Abstract
Background
Inherited red-green colour vision defects are quite common, affecting nearly 1 in 10 males, but are less common in women, affecting about 1 in 250. However because red-green defects are X-linked, nearly 15% of females are heterozygous carriers of red-green colour deficiency. In addition, about 1 in 150 females are “double carriers”, where both of their X chromosomes have L/M gene arrays encoding a red-green defect. If a woman carries the same type of colour vision defect on each X-chromosome, she herself will be red-green colour deficient, whereas if she carries opposing defects (protan vs. deutan) on each X chromosome she will be trichromatic, owing to the process of X-inactivation. These women are referred to as compound heterozygotes, though very few have been reported. Moreover, questions remain as to whether the colour vision capacity of these women is comparable to that of “normal” trichromats.
Methods
We examined a compound heterozygote carrier of both protanopia and deuteranomaly. We also examined male members of her family representing both forms of red-green defect carried by the female proband. Complete colour vision testing was done, including Rayleigh matches, pseudoichromatic plates, unique hue measurements, and 100-Hue tests. Flicker-photometric ERG estimates of L:M cone ratio were obtained, as were Medmont C100 settings.
Results
Genetic analyses provided direct confirmation of compound heterozygosity. The compound heterozygote showed Schmidt’s sign, consistent with an extreme skew in her L:M cone ratio, and usually associated with protan carrier status.
Conclusion
Apart from Schmidt’s sign, we found the colour vision of the compound heterozygote to be indistinguishable from that of a normal trichromat.
Keywords: Color Vision, Retina, Protan, Deutan
INTRODUCTION
Normal human colour vision is trichromatic, based on the presence of three spectrally-distinct types of cone photoreceptors in the retina (long-, middle-, and short-wavelength sensitive; L-, M-, and S-, respectively). The severe form of congenital red-green colour vision deficiency, dichromacy, is associated with a loss of L- or M-cone function, while a less severe form of color defect, known as anomalous trichromacy, occurs when the L or M pigment is altered in its spectral sensitivity. There are two broad categories of “red-green” defects, protan and deutan. Protan defects are characterized by an absence or anomaly of L-cone function, while deutan defects are characterized by an absence or anomaly of M-cone function. Both protan and deutan defects are associated with disruptions in the X-linked L/M gene array, which can include an absence of L or M genes or mutations in L or M genes encoding anomalous or even nonfunctional pigments.
About 8% of males have a red-green colour vision deficiency, and these defects are transmitted in an X-linked recessive fashion. As females possess two X-chromosomes, they can “carry” a red-green colour vision deficiency – that is, one of their L/M gene arrays encodes a colour vision defect. In fact, some 15% of women are heterozygous carriers of colour vision deficiency. The quality of colour discrimination of these carriers has been of high interest – some evidence suggests they may have compromised colour vision 1–3, while others suggest their colour vision is indistinguishable from normal4. There even exists the hypothesis that they have better colour discrimination, owing to the fact that they may possess the photopigment basis for tetrachromatic colour vision 5.
Females can also possess two defective L/M gene arrays (one on each X chromosome). If the two arrays encode the same type of defect (e.g., protan or deutan), the female will manifest the corresponding type of defect. However, only about 0.4% of females actually have a red-green defect. If the two arrays encode opposing types of defect, the female should have normal colour vision, as the two-locus theory implies that the two defects complement one another. These women are referred to as double carriers or compound heterozygotes. It has generally been concluded that their colour vision is normal, however the reports are sparse 6–13 and there are no reports with both complete genetic data and thorough assessment of their colour discrimination. We present here a complete description of the colour vision in a woman carrying genes encoding both a protan and deutan defect, as well as detailed genetic basis of the defects. This individual provides an excellent opportunity to compare the trichromacy that comes from a retina in which the L and M cone submosaics arise by X-inactivation, to the routine trichromacy that most people have in which the L and M cones are specified by two different genes on one chromosome.
