Abstract
In this study we asked whether Helicobacter pylori whole cells and lipopolysaccharide (LPS) utilize sugar moieties of Lewis (Le) antigenic determinants to interact with DC-SIGN (dendritic cell specific ICAM grabbing nonintegrin) receptor on dendritic cells (DCs). For this purpose the soluble DC-SIGN/Fc adhesion assay and the THP-1 leukemia cells with induced expression of DC-SIGN were used. We showed that the binding specificity of DC-SIGN with H. pylori LeX/Y positive whole cells and H. pylori LPS of LeX/Y type was fucose dependent, whereas in LeXY negative H. pylori strains and LPS preparations without Lewis determinants, this binding was galactose dependent. The binding of soluble synthetic LeX and LeY to the DC-SIGN-like receptor on THP-1 cells was also observed. In conclusion, the LeXY dependent as well as independent binding of H. pylori whole cells and H. pylori LPS to DC-SIGN was described. Moreover, we demonstrated that THP-1 cells may serve as an in vitro model for the assessment of H. pylori-DC-SIGN interactions mediated by LeX and LeY determinants.
1. Introduction
Helicobacter pylori is a causative agent of chronic gastritis, gastroduodenal ulcers, and gastric cancers. The correlation between H. pylori-infection and gastroduodenal diseases was described in 1983 by Marshall and Warren [1]. The course of H. pylori infection depends on the host immune responses towards this pathogen, both innate and adaptive. In H. pylori infected individuals the gastric mucosa is massively infiltrated with immunocompetent cells, which interact in a complex way with bacterial cells. Such interactions are responsible for gastric pathologies but they are also involved in the elimination of these pathogens from the gastric mucosa [2]. During the first stages of the infection various H. pylori compounds, for example, urease, vacuolating cytotoxin-VacA, or cytotoxin associated gene A antigen (CagA), initiate an acute inflammatory response in the gastric epithelium, which later becomes chronic [3–5]. Long-lasting inflammation results in many pathological disorders in the mucus layer and diminished ability of the immune cells to fight the infection [6–10].
Although a lipopolysaccharide (LPS) is an important proinflammatory compound of gram-negative bacteria [11], the structure of H. pylori lipid A probably evolved in the mode which promoted persistence of the infection. It was shown that H. pylori LPS regulates the expression of adhesins and it can diminish the secretion of inflammatory cytokines by host immune cells [12]. Recently, antiphagocytic and antiproliferative properties of H. pylori LPS were also detected [13, 14]. Downregulation of the natural cytotoxic capacity of lymphocytes in response to H. pylori LPS was correlated with the modulation of IFN-γ, interleukin 2 (IL-2) and IL-10 secretion by the immune cells [15]. It was suggested that H. pylori LPS, through the activation of immunocompetent cells diminish the number of bacteria in the gastric tissue and thus prolong the infection [16].
The majority of H. pylori strains produce LPS with Lewis (Le) blood group antigens in O-specific chains: LeX, LeY, H type 1, Lea, Leb, i-antigen, and sialyl LeX [17–22]. The sugar residues in the O-specific chains, which are similar to Le determinants of the host, influence the activity of H. pylori LPS. The expression of Le determinants by H. pylori results in better attachment of the bacteria to the host epithelial cells, modulation of the inflammatory response, and evasion of the bacteria due to mimicking blood group antigens present on the gastric mucosa [23, 24]. The epitope mimicry may contribute to the pathological, autoreactive responses during H. pylori infections [25, 26]. The Lewis expression on H. pylori cells is closely related to the epithelial area and the stage of disease [27].
The interactions of LPS with host cells are mediated by both, cellular and soluble molecules involved in cell signaling via Toll-like receptor 4 (TLR 4) [28–30]. Analyses of the interactions between purified H. pylori LPS and TLRs revealed that, in contrast to LPSs from other gram-negative bacteria, the LPS of H. pylori is not effectively recognized by TLR4. The localization of TLRs on the basolateral poles of epithelial cells reduces the likelihood of H. pylori being recognized by these receptors [31, 32]. However, it was suggested that the phase-variable expression of Lewis antigens allows the bacteria to modulate the host adaptive immune response through interactions with DC-SIGN (dendritic cell-specific ICAM-grabbing nonintegrin) on dendritic cells (DCs) and macrophage subpopulations [33]. DCs are highly specialized antigen-presenting cells, capable of activating naive and memory T lymphocytes. A number of adhesive or cytokine receptor-mediated interactions between DCs and T lymphocytes are important for proper T lymphocyte activation [34]. DC-SIGN is a C-type lectin representing calcium-dependent carbohydrate binding molecules. DCs expressing the DC-SIGN receptor are present on all mucosal surfaces and lymphoid organs. Although no antigenic stimulation is required to induce the expression of DC-SIGN on DCs, macrophages need an environmental signal for DC-SIGN induction [35].
DCs and macrophages are the main targets for LPS, thus participating in the immune response to gram-negative bacteria. It is possible that the Le epitope mimicry may contribute to a different effectiveness of IL-8 and tumor necrosis factor (TNF) secretion by peripheral blood mononuclear leukocytes in response to H. pylori LPS. It was shown that macrophages stimulated with H. pylori LPS of LeX or LeY type produce cytokines more effectively than those cultured in the presence of H. pylori LPS without these determinants [36]. The presence of LeX or LeY moieties in H. pylori LPS promotes a production of potentially self-destructive anti-LeX and anti-LeY IgG in patients with coronary heart disease, seropositive for anti-H. pylori antibodies [26]. Appelmelk et al. reported that the epitope which was most commonly recognized by anti-LPS antibodies in the sera from H. pylori-infected patients was the LeX blood group antigen [25].
