Abstract
Neurons in layer IV of the rodent whisker somatosensory cortex are tangentially organized in periodic clusters called barrels, each of which is innervated by thalamocortical axons transmitting sensory information from a single principal whisker, together forming a somatotopic map of the whisker pad. Proper thalamocortical innervation is critical for barrel formation during development, but the molecular mechanisms controlling layer IV neuron clustering are unknown. Here, we investigate the role in this mapping of the nuclear orphan receptor RORβ, which is expressed in neurons in layer IV during corticogenesis. We find that RORβ protein expression specifically increases in the whisker barrel cortex during barrel formation and that in vivo overexpression of RORβ is sufficient to induce periodic barrel-like clustering of cortical neurons. Remarkably, this clustering can be induced as early as E18, prior to innervation by thalamocortical afferents and whisker derived-input. At later developmental stages, these ectopic neuronal clusters are specifically innervated by thalamocortical axons, demonstrated by anterograde labeling from the thalamus and by expression of thalamocortical-specific synaptic markers. Together, these data indicate that RORβ expression levels control cytoarchitectural patterning of neocortical neurons during development, a critical process for the topographical mapping of whisker input onto the cortical surface.
Keywords: barrel cortex, cortical patterning, cytoarchitecture, RORβ, thalamocortical innervation
Introduction
The richness and diversity of our sensory perceptions and motor actions largely originate in the neuronal networks formed by the diverse subtypes of neurons of the neocortex. Along its tangential plane, the neocortex is patterned into distinct functional regions, including the motor, somatosensory, visual, and auditory cortical areas, in which distinct neuronal subtypes form precise area-specific circuits (Krubitzer 2007; O'Leary and Sahara 2008). The generation and differentiation of the distinct subtypes of cortical neurons in each of these areas during development results from combinatorial interactions between neuron type–specific and area-specific programs of gene expression. These molecular programs give rise to the area-specific cytoarchitectural, hodological, and functional features of the adult neocortex (reviewed in Molyneaux et al. 2007). Over the past few years, we and others have made substantial progress toward identifying molecular mechanisms that control both subtype-specific and area-specific differentiation of cortical projection neuron and interneuron (Arlotta et al. 2005, 2008; Molyneaux et al. 2005, 2007, 2009; Ozdinler and Macklis 2006; Alcamo et al. 2008; Chen et al. 2008; Joshi et al. 2008; Lai et al. 2008; Azim, Jabaudon, et al. 2009; Azim, Shnider, et al. 2009; Tomassy et al. 2010). During cortical arealization, the Studer lab and ours have shown that the transcription factor Coup-TFI acts in the developing somatosensory cortex to repress default corticospinal motor neuron differentiation programs, thereby imparting this area with sensory features (Armentano et al., Tomassy et al. 2010). Similarly, the Gan lab and our own showed that the transcription factor Bhlhb5 critically controls postmitotic fate acquisition in projection neurons of layers II–V in an area-specific manner (Joshi et al. 2008). However, despite recent progress in understanding molecular controls over area-specific differentiation of distinct subtypes of cortical neurons, how these neurons assemble to form area-specific circuits with distinctive cytoarchitectural features remains unknown.
Two main hypotheses have been put forth to explain how cortical areas are specified during development. The protomap hypothesis postulates that area identities are specified in neocortical progenitors at early stages of development in response to morphogens secreted by signaling centers in the telencephalon. This information is translated into a spatial map in postmitotic neurons through regulation of proliferation, differentiation, and migration (Rakic 1988, 2009). In contrast, the protocortex (or “tabula rasa”) hypothesis states that the spatial identity of neocortical neurons is established by cues from thalamic afferents innervating specific areas in a modality-specific manner (O'Leary 1989; Mallamaci and Stoykova 2006). Recently, both hypotheses have been integrated into a single model in which intrinsic and extrinsic factors work in combination to specify area identity in 2 developmental phases. At early stages, prior to innervation from thalamocortical afferents, areal identity is established cell-intrinsically in the progenitors and postmitotic neurons, whereas at later stages, extrinsic input refines and sharpens areal boundaries. These stages are mirrored by changes in expression of area identity genes from broad gradients to sharp boundaries of expression.
Area-specific cytoarchitectural features are particularly striking in the rodent whisker somatosensory cortex, where neurons in layer IV assemble into periodic clusters called barrels. Barrels are dominated by input from a single whisker and are formed by columnar clusters of layer IV neurons surrounding the fasciculated thalamocortical axons originating in neurons of the ventral posterior medial (VPM) nucleus of the thalamus. Barrels develop rapidly during the first few postnatal days and are severely disorganized by lesions to whiskers or their afferent pathways during this critical period of development (reviewed in Erzurumlu and Kind 2001; López-Bendito and Molnár 2003). Although the whisker-to-barrel system has been widely used to study the development, topography, and plasticity of thalamocortical connectivity, the molecular mechanisms that underlie the whisker-specific clustering of layer IV cortical neurons are essentially unknown. In accordance with the protomap hypothesis described above, while the initial specification of the barrel fields is initially cell intrinsic, this cytoarchitecture after birth is sculped by sensory input from the periphery (i.e., thalamocortical axons), which are attracted specifically to this particular area and are essential for full differentiation of the barrels (Gitton et al. 1999).
Here, we show that RORβ, a nuclear orphan receptor of previously unknown function in the neocortex, functions in regulating neuronal patterning during cortical development. RORβ is expressed at progressively increasing levels by neurons in layer IV in the whisker somatosensory cortex during barrel formation. Overexpression of RORβ during cortical development is sufficient to induce the periodic clustering of cortical neurons in vivo, forming structures with characteristics of barrels that receive synaptic input specifically from thalamocortical neurons. Together, these data reveal a central cell-intrinsic function for RORβ in regulating neuronal patterning in the developing neocortex and suggest that this orphan receptor contributes centrally to the cytoarchitectural patterning of layer IV neurons into barrels during somatosensory cortex development.
Materials and Methods
Animals
The day of vaginal plug detection was designated as E0.5. The day of birth was designated as P0. All mouse studies were approved by the Massachusetts General Hospital IACUC and were performed in accordance with institutional and federal guidelines. Barrelless mice were a generous gift from Egbert Welker, Lausanne University, Switzerland (Welker et al. 1996)
Immunocytochemistry
Brains were fixed and stained using standard methods. For immunofluorescence studies, brain sections were blocked in a 0.3% bovine serum albumin (Sigma-Aldrich Chemicals), 8% goat or donkey serum, 0.3% Triton X-100 (Sigma-Aldrich Chemicals), and phosphate-buffered saline (PBS) azide (0.025%) solution for 1 h at room temperature, before incubation in primary antibody. Primary antibodies and dilutions used were rabbit anti-RORβ, gift of H. Stunnenberg (1:2000); mouse anti-SATB2 (1:200; Abcam), rabbit anti-GFP (1:1000; Molecular Probes); mouse anti-synaptophysin (1:500; Chemicon); and mouse anti-VGLUT2 (1:200; Chemicon). Appropriate secondary antibodies were obtained from the Molecular Probes Alexa series and used at dilutions of 1:500.
Cytochrome Oxidase Staining
Freshly prepared brain sections were incubated in a developing solution containing 10% (w/v) sucrose, 0.0005% (w/v) cytochrome C (Sigma-Aldrich Chemicals), 0.00025% (w/v) of 3,3′-diaminobenzidine tetrahyrochloride in 0.1 M phosphate buffer at 37 °C until optimal staining intensity was achieved. Brain sections were then rinsed 3 times in PBS and subsequently mounted onto slides for imaging.
