Abstract
Accumulating evidence from structural brain imaging studies on individuals with fetal alcohol spectrum disorder (FASD) has supported links between prenatal alcohol exposure and brain morphological deficits. Although global and regional volumetric reductions appear relatively robust, the effects of alcohol exposure on cortical thickness and relationships with facial dysmorphology are not yet known. The structural magnetic resonance imaging data from 69 children and adolescents with FASD and 58 nonexposed controls collected from 3 sites were examined using FreeSurfer to detect cortical thickness changes across the entire brain in FASD and their associations with facial dysmorphology. Controlling for brain size, subjects with FASD showed significantly thicker cortices than controls in several frontal, temporal, and parietal regions. Analyses conducted within site further revealed prominent group differences in left inferior frontal cortex within all 3 sites. In addition, increased inferior frontal thickness was significantly correlated with reduced palpebral fissure length. Consistent with previous reports, findings of this study are supportive of regional increases in cortical thickness serving as a biomarker for disrupted brain development in FASD. Furthermore, the significant associations between thickness and dysmorphic measures suggest that the severity of brain anomalies may be reflected by that of the face.
Keywords: cortical thickness, developmental, fetal alcohol exposure, MRI
Introduction
Fetal alcohol syndrome (FAS) is caused by excessive maternal alcohol consumption during pregnancy and is characterized by permanent birth defects including facial dysmorphology, growth deficiency, and dysfunction of the central nervous system (CNS), among other anomalies. The umbrella term of fetal alcohol spectrum disorders (FASDs) was coined to characterize a spectrum of outcomes resulting from heavy prenatal alcohol exposure including varying degrees of anomalies characteristic of those with the full FAS. The CNS defects in FASD range from microcellular and neurochemical aberrations to gross structural brain abnormalities (Norman et al. 2009). Additionally, cognitive and behavioral dysfunction is also evidenced by reports of lowered IQ, hyperactivity, and deficits in motor function, attention, verbal learning, expressive and receptive language, executive function, and visuospatial skills in individuals with FASD (Mattson and Riley 1998; Kodituwakku 2007; Norman et al. 2009).
Accelerated research efforts from projects such as the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which encourages the collaboration of basic, behavioral, and clinical investigators around the world to use novel and cutting-edge techniques coordinately to develop effective interventions and treatments for FASD (Mattson et al. 2010), have led to accumulated evidence supporting a potential link between brain structural deficits and prenatal alcohol exposure, in line with observations from previous postmortem reports (Norman et al. 2009). Among the most consistent findings in brain imaging studies of FASD is that of overall brain volume reductions (Mattson et al. 1994; Johnson et al. 1996; Swayze et al. 1997; Archibald et al. 2001; Lebel et al. 2008; Li et al. 2008; Willoughby et al. 2008; Norman et al. 2009). These volume decreases are widespread throughout the brain, with frontal, temporal, and parietal lobes showing the most pronounced effects in individuals with FASD as compared with nonexposed controls (Archibald et al. 2001; Sowell, Thompson, Mattson, et al. 2002). Additionally, regional abnormalities in the corpus callosum (Riley et al. 1995; Sowell et al. 2001; Bookstein, Sampson, et al. 2002; Bookstein, Streissguth, et al. 2002; Bookstein et al. 2007), cerebellum (Mattson et al. 1992, 1994; Archibald et al. 2001; Autti-Ramo et al. 2002), basal ganglia (Mattson et al. 1992, 1996; Archibald et al. 2001), and hippocampus (Riikonen et al. 1999; Autti-Ramo et al. 2002) have also been documented.
Despite the increasing number of studies reporting gross and regional volumetric alterations FASD, relatively little is known regarding finer-scale cortical abnormalities assessed across the whole brain that may more closely relate with the clinical and behavioral features of the disorder. Using voxel-based morphometry and surface-based analysis methods, studies by our group (Sowell et al. 2001; Sowell, Thompson, Mattson, et al. 2002; Sowell, Thompson, Peterson, et al. 2002) revealed that the temporal and parietal lobes of alcohol-exposed subjects tend to have increased gray matter density in combination with cortical surface alterations. Specifically, we found an estimated 15% increase in cortical gray matter in inferior parietal and perisylvian cortex bilaterally in alcohol exposed compared with control subjects (Sowell, Thompson, Mattson, et al. 2002). In addition, significant surface deformations in the inferior parietal/perisylvian, anterior frontal, and orbital frontal regions were observed in alcohol-exposed subjects. In a later study, increased cortical thickness in bilateral temporal and inferior parietal and right inferior frontal regions of another subset of FASD patients (N = 21) was reported (Sowell et al. 2008). Although these findings have shed light on the neuropathology underlying FASD, characterization of cortical thickness abnormalities of FASD requires further clarification and confirmation in independent samples. Furthermore, although diagnostic of FAS, few studies have addressed the structural correlates of facial dysmorphology, such as short palpebral fissure length (PFL), which may provide further insight into the neurodevelopmental disturbances associated with prenatal exposure to alcohol.
To extend the prior findings concerning cortical thickness abnormalities in FASD from independent samples, this study applied FreeSurfer analysis methods to the structural magnetic resonance imaging (MRI) data collected for a CIFASD project from a cohort of children and adolescents prenatally exposed to alcohol and unexposed controls (Mattson et al. 2010) recruited from 3 study sites (i.e., University of California Los Angeles, University of Cape Town, San Diego State University). Measures of facial morphology, including PFL and appearance of the philtrum, were also assessed for a subset of subjects at each of the 3 study sites. Based on our previous study (Sowell et al. 2008), it was predicted that children and adolescents with FASD would have increased thickness in the perisylvian region, specifically the inferior frontal, superior temporal, middle temporal, and the inferior parietal cortices. Additionally, greater facial dysmorphology as reflected in PFL and philtral deficiency was expected to directly correlate with increased cortical thickness in regions that best differentiated the exposed from the control groups.
Materials and Methods
Subjects
Structural MRI data was collected from 127 subjects at 3 sites: Los Angeles (LA, age range 8–16), Cape Town, South Africa (SA, age range 13–15), and San Diego (SD, age range 10–14). Subjects included 69 children and adolescents with FASD (23 from LA, 31 from SA, and 15 from SD) and 58 nonexposed controls (18 from LA, 22 from SA, and 18 from SD). As previously reported (Roussotte et al. 2011) in a subset of participants studied in the present report, the ethnic composition of participants varied by site. In LA and SD, although the majority of participants were classified as non-Hispanic White (N = 38; 19 from LA and 19 from SD), several subjects classified as Asians (N = 3; all from LA), Native Hawaiians (N = 1; from SD), African Americans (N = 12; 8 from LA and 4 from SD) and more than one race (N = 5; 4 from LA and 1 from SD) were also included. In SA, all but one participant with ethnicity information were classified as “Cape Colored,” a racial category comprising people of mixed ancestry from intermarriages of Black African populations (primarily of Khoi and San tribal origins), European whites, and some Asians (May et al. 2007).