The interaction between H. pylori LPS and DCs is poorly understood. In this study, we asked whether H. pylori targets DC-SIGN using its LPS and whether this binding occurs via LeX and LeY determinants or not. The binding of H. pylori to DC-SIGN may result in functional consequences especially regarding the ability of DCs to produce and secrete cytokines. This could be of great importance for the control of H. pylori infections since both direct and indirect stimulation of T lymphocytes by H. pylori LPS is very likely. Furthermore, activated lymphocytes more effectively control the bacterial growth and might diminish gastric mucosa inflammation by releasing cytokines, possibly of Th2 type. Weak inflammatory response helps the bacteria to survive in the host tissue.
In this study, we estimated the binding of H. pylori whole cells and H. pylori LPS preparations with or without LeX/Y determinants to the DC-SIGN receptor in a solid-phase binding assay. We have also made an attempt to examine whether LeX and LeY antigens are able to associate with native, endogenously expressed DC-SIGN. For this purpose we adopted the THP-1 monocyte-macrophage leukemia cell model pretreated with PMA, GM-CSF, and IL-4 to induce the expression of DC-SIGN.
2. Materials and Methods
2.1. Bacterial Strains
The structure of O-antigen chain and core oligosaccharide regions of H. pylori strains with or without LeX, LeY, and LeXY determinants was analysed as previously described [17, 18]. The bacteria were stored at −70°C in tryptic soy broth containing 10% glicerol. Before being used in experiments the bacteria were cultured for 48 h, at 37°C in microaerophilic conditions in the GasPack EZ Campy Container System (Becton, Dickinson and Company, Sparks, USA) on Columbia blood agar with 10% heat inactivated foetal calf serum and washed in phosphate buffered saline (PBS), pH 7.2. The expression of Le determinants on H. pylori cells was confirmed by ELISA (enzyme-linked immunosorbent assay) using mouse monoclonal anti-LeX (mAb anti- LeX) and anti-LeY (mAb anti- LeY) antibodies (Merck, Darmstadt, Germany), and control antigens: synthetic LeX and LeY determinants (Merck) or LeX-BSA (Dextra Laboratories Ltd, Reading, UK), as previously described [37].
2.2. H. pylori Lipopolysaccharides
The H. pylori LPS preparations were classified chemically and serologically as LeX(+), LeY(+), LeXY(+), or LeXY(−) [17, 18]. The H. pylori LPS was obtained by the hot phenol-water extraction method after pretreatment of bacterial biomass with protease. The LPS crude preparation was then treated with RNase, DNase, protein kinase, and ultracentrifugation, as previously described [38].
2.3. The Estimation of H. pylori Whole Cells and H. pylori LPS Binding to Soluble DC-SIGN in the Solid-Phase Assay
The binding of H. pylori whole cells and H. pylori LPS preparations to DC-SIGN was estimated by ELISA, as recommended by Geijtenbeek et al. [34]. The ELISA plates were coated in triplicate with antigens: 1 × 107 bacterial cells/well, 2 μg/mL of LPS, and 1 μg/mL of synthetic LeX-BSA determinants (Dextra Laboratories Ltd) in the carbonate buffer pH 9.6, 18 h, 4°C. After blocking (3% bovine serum albumin in PBS supplemented with 0.05% Tween 80-PBS/BSA/Tween, 2 h at 20°C) and washing (Tris-saline-magnesium buffer-TSM, pH 7.4–8.0), the plates were incubated with 1 μg/well of the recombinant DC-SIGN/Fc chimera—rhDC-SIGN/Fc (R&D Systems, Minneapolis, USA) in TSM, 1 h, 37°C, and washed again. The immunocomplexes were detected using anti-human IgG antibodies conjugated with HRP—horseradish peroxidase (Dako, Glostrup, Denmark). For color development, chromogen o-phenylenediamine dihydrochloride (Sigma, St. Louis, USA) was added in the concentration of 1 mg/mL in the citric phosphate buffer, pH 5.0, with 0.005 mL of 30% H2O2/mL. The results were expressed as OD450 values. In each ELISA test the control wells were included for the evaluation of unspecific reactions. The ELISA cut-off value was defined as twice mean OD for the control wells coated with only one type of antigen or rhDC-SIGN/Fc, incubated with an HRP-conjugated secondary antibody and chromogen-substrate detection solution. The cut-off OD values were in the range of 0.1-0.2.
2.4. The Binding Specificity of H. pylori Whole Cells and H. pylori LPS to Soluble DC-SIGN Detected in the Solid-Phase Inhibition Assay
In order to identify the specificity of DC-SIGN binding, the inhibition of a solid-phase binding assay was performed using various blocking agents: mouse monoclonal antibodies against LeX and LeY determinants, 2 μg/well (Merck), lipopolysaccharide binding protein (LBP), 2 μg/well (HyCult Biotechnology, Uden, Holland), rabbit antibodies against the LPS core region 1 : 500, and fucose or galactose, 25 μg/well (Sigma). These inhibitors were added into the selected wells coated with H. pylori LPSs or LewisX-BSA and the plates were incubated for 30 min, 37°C. After washing, the ELISA assay was performed as previously described. Specificity of DC-SIGN binding to H. pylori whole cells was established by pretreatment of bacterial cells (30 min, 37°C) with monoclonal anti-LeX and anti-LeY antibodies, LBP and fucose or galactose. The binding specificity was estimated by comparing OD values for the ELISA assay without inhibitors to the results obtained in the assay developed using inhibitory agents.
2.5. THP-1 Cell Culture
The acute monocytic leukemia cell line THP-1 was obtained from the American Type Culture Collection (ATCC, Rockville, MD). Cells were cultured in RPMI 1640 medium with 10% foetal calf serum and maintained at 5 × 105 cell/mL. THP-1 cell differentiation was induced by the addition of phorbol myristate acetate (PMA) in the final concentration of 50 nM. After 24 h incubation, a granulocyte-macrophage colony-stimulating factor—GM-CSF (25 ng/mL)—either alone or in combination with interleukin 4-IL-4 (10 ng/mL) was added, and the cell cultures were continued for 5 days, at 37°C, 5% CO2. For subsequent analysis, differentiated THP-1 cells were detached from culture plates by incubation with PBS 5 mM EDTA on ice.
