Abstract
Although sensory feedback from the urethra plays an integral role in the regulation of lower urinary tract function, little is known about the properties of flow-responsive primary afferent neurons. The purpose of this study was to characterize the activity of sacral afferents that respond to fluid flow through the urethra. Single neuron action potentials were recorded extracellularly from the S1 and S2 dorsal root ganglia in eight cats anesthetized with α-chloralose. 21 of 116 cells responded to urethral flow but not to mechanical palpation of the perineum, 22 responded to both urethral flow and palpation, and 27 responded to palpation only. 34 of the 43 flow-responsive cells exhibited a firing response to 10 ml flow boluses that could be fit using a power function: FR (t) = a * (t)b + c, where FR is firing rate, t is time, and a, b and c are constants. In all 34 cells the `b' term was negative, indicating that the firing rate slowed over the time course of the urethral flow. In 16 of the 24 cells that were recorded during at least four different flow rates, a power function provided a good fit of the relationship between firing rate and flow rate: FR (flow)= k * (flow)p + q, where k, p and q are constants. In each of these 16 cells the `p' term was positive, indicating that the firing rate tended to increase with increases in flow rate. These are the first data to characterize the properties of flow-responsive afferents in the cat, and reveal properties that parallel those of other afferents.
INTRODUCTION
The urinary bladder accumulates and stores urine (continence) and evacuates urine at an appropriate time and place (micturition or voiding). During voiding, the bladder (detrusor) contracts and the external urethral sphincter (EUS) relaxes to allow urine flow. Although coordinated contraction of the bladder and EUS persists following lumbosacral de-afferentation [28], afferent feedback contributes to generating large amplitude voiding contractions [20], and sensory feedback from the urethra plays a pivotal role in efficient bladder emptying [20]. The objective of the present study was to record the activity of primary afferent neurons that respond to fluid flow through the urethra and could provide sensory feedback from the urethra.
Sensory signals originating from the urethra can initiate bladder contractions in the quiescent bladder and or augment ongoing contractions in cat [2, 3, 14], ewe [7, 25], rat [17, 22, 23], and human [15, 27, 36]. Conversely, silencing urethral afferents with anesthesia reduces bladder contraction frequency [17] and bladder emptying efficiency in rats [23] and humans [27]. However, there is a paucity of recordings from flow-responsive afferent neurons [12, 32, 33], and the properties of afferents responsive to urethral flow are largely unknown. The purpose of the present study was to identify sacral afferents that respond to fluid flow through the urethra and to quantify the firing patterns of these neurons. This information provides a characterization of a new class of sensory receptors and the foundation to understand the role of this feedback in the physiology and pathophysiology of the lower urinary tract.
MATERIAL AND METHODS
Extracellular recordings from single sacral afferent neurons were conducted in eight sexually intact adult male cats initially anesthetized with ketamine HCl (Ketaset, 25–35 mg/kg, IM) and maintained with α-chloralose (Sigma, 60 mg/kg, IV, supplemented at 15 mg/kg). All experimental procedures were reviewed and approved by the Duke University Institutional Animal Care and Use Committee. Blood pressure was measured through a catheter in the carotid artery, and a surgical plane of anesthesia was maintained by monitoring blood pressure, heart rate, and lack of blink and withdrawal reflexes. Animals were intubated and respired artificially to maintain end tidal CO2 at 3–4%, body temperature was maintained at 37°–39° C, and 0.9% saline with 8.4 mg/ml sodium bicarbonate and 5% dextrose added was administered IV (10 – 15 ml/kg/hr).
A ventral midline incision was made to expose the bladder, and a 3.5 Fr (1.17 mm) catheter was inserted through the bladder, tied into the neck of the urethra, and used to administer saline through the urethra. A second suprapubic catheter was secured in the dome of the bladder and drained continuously to maintain the bladder empty. Urethral flow was introduced either with an infusion pump for fixed flow rates from 1 – 30 ml/min or via a 10 ml bolus of saline manually injected using a syringe to approximate flow rates of ~ 60 ml/min. We estimated the urethral flow rate in male cats to be between 22 and 40 ml/min during voiding [4, 31]. The pudendal nerve was exposed and instrumented with a bipolar nerve cuff electrode to deliver charge-balanced biphasic stimuli.