In LA, exposed subjects were recruited primarily through the University of California Los Angeles Fetal Alcohol and Related Disorders Clinic. Exposure status of FASD subjects recruited at this location was established through extensive interviews administered to the parents/guardians and the use of collateral information such as social, medical, and/or legal records when available. In SA, exposed subjects were recruited through the University of Cape Town, from small towns and rural settlements in the Western Cape Province that has one of the highest documented rates of FASD in the world (May et al. 2007). All of the subjects from SA site had been diagnosed previously as FAS or partial FAS from a population-based study of the epidemiology of FASD among first graders (May et al. 2007), and their diagnoses were reconfirmed for this study through maternal interview and questionnaires. In SD, exposed participants were recruited through the San Diego State University Center for Behavioral Teratology and exposure status was determined through maternal reports and the review of medical, legal, and social service records when available. The number of subjects who received a full FAS diagnosis from each site was listed in Table 1. At all sites, participants were excluded from the FASD group upon evidence of other known causes of mental deficiency, such as chromosomal abnormalities.
Table 1.
Demographic, clinical, and MRI measures of the sample
| Across sites |
Within sites |
|||||||
| Los Angeles |
South Africa |
San Diego |
||||||
| FASD | Nonexposed controls | FASD | Nonexposed controls | FASD | Nonexposed controls | FASD | Nonexposed controls | |
| (N = 69) | (N = 58) | (N = 23) | (N = 18) | (N = 31) | (N = 22) | (N = 15) | (N = 18) | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Demographic measures | ||||||||
| Age | 13.4 (1.9) | 13.0 (2.0) | 12.7 (2.3) | 11.8 (2.4) | 14.7 (0.6) | 14.6 (0.4) | 12.1 (1.1) | 12.1 (1.4) |
| Gender (male/female) | 43/26 | 28/30 | 14/9 | 7/11 | 18/13 | 11/11 | 11/4 | 10/8 |
| Ethnicitya (white/other) | 21/45 | 17/32 | 11/9 | 8/9 | 0/31 | 0/22 | 10/5 | 9/1 |
| FSIQb | 64.5 (27.6) | 93.6 (26.1) | 83.1 (21.1) | 111.2 (20.7) | 44.5 (17.8) | 68.4 (12.1) | 78.3 (26.1) | 107.5 (17.6) |
| Clinical measures | ||||||||
| PFLc | 2.5 (0.3) | 2.6 (0.1) | 2.6 (0.2) | 2.7 (0.2) | 2.4 (0.3) | 2.6 (0.1) | 2.6 (0.2) | 2.6 (0.1) |
| Lipometerd | 3.5 (0.6) | 3.2 (0.8) | 3.4 (0.6) | 3.2 (0.6) | 3.7 (0.7) | 3.3 (1.1) | 3.4 (0.5) | 3.0 (0.5) |
| Full FASe (yes/no) | 21/21 | 0/58 | 3/10 | 0/18 | 15/6 | 0/22 | 3/5 | 0/18 |
| MRI measures | ||||||||
| Total brain volume (cm3) | 1180.6 (150.8) | 1290.7 (132.0) | 1259.8 (167.8) | 1349.4 (127.6) | 1118.6 (119.8) | 1232.7 (96.6) | 1187.2 (128.8) | 1302.9 (149.7) |
Note: SD, standard deviation; PFL, palpebral fissure length.
Ethnicity information was available for only 115 subjects.
FSIQ scores were available for 125 subjects (68 FASD and 57 CC subjects) including 41 subjects from LA (23 FASD and 18 CC subjects), all 53 subjects from SA, and 31 subjects from SD (14 FASD and 17 CC subjects).
FPL measure was only available in 95 subjects (61 FASD and 34 nonexposed control subjects) including 29 subjects from LA (16 FASD and 13 nonexposed control subjects), 41 subjects from SA (30 FASD and 11 nonexposed control subjects), and 25 subjects from SD (15 FASD and 10 nonexposed control subjects).
Lipometer measure was available for all 95 subjects with FPL measures except one nonexposed control subject from LA.
Full FAS diagnosis was available for only 199 subjects.
Nonexposed control subjects were recruited from ongoing studies at each site or for this study specifically via advertisements and word of mouth and occasionally through other recruiting strategies such as the use of national registers (Roussotte et al. 2011). Control participants were excluded if they were prenatally exposed to more than 1 drink per week on average or more than 2 drinks on any one occasion during pregnancy. Additional exclusion criteria for both the control and the FASD group included significant head injury with loss of consciousness for more than 30 min or significant physical or psychiatric disability sufficient to preclude participation in neurocognitive and neuroimaging assessments.
Facial and Cognitive Evaluations
Standardized methodology was used to obtain facial dysmorphology scores on both FASD and nonexposed subjects by a trained member of the CIFASD (Hoyme et al. 2005; Jones et al. 2006; Roussotte et al. 2011). In brief, PFL was measured using a rigid ruler marked in millimeters held against the lower eyelid (Hoyme et al. 2005). The morphological characteristics of the philtrum were assessed and scored with a previously described and verified lip/philtrum guide (“lipometer”) (Astley and Clarren 2000). Participants were assigned a score of 1 through 5, with a higher score representing greater dysmorphology of the upper lip. Whenever possible, examiners were blind to the diagnostic status of subjects. Of the 127 subjects who were studied with structural MRI, 95 subjects (61 FASD and 34 nonexposed subjects) received a PFL measurement and 94 subjects (61 FASD and 33 nonexposed subjects) received philtrum lipometer scores. The 61 FASD subjects who received both scores were included in the facial dysmorphology correlational analysis described below. As described in detail previously (Roussotte et al. 2011), all subjects eligible for neuropsychological testing were given a standardized test battery by trained examiners, including the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV; Wechsler 2003), from which a Full Scale IQ (FSIQ) score was derived. The scores were obtained by the examiners and checked for accuracy by a second rater, and were available for all but 1 FASD and 1 control subject from SA.
MRI Data Acquisition
In LA, high-resolution T1-weigthed sagittal volumes were collected from a 1.5-T Siemens Sonata magnet, with repetition time (TR) = 1900 ms, echo time (TE) = 4.38 ms, flip angle = 15°, matrix size = 256 × 256 × 160 mm, field of view (FOV) = 256 mm, voxel size = 1 × 1 × 1 mm, and acquisition time = 8 min 8 s. In SA, high-resolution T1-weigthed sagittal volumes were collected from a 3-T Siemens Allegra scanner, with TR = 2200 ms, TE = 5.16 ms, flip angle = 12°, matrix size = 256 × 256 × 160 mm, FOV = 256 mm, voxel size = 1 × 1 × 1 mm, and acquisition time = 7 min 4 s. In SD, high-resolution T1-weigthed sagittal volumes were collected from a 3-T GE Signa Excite scanner, with TR = 7.8 ms, TE = 3.0 ms, flip angle = 12°, matrix size = 256 × 256 × 192 mm, FOV = 240 mm, voxel size = 0.938 × 0.938 × 1 mm, and acquisition time = 7 min 26 s.
