Abstract
Involuntary visual spatial attention is captured when a salient cue appears in the visual field. If a target appears soon after the cue, response times to targets at the cue location are faster relative to other locations. However, after longer cue–target intervals, responses to targets at the cue location are slower, due to inhibition of return (IOR). IOR depends on striatal dopamine (DA) levels: It varies with different alleles of the DA transporter gene DAT1 and is reduced in patients with Parkinson’s disease, a disease characterized by reduced striatal dopaminergic transmission. We examined the role of DA in involuntary attention and IOR by administering the DA D2 receptor-specific agonist bromocriptine to healthy human subjects. There was no effect of either DAT1 genotype or bromocriptine on involuntary attention, but participants with DAT1 alleles predicting higher striatal DA had a larger IOR. Furthermore, bromocriptine increased the magnitude of IOR in participants with low striatal DA but abolished the IOR in subjects with high striatal DA. This inverted U-shaped pattern resembles previously described relationships between DA levels and performance on cognitive tasks and suggests an involvement of striatal DA in IOR that does not include a role in involuntary attention.
Keywords: DAT1, dopamine, inhibition of return, striatum, visual attention
Introduction
When a salient event occurs in the visual field, involuntary visual spatial attention is captured at that location (Yantis and Jonides 1990). As a consequence, performance on discrimination tasks is facilitated and response times (RTs) are faster when a target appears in the cued location, relative to other locations (Prinzmetal et al. 2005). This effect of cueing, due to capture of involuntary attention, develops quickly but is transient (Posner and Cohen 1984). On the other hand, with longer delays between cue and target, involuntary attention dissipates before target presentation, and the opposite effect is observed: RTs are faster for targets presented at noncued locations, relative to the cue location (Posner and Cohen 1984). This phenomenon is known as the inhibition of return (IOR; for a review, see Klein (2000)).
The physiological mechanisms underlying the IOR are only partially understood. Brain imaging studies suggest that IOR involves frontal and posterior parietal cortical regions (Lepsien and Pollmann 2002; Mayer et al. 2004). However, other results implicate subcortical structures in IOR (Sapir et al. 1999; Fecteau and Munoz 2005). Although physiological (Fecteau and Munoz 2005), neuropsychological (Sapir et al. 1999), and behavioral (Ro and Rafal 1999) studies suggest that involuntary attention and IOR can occur independently, it is unclear to what extent these 2 phenomena depend on the same neural substrates.
Here, we examined the role of the neurotransmitter dopamine (DA) in both involuntary attention and IOR. DA is involved in a variety of cognitive functions, and 3 lines of evidence suggest that it also modulates IOR. First, patients with Parkinson’s disease (PD), a disease characterized by reduced dopaminergic transmission in the striatum, have reduced IOR magnitude, relative to healthy controls (Filoteo et al. 1997; Yamaguchi and Kobayashi 1998; Possin et al. 2009). Second, even in healthy individuals, genetic differences in striatal DA transmission predict differences in IOR. In particular, the gene DAT1 codes for a DA transporter which facilitates reuptake of DA in the striatum (Sesack et al. 1998), and this gene has different alleles that are associated with different levels of DA clearance from synapses (Mill et al. 2002). Subjects with a DAT1 allele that predicts higher levels of striatal DA have a larger IOR for short cue–target intervals (less than 750 ms), relative to subjects with a DAT1 allele that predicts lower levels of striatal DA (Colzato et al. 2010). Finally, DA D2 receptors (DRD2) are enriched in the human striatum (Camps et al. 1989; Meador-Woodruff et al. 1996), and long-term cocaine use, which leads to reductions in DRD2 (Volkow et al. 1999), abolishes the IOR (Colzato and Hommel 2009).
Taken together, these results suggest that increased striatal DA transmission is associated with larger IOR. In order to delineate a causal role of striatal DA transmission in the IOR, pharmacological methods can be used. A previous study has shown that the temporal extent of the IOR is increased in a dose-dependent manner by the administration of d-amphetamine (Fillmore et al. 2005), a drug that increases extracellular DA levels. However, the actions of d-amphetamine are not specific to a particular type of DA receptor. Moreover, d-amphetamine also increases levels of extracellular noradrenaline in the central nervous system (Heal et al. 2009).