The lumbosacral spinal cord, dorsal root ganglia (DRG), and spinal roots were exposed by a L7 – S3 laminectomy. The S1 and S2 DRG were identified, and a rigidly fixed cork platform was placed under the DRG to provide support for electrode insertion. Single neuron action potentials were recorded extracellularly with a 16-channel multisite electrode (NeuroNexus Technologies, Inc., Ann Arbor, MI; 413 μm2 sites, 50 μm site spacing) inserted into the S1 or S2 DRG. The electrode was positioned within the DRG and then search stimuli including pudendal nerve stimulation at 1 Hz, repeated urethral bolus infusions, and perineal palpation were used to identify cells. Following characterization of units from one electrode position, the electrode was repositioned and the search stimuli were repeated. Thus, this was not an unbiased sample, but rather biased recordings to units that were responsive to the stimuli of interest.
Activity of single neurons was measured during urethral flow introduced through syringe boluses for all cells, and using the infusion pump for 90 / 116 cells. Neural activity was also recorded during periods of no fluid flow (control, all cells), during pudendal nerve stimulation at 1 Hz (85/116 cells), and during tactile palpation of the genital and anal regions of the cat with either a swirling motion or direct pressure using a cotton tipped applicator (114/116 cells).
Single neuron action potentials were sorted offline using multiple features (including three principal components, peak and valley voltage, energy level) to cluster the data as tightly as possible into individual units (Offline Sorter, Plexon, Inc., Dallas, TX). Auto- and cross-correlograms were conducted for identified units to ensure that sufficient time passed between spikes to account for refractory periods and that units identified as separate did not overlap temporally. Neural firing rate and other firing patterns were quantified using NeuroExplorer (Plexon, Inc.).
RESULTS
Of 116 neurons recorded from the S1 (93 neurons) and S2 (23 neurons) DRG, 70 responded to urethral flow and/or perineal palpation: 21 to flow only, 22 to both flow and palpation, and 27 to palpation only. Of the remaining 46 cells that did not respond to urethral flow or perineal palpation, 27 were spontaneously active, 10 fired tonically, and nine responded to pudendal nerve stimulation. There were no apparent differences between the properties of the 21 units that responded to flow only and the 22 units that responded to both flow and palpation, and these two populations were grouped together for further analysis. The 43 cells that responded to flow were analyzed to identify temporal and flow rate-dependent activity. 24 of the flow responsive afferents were among those tested using pudendal nerve stimulation, and 15 of those cells were confirmed as pudendal afferents via recording of antidromically evoked action potentials and had conduction velocities of 24 m/s ± 2 m/s (mean ± s.e.m.). No differences were apparent between units that were confirmed to originate in the pudendal nerve and those that were not.
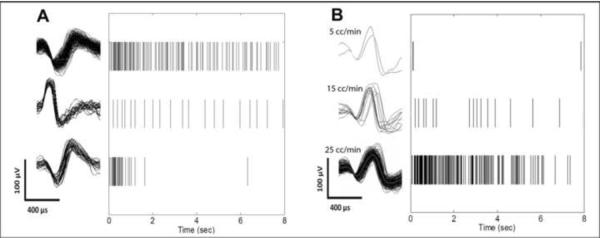
Example spike trains evoked in three cells by injection of a 10 cc bolus of saline through the urethra are shown in Fig 1A, and example spike trains evoked in one cell by infusions at three different flow rates are shown in Fig 1B. The highest mean firing rates (across all tested flow rates) of the 43 flow responsive cells varied from < 1 – 65 spikes/sec, and the maximum number of spikes per one second bin varied from 2 – 152 spikes across cells.
Figure 1.
Sorted spike waveforms (left) and spike train raster plots (right) of single afferent neurons recorded in the cat sacral dorsal root ganglia in response to urethral fluid flow. A. Response of three different primary afferent neurons to a 10 ml bolus of saline injected through the urethra. B. Response of one primary afferent neuron to three different rates of saline flow through the urethra.