Image Processing
Cortical thickness was estimated using FreeSurfer software (FreeSurfer 4.0.5, http://surfer.nmr.mgh.harvard.edu) (Dale et al. 1999; Fischl et al. 1999), a widely documented and automated program for the analysis of brain structure (Fischl and Dale 2000). In short, FreeSurfer processing streams included the removal of nonbrain tissue, the segmentation of gray–white matter, and the alignment of each image volume to a standardized space (i.e., the Montreal Neurological Institute space). For the estimation of cortical thickness specifically, after intensity normalization, gray–white tissue segmentation was used as the starting point for a deformable surface algorithm that was applied to extract the pial and gray–white cortical surfaces (Dale et al. 1999). The entire cortex of each subject was then visually inspected for accuracies and manually corrected if necessary, following previously established procedures (Yang et al. 2009, 2011; Yang, Nuechterlein, et al. 2010; Yang, Raine, et al. 2010; Roussotte et al. 2011). This additional quality control step ensures the accuracy of gray/white matter segmentation and the effective removal of scalp and meninges and inclusion of brain tissue and was performed on a slice-by-slice basis on each brain slice through each image volume by one rater (EK), who was blinded to group membership. After spatial normalization of the data to a cortical surface-based atlas, local cortical thickness was measured by estimating the shortest distance between the position of spatially equivalent surface points on the pial surface and the gray–white matter boundary and vice versa and averaging these 2 values (Fischl and Dale 2000). A 10-mm full-width at half-maximum Gaussian kernel was then applied to smooth the data. Because the generated cortical maps are not limited to the voxel resolution of the original data, FreeSurfer has relatively high sensitivity in detecting submillimeter differences between groups. Last, total brain volume for each individual was also estimated using FreeSurfer segmentations (i.e., the sum of all white and gray matter regions of interest [ROIs] Buckner et al. 2004).
Statistical Analysis
For demographic cognitive and facial morphology variables, statistical analyses were conducted using SPSS 18.0. Age, total brain volume, and FSIQ were compared between groups using independent 2-sample t-tests and difference in gender was evaluated with Pearson chi-square tests. To examine cortical thickness variations across the whole brain between the FASD and nonexposed control groups within site, vertex-by-vertex analysis was performed using the general linear model, in which multiple regression analyses were conducted with cortical thickness as dependent variable and group as independent variable while age, gender, and cubed root of brain volume were included as covariates. Although there were no significant group-by-site interactions for the ROI thickness (all P > 0.23), there was a significant main effect for site for left and right middle temporal (P = 0.001, P = 0.026, respectively) and inferior temporal cortex (both P < 0.001) with SA subjects showing thinner cortex in these regions than LA and SD subjects. Thus, site was included as categorical predictor in all analyses conducted across sites to control for any potential confounding effects. Permutation analyses with a threshold of 0.05 were used to confirm the significance of multiple spatially correlated comparisons performed at high spatial density across each hemisphere (Thompson et al. 2003).
Post Hoc Analyses
For descriptive purposes and to confirm the location of the observed effects, additional group comparisons were conducted on average cortical thickness for the a priori regions. Specifically, the a priori regions were selected based on findings of an independent sample reported previously by our group (Sowell et al. 2008), in which the abnormal thickness increases were observed in perisylvian region, specifically the posterior part of the inferior frontal (pars opercularis), superior temporal, middle temporal, and inferior parietal gyrus, in subjects with FASD. Thus, average thickness of these ROIs was estimated using the Desikan template (Desikan et al. 2006) and compared between the 2 groups using general linear models including total brain volume, age, and gender for the within-sites analyses, whereas site was added as a covariate for the combined-site analyses. Further analyses were conducted on the subsample of 42 FASD subjects of which the FAS diagnosis data was available to assess the cortical thickness of these ROIs in FAS and nondysmorphic FASD separately. Finally, to confirm the disease effect on the inferior frontal thickness observed both within and across sites, we further conducted multiple regression analysis to assess the relationship between the average thickness in the inferior frontal gyrus and measures of facial dysmorphology within the FASD groups.
Results
Demographic, Cognitive, and Clinical Measures
Table 1 shows demographic, cognitive, and clinical details of all subjects. Across and within scan sites, FASD and control groups did not differ from each other in age or gender (all P > 0.15). The age of subjects differed between scan locations (F2,126 = 40.48, P < 0.001), with the SA subjects being significantly older than both the LA and the SD participants (both P < 0.001). However, the LA and SD sites did not differ from each other in mean age (P = 0.63). FASD subjects also had significantly lower FSIQ compared with control subjects both across and within scan sites (all P < 0.001). For clinical measures, FASD subjects showed significantly smaller PFL measures and increased lipometer scores compared with nonexposed control subjects (F1,94 = 3.99, P = 0.049; F1,93 = 6.89, P = 0.01, respectively) across sites. However, no significant group difference in clinical measures was observed within sites when examined alone (all P > 0.06).
Across sites, FASD subjects had smaller total brain volumes than nonexposed control subjects (F1,126 = 18.8, P < 0.001) while controlling for age and gender. Within sites, the total brain volumes of FASD subjects were found to be smaller than that of nonexposed control subjects within LA (F1,41 = 7.74, P = 0.008), SA (F1,53 = 22.55, P < 0.001), and SD (F1,33 = 9.57, P = 0.004) samples. Thus, all analyses of cortical thickness were conducted including total brain volume as a covariate, in addition to age, gender, and site (when compared across sites). Since it has been previously shown that FSIQ may influence findings of structural brain alterations in FASD, group comparisons were additionally performed including FSIQ as covariate in the statistical models to ensure that results were not fully attributable to such effects.