In the present study, we administered the DRD2-specific agonist bromocriptine to a group of young healthy participants and tested their performance in a cued visual discrimination task. Bromocriptine is used to treat PD (Radad et al. 2005), and in healthy young participants, it can increase performance on tasks requiring spatial working memory (reviewed in Mehta and Riedel (2006)). However, these findings are mixed, with some studies failing to replicate increased spatial working memory performance following bromocriptine administration or replicating them only at lower doses. One possible explanation of these discrepancies is the inverted U-shaped effect of DA transmission on cognitive functions that has been observed in several different contexts (Cools and Robbins 2004; Seamans and Yang 2004; Cools and D'Esposito 2011), including following bromocriptine administration (Kimberg et al. 1997; Cools et al. 2007, 2009). Based on the inverted U-shaped effects of DA, we predicted that participants would respond to pharmacological activation of DRD2 in a non-monotonic fashion, depending on their genetic background. Specifically, we predicted that participants with low baseline levels of striatal DA would show an increase in the magnitude of the IOR following bromocriptine administration, whereas bromocriptine would decrease IOR magnitude in participants with high baseline levels of striatal DA.
Materials and Methods
Subjects
Twenty-one healthy adults (11 females; age: 19.9 ± 1.7) participated in the study. The experimental procedures were approved by the Committee for the Protection of Human Subjects at the University of California, Berkeley, and all experiments were conducted with the written consent of each subject. Two subjects experienced adverse effects of the drug and did not complete the task.
Task
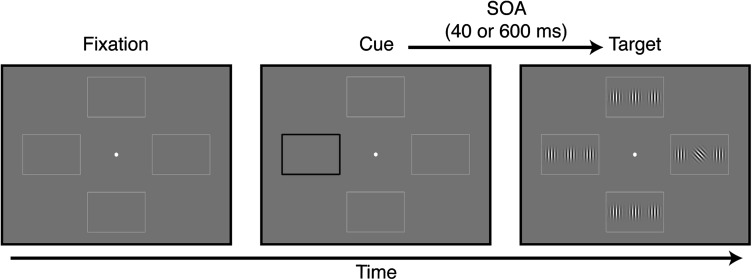
Each trial began with a 200 ms cue: one of the peripheral rectangular frames (Fig. 1) became black and thicker and, after a variable stimulus onset asynchrony (SOA), a target display appeared for 240 ms. The target display contained 12 Gabor patches (100% contrast, spatial frequency: 3 cycles/degree of visual angle; space constant: 1 degree of visual angle), 3 within each frame. The target (always the central of the 3 Gabor patches) was tilted ±45° away from vertical, and all other patches were vertically oriented (Rokem et al. 2010). The tilted grating appeared in one of the 4 locations with equal probability (25% of the trials), independent of the cue location, and subjects were told that the cue did not contain any information about the subsequent target location. Subjects reported the direction of tilt of the target by pressing one of 2 buttons as quickly and accurately as they could. Auditory feedback on performance was provided at the end of each trial. In different blocks, the SOA between cue and target appearance was either 40 or 600 ms. SOA blocks were interleaved, and the order was counterbalanced between subjects, such that all combinations of order of SOA and order of drug administration were approximately equally represented.
Figure 1.
Visual cueing task. At the beginning of each trial, one of the 4 peripheral rectangular frames became black and thicker. Following a SOA of either 40 or 600 ms, the target appeared in one of the 4 locations (25% probability at each location). The target was a Gabor patch oriented ±45° relative to vertical. Subjects indicated target orientation as quickly and accurately as they could by pressing one of 2 buttons.
Procedure
A crossover design was employed: each subject received placebo prior to one experimental session and 1.25 mg bromocriptine prior to the other. Drug administration was double blind. Testing was conducted approximately 3–4 h after bromocriptine administration (drug plasma levels peak ca. 100 min after oral administration and remain significantly elevated for several hours; Price et al. (1978)). Subjects first conducted a brief block of training (20 trials) to acquaint them with the task, followed by 4 blocks of 80 trials each. They were instructed to fixate on a central point, and eye movements were monitored using a camera placed in front of their eyes. Auditory feedback was provided at the end of a trial if fixation was not maintained, and trials containing eye movements were excluded from further analysis. The proportion of trials in which eye movements occurred was low (ca. 0.3% of all trials) and did not differ between drug and placebo sessions (F1,15 = 0.21, P = 0.65).
Genetic Testing
Using the Oragene DNA Self-Collection Kit (DNA Genotek Inc., Ottawa, Ontario, Canada), we collected saliva samples from each subject, and the variable number of tandem repeats (VNTR) for the DAT1 gene was determined by polymerase chain reaction, using primers designed specifically for the 40-bp VNTR polymorphism in the 3′-untranslated region (Creative Genomics, Port Jefferson Station, NY). Of the 19 subjects that completed the study, 10 were homozygous for the 10-repeat allele of this gene (10R), 8 were heterozygous (one copy each of the 10-repeat and 9-repeat alleles), and 1 was homozygous for the 9-repeat (9R) allele. Following Colzato et al. (2010), subjects carrying at least one copy of the 9R allele were grouped together and referred to collectively as 9R.
