Abstract
A growing body of evidence suggests that autism spectrum disorders (ASDs) are related to altered communication between brain regions. Here, we present findings showing that ASD is characterized by a pattern of reduced functional integration as well as reduced segregation of large-scale brain networks. Twenty-three children with ASD and 25 typically developing matched controls underwent functional magnetic resonance imaging while passively viewing emotional face expressions. We examined whole-brain functional connectivity of two brain structures previously implicated in emotional face processing in autism: the amygdala bilaterally and the right pars opercularis of the inferior frontal gyrus (rIFGpo). In the ASD group, we observed reduced functional integration (i.e., less long-range connectivity) between amygdala and secondary visual areas, as well as reduced segregation between amygdala and dorsolateral prefrontal cortex. For the rIFGpo seed, we observed reduced functional integration with parietal cortex and increased integration with right frontal cortex as well as right nucleus accumbens. Finally, we observed reduced segregation between rIFGpo and the ventromedial prefrontal cortex. We propose that a systems-level approach—whereby the integration and segregation of large-scale brain networks in ASD is examined in relation to typical development—may provide a more detailed characterization of the neural basis of ASD.
Keywords: amygdala, connectivity, default mode network, face processing, mirror neuron system
Introduction
Autism spectrum disorders (ASDs) are pervasive neurodevelopmental disorders characterized by atypical social behavior, delayed and/or abnormal verbal and nonverbal communication, as well as unusual patterns of repetitive behaviors and restricted interests. While the neurobiological basis for ASD remains largely unknown, it has been hypothesized that disruption of the initial architecture and connectivity of local circuits in individuals with ASD may alter the experience-dependent maturation of large-scale brain networks required for integrative processing (Belmonte et al. 2004; Just et al. 2004; Courchesne and Pierce 2005; Geschwind and Levitt 2007; Mundy et al. 2009). Thus, developmental abnormalities in ASD may prevent the typical reorganization of neuronal connections into functionally integrated networks that are crucial for facilitating complex social behavior.
Functional neuroimaging studies have generally focused on differences in regional brain activation among individuals with ASD while processing social and emotional stimuli. More specifically, abnormal activity has been reported in specialized regions or networks such as the amygdala (emotion processing; e.g., Baron-Cohen et al. 1999; Dalton et al. 2005), fusiform gyrus (face processing; e.g., Pierce et al. 2004; Schultz 2005), superior temporal sulcus (biological motion; e.g., Pelphrey and Carter 2008), inferior frontal gyrus (mirror neuron system [MNS]; e.g., Dapretto et al. 2006; Oberman and Ramachandran 2007), medial prefrontal cortex (theory of mind; e.g., Castelli et al. 2002; Wang et al. 2007), and precuneus (default mode network [DMN]; e.g., Kennedy and Courchesne 2008a). A recent meta-analysis of functional activation studies in ASD identified areas consistently hypoactivated during social and emotional information processing which included amygdala, inferior frontal gyrus, and higher order association areas such as medial prefrontal cortex (Di Martino et al. 2009). Additionally, the meta-analysis found that individuals with ASD tended to hyperactivate primary sensory areas such as postcentral gyrus, superior temporal gyrus, and inferior occipital gyrus. Hyperactivation of primary sensory areas and hypoactivation of association areas may reflect a bottleneck of information flow that prevents appropriate integration of incoming sensory information important for social behavior. While regional characterizations of brain dysfunction in ASD have informed our initial understanding of the neurobiological underpinnings of this disorder, a systems-level approach that characterizes communication within and between different brain networks should provide further insight.
In recent years, neuroimaging studies of autism have begun to focus on functional connectivity between different brain networks. In an early baseline glucose metabolism study using positron emission tomography (PET), Horwitz et al. (1988) first reported reduced correlations among frontal and parietal cortices in individuals with autism. Later, in a theory of mind PET study involving mental attributions of animated shapes, Castelli et al. (2002) found reduced connectivity between extrastriate visual cortex and the superior temporal sulcus in adults with ASD. They suggested that dysfunction in the interaction between higher and lower order perceptual processes may be related to the behavioral manifestations of autism. In a later functional magnetic resonance imaging (fMRI) study, Just et al. (2004) found reductions in connectivity strengths between higher level association areas engaged during a sentence comprehension task in high-functioning individuals with ASD. Although their analyses found reduced connectivity in relatively few of the many pairwise connections examined, the authors proposed that the underdevelopment of integrative circuitry as indexed by widespread anterior–posterior underconnectivity could be responsible for all higher level cognitive deficits in autism. Further studies in individuals with ASD have supported this claim, reporting evidence of underconnectivity between task-activated regions, particularly for frontoparietal connections, during tasks involving working memory (Koshino et al. 2005), visuomotor coordination (Villalobos et al. 2005), visual imagery (Kana et al. 2006), executive functioning (Just et al. 2007), response inhibition (Kana et al. 2007), face processing (Kleinhans et al. 2008), and theory of mind (Kana et al. 2009).
Recent imaging studies performed in the absence of an overt cognitive task (i.e., during “resting-state”) have established that synchronized low frequency (<0.1 Hz) spontaneous fluctuations in neuronal activity are present across different brain regions (Biswal et al. 1995; see Fox and Raichle 2007, for review). These findings suggest that the brain is intrinsically organized into several large-scale interactive functional networks during both rest and task conditions (Calhoun et al. 2008; Smith et al. 2009). A growing number of resting-state studies examining the DMN (Raichle et al. 2001) have reported reduced connectivity in this network in individuals with ASD (Cherkassky et al. 2006; Kennedy and Courchesne 2008b; Monk et al. 2009; Assaf et al. 2010; Weng et al. 2010). Dysfunction of the DMN in ASD is consistent with the social deficits observed in ASD and the DMN’s known role in social cognition (e.g., Iacoboni et al. 2004; Andrews-Hanna et al. 2010). However, the DMN does not function independently of other systems and is unlikely to be the only affected system in autism given the array of both social and cognitive impairments as well as non-DMN brain regions implicated in the disorder.
Although there have been consistent reports of reduced frontoparietal connectivity in ASD during both rest and task (e.g., Just et al. 2007; Kennedy and Courchesne 2008b), other studies have found evidence of overconnectivity within thalamocortical (Mizuno et al. 2006) and striatocortical circuits (Turner et al. 2006; Di Martino et al. 2010), as well as greater corticocortical connectivity (Noonan et al. 2009; Shih et al. 2010). Thus, in contrast with a general underconnectivity theory, it has been argued that underconnectivity is likely not a general feature of the autistic brain; rather, it may depend on the specific regions and networks being examined as well as the networks engaged by the task being performed.
In parallel with reports of altered functional connectivity in autism, investigators have begun to map the typical development of functional brain networks (see Uddin et al. 2010, for review). Using a variety of methodological approaches, several groups have reported that during development, functional brain networks show increases in long-range functional connectivity among nodes of a given network (i.e., functional integration) as well as reduced local (i.e., intralobar) positive connectivity among nodes in different networks (i.e., functional segregation; Fair, Dosenbach, et al. 2007; Fair et al. 2008, 2009; Kelly et al. 2009; Stevens et al. 2009; Supekar et al. 2009; Dosenbach et al. 2010). Studies in neurotypical individuals have also highlighted the role of functional segregation as measured through anticorrelations between distinct brain networks (Fox et al. 2005, 2009; Fransson et al. 2005; Kelly et al. 2008; Stevens et al. 2009). Most prominently, the internally directed default mode or “task negative” network has been shown to display an anticorrelated relationship with the externally directed “task positive” or attentional control network. Increased anticorrelations (i.e., reduced connectivity), interpreted as increasing segregation, between these networks have been observed across typical development (Stevens et al. 2009) and in adults who displayed superior behavioral performance (Kelly et al. 2008; Hampson et al. 2010). Despite recent controversy regarding the proper interpretation of anticorrelations (Murphy et al 2009, Fox et al. 2009; see a discussion of this issue in Supplementary Material), it has been suggested that investigating functional segregation, as measured by antagonistic relationships between large-scale networks, such as the default mode and task-positive networks, may provide a better understanding of the neural basis of social communication deficits in ASD than functional integration alone (Uddin and Menon 2009). However, this hypothesis has largely been unexplored (Kennedy and Courchesne 2008b).
Although resting-state studies have become the standard way to probe functional connectivity, examining connectivity of networks engaged during cognitive tasks should provide information about network functioning that is complementary to resting-state and task activation studies (Stevens 2009). In the present study, we sought to examine functional integration and segregation of large-scale brain networks involved in social and emotional information processing in children and adolescents with ASD by performing whole-brain connectivity analyses of fMRI data acquired during an emotion processing task using seed regions that have previously been reported to display aberrant activation during socially relevant tasks.