Cortical Thickness Measures
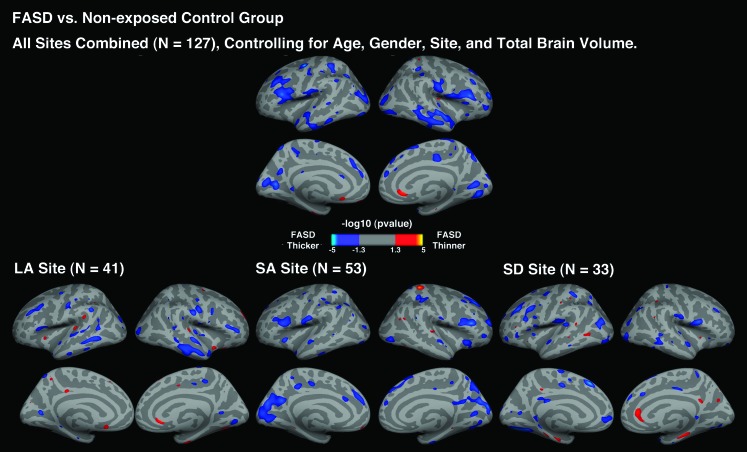
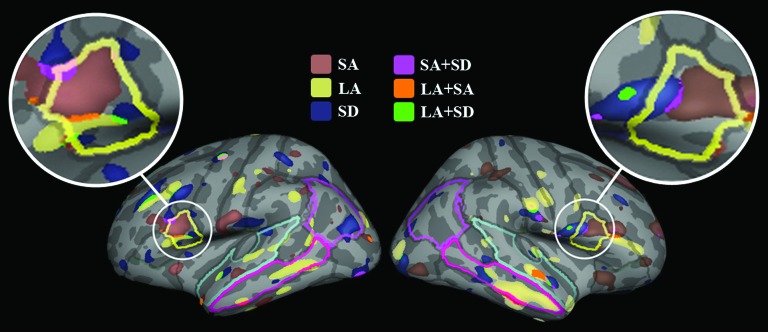
Across and within sites, FASD subjects showed an overall pattern of increased cortical thickness compared with nonexposed control subjects (left hemisphere: permutation corrected P = 0.028; right hemisphere: permutation corrected P = 0.019; see Fig. 1). The surface analysis of cortical thickness showed that thicker cortex appeared in several a priori regions, including bilateral inferior frontal, superior temporal, inferior temporal, and right middle temporal but also other regions including lateral occipital, pericalcarine, postcentral, and left inferior parietal, right lingual gyrus in FASD subjects compared with nonexposed control subjects. For the LA site, FASD subjects showed increased cortical thickness in the a priori regions most prominently in the right middle temporal cortex compared with nonexposed control subjects. For the SA site, significantly increased cortical thickness in the a priori regions was observed in the bilateral inferior frontal and right superior frontal compared with nonexposed control subjects. For the SD site, FASD subjects showed significantly increased cortical thickness in the left inferior frontal and right inferior temporal cortices compared with nonexposed control subjects. No significant cortical thinning was found in FASD subjects compared with nonexposed control subjects. Overall, increased cortical thickness in the FASD subjects appeared to be most prominently in the inferior frontal gyrus both across and within sites (see Fig. 2).
Figure 1.
Uncorrected P maps representing the significant group difference in cortical thickness for the FASD versus control comparisons across the entire sample of 127 subjects and within LA, SA, and SD site, controlling for age, gender, and total brain volume.
Figure 2.
Illustrations of the significant inferior frontal thickness increases in FASD compared with nonexposed control group both within and across sites. The ROIs were also highlighted as follows: inferior frontal gyrus (IFG; yellow), superior temporal gyrus (STG; pal blue), middle temporal gyrus (MTG; bright pink), and inferior parietal gyrus (IPG; pink).
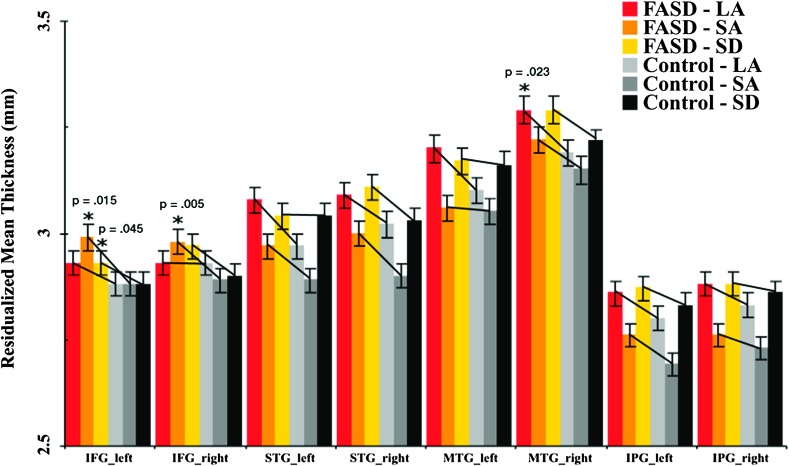
To compliment the whole-brain surface thickness analyses, group differences in mean cortical thickness within ROIs were also analyzed. As expected, findings were found to be largely reflective of those from surface analysis. Cortical thickness increases were observed in the left and right inferior frontal (F1,118 = 13, P = 0.011; F1,118 = 6.72, P < 0.001, respectively), right middle temporal (F1,118 = 5.84, P = 0.017), and right superior temporal (F1,118 = 4.39, P = 0.038) in FASD subjects compared with nonexposed control subjects in the combined-site analyses (Fig. 3). Upon further examination of the age effects, the ROI thickness analysis on subjects under 10 years old showed significantly increased cortical thickness in the right inferior frontal (P = 0.38) and right superior temporal cortex (P = 0.005), while subjects over 13 years old showed significantly increased thickness in the left and right inferior frontal (P = 0.007, P = 0.038, respectively), left inferior temporal (P = 0.016), and right superior temporal cortex (P = 0.026). However, we only had 8 subjects under 10 years old and they were all from the LA site, thus these results should be interpreted with caution. Additional analyses were performed including FSIQ as covariates, which revealed similar results for left and right inferior frontal (P < 0.001, P = 0.025, respectively) and right middle temporal cortex (P = 0.004). Further analysis revealed that, when separating those with full FAS diagnosis and those without, both FAS and nondysmorphic FASD subjects showed increased thickness in the left inferior frontal (P = 0.021, P = 0.002, respectively) and right middle temporal cortex (P = 0.008, P = 0.004, respectively) compared with nonexposed control subjects, whereas the 2 groups of subjects did not differ significantly from each other (all P > 0.25).
Figure 3.
Average thickness of the left and right inferior frontal gyrus (IFG), superior temporal gyrus (STG), middle temporal gyrus (MTG), and the inferior parietal gyrus (IPG) of the FASD and control subjects within each site, corrected for age, gender, and total brain volume. The vertical lines represent the standard error bars.
Significant or trend-level cortical thickness increases were observed in bilateral inferior frontal ROIs in all 3 independent samples. Specifically, for the LA site, group differences in cortical thickness were confirmed for the right middle temporal cortex (F1,36 = 5.65, P = 0.023) with a trend-level significance observed in the left inferior frontal cortex (F1,36 = 3.79, P = 0.06). For the SA site, group differences in cortical thickness were confirmed for the left and right inferior frontal cortex (F1,48 = 6.32, P = 0.015; F1,48 = 8.48, P = 0.005, respectively). For the SD site, group differences in cortical thickness were significant in the left inferior frontal cortex (F1,28 = 4.4, P = 0.045), with a trend-level significance found in the right inferior frontal cortex (F1,28 = 3.26, P = 0.082).
