pre-specified baseline factors, age and BMI.
In sensitivity analyses the mixed effects analyses for the monthly outcomes were repeated for the Nocturnal Trial after excluding patients with baseline residual renal clearance (defined as the average of urea and creatinine clearance) < 1.70 ml/min, the median baseline value for the nocturnal trial. We estimated the effect of hemodilution associated with the interdialytic interval on the predialysis serum albumin concentrations by extending the mixed effects models in each trial to relate the predialysis albumin to the inter-dialytic interval preceding the blood draw after controlling for clinical center (Daily trial only) and treatment assignment, the interaction between treatment assignment and visit month, diabetes, age, baseline GFR and clinical center (both trials). We then applied the estimated regression coefficients from these models to the mean interdialytic intervals in the respective treatment groups to assess the influence of different average interval lengths on comparisons of serum albumin between the 6x per week and 3x per week treatment groups. All analyses were performed without formal adjustment for multiple comparisons using SAS version 9.2. Two-tailed P-values <0.05 were considered statistically significant.
Acknowledgements
Members of the FHN Trial Group are Achinger S, Anderson S, Appel L, Apruzzes R, Atwal J, Augustine B, Ayus J, Bardsley J, Bay W, Beach S, Beck G, Bharti B, Briggs J, Bullas R, Burkart J, Burrowes J, Cabezon E, Callegari J, Carter M, Champagne J, Chan C, Chan W, Chang J, Chertow G, Cheung A, Copland M, Coplon N, Coppley A, Daugirdas J, Dellagrottaglie S, Depner T, Derse A, Dominguez A, Doss S, Eggers P, Eknoyan G, Escalada R, Fensterer A, Finkelstein F, Fofie Y, Franzwa B, Frome R, Fu Z, Garg A, Gassman J, Gayda P, Geller N, Geronemus R, Goodman W, Gorodetskaya I, Gotch F, Greene T, Greenwood R, Grimm R, Gutierrez M, Hall Y, Handelman G, Henderson L, Hernandez A, Higgins H, Hilkin A, Hostetter T, Hoy C, Humphreys M, Hunsicker L, James S, Kariisa M, Kaufman A, Kaufman T, Kaysen G, Ke S, Keene R, Kimmel P, Kliger A, Kotanko P, Kramer C, Kuhlmann M, Kwan S, Kwok S, Lacson E, Larive B, Leavell E, Lemus D, Levin A, Levin N, Li M, Lilli K, Lindsay R, Lockridge R, Luan J, MacKrell J, Manaster R, Mandaci O, Mathew R, Mauck V, Mazzorato A, McCulloch C, McGrath-Chong M, McLeroy S, Mehta R, Meisels I, Miller B, Mohr P, Moossavi S, Nabali A, Narva A, Nissenson A, Ornt D, Painter P, Pepas J, Peterson C, Pierratos A, Pipkin M, Prichard S, Rajagopalan S, Ramos R, Rashid M, Rastogi A, Regozo K, Riley J, Rivas M, Rocco M, Rodriquez R, Roecker E, Roger D, Rogers J, Salusky I, Sanz G, Sanz J, Schiller-Moran B, Schlarb J, Schuessler R, Schulman G, Schweitzer S, Sergeyeva O, Shah S, Sherer S, Sika M, Sioson L, Skelton R, Smith M, Snell C, Somers D, Sonico J, Spanner E, Star R, Steigerwald D, Stokes J, Suri R, Suter M, Tamura M, Tarallo M, Tichy M, Ting G, Tran T, Ulloa D, Unruh M, Vassalotti J, Wallace W, Waterman E, Wei J, Weiss B, West J, Wiggins K, Winchester J.
Support: The FHN trials were supported by the National Institutes of Health (NIH), National Institutes of Diabetes and Digestive and Kidney Diseases, the Centers for Medicare and Medicaid Services, and the NIH Research Foundation. Contributors to the NIH Foundation in support of the FHN trials included Amgen, Baxter and Dialysis Clinics. Additional support was provided by DaVita, Dialysis Clinics, Fresenius Medical Care, Renal Advantage, Renal Research Institute, and Satellite Healthcare.