It is well established that the amygdala plays a central role in emotion processing (LeDoux 2000). Findings of dysfunctional emotional face processing (Baron-Cohen et al. 2000; Adolphs et al. 2001), as well as diminished amygdala functional activation (Baron-Cohen et al. 1999; Critchley et al. 2000) led to an early theory of amygdala dysfunction in autism (Baron-Cohen et al. 2000). Although initial studies documented amygdala hypoactivation, later functional studies have found amygdala hyperactivation (Dalton et al. 2005; Monk et al. 2010), which was related to eye fixation patterns (Dalton et al. 2005). Additional studies have documented structural abnormalities (Munson et al. 2006; Nacewicz et al. 2006; Schumann et al. 2009) as well as reduced functional connectivity between the amygdala and both the fusiform face area (Kleinhans et al. 2008) and the anterior insula (Ebisch et al. 2010). Thus, further characterization of amygdala connectivity may be useful for understanding emotion processing deficits in ASD.
In addition to the amygdala’s role in emotion processing, it is known that higher order networks, including the MNS and the salience network, are involved in social and emotional information processing. The salience or cinguloopercular network (Dosenbach et al. 2007; Seeley et al. 2007) is a task-positive network that includes the anterior cingulate and anterior insula and has been described as being involved in regulating behavior through monitoring homeostatic and sensory stimuli (Seeley et al. 2007; Craig 2009). The MNS, which includes the inferior frontal gyrus, pars opercularis (IFGpo) and inferior parietal lobule (IPL), is believed to allow for simulation of shared motor representations between self and others (see Rizzolati and Fabbri-Destro 2010, for review). Consistent with the hypothesized role of the salience network, the MNS has been hypothesized to connect higher level association areas with the limbic system (including the amygdala) through the anterior insula (Jabbi and Keysers 2008), allowing for an intuitive understanding of one’s own and others’ emotions (Carr et al. 2003; Leslie et al. 2004). Measures of empathetic behavior and interpersonal competence have been found to positively correlate with activity in the IFGpo, anterior insula, and amygdala (Pfeifer et al. 2008). Numerous studies have reported structural and functional abnormalities in these higher order networks in individuals with ASD (e.g., Villalobos et al. 2005; Dapretto et al. 2006; Hadjikani et al. 2006, 2007; Ebisch et al. 2010).
Given the known roles of the amygdala and IFGpo in social and emotional information processing, as well as consistent reports of dysfunction of these regions and associated systems in ASD (Di Martino et al. 2009), we used these structures as seeds for whole-brain connectivity analyses in children and adolescents with ASD. We chose to examine connectivity during an emotional face processing task known to engage these networks in order to further tap into the functioning of this circuitry. We predicted that children and adolescents with ASD would show reduced long-range functional connectivity/integration of networks examined using these seed regions. We also predicted that there would be reduced segregation between distinct functional networks in individuals with ASD as measured by increased local connectivity as well as reduced anticorrelations.
Materials and Methods
Participants
High-functioning children and adolescents with ASDs and typically developing (TD) children and adolescents were recruited through referrals from the University of California, Los Angeles (UCLA) Autism Evaluation Clinic and/or through flyers posted throughout the greater Los Angeles area. Six subjects (20% of the total sample) with ASD and 3 TD subjects (10% of the total sample) were scanned but not included in analyses due to excessive head motion (>3 mm maximum relative head motion and/or no activation in primary visual cortices). The final matched groups consisted of 23 high-functioning children and adolescents (2 females) with ASD and 25 TD children and adolescents (3 females). The groups did not differ significantly in age, head motion, Full Scale IQ, Verbal IQ, or Performance IQ as assessed by the Wechsler Abbreviated Scale of Intelligence—Revised (Wechsler 1999) or Wechsler Intelligence Scale for Children—Third Edition (see Table 1 for sample characteristics). Although the groups did not significantly differ by age or IQ, these variables were included as covariates in additional between-group analyses to examine how they may have affected our results; these analyses showed that covarying for age or IQ did not alter any of the observed between-group differences, thus these results are not reported. For the ASD group, prior clinical diagnosis of autism or ASD was confirmed by the Autism Diagnostic Observation Scale (ADOS-G) and/or Autism Diagnosis Interview (ADI-R; Lord et al. 1994, 2000). Nineteen of the children with ASD met criteria for autism as defined by the ADI-R and all children in the ASD group met criteria for autism or autism spectrum disorder as defined by the ADOS. Seventeen participants with ASD and all TD participants reported no current medication use. For the remaining 6 participants with ASD, medication use was unknown. All participants reported no history of neurologic disorders (including head injury, stroke, tumor, and seizures), psychiatric disorders (e.g., schizophrenia, attention-deficit/hyperactivity disorder), or other brain abnormalities. Subjects and parents provided written consent according to the guidelines specified by the Institutional Review Board at the University of California, Los Angeles.
Table 1.
Mean, standard deviation, and range of sample descriptives
| Characteristic | Typically developing | Autism spectrum | P value |
| Age | 13.3 ± 0.96, 10.8−15.7 | 12.6 ± 2.83, 8.2−17.4 | 0.28 |
| Verbal IQ | 105.6 ± 10.5, 89−126 | 97.5 ± 20.9, 69−138 | 0.09 |
| Performance IQ | 105.9 ± 10.3, 88−121 | 105.6 ± 15.9, 75−142 | 0.94 |
| Full scale IQ | 106.4 ± 8.2, 92−116 | 100.7 ± 18.6, 70−134 | 0.19 |
| Mean relative head motion (mm) | 0.12 ± 0.10, 0.03−0.27 | 0.15 ± 0.10, 0.05−0.41 | 0.38 |
| Maximum relative head motion (mm) | 0.97 ± 0.85, 0.07−2.83 | 0.97 ± 0.73, 0.09−2.89 | 1 |
| Social responsiveness scale total | 17 ± 12, 3−51 | 109 ± 30, 60−162 | <0.00 |
| ADOS (Comm + Soc) | N/A | 12.9 ± 4.1, 7−20 | N/A |
| ADI total | N/A | 47.0 ± 11.2, 23−62 | N/A |
N/A, not applicable.
Experimental Design
Participants underwent a rapid event-related fMRI paradigm in which faces with different emotional expressions were displayed. Subjects were asked to “just look at the expression on each face.” The experimental design was the same as the observation run used in our previous studies in children with ASD (Dapretto et al. 2006) and TD children (Pfeifer et al. 2008, 2011). Ten of the children with ASD and 5 of the TD children from Dapretto et al. (2006) overlapped with subjects used in this study. Subjects were presented with 80 full color faces from the NimStim facial expressions stimulus set (Tottenham et al. 2009). The scan consisted of 96 events whereby each emotion (neutral, happy, sad, fearful, and angry) as well fixation crosses (null events) were displayed for 2 s with an average intertrial interval of 3 s. The fixation crosses were displayed at eye level in order to direct attention to the eyes as this has previously been shown to increase fixation to the eyes and normalize activity in both amygdala and fusiform gyrus (e.g., Hadjikhani et al. 2004). The order of presentation of all events was optimized and jittered (jitter ranging from 500 to 1500 ms in 125 ms increments) to maximize contrast detection efficiency (Wager and Nichols 2003).
The patterns of regional activity observed in both TD and ASD children (see Supplementary Fig. 1) closely resembled those previously obtained in prior studies using the same activation paradigm (Dapretto et al. 2006; Pfeifer et al. 2008, 2011), including robust activity in visual cortices, amygdala, IFG, and hippocampus.
Data Acquisition
The MRI data were acquired on a Siemens 3.0 T Allegra MRI scanner. Stimuli were presented through a computer connected to a high-resolution magnet-compatible audio and goggle system (Resonance Technology, Inc.). A 2D spin-echo scout localizing scan (time repetition [TR] = 4000 ms, time echo [TE] = 40 ms, matrix size 256 × 256, 4 mm thick, 1 mm gap) was acquired and used for graphic prescription. A matched bandwidth T2-weighted high-resolution echo planar scan (TR = 5000 ms, TE = 33 ms, matrix size 128 × 128, field of view [FoV] = 20 cm, 36 slices, yielding an in-plane voxel dimension of 1.5 × 1.5 mm, with 4-mm thick slices) was acquired coplanar to the functional scan in order to ensure identical distortion characteristics to the fMRI scan. The functional blood oxygenation level–dependent MRI scan lasted 4 min 54 s (TR = 3000 ms, TE = 28 ms, matrix size 128 × 128, FoV = 20 cm, 36 slices yielding an in-plane voxel dimension of 3 × 3 mm with 4-mm thick axial slices).