In Utero Electroporation
Experiments were performed essentially as described in Lai et al. (2008). Briefly, timed pregnant CD1 females carrying embryonic day 12.5 (E12.5) embryos were anesthetized via an intraperitoneal injection of 0.015 cc/g body weight of Avertin (1.25% of 2,2,2-tribromoethanol) in a solvent containing 0.63% isoamyl alcohol by weight (in double-distilled H2O) together with 0.3 mL of MgSO4 (5 mg/mL in 0.9% NaCl) for tocolytic purposes. Single embryos at a time were then removed and gently pulled through a preprepared embryo-holding chamber filled with prewarmed PBS. A total of 1 μL of RORβ or FEZF2 overexpression (CMV-βactin-RORβ-IRES-GFP or CMV-βactin-FEZF2-IRES-GFP) or control (CMV-βactin-IRES-GFP) DNA plasmid vector (1.0 μg/μl DNA plasmid mixed with 0.005% (v/w) Fast Green in sterile 0.1 M phosphate buffer) was then injected into either lateral ventricle under ultrasound guidance (Vevo 770, VisualSonics) and electroporated into ventricular progenitor cells with five 30 V pulses of 50-m duration at 1-s intervals using 0.5-cm-diameter platinum electrodes and an ECM 830 Square Wave electroporator (BTX-Harvard Apparatus). Injected embryos were then returned to the abdominal cavity and allowed to develop normally until processed at the appropriate age for immunocytochemical or labeling analysis.
Anterograde Thalamocortical Labeling
For anterograde tracing of thalamocortical axons, brains of pups previously electroporated at E12.5 were collected at P3 and fixed overnight in 4% paraformaldehyde at 4 °C. Brains were then hemidissected, and, starting at the rostral portion of the dorsal thalamus, a transverse incision was made at the level of the VPM nucleus. A small crystal of 1,1′-dioctadecyl-3,3,3′,3′ tetra-methyl-indocarbocyanine perchlorate (DiI) was inserted into the VPM under stereomicroscopic guidance. Brains were then placed in PBS and left for 9 days at 37 °C to allow for DiI diffusion. Following diffusion, the brains were sectioned coronally on a vibrating microtome (Leica) at 80 μm thickness and analyzed as “wet-mounts” for appropriate DiI crystal placement under a Nikon Eclipse E1000 epifluorescence microscope. Only those brains with confirmed placement of DiI into the VPM were included for subsequent confocal microscopic analysis.
Light and Confocal Microscopy
Slides were visualized using a Nikon Eclipse E1000 fluorescence microscope equipped with an X-Cite 120 illuminator (EXF0), and images were acquired using a Q-imaging Retiga EX camera (Q-Imaging Corporation, Surrey, Canada). Confocal images were collected using a BioRad Radiance 2100 Rainbow laser-scanning confocal microscope based on a Nikon E800 microscope. Images were assembled in Adobe Photoshop (v. 10), with adjustments for contrast, brightness, and color balance to obtain optimal visual reproduction of data.
Results
RORβ Expression Increases in Neurons in Layer IV of the Developing Somatosensory Cortex during Thalamocortical Afferent Innervation
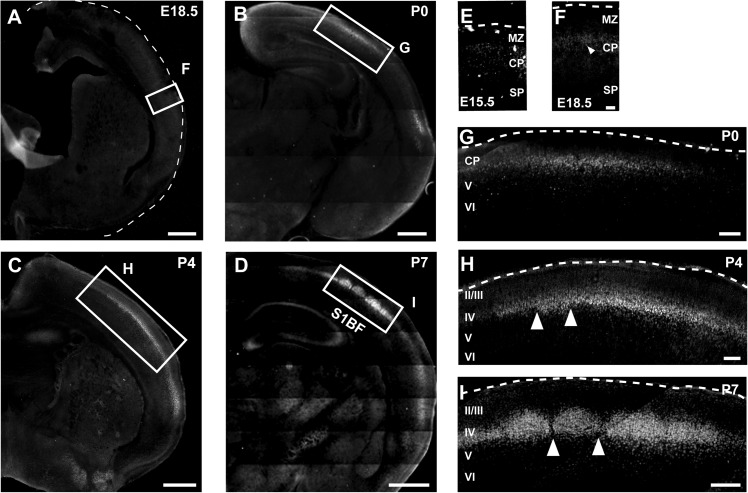
RORβ is known to be expressed at high levels in layer IV of the somatosensory cortex, but the roles of this orphan receptor during corticogenesis were previously unknown. To investigate whether RORβ might function in the differentiation of neurons in layer IV, we first investigated the temporal and regional expression of RORβ in the developing mouse brain. Immunocytochemical analysis reveals that RORβ protein is expressed in the lateral cortical plate at E18.5 (Fig. 1A,F) and is only very weakly expressed by cortical neurons at E15.5 (Fig. 1E). RORβ protein expression likely follows Rorβ messenger RNA (mRNA) expression by a few days since expression of the transcript in the cortex has been reported to begin at about E14.5 (Schaeren-Wiemers et al. 1997; Azadi et al. 2002; Nakagawa and O'Leary 2003). At P0, as thalamocortical axons invade the cortical plate and establish synapses with cortical neurons in primary sensory areas (López-Bendito and Molnár 2003), RORβ expression progressively increases in neurons in layer IV of the prospective somatosensory, visual, and auditory cortices (Fig. 1B–D,G–I). RORβ is also expressed by scattered neurons in layer V in these areas, but at much lower levels. The increase in RORβ expression is particularly striking in the whisker barrel cortex of the somatosensory cortex, where sharp expression domains in layer IV are clearly visible by P4 (Fig. 1H), and distinct barrels are present by P7 (Fig. 1D,I). These results demonstrate that expression of RORβ protein is precisely spatially and temporally regulated during cortical development, as has been reported for Rorβ mRNA (Nakagawa and O'Leary 2003). These data reveal that RORβ protein expression becomes progressively restricted primarily to neurons in layer IV of the sensory cortices, with a striking postnatal increase in expression in the somatosensory cortex.
Figure 1.
RORβ protein expression increases in neurons in layer IV as cortical barrels are forming. Immunocytochemistry on coronal hemisections at E18.5 (A,F), P0 (B,G), P4 (C,H), and P7 (D,I) showing rapid and restricted increase in RORβ protein expression in neurons in layer IV postnatally, coinciding with the period of barrel formation. Boxed areas in A–D (putative barrel field) are magnified in F–I. RORβ is initially only weakly and diffusely expressed at E15.5 (E) but increases rapidly in putative layer IV neurons between E18.5 (A,F, arrowhead in F indicates labeled neuron) and P7 (D,I), a time when individual barrels are clearly distinguishable (arrowheads in I indicate barrel septae, emerging in H). Abbreviations: S1BF: S1 barrel field; SP: subplate; CP: cortical plate; MZ: marginal zone; II/III-VI: neocortical layers II/III-VI. Scale bars: 500 μm (A–D), 50 μm (E,F), 100 μm (G–I).
Postnatal Increase in RORβ Expression in the Somatosensory Cortex Is Regulated by Thalamocortical Input
RORβ expression in neurons in layer IV of the whisker barrel cortex sharply increases between P0 and P4, while axons of thalamocortical neurons located in the VPM nucleus are invading the neocortex. We hypothesized that the increase in RORβ expression during the first few postnatal days might at least partially depend on innervation of neurons in layer IV by thalamocortical axons.