Analysis
Correct responses were well above 90% in all conditions, and there was no main effect of the drug on the percentage of correct responses (F1,15 = 0.97, P = 0.34). RTs from incorrect trials and trials with RTs faster than 100 ms or slower than 1500 ms were excluded from the analysis, as were trials with RTs more than 3 standard deviations (SDs) away from each participant’s mean performance in a given condition (combination of drug/SOA/target location for that subject).
Results
In order to measure behavioral effects of the allocation of involuntary attention and the IOR, we measured RT in a visual discrimination task. In each trial, a cue appeared with equal probability in one of 4 locations in the visual field (Fig. 1). The location of the cue was not predictive of subsequent target location, which was also randomly selected on each trial. The effects of cueing were assessed by comparing RTs from trials in which the target appeared in the cue location (25% of trials) with RTs from trials in which targets appeared in other locations (75% of trials). Separate sets of blocks with different cue-to-target SOAs were used to assess involuntary attention and IOR. In half of the blocks, a 40 ms SOA was used, consistent with the SOA required for allocation of involuntary attention (Posner and Cohen 1984). In the other half of the blocks, a 600 ms SOA was used, consistent with the time course of the IOR (Posner and Cohen 1984). To test the effects of the DRD2 agonist bromocriptine, we employed a placebo-controlled, double blind crossover design in which each subject participated in 2 sessions: one with a placebo and the other after taking a pill containing bromocriptine.
In order to assess the effects of cueing, SOA and bromocriptine, we conducted a mixed-model analysis of variance (ANOVA) on mean RTs, with target location (cued vs. other), SOA (short vs. long), and drug (bromocriptine vs. placebo) as within-subject factors. In addition, DAT1 genotype (10R, or low striatal DA, vs. 9R, or high striatal DA) was also entered as a between-subjects factor in the ANOVA to test the effects of individual differences in baseline striatal DA levels. Finally, to account for effects of learning between the 2 sessions, order of drug administration (bromocriptine first vs. placebo first) was also entered as a between-subjects factor.
We did not find a main effect of bromocriptine on RT (F1,15 = 0.01, P = 0.9), suggesting that the drug did not have an overall effect on motor response or arousal. In addition, there was no main effect of DAT1 genotype on RT (F1,15 = 1.41, P = 0.25), suggesting that overall task performance was not determined by baseline striatal DA levels. However, there was a significant interaction of the effects of drug and order of drug administration (F1,15 = 23.23, P < 0.01). Specifically, if participants were administered placebo in the first session and bromocriptine in the second session, they were faster in the bromocriptine session. If they were administered bromocriptine and then placebo, they were faster in the placebo session. This suggests that participants’ performance improved through their experience with the task and that they were generally faster in the second testing session, regardless of whether bromocriptine or placebo was administered in this session. We controlled for this order effect by counterbalancing the order of drug and placebo sessions between subjects.
Collapsing across both SOA conditions and drug and placebo sessions, we found no main effect of target location (cued vs. other, F1,15 = 2.95, P = 0.1). However, there was a main effect of SOA (F1,15 = 6.60, P < 0.05): subjects were faster in 600 ms compared with 40 ms SOA blocks. This reflects the fact that subjects have more time to prepare their response in long SOA trials. In addition, there was a significant interaction of target location and SOA (F1,15 = 14.37, P < 0.01), indicating that, as predicted, the cue had opposite effects in the 2 SOA conditions. Specifically, RTs were faster for trials in which the cue and target location were the same for the short SOA condition (involuntary attention), but they were slower for these trials in the long SOA condition (IOR). Therefore, we will separately examine the effects observed in each SOA condition.