2.6. The Immunofluorescence Assay for the Detection of DC- SIGN Expression on THP-1 Cells and Its Binding to Soluble LeX or LeY Determinants
The phenotypic analysis of the cells was carried out by an indirect immunofluorescence assay using mouse monoclonal anti-DC-SIGN antibodies and FITC-conjugated polyclonal sheep antibodies against mouse immunoglobulins. The first incubation was performed in the presence of 1% inactivated sheep serum solution in PBS. The fluorescence intensity was estimated using the Victor 2 reader (Wallac, Oy, Turku, Finland) and cell imaging was performed using JuLI Smart fluorescent cell analyzer (Digital Bio Technology, Boston, USA). To verify whether DC-SIGN on THP-1 cells binds to LeX or LeY determinants, the cells were incubated with soluble, synthetic LeX or LeY, 20 μg/mL (Merck), and then treated with mouse monoclonal antibodies against DC-SIGN, followed by the treatment with FITC-conjugated polyclonal sheep antibodies to mouse immunoglobulins.
The interaction of soluble DC-SIGN (R&D Systems) with LeX or LeY determinants expressed on the surface of THP-1 cells was also estimated. The fluorescence values for the THP-1 cells incubated with mouse mAb anti-LeX or anti-LeY detected with FITC-labeled sheep anti-mouse IgG were compared to the fluorescence intensity values obtained in the inhibition assay where DC-SIGN was used before the incubation with mouse mAb anti-LeX or anti-LeY and then with FITC-labeled sheep anti-mouse IgG.
3. Results
3.1. Binding of H. pylori Whole Cells to rhDC-SIGN/Fc
The solid-phase binding assay results presented in Figure 1 show that H. pylori whole cells containing LPS with LeX, LeY or LeXY determinants bound to rhDC-SIGN/Fc. The binding of H. pylori whole cells expressing LeX to rhDC-SIGN was inhibited by mAb anti- LeX and fucose but not with anti- LeY mAb. By comparison the binding of H. pylori whole cells with LeY expression was inhibited by anti- LeY mAb and fucose but not with anti- LeX mAb. The binding of H. pylori LewisXY positive whole cells to DC-SIGN was partially decreased when anti-LeX mAb and anti-LeY mAb were used separately and almost completely when anti-LeX and anti-LeY mAb were used simultaneously. The binding was successfully inhibited by fucose moieties as well. In addition we showed that bacteria bearing LPS without LeXY determinants were also able to bind rhDC-SIGN. The binding affinity of H. pylori whole cells which do not carry LeX and LeY determinants to rhDC-SIGN was inhibited neither with mAb anti-LeX and mAb anti-LeY nor with polyclonal antibodies to the core region of LPS. Similarly, the LBP molecule which binds LPS via lipid A had no inhibitory effect. The binding of H. pylori whole cells producing LPS without LeXY determinants to rhDC-SIGN/Fc was successfully inhibited by galactose (Figure 2).
Figure 1.
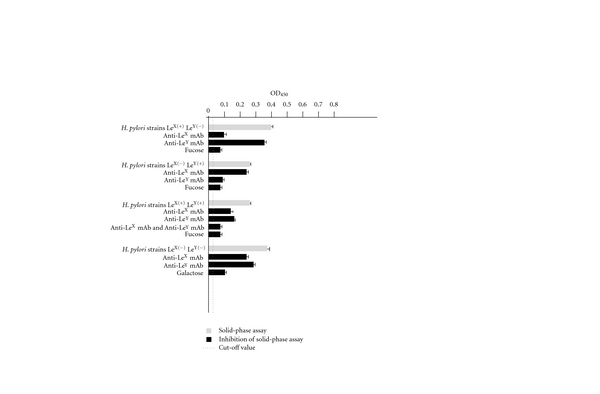
The binding of H. pylori whole cells positive or negative for LeX, LeY, or LeXY determinants with a soluble DC-SIGN receptor. The binding estimated by the solid-phase assay (grey bars). The specificity of binding estimated by the solid-phase inhibition assay (black bars).
Figure 2.
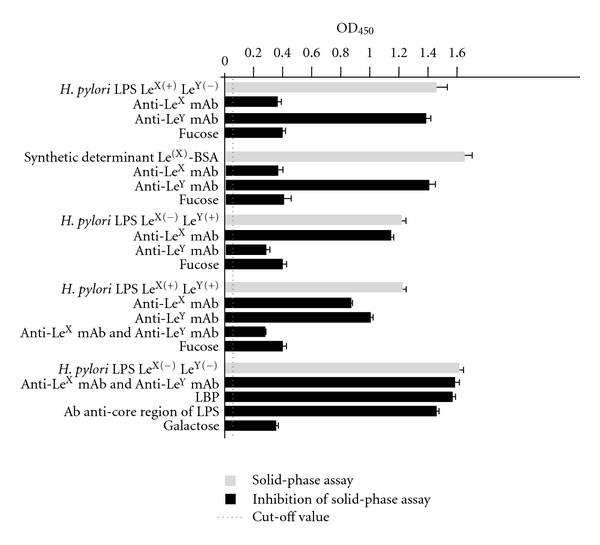
The binding of H. pylori LPS with or without expression of LeXY determinants and synthetic LeX-BSA with a soluble DC-SIGN receptor. The binding estimated by the solid-phase assay (grey bars). The specificity of binding estimated by the solid-phase inhibition assay (black bars).