METHODS
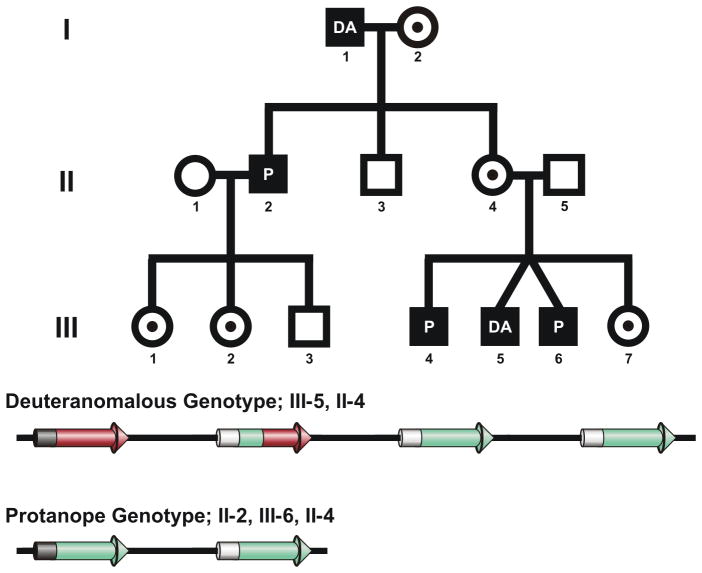
A complete pedigree of the family studied is shown in Figure 1. The brother (II-2) of the proband (II-4) was seen, having volunteered for a study on red-green colour vision deficiency. He presented with a classic protanope phenotype (see Table 1). When taking a family history, he mentioned that his father also had a colour vision defect, and that they often disagreed on the appearance of certain objects. Recalling that red-green defects are X-linked, father-to-son transmission is not possible. Further questioning revealed he had a sister (II-4) with colour deficient sons. We mailed copies of the Neitz Test of Colour Vision (Western Psychological Services, Los Angeles, CA) 14 and the AO-HRR (Richmond Products, Albuquerque, NM) 15, along with instructions for administration. Results revealed two of her sons (III-4, III-6) were protan and the other (III-5) was deutan.
Figure 1.
Genetic analysis of a family with two types of colour vision defect. Top, Pedigree of a heterozygous female. I-2, III-1, III-2, and III-7 are all obligate carriers. They had normal colour vision but were untested genetically. I-1 and III-4 (also untested genetically) tested as deutan and protan, respectively, on the AO-HRR & Neitz Colour Vision Test. Bottom, Color defective genotypes present in the family. A complete genetic analysis and colour vision testing was done on II-2, II-4, III-5, and III-6. Results revealed a deuteranomalous geneotype in III-5 where the first two genes in the L/M array encoded L-type pigments differing slightly in spectral sensitivity. II-2 and III-6 had an L/M gene array predictive of a protanopic phenotype, as there were no L genes and the remaining M genes encoded pigments with identical spectral sensitivity. The proband (II-4) had both the deuteranomalous and protanopic gene arrays.
Table 1.
Results from Colour Vision Tests
| C-100† | FM-100‡ | Rayleigh§ | AO-HRR¶ | D-15†† | Desat D-15‡‡ | |
|---|---|---|---|---|---|---|
|
|
||||||
| II-2 (protanope) | −4.25 | 112 | Match All | >0.081 | Not Done | Not Done |
| II-4 (double carrier/proband) | −2.3 | 8 | 41–44 | None | None | None |
| III-5 (deuteranomalous) | 1 | Not Done | 23–35 | 0.051 | Deutan 2 d.c. 1.52 | Non-Specific 1.5 d.c 1.71 |
| III-6 (protanope) | Not Done | Not Done | Match All | >0.081 | Protan 8.5 d.c. 3.11 | Protan 6.5 d.c. 3.86 |
Average of 10 settings on the Medmont C-100
Average error score from two administrations
Limits of the match range on a Model 1 Nagel Anomaloscope
D values are reported, indicating the most difficult plate read by the subject. D value quantitatively expresses the difference in chromaticity between the symbol and the gray background, measured as the distance (D) in the CIE diagram between the symbol and the background.