Short SOA: Involuntary Attention Is Unaffected by Striatal DA Transmission
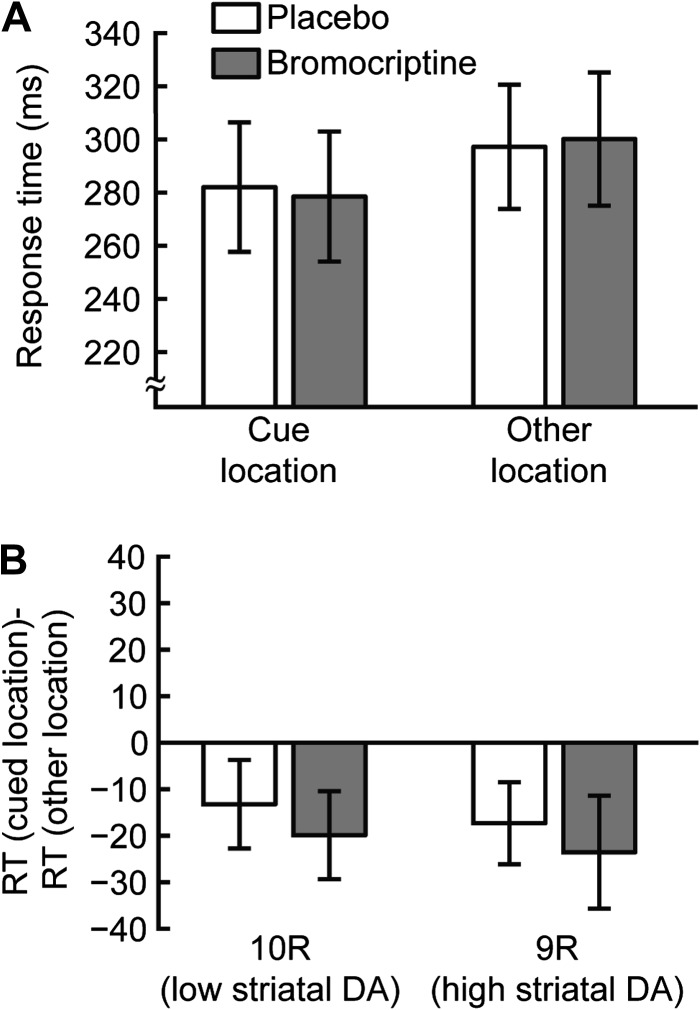
In 40 ms SOA trials, capture of involuntary attention occurred at the cue location, and RTs (placebo sessions, combining both genotype groups) were faster when the target appeared in this cued location versus other locations (cue: 282 ms, SD 103; other: 297 ms SD 99, Fig. 2A). As there is substantial between-subject variance in mean RT in these measurements, we computed within-subject cueing effects in order to eliminate variability due to overall RT differences between subjects. The average cueing effect, defined as the difference in RT between trials in which the target was in the cue location and trials in which the target appeared in one of the other locations, was significantly less than zero (−15 ms, within-subject 2-tailed t-test: t18 = 2.38, P < 0.05) and was negative for 14 of the 19 subjects in the placebo sessions. In the bromocriptine sessions, subjects were also faster to respond to targets presented at the cued location than other locations (cue location: 278 ms, SD 104, other locations: 300 ms, SD 106, Fig. 2A), again resulting in a significant cueing effect due to involuntary attention (−22 ms, within-subject 2-tailed t-test: t18 = 2.92, P < 0.01). There was no significant difference between the cueing effect observed in the placebo sessions and the cueing effect in the bromocriptine sessions (within-subject 2-tailed t-test: t18 = 0.88, P = 0.39). Additionally, involuntary attention cueing effects were not significantly different for 9R and 10R subjects in either placebo (9R: −17 ms, 10R: −13 ms, t17 = 0.3, P = 0.76) or bromocriptine (9R: −24 ms, 10R: −20 ms, t17 = 0.57, P = 0.58, Fig. 2B) sessions. We conclude that differences in DA transmission in the striatum, resulting either from individual genetic differences or from bromocriptine administration, do not affect involuntary attention.
Figure 2.
Short SOA blocks. (A) Average RTs for 40 ms SOA blocks. RTs are shown for trials in which the target appeared in the cue location (25% of trials, left) and for trials in which the target appeared in one of the other locations (75% of trials, right). (B) The cueing effect is defined for each subject as the mean RT of trials in which the target appeared in the cue location minus the mean RT of trials in which the target appeared in one of the other locations. Cueing effects are shown separately for 10R (left) and 9R subjects (right) and for placebo (white) and bromocriptine (gray) sessions. The negative values indicate a reduction in RT for trials in which the target appeared in the cue location, reflecting capture of involuntary attention. This involuntary attention effect was not affected by either bromocriptine administration or DAT1 genotype. Error bars are standard error of the mean within group/condition.