Functional Connectivity MRI Data Analysis
Data were analyzed using FSL version 4.1.4 (FMRIB's Software Library, http://www.fmrib.ox.ac.uk/fsl; Smith et al. 2004) and AFNI (Analysis of Functional NeuroImages; Cox 1996). Structural and functional images were skull stripped using AFNI (3dskullstrip and 3dautomask, respectively). Functional volumes were motion-corrected to the average functional volume with MCFLIRT using a normalized correlation ratio cost function and sinc interpolation (Jenkinson et al. 2002). Translations and rotations in the x, y, and z dimensions were calculated from volume to volume and then collapsed into mean absolute (compared with the average functional volume) and relative (compared with the previous volume) displacements. Images were spatially smoothed (full-width at half maximum 5 mm) and temporally high pass filtered (t > 0.01 Hz).
Time-series statistical analysis was carried out according to the general linear model using FEAT (FMRI Expert Analysis Tool), Version 5.98. For each subject, we first regressed out nuisance covariates, including 6 rigid body motion parameters and average white matter (WM), cerebrospinal fluid (CSF), and global time-series. The WM and CSF time-series reflected signal from subject-specific regions of interest (ROIs) created using FAST (FSL’s Automatic Segmentation Tool). The residuals from the previous step were aligned to high-resolution coplanar images via an affine transformation with 6 degrees of freedom and then aligned to the standard Montreal Neurological Institute (MNI) average of 152 brains using an affine transformation with 12 degrees of freedom using FMRIB’s Linear Image Registration Tool (FLIRT). In order to examine whole-brain connectivity with nodes of interest, we used anatomically based ROIs from the Harvard–Oxford probabilistic atlas (thresholded at 25% probability): the bilateral amygdala (Fig. 1 top panel, A) and the right IFGpo (rIFGpo) (BA 44; Fig. 1 bottom panel, A). We chose to focus on the right IFGpo given that previous studies on the processing of facial affect reported greater activity and/or more robust group differences in this region (Dapretto et al. 2006; Hadjikhani et al. 2007; Uddin et al. 2008). We extracted time-series from our ROIs for each subject and correlated them with every voxel in the brain to generate connectivity maps for each subject and ROI. Individual correlation maps were converted into z-statistic maps using Fischer’s r to z transformation and then combined at the group level using the ordinary least squares method. For a complete list of anatomical regions positively and negatively connected to the seeds, as well as the peak voxel in MNI coordinates and Z-statistics, see Tables 2 and 3.
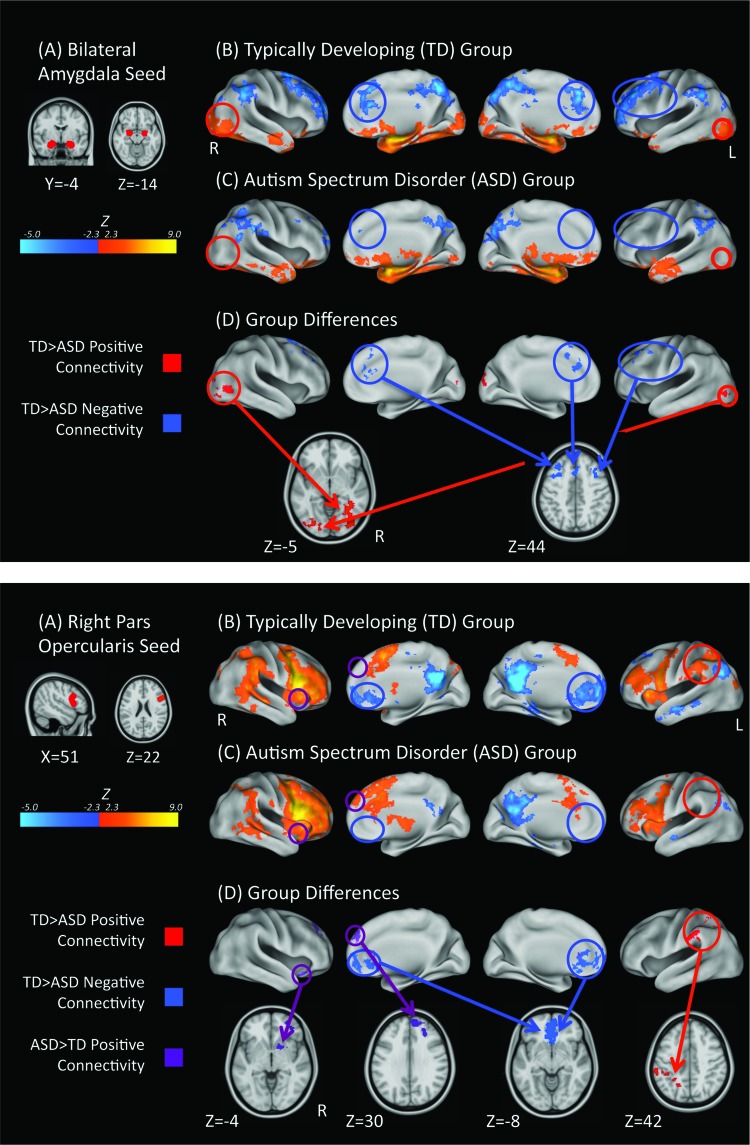
Figure 1.
Bilateral amygdala (top panel) and rIFGpo (bottom panel) connectivity. (A) The Harvard–Oxford bilateral amygdala (25% probability) and rIFGpo (25% probability) used as seed regions and displayed on the 1mm MNI152 T1 standard brain. (B) TD within-group connectivity maps, (C) ASD within-group connectivity maps, and (D) direct between-group contrasts rendered on the Inflated PALS B12 brain using CARET and on the 1mm MNI152 T1 standard brain using AFNI. Maps are thresholded at Z > 2.3 (P < 0.01) with correction for multiple comparisons applied at the cluster level (P < 0.05). Red circles highlight areas of greater positive connectivity with the seed region for the TD group. Blue circles highlight areas of greater negative connectivity with the seed region for the TD group. Purple circles highlight areas of greater positive connectivity with the seed region for the ASD group.
Table 2.
Bilateral amygdala
| TD |
ASD |
TD > ASD |
ASD > TD |
|||||||||||||
| MNI peak (mm) |
Max | MNI peak (mm) |
Max | MNI peak (mm) |
Max | MNI peak (mm) |
Max | |||||||||
| x | y | z | Z | x | y | z | Z | x | y | z | Z | x | y | z | Z | |
| Positive connectivity | ||||||||||||||||
| R amygdala | 30 | −8 | −16 | 8.2 | 14 | −4 | −18 | 7.54 | ||||||||
| L amygdala | −16 | −10 | −16 | 7.7 | −18 | −10 | −16 | 7.52 | ||||||||
| R hippocampus | 26 | −14 | −18 | 7.3 | 28 | −16 | −18 | 6.45 | ||||||||
| L hippocampus | −20 | −18 | −18 | 6.6 | −24 | −12 | −26 | 6.79 | ||||||||
| R putamen | 30 | −14 | −8 | 4.8 | 30 | −6 | −8 | 4.73 | ||||||||
| L putamen | −32 | −12 | −10 | 3.6 | −20 | 2 | −8 | 5.14 | ||||||||
| R pallidum | 22 | −10 | −6 | 4.1 | 22 | −12 | 6 | 3.87 | ||||||||
| L pallidum | −16 | −8 | −8 | 4.2 | −16 | 0 | −8 | 4.47 | ||||||||
| R accumbens | 6 | 8 | −4 | 4.9 | 6 | 6 | −6 | 4.40 | ||||||||
| L accumbens | −8 | 10 | −4 | 3.7 | −6 | 6 | −6 | 4.02 | ||||||||
| R thalamus | 24 | −30 | 0 | 5.8 | 8 | −30 | 2 | 5.14 | ||||||||
| L thalamus | −22 | −34 | −2 | 4.2 | −24 | −26 | 2 | 4.14 | ||||||||
| Hypothalamus | 4 | −6 | −4 | 5.3 | −4 | −10 | 2 | 4.67 | ||||||||
| R parahippocampal gyrus | 18 | −16 | −22 | 5.3 | 22 | −32 | −20 | 5.90 | ||||||||
| L parahippocampal gyrus | −16 | −14 | −24 | 5.1 | −20 | −14 | −26 | 6.48 | ||||||||
| R insula | 34 | 8 | −16 | 4.9 | 30 | 10 | −16 | 5.19 | ||||||||
| L insula | −38 | 0 | −14 | 5.2 | −38 | 2 | −18 | 4.46 | ||||||||
| Ventral anterior cingulate | 0 | 18 | −8 | 4.15 | ||||||||||||
| Frontal medical gyrus | 4 | 60 | −14 | 3.6 | 4 | 68 | −6 | 3.56 | ||||||||
| Medical orbital gyrus | 0 | 34 | −18 | 4.9 | −2 | 40 | −20 | 4.65 | ||||||||
| R inferior frontal gyrus, pars orbitalis | 38 | 26 | −18 | 3.96 | 44 | 38 | −18 | 4.53 | ||||||||
| L inferior frontal gyrus, pars orbitalis | −44 | 20 | −14 | 3.54 | −46 | 22 | −10 | 3.87 | ||||||||
| R temporal pole | 54 | 10 | −34 | 3.05 | 28 | 12 | −36 | 5.40 | ||||||||
| L temporal pole | −32 | 26 | −34 | 3.56 | −24 | 4 | −40 | 5.15 | ||||||||
| R fusiform | 28 | 4 | −38 | 5.55 | 38 | −44 | −16 | 4.73 | 40 | −44 | −6 | 5.13 | ||||
| L fusiform | −34 | −50 | −12 | 5.43 | ||||||||||||
| R middle temporal gyrus | 66 | −12 | −22 | 5.07 | 62 | −4 | −14 | 3.06 | ||||||||
| L middle temporal gyrus | −62 | −18 | −18 | 4.51 | ||||||||||||
| R inferior temporal gyrus | 48 | −46 | −26 | 4.27 | 48 | −18 | −28 | 4.33 | ||||||||
| L inferior temporal gyrus | −48 | −44 | −26 | 3.48 | −44 | −26 | −22 | 4.99 | ||||||||
| R lingual gyrus | 26 | −48 | −12 | 4.81 | 8 | −86 | −4 | 3.89 | ||||||||
| L lingual gyrus | −16 | −70 | −10 | 4.09 | −28 | −42 | −8 | 4.25 | ||||||||