Farnsworth D15; orientation, average number of diametrical crossings (d.c.) from 2 administrations, and average Color Confusion Index based on Bowman scoring method 29.
Lanthony’s Desaturated D15; orientation, average number of diametrical crossings (d.c.) from 2 administrations, and average Color Confusion Index based on Lanthony scoring method 30.
We then arranged for a subset of the family (II-4, III-5, and III-6) to travel to the lab for detailed color vision testing. The results, shown in Table 1, include the Farnsworth Dichotomous Test for Colour Blindness (D-15)(Psychological Corporation, New York), Lanthony’s Desaturated D-15 (Luneau Ophthalmologie, France), the total error score on the Farnsworth-Munsell 100 Hue Test (FM 100)(Macbeth Color and Photometry Division, Baltimore, Md) 16, the limits of the Rayleigh match range on a Nagel Model 1 Anomaloscope (Schmidt Haensch), the average of 10 settings on the Medmont C-100 17 and the D values (used to quantitatively express the color difference between the symbol and it’s gray background) for the AO-HRR (2002). Neither son was tested on the FM 100, nor was the protanopic son tested on the Medmont C-100. The proband’s (II-4) unique hues (blue, green, yellow) were also measured using a two alternative forced choice paradigm 18.
Using a flicker-photometric electroretinogram (ERG), spectral sensitivity was measured over a range of 480–680 nm in 10 nm increments for II-4, III-5, and III-6. In dichromats, this reveals the spectral sensitivity of the single functional cone type in the retina, whereas individuals with both L and M cones, it reflects some combined response of L and M cone function. By fitting such a spectral sensitivity curve to a weighted sum of an L and an M cone spectral sensitivity curve, it is possible to derive an estimate of the relative contribution of the L and M cone populations to the ERG19,20. This is inferred to represent the relative numerosity of the L and M cones (L:M cone ratio), and evidence to date supports this conclusion 21,22. Genetic analysis was also performed on II-2, II-4, III-5, and III-6. Here, DNA was extracted from whole blood or buccal swabs as described previously 23 and used in a previously described real-time quantitative polymerase chain reaction (PCR) assay to estimate the relative number of L and M genes in the X-chromosome visual pigment gene array 14. The L and M genes were selectively amplified by long-distance PCR, and the product obtained was subsequently used to amplify separately exons 2, 3, and 4 of L and of M genes for direct DNA sequence analysis. The primers and thermal cycling parameters for all amplifications were reported previously 20.
The mother’s discrimination was tested on a modified version of the Cambridge Colour Test24,25. The stimuli resemble the plates of a traditional pseudoisochromatic test in which the target and background are made up of many discrete disks that vary in size and luminance. These were generated by a VSG 2/3 video display card (Cambridge Research Systems Ltd., England), presented on a Sony Trinitron 21 inch monitor, and calibrated using a ColorCal colorimeter (Cambridge Research Systems Ltd., England). We modified the test to allow reductions in stimulus size and a greater range of stimulus durations. Chromatic contrast sensitivity was measured along three axes in u′v′ colour space. Coloured stimuli were viewed against a background field array of grey dots that had the coordinates of u′= 0.1970, v′= 0.4690. Stimulus presentation was 60 msec, and the outer diameter and gap of the ‘C’ subtended 0.68 deg. A staircase method for measuring chromatic contrast threshold was used, where 2 correct responses were required before challenging with a stimulus of lesser vector length. This diminished the impact of guessing on the threshold determination.
Informed consent was obtained from all adult subjects participating in the investigation as well as from parents of participating minors. Research on human subjects followed the tenets of the Declaration of Helsinki and was approved by the Institutional Review Boards at the University of Rochester and the Medical College of Wisconsin.