Long SOA: IOR Depends on Bromocriptine Administration and Baseline Levels of Striatal DA
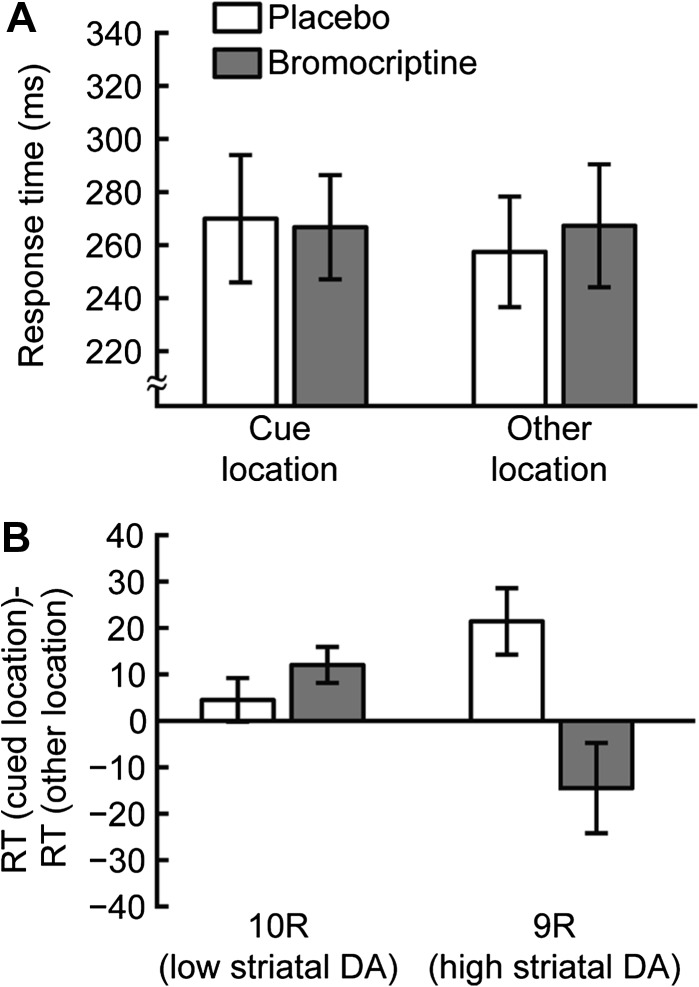
In 600 ms SOA blocks (placebo sessions, combining both genotype groups), RTs were slower when the target appeared in the cue location compared with one of the other locations (cue: 267 ms, SD 101; other: 257 ms, SD 89, Fig. 3A). This resulted in a significant mean positive cueing effect of 13 ms (within-subject 2-tailed t-test: t18 = 2.76, P < 0.05), indicating IOR (Posner and Cohen 1984). This positive cueing effect was found in 13 of 19 subjects in the placebo sessions. Differences in striatal DA transmission, as indicated by subjects’ DAT1 genotype, have previously been found to predict differences in IOR for SOAs less than 750 ms (Colzato et al. 2010). We also found a significant difference of DAT1 genotype on the magnitude of the IOR in the placebo sessions, with 9R participants (higher striatal DA) having a greater IOR (9R: 21 ms, 10R: 5 ms, between-subject one-tailed t-test: t17 = 2.02, P < 0.05).
Figure 3.
Long SOA blocks. (A) Average RTs for 600 ms SOA blocks. RTs are shown for the cue location (left) and other locations (right) for sessions in which placebo (white) or bromocriptine (gray) was administered. (B) Cueing effects. Data are plotted separately for 10R (left) and 9R subjects (right) for sessions in which placebo (white) or bromocriptine (gray) was administered. Positive values indicate IOR. In placebo sessions, 9R subjects had greater IOR than 10R subjects. Bromocriptine increased IOR in 10R participants and abolished IOR in 9R participants. Error bars are standard error of the mean within group/condition.
In the bromocriptine session, the RT in 600 ms SOA blocks for all subjects was not significantly different for cue versus other target locations (cue: 266 ms, SD 84; other: 267 ms, SD 98; cueing effect: 1 ms, within-subject 2-tailed t-test: t18 = 0.1, P = 0.93, Fig. 3A). However, when the cueing effects were separately analyzed for the 2 DAT1 genotypes, significant differences were found, indicating that bromocriptine had different effects on IOR, depending on DAT1 genotype (drug by target location by DAT1 interaction: F1,15 = 4.85, P < 0.05; drug by target location by SOA by DAT1 interaction: F1,15 = 4.85, P < 0.05). Specifically, the 10R subjects (lower striatal DA) showed a numerically higher IOR in bromocriptine compared with placebo sessions, although this was not statistically significant (placebo: 5 ms, bromocriptine: 12 ms, within-subject 2-tailed t-test: t9 = 1.25, P = 0.24, Fig. 3B). On the other hand, participants with the 9R allele of DAT1 (higher striatal DA) exhibited larger IOR than 10R subjects under placebo (see above) as well as a significant decrease in the magnitude of IOR following bromocriptine administration (placebo: 21 ms, bromocriptine: −15 ms, within-subject 2-tailed t-test: t8 = 2.88, P < 0.05, Fig. 3B).
To summarize the results, although an involuntary attention cueing effect was observed with a short SOA, we did not find any effects of either bromocriptine administration or DAT1 genotype on involuntary attention. In addition, we replicated previous results (Colzato et al. 2010) showing that for an SOA of 600 ms, IOR is larger for 9R (higher striatal DA) subjects than for 10R (lower striatal DA) subjects. Moreover, bromocriptine administration had differential effects on IOR, depending on DAT1 genotype: bromocriptine increased IOR in 10R subjects and abolished it in 9R subjects.