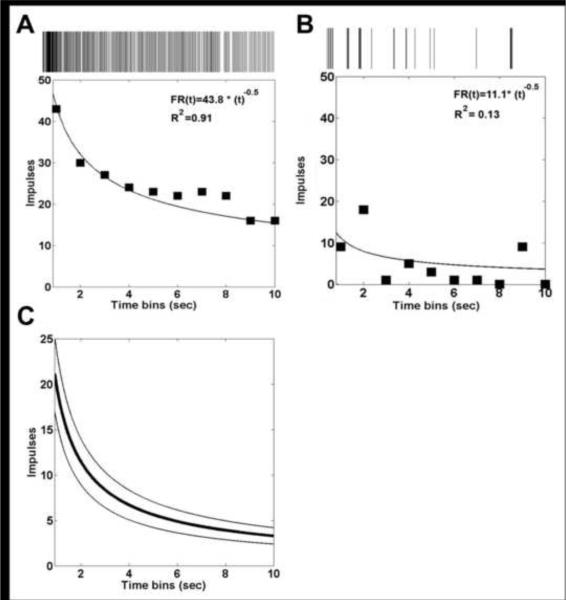
34 of 43 cells exhibited a firing pattern during the 10 ml bolus that was well fit as a function of time using a power function (Fig 2A) with R2 > 0.50 (R2 from 0.51 to 1.0, R2average = 0.81): FR (t)= a * (t)b + c where FR is firing rate, t is time, a is a constant term that reflects the initial firing rate (range: 2.4 to 116.1, mean: 24.2), b is a constant that describes how the firing rate changes over time (range: −12.7 to −0.4, mean: −1.75), and c is a constant offset term (range: 0 to 0.6, mean: 0.02). The exponent, b, was negative for all 34 cells, indicating that the firing rate tended to slow during the period of urethral flow (Fig 2C). Each of the nine cells that were not well fit with the power function (R2 < 0.5) exhibited inconsistent activity during urethral flow (Fig 2B).
Figure 2.
Temporal properties of response of sacral afferents to urethral flow. A, B Firing rate (FR) profiles of two different primary afferent neurons in response to 10 ml boluses of fluid injected through the urethra. C. Average firing rate profile (mean ± standard error) of all 34 cells that had a temporal profile of firing rate response to a 10 ml bolus of fluid flow injected through the urethra that was well fit with a power function.
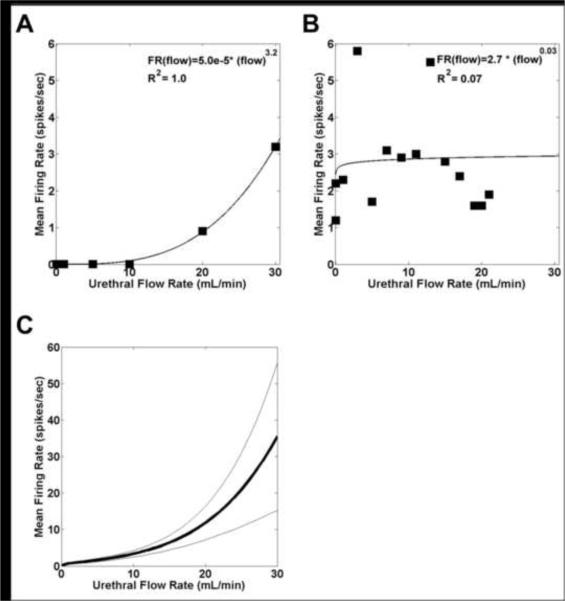
The firing rates of 24 of the flow-responsive cells were recorded during at least 4 different controlled flow rates of 1 – 30 cc/min (Fig 3A). In 16 / 24 cells, a power function provided a good fit (R2 ranged from 0.51 to 1.0, R2average= 0.75) of the relationship between the mean firing rate and flow rate: FR (flow) = k* (flow)p + q, where k (range: 0.0 to 3.7, mean: 0.6), p (range: 0.1 to 4.1, mean: 1.5), and q (range: 0.0 to 2.1, mean 0.1) are constants. In all 16 cells k was near 0 reflecting that cells were silent at control but fired in response to flow, while p > 0 indicated that the firing rate increased with increases in flow rate (Fig 3C). The eight cells not well fit by a power function all had peak firing rates at lower flow rates that were higher than their firing rates at higher flow rates (Fig 3B).