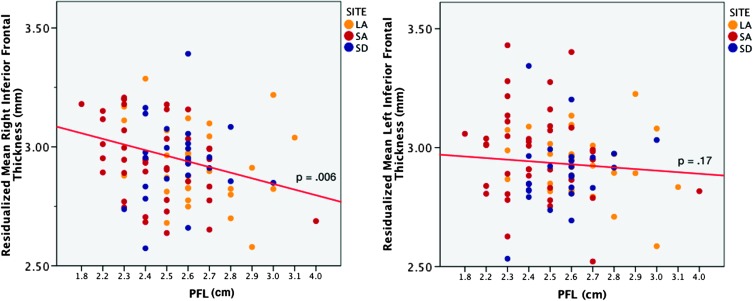
Finally, we conducted post hoc analysis to evaluate the relationship between cortical thickness in inferior frontal regions that best differentiated FASD from control subjects (in all 3 independent samples) and facial morphological measures. Within the exposed group combined across sites, increased cortical thickness was associated with smaller PFL in the right inferior frontal cortex (r = −0.36, P = 0.006) but not the left (P = 0.17) (see Fig. 4). However, no similar correlations were observed for lipometer scores (all P > 0.11).
Figure 4.
Relationships between average thickness in left and right inferior frontal gyrus and PFL scores after controlling for age, gender, site, and total brain volume in FASD subjects. The partial correlation coefficient between left and right inferior frontal thickness and PFL partialling out age, gender, site, and total brain volume is −0.36 and −0.18, respectively.
Discussion
Our results confirm abnormal cortical thickness patterns in a relatively large sample of children with FASD with increased cortical thickness observed in a regionally similar pattern across 3 independent samples of children studied at 3 data collection sites. Specifically, increases in cortical thickness were observed in the perisylvian cortices of the frontal and temporal lobes, most prominently in the inferior frontal, superior temporal, and middle temporal cortices, consistent with our a priori hypotheses. Our novel findings of significant relationships between increased cortical thickness and decreased PFL support the notion that abnormally thick cortex may serve as a biomarker for disrupted brain development in individuals prenatally exposed to alcohol. Our findings further suggest that the severity of such brain anomalies may be reflected in the severity of facial dysmorphology.
The cortical thickness findings in this study are highly consistent with those from a previous report involving an independent sample of 21 individuals with FASD and 21 nonexposed controls, where increased cortical thickness that was most pronounced in areas surrounding the perisylvian cortices were described (Sowell et al. 2008). Numerous studies of typical brain development have shown that the majority of brain regions attain peak cortical thickness around ages 8–10 years and thickness declines subsequently thereafter, with the greatest thinning occurring during early adolescence (Giedd et al. 1999; Sowell et al. 2003; Shaw et al. 2006). This systematic cortical thinning is likely due to synaptic pruning in the form of activity-dependent selective elimination (Bourgeois and Rakic 1993; Huttenlocher and Dabholkar 1997; Shaw et al. 2008; Tamnes, Østby, Fjell, et al. 2010) and in increased myelination (Huttenlocher 1990), which are thought to serve as a biological indicators of increased efficiency in cognition, emotion, and behavior that occur during this period of development (Hensch 2004; Knudsen 2004). The argument that prenatal exposure to alcohol may disrupt this pruning process is supported by the fact that alcohol disrupts the activity of γ-aminobutyric acid and its receptors in modulating progenitor cell proliferation, cell migration, and neurite growth during early neuronal development (Schwartz 1992; Borodinsky et al. 2003). Previous investigations have linked prenatal alcohol exposure to decreased white matter concentration, delayed neuronal migration, and decreased myelination (Shetty and Phillips 1992; Barrow Heaton et al. 1999; Ozer et al. 2000; Archibald et al. 2001; Redila et al. 2006). Although the current study lacks sufficient power to assess whether early developmental events such as neurogenesis or later ones such as pruning have more influences in FASD subjects, we observed increased cortical thickness in both younger (under 10 years old) and older subjects (over 13 years old) with older subjects showing more widespread increases, providing support for a greater involvement of disrupted pruning process in the brain development of FASD. Nonetheless, it remains a possibility that earlier developmental events may also be affected in children with FASD.
The regional specificity of our findings is also consistent with previous reports of functional disturbances observed in FASD (Mattson et al. 1997, 1999, 2006; Mattson and Riley 1998; Mattson and Roebuck 2002; Reobuck-Spencer and Mattson 2004; Streissguth et al. 2004; Burden et al. 2005; Riley and McGee 2005; Rasmussen et al. 2006; Greenbaum et al. 2009). In this study, we revealed increases in cortical thickness in several frontal and temporal regions but most prominent in the inferior frontal gyrus; a region critically involved in response inhibition, attention, social cognition, and executive functioning. The prominence of the thicker inferior frontal cortex may be partially explained by the prefrontal–striatal circuitry that has found to be disrupted by prenatal alcohol exposure (Roussotte et al. 2011). Specifically, it may be argued that the prenatal insult to the striatal structures due to heavy prenatal alcohol exposure, as observed in previous studies (Norman et al. 2009; Lebel et al. 2011), may result in the downstream effects on later cortical development of the inferior frontal gyrus long after the insult has occurred in utero. On the other hand, several recent functional MRI studies have shown abnormal, often excessive, activation in the inferior frontal cortex and associated regions (such as the striatum) during spatial and verbal working memory (Malisza et al. 2005; Spadoni et al. 2009; Roussotte et al. 2011), number processing (Santhanam et al. 2009), and response inhibition (Fryer et al. 2007) in subjects exposed to alcohol prenatally. Taken together the evidence described in previous studies that have directly linked thinner cortex to better performance in typically developing populations (Sowell et al. 2004; Shaw et al. 2006; Lu et al. 2007; Tamnes, Østby, Walhovd, et al. 2010), it seems reasonable to speculate that the thicker cortex, especially in the inferior frontal region, observed in this study may reflect less efficient neural processing in affected areas among children with FASD.
Another key finding of this study is the significant association between facial dysmorphology measures and cortical thickness. Specifically, increased cortical thickness in the right inferior frontal gyrus was found to correlate with shorter PFLs. Although very few studies have evaluated the association between facial morphology and brain structural measures, the current results are in keeping with the premise that deficiencies in the upper midface features (i.e., PFL) are more predictive of underlying cortical dysmorphology than lower features (i.e., lipometer) (DeMeyer 1975). Those clinical FASD face/brain studies that have been reported have shown facial anomalies to be significantly associated with smaller frontal, ventral diencephalon, and several subcortical brain structures (Astley et al. 2009; Roussotte et al. 2011). These findings are consistent with animal studies showing concurrent insult to the upper midface, eyes, and brain following alcohol exposure at discrete early stages of prenatal development (Sulik 2005; Parnell et al. 2009; Godin, Dehart, et al. 2010; Godin, O'Leary-Moore, et al. 2010; Roussotte et al. 2011). Furthermore, evidence from functional neuroimaging and lesion studies suggest that the right inferior frontal gyrus is heavily involved in cognitive control processes related to response inhibition and stimulus-driven attention (Aron et al. 2003, 2004, 2007; Westlye et al. 2011), which have been found impaired in FASD subjects (Fryer et al. 2007; Chlodo et al. 2010; Burden et al. 2011). Thus, considering the specialized hemispheric functioning of the right inferior frontal cortex, the face–brain association observed in this study may have further validated the measure of PFL as a critical diagnostic feature of FASD.