RESULTS
Molecular genetic analysis of the family shown in Figure 1 revealed a deuteranomalous genotype in III-5, and a protanopic genotype in II-2 & III-6. The proband (II-4) possessed both gene arrays, confirming her identity as a compound heterozygote. The genetic results were complimented by the subjects’ performance on a battery of psychophysical tests (Table 1). The performance of the proband (II-4) on the Medmont C-100 was similar to that reported for protan carriers 26,27, that is, she required more red light in her heterochromatic flicker-photometric match. Nevertheless, her unique hues were tested and unique yellow was 576.8 nm, unique green was 525.9 nm, and unique blue was 468.1 nm, all within normal ranges 28. She also performed normally on the remaining colour vision tests; specifically, the Rayleigh match, Lanthony D-15, Farnsworth Munsell 100, and AO-HRR.
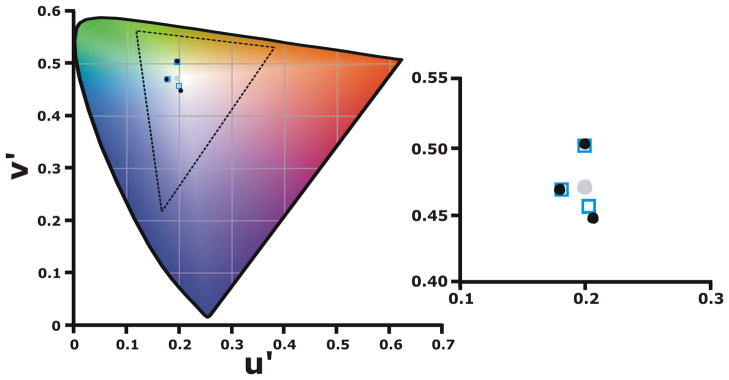
In order to evaluate her discrimination, we measured chromatic thresholds using a modified version of the Cambridge Colour test. Results from this modified version of the Cambridge Colour Test are shown in Figure 2, which show the proband (filled circles) as having comparable discrimination to controls (open squares). In this test, the further the result is from the gray background (filled circle), the worse the discrimination of the test subject.
Figure 2.
Cambridge Colour Test results. The filled gray circle represents the chromaticity of the background, filled black circles are the results from the double carrier proband (II-4), and the open squares are the average results from 11 normal subjects. The axis of one of the hues tested was along a tritan confusion line from background gray to violet (u′= 0.1990, v′= 0.5470, Tritan). The second hue tested was along a line from an average unique green for our subjects (u′=0.2230, v′= 0.3870, Deutan) to gray. The third was from an average unique blue to gray (u′=0.1210, v′= 0.4690, Protan). Shown on the right is a magnified view of the data, demonstrating the coincidence of the proband’s data with that of the controls.