Figure 3.
Urethral flow rate dependent responses of sacral afferents. A, B Mean firing rate (FR) as a function of urethral flow rate in two different cells. C. Average firing rate (mean ± standard error) as a function of urethral flow rate of all 16 cells that could that had a firing rate response to different urethral flow rates that was well fit with a power function.
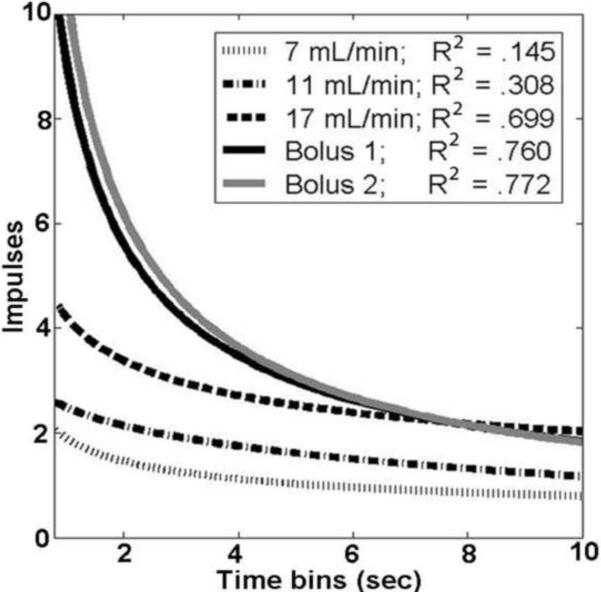
Of the 24 flow-responsive cells recorded during multiple flow rates, 18 had at least 1 response to an infusion pump-controlled flow rate that could be fit by a power function as a function of time with R2 > 0.5. Across those 18 cells, 133 trials contained a firing rate response to flow at a fixed rate (out of 165 flow rates tested), but only 42 of those 133 trials could be fit as a function of time with a power function. The probability that a firing rate trial could be fit with a power function increased as the flow rate increased. 25% (17 of 68) of trials with flow rates under 10 ml/min, 34% (13 of 38) of trials between 10 and 20 ml/min, 46% (12 of 26) of trials between 20 and 30 ml/min, and 82% (27 of 33) of trials from the high flow rate 10 ml boluses could be fit with a power function (Fig 4).
Figure 4.
Firing rate as a function of time from a sacral neuron in response to several different flow rates.
DISCUSSION
Sensory feedback of urethral flow plays an integral role in the regulation of micturition, but little is known about the properties of flow-responsive primary afferent neurons. The purpose of this study was to characterize sacral afferents that responded to fluid flow through the urethra. 34 of 43 flow-responsive cells exhibited a firing pattern that could be fit as a function of time using a power function with a negative exponent, which reflected that the firing rate tended to slow over the time course of the urethral flow (adaptation). In 16 of the 24 flow-responsive cells that were recorded during at least four different flow rates, a power function with a positive exponent provided a good fit of the relationship between firing rate and flow rate. The probability that an afferent firing pattern could be fit with a power function of time increased as the urethral flow rate increased. Distal stimulation of the pudendal nerve was used to identify 15 of the flow responsive cells as pudendal afferents with conduction velocities of 24 m/s, consistent with conduction velocities in the literature [1, 9]. Although we recorded from units that responded to both flow and palpation, the palpation was done with sufficient force that the distal urethra could be deformed, suggesting that these afferents innervated the distal urethra, and based on anatomical tracing studies [39], it is unlikely that afferents are branching to innervate both the urethra and perineal skin.