Discussion
The neurotransmitter DA is involved in a variety of cognitive functions through its activity in multiple brain areas, including the prefrontal cortex (PFC) and striatum (Cools and Robbins 2004; Cools and D'Esposito 2009). In this study, we focused on the role of DA transmission in the striatum in modulating visual discrimination performance following a nonpredictive cue. This type of cue leads to capture of involuntary attention for short cue-to-target SOAs and causes IOR for longer SOAs (Posner and Cohen 1984).
We manipulated striatal DA transmission by administering the DRD2 receptor–specific agonist bromocriptine to healthy participants. DRD2 levels are much higher in striatum than in other parts of the human brain, including PFC, where DRD1 is more abundant (Camps et al. 1989; Meador-Woodruff et al. 1996). A non-monotonic effect of DA transmission levels on cognitive functions has been identified in many different contexts (Cools and Robbins 2004; Seamans and Yang 2004), and the effects of bromocriptine on a variety of measures can be described by an inverted U-shaped function of DA levels (Cools and D'Esposito 2011). Thus, bromocriptine benefits performance on cognitive tasks in subjects with low memory spans (who have lower baseline striatal DA; Cools et al. (2008)), while subjects with high memory spans are impaired on these tasks following bromocriptine administration (Kimberg et al. 1997). Similarly, bromocriptine reduces the behavioral costs of task switching and their neural correlates in the striatum in high-impulsive but not in low-impulsive subjects (Cools et al. 2007), and impulsivity is a personality trait that is linked to low-binding availability of striatal DA D2/D3 receptors (Dalley et al. 2007). In addition, an inverted U-shaped curve accounts for the differential effects of bromocriptine on reversal learning as a function of baseline striatal DA synthesis capacity, as measured using positron emission tomography (Cools et al. 2009). These findings suggest that cognitive functions are most efficiently performed at intermediate levels of striatal DA activation and that higher or lower levels of DA transmission at striatal synapses may lead to suboptimal performance.
In order to examine individual differences in drug effects as a function of baseline striatal DA levels, we determined the genotype of DAT1, a DA transporter that is enriched in the striatum (Sesack et al. 1998), in every subject. Replicating previous results (Colzato et al. 2010), we found that carriers of the 9R allele (higher striatal DA) have a larger IOR in 600 ms SOA blocks. In addition, we found a differential effect of bromocriptine, resulting in reduced IOR in 9R subjects and increased IOR in 10R subjects. These results are consistent with an inverted U-shaped function of the effects of striatal DA transmission on IOR magnitude.
Brain imaging studies suggest that IOR involves regions of the frontal and posterior parietal cerebral cortex (Lepsien and Pollmann 2002; Mayer et al. 2004), but the pattern of deficits in a human patient with a focal midbrain lesion (Sapir et al. 1999), as well as electrophysiological evidence from non-human primates (Dorris et al. 2002; Fecteau and Munoz 2005), suggest that the IOR is also mediated by the superior colliculus (SC), a brainstem structure involved in oculomotor control and in allocation of visual spatial attention (Cavanaugh and Wurtz 2004). Furthermore, functional imaging studies have shown that bromocriptine modulates striatal and prefrontal cortical activity (Cools et al. 2007) as well as the functional connectivity between these regions (Wallace et al. 2011). Therefore, despite the systemic administration of bromocriptine in our study, the known anatomical distribution of DRD2, the observed effects of DAT1 genotype, and previous functional magnetic resonance imaging results are all consistent with a striatal site of action of this drug.
The evidence presented above suggests that both the SC and the striatum are components of the neural circuit mediating the IOR. Striatal signals are known to influence activity in the SC (Hikosaka et al. 2000). A substantial proportion of neurons in the caudate nucleus exhibit spatially specific responses to visual stimuli, eye movements, and allocation of attention (Hikosaka et al. 1989). These caudate neurons affect SC activity through 2 parallel pathways: one that excites SC neurons by disinhibition via the substantia nigra pars reticulata and the other inhibitory to the SC, via the external globus pallidus. The balance between these 2 pathways is regulated by the relative levels of activation of DRD1 (excitatory pathway) and DRD2 (inhibitory pathway) in the caudate by projections from the substantia nigra pars compacta (Gerfen and Surmeier 2011). Importantly, nigrostriatal projections are affected in PD (Davie 2008). Previous studies in patients with PD have shown that for short cue–target intervals, no impairment is observed in the allocation of visual spatial attention (Rafal et al. 1984). However, longer intervals reveal reduced magnitude of IOR in patients with PD (Filoteo et al. 1997; Yamaguchi and Kobayashi 1998; Possin et al. 2009).