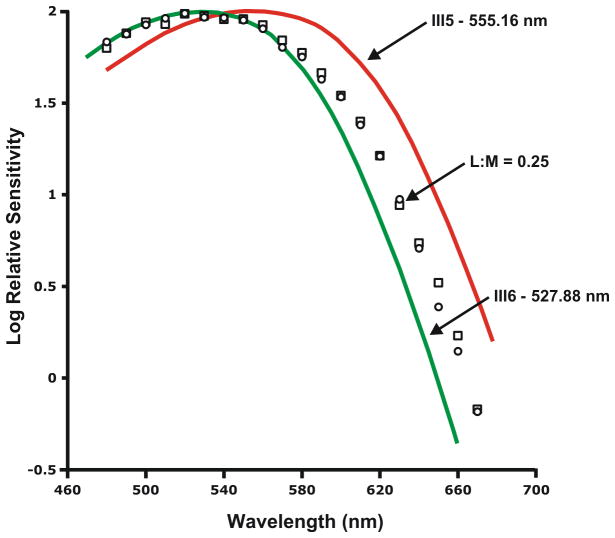
We used the flicker-photometric ERG to measure the spectral sensitivity of the proband (II-4) and two of her sons (III-5 & III-6). Subject III-5 had a peak sensitivity of 555.16 nm, consistent with no M-cone contribution to the ERG, while subject III-6 had a peak sensitivity of 527.88 nm, consistent with no L-cone contribution to the ERG. These values are consistent with in vitro data on isolated L and M photopigment, respectively. Normal trichromats have both L- and M-cone contribution to the ERG, so it is typical to fit their data to a weighted sum of an L and M cone fundamental. However here, rather than using average L and M spectra, we used the sons measured spectral sensitivities to interpret the mother’s data. This analysis produces an L:M ratio of about 0.25 (20 %L), as shown in Figure 3. We have shown previously that using “individualized” L and M cone curves to fit trichromat data is more accurate than using average L/M fundamentals 19. The left and right eyes were tested independently and the results were consistent. Moreover, this method provides a highly reliable estimate of L:M ratio, repeated measures in males deviate by about 2–3%L 20. The skewed L:M ratio is a result of a skew in X-inactivation; and has been observed in female New World primates who derive their trichromatic colour vision via the same process 22. Moreover, this skew in L:M ratio explains her seemingly aberrant performance on the Medmont C-100, which is typically reserved for protan carriers.
Figure 3.
L:M cone ratio. Flicker-photometric ERG was used to measure the spectral sensitivity of the compound heterozygote (II-4), and her sons’ (III-5 and III-6) measured spectral sensitivities were used to interpret their mother’s L:M ratio. The red and green curves are the ERG derived spectral sensitivity of III-5, and III-6 respectively. Open circles and open squares represent data from the mother’s right and left eye respectively and indicate an L:M ratio of about 0.25 (20%L).
DISCUSSION
This report provides a detailed description of an often-overlooked population of females, those who are double carriers of red-green colour vision deficiency and for the first time provides a complete genetic and psychophysical classification of one such compound heterozygote and her family. The behavioral and genetic data obtained supports the conclusion that each of the compound heterozygote’s X-chromosomes encodes by itself a colour vision deficiency; however, perceptually she has normal trichromatic vision. In fact, the only significant difference between the proband (II-4) and an average female is a biased cone ratio (detected on both the Medmont C-100 and the ERG). However, even normal males can have skewed L:M ratios21, although this variability is believed to be caused by a genetically separate mechanism. Nevertheless some males with skewed L:M cone ratios perform as female carriers on the Medmont C-100 (data not shown), demonstrating the potential confound introduced by the magnificent variability in the “normal” L/M cone mosaic.
This study reinforces the fact that taking a thorough family history is important when doing colour vision testing, as is asking for and recording qualitative impressions of their colour vision as well as that of family members. Without fully investigating family history and doing genetic analysis, there would be no reliable way to distinguish a compound heterozygote from a normal female, but such distinction is important in predicting the potential impact on the woman’s children. Male children of a compound heterozygote will manifest a colour vision deficiency, and female children will be obligate carriers of colour vision deficiency. Finally, the diagnostic utility the C-100 to identify carriers of color vision deficiencies bears thinking about, as individuals with skewed L:M cone ratios often perform abnormally, while some carriers with near equal L:M ratios can go undetected.
Acknowledgments
The authors thank Phyl Summerfelt, Matt Mauck, Debbie Conklyn, David Williams, Jay Neitz, and Maureen Neitz for their assistance in this study.
Statement on sources of funding: This study was supported by the Harry J. Heeb Foundation, an unrestricted grant from Research to Prevent Blindness, and the NEI (F32 EY014749).
Footnotes
Statement of conflict of interest: The authors have no association with any commercial organization or any financial interest in a product described or reported on in this manuscript.
Contributor Information
Diane M. Tait, Department of Ophthalmology, Medical College of Wisconsin.
Joseph Carroll, Departments of Ophthalmology and Biophysics, Medical College of Wisconsin.