The properties of the flow responsive afferents parallel those of cutaneous and muscle spindle afferents reported in previous studies. Two types of mechanoreceptive afferents to sustained displacement had been previously identified in skin regions of each of the cat, macaque, and human: rapid adapting (RA) and slow adapting (SA) [5, 16, 19]. In the present study, several cells responded to sustained flow in a way consistent with an RA response (Fig 1A, bottom), while others were consistent with an SA response (Fig 1A top, middle; Fig 2A). Penile mechanoreceptors in the cat respond to changes in displacement amplitude with firing rate changes that can be fit as a power function with a positive exponent [16], similar to the response of the majority (16 of 24) of flow-responsive cells (Fig 3). Similarly, muscle spindles in the cat respond to velocity in a way that can be fit with a power function [21, 24], further supporting the notion that the power-law relationship is a plausible model for afferent coding of physiological inputs. All innervation was intact during the present study, and urethral reflexes, whereby flow through the urethra leads to changes in activity in urethral musculature [11], could have influenced the activity of urethral afferents.
In persons with spinal cord injury (SCI), the spino-bulbo-spinal micturition reflex is interrupted [6, 8, 10, 13], but a spinal reflex mediated by pudendal afferents evokes bladder contractions after SCI in the cat [29, 30, 34, 35] and human [15, 18, 36, 37]. This reflex is mediated by afferents that respond to flow through the urethra [14, 33], and thus a better understanding of the mechanisms involved in this urethral flow-modulated response is important to developing more effective approaches to restore bladder emptying. For example, the temporal patterns of activity during urethral flow could serve as templates for biomimetic patterns of stimulation of pudendal afferents for neural prosthetic restoration of bladder emptying. The stimulation frequencies that generate bladder contractions [26, 29, 30, 34, 35, 38] are similar to the mean firing rates of the flow-responsive afferent recorded in the present study.
HIGHLIGHTS
Recorded response of sacral afferents to urethral fluid flow in anesthetized cats
Single unit firing rates declined during flow with a power law dependence on time
Firing rates increased with flow rates exhibiting a power law dependence on rate
First characterization of a new class of sensory receptors
Advances understanding role of urethral feedback in control of lower urinary tract
ACKNOWLEDGMENTS
This work was supported by NIH Grant F32 DK082175 and NIH R01 NS050514. The authors thank Gilda Mills, John P. Woock, and Meredith J. McGee for assistance during conduct of the experiments.
This work was supported by NIH grant F32 DK082175 and NIH R01 NS050514.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Bahns E, Halsband U, Janig W. Responses of sacral visceral afferents from the lower urinary tract, colon and anus to mechanical stimulation. Pflugers Arch. 1987;410:296–303. doi: 10.1007/BF00580280. [DOI] [PubMed] [Google Scholar]
- [2].Barrington F. The component reflexes of micturition in the cat, I and II. Brain. 1931;54:177–188. [Google Scholar]
- [3].Barrington F. The component reflexes of micturition in the cat, III. Brain. 1941;64:239–243. [Google Scholar]
- [4].Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Bladder emptying by intermittent electrical stimulation of the pudendal nerve. J Neural Eng. 2006;3:43–51. doi: 10.1088/1741-2560/3/1/005. [DOI] [PubMed] [Google Scholar]
- [5].Bolanowski SJ, Jr., Gescheider GA, Verrillo RT, Checkosky CM. Four channels mediate the mechanical aspects of touch. J Acoust Soc Am. 1988;84:1680–1694. doi: 10.1121/1.397184. [DOI] [PubMed] [Google Scholar]