| R middle occipital gyrus | 40 | −68 | −10 | 5.12 | 24 | −44 | −14 | 4.07 | 50 | −68 | 4 | 4.26 | ||||
| L middle occipital gyrus | −32 | −78 | 2 | 5.32 | −32 | −88 | −14 | 3.38 | −32 | −78 | 2 | 4.69 | ||||
| R occipital pole | 8 | −98 | 0 | 4.23 | ||||||||||||
| L occipital pole | −14 | −98 | 16 | 4.84 | −6 | −92 | 22 | 3.38 | ||||||||
| L cerebellum | −4 | −52 | −18 | 4.33 | −26 | −54 | −26 | 3.09 | ||||||||
| Negative connectivity | ||||||||||||||||
| Precuneus | 0 | −52 | 56 | 5.61 | −12 | −62 | 42 | 5.07 | ||||||||
| Posterior cingulate | 6 | −34 | 46 | 4.40 | 8 | −40 | 44 | 3.22 | ||||||||
| R supramarginal gyrus | 66 | −34 | 42 | 3.52 | 64 | −36 | 32 | 4.55 | ||||||||
| L supramarginal gyrus | −48 | −52 | 36 | 4.98 | −62 | −50 | 34 | 4.10 | ||||||||
| R angular gyrus | 44 | −46 | 38 | 5.36 | 58 | −52 | 46 | 4.45 | ||||||||
| L angular gyrus | −42 | −56 | 56 | 5.17 | −44 | −54 | 56 | 4.57 | ||||||||
| R superior parietal lobule | 44 | −48 | 58 | 3.52 | 32 | −46 | 54 | 3.98 | ||||||||
| L superior parietal lobule | −24 | −46 | 46 | 2.43 | ||||||||||||
| R superior occipital gyrus | 50 | −70 | 36 | 3.80 | 42 | −72 | 38 | 3.05 | ||||||||
| L superior occipital gyrus | −40 | −84 | 32 | 4.55 | −38 | −76 | 48 | 2.84 | ||||||||
| Superior frontal gyrus medial | −2 | 32 | 36 | 5.29 | −10 | 32 | 26 | 4.24 | ||||||||
| R superior frontal gyrus | 18 | 8 | 58 | 5.39 | 20 | 56 | 22 | 4.89 | 18 | 8 | 62 | 4.35 | ||||
| L superior frontal gyrus | −18 | 10 | 62 | 3.49 | ||||||||||||
| R inferior frontopolar gyrus | 20 | 60 | 0 | 5.04 | 32 | 60 | 6 | 2.79 | ||||||||
| L inferior frontopolar gyrus | −22 | 64 | −4 | 3.53 | ||||||||||||
| R middle frontal gyrus | 34 | 24 | 38 | 5.36 | 50 | 20 | 36 | 3.41 | 28 | 22 | 38 | 4.06 | ||||
| L middle frontal gyrus | −38 | 34 | 36 | 5.33 | −32 | 28 | 44 | 3.77 | ||||||||
| Anterior cingulate | 10 | 34 | 16 | 4.22 | 10 | 26 | 22 | 4.02 | ||||||||
| R precentral gyrus | 36 | 4 | 36 | 3.23 | ||||||||||||
| L precentral gyrus | −50 | 0 | 36 | 3.62 | ||||||||||||
| R postcentral gyrus | 54 | −20 | 24 | 3.71 | ||||||||||||
Table 3.
Right pars opercularis
| TD |
ASD |
TD > ASD |
ASD > TD |
|||||||||||||
| MNI peak (mm) |
Max | MNI peak (mm) |
Max | MNI peak (mm) |
Max | MNI peak (mm) |
Max | |||||||||
| x | y | z | Z | x | y | z | Z | x | y | z | Z | x | y | z | Z | |
| Positive connectivity | ||||||||||||||||
| R inferior frontal gyrus, pars opercularis | 58 | 16 | 14 | 8.72 | 56 | 14 | 16 | 8.85 | ||||||||
| L inferior frontal gyrus, pars opercularis | −56 | 10 | 18 | 6.33 | 50 | 8 | 4 | 5.94 | ||||||||
| R precentral gyrus | 50 | 4 | 42 | 7.45 | 50 | 6 | 40 | 6.74 | ||||||||
| L precentral gyrus | −52 | 4 | 42 | 4.61 | −40 | 0 | 30 | 5.58 | ||||||||
| R inferior frontal gyrus, pars orbitalis | 46 | 44 | −4 | 5.91 | 50 | 30 | −6 | 6.63 | 30 | 24 | −16 | 3.72 | ||||
| L inferior frontal gyrus, pars orbitalis | −44 | 40 | 18 | 5.91 | ||||||||||||
| R middle frontal gyrus | 38 | 40 | 28 | 5.73 | 50 | 32 | 26 | 6.18 | ||||||||
| L middle frontal gyrus | −26 | −2 | 54 | 4.02 | −42 | 40 | 20 | 4.96 | ||||||||
| R anterior insula | 38 | 20 | −2 | 6.56 | 36 | 14 | 6 | 6.14 | ||||||||
| L anterior insula | −32 | 22 | 2 | 7.11 | −32 | 22 | 2 | 5.47 | ||||||||
| Superior frontal gyrus, medial part | 4 | 14 | 50 | 6.65 | −2 | 16 | 50 | 3.89 | ||||||||
| R superior frontal gyrus, lateral part | 16 | 4 | 62 | 4.54 | 22 | 36 | 40 | 4.64 | ||||||||
| Anterior cingulate cortex | 10 | 18 | 30 | 4.88 | 10 | 18 | 32 | 5.81 | 4 | 28 | 2 | 3.97 | ||||
| R supramarginal gyrus | 64 | −32 | 30 | 5.73 | 44 | −42 | 44 | 4.95 | ||||||||
| L supramarginal gyrus | −50 | −42 | 52 | 4.80 | −62 | −32 | 42 | 3.99 | ||||||||
| R superior parietal lobule | 26 | −52 | 50 | 3.95 | 30 | −56 | 44 | 4.33 | ||||||||
| L superior parietal lobule | −30 | −48 | 42 | 4.88 | −30 | −48 | 54 | 3.95 | ||||||||
| R Middle occipital gyrus | 34 | −80 | 24 | 4.60 | 30 | −68 | 36 | 3.27 | ||||||||
| L middle occipital gyrus | −36 | −88 | 20 | 4.41 | ||||||||||||
| R superior occipital gyrus | 16 | −78 | 54 | 3.96 | 32 | −62 | 46 | 3.85 | ||||||||
| L superior occipital gyrus | −22 | −60 | 46 | 5.35 | −14 | −62 | 46 | 3.47 | ||||||||
| R superior temporal gyrus | 54 | −44 | 10 | 5.69 | 48 | −32 | 2 | 4.13 | ||||||||
| R middle temporal gyrus | 48 | −24 | −6 | 5.49 | 64 | −48 | −6 | 4.33 | ||||||||
| R inferior temporal gyrus | 44 | −50 | −10 | 3.18 | ||||||||||||
| R lateral orbital gyrus | 30 | 52 | −10 | 4.59 | 36 | 46 | −4 | 3.07 | ||||||||
| R medial orbital gyrus | 16 | 42 | −18 | 3.37 | ||||||||||||
| R caudate | 18 | 0 | 14 | 4.75 | 16 | 2 | 14 | 4.82 | ||||||||
| R putamen | 20 | 2 | 8 | 4.13 | 22 | 10 | −8 | 4.30 | 20 | 10 | −2 | 2.79 | ||||
| L putamen | −18 | −2 | 6 | 2.89 | ||||||||||||
| R accumbens | 12 | 12 | −8 | 3.08 | 12 | 14 | −6 | 3.42 | ||||||||
| R thalamus | 6 | −18 | 10 | 3.41 | ||||||||||||
| Negative connectivity | ||||||||||||||||
| Precuneus | −4 | −54 | 10 | 6.30 | −6 | −66 | 14 | 5.06 | ||||||||
| Posterior cingulate | −2 | −48 | 32 | 6.71 | −4 | −40 | 34 | 4.66 | ||||||||
| L angular gyrus | −42 | −64 | 32 | 5.10 | −44 | −60 | 34 | 4.18 | ||||||||
| L parahippocampal gyrus | −22 | −26 | −26 | 4.35 | −24 | −26 | −26 | 4.25 | ||||||||
| L middle temporal gyrus | −56 | −14 | −18 | 5.50 | −50 | −12 | −22 | 3.62 | ||||||||
| L middle frontal gyrus | −32 | 24 | 52 | 4.26 | ||||||||||||
| L temporal pole | −42 | 20 | −36 | 4.06 | ||||||||||||
| L cerebellum | −20 | −52 | −30 | 3.37 | −14 | −58 | −18 | 4.26 | ||||||||
| R cerebellum | 20 | −58 | −28 | 3.31 | ||||||||||||
| Ventral anterior cingulate | −2 | 32 | −4 | 4.34 | 0 | 30 | 4 | 4.14 | ||||||||
| Middle frontopolar gyrus | −24 | 60 | 4 | 3.72 | −4 | 60 | 14 | 2.75 | ||||||||
| Frontal medial gyrus | 0 | 60 | −2 | 5.17 | 6 | 54 | −4 | 3.84 | ||||||||
Analyses without Global Signal Regression and with Task Regression
To address the methodological concern related to anticorrelations and using global signal regression as a preprocessing step (see Murphy et al. 2009; Jones et al. 2010), we performed all analyses with and without global signal regression. Each of the major patterns of between-group differences was also present when global signal regression was not used (see Supplementary Material and Figs. 2 and 3 for a more thorough discussion of the analysis without global signal regression).