3.2. Specificity of H. pylori LPS Binding to rhDC-SIGN
The results presented in Figure 2 show that H. pylori-rhDC-SIGN/Fc binding occurs via LPS. The inhibition of LPS-rhDC-SIGN/Fc interaction with mAb anti-LeX and/or mAb anti-LeY or fucose indicated that rhDC-SIGN/Fc bound to Le determinants in LPS molecules: LeX, LeY, or both in fucose-dependent manner. The role of LeX determinants in the interaction with DC-SIGN was confirmed in the solid-phase binding and binding inhibition assays using the plates coated with synthetic LeX-BSA and several blocking agents: anti-LeX mAb, anti-LeY mAb, or fucose. We showed that the LPSs without LeX or LeY determinants also effectively bound to rhDC-SIGN/Fc. The interaction via the core region or lipid A of the LPS was rather excluded due to the lack of the inhibitory effect when anti-LPS core region antibodies or LBP were used, respectively. The binding of purified H. pylori LPS without LeX and LeY determinants was blocked by galactose, indicating that this sugar moiety in H. pylori LPS lacking LeX or LeY determinants mediated the binding of LeX/Y negative LPS to rhDC-SIGN.
3.3. Detection of DC-SIGN on the Surface of THP-1 Cells and Its Binding to Soluble LeX and LeY Determinants in the Indirect Immunofluorescence Assay
The surface expression of DC-SIGN on THP-1 cells was induced by PMA, GM-CSF, and IL-4, treatment. We verified this by quantitative fluorescence measurement of THP-1 cells labeled with mouse anti-DC-SIGN mAb and then with FITC-conjugated sheep antibodies against mouse Igs before and after PMA, GM-CSF, and IL-4 treatment. The unspecific fluorescence intensity of control cells, which were not treated with PMA, GM-CSF and IL-4 reached the value of 240 RFU, whereas the unspecific fluorescence intensity of PMA, GM-CSF and IL-4 treated cells was equal to 545 RFU (Figures 3(a), 3(b)). The fluorescence of nondifferentiated THP-1 cells labeled with anti-DC-SIGN mAb reached 900 RFU versus 4400 RFU for differentiated THP-1 cells (Figure 3(c), 3(d)). Pretreatment of differentiated THP-1 cells with synthetic LeX, before the incubation with anti-DC-SIGN antibodies, diminished the fluorescence emission of the cells to 1270 RFU (Figure 3(e)) which accounted for 70% decrease in fluorescence intensity. Similarly, pretreatment of differentiated THP-1 cells with synthetic LeY before the incubation of the cells with anti-DC-SIGN mAb, diminished the fluorescence emission to 2800 RFU which constitute for 36% inhibition (Figure 3(f)).
Figure 3.
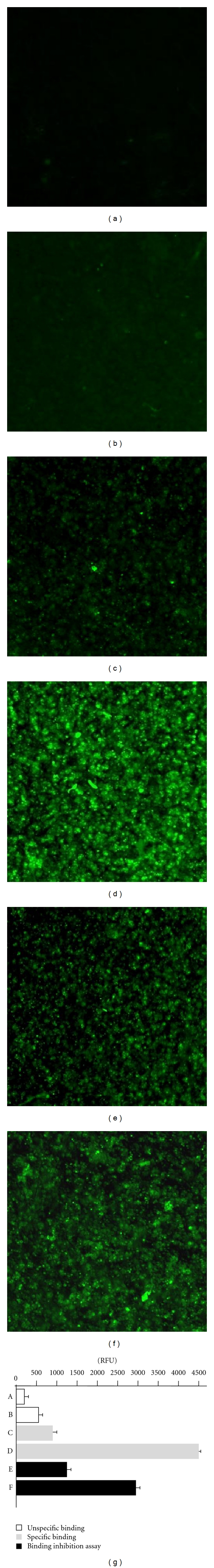
The cell imaging and fluorescence intensity reflecting the interactions between soluble synthetic LeX and LeY determinants with DC-SIGN receptor on THP-1 leukemia cells, in the immunofluorescence assay. Control THP-1 cells, nondifferentiated (a) or differentiated (b) with PMA, GM-CSF and IL-4 stained with FITC-conjugated sheep antibodies against mouse Igs (secondary Ab). Nondifferentiated (c) and differentiated (d) THP-1 cells stained with mouse anti-DC-SIGN monoclonal antibodies (mAb) and with FITC-conjugated secondary Ab. Differentiated THP-1 cells preincubated with synthetic LeX (e) or LeY (f) determinants before the staining of the cells with anti- DC-SIGN mAb followed by the treatment with FITC-conjugated secondary Ab.
3.4. Detection of LeX and LeY Determinants on the Surface of THP-1 Cells and Their Binding to Soluble DC-SIGN in the Indirect Immunofluorescence Assay
The unspecific fluorescence intensity of nondifferentiated and differentiated THP-1 cells is shown on Figures 4(a) and 4(b), respectively. The presence of LeX determinants on the surface of THP-1 cells was verified by the preincubation of the cells with mouse mAb anti-LeX followed by the treatment of the cells with FITC-conjugated antibodies against mouse Igs. The fluorescence intensity of the cells treated in this way was equal to 2388 RFU (Figure 4(c)). By comparison, the pretreatment of the cells with soluble DC-SIGN before the incubation with a mouse mAb anti-LeX and with FITC-conjugated antibodies against mouse Igs caused a decrease in the fluorescence intensity from 2388 RFU to 1282 RFU (46% reduction) (Figure 4(d)). The LeY exposition on the surface of THP1 was visualized by treatment of the cells with anti-LeY mAb followed by the incubation with secondary antibody conjugated with FITC. The fluorescence intensity of THP-1 cells stained with anti- LeY mAb was equal to 913 RFU (Figure 4(e)) and was not diminished (971 RFU) after treatment of the cells with soluble DC-SIGN (Figure 4(f)).
Figure 4.