- [6].Chai TC, Steers WD. Neurophysiology of micturition and continence. Urol Clin North Am. 1996;23:221–236. doi: 10.1016/s0094-0143(05)70306-2. [DOI] [PubMed] [Google Scholar]
- [7].Combrisson H, Allix S, Robain G. Influence of temperature on urethra to bladder micturition reflex in the awake ewe. Neurourol Urodyn. 2007;26:290–295. doi: 10.1002/nau.20311. [DOI] [PubMed] [Google Scholar]
- [8].Craggs M, McFarlane J. Neuromodulation of the lower urinary tract. Exp Physiol. 1999;84:149–160. doi: 10.1111/j.1469-445x.1999.tb00080.x. [DOI] [PubMed] [Google Scholar]
- [9].Cueva-Rolon R, Munoz-Martinez EJ, Delgado-Lezama R, Raya JG. The cat pudendal nerve: afferent fibers responding to mechanical stimulation of the perineal skin, the vagina or the uterine cervix. Brain Res. 1994;655:1–6. doi: 10.1016/0006-8993(94)91589-x. [DOI] [PubMed] [Google Scholar]
- [10].de Groat WC. Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol. 2006;147(Suppl 2):S25–40. doi: 10.1038/sj.bjp.0706604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].de Groat WC, Booth AM. Physiology of the urinary bladder and urethra. Ann Intern Med. 1980;92:312–315. doi: 10.7326/0003-4819-92-2-312. [DOI] [PubMed] [Google Scholar]
- [12].Feber JL, van Asselt E, van Mastrigt R. Neurophysiological modeling of voiding in rats: urethral nerve response to urethral pressure and flow. Am J Physiol. 1998;274:R1473–1481. doi: 10.1152/ajpregu.1998.274.5.R1473. [DOI] [PubMed] [Google Scholar]
- [13].Fowler CJ. Integrated control of lower urinary tract--clinical perspective. Br J Pharmacol. 2006;147(Suppl 2):S14–24. doi: 10.1038/sj.bjp.0706629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Garry RC, Roberts TD, Todd JK. Reflexes involving the external urethral sphincter in the cat. J Physiol. 1959;149:653–665. doi: 10.1113/jphysiol.1959.sp006366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gustafson KJ, Creasey GH, Grill WM. A urethral afferent mediated excitatory bladder reflex exists in humans. Neurosci Lett. 2004;360:9–12. doi: 10.1016/j.neulet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- [16].Johnson RD, Kitchell RL, Gilanpour H. Rapidly and slowly adapting mechanoreceptors in the glans penis of the cat. Physiol Behav. 1986;37:69–78. doi: 10.1016/0031-9384(86)90386-0. [DOI] [PubMed] [Google Scholar]
- [17].Jung SY, Fraser MO, Ozawa H, Yokoyama O, Yoshiyama M, De Groat WC, Chancellor MB. Urethral afferent nerve activity affects the micturition reflex; implication for the relationship between stress incontinence and detrusor instability. J Urol. 1999;162:204–212. doi: 10.1097/00005392-199907000-00069. [DOI] [PubMed] [Google Scholar]
- [18].Kennelly MJ, Arena KC, Shaffer N, Bennett ME, Grill WM, Grill JH, Boggs JW. Electrical stimulation of the urethra evokes bladder contractions in a woman with spinal cord injury. J Spinal Cord Med. 33:261–265. doi: 10.1080/10790268.2010.11689704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim SS, Sripati AP, Bensmaia SJ. Predicting the timing of spikes evoked by tactile stimulation of the hand. J Neurophysiol. 104:1484–1496. doi: 10.1152/jn.00187.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kruse MN, Mallory BS, Noto H, Roppolo JR, de Groat WC. Properties of the descending limb of the spinobulbospinal micturition reflex pathway in the cat. Brain Res. 1991;556:6–12. doi: 10.1016/0006-8993(91)90541-3. [DOI] [PubMed] [Google Scholar]
- [21].Mileusnic MP, Brown IE, Lan N, Loeb GE. Mathematical models of proprioceptors. I. Control and transduction in the muscle spindle. J Neurophysiol. 2006;96:1772–1788. doi: 10.1152/jn.00868.2005. [DOI] [PubMed] [Google Scholar]
- [22].Peng CW, Chen JJ, Cheng CL, Grill WM. Improved bladder emptying in urinary retention by electrical stimulation of pudendal afferents. J Neural Eng. 2008;5:144–154. doi: 10.1088/1741-2560/5/2/005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Peng CW, Chen JJ, Cheng CL, Grill WM. Role of pudendal afferents in voiding efficiency in the rat. Am J Physiol Regul Integr Comp Physiol. 2008;294:R660–672. doi: 10.1152/ajpregu.00270.2007. [DOI] [PubMed] [Google Scholar]