We propose that for subjects with low baseline striatal DA levels, increasing DRD2 transmission with bromocriptine enhances caudate-mediated inhibition of the SC, thereby increasing IOR magnitude. However, DRD2 are also located presynaptically in nigrostriatal projections, where they function as autoreceptors and negatively regulate DA release in striatum (Gonon and Buda 1985). One possibility is that for subjects with high baseline striatal DA levels, further increase of DRD2 signaling by bromocriptine could reduce striatal DA release via nigrostriatal autoreceptors. Indeed, behavioral and microdialysis studies in rats suggest that under conditions of low extracellular DA in the striatum, bromocriptine acts mainly on postsynaptic DRD2, while in the presence of high striatal extracellular DA, bromocriptine has primarily presynaptic DRD2 effects (Maruya et al. 2003). Such a differential activation of pre- and postsynaptic DRD2 by bromocriptine, depending on baseline striatal DA levels, could account for the inverted U-shaped effects of striatal DA on IOR that we have observed.
Finally, we found that involuntary attention cueing effects were not affected by either bromocriptine administration or DAT1 genotype, suggesting that involuntary attention is not likely to be substantially influenced by striatal DA transmission. These results provide further evidence that the allocation of involuntary attention and the IOR rely on different neural mechanisms.
Funding
National Institutes of Health (R01-DA20600 to M.D. and NEI CORE grant EY003176).
Acknowledgments
Jon Kelvey and Alexandra Carstensen helped collect the data. Conflict of Interest: None declared.
References
- Camps M, Cortés R, Gueye B, Probst A, Palacios JM. Dopamine receptors in human brain: autoradiographic distribution of D2 sites. Neuroscience. 1989;28:275–290. doi: 10.1016/0306-4522(89)90179-6. [DOI] [PubMed] [Google Scholar]
- Cavanaugh J, Wurtz RH. Subcortical modulation of attention counters change blindness. J Neurosci. 2004;24:11236–11243. doi: 10.1523/JNEUROSCI.3724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato LS, Hommel B. Recreational use of cocaine eliminates inhibition of return. Neuropsychology. 2009;23:125–129. doi: 10.1037/a0013821. [DOI] [PubMed] [Google Scholar]
- Colzato LS, Pratt J, Hommel B. Dopaminergic control of attentional flexibility: inhibition of return is associated with the dopamine transporter gene (DAT1) Front Hum Neurosci. 2010;4:53. doi: 10.3389/fnhum.2010.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, D'Esposito M. Dopaminergic modulation of flexible cognitive control in humans. In: Björklund A, Dunnett SB, Iversen LL, Iversen SD, editors. Dopamine handbook. Oxford: Oxford University Press; 2009. [Google Scholar]
- Cools R, D'Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D'Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci. 2009;29:1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Gibbs SE, Miyakawa A, Jagust W, D'Esposito M. Working memory capacity predicts dopamine synthesis capacity in the human striatum. J Neurosci. 2008;28:1208–1212. doi: 10.1523/JNEUROSCI.4475-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Robbins TW. Chemistry of the adaptive mind. Philos Transact A Math Phys Eng Sci. 2004;362:2871–2888. doi: 10.1098/rsta.2004.1468. [DOI] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D'Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci. 2007;27:5506–5514. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie CA. A review of Parkinson's disease. Br Med Bull. 2008;86:109–127. doi: 10.1093/bmb/ldn013. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Klein RM, Everling S, Munoz DP. Contribution of the primate superior colliculus to inhibition of return. J Cogn Neurosci. 2002;14:1256–1263. doi: 10.1162/089892902760807249. [DOI] [PubMed] [Google Scholar]
- Fecteau JH, Munoz DP. Correlates of capture of attention and inhibition of return across stages of visual processing. J Cogn Neurosci. 2005;17:1714–1727. doi: 10.1162/089892905774589235. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, Abroms BD. d-Amphetamine-induced enhancement of inhibitory mechanisms involved in visual search. Exp Clin Psychopharmacol. 2005;13:200–208. doi: 10.1037/1064-1297.13.3.200. [DOI] [PubMed] [Google Scholar]
- Filoteo JV, Delis DC, Salmon DP, Demadura T, Roman MJ, Shults CW. An examination of the nature of attentional deficits in patients with Parkinson's disease: evidence from a spatial orienting task. J Int Neuropsychol Soc. 1997;3:337–347. [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonon FG, Buda MJ. Regulation of dopamine release by impulse flow and by autoreceptors as studied by in vivo voltammetry in the rat striatum. Neuroscience. 1985;14:765–774. doi: 10.1016/0306-4522(85)90141-1. [DOI] [PubMed] [Google Scholar]