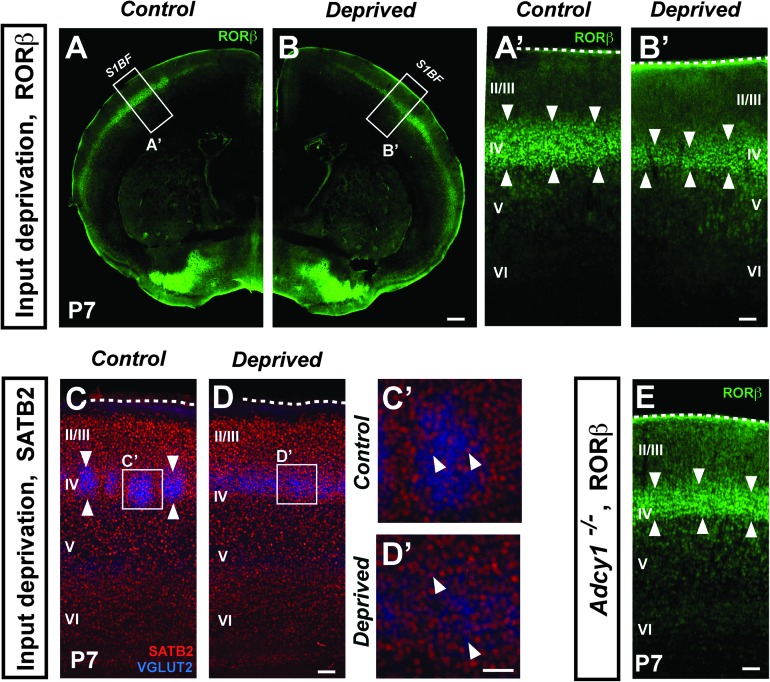
We find that this is indeed the case, using 2 complementary strategies to specifically disrupt the normal innervation of layer IV neurons by VPM thalamocortical afferents. First, we performed a neonatal unilateral section of the infraorbital nerve, which prevents sensory input from the whiskers from reaching the thalamus, and which dramatically disrupts VPM thalamocortical axon pathfinding and cortical barrel formation (Van der Loos and Woolsey 1973; Erzurumlu and Kind 2001). These experiments reveal that neurons in layer IV in whisker input-deprived cortex express reduced levels of RORβ as compared with the contralateral undeprived neurons (Fig. 2A–B′). Importantly, this decrease in RORβ expression does not reflect a nonspecific effect on layer IV neuron protein synthesis, or increased cell death, since expression of SATB2, a transcription factor expressed by neurons in layer IV (Alcamo et al. 2008; Britanova et al. 2008), is unaffected by input deprivation following infraorbital nerve section (Fig. 2C–D′).
Figure 2.
Thalamocortical input specifically regulates postnatal RORβ protein expression. Immunocytochemical analysis of bilateral coronal hemisections showing RORβ protein expression in neurons in layer IV of the whisker barrel cortices in a P7 mouse after perinatal unilateral section of the left infraorbital nerve (A–B′). RORβ expression in layer IV neurons of the control right whisker barrel field (A,A′) is much stronger than in the left barrel field that underwent ION section and subsequent deprivation of vibrissal input (B,B′). In contrast, levels of another layer IV–expressed gene, SATB2, are unaffected by IO nerve section (C–D′). In “barrelless” (Adcy1−/−) transgenic mice (E), RORβ expression is modestly reduced (compare with A′). Arrowheads delineate the area where most RORβ expressing neurons are located (layer IV). Abbreviations: S1BF, S1 barrel field; II/III-VI: neocortical layers II/III-VI. Scale bars: 500 μm (A,B), 100 μm (A′–D,E), 50 μm (C′,D′).
A second set of experiments, using transgenic mice with abnormal thalamocortical innervation (the “barrelless” mice strain, which lacks Adcy1 gene function; Welker et al. 1996; Abdel-Majid et al. 1998), demonstrates decreased cortical RORβ expression, but to a lesser extent than following infraorbital nerve section (Fig. 2E). These barrelless mice also express RORβ more broadly in layers V, IV, and II/III. Whereas the “barrelless” genetic manipulation affects thalamocortical neurons throughout their differentiation, infraorbital nerve lesions produce an acute disruption of thalamocortical input, which might cause a stronger effect on layer IV RORβ expression. Together, these findings indicate that the postnatal increase in RORβ protein expression in neurons in layer IV of the primary somatosensory cortex is at least partially non-cell autonomous and depends on thalamocortical axon innervation.
Overexpression of RORβ during Corticogenesis Induces Barrel-Like Periodic Clustering of Neurons
Neurons in layer IV of the whisker barrel cortex, which express the highest levels of RORβ protein of all cortical neurons, are not distributed homogenously but instead aggregate into periodic clusters to form the barrels. Our observations suggest that RORβ might critically regulate layer IV neuron clustering since 1) the rapid postnatal increase in RORβ expression in the whisker barrel cortex temporally coincides with barrel formation (Fig. 1); and 2) reduced levels of RORβ expression in the somatosensory cortex after infraorbital nerve section or genetic manipulation are associated with abnormal barrel formation (Fig. 2B,E; Van der Loos and Woolsey 1973; Welker et al. 1996).
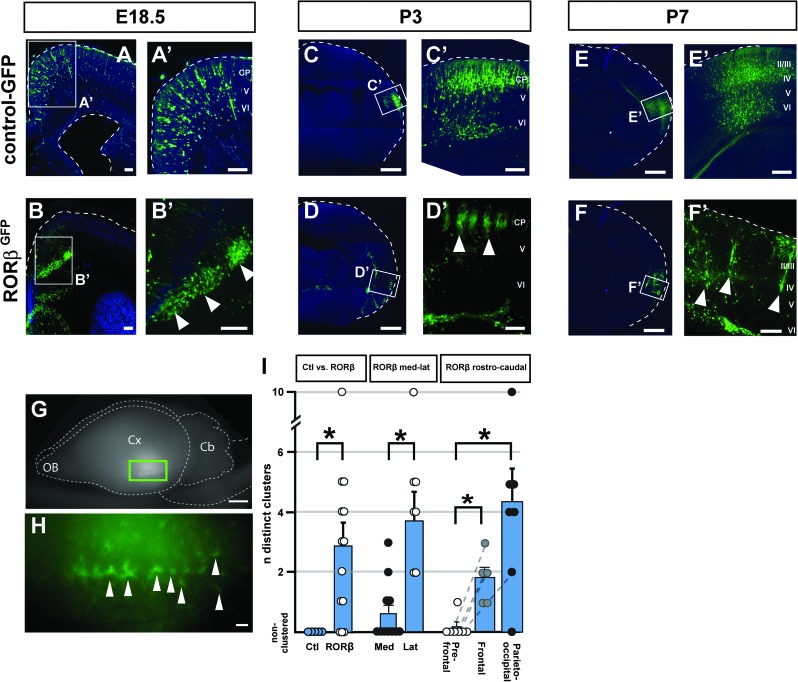
We investigated whether direct manipulation of RORβ expression in neocortical neurons during corticogenesis might modify neuronal distribution and clustering. To this end, we focally overexpressed RORβ in cortical neurons via in utero electroporation of an RORβ-expressing plasmid into cortical progenitors at embryonic day (E) 12.5, as deep layer cortical neurons are being generated, well before the normal developmental period of barrel formation. These experiments reveal that RORβ overexpression dramatically affects neuronal distribution in the neocortex and leads to periodic, focal clustering of the transfected neurons (Fig. 3). Whereas neurons expressing the control GFP vector become distributed evenly throughout the electroporated area (Fig. 3A,A′,C,C′,E,E′), RORβGFP-expressing neurons become largely distributed into discrete periodic clusters (Fig. 3B,B′,D,D′,F,F′). These clusters are already present by E18.5, before maturation of the whisker somatosensory circuits. At E18.5, the clusters are found mostly at the border between the subcortical white matter and developing cortical plate (Fig. 3B,B′). Over the next few days, many of the RORβ-overexpressing neurons migrate into the cortical plate and become even more strikingly clustered by P3 (Fig. 3D,D′), retaining a periodic columnar organization at P7 (Fig. 3F,F′). Using a whole-mount preparation, the neuronal clusters were visible as periodically displayed at regular intervals, along a mostly anteroposterior axis (Fig. 3G,H). Interestingly, despite their ectopic location, these RORβ overexpressing neurons appear to retain central elements of proper molecular identity; for example, they express SATB2 and do not express CITP2, appropriate for layer IV neurons (Arlotta et al. 2005; Supplementary Fig. 1).
Figure 3.