References
- 1.Cohn SA, Emmerich DS, Carlson EA. Differences in the responses of heterozygous carriers of colorblindness and normal controls to briefly presented stimuli. Vision Research. 1989;29:255–62. doi: 10.1016/0042-6989(89)90129-6. [DOI] [PubMed] [Google Scholar]
- 2.Lang A, Good GW. Color discrimination in heterozygous deutan carriers. Optometry Vision Science. 2001;78:584–8. doi: 10.1097/00006324-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Feig K, Ropers H. On the incidence of unilateral and bilateral colour blindness in heterozygous females. Human Genetics. 1978;41:313–23. doi: 10.1007/BF00284765. [DOI] [PubMed] [Google Scholar]
- 4.Nagy AL, MacLeod DIA, Heyneman NE, Eisner A. Four cone pigments in women heterozygous for color deficiency. Journal of the Optical Society of America. 1981;71:719–22. doi: 10.1364/josa.71.000719. [DOI] [PubMed] [Google Scholar]
- 5.Jordan G, Mollon JD. A study of women heterozygous for colour deficiencies. Vision Research. 1993;33:1495–508. doi: 10.1016/0042-6989(93)90143-k. [DOI] [PubMed] [Google Scholar]
- 6.Brunner W. Ueber den Vererbungsmodus der verschiedenen Typen der angeborenen Rotgruenblindheit. Albrecht von Graefes Archiv für Klinische und Experimentelle Ophthalmologie. 1932;124:1–52. [Google Scholar]
- 7.Arias S, Rodríguez A. An informative large pedigree with four compound hemizygotes of three combinations of deutan and protan genes. Acta Científica Venezolana. 1973;24:44–52. [PubMed] [Google Scholar]
- 8.Drummond-Borg M, Deeb S, Motulsky AG. Molecular basis of abnormal red-green color vision: A family with three types of color vision defects. American Journal of Human Genetics. 1988;43:675–83. [PMC free article] [PubMed] [Google Scholar]
- 9.Franceschetti A, Klein D. Two families with parents of different types of red-green blindness. Acta genetica et statistica medica. 1957;7:255–9. doi: 10.1159/000150979. [DOI] [PubMed] [Google Scholar]
- 10.Kondo T. Untersuchungen bei angeborenen Farbensinn-Anomalien. Ueber das Zustandekommen und Wesen der angeborenen Farbensinn-Anomalien. Acta Societatis Ophthalmologicae Japonicae. 1941;45:659. [Google Scholar]
- 11.Tanabe S, Ichikawa K, Hukami K, Nakashima S. A family with protanomaly and deuteranomaly. Color Research and Application. 2001;26:S93–5. [Google Scholar]
- 12.Roth A, Klein D, Paccolat F, Hermes D, Pelizzone M, Mandel JL, Feil R. The progeny of the two protan and deutan families described by Franceschetti and Klein (1949, 1956), one generation later. Genealogy, color vision and genomic DNA. Ophtalmologie. 1989;3:275–8. [PubMed] [Google Scholar]
- 13.Vanderdonck R, Verriest G. Femme protanomale et heterozygote mixte (genes de la protanomalie et de la deuteranopie en position de repulsion) ayant deux fils deuteranopes, un fils protanomal et deux fils normaux. Biotypologie. 1960;21:110–20. [Google Scholar]
- 14.Neitz M, Neitz J. A new mass screening test for color-vision deficiencies in children. Color Research and Application. 2001;26:S239–S49. [Google Scholar]
- 15.Bailey JE, Neitz M, Tait DM, Neitz J. Evaluation of an updated HRR color vision test. Visual Neuroscience. 2004;21:431–6. doi: 10.1017/s0952523804213463. [DOI] [PubMed] [Google Scholar]