Additionally, we wanted to examine whether we could isolate connectivity differences that were task-dependent versus those that were due to underlying intrinsic connectivity. In order to do this, we ran all analyses using residuals from a general linear model that included task stimulus timings for each type of emotional expression convolved with 4 basis functions generated with FLOBS (FSL’s Linear Optimal Basis Function) and by applying a low pass filter (t < 0.1 Hz; Fair, Schlaggar, et al. 2007; Jones et al. 2010). Again, we did not observe substantial qualitative differences with this approach (see Supplementary Fig. 4), which may reflect the fact that this method does not completely remove task effects and/or that measured connectivity may largely be due to intrinsic connectivity (see Supplementary Material). Given that the results may be partially driven by the task regardless of this approach, we present all results without task regression or low pass filtering (and global signal regression) and discuss group differences as a combination of task-related and intrinsic connectivity.
Thresholding and Visualization of Segregated Networks
All within- and between-group connectivity maps were thresholded at Z > 2.3 (P < 0.01) and corrected for multiple comparisons at the cluster level (P < 0.05) using Gaussian random field theory. For between-group comparisons of connectivity maps, ASD > TD for positive connectivity is the same as TD > ASD for negative connectivity. Therefore, in order to identify and interpret 4 potential types of group differences (TD > ASD for regions of positive connectivity, TD > ASD for regions of negative connectivity, ASD > TD for regions of positive connectivity, ASD > TD for regions of negative connectivity), we masked the group difference maps by the respective TD and ASD within-group positive and negative connectivity maps.
Results
Bilateral Amygdala Seed
Within-Group Positive Connectivity
In the TD group, activity in bilateral amygdala was positively correlated with activity in portions of the temporal lobe including the hippocampus, inferior temporal gyrus, and fusiform gyrus as well as basal ganglia and thalamus (Fig. 1 top panel, B; Table 2). Bilateral amygdala activity was also correlated with activity in frontal regions including the orbitofrontal gyrus and IFG pars orbitalis, as well as occipital regions including the lingual gyrus, occipital poles, and MT/V5 complex (Fig. 1 top panel, B; Table 2). For the ASD group, activity in bilateral amygdala was positively correlated with a similar network as the TD group except for the bilateral occipital poles and left fusiform gyrus (Fig. 1 top panel, C; Table 2). The ASD group also displayed positive connectivity between the bilateral amygdala and the left middle temporal gyrus (Fig. 1 top panel, C; Table 2).
Within-Group Negative Connectivity
In the TD group, activity in bilateral amygdala was negatively correlated with posterior regions including the precuneus/posterior cingulate, inferior and superior parietal lobules, and frontal regions including dorsolateral prefrontal frontal cortex (DLPFC), and dorsal anterior cingulate cortex (ACC) (Fig. 1 top panel, B; Table 2). For the ASD group, activity in the bilateral amygdala was negatively correlated with a less extensive network that included the same regions as the TD group except the DLPFC and dorsal ACC (Fig. 1 top panel, C; Table 2).
Between-Group Analyses
The TD group showed significantly more positive connectivity with bilateral MT/V5 complex and inferior temporal/fusiform cortex (red circles in Fig. 1 top panel, D; Table 2). The TD group also showed greater negative connectivity with DLPFC and dorsal ACC (blue circles in Fig. 1 top panel, D; Table 2). Relative to the TD group, the ASD group did not show stronger positive or negative connectivity with any regions.
rIFGpo Seed
Within-Group Positive Connectivity
In the TD group, activity in the rIFGpo was positively correlated with activity in other frontal regions, including the precentral gyrus, medial superior frontal gyrus, anterior insula, and ACC, as well as with activity in the inferior and superior parietal lobules (Fig. 1 bottom panel, B; Table 3). Activity in the rIFGpo was also correlated with activity in the caudate and putamen. In the ASD group, activity in the rIFGpo was positively correlated with a similar network as the TD group that did not include the left inferior and superior parietal lobules (Fig. 1 bottom panel, C; Table 3). In the ASD group, activity in the rIFGpo was also positively correlated with activity in right lateral frontal regions as well as the right nucleus accumbens and thalamus (Fig. 1 bottom panel, C; Table 3).
Within-Group Negative Connectivity
In the TD group, activity in the rIFGpo was negatively correlated with activity in regions including the ventral medial prefrontal cortex (VMPFC), precuneus/posterior cingulate, left angular gyrus, left middle temporal gyrus, and left parahippocampal gyrus (Fig. 1 bottom panel, B; Table 2). For the ASD group, activity in rIFGpo was negatively correlated with a similar but less extensive network of regions that did not include the VMPFC (Fig. 1 bottom panel, C; Table 2).
Between-Group Analyses
The TD group showed significantly more positive connectivity with the left inferior and superior parietal lobules (red circles in Fig. 1 bottom panel, D; Table 3). The ASD group showed significantly more positive connectivity with right frontal regions and right nucleus accumbens (purple circles in Fig. 1 bottom panel, D; Table 3). The TD group showed greater negative connectivity with regions in the VMPFC (blue circles in Fig. 1 bottom panel, D; Table 3). There were no regions showing greater negative connectivity in the ASD group.
Discussion
Here, we examined the functional organization of brain networks in children and adolescents with ASD, as compared with matched controls, during a passive emotional face processing task. We used the bilateral amygdala and the right pars opercularis as seed regions for whole-brain connectivity analyses since these areas have been implicated in atypical socioemotional and face processing in ASD. Overall, the pattern of altered connectivity we observed in ASD for both seeds suggest that ASD is characterized by reduced functional integration and segregation of large-scale brain networks. Specifically, the ASD group showed reduced integration between amygdala and secondary visual areas and between rIFGpo and parietal cortex as well as increased positive connectivity between rIFGpo and several regions in right frontal cortex. Additionally, the ASD group displayed weaker negative correlations (i.e., reduced functional segregation) between amygdala and dorsal anterior cingulate/dorsolateral prefrontal cortex as well as between rIFGpo and ventromedial prefrontal cortex.
We interpret these findings in accordance with recent studies that have begun to chart the typical maturation of functional brain networks across development as well as in terms of the known functional roles of the regions/networks positively and negatively connected with each of our seed regions. Additionally, given that group differences may be due to a combination of intrinsic and task-driven connectivity, we interpret our results in the context of previous studies that have carefully characterized differences in intrinsic connectivity as well as differences in task-related regional activation and connectivity in ASD during emotional face processing.