References
- 1.Johansen KL, Kaysen GA, Young BS, et al. Longitudinal study of nutritional status, body composition, and physical function in hemodialysis patients. Am J Clin Nutr. 2003;77:842–846. doi: 10.1093/ajcn/77.4.842. [DOI] [PubMed] [Google Scholar]
- 2.Williams AJ, Dittmer ID, McArley A, Clarke J. High bicarbonate dialysate in haemodialysis patients: effects on acidosis and nutritional status. Nephrol Dial Transplant. 1997 Dec;12(12):2633–7. doi: 10.1093/ndt/12.12.2633. [DOI] [PubMed] [Google Scholar]
- 3.Pickering WP, Price SR, Bircher G, et al. Nutrition in CAPD: serum bicarbonate and the ubiquitin-proteasome system in muscle. Kidney Int. 2002 Apr;61(4):1286–92. doi: 10.1046/j.1523-1755.2002.00276.x. [DOI] [PubMed] [Google Scholar]
- 4.Johansen KL, Shubert T, Doyle J, et al. Muscle atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, and physical function. Kidney Int. 2003 Jan;63(1):291–7. doi: 10.1046/j.1523-1755.2003.00704.x. [DOI] [PubMed] [Google Scholar]
- 5.Mitch WE, Hu Z, Lee SW, Du J. Strategies for suppressing muscle atrophy in chronic kidney disease: mechanisms activating distinct proteolytic systems. J Ren Nutr. 2005 Jan;15(1):23–7. doi: 10.1053/j.jrn.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Pupim LB, Flakoll PJ, Levenhagen DK, Ikizler TA. Exercise augments the acute anabolic effects of intradialytic parenteral nutrition in chronic hemodialysis patients. Am J Physiol Endocrinol Metab. 2004 Apr;286(4):E589–97. doi: 10.1152/ajpendo.00384.2003. [DOI] [PubMed] [Google Scholar]
- 7.Duenhas MR, Draibe SA, Avesani CM, et al. Influence of renal function on spontaneous dietary intake and on nutritional status of chronic renal insufficiency patients. Eur J Clin Nutr. 2003 Nov;57(11):1473–8. doi: 10.1038/sj.ejcn.1601713. [DOI] [PubMed] [Google Scholar]
- 8.Chazot C, Laurent G, Charra B, et al. Malnutrition in long-term haemodialysis survivors. Nephrol Dial Transplant. 2001;16(1):61–69. doi: 10.1093/ndt/16.1.61. [DOI] [PubMed] [Google Scholar]
- 9.Chertow G, Johansen K, Lew N, et al. Vintage, nutritional status, and survival in hemodialysis patients. Kidney Int. 2000;57:1176–81. doi: 10.1046/j.1523-1755.2000.00945.x. [DOI] [PubMed] [Google Scholar]
- 10.Rocco MV, Dwyer JT, Larive B, et al. The effect of dialysis dose and membrane flux on nutritional parameters in hemodialysis patients: results of the HEMO Study. Kidney Int. 2004 Jun;65(6):2321–34. doi: 10.1111/j.1523-1755.2004.00647.x. [DOI] [PubMed] [Google Scholar]
- 11.Galland R, Traeger J, Arkouche W, et al. Short daily hemodialysis and nutritional status. Am J Kidney Dis. 2001b;37:S95–S98. doi: 10.1053/ajkd.2001.20758. [DOI] [PubMed] [Google Scholar]
- 12.Galland R, Traeger J, Arkouche W, et al. Short daily hemodialysis rapidly improves nutritional status in hemodialysis patients. Kidney Int. 2001a;60:1555–1560. doi: 10.1046/j.1523-1755.2001.00959.x. [DOI] [PubMed] [Google Scholar]
- 13.Spanner E, Suri R, Heidenheim AP, Lindsay RM. The impact of quotidian hemodialysis on nutrition. Am J Kidney Dis. 2003;42:30–35. doi: 10.1016/s0272-6386(03)00535-3. [DOI] [PubMed] [Google Scholar]
- 14.Suri RS, Garg AX, Chertow GM, et al. Frequent Hemodialysis Network Trial Group Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney Int. 2007 Feb;71(4):349–59. doi: 10.1038/sj.ki.5002032. Epub 2006 Dec 13. [DOI] [PubMed] [Google Scholar]
- 15.FHN Trial Group. Chertow GM, Levin NW, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010 Dec 9;363(24):2287–300. doi: 10.1056/NEJMoa1001593. Epub 2010 Nov 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocco MV, Lockridge RS, Jr, Beck GJ, et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Group Kidney Int. 2011 Nov;80(10):1080–1091. doi: 10.1038/ki.2011.213. doi: 10.1038/ki.2011.213. Epub 2011 Jul 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galland R, Traeger J, Arkouche W, et al. Short daily hemodialysis and nutritional status. Am J Kidney Dis. 2001b;37:S95–S98. doi: 10.1053/ajkd.2001.20758. [DOI] [PubMed] [Google Scholar]