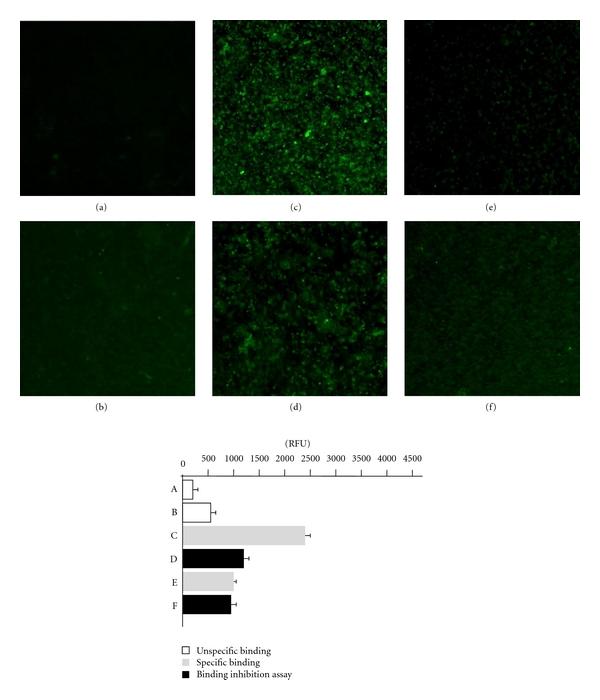
The cell imaging and fluorescence intensity reflecting the interactions between soluble DC-SIGN receptor with the LeX and LeY determinants exposed on the surface THP-1 leukemia cells, in the immunofluorescence assay. Control THP-1 cells, nondifferentiated (a) or differentiated (b) with PMA, GM-CSF and IL-4 stained with FITC-conjugated sheep antibodies against mouse Igs (secondary Ab). Differentiated THP-1 cells treated with anti-LeX mAb and FITC-conjugated secondary Ab (c) or firstly treated with soluble DC-SIGN followed by treatment as above (d). Differentiated THP-1 cells treated with anti-LeY mAb and FITC-conjugated secondary Ab (e) or firstly treated with soluble DC-SIGN followed by the treatment as above (f).
4. Discussion
Geijtenbeek et al. demonstrated that DC-SIGN is exclusively expressed on DCs and it mediates the interaction between DCs and resting T cells via ICAM-3, which is required for DCs-T-cell clustering and for DC-induced proliferation of resting T cells [34]. Several pathogens are able to target DC-SIGN and modulate DC functions and, due to this, escape immune response of the host [39–42]. Little attention was paid to the role of DCs in H. pylori infection, and how this pathogen is able to persistently colonize so many hosts is poorly understood. DC-SIGN recognizes both internal branched mannose residues and terminal di-mannoses, α-1-3 and α-1-4 fucosylated glycan structures as well as certain N-acetyloglucosamine containing molecules on self proteins/or pathogens [43–46]. It was suggested that H. pylori binds to DC-SIGN via common pathogen recognition patterns such as high mannose and/or Lewis carbohydrates [33]. The presence of Lewis epitopes in the O-specific region of LPS possibly enables these bacteria to omit the immune responses, resulting in the phenomenon of antigenic mimicry. It was assumed that the differences in H. pylori binding affinity to the DC-SIGN receptor is determined by the Le phase variation. This ability of H. pylori might allow the bacteria to modulate the host immune responses and in consequence contributes to the persistent character of this infection [33].
The results obtained in this study indicate that H. pylori whole cells with antigenic LeX and/or LeY determinants bind to the recombinant DC-SIGN receptor. Similarly, H. pylori LPS of LeX, LeY, LeXY type was involved in rhDC-SIGN/Fc binding. The fucose residues present in the structure of LeX and LeY determinants were responsible for binding specificity of H. pylori cells containing LPS of LeX, LeY, or LeXY to DC-SIGN receptor. This was shown in binding inhibition assay with monoclonal anti-LeX and/or anti-LeY antibodies, and fucose. Bergman et al. demonstrated that DC-SIGN recognizes H. pylori and the binding of H. pylori whole cells or purified LPS to DC-SIGN was blocked with anti-DC-SIGN antibodies, mannan, or by ethylene glycol tetraacetic acid (EGTA)-Ca2+ chelating agent. H. pylori expressing LeY or short chains of LeX (monomeric LeX up to (LeX)4), but not polymeric LeX, were able to bind DC-SIGN. Double knockout H. pylori mutants without α-3-fucosyltransferases FutA and FutB unable to produce LeY and monomeric LeX did not bind DC-SIGN. Also steric organization of the sugar residues of Le antigens influences their binding affinity to DC-SIGN [33].
In this study H. pylori whole cells and LPS preparations without LeXand LeY determinants were also found to be able to bind DC-SIGN. The binding was successfully inhibited with galactose but not with anti-LeX/Y or anti-LPS core region antibodies. Guo et al. showed that LeX oligosaccharide primarily binds to DC-SIGN through the fucose moieties. However, galactose is involved in the stabilization of this interaction [44]. The H. pylori LeXY negative strain used in this study contained a galE gene encoding UDP-galactose 4-epimerase, which mediates the incorporation of galactose in the O-side chain of LPS. To elucidate the role of galactose in H. pylori LPS binding to DC-SIGN, the construction of gale-deficient mutant would be of major benefit. Recently, Klena et al. demonstrated that only E. coli strains bearing the complete core region (without O-antigen) are able to mediate binding to DC-SIGN and, in consequence, further phagocytosis of bacteria by macrophages [47]. Previous studies demonstrated that DC-SIGN, besides binding to mannose and fucose, can also interact with glucose, α-1,4-diglucosyl (maltose) structures, and galactose [48]. The binding of galactose to mannose-specific C-type carbohydrate recognition domains (CRDs) can occur through interactions with the C-1 and C-2 hydroxyl groups of the free sugar [49]. Recently α-glucans were identified as new ligands for DC-SIGN [50].