Bilateral Amygdala Seed
Positive Connectivity
Consistent with prior reports (Pezawas et al. 2005; Stein et al. 2007; Roy et al. 2009), using the bilateral amygdala as a seed region for a whole-brain functional connectivity analysis, we found that, in TD children, the bilateral amygdala was positively connected to a network of regions which included the hippocampus/parahippocampal gyrus, basal ganglia, thalamus, fusiform gyrus, orbitofrontal gyrus, and ventral ACC (Fig. 1 top panel, B). In the TD group, the amygdala was also positively connected with visual regions including the lingual gyrus, occipital poles, and V5/MT complex. Although the first set of regions listed above was functionally connected with the amygdala in a resting-state study of neurotypical adults (Roy et al. 2009), visual regions reported here were not correlated with amygdala activity in that study (in fact, they were found to be mostly anticorrelated with amygdala activity). This difference may reflect coactivation between the amygdala and visual areas induced by the task, considering that these regions are strongly engaged by the emotional faces presented in this study (Dapretto et al. 2006; Pfeifer et al. 2008; see Supplementary Material).
Children with ASD displayed connectivity between the bilateral amygdala and each of the same regions as the TD group, except that they exhibited reduced connectivity with secondary visual areas including bilateral V5/MT complex and the right fusiform gyrus (see red circles in Fig. 1 top panel, D). Reduced connectivity between secondary visual areas and amygdala in the ASD group is consistent with reduced connectivity between fusiform face area and amygdala found by Kleinhans et al. (2008). In previous face processing activation studies, hypoactivation of the amygdala (Critchley et al. 2000; Wang et al. 2004) as well as fusiform gyrus (e.g., Schultz et al. 2000; Pierce et al. 2001) have been consistently reported. However, amygdala and fusiform hypoactivation have been shown to be highly dependent on task demands (Hadjikhani et al. 2004; Wang et al. 2004), familiarity (Pierce et al. 2004), or time fixating on eyes (Dalton et al. 2005). In the present study (see Supplementary Fig. 1), TD children showed heightened activity in the fusiform gyrus and amygdala; however, post hoc analyses found no correlations between task-related activation in either the fusiform gyrus or amygdala and the level of fusiform–amgydala connectivity. Furthermore, reduced amygdala–V5/MT/fusiform connectivity was still observed in analyses where task-related activity was regressed out (see Supplementary Material and Fig. 4). Thus, it is unlikely that between-group differences in task-related activity is driving the observed connectivity effect. Reduced connectivity between amygdala and secondary visual areas fits well with the general underconnectivity theory (Just et al. 2004) and highlights a pattern of reduced functional integration in a distributed network involved in processing facial affect.
Negative Connectivity
A network of regions was found to be negatively correlated (i.e., segregated) with activity in the amygdala. In the TD group, this network largely overlapped with regions previously reported to be anticorrelated with activity in the amygdala (Pezawas et al. 2005; Stein et al. 2007; Roy et al. 2009; Fig. 1 top panel, B) including the precuneus/posterior cingulate, dorsal ACC, and DLPFC. Frontal regions including the dorsal ACC and DLPFC have been associated with cognitive processes (e.g., reasoning and rationalizing) that are deemed important for regulating emotional reactions stemming from the amygdala and limbic system (e.g., Hariri et al. 2000, 2003; Phillips et al. 2003). In a structural equation modeling study performed during an emotion processing task, the precuneus/anterior cingulate and dorsal ACC were shown to have a negative influence on amygdala activity (Stein et al. 2007). Reduced negative connectivity between these frontal regions and the amygdala has been found in affective disorders such as major depression and bipolar disorder (Almeida et al. 2009; Chepenik et al. 2010).
Interestingly, while children with ASD did show a similar, although relatively weaker, network of regions anticorrelated with amygdala activity, they did not show anticorrelated activity between the bilateral amygdala and the dorsal ACC and DLPFC (see blue circles in Fig. 1 top panel, D). This between-group difference remained in analyses conducted without global signal regression (see Supplementary Material and Figs. 2 and 3). Although stronger negative connectivity for the TD group could also be interpreted as stronger positive connectivity for the ASD group, given the previous literature on frontal regulation of the limbic system (e.g., Hariri et al. 2003; Stein et al. 2007), we take this difference to more likely reflect stronger frontolimbic decoupling in TD children. Future studies using methods better suited for capturing regulatory interactions (such as psychophysiological interaction) may be able to more definitively address these competing accounts.
rIFGpo Seed
Positive Connectivity
Using the rIFGpo as a seed ROI for whole-brain functional connectivity, we found that, in the TD group, the rIFGpo was positively connected to a network of regions largely resembling the task-positive network that has been associated with externally directed, attentionally demanding tasks (Fox et al. 2005; Fig. 1 bottom panel, B). This network includes the precentral gyrus, anterior insula, anterior cingulate, medial superior frontal gyrus, inferior/superior parietal lobule, and lateral occipital gyrus. Although a finer classification of the task-positive network might place the rIFGpo into the salience/cingulo-opercular subnetwork, as opposed to the frontoparietal executive subnetwork (Dosenbach et al. 2007; Seeley et al. 2007), our seed-based approach, using the rIFGpo as a seed region, generated connectivity maps resembling the entire task-positive network.
While children with ASD also displayed connectivity between the rIFGpo and a similar network as observed in the TD group, there was notably decreased connectivity with regions increasingly distant from the seed (Fig. 1 bottom panel, C). Group differences were significant for regions in the contralateral parietal lobe, which included the inferior and superior parietal lobules (see red circles in Fig. 1 bottom panel, D). This finding is consistent with multiple task-based studies (e.g., Just et al. 2004; Koshino et al. 2005; Kana et al. 2007) which reported reduced connectivity between frontal and parietal regions in individuals with ASD, supporting the underconnectivity theory (e.g., Just et al. 2004, 2007) as well as dysfunction of the canonical MNS (Nishitani et al. 2004; Dapretto et al. 2006; Oberman and Ramachandran 2007). Reduced connectivity along this anterior–posterior axis is also strongly associated with an immature pattern of functional integration in neurotypical individuals (Fair, Dosenbach, et al. 2007; Fair et al. 2008, 2009; Kelly et al. 2009; Dosenbach et al. 2010). Given that the IFGpo is part of the functionally significant cingulo-opercular/salience network (Dosenbach et al. 2007; Seeley et al. 2007), our findings provide support for the notion that this network may be a hub of dysfunction in autism (Uddin and Menon 2009; Ebisch et al. 2010) and, more specifically, that altered connectivity within this network may be related to socio-emotional functioning.
The ASD group showed a pattern of greater intralobar or “local” connectivity with frontal regions relatively proximal to the seed that included right superior frontal cortex and right lateral orbital cortex (see purple circles in Fig. 1 bottom panel, D). Greater connectivity between the rIFGpo and other right frontal regions fits well with theoretical accounts of greater local connectivity in ASD (Belmonte et al. 2004; Courchesne and Pierce 2005; Geschwind and Levitt 2007), as well as empirical findings of corticocortical overconnectivity in adults with ASD (Welchew et al. 2005; Noonan et al. 2009; Shih et al. 2010). Consistent with our findings, Shih et al. (2010) examined connectivity in the imitation network (Iacoboni et al. 1999; Nishitani et al. 2004) and found aberrantly stronger connectivity between IFGpo and superior frontal cortex. They discussed aberrantly increased corticocortical connectivity as either reflecting a compensatory mechanism in ASD or being related to early brain growth anomalies that lead to aberrant segregation of functional networks. In studies of typical development, increased functional segregation between networks as measured by reduced local connectivity has been consistently found in adults compared to children (e.g., Kelly et al. 2008; Fair et al. 2009; Dosenbach et al. 2010). Moreover, weakening connections between nodes of different networks were found to be twice as powerful at predicting brain maturity than increasing functional integration within networks (Dosenbach et al. 2010).
In addition to greater local frontal connectivity, the ASD group also showed greater connectivity than the TD group between the rIFGpo and right nucleus accumbens (see purple circle in Fig. 1 bottom panel, D). Greater striatocortical (Turner et al. 2006; Di Martino et al. 2010) and thalamocortical (Mizuno et al. 2006; Di Martino et al. 2010) connectivity have previously been found in adults with ASD. Thus, greater corticosubcortical connectivity appears to be a robust finding in ASD that does not fit with a pattern of local/intralobar overconnectivity. Turner et al. (2006) hypothesized that increased connectivity in basal ganglia–cortical circuits in ASD is related to executive impairments and may also reflect a compensatory mechanism associated with repetitive and stereotypic behaviors. Mizuno et al. (2006) speculated that increased thalamocortical connectivity in ASD might be related to increased arousal and sensory hypersensitivity as well as reduced sensory gating, although there is little evidence directly relating these alterations to specific ASD symptomatology. Interestingly, a recent developmental connectivity study found that subcortical–cortical connectivity is stronger in children compared to adults (Supekar et al. 2009). Thus, a parsimonious, although not mutually exclusive, explanation for each of the major patterns of aberrant connectivity reported in the literature, including the present study, is that they may altogether reflect the relatively “immature” integration and segregation of functional brain networks in ASD. While increased subcorticocortical connectivity has been found in typical children compared to adults, it is still unclear to what extent increased subcortical–cortical connectivity in ASD may reflect immature versus aberrant patterns of connectivity (Di Martino et al. 2010).