RORβ overexpression induces area-specific periodic neuronal clustering. In utero overexpression of RORβ at E12.5 induces periodic neuronal clustering in the cortex and subcortical white matter at E18.5 (B,B′, compare with A,A′, where a control GFP-expressing plasmid was used), P3 (D,D′, compare with C,C′), and P7 (F,F′, compare with E,E′). An RORβ-IRES-GFP or control-GFP plasmid construct was electroporated so that RORβ-overexpressing neurons or control neurons can be visualized in green using anti-GFP immunocytochemistry. Arrowheads in B′, D′, and F′ highlight individual clusters of RORβ-overexpressing neurons. The dashed lines outline the cortical surface (and also the ventricular surface in A). Using a whole-mount preparation (G,H; same brain shown in F,F′), the neuronal clusters can be seen as periodically aligned along a mostly anteroposterior axis (H). Quantification of the electroporation results (I) reveals that RORβ overexpression in posterior and lateral cortical areas is most efficient in generating multiple clusters. Circles indicate individual measurements; dotted lines connect measurements within the same brain, error bar: SEM, *P < 0.05 (Mann–Whitney test) Abbreviation: CP: cortical plate. OB: olfactory bulb, Cb: cerebellum, Cx: cerebral cortex, V,VI: neocortical layers V,VI. Scale bars: 500 μm (C,D,E,F), 100 μm (A,B,A′-F′), 2 mm (G), 200 μm (H).
Remarkably, RORβ-induced periodic neuronal clustering is an area-dependent process, in which neurons in lateral and posterior cortical areas give rise to more periodic clusters than in frontal and medial regions (Fig. 3I). While RORβ overexpression generates on average 3 clusters (up to 10; n = 14 RORβ-electroporated embryos), neuronal clusters are never seen in control electroporations. RORβ overexpression in lateral neocortical regions always gives rise to multiple clusters (7/7 electroporation sites) while this is the case for only 4/11 medial electroporation sites (P < 0.05, Mann–Whitney test). Similarly, periodic clusters are rarely seen in neurons from prefrontal areas (1/7 sites), while progressively more clusters are seen in frontal and parieto-occipital regions (6/7 sites in the parieto-occipital cortex, P < 0.05). These results suggest an area-specific heterogeneity in RORβ-overexpressing neurons acting to regulate clustering, or that non-cell autonomous, area-specific factors contribute to cluster generation. Together, these data indicate that increasing RORβ expression in differentiating cortical neurons is sufficient to induce an area-dependent clustering into barrel-like structures, bypassing the requirement for functional thalamocortical innervation and connectivity between the whiskers, the thalamus, and the somatosensory cortex.
RORβ-Induced Neuronal Clusters Are Specifically Targeted by Developing Thalamocortical Axons
Neurons in layer IV, which during neocortical development express the highest levels of RORβ, are the main postsynaptic targets of neurons located in the principal sensory thalamic nuclei. We thus investigated whether the ectopic clusters of neurons induced by RORβ overexpression might be specifically innervated by thalamocortical axons, potentially indicating an instructive role for RORβ in the assembly of thalamocortical circuits during development.
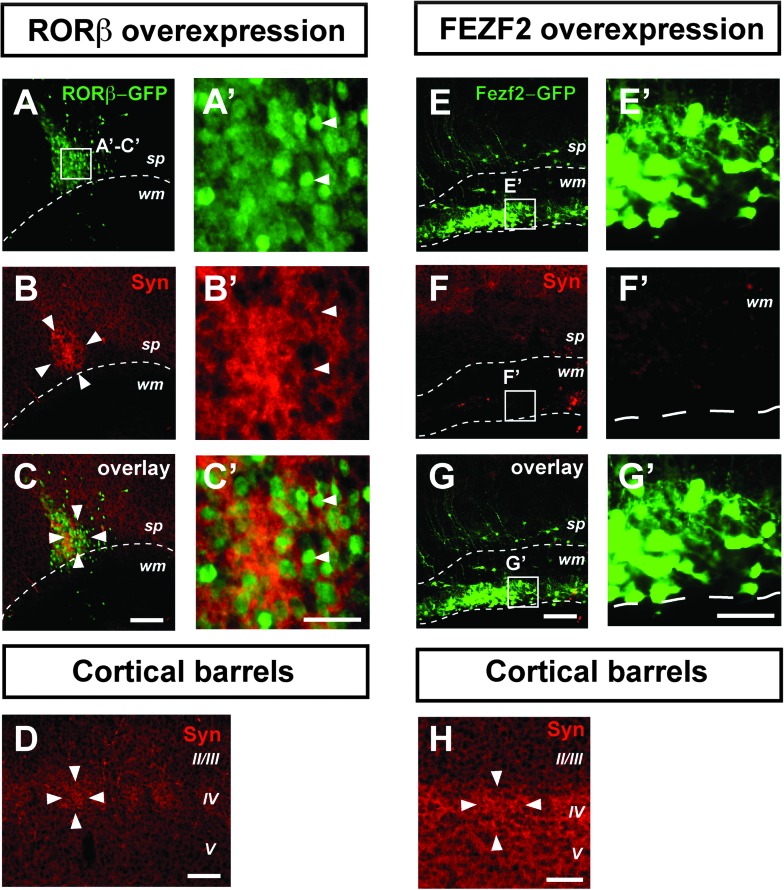
To assess the synaptic integration of clustered RORβ-overexpressing neurons within the cortical circuitry, we first investigated whether these neurons receive synaptic input by analyzing the distribution of the presynaptic marker synaptophysin (Stettler et al. 1996). This analysis reveals that the clusters of RORβ-overexpressing neurons are synapse-rich (Fig. 4A–C′), with levels of synaptophysin expression surpassing those found in genuine cortical barrels (Fig. 4D). Neurons in layer IV are mostly excitatory interneurons, which normally establish short-range connections within a single barrel column, thus contributing to the high synaptic density of barrels (Schubert et al. 2007). In order to examine whether the high synaptic density within the neuronal clusters was specifically induced by RORβ overexpression, we next used FEZF2 overexpression to generate ectopic corticospinal projection neurons in the subcortical white matter (Molyneaux et al. 2005) and examined the synaptic density of these long-range projecting ectopic neurons (Fig. 4E–G′). In contrast to RORβ-overexpressing neurons and despite a similar density of transfected neurons, there are very few synapses onto FEZF2-overexpressing neurons and overall synaptic density is well below that of the corresponding barrel cortex (Fig. 4H). Therefore, the barrel-like synaptic features of the periodic neuronal clusters are specifically induced by RORβ overexpression and correspond to the normal synaptic features of layer IV excitatory interneurons.
Figure 4.
Clusters of RORβ-overexpressing neurons are synapse rich. Clustered RORβ-overexpressing neurons after in utero electroporation at E12.5 with an RORβ-IRES-GFP plasmid have a high synaptic content, as indicated by strong synaptophysin immunoreactivity (A–C′), even exceeding levels found in genuine cortical barrels (D). In contrast, ectopic neurons generated by overexpression of a FEZF2-IRES-GFP plasmid are synapse-poor (E–G′) (H) Cortical barrel in the same brain and using the same settings as in (E–G′), showing high synaptic density. Arrowheads point to GFP-RORβ positive neurons. Abbreviations: Cx: cortex; sp: subplate; Str: striatum; S1: primary somatosensory cortex; Syn: synaptophysin; wm: white matter; II/III-V: neocortical layers II/III-V. Scale bars: 100 μm (A–H), 50 μm (A’–G’).