DC-SIGN expression is induced de novo during the generation of monocyte-derived cells and is normally considered as a DC-specific phenotypic marker. However, DC-SIGN was also detected on synovial, placenta, and alveolar macrophages [34]. The expression of DC-SIGN depends on IL-4 which drives monocyte/macrophages into the “alternative activation” pathway or results in the generation of monocyte-derived dendritic cells if combined with GM-CSF. It was shown that leukemic THP-1 cells, widely used as a model for monocyte-macrophage differentiation, express very low, basal amounts of DC-SIGN and that expression of DC-SIGN in THP-1 cells is regulated during differentiation induced by PMA, GM-CSF, and IL-4 [51]. These results demonstrate that DC-SIGN is a marker for both DCs and alternatively activated macrophages and is present on THP-1 cells, which may be considered a useful cellular system to characterize the pathogen binding capacities. In this study, we asked whether THP-1 cells with the surface expression of DC-SIGN induced by PMA, GM-CSF, and IL-4 can bind to synthetic LeX and LeY determinants. Considering the fact that the role of DCs and DC-SIGN receptors in the course of H. pylori infections is poorly understood, the detection of interactions between H. pylori antigens and DC-SIGN, especially in the context of LeX and LeY determinants in H. pylori LPS structure, could be of great importance. This study was focused on the development of a cellular model to estimate the interactions between H. pylori expressing LeX and/or LeY antigens and the DC-SIGN receptor in a solid-phase assay. The upregulation of the DC-SIGN receptor on the surface of THP-1 cells was induced by the stimulation of the cells with PMA, GM-CSF, and IL-4 as recommended by Puig-Kröger et al. and assessed quantitatively by an immunofluorescence assay, using monoclonal anti-DC-SIGN antibodies and FITC-conjugated anti-mouse Igs [51]. Our results demonstrated that DC-SIGN-like receptor is present on the surface of THP-1 cells and that this receptor is able to bind synthetic LeX and LeY determinants. This enables the assessment of molecular effects of the H. pylori-DC-SIGN interactions via Le determinants using the THP-1 cellular model. Previously, Perez-Perez et al., using PMA differentiated THP-1 cells, showed that the lipid A from H. pylori LPS has a low ability to mediate macrophage activation [52]. We expect that the in vitro THP-1 cellular model could help to elucidate how leukemic cells, especially of monocytic lineage, respond to H. pylori polysaccharide stimuli, regarding the expression of HLA-DR, CD40, CD80 and CD86, as well as the antigen uptake ability and potency in inducing allogenic T-cell proliferation. This cellular tool could reveal new facts regarding immune response elicited by H. pylori compounds, especially in the context of its activity as a bacterial carcinogen classified to IInd group. Recently, Chan et al. showed that Ganoderma lucidum polysaccharides can induce transformation of THP-1 cells into dendritic cells with an immune-stimulatory function [53]. It was also demonstrated that, apart from α1β2 integrin, DC-SIGN on macrophages and DCs is an additional ligand for the lymphocyte ICAM-3 molecule which mediates the contact between T cells and antigen-presenting cells. ICAM-3 also assists in the interaction of granulocytes with DC-SIGN of DCs [54].
We also asked whether the expression of LeX and LeY determinants on the surface of THP-1 cells makes this model suitable for the estimation of interactions with soluble DC-SIGN.
In this study we demonstrated binding of soluble DC-SIGN receptor with LeX determinants on THP-1 cells. By comparison using this model we could not show successful binding of soluble DC-SIGN with LeY due to low expression of such determinants on THP-1 cells. In this case the THP-1 cellular model may selectively mimic the interactions between surface-exposed LeX determinants and DC-SIGN molecule thus it opens new experimental possibilities. On the other hand, the results demonstrating the presence of a DC-SIGN-like receptor on the surface of THP-1 cells show that this cellular model can be used for the assessment of molecular effects due to H. pylori-DC-SIGN interactions via surface exposed or soluble Lewis compounds, which can be released during bacterial cell lysis in the inflammatory milieu of H. pylori colonized gastric mucosa. This idea correlates with the results obtained in the solid-phase assay showing that LeX, LeY, and LeXY determinants on H. pylori whole cells and H. pylori LPS preparations are involved in binding with DC-SIGN.
Conflict of Interests
The authors have no conflicting financial interests.
Acknowledgments
This paper is supported by The Polish Ministry of Science and Higher Education (Grant N N401 015 136), The European Union Project “Stipends supporting innovative research projects for PhD students.”
References
- 1.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. The Lancet. 1984;1(8390):1311–1314. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Chmiela M, Michetti P. Inflammation, immunity, vaccines for Helicobacter infection. Helicobacter. 2006;11:21–26. doi: 10.1111/j.1478-405X.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- 3.Crabtree JE. Mucosal immune responses to Helicobacter pylori. European Journal of Gastroenterology and Hepatology. 1993;5(2):S30–S32. [Google Scholar]
- 4.Gebert B, Fischer W, Weiss E, Hoffmann R, Haas R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science. 2003;301(5636):1099–1102. doi: 10.1126/science.1086871. [DOI] [PubMed] [Google Scholar]
- 5.Cover TL, Blaser MJ. Helicobacter pylori in Health and Disease. Gastroenterology. 2009;136(6):1863–1873. doi: 10.1053/j.gastro.2009.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paziak-Domańska B, Chmiela M, Jarosińska A, Rudnicka W. Potential role of cagA in the inhibition of T cell reactivity in Helicobacter pylori infections. Cellular Immunology. 2000;202(2):136–139. doi: 10.1006/cimm.2000.1654. [DOI] [PubMed] [Google Scholar]