Negative Connectivity
A network of regions that closely resembles the task-negative network or DMN (Raichle et al. 2001; Greicius et al. 2003) was found to be anticorrelated (i.e., functionally segregated) with the rIFGpo. In TD children, the network of regions anticorrelated with activity in the rIFGpo included the precuneus/posterior cingulate, left angular gyrus, left parahippocampal gyrus, and VMPFC. The DMN and task-positive network have been shown to consistently display an anticorrelated relationship (Fox et al. 2005, 2009; Fransson et al. 2005; Chang and Glover 2009) and despite the controversy regarding global signal regression and anticorrelated networks (Murphy et al 2009; Fox et al. 2009; see Supplementary Material) recent work has suggested that the degree of anticorrelation between these networks is biologically meaningful (Kelly et al. 2008; Whitfield-Gabrieli et al. 2009; Hampson et al. 2010).
Children with ASD showed a similar, albeit weaker, network of regions anticorrelated with the right IFGpo, which included the posterior components of the DMN. In particular, the ASD group lacked a significant negative relationship between activity in the rIFGpo and VMPFC (see blue circles in Fig. 1 bottom panel, D). Reduced anticorrelation (or increased positive correlation) between the task-positive network and the DMN is consistent with reduced functional segregation between these two networks. In a developmental study, Stevens et al. (2009) found reductions in positive interactions between a task-positive executive control network and the DMN in children as compared with adults. The authors suggested that increased segregation between these two networks might allow for more flexible processing as a function of development. Therefore, reduced segregation between these networks found here in ASD is also consistent with the notion that ASD is characterized by an “immature” pattern of functional segregation between major cognitive networks. It is interesting to note that Iacoboni (2006) and Uddin et al. (2007) have previously discussed how the MNS and DMN are “two sides of the same coin,” whereby the MNS is involved with simulation of physical and external aspects of self (and others), whereas the DMN is related to more internal and higher level mental-state attribution aspects of self (and others). Reduced segregation between these systems reported here in ASD and during typical development (Stevens et al. 2009) may therefore reflect immature development of one’s internal and external representations of self and others.
Our finding that the ASD group showed an anticorrelated relationship between the rIFGpo and the posterior portion of the DMN but not the VMPFC also suggests that the DMN itself is not as well connected in the ASD group. In fact, post hoc analyses (not shown) that used the VMPFC as an additional seed region confirmed that there was reduced functional integration between this frontal DMN region and the posterior parietal components of the DMN in our sample. In line with recent resting-state studies (Kennedy and Courchesne 2008b; Monk et al. 2009; Weng et al. 2010; Assaf et al. 2010), our results provide additional evidence of dysfunction within the DMN in ASD and further suggest that the frontal cortex may be the most immature link of this network in ASD.
Conclusions and Future Directions
By using a seed-based connectivity approach to examine communication between brain regions implicated in the processing of facial affect, we found several patterns of altered connectivity suggesting that brain networks in ASD are characterized by reduced functional integration and segregation. To our knowledge, this is the first study to report both decreased long-range connectivity and reduced functional segregation as indexed by increased local connectivity and reduced anticorrelations, respectively. Our findings fit well with theoretical accounts of altered connectivity in ASD and provide a framework whereby connectivity disturbances in ASD can be understood in terms of multiple interacting systems across development. By carefully considering methodological concerns and characterizing connectivity both in terms of integration and segregation, our study may help explain previously conflicting reports of decreased (e.g., Just et al. 2004; Weng et al. 2010) but also increased connectivity in autism (e.g., Noonan et al. 2009; Shih et al. 2010). More generally, our findings suggest that measuring connectivity of neural systems—during task performance and/or resting state—may provide a more sensitive marker of abnormalities in brain function in autism than focusing exclusively on regional activation patterns.
Reduced functional integration and segregation of corticocortical and corticosubcortical networks in ASD are perhaps not surprising given that ASD is a neurodevelopmental disorder associated with reduced engagement in social interactions. However, the extent to which reduced functional integration and segregation may simply reflect immature or delayed connectivity, as opposed to, altered connectivity that is specific to autism and/or related to compensatory mechanisms, remains to be determined. A recent basal ganglia resting-state study in ASD found that although some increased corticosubcortical connectivity in ASD appeared “immature,” most of the altered connectivity appeared to reflect “developmental derangement” or aberrantly high subcorticocortical connectivity (Di Martino et al. 2010). Future experiments, including longitudinal studies, should also focus on disentangling how a history of altered engagement with the environment may affect connectivity versus how early brain abnormalities may directly lead to altered connectivity patterns.
A more careful examination of how corticocortical and corticosubcortical connectivity changes across typical development as compared with ASD is also needed. The majority of previous connectivity studies in ASD were performed with adults with ASD, whereas our sample consisted of children and adolescents. A greater reduction in connectivity for adolescents with ASD as compared with adults with ASD has recently been observed in the DMN (Monk et al. 2009; Weng et al. 2010), suggesting that the development of intrinsic connectivity networks shows a more protracted development in ASD and that differences may become subtler by adulthood. Future studies should more carefully investigate this possibility as well as focus on typical and atypical development in even younger populations, given that measures of connectivity can easily be acquired during resting-state scans with little concern about adequate task performance. Importantly, resting-state connectivity could also be examined in lower functioning individuals, a highly understudied population in the existing neuroimaging literature.
One limitation of the present study, as well as of prior work, is the difficulty of teasing apart the relative contributions of task-related and intrinsic connectivity in determining the observed between-group differences. Although we attempted to control for attention and alertness, we cannot completely rule out the possibility that subtle differences in these behavioral variables may have contributed to the connectivity differences observed between TD children and children with ASD. It should also be noted that, although resting-state studies minimize task-induced connectivity, differences in covert cognition might partially drive group differences (Kennedy and Courchesne 2008b). Future studies that directly compare measures of connectivity acquired during resting state versus task performance should prove useful in this respect. Furthermore, the extent to which aberrant functional connectivity in ASD is related to underlying cortical development such as synaptic pruning, myelination, or other processes remains largely unknown. Despite growing evidence that predisposing genetic and environmental factors may lead to altered neuronal migration and synaptic formation (Betancur et al. 2009), dysfunctional microcircuitry (Casanova et al. 2002), early brain overgrowth (Courchesne et al. 2003), and disordered structural connections (e.g., Herbert et al. 2004; Sundaram et al. 2008), little work thus far has directly linked any of these findings with altered functional connectivity in ASD (Scott-Van Zeeland et al. 2010). Finally, and perhaps most importantly, future studies in this line of research should also strive to be directly relevant to clinical outcomes (Fox and Greicius 2010). For example, characterizing functional brain networks in individuals at risk for ASD may eventually be used for earlier diagnosis or for developing individualized behavioral and pharmacological interventions.
Funding
National Institute of Child Health and Human Development (PO1 HD055784), National Institute of Mental Health (1R01 HD065280-01), Autism Speaks, UCLA’s Medical Scientist Training Program (T32 GM008044), as well as UCLA’s Training Program in Neurobehavioral Genetics (T32 MH073526-05). This project was also in part supported by grants (RR12169, RR13642, and RR00865) from the National Center for Research Resources, a component of the National Institutes of Health (NIH); its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Research or NIH.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Acknowledgments
We want to thank all of the children and families who graciously participated in the study. For generous support, we also wish to thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, and North-star Fund. We also thank Jennifer Pfiefer, Kristin McNeally, Larissa Borofsky, Susan Lee, Mari Davies, and Ashley Scott for help with experimental design and data collection and Salvatore Torrisi, Jesse Brown, and Elizabeth Losin for reading and commenting on drafts of the manuscript. Conflict of Interest : None declared.