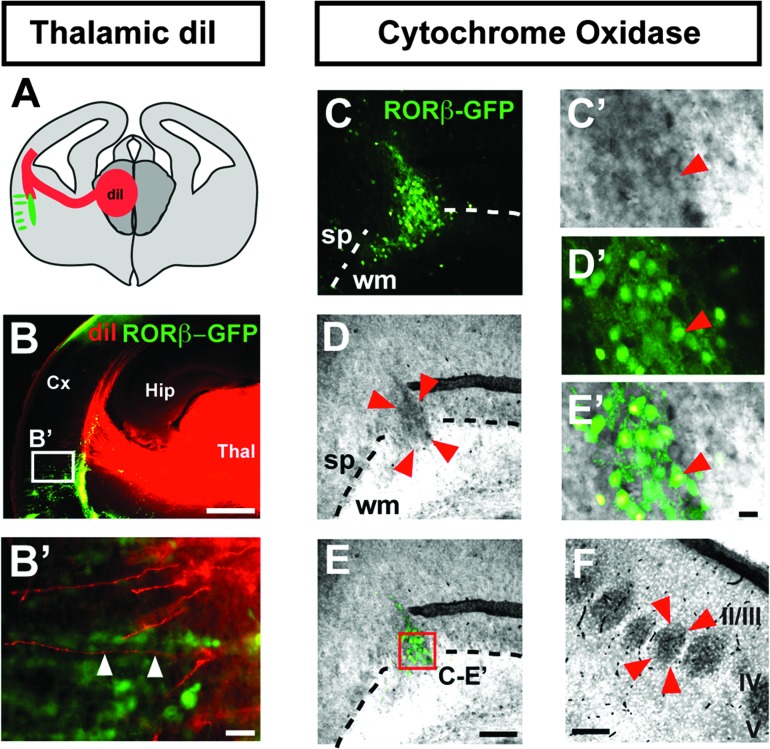
We next examined whether at least some of the afferent synaptic contacts within the RORβ-induced neuronal clusters might be established by neurons located in the thalamus, a possibility suggested by the observation that a subset of these synapses (labeled by synaptophysin) do not express RORβ-GFP (Fig. 4A–C′). Anterograde labeling from the thalamus with DiI supports this hypothesis and reveals that thalamocortical axons project along ectopic RORβ-expressing neurons (Fig. 5A–B′), often in close apposition to clusters of RORβ-positive neurons (Fig. 5B′, arrowheads). Innervation of RORβ-overexpressing neurons by thalamocortical afferents is further suggested by positive staining of the neuronal clusters for the mitochondrial enzyme cytochrome oxidase (Fig. 5C–E′). Since thalamocortical axon terminals are rich in mitochondria, this method is widely used to identify cortical barrels (Wong-Riley and Welt 1980), and this finding supports the presence of thalamocortical afferents in the RORβ-overexpressing clusters (Fig. 5F).
Figure 5.
Clusters of RORβ-overexpressing neurons colocalize with thalamocortical axons. Schematic representation of the photomicrograph in B, showing a coronal brain section with anterograde labeling of thalamocortical afferents by a crystal of DiI placed in the thalamus (red) and the location of the clusters of RORβ-overexpressing neurons (green) (A). Anterograde labeling of thalamocortical afferents shows apposition of thalamocortical axons and RORβ-overexpressing neurons (arrowheads in B′, magnified from the boxed area in B) (B,B′). Cluster of RORβ-overexpressing neurons (delineated by 4 arrowheads), enriched in cytochrome oxidase-positive presynaptic terminals (C-E, magnified in C′-E′). This brain was electroporated in utero at E12.5 with an RORβ-IRES-GFP plasmid (C–E). Genuine cortical barrels are shown in F (barrel delineated by 4 arrowheads). Abbreviations: Cx: cortex; Hip: hippocampus; sp: subplate; Thal: thalamus; wm: white matter; II/III–V: neocortical layers II/III–V. Scale bars: 500 μm (B), 100 μm (C–E,F), 10 μm (B′,C’–E’).
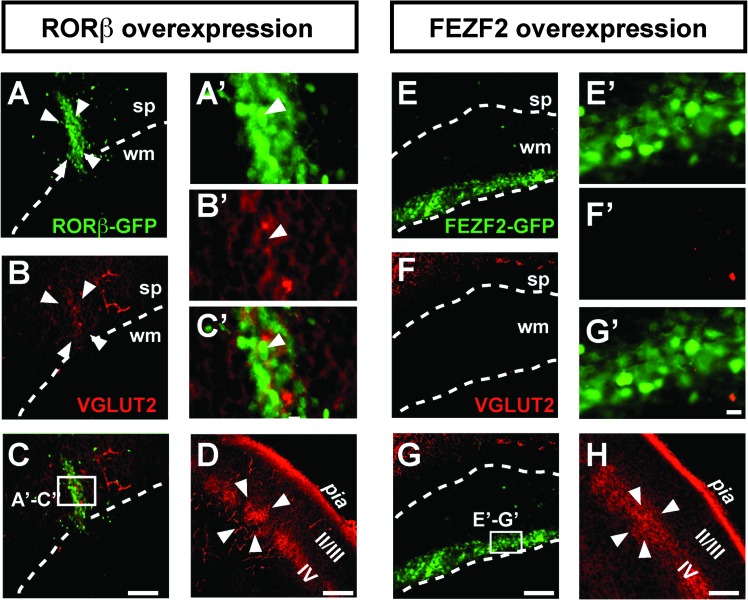
However, DiI labeling does not reliably enable identification of presynaptic terminals, and positive cytochrome oxidase staining could reflect a high density of cell bodies, and thus mitochondria. We therefore next directly examined whether presynaptic terminals of thalamocortical axons are located in the neuronal clusters by assessing expression of the vesicular glutamate transporter 2 (VGLUT2), which in the cortex is exclusively expressed at presynaptic thalamocortical terminals (Fujiyama et al. 2001; Nakamura et al. 2005; Liguz-Lecznar and Skangiel-Kramska 2007). This approach reveals that the RORβ-expressing neuronal clusters contain high densities of thalamocortical synapses (Fig. 6A–C′), comparable to those found in genuine cortical barrels (Fig. 6D). Remarkably, thalamocortical innervation is specifically induced by RORβ overexpression since FEZF2-overexpressing neuronal heterotopies were devoid of VGLUT2 immunoreactivity, despite a similar density of transfected neurons (Fig. 6E–H). Together, these results indicate that RORβ-expressing neurons are specifically targeted by axons of thalamocortical neurons, strongly suggesting that this orphan receptor plays a role in the guidance of thalamocortical axons to their proper cortical target during cortical development.
Figure 6.
RORβ-overexpressing neurons specifically receive thalamocortical afferents. Clustered RORβ-overexpressing neurons after in utero electroporation at E12.5 (A–C′) showing immunoreactivity for the thalamocortical synapse marker VGLUT2 (B,B’). Expression of VGLUT2 in genuine cortical barrels is shown in D (barrel delineated by 4 arrowheads). The cluster of RORβ-overexpressing neurons (delineated by 4 arrowheads) is enriched in VGLUT2 positive (i.e., thalamocortical) presynaptic terminals (B,C, magnified in B′,C′). In contrast, in areas where ectopic neurons generated by overexpression of a FEZF2-IRES-GFP plasmid are located, VGLUT2 immunoreactivity is absent (E-G′), indicating lack of thalamocortical innervation. (H) Cortical barrel in the same brain and using the same settings as in (E–G’), showing strong VGLUT2 immunoreactivity. Abbreviations: sp: subplate; Str: striatum; S1: primary somatosensory cortex; Syn: synaptophysin; wm: white matter; II/III,IV: neocortical layers II/III,IV. Scale bars: 100 μm (A–H), 10 μm (A′–G′).
Discussion
Topographical mapping of sensory input and motor output onto the neocortical surface is a fundamental organizational principle of the mammalian neocortex and can be detected at the molecular, cellular, anatomical, physiological, and behavioral levels of nervous system organization (Krubitzer 2007; O'Leary et al. 2007). Topographical mapping of sensory input is particularly striking in the rodent somatosensory whisker cortex, where distinct periodic clusters of layer IV neurons receive input from single principal whiskers to form structures called barrels, which are easily identified in coronal brain sections even without specialized staining. The cortical mapping of the whisker pad is somatotopical; neighboring barrels represent neighboring whiskers, and the size of each barrel is proportional to the density of innervation of its corresponding whisker follicle. Within barrels, neurons in layer IV have been previously shown to express high levels of the nuclear orphan receptor RORβ, but though this specific expression has been employed to identify these neurons for years, the functions of this nuclear receptor during corticogenesis have previously remained unknown (Jetten and Joo 2006).
The experiments presented here demonstrate that early RORβ expression in the somatosensory cortex precedes thalamocortical innervation and barrel formation and that overexpression of RORβ in cortical neurons during development is sufficient to induce periodic neuronal clusters reminiscent of barrels. These results suggest that RORβ acts in a dose-dependent manner to regulate barrel formation upon innervation of layer IV neurons by thalamocortical axons.