Finally, there was noticeable variability among regional group effects from the within site analyses, and we cannot dismiss the possibility that the differences in demographic and clinical characteristics may have impacted the brain imaging results. For example, in normally developed children and adolescents, FSIQ has been found to correlate positively with thicker cortex, specifically in the temporal lobes (Choi et al. 2008). The subjects in SA not only had the greatest facial dysmorphology and highest prevalence of full FAS diagnosis but also the lowest FSIQ scores among the 3 sites, which may contribute to the stronger FASD effects of thicker cortex but the lack of findings in the temporal cortex within this site. On the other hand, the US samples from LA and SD were significantly younger (averaged ∼12 years old) compared with the SA sample (average ∼14 years old). As discussed earlier, systematic synaptic pruning occurs more rapidly in adolescence (13 years or older), and this may explain why we observed less significant thickness abnormality in these samples. Despite the variations among sites, the discovery of notable overlaps between sites in patterns of cortical thickness in the alcohol-exposed cohort, especially in the inferior frontal gyrus, makes it unlikely that differences in population characteristics across scan locations represent a critical confounding factor.
One limitation of this study is the potential influence of concurrent prenatal exposure to other teratogenic substances in addition to alcohol in the clinical population assessed. Indeed, maternal alcohol use is often co-occurring with smoking and the use of illicit drugs (Astley et al. 2009), both of which have been linked to neurogenesis defects in animal models (Slotkin 1998; Bruin et al. 2010). While these or similar confounding exposures cannot be completely ruled out, well-controlled animal studies have demonstrated that prenatal alcohol exposure alone is sufficient to induce both structural brain abnormalities and facial dysmorphology (Sulik 2005). Another limitation of this study is the lack of detailed information on the timing and frequency of alcohol consumption during pregnancy. It has been shown that the timing of heavy episodes of alcohol consumption during pregnancy may determine which functional system may be affected depending on the cytoarchitectonic areas that are at the peak of neuronal migration at that period (Sidman and Rakic 1973). Furthermore, the timing of the prenatal alcohol exposure may alter gene expression (Schneider et al. 2009; Hashimoto-Torii et al. 2011) and differentially affect both neural migration and differentiation. Future studies with detailed information on the amount, frequency, and timing of prenatal alcohol use can advance our understanding on the teratogenic effect of alcohol on the brain, though this information is rarely available in human populations.
Last, despite the larger sample included in this study, the abnormally thicker cortex observed in FASD was similar but of lesser magnitude in both the effect and the extent of regional alteration in comparison to the effects observed in a prior report with a smaller sample of 21 FASD subjects (Sowell et al. 2008). Although the sample size of this study was larger than that of our prior report, there were also greater demographic variations in age and ethnicity among the samples studied here. In addition, the inclusion of an SA sample in this study, which had lower mean FSIQ and perhaps different drinking patterns during pregnancy, may have further contributed to the less significant results. It is also possible that the FreeSurfer methods employed in this study, which inflates the cortical surface, are less influenced by potential difference in sulcal folding patterns between groups than other methods. Thus, the FreeSurfer methods have allowed us to produce a different though possibly more accurate estimation of the gray matter thickness at each surface location though may also be attributable to the difference in findings between this and the prior study.
Despite the limitations inherent in a clinical study of prenatal exposure to alcohol, the results of this study revealed abnormal cortical thickness occurs in FASD across populations in this unique multisite cohort sample. Specifically, results are consistent with findings of a previous more limited study (Sowell et al. 2008) confirming that significantly thicker cortex exist in children and adolescents with FASD in comparison with their nonexposed peers. The findings of thicker frontal and temporal cortex, particularly the inferior frontal gyrus, may suggest that these regions are especially vulnerable to alcohol neurotoxicity during cortical maturation throughout childhood, and that this involvement may reflect the functional impairments observed in FASD. Certainly additional clinical and basic research is needed as a follow up to this investigation, with pertinent structure/function correlations and mechanistic studies of alcohol-mediated cortical thickening being indicated.
Funding
All or part of this work was performed in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol and Alcohol Abuse. Additional information about CIFASD can be found at www.cifasd.org. This work was also supported by National Institute of Mental Health (K99 MH093388 to Y.Y.); National Institute on Alcohol Abuse and Alcoholism (U01 AA017122-01 and U24AA014811 to E.R.S., U01 AA014834 to S.N.M., and U01 AA 11685 and R01 AA15134 to P.A.M.); National Institute of Drug Abuse (R01DA017831 to E.R.S.); National Institute of Child Health and Human Development (R01 HD053893 to E.R.S.); and March of Dimes (6-FY2008-50 to E.R.S.).
Acknowledgments
We would like to acknowledge the efforts in data collection of Suzanne Houston, Ariel Starr, and Genevieve Rodriguez in Los Angeles; Tania Badenhorst, Dominique Brand, Claire Corbett, Gosia Lapinska, and Karen Van Eden in Cape Town, South Africa; and Andria Norman, Jessica O'Brien, Kristina Hubbard, and Delilah Bolo in San Diego. We would also like to thank John Colby for technical assistance. Conflict of Interest : None declared.