- 16.Kinnear PR, Sahraie A. New Farnsworth-Munsell 100 hue test norms of normal observers for each year of age 5–22 and for decades 30–70. British Journal of Ophthalmology. 2002;86:1408–11. doi: 10.1136/bjo.86.12.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metha AB, Vingrys AJ. The C-100: a new dichotomiser of colour vision defectives. Clinical and Experimental Optometry. 1992:114–23. [Google Scholar]
- 18.Neitz J, Carroll J, Yamauchi Y, Neitz M, Williams DR. Color perception is mediated by a plastic neural mechanism that is adjustable in adults. Neuron. 2002;35:783–92. doi: 10.1016/s0896-6273(02)00818-8. [DOI] [PubMed] [Google Scholar]
- 19.Carroll J, Neitz M, Neitz J. Estimates of L:M cone ratio from ERG flicker photometry and genetics. Journal of Vision. 2002;2:531–42. doi: 10.1167/2.8.1. [DOI] [PubMed] [Google Scholar]
- 20.Carroll J, McMahon C, Neitz M, Neitz J. Flicker-photometric electroretinogram estimates of L:M cone photoreceptor ratio in men with photopigment spectra derived from genetics. Journal of the Optical Society of America A. 2000;17:499–509. doi: 10.1364/josaa.17.000499. [DOI] [PubMed] [Google Scholar]
- 21.Hofer H, Carroll J, Neitz J, Neitz M, Williams DR. Organization of the human trichromatic cone mosaic. Journal of Neuroscience. 2005;25:9669–79. doi: 10.1523/JNEUROSCI.2414-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs GH, Williams GA. L and M cone proportions in polymorphic New World monkeys. Visual Neuroscience. 2006;23:365–70. doi: 10.1017/S0952523806233066. [DOI] [PubMed] [Google Scholar]
- 23.Neitz M, Neitz J, Grishok A. Polymorphism in the number of genes encoding long-wavelength sensitive cone pigments among males with normal color vision. Vision Research. 1995;35:2395–407. [PubMed] [Google Scholar]
- 24.Reffin J, Astell S, Mollon J. Trials of a computer-controlled colour vision test that preserves the advantages of pseudoisochromatic plates. In: Drum B, Moreland JD, Serra A, editors. Colour Vision Deficiencies X: Proceedings of the tenth Symposium of the International Research Group on Color Vision Deficiencies. Boston: Kluwer Academic Publishers; 1991. pp. 69–76. [Google Scholar]
- 25.Regan BC, Reffin JP, Mollon JD. Luminance noise and the rapid determination of discrimination ellipses in colour deficiency. Vision Research. 1994;34:1279–99. doi: 10.1016/0042-6989(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 26.Harris RW, Cole BL. Diagosing protan heterozygosity using the Medmont C-100 colour vision test. Clinical and Experimental Optometry. 2005;88:240–7. doi: 10.1111/j.1444-0938.2005.tb06702.x. [DOI] [PubMed] [Google Scholar]
- 27.Robbins HG. Silent substitution tests like the OSCAR and the Medmont C100 can identify protan and deutan carriers of abnormal colour vision. Clinical and Experimental Optometry. 2005;88:426–7. doi: 10.1111/j.1444-0938.2005.tb05111.x. [DOI] [PubMed] [Google Scholar]
- 28.Philipona DL, O’Regan JK. Color naming, unique hues, and hue cancellation predicted from singularities in reflection properties. Visual Neuroscience. 2006;23:331–9. doi: 10.1017/S0952523806233182. [DOI] [PubMed] [Google Scholar]
- 29.Bowman KJ. A method for quantitative scoring of the Farnsworth Panel D-15. Acta Ophthalmol (Copenh) 1982;60:907–16. doi: 10.1111/j.1755-3768.1982.tb00621.x. [DOI] [PubMed] [Google Scholar]
- 30.Lanthony P. Evaluation of the desaturated Panel D-15. I. Method of quantification and normal scores. J Fr Ophtalmol. 1986;9:843–7. [PubMed] [Google Scholar]