This model is in accordance with the findings of the Wassef group, where specification of the barrel cortex is initially cell-autonomous (as measured by expression of the somatosensory cortex-specific transgene H-2Z1), and only after birth can this gene expression be regulated by thalamocortical axons, which are attracted specifically to this particular area (Gitton et al. 1999). Here, we show that baseline RORβ expression is initially cell-autonomous but is increased at postnatal stages by arrival of thalamocortical afferents. Increases in RORβ expression cause clustering of neurons in layer IV and likely participates in the guidance of thalamocortical axons to their targets, as shown by the results of our overexpression experiments. Consistent with this possibility, recent findings in the Reeler mouse, where the laminar organization of the cortex is roughly inverted, show that RORβ-expressing neurons, while loosely distributed, form barrel equivalent-columns that specifically receive thalamocortical input (Wagener et al. 2010).
Taken together, these experiments reveal a novel and instructive function for RORβ in regulating the spatial distribution of neurons in layer IV during corticogenesis and identify a novel molecular component that controls the topographical organization and patterning of sensory areas during development.
Activity-Dependent Regulation of RORβ Expression
A first indication from these results that RORβ expression in neurons in layer IV of the somatosensory cortex might function in barrel formation and thalamocortical afferentation emerged from the observation that the sharp postnatal increase in RORβ expression levels specifically in the somatosensory cortex temporally coincides with the period of cortical invasion by axons of thalamocortical neurons located in the VPM nucleus (P0–P4) and immediately precedes cortical barrel formation (P4–P6) (Schaeren-Wiemers et al. 1997; Azadi et al. 2002; Nakagawa and O'Leary 2003). Since layer IV neurons are the main postsynaptic targets of thalamocortical neurons, we hypothesized that there might be a causal relationship between the increase in RORβ expression and cortical barrel formation. We find strong evidence for interactions between RORβ expression, thalamocortical innervation, and barrel formation since either surgical or genetic interference with thalamocortical innervation disrupts the normal postnatal increase in layer IV neuron RORβ expression (and barrel formation) (Fig. 2), and RORβ overexpression is sufficient to induce neuronal clusters with features reminiscent of barrels prior to maturation of the thalamocortical system (Fig. 3).
These converging findings suggest a model in which thalamocortical input acts postnatally in an activity-dependent manner to increase baseline levels of RORβ in target neurons in layer IV, which in turn leads to dose-dependent neuronal aggregation and barrel formation. Supporting this interpretation, acute deafferentation of layer IV neurons via infraorbital nerve section affects RORβ expression to a much greater extent than genetically disrupting thalamocortical patterning in “barrelless” Adcy1 loss-of-function mice, suggesting that activity-dependent mechanisms play a critical role in controlling RORβ levels. Interestingly, RORβ expression in layers II/III in the barrelless cortex is comparable to that of the input-deprived cortex, further suggesting that thalamocortical axons may act to maintain and restrict RORβ high expression levels to neurons in layer IV. These experiments also reveal that overexpression of RORβ at high levels in developing cortical neurons obviates the requirement for thalamocortical afferentation and causes neuronal clustering. Interestingly, neurons from caudolateral cortical regions, where sensory areas are located, were the most susceptible to the clustering effects of RORβ overexpression, suggesting either an area-specific heterogeneity in differentiating neurons with regard to clustering, or the presence of non-cell autonomous area-specific factors regulating cluster generation. Finally, in further support of RORβ expression regulating neuronal clustering in a dose-dependent manner, layer Vb neurons normally express RORβ at low levels and are sparsely innervated by collaterals from the VPM thalamocortical axons that innervate layer IV neurons (Jensen and Killackey 1987) but do not display either increase in RORβ expression or clustering upon postnatal afferentation.
Potential Instructive Role for RORβ in the Clustering of Thalamocortical Axon Terminals
Interestingly, our data suggest that, in addition to its permissive role in controlling neuronal clustering in response to presynaptic signals from thalamocortical axons, RORβ likely plays an instructive role in guiding these axon terminals to their proper neuronal targets. Indeed, induced clusters of RORβ-overexpressing neurons are richly innervated by thalamocortical axons, as demonstrated by high-density VGLUT2 immunoreactivity. Remarkably, we demonstrate that this innervation is specific to RORβ-overexpressing neurons since it is not seen with FEZF2 overexpression (Fig. 6). Such reassignment in thalamocortical connectivity has been reported following manipulation of molecular gradients in cortical neurons, most strikingly following ectopic expression of FGF8, which leads to a duplication of the whisker barrel cortex (Fukuchi-Shimogori and Grove 2001) but also after more global shifts in anteroposterior or mediolateral gradients of gene expression. Examples of the latter include the areal shifts in thalamocortical connectivity that we and others have identified with loss of function of Coup-TFI, Bhlhb5, Emx1, or Pax6 function (Stoykova et al. 2000; Bishop et al. 2003; Joshi et al. 2008; Tomassy et al. 2010). Interestingly, in COUP-TFI mutant mice, there is a congruent shift in the pattern of RORβ expression and thalamocortical connectivity of VPM neurons (Armentano et al. 2007), while in FGF8 hypomorphs, in which RORβ expression is unchanged, the topography of projections between the dorsal thalamus and rostral neocortex remains unaffected (Garel et al. 2003). Together, these results strongly support an instructive role for RORβ in thalamocortical axon guidance. Furthermore, these results suggest that the tropism of thalamocortical axons for the induced heterotopic clusters of RORβ-misexpressing neurons reflects erroneous positional information provided to thalamocortical axons by these neuronal clusters.
Remarkably, axons of thalamocortical neurons are able to specifically innervate RORβ-expressing neuronal clusters despite of the heterotopic location of these clusters in the subcortical white matter and deep cortical layers. Similarly, in the Reeler mouse cortex, thalamocortical terminals revealed by VGLUT2 are distributed trough the cortical thickness but focally enriched in a patchy fashion resembling the distribution of RORβ-expressing cells (Wagener et al. 2010).
Interactions with subplate neurons are thought to play a critical role as intermediate targets for thalamocortical axons on their way to their principal neuronal targets in layer IV (McConnell et al. 1989; Allendoerfer and Shatz 1994; Kanold 2009). However, interactions between the subplate and thalamocortical neurons do not appear to play a role in setting the directionality of pathfinding, but, rather, seems to be required to establish responsiveness to intracortical area-specific attractant cues (Molnár et al. 1998; Shimogori and Grove 2005). This is the case in transgenic mice in which subplate neurons are abnormally located in superficial cortical layers, such as after preplate splitting defects, or in the Reeler mouse, in which thalamocortical neurons are still able to reach their normal targets in layer IV (Terashima et al. 1987; Rakic et al. 2006). In agreement with these prior interpretations, although our experiments using DiI anterograde labeling from the thalamus lack the resolution to identify functional thalamocortical synapses onto RORβ-overexpressing neurons, they reveal an abundant network of thalamocortical axons within the subplate (Fig. 5B), where axonal sorting might take place. This subplate sorting process likely accounts for the subsequent normal targeting to neurons in layer IV and abnormal targeting of heterotopic clustered RORβ-overexpressing neurons.
Mechanisms of RORβ Action
The periodic clustering of RORβ-overexpressing neurons and the specific thalamocortical afferentation of these clusters suggest that RORβ functions to regulate cell–cell interactions and axon targeting. In contrast, the heterotopic location of RORβ-overexpressing neurons is likely to be a nonspecific effect on neuronal migration and neuronal differentiation (as seen with the diffuse neuronal heterotopias occurring after overexpression of Fezf2). RORβ might function to regulate receptors and/or ligands involved in cell–cell and axon–cell interactions. For example, EphA receptors and ephrin-A ligands have recently been shown to similarly regulate the lateral dispersion of migrating cortical neurons, and overexpression of EphA7 leads to columnar aggregates of neurons throughout all cortical layers (Torii et al. 2009). Interestingly, another ephrin, Ephrin A5, has been shown to play a role in the guidance of thalamocortical axons toward superficial cortical layers (Maruyama et al. 2008). It would therefore be interesting in future studies to investigate whether RORβ might regulate expression of Eph/Ephrin family members. Interestingly, although the periodic clustering may in principle be initiated at the level of the progenitors, this is not likely a central mechanism since a discontinuous/periodic pattern of staining in the ventricular and subventricular zone, where the progenitors are located, was never observed.