- Heal D, Cheetham S, Smith S. The neuropharmacology of ADHD drugs in vivo: insights on efficacy and safety. Neuropharmacology. 2009;57:608–618. doi: 10.1016/j.neuropharm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. II. Visual and auditory responses. J Neurophysiol. 1989;61:799–813. doi: 10.1152/jn.1989.61.4.799. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, D'Esposito M, Farah MJ. Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport. 1997;8:3581–3585. doi: 10.1097/00001756-199711100-00032. [DOI] [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. Trends Cogn Sci. 2000;4:138–147. doi: 10.1016/s1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Pollmann S. Covert reorienting and inhibition of return: an event-related fMRI study. J Cogn Neurosci. 2002;14:127–144. doi: 10.1162/089892902317236795. [DOI] [PubMed] [Google Scholar]
- Maruya H, Watanabe Y, Okita M, Lawlor GF, Utsumi H, Niitsuma T. Inhibitory effects of D2 agonists by striatal injection on excessive release of dopamine and hyperactivity induced by Bay K 8644 in rats. Neuroscience. 2003;118:1091–1098. doi: 10.1016/s0306-4522(02)00807-2. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Seidenberg M, Dorflinger JM, Rao SM. An event-related fMRI study of exogenous orienting: supporting evidence for the cortical basis of inhibition of return? J Cogn Neurosci. 2004;16:1262–1271. doi: 10.1162/0898929041920531. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Damask SP, Wang J, Haroutunian V, Davis KL, Watson SJ. Dopamine receptor mRNA expression in human striatum and neocortex. Neuropsychopharmacology. 1996;15:17–29. doi: 10.1016/0893-133X(95)00150-C. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Riedel WJ. Dopaminergic enhancement of cognitive function. Curr Pharm Des. 2006;12:2487–2500. doi: 10.2174/138161206777698891. [DOI] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D'Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3' UTR VNTR: evidence from brain and lymphocytes using quantitative RT-PCR. Am J Med Genet. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of visual orienting. In: Bouma H, Bouwhais DG, editors. Attention and performance X: control of language processes. Hillsdale (NJ): Erlbaum; 1984. pp. 531–556. [Google Scholar]
- Possin KL, Filoteo JV, Song DD, Salmon DP. Space-based but not object-based inhibition of return is impaired in Parkinson's disease. Neuropsychologia. 2009;47:1694–1700. doi: 10.1016/j.neuropsychologia.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P, Debono A, Parkes JD, Marsden CD, Rosenthaler J. Plasma bromocriptine levels, clinical and growth hormone responses in Parkinsonism. Br J Clin Pharmacol. 1978;6:303–309. doi: 10.1111/j.1365-2125.1978.tb00856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinzmetal W, McCool C, Park S. Attention: reaction time and accuracy reveal different mechanisms. J Exp Psychol Gen. 2005;134:73–92. doi: 10.1037/0096-3445.134.1.73. [DOI] [PubMed] [Google Scholar]
- Radad K, Gille G, Rausch WD. Short review on dopamine agonists: insight into clinical and research studies relevant to Parkinson's disease. Pharmacol Rep. 2005;57:701–712. [PubMed] [Google Scholar]
- Rafal RD, Posner MI, Walker JA, Friedrich FJ. Cognition and the basal ganglia. Separating mental and motor components of performance in Parkinson's disease. Brain. 1984;107(Pt 4):1083–1094. doi: 10.1093/brain/107.4.1083. [DOI] [PubMed] [Google Scholar]
- Ro T, Rafal RD. Components of reflexive visual orienting to moving objects. Percept Psychophys. 1999;61:826–836. doi: 10.3758/bf03206900. [DOI] [PubMed] [Google Scholar]
- Rokem A, Landau AN, Garg D, Prinzmetal W, Silver MA. Cholinergic enhancement increases the effects of voluntary attention but does not affect involuntary attention. Neuropsychopharmacology. 2010;35:2538–2544. doi: 10.1038/npp.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir A, Soroker N, Berger A, Henik A. Inhibition of return in spatial attention: direct evidence for collicular generation. Nat Neurosci. 1999;2:1053–1054. doi: 10.1038/15977. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J Psychopharmacol. 1999;13:337–345. doi: 10.1177/026988119901300406. [DOI] [PubMed] [Google Scholar]
- Wallace DL, Vytlacil JJ, Nomura EM, Gibbs SE, D'Esposito M. The dopamine agonist bromocriptine differentially affects fronto-striatal functional connectivity during working memory. Front Hum Neurosci. 2011;5:32. doi: 10.3389/fnhum.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Kobayashi S. Contributions of the dopaminergic system to voluntary and automatic orienting of visuospatial attention. J Neurosci. 1998;18:1869–1878. doi: 10.1523/JNEUROSCI.18-05-01869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S, Jonides J. Abrupt visual onsets and selective attention: voluntary versus automatic allocation. J Exp Psychol Hum Percept Perform. 1990;16:121–134. doi: 10.1037//0096-1523.16.1.121. [DOI] [PubMed] [Google Scholar]