- 18.Galland R, Traeger J, Arkouche W, et al. Short daily hemodialysis rapidly improves nutritional status in hemodialysis patients. Kidney Int. 2001a;60:1555–1560. doi: 10.1046/j.1523-1755.2001.00959.x. [DOI] [PubMed] [Google Scholar]
- 19.Spanner E, Suri R, Heidenheim AP, Lindsay RM. The impact of quotidian hemodialysis on nutrition. Am J Kidney Dis. 2003;42:30–35. doi: 10.1016/s0272-6386(03)00535-3. [DOI] [PubMed] [Google Scholar]
- 20.Heidenheim AP, Muirhead N, Moist L, Lindsay RM. Patient quality of life on quotidian hemodialysis. Am J Kidney Dis. 2003;42:36–41. doi: 10.1016/s0272-6386(03)00536-5. [DOI] [PubMed] [Google Scholar]
- 21.Chumlea WC, Dwyer J, Bergen C, et al. Hemodialysis Study Group Nutritional status assessed from anthropometric measures in the HEMO study. J Ren Nutr. 2003 Jan;13(1):31–8. doi: 10.1053/jren.2003.50003. [DOI] [PubMed] [Google Scholar]
- 22.Goldwasser P, Kaldas AI, Barth RH. Rise in serum albumin and creatinine in the first half year on hemodialysis. Kidney Int. 1999 Dec;56(6):2260–8. doi: 10.1046/j.1523-1755.1999.00768.x. [DOI] [PubMed] [Google Scholar]
- 23.Woods JD, Port FK, Orzol S, et al. Clinical and biochemical correlates of starting “daily” hemodialysis. Kidney Int. 1999 Jun;55(6):2467–76. doi: 10.1046/j.1523-1755.1999.00493.x. [DOI] [PubMed] [Google Scholar]
- 24.Johansen KL, Young B, Kaysen GA, Chertow GM. Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr. 2004 Aug;80(2):324–32. doi: 10.1093/ajcn/80.2.324. [DOI] [PubMed] [Google Scholar]
- 25.Löfberg E, Gutierrez A, Anderstam B, et al. Effect of bicarbonate on muscle protein in patients receiving hemodialysis. Am J Kidney Dis. 2006;48(3):419–29. doi: 10.1053/j.ajkd.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 26.Mitch WE. Metabolic and clinical consequences of metabolic acidosis. J Nephrol. 2006;19(Suppl 9):S70–5. [PubMed] [Google Scholar]
- 27.Brinkley TE, Leng X, Miller ME, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64:455–61. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taaffe DR, Harris TB, Ferrucci L, et al. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 55:M709–152000. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 29.West DW, Burd NA, Staples AW, Phillips SM. Human exercise-mediated skeletal muscle hypertrophy is an intrinsic process. Int J Biochem Cell Biol. 2010 Sep;42(9):1371–5. doi: 10.1016/j.biocel.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Johansen KL, Mulligan K, Schambelan M. Anabolic effects of nandrolone decanoate in patients receiving dialysis: a randomized controlled trial. JAMA. 1999 Apr 14;281(14):1275–81. doi: 10.1001/jama.281.14.1275. [DOI] [PubMed] [Google Scholar]
- 31.Johansen KL, Painter PL, Sakkas GK, et al. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: A randomized, controlled trial. J Am Soc Nephrol. 2006 Aug;17(8):2307–14. doi: 10.1681/ASN.2006010034. [DOI] [PubMed] [Google Scholar]
- 32.Lesser GT, Markofsky J. Body water compartments with human aging using fat-free mass as the reference standard. Am J Physiol. 1979 Mar;236(3):R215–20. doi: 10.1152/ajpregu.1979.236.3.R215. [DOI] [PubMed] [Google Scholar]
- 33.Chamney PW, Wabel P, Moissl UM, et al. A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am J Clin Nutr. 2007 Jan;85(1):80–9. doi: 10.1093/ajcn/85.1.80. [DOI] [PubMed] [Google Scholar]
- 34.Kotler DP, Tierney AR, Wang J, Pierson RN., Jr. Magnitude of body-cell-mass depletion and the timing of death from wasting in AIDS. Am J Clin Nutr. 1989;50:444–447. doi: 10.1093/ajcn/50.3.444. [DOI] [PubMed] [Google Scholar]
- 35.Moore FD, Olesen KH, McMurray JD, et al. Body Composition in Health And Disease. Saunders; Philadelphia, PA: 1963. The Body Cell Mass and Its Supporting Environment. [Google Scholar]
- 36.Wang Z, St-Onge MP, Lecumberri B, et al. Body cell mass: model development and validation at the cellular level of body composition. Am J Physiol Endocrinol Metab. 2004;286(1):E123–8. doi: 10.1152/ajpendo.00227.2003. [DOI] [PubMed] [Google Scholar]
- 37.Fitzmaurice GM, Laird NM, Ware JH. Covariance Pattern Models. John Wiley & Sons; Philadelphia, PA: 2004. Applied Longitudinal Analysis; pp. 166–173. [Google Scholar]