RORα, a related member of the ROR family, is expressed at high levels in Purkinje cells of the developing cerebellum, in which it is critical for not only neuronal differentiation and survival but also for the proper targeting of afferent innervation. Lack of RORα expression leads to the absence of parallel fiber input and the persistence of innervation from multiple climbing fibers (Mariani 1982; Boukhtouche et al. 2006; Janmaat et al. 2009). These RORα results suggest the possibility that regulation of expression of target-derived pathfinding molecules might be a feature shared by multiple ROR family members. Rorβ knockout mice have been reported to display retinal degeneration and a duck-like gait but specific alterations in the connectivity or distribution of cortical neurons have, to the best of our knowledge, not been reported (André, Conquet, et al. 1998; Andre, Gawlas et al. 1998; Jetten and Joo 2006; Masana et al. 2007). Given the high degree of homology between ROR family members, and partially overlapping patterns of expression (RORα is also expressed in the neocortex and thalamus; Nakagawa and O’Leary 2003), compensatory mechanisms might have occluded the consequences of loss of cortical RORβ function in these studies.
In contrast, the gain-of-function strategy used here is comparatively resistant to compensatory up- or downregulation of other genes with similar functions, enabling identification of the function of RORβ in neuronal clustering and barrel formation. Given the potential functional overlap between members of the ROR protein family, combined loss of RORα and RORβ might provide valuable information regarding the molecular mechanisms controlling cortical barrel formation.
In summary, the results reported here demonstrate that the nuclear orphan receptor RORβ functions in the cytoarchitectural patterning of neocortical neurons and guidance of thalamocortical axons to their cellular targets, potentially acting on cortical barrel formation through activity-dependent regulation of expression by these thalamocortical afferents. Future investigation into the transcriptional networks activated by RORβ expression might provide important insights into molecular controls over topographical mapping in the neocortex.
Funding
National Institutes of Health (NIH) (NS49553, NS45523 to J.D.M.; additional infrastructure supported by grant number NS41590 to J.D.M.); Harvard Stem Cell Institute; Spastic Paraplegia Foundation; Travis Roy Foundation; Massachusetts Spinal Cord Injury Research Fund; Jane and Lee Seidman Fund for CNS Research; and Emily and Robert Pearlstein Fund for Nervous System Repair to J.D.M. The Swiss National Science Foundation and Pierre Mercier Science Foundation partially supported D.J.; NIH predoctoral NRSA (F31 NS063516) partially supported S.J.S.; Harvard Stem Cell Institute Internship Program and a Mary Gordon Roberts Fellowship partially supported D.T.; a Caja Madrid Foundation fellowship partially supported M.J.G.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Acknowledgments
We thank L. Pasquina, D. Schuback, E. Sievert, and T. Yamamoto for superb technical assistance; L. Frangeul for excellent technical and scientific assistance; Dr E. Welker for generous sharing of the “barrelless” mice; and past and present members of our laboratory for helpful suggestions. Conflict of Interest : None declared.
References
- Abdel-Majid RM, Leong WL, Schalkwyk LC, Smallman DS, Wong ST, Storm DR, Fine A, Dobson MJ, Guernsey DL, Neumann PE. Loss of adenylyl cyclase I activity disrupts patterning of mouse somatosensory cortex. Nat Genet. 1998;19:289–291. doi: 10.1038/980. [DOI] [PubMed] [Google Scholar]
- Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Fariñas I, Grosschedl R, McConnell SK. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–377. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- André E, Conquet F, Steinmayr M, Stratton SC, Porciatti V, Becker-Andre M. Disruption of retinoid-related orphan receptor beta changes circadian behavior, causes retinal degeneration and leads to vacillans phenotype in mice. EMBO J. 1998;17:3867–3877. doi: 10.1093/emboj/17.14.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André E, Gawlas K, Becker-Andre M. A novel isoform of the orphan nuclear receptor RORbeta is specifically expressed in pineal gland and retina. Gene. 1998;216:277–283. doi: 10.1016/s0378-1119(98)00348-5. [DOI] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Jabaudon D, Yoshida Y, Macklis JD. Ctip2 controls the differentiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. J Neurosci. 2008;28:622–632. doi: 10.1523/JNEUROSCI.2986-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentano M, Chou S-J, Tomassy GS, Leingartner A, O'Leary DDM, Studer M. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10:1277–1286. doi: 10.1038/nn1958. [DOI] [PubMed] [Google Scholar]
- Azadi S, Zhang Y, Caffé AR, Holmqvist B, van Veen T. Thyroid-beta2 and the retinoid RAR-alpha, RXR-gamma and ROR-beta2 receptor mRNAs; expression profiles in mouse retina, retinal explants and neocortex. Neuroreport. 2002;13:745–750. doi: 10.1097/00001756-200205070-00003. [DOI] [PubMed] [Google Scholar]
- Azim E, Jabaudon D, Fame RM, Macklis JD. SOX6 controls dorsal progenitor identity and interneuron diversity during neocortical development. Nat Neurosci. 2009;12:1238–1247. doi: 10.1038/nn.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim E, Shnider GJ, Cederquist GY, Sohur US, Macklis JD. Lmo4 and Clim1 progressively delineate cortical projection neuron subtypes during development. Cer Cortex. 2009;19(Suppl 1):i62–i69. doi: 10.1093/cercor/bhp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop KM, Garel S, Nakagawa Y, Rubenstein JLR, O'Leary DDM. Emx1 and Emx2 cooperate to regulate cortical size, lamination, neuronal differentiation, development of cortical efferents, and thalamocortical pathfinding. J Comp Neurol. 2003;457:345–360. doi: 10.1002/cne.10549. [DOI] [PubMed] [Google Scholar]
- Boukhtouche F, Janmaat S, Vodjdani G, Gautheron V, Mallet J, Dusart I, Mariani J. Retinoid-related orphan receptor alpha controls the early steps of Purkinje cell dendritic differentiation. J Neurosci. 2006;26:1531–1538. doi: 10.1523/JNEUROSCI.4636-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britanova O, de Juan Romero C, Cheung AFP, Kwan KY, Schwark M, Gyorgy A, Vogel T, et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57(3):378–392. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Chen B, Wang SS, Hattox AM, Rayburn H, Nelson SB, McConnell SK. The Fezf2-Ctip2 genetic pathway regulates the fate choice of subcortical projection neurons in the developing cerebral cortex. Proc Natl Acad Sci U S A. 2008;105:11382–11387. doi: 10.1073/pnas.0804918105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Kind PC. Neural activity: sculptor of ‘barrels' in the neocortex. Trends Neurosci. 2001;24:589–595. doi: 10.1016/s0166-2236(00)01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama F, Furuta T, Kaneko T. Immunocytochemical localization of candidates for vesicular glutamate transporters in the rat cerebral cortex. J Comp Neurol. 2001;435:379–387. doi: 10.1002/cne.1037. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- Garel S, Huffman KJ, Rubenstein JL. Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development. 2003;130:1903–1914. doi: 10.1242/dev.00416. [DOI] [PubMed] [Google Scholar]
- Gitton Y, Cohen-Tannoudji M, Wassef M. Role of thalamic axons in the expression of H-2Z1, a mouse somatosensory cortex specific marker. Cereb Cortex. 1999;9:611–620. doi: 10.1093/cercor/9.6.611. [DOI] [PubMed] [Google Scholar]
- Janmaat S, Frederic F, Sjollema K, Luiten P, Mariani J, van der Want J. Formation and maturation of parallel fiber-Purkinje cell synapses in the Staggerer cerebellum ex vivo. J Comp Neurol. 2009;512:467–477. doi: 10.1002/cne.21910. [DOI] [PubMed] [Google Scholar]