References
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43:148–154. [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Aylward EH, Olsen HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T, et al. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2009;33:1671–1689. doi: 10.1111/j.1530-0277.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley SJ, Clarren SK. Diagnosing the full spectrum of fetal alcohol-exposed individuals: introducing the 4-digit diagnostic code. Alcohol Alcohol. 2000;35:400–410. doi: 10.1093/alcalc/35.4.400. [DOI] [PubMed] [Google Scholar]
- Autti-Ramo I, Autti T, Korkman M, Kettunen S, Salonen O, Valanne L. MRI findings in children with school problems who had been exposed prenatally to alcohol. Dev Med Child Neurol. 2002;44:98–106. doi: 10.1017/s0012162201001748. [DOI] [PubMed] [Google Scholar]
- Barrow Heaton MB, Kidd K, Bradley D, Palva M, Mitchell J, Walker DW. Prenatal ethanol exposure reduces spinal cord motoneuron number in the fetal rat but does not affect GDNF target tissue protein. Dev Neurosci. 1999;21:444–452. doi: 10.1159/000017412. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Connor PD, Huggins JE, Barr HM, Pimentel KD, Streissguth AP. Many infants prenatally exposed to high levels of alcohol show one particular anomaly of the corpus callosum. Alcohol Clin Exp Res. 2007;31:868–879. doi: 10.1111/j.1530-0277.2007.00367.x. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Sampson PD, Connor PD, Streissguth AP. Midline corpus callosum is a neuroanatomical focus of fetal alcohol damage. Anat Rec Part B New Anat. 2002;269:162–174. doi: 10.1002/ar.10110. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Streissguth AP, Sampson PD, Connor PD, Barr HM. Corpus callosum shape and neuropsychological deficits in adult males with heavy fetal alcohol exposure. Neuroimage. 2002;15:233–251. doi: 10.1006/nimg.2001.0977. [DOI] [PubMed] [Google Scholar]
- Borodinsky LN, O'Leary D, Meale JH, Vicini S, Cosco OA, Fiszman ML. GABA-induced neurite outgrowth of cerebellar granule cells is mediated by GABA(A) receptor activation, calcium influx and CaMKII and erk1/2 pathways. J Neurochem. 2003;84:1411–1420. doi: 10.1046/j.1471-4159.2003.01638.x. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruin JE, Gerstein HC, Holloway AC. Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicol Sci. 2010;116:364–374. doi: 10.1093/toxsci/kfq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Jacobson SW, Jacobson JL. Relation of prenatal alcohol exposure to cognitive processing speed and efficiency in childhood. Alcohol Clin Exp Res. 2005;29:1473–1483. doi: 10.1097/01.alc.0000175036.34076.a0. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Westerlund A, Muckle G, Dodge N, Dewailly E, Nelson CA, Jacobson SW, Jacobson JL. The effects of maternal binge drinking during pregnancy on neural correlates of response inhibition and memory in childhood. Alcohol Clin Exp Res. 2011;35:69–82. doi: 10.1111/j.1530-0277.2010.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlodo LM, de Costa DE, Hannigan JH, Covington CY, Sokoi RJ, Janisse J, Greenwald M, Ager J, Delaney-Black V. The impact of maternal age on the effects of prenatal alcohol exposure on attention. Alcohol Clin Exp Res. 2010;34:1813–1821. doi: 10.1111/j.1530-0277.2010.01269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YY, Shamosh NA, Cho SH, DeYoung CG, Lee MJ, Le JM, Kim SI, Cho ZH, Kim K, Gray JR, et al. Multiple bases of human intelligence revealed by cortical thickness and neural activation. J Neurosci. 2008;28:10323–10329. doi: 10.1523/JNEUROSCI.3259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis, I: segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- DeMeyer W. Median facial malformations and their implications for brain malformations. In: Bergsma D, editor. Morphogenesis and malformation of face and brain. Birth defects: original article series. 7. Vol. 11. White Plains (NY): The National Foundation—March of Dimes; 1975. pp. 155–181. [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP. Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcohol Clin Exp Res. 2007;31:1415–1424. doi: 10.1111/j.1530-0277.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Godin EA, Dehart DB, Parnell SE, O'Leary-Moore SK, Sulik KK. Ventromedian forebrain dysgenesis follows early prenatal ethanol exposure in mice. Neurotoxicol Teratol. 2009;33:231–239. doi: 10.1016/j.ntt.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin EA, O'Leary-Moore SK, Khan AA, Parnell SE, Ament JJ, Dehart DB, Johnson BW, Allan Johnson G, Styner MA, Sulik KK. Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: effects of acute insult on gestational day 7. Alcohol Clin Exp Res. 2010;34:98–111. doi: 10.1111/j.1530-0277.2009.01071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum RL, Stevens SA, Nash K, Koren G, Rovet J. Social cognitive and emotion processing abilities of children with fetal alcohol spectrum disorders: a comparison with attention deficit hyperactivity disorder. Alcohol Clin Exp Res. 2009;33:1656–1670. doi: 10.1111/j.1530-0277.2009.01003.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Torii K, Kawasawa YI, Kuhn A, Rakic P. Combined transcriptome analysis of fetal human and mouse cerebral cortex exposed to alcohol. Proc Natl Acad Sci U S A. 2011;108(10):4212–4217. doi: 10.1073/pnas.1100903108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Johnson VP, Swayze VW, II, Sato Y, Andreasen NC. Fetal alcohol syndrome: craniofacial and central nervous system manifestations. Am J Med Genet. 1996;61:329–339. doi: 10.1002/(SICI)1096-8628(19960202)61:4<329::AID-AJMG6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Jones KL, Robinson LK, Bakhireva LN, Marintcheva G, Storojev V, Strahova A, Sergeevskaya S, Budantseva S, Mattson SN, Riley EP, et al. Accuracy of the diagnosis of physical features of fetal alcohol syndrome by pediatricians after specialized training. Pediatrics. 2006;118:e1734–e1738. doi: 10.1542/peds.2006-1037. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: a review. Neurosci Biobehav Rev. 2007;31:192–201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Li Z, Ma X, Peltier S, Hu X, Coles CD, Lynch ME. Occipital-temporal reduction and sustained visual attention deficit in prenatal alcohol exposed adults. Brain Imaging Behav. 2008;2:39–48. doi: 10.1007/s11682-007-9013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Rasmussen C, Wyper K, Walker L, Andrew G, Yager J, Beaulieu C. Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2008;32:1732–1740. doi: 10.1111/j.1530-0277.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- Lebel C, Roussotte F, Sowell ER. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsyhcol Rev. 2011;21:102–118.. doi: 10.1007/s11065-011-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Leonard C, Thompson P, Kan E, Jolley J, Welcome S, Toga A, Sowell E. Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: a longitudinal MRI analysis. Cereb Cortex. 2007;17:1092–1099. doi: 10.1093/cercor/bhl019. [DOI] [PubMed] [Google Scholar]
- Malisza KL, Allman AA, Shiloff D, Jakobson L, Longstaffe S, Chudley AE. Evaluation of spatial working memory function in children and adults with fetal alcohol spectrum disorders: a functional magnetic resonance imaging study. Pediatr Res. 2005;58:1150–1157. doi: 10.1203/01.pdr.0000185479.92484.a1. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Calarco KE, Lang AR. Focused and shifting attention in children with heavy prenatal alcohol exposure. Neuropsychology. 2006;20:361–369. doi: 10.1037/0894-4105.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Foroud T, Sowell ER, Jones KL, Coles CD, Fagerlund A, Autti-Rämö I, May PA, Adnams CM, Konovalova V, et al. Collaborative initiative on fetal alcohol spectrum disorders: methodology of clinical projects. Alcohol. 2010;44:635–641. doi: 10.1016/j.alcohol.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 1999;23:1808–1815. [PubMed] [Google Scholar]