- 7.Zabaleta J, McGee DJ, Zea AH, et al. Helicobacter pylori arginase inhibits T cell proliferation and reduces the expression of the TCR ζ-chain (CD3ζ) Journal of Immunology. 2004;173(1):586–593. doi: 10.4049/jimmunol.173.1.586. [DOI] [PubMed] [Google Scholar]
- 8.Sundrud MS, Torres VJ, Unutmaz D, Cover TL. Inhibition of primary human T cell proliferation by Helicobacter pylori vacuolating toxin (VacA) is independent of VacA effects on IL-2 secretion. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(20):7727–7732. doi: 10.1073/pnas.0401528101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerhard M, Schmees C, Voland P, et al. A secreted low-molecular-weight protein from Helicobacter pylori induces cell-cycle arrest of T cells. Gastroenterology. 2005;128(5):1327–1339. doi: 10.1053/j.gastro.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Torres VJ, VanCompernolle SE, Sundrud MS, Unutmaz D, Cover TL. Helicobacter pylori vacuolating cytotoxin inhibits activation-induced proliferation of human T and B lymphocyte subsets. Journal of Immunology. 2007;179(8):5433–5440. doi: 10.4049/jimmunol.179.8.5433. [DOI] [PubMed] [Google Scholar]
- 11.Alexander Ch, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. Journal of Endotoxin Research. 2001;7(3):167–202. [PubMed] [Google Scholar]
- 12.Muotiala A, Helander IM, Pyhala L, Kosunen TU, Moran AP. Low biological activity of Helicobacter pylori lipopolysaccharide. Infection and Immunity. 1992;60(4):1714–1716. doi: 10.1128/iai.60.4.1714-1716.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grębowska A, Moran AP, Matusiak A, et al. Anti-phagocytic activity of Helicobacter pylori lipopolysaccharide (LPS)-possible modulation of the innate immune response to these bacteria. Polish Journal of Microbiology. 2008;57(3):185–192. [PubMed] [Google Scholar]
- 14.Grębowska A, Moran AP, Bielanski W, et al. Helicobacter pylori lipopolysaccharide activity in human peripheral blood mononuclear leukocyte cultures. Journal of Physiology and Pharmacology. 2010;61(4):437–442. [PubMed] [Google Scholar]
- 15.Rudnicka K, Włodarczyk M, Moran AP, et al. Immunomodulatory activity of H. pylori LPS-possible reason of chronic infections. Helicobacter. 2009;14(4):p. 10. [Google Scholar]
- 16.Rudnicka W, Jarosinska A, Bak-Romaniszyn L, et al. Helicobacter pylori lipopolysaccharide in the IL-2 milieu activates lymphocytes from dyspeptic children. FEMS Immunology and Medical Microbiology. 2003;36(3):141–145. doi: 10.1016/S0928-8244(03)00023-3. [DOI] [PubMed] [Google Scholar]
- 17.Aspinall GO, Monteiro MA, Pang H, Walsh EJ, Moran AP. Lipopolysaccharide of the Helicobacter pylori strains P466 and M019: structures of the O antigen and core oligosaccharide regions. Biochemistry. 1996;35(7):2498–2504. doi: 10.1021/bi951853k. [DOI] [PubMed] [Google Scholar]
- 18.Aspinall GO, Monteiro MA, Pang H, Walsh EJ, Moran AP. Lipopolysaccharide of the Helicobacter pylori type strain NCTC 11637 (ATCC 43504): structure of the O antigen chain and core oligosaccharide regions. Biochemistry. 1996;35(5):2489–2497. doi: 10.1021/bi951852s. [DOI] [PubMed] [Google Scholar]
- 19.Simoons-Smit IM, Appelmelk BJ, Verboom T, et al. Typing of Helicobacter pylori with monoclonal antibodies against Lewis antigens in lipopolysaccharide. Journal of Clinical Microbiology. 1996;34(9):2196–2200. doi: 10.1128/jcm.34.9.2196-2200.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appelmelk BJ, Shiberu B, Trinks C, et al. Phase variation in Helicobacter pylori lipopolysaccharide. Infection and Immunity. 1998;66(1):70–76. doi: 10.1128/iai.66.1.70-76.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monteiro MA, Zheng PY, Ho B, et al. Expression of histo-blood group antigens by lipopolysaccharides of Helicobacter pylori strains from Asian hosts: the propensity to express type 1 blood-group antigens. Glycobiology. 2000;10(7):701–713. doi: 10.1093/glycob/10.7.701. [DOI] [PubMed] [Google Scholar]
- 22.Rasko DA, Keelan M, Wilson TJM, Taylor DE. Lewis antigen expression by Helicobacter pylori. Journal of Infectious Diseases. 2001;184(3):315–321. doi: 10.1086/322025. [DOI] [PubMed] [Google Scholar]
- 23.Edwards NJ, Monteiro MA, Faller G, et al. Lewis X structures in the O antigen side-chain promote adhesion of Helicobacter pylori to the gastric epithelium. Molecular Microbiology. 2000;35(6):1530–1539. doi: 10.1046/j.1365-2958.2000.01823.x. [DOI] [PubMed] [Google Scholar]
- 24.Heneghan MA, McCarthy CF, Moran AP. Relationship of blood group determinants on Helicobacter pylori lipopolysaccharide with host Lewis phenotype and inflammatory response. Infection and Immunity. 2000;68(2):937–941. doi: 10.1128/iai.68.2.937-941.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Appelmelk BJ, Simoons-Smit I, Negrini R, et al. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infection and Immunity. 1996;64(6):2031–2040. doi: 10.1128/iai.64.6.2031-2040.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grȩbowska A, Rechciński T, Moran A, et al. Increased levels of immunoglobuline and non-immunoglobuline markers of host response to H. pylori LPS in the patients with coronary artery disease (CAD) Current Trends in Immunology. 2006;7:85–96. [Google Scholar]
- 27.Cambi A, Figdor CG. Levels of complexity in pathogen recognition by C-type lectins. Current Opinion in Immunology. 2005;17(4):345–351. doi: 10.1016/j.coi.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brightbill HD, Modlin RL. Toll-like receptors: molecular mechanisms of the mammalian immune response. Immunology. 2000;101(1):1–10. doi: 10.1046/j.1365-2567.2000.00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krutzik SR, Sieling PA, Modlin RL. The role of Toll-like receptors in host defense against microbial infection. Current Opinion in Immunology. 2001;13(1):104–108. doi: 10.1016/s0952-7915(00)00189-8. [DOI] [PubMed] [Google Scholar]