- Jensen KF, Killackey HP. Terminal arbors of axons projecting to the somatosensory cortex of the adult rat. I. The normal morphology of specific thalamocortical afferents. J Neurosci. 1987;7:3529–3543. doi: 10.1523/JNEUROSCI.07-11-03529.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten A, Joo J. Retinoid-related orphan receptors (RORs): roles in cellular differentiation and development. Adv Dev Biol. 2006;16:313–355. doi: 10.1016/S1574-3349(06)16010-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi PS, Molyneaux BJ, Feng L, Xie X, Macklis JD, Gan L. Bhlhb5 regulates the postmitotic acquisition of area identities in layers II-V of the developing neocortex. Neuron. 2008;60:258–272. doi: 10.1016/j.neuron.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold P. Subplate neurons: crucial regulators of cortical development and plasticity. Front Neuroanat. 2009;3:16. doi: 10.3389/neuro.05.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer L. The magnificent compromise: cortical field evolution in mammals. Neuron. 2007;56:201–208. doi: 10.1016/j.neuron.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Lai T, Jabaudon D, Molyneaux BJ, Azim E, Arlotta P, Menezes JRL, Macklis JD. SOX5 controls the sequential generation of distinct corticofugal neuron subtypes. Neuron. 2008;57:232–247. doi: 10.1016/j.neuron.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Liguz-Lecznar M, Skangiel-Kramska J. Vesicular glutamate transporters VGLUT1 and VGLUT2 in the developing mouse barrel cortex. Int J Dev Neurosci. 2007;25:107–114. doi: 10.1016/j.ijdevneu.2006.12.005. [DOI] [PubMed] [Google Scholar]
- López-Bendito G, Molnár Z. Thalamocortical development: how are we going to get there? Nat Rev Neurosci. 2003;4:276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- Mallamaci A, Stoykova A. Gene networks controlling early cerebral cortex arealization. Eur J Neurosci. 2006;23:847–856. doi: 10.1111/j.1460-9568.2006.04634.x. [DOI] [PubMed] [Google Scholar]
- Mariani J. Extent of multiple innervation of Purkinje cells by climbing fibers in the olivocerebellar system of weaver, reeler, and staggerer mutant mice. J Neurobiol. 1982;13:119–126. doi: 10.1002/neu.480130204. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Matsuura M, Suzuki K, Yamamoto N. Cooperative activity of multiple upper layer proteins for thalamocortical axon growth. Dev Neurobiol. 2008;68:317–331. doi: 10.1002/dneu.20592. [DOI] [PubMed] [Google Scholar]
- Masana MI, Sumaya IC, Becker-Andre M, Dubocovich ML. Behavioral characterization and modulation of circadian rhythms by light and melatonin in C3H/HeN mice homozygous for the RORbeta knockout. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2357–R2367. doi: 10.1152/ajpregu.00687.2006. [DOI] [PubMed] [Google Scholar]
- McConnell SK, Ghosh A, Shatz CJ. Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science. 1989;245:978–982. doi: 10.1126/science.2475909. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Adams R, Blakemore C. Mechanisms underlying the early establishment of thalamocortical connections in the rat. J Neurosci. 1998;18:5723–5745. doi: 10.1523/JNEUROSCI.18-15-05723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Fame RM, MacDonald JL, MacQuarrie KL, Macklis JD. Novel subtype-specific genes identify distinct subpopulations of callosal projection neurons. J Neurosci. 2009;29:12343–12354. doi: 10.1523/JNEUROSCI.6108-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–831. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JRL, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, O'Leary DD. Dynamic patterned expression of orphan nuclear receptor genes RORalpha and RORbeta in developing mouse forebrain. Dev Neurosci. 2003;25:234–244. doi: 10.1159/000072271. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hioki H, Fujiyama F, Kaneko T. Postnatal changes of vesicular glutamate transporter (VGluT)1 and VGluT2 immunoreactivities and their colocalization in the mouse forebrain. J Comp Neurol. 2005;492:263–288. doi: 10.1002/cne.20705. [DOI] [PubMed] [Google Scholar]
- O'Leary DD. Do cortical areas emerge from a protocortex? Trends Neurosci. 1989;12:400–406. doi: 10.1016/0166-2236(89)90080-5. [DOI] [PubMed] [Google Scholar]
- O'Leary DD, Chou SJ, Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56:252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- O'Leary DDM, Sahara S. Genetic regulation of arealization of the neocortex. Curr Opin Neurobiol. 2008;18:90–100. doi: 10.1016/j.conb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdinler PH, Macklis JD. IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat Neurosci. 2006;9:1371–1381. doi: 10.1038/nn1789. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rakic P, Ayoub AE, Breunig JJ, Dominguez MH. Decision by division: making cortical maps. Trends Neurosci. 2009;32:291–301. doi: 10.1016/j.tins.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic S, Davis C, Molnar Z, Nikolic M, Parnavelas JG. Role of p35/Cdk5 in preplate splitting in the developing cerebral cortex. Cereb Cortex. 2006;16(Suppl 1):i35–i45. doi: 10.1093/cercor/bhj172. [DOI] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, André E, Kapfhammer JP, Becker-Andre M. The expression pattern of the orphan nuclear receptor RORbeta in the developing and adult rat nervous system suggests a role in the processing of sensory information and in circadian rhythm. Eur J Neurosci. 1997;9:2687–2701. doi: 10.1111/j.1460-9568.1997.tb01698.x. [DOI] [PubMed] [Google Scholar]
- Schubert D, Kötter R, Staiger JF. Mapping functional connectivity in barrel-related columns reveals layer- and cell type-specific microcircuits. Brain Struct Funct. 2007;212:107–119. doi: 10.1007/s00429-007-0147-z. [DOI] [PubMed] [Google Scholar]
- Shimogori T, Grove EA. Fibroblast growth factor 8 regulates neocortical guidance of area-specific thalamic innervation. J Neurosci. 2005;25:6550–6560. doi: 10.1523/JNEUROSCI.0453-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler O, Tavitian B, Moya KL. Differential synaptic vesicle protein expression in the barrel field of developing cortex. J Comp Neurol. 1996;375:321–332. doi: 10.1002/(SICI)1096-9861(19961111)375:2<321::AID-CNE10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Stoykova A, Treichel D, Hallonet M, Gruss P. Pax6 modulates the dorsoventral patterning of the mammalian telencephalon. J Neurosci. 2000;20:8042–8050. doi: 10.1523/JNEUROSCI.20-21-08042.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima T, Inoue K, Inoue Y, Mikoshiba K. Thalamic connectivity of the primary motor cortex of normal and reeler mutant mice. J Comp Neurol. 1987;257:405–421. doi: 10.1002/cne.902570309. [DOI] [PubMed] [Google Scholar]
- Tomassy GS, De Leonibus E, Jabaudon D, Lodato S, Alfano C, Mele A, Macklis JD, Studer M. Area-specific temporal control of corticospinal motor neuron differentiation by COUP-TFI. Proc Natl Acad Sci U S A. 2010;107:3576–3581. doi: 10.1073/pnas.0911792107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M, Hashimoto-Torii K, Levitt P, Rakic P. Integration of neuronal clones in the radial cortical columns by EphA and ephrin-A signalling. Nature. 2009;461:524–528. doi: 10.1038/nature08362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Loos H, Woolsey TA. Somatosensory cortex: structural alterations following early injury to sense organs. Science. 1973;179:395–398. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- Wagener RJ, David C, Zhao S, Haas CA, Staiger JF. The somatosensory cortex of reeler mutant mice shows absent layering but intact formation and behavioral activation of columnar somatotopic maps. J Neurosci. 2010;30:15700–15709. doi: 10.1523/JNEUROSCI.3707-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker E, Armstrong-James M, Bronchti G, Ourednik W, Gheorghita-Baechler F, Dubois R, Guernsey DL, Van der Loos H, Neumann PE. Altered sensory processing in the somatosensory cortex of the mouse mutant barrelless. Science. 1996;271:1864–1867. doi: 10.1126/science.271.5257.1864. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Welt C. Histochemical changes in cytochrome oxidase of cortical barrels after vibrissal removal in neonatal and adult mice. Proc Natl Acad Sci U S A. 1980;77:2333–2337. doi: 10.1073/pnas.77.4.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.