- [24].Prochazka A, Gorassini M. Models of ensemble firing of muscle spindle afferents recorded during normal locomotion in cats. J Physiol. 1998;507(Pt 1):277–291. doi: 10.1111/j.1469-7793.1998.277bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Robain G, Combrisson H, Mazieres L. Bladder response to urethral flow in the awake ewe. Neurourol Urodyn. 2001;20:641–649. doi: 10.1002/nau.1014. [DOI] [PubMed] [Google Scholar]
- [26].Schultz-Lampel D, Jiang C, Lindstrom S, Thuroff JW. Experimental results on mechanisms of action of electrical neuromodulation in chronic urinary retention. World J Urol. 1998;16:301–304. doi: 10.1007/s003450050071. [DOI] [PubMed] [Google Scholar]
- [27].Shafik A, Shafik AA, El-Sibai O, Ahmed I. Role of positive urethrovesical feedback in vesical evacuation. The concept of a second micturition reflex: the urethrovesical reflex. World J Urol. 2003;21:167–170. doi: 10.1007/s00345-003-0340-5. [DOI] [PubMed] [Google Scholar]
- [28].Shefchyk SJ. The effects of lumbosacral deafferentation on pontine micturition centre-evoked voiding in the decerebrate cat. Neurosci Lett. 1989;99:175–180. doi: 10.1016/0304-3940(89)90285-1. [DOI] [PubMed] [Google Scholar]
- [29].Tai C, Smerin SE, de Groat WC, Roppolo JR. Pudendal-to-bladder reflex in chronic spinal-cord-injured cats. Exp Neurol. 2006;197:225–234. doi: 10.1016/j.expneurol.2005.09.013. [DOI] [PubMed] [Google Scholar]
- [30].Tai C, Wang J, Wang X, de Groat WC, Roppolo JR. Bladder inhibition or voiding induced by pudendal nerve stimulation in chronic spinal cord injured cats. Neurourol Urodyn. 2007;26:570–577. doi: 10.1002/nau.20374. [DOI] [PubMed] [Google Scholar]
- [31].Tai C, Wang J, Wang X, Roppolo JR, de Groat WC. Voiding reflex in chronic spinal cord injured cats induced by stimulating and blocking pudendal nerves. Neurourol Urodyn. 2007;26:879–886. doi: 10.1002/nau.20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Talaat M. Afferent impulses in the nerves supplying the urinary bladder. J. Physiol. 1936;89:1–13. doi: 10.1113/jphysiol.1937.sp003458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Todd JK. Afferent Impulses in the Pudendal Nerves of the Cat. Q J Exp Physiol Cogn Med Sci. 1964;49:258–267. doi: 10.1113/expphysiol.1964.sp001730. [DOI] [PubMed] [Google Scholar]
- [34].Woock JP, Yoo PB, Grill WM. Activation and inhibition of the micturition reflex by penile afferents in the cat. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1880–1889. doi: 10.1152/ajpregu.00029.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Woock JP, Yoo PB, Grill WM. Intraurethral stimulation evokes bladder responses via 2 distinct reflex pathways. J Urol. 2009;182:366–373. doi: 10.1016/j.juro.2009.02.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yoo PB, Horvath EE, Amundsen CL, Webster GD, Grill WM. Multiple pudendal sensory pathways reflexly modulate bladder and urethral activity in patients with spinal cord injury. J Urol. 2011;185:737–743. doi: 10.1016/j.juro.2010.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yoo PB, Klein SM, Grafstein NH, Horvath EE, Amundsen CL, Webster GD, Grill WM. Pudendal nerve stimulation evokes reflex bladder contractions in persons with chronic spinal cord injury. Neurourol Urodyn. 2007;26:1020–1023. doi: 10.1002/nau.20441. [DOI] [PubMed] [Google Scholar]
- [38].Yoo PB, Woock JP, Grill WM. Bladder activation by selective stimulation of pudendal nerve afferents in the cat. Exp Neurol. 2008;212:218–225. doi: 10.1016/j.expneurol.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yoo PB, Woock JP, Grill WM. Somatic innervation of the feline lower urinary tract. Brain Res. 2008;1246:80–87. doi: 10.1016/j.brainres.2008.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]