- 30.Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nature Reviews Microbiology. 2005;3(1):36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- 31.Bäckhed F, Rokbi B, Torstensson E, et al. Gastric mucosal recognition of Helicobacter pylori is independent of Toll-like receptor 4. Journal of Infectious Diseases. 2003;187(5):829–836. doi: 10.1086/367896. [DOI] [PubMed] [Google Scholar]
- 32.Smith MF, Jr, Mitchell A, Li G, et al. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-κB activation and chemokine expression by epithelial cells. Journal of Biological Chemistry. 2003;278(35):32552–32560. doi: 10.1074/jbc.M305536200. [DOI] [PubMed] [Google Scholar]
- 33.Bergman MP, Engering A, Smits HH, et al. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. Journal of Experimental Medicine. 2004;200(8):979–990. doi: 10.1084/jem.20041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geijtenbeek TBH, Engering A, Van Kooyk Y. DC-SIGN, a C-type lectin on dendritic cells that unveils many aspects of dendritic cell biology. Journal of Leukocyte Biology. 2002;71(6):921–931. [PubMed] [Google Scholar]
- 35.Soilleux EJ, Morris LS, Leslie G, et al. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. Journal of Leukocyte Biology. 2002;71(3):445–457. [PubMed] [Google Scholar]
- 36.Grębowska A, Moran AP, Czkwianianc E, et al. A modulation of macrophage response to H. pylori LPS with or without Lewis XY determinants. Helicobacter. 2004;9(5):p. 16. [Google Scholar]
- 37.Rudnicka W, Czkwianianc E, Płaneta-Małecka I, et al. A potential double role of anti-Lewis X antibodies in Helicobacter pylori-associated gastroduodenal diseases. FEMS Immunology and Medical Microbiology. 2001;30(2):121–125. doi: 10.1111/j.1574-695X.2001.tb01559.x. [DOI] [PubMed] [Google Scholar]
- 38.Moran AP, Helander IM, Kosunen TU. Compositional analysis of Helicobacter pylori rough-form lipopolysaccharides. Journal of Bacteriology. 1992;174(4):1370–1377. doi: 10.1128/jb.174.4.1370-1377.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geijtenbeek TBH, Kwon DS, Torensma R, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100(5):587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 40.van Kooyk Y, Geijtenbeek TBH. DC-SIGN: escape mechanism for pathogens. Nature Reviews Immunology. 2003;3(9):697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- 41.Ludwig IS, Lekkerkerker AN, Depla E, et al. Hepatitis C virus targets DC-SIGN and L-SIGN to escape lysosomal degradation. Journal of Virology. 2004;78(15):8322–8332. doi: 10.1128/JVI.78.15.8322-8332.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehlers S. DC-SIGN and mannosylated surface structures of Mycobacterium tuberculosis: a deceptive liaison. European Journal of Cell Biology. 2010;89(1):95–101. doi: 10.1016/j.ejcb.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Feinberg H, Mitchell DA, Drickamer K, Weis WI. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science. 2001;294(5549):2163–2166. doi: 10.1126/science.1066371. [DOI] [PubMed] [Google Scholar]
- 44.Guo Y, Feinberg H, Conroy E, et al. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nature Structural and Molecular Biology. 2004;11(7):591–598. doi: 10.1038/nsmb784. [DOI] [PubMed] [Google Scholar]
- 45.van Liempt E, Bank CMC, Mehta P, et al. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Letters. 2006;580(26):6123–6131. doi: 10.1016/j.febslet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Bogoevska V, Horst A, Klampe B, Lucka L, Wagener C, Nollau P. CEACAM1, an adhesion molecule of human granulocytes, is fucosylated by fucosyltransferase IX and interacts with DC-SIGN of dendritic cells via Lewis x residues. Glycobiology. 2006;16(3):197–209. doi: 10.1093/glycob/cwj057. [DOI] [PubMed] [Google Scholar]
- 47.Klena J, Zhang P, Schwartz O, Hull S, Chen T. The core lipopolysaccharide of Escherichia coli is a ligand for the dendritic-cell-specific intercellular adhesion molecule nonintegrin CD209 receptor. Journal of Bacteriology. 2005;187(5):1710–1715. doi: 10.1128/JB.187.5.1710-1715.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitchell DA, Fadden AJ, Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. Journal of Biological Chemistry. 2001;276(31):28939–28945. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- 49.Kolatkar AR, Weis WI. Structural basis of galactose recognition by C-type animal lectins. Journal of Biological Chemistry. 1996;271(12):6679–6685. [PubMed] [Google Scholar]
- 50.Geurtsen J, Chedammi S, Mesters J, et al. Identification of mycobacterial α-glucan as a novel ligand for DC-SIGN: involvement of mycobacterial capsular polysaccharides in host immune modulation. Journal of Immunology. 2009;183(8):5221–5231. doi: 10.4049/jimmunol.0900768. [DOI] [PubMed] [Google Scholar]
- 51.Puig-Kröger A, Serrano-Gomez D, Caparros E, et al. Regulated expression of the pathogen receptor dendritic cell-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonitegrin in THP-1 human leukemic cells, monocytes and macrophages. The Journal of Biological Chemistry. 2004;279(24):25680–25688. doi: 10.1074/jbc.M311516200. [DOI] [PubMed] [Google Scholar]
- 52.Perez-Perez GI, Shepherd VL, Morrow JD, Blaser MJ. Activation of human THP-1 cells and rat bone marrow-derived macrophages by Helicobacter pylori lipopolysaccharide. Infection and Immunity. 1995;63(4):1183–1187. doi: 10.1128/iai.63.4.1183-1187.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan WK, Cheung CC, Law HK, Lau YL, Chan GC. Ganoderma lucidum polysaccharides can induce human monocytic leukemia cells into dendritic cells with immuno-stimulatory function. Journal of Hematology & Oncology. 2008;1:p. 9. doi: 10.1186/1756-8722-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bogoevska V, Nollau P, Lucka L, et al. DC-SIGN binds ICAM-3 isolated from peripheral human leukocytes through Lewis x residues. Glycobiology. 2007;17(3):324–333. doi: 10.1093/glycob/cwl073. [DOI] [PubMed] [Google Scholar]