References
- Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. J Cogn Neurosci. 2001;13:232–240. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- Almeida JR, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, Phillips ML. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry. 2009;66:451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network's role in spontaneous cognition. J Neurophysiol. 2010;104:322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, O'Boyle JG, Schultz RT, Pearlson GD. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 2010;53:247–256. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24:355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, Williams SC. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur C, Sakurai T, Buxbaum JD. The emerging role of synaptic cell-adhesion pathways in the pathogenesis of autism spectrum disorders. Trends Neurosci. 2009;32:402–412. doi: 10.1016/j.tins.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp. 2008;29:828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci U S A. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Brown C. Clinical and macroscopic correlates of minicolumnar pathology in autism. J Child Neurol. 2002;17:692–695. doi: 10.1177/088307380201700908. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage. 2009;47:1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepenik LG, Raffo M, Hampson M, Lacadie C, Wang F, Jones MM, Pittman B, Skudlarski P, Blumberg HP. Functional connectivity between ventral prefrontal cortex and amygdala at low frequency in the resting state in bipolar disorder. Psychiatry Res. 2010;182:207–210. doi: 10.1016/j.pscychresns.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, Williams SC, Van Amelsvoort T, Robertson DM, Rowe A, Phillips M, McAlonan G, Howlin P, et al. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123(Pt 11):2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, Davidson RJ. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Kelly C, Grzadzinski R, Zuo XN, Mennes M, Mairena MA, Lord C, Castellanos FX, Milham MP. Abberant striatal functional connectivity in children with autism. Biol Psychiatry. 2010;69:847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–3161. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisch SJ, Gallese V, Willems RM, Mantini D, Groen WB, Romani GL, Buitelaar JK, Bekkering H. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum Brain Mapp. 2010;32:1013–1028. doi: 10.1002/hbm.21085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain's default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Abnormal activation of the social brain during face perception in autism. Hum Brain Mapp. 2007;28:441–449. doi: 10.1002/hbm.20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex. 2006;16:1276–1282. doi: 10.1093/cercor/bhj069. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Chabris CF, Clark J, Steele S, McGrath L, Vangel M, Aharon I, Feczko E, et al. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage. 2004;22:1141–1150. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen N, Roth JK, Gore JC, Constable RT. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn Reson Imaging. 2010;28:1051–1057. doi: 10.1016/j.mri.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, Sanders HA, Kennedy DN, Caviness VS. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Grady CL, Rapoport SI. The cerebral metabolic landscape in autism. Intercorrelations of regional glucose utilization. Arch Neurol. 1988;45:749–755. doi: 10.1001/archneur.1988.00520310055018. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Failure to deactivate in autism: the co-constitution of self and other. Trends Cogn Sci. 2006;10:431–433. doi: 10.1016/j.tics.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Lieberman MD, Knowlton BJ, Molnar-Szakacs I, Moritz M, Throop CJ, Fiske AP. Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. Neuroimage. 2004;21:1167–1173. doi: 10.1016/j.neuroimage.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Keysers C. Inferior frontal gyrus activity triggers anterior insula response to emotional facial expressions. Emotion. 2008;8:775–780. doi: 10.1037/a0014194. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jones TB, Bandettini PA, Kenworthy L, Case LK, Milleville SC, Martin A, Birn RM. Sources of group differences in functional connectivity: an investigation applied to autism spectrum disorder. Neuroimage. 2010;49:401–414. doi: 10.1016/j.neuroimage.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Soc Neurosci. 2009;4:135–152. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biol Psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. Functional abnormalities of the default network during self- and other-reflection in autism. Soc Cogn Affect Neurosci. 2008a;3:177–190. doi: 10.1093/scan/nsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008b;39:1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, Greenson J, Dawson G, Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leslie KR, Johnson-Frey SH, Grafton ST. Functional imaging of face and hand imitation: towards a motor theory of empathy. Neuroimage. 2004;21:601–607. doi: 10.1016/j.neuroimage.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mizuno A, Villalobos ME, Davies MM, Dahl BC, Müller RA. Partially enhanced thalamocortical functional connectivity in autism. Brain Res. 2006;1104:160–174. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Weng SJ, Wiggins JL, Kurapati N, Louro HM, Carrasco M, Maslowsky J, Risi S, Lord C. Neural circuitry of emotional face processing in autism spectrum disorders. J Psychiatry Neurosci. 2010;35:105–114. doi: 10.1503/jpn.090085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Sullivan L, Mastergeorge AM. A parallel and distributed-processing model of joint attention, social cognition and autism. Autism Res. 2009;2:2–21. doi: 10.1002/aur.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson J, Dawson G, Abbott R, Faja S, Webb SJ, Friedman SD, Shaw D, Artru A, Dager SR. Amygdalar volume and behavioral development in autism. Arch Gen Psychiatry. 2006;63:686–693. doi: 10.1001/archpsyc.63.6.686. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacewicz BM, Dalton KM, Johnstone T, Long MT, McAuliff EM, Oakes TR, Alexander AL, Davidson RJ. Amygdala volume and nonverbal social impairment in adolescent and adult males with autism. Arch Gen Psychiatry. 2006;63:1417–1428. doi: 10.1001/archpsyc.63.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani N, Avikainen S, Hari R. Abnormal imitation-related cortical activation sequences in Asperger's syndrome. Ann Neurol. 2004;55:558–562. doi: 10.1002/ana.20031. [DOI] [PubMed] [Google Scholar]
- Noonan SK, Haist F, Müller RA. Aberrant functional connectivity in autism: evidence from low-frequency BOLD signal fluctuations. Brain Res. 2009;1262:48–63. doi: 10.1016/j.brainres.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LM, Ramachandran VS. The simulating social mind: the role of the mirror neuron system and simulation in the social and communicative deficits of autism spectrum disorders. Psychol Bull. 2007;133:310–327. doi: 10.1037/0033-2909.133.2.310. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Carter EJ. Brain mechanisms for social perception: lessons from autism and typical development. Ann N Y Acad Sci. 2008;1145:283–299. doi: 10.1196/annals.1416.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Iacoboni M, Mazziotta JC, Dapretto M. Mirroring others' emotions relates to empathy and interpersonal competence in children. Neuroimage. 2008;39:2076–2085. doi: 10.1016/j.neuroimage.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Moore WE, Oswald TM, Mazziotta JC, Iacoboni M, Dapretto M. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69:1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pierce K, Haist F, Sedaghat F, Courchesne E. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004;127:2703–2716. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- Pierce K, Müller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Fabbri-Destro M. Mirror neurons: from discovery to autism. Exp Brain Res. 2010;200:223–237. doi: 10.1007/s00221-009-2002-3. [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, Skudlarski P, Lacadie C, Cohen DJ, Gore JC. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Barnes CC, Lord C, Courchesne E. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biol Psychiatry. 2009;66:942–949. doi: 10.1016/j.biopsych.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Abrahams BS, Alvarez-Retuerto AI, Sonnenblick LI, Rudie JD, Ghahremani D, Mumford JA, Poldrack RA, Dapretto M, Geschwind DH, et al. Altered functional connectivity in frontal lobe circuits is associated with variation in the autism risk gene CNTNAP2. Sci Transl Med. 2010;2:56ra80. doi: 10.1126/scitranslmed.3001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih P, Shen M, Ottl B, Keehn B, Gaffrey MS, Müller RA. Atypical network connectivity for imitation in autism spectrum disorder. Neuropsychologia. 2010;48:2931–2939. doi: 10.1016/j.neuropsychologia.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Stevens MC. The developmental cognitive neuroscience of functional connectivity. Brain Cogn. 2009;70:1–12. doi: 10.1016/j.bandc.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum Brain Mapp. 2009;30:2356–2366. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb Cortex. 2008;18:2659–2665. doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner KC, Frost L, Linsenbardt D, McIlroy JR, Müller RA. Atypically diffuse functional connectivity between caudate nuclei and cerebral cortex in autism. Behav Brain Funct. 2006;2:34. doi: 10.1186/1744-9081-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Menon V. The anterior insula in autism: under-connected and under-examined. Neurosci Biobehav Rev. 2009;33:1198–1203. doi: 10.1016/j.neubiorev.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Davies MS, Scott AA, Zaidel E, Bookheimer SY, Iacoboni M, Dapretto M. Neural basis of self and other representation in autism: an FMRI study of self-face recognition. PLoS One. 2008;3:e3526. doi: 10.1371/journal.pone.0003526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn Sci. 2007;11:153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Front Syst Neurosci. 2010;4:21. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Müller RA. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage. 2005;25:916–925. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Nichols TE. Optimization of experimental design in fMRI: a general framework using a genetic algorithm. Neuroimage. 2003;18:293–309. doi: 10.1016/s1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- Wang AT, Dapretto M, Hariri AR, Sigman M, Bookheimer SY. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Wang AT, Lee SS, Sigman M, Dapretto M. Reading affect in the face and voice: neural correlates of interpreting communicative intent in children and adolescents with autism spectrum disorders. Arch Gen Psychiatry. 2007;64:698–708. doi: 10.1001/archpsyc.64.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio (TX): Psychological Corporation; 1999. [Google Scholar]
- Welchew DE, Ashwin C, Berkouk K, Salvador R, Suckling J, Baron-Cohen S, Bullmore E. Functional disconnectivity of the medial temporal lobe in Asperger's syndrome. Biol Psychiatry. 2005;57:991–998. doi: 10.1016/j.biopsych.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Weng SJ, Wiggins JL, Peltier SJ, Carrasco M, Risi S, Lord C, Monk CS. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2010;1313:202–214. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.