- Mattson SN, Jernigan TL, Riley EP. MRI and prenatal alcohol exposure: images provide insight into FAS. Alcohol Health Res World. 1994;18:49–52. [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. J Pediatr. 1997;131:718–721. doi: 10.1016/s0022-3476(97)70099-4. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Jernigan TL, Ehlers CL, Dells DC, Jones KL, Stern C, Johnson KA, Hesselink JR, Bellugi U. Fetal alcohol syndrome: a case report of neuropsychological. MRI and EEG assessment of two children. Alcohol Clin Exp Res. 1992;16:1001–1003. doi: 10.1111/j.1530-0277.1992.tb01909.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Sowell ER, Jernigan TL, Sobel DF, Jones KL. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20:1088–1093. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Roebuck TM. Acquisition and retention of verbal and nonverbal information in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2002;26:875–882. [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, Adnams CM, Hoyme HE, Jones KL, Robinson LK, Khaole NC, Snell C, Kalberg WO, et al. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. 2007;88:259–271. doi: 10.1016/j.drugalcdep.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AL, Crocker N, Mattson SN, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Dev Disabil Res Rev. 2009;15:209–217. doi: 10.1002/ddrr.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer E, Sarioglu S, Gure A. Effects of prenatal ethanol exposure on neuronal migration, neuronogenesis and brain myelination in the mice brain. Clin Neuropathol. 2000;19:21–25. [PubMed] [Google Scholar]
- Parnell SE, O'Leary-Moore SK, Godin EA, Dehart DB, Johnson BM, Johnson AG, Styner MA, Sulik KK. Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: effects of acute insult on gestational day 8. Alcohol Clin Exp Res. 2009;33:1001–1011. doi: 10.1111/j.1530-0277.2009.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen C, Horne K, Witol A. Neurobehavioral functioning in children with fetal alcohol spectrum disorder. Child Neuropsychol. 2006;12:453–468. doi: 10.1080/09297040600646854. [DOI] [PubMed] [Google Scholar]
- Redila VA, Olson AK, Swann SE, Mohades G, Webber AJ, Weinberg J, Christie BR. Hippocampal cell proliferation is reduced following prenatal ethanol exposure but can be rescued with voluntary exercise. Hippocampus. 2006;16:305–311. doi: 10.1002/hipo.20164. [DOI] [PubMed] [Google Scholar]
- Reobuck-Spencer TM, Mattson SN. Implicit strategy affects learning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2004;28:1424–1431. doi: 10.1097/01.alc.0000139826.25247.5b. [DOI] [PubMed] [Google Scholar]
- Riikonen RS, Salonen I, Partanen K, Verho S. Brain perfusion SPECT and MRI in fetal alcohol syndrome. Dev Med Child Neurol. 1999;41:652–659. doi: 10.1017/s0012162299001358. [DOI] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res. 1995;19:1198–1202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med. 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Roussotte FF, Bramen JE, Nunez SC, Quandt LC, Smith L, O'Connor MJ, Bookheimer SY, Sowell ER. Abnormal brain activation during working memory in children with prenatal exposure to drugs of abuse: the effects of methamphetamine, alcohol, and polydrug exposure. Neuroimage. 2011;54:3067–3075. doi: 10.1016/j.neuroimage.2010.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussotte FF, Sulik KK, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, O'Connor MJ, Narr KL, Sowell ER. Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21260. doi: 10.1002/hbm.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam P, Li Z, Hu X, Lynch ME, Coles CD. Effects of prenatal alcohol exposure on brain activation during an arithmetic task: an fMRI study. Alcohol Clin Exp Res. 2009;33:1901–1908. doi: 10.1111/j.1530-0277.2009.01028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Larson JA, Barr CS, Dejesus OT, Roverts AD. Timing of moderate level prenatal alcohol exposure influences gene expression of sensory processing behavior in rhesus monkeys. Front Integr Neurosci. 2009;3:30. doi: 10.3389/neuro.07.030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JP. Neurotransmitters as neurotrophic factors: a new set of functions. Int Rev Neurobiol. 1992;34:1–23. doi: 10.1016/s0074-7742(08)60096-3. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Phillips DE. Effects of prenatal ethanol exposure on the development of Bergmann glia and astrocytes in the rat cerebellum: an immunohistochemical study. J Comp Neurol. 1992;321:19–32. doi: 10.1002/cne.903210103. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973;62(1):1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285:931–945. [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Kan E, Thompson PM, Riley EP, Toga AW. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb Cortex. 2008;18:136–144. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Thompson PM, Jernigan TL, Riley EP, Toga AW. Mapping callosal morphology and cognitive correlates: effects of heavy prenatal alcohol exposure. Neurology. 2001;57:235–244. doi: 10.1212/wnl.57.2.235. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb Cortex. 2002;12:856–865. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Peterson BS, Mattson SN, Welcome SE, Henkenius AL, Riley EP, Jernigan TL, Toga AW. Mapping cortical gray matter asymmetry patterns in adolescents with heavy prenatal alcohol exposure. Neuroimage. 2002;17:1807–1819. doi: 10.1006/nimg.2002.1328. [DOI] [PubMed] [Google Scholar]
- Spadoni AD, Bazinet AD, Fryer SL, Tapert SF, Mattson SN, Riley EP. BOLD response during spatial working memory in youth with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2009;33:2067–2076. doi: 10.1111/j.1530-0277.2009.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Sulik KK. Genesis of alcohol-induced craniofacial dysmorphism. Exp Biol Med (Maywood) 2005;230:366–375. doi: 10.1177/15353702-0323006-04. [DOI] [PubMed] [Google Scholar]
- Swayze VW, II, Johnson VP, Hanson JW, Piven J, Sato Y, Giedd JN, Mosnik D, Andreasen NC. Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics. 1997;99:232–240. doi: 10.1542/peds.99.2.232. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Østby Y, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Østby Y, Walhovd KB, Westlye LT, Due-Tønnessen P, Fjell AM. Neuroanatomical correlates of executive functions in children and adolescents: a magnetic resonance imaging (MRI) study of cortical thickness. Neuropsychologia. 2010;48:2496–2508. doi: 10.1016/j.neuropsychologia.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, Herman D, Hong MS, Dittmer SS, Doddrell DM, et al. Dynamics of gray matter loss in Alzheimer's disease. J Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler intelligence scale for children. 4th ed. San Antonio (TX): The Psychological Corporation; 2003. [Google Scholar]
- Westlye LT, Grydeland H, Walhovd KB, Fjell AM. Associations between regional cortical thickness and attentional networks as measured by the attention network test. Cereb Cortex. 2011;21:345–356. doi: 10.1093/cercor/bhq101. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J Int Neuropsychol Soc. 2008;14:1022–1033. doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]
- Yang Y, Nuechterlein KH, Phillips O, Hamilton LS, Subotnik KL, Asarnow RF, Toga AW, Narr KL. The contributions of disease and genetic factor towards regional cortical thinning in schizophrenia: the UCLA family study. Schizophr Res. 2010;123(2–3):116–125. doi: 10.1016/j.schres.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Abnormal temporal and prefrontal cortical gray matter thinning in psychopaths. Mol Psychiatry. 2009;14:561–562. doi: 10.1038/mp.2009.12. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Morphological alterations in the prefrontal cortex and the amygdala in unsuccessful psychopaths. J Abnorm Psychol. 2010;119(3):546–554. doi: 10.1037/a0019611. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Abnormal structural correlates of response perseveration in individuals with psychopathy. J Neuropsychiatry Clin Neurosci. 2011;23(1):107–110. doi: 10.1176/appi.neuropsych.23.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]