The ER chaperone GRP78/BiP is crucial for the development of rheumatoid arthritis.
Abstract
An accumulation of misfolded proteins can trigger a cellular survival response in the endoplasmic reticulum (ER). In this study, we found that ER stress–associated gene signatures were highly expressed in rheumatoid arthritis (RA) synoviums and synovial cells. Proinflammatory cytokines, such as TNF and IL-1β, increased the expression of GRP78/BiP, a representative ER chaperone, in RA synoviocytes. RA synoviocytes expressed higher levels of GRP78 than osteoarthritis (OA) synoviocytes when stimulated by thapsigargin or proinflammatory cytokines. Down-regulation of Grp78 transcripts increased the apoptosis of RA synoviocytes while abolishing TNF- or TGF-β–induced synoviocyte proliferation and cyclin D1 up-regulation. Conversely, overexpression of the Grp78 gene prevented synoviocyte apoptosis. Moreover, Grp78 small interfering RNA inhibited VEGF165-induced angiogenesis in vitro and also significantly impeded synoviocyte proliferation and angiogenesis in Matrigel implants engrafted into immunodeficient mice. Additionally, repeated intraarticular injections of BiP-inducible factor X, a selective GRP78 inducer, increased synoviocyte proliferation and angiogenesis in the joints of mice with experimental OA. In contrast, mice with Grp78 haploinsufficiency exhibited the suppression of experimentally induced arthritis and developed a limited degree of synovial proliferation and angiogenesis. In summary, this study shows that the ER chaperone GRP78 is crucial for synoviocyte proliferation and angiogenesis, the pathological hallmark of RA.
Rheumatoid arthritis (RA) is characterized by tumor-like expansion of the synovium and the subsequent destruction of adjacent articular cartilage and bone (Feldmann et al., 1996; Firestein, 1996). In RA synovium, fibroblast-like synoviocytes (FLSs), the major cell population in invasive pannus, actively participate in the inflammatory processes of RA (Firestein, 1996). They produce not only several matrix metalloproteinases but also proinflammatory cytokines such as IL-1 and IL-6 (Firestein, 1996) and angiogenic factors such as vascular endothelial growth factor (VEGF; Fava et al., 1994). In addition, RA FLSs proliferate abnormally, show resistance to Fas-mediated apoptosis (Okamoto et al., 2000), and are able to induce cartilage destruction in the absence of other immune cells when adoptively transferred into SCID mice (Müller-Ladner et al., 1996). In contrast, angiogenesis, the process of new blood vessel formation, is also highly active in RA, particularly during the onset of the disease (Koch, 1998). Furthermore, newly formed vessels can transport inflammatory cells to synovitis sites and supply nutrients and oxygen to the pannus and thus maintain a chronic inflammatory state (Firestein, 1999).
ER stress is a cellular danger signal, which is triggered by a failure to fold newly synthesized ER proteins. Diverse conditions including hypoxia and low glucose, which are frequently observed in the RA joints (Stevens et al., 1991), may act as an ER stress to the cells, although evidence of this is lacking. Unless two compensatory mechanisms of unfolded protein response (UPR) and ER-associated degradation (ERAD) work properly, ER stress causes cell damage and eventually cell death (Rutkowski and Kaufman, 2004; Schröder and Kaufman, 2005). It has become apparent that dysregulated UPR plays an important role in the pathogenesis of some human diseases (Kaufman, 2002; Pfaffenbach and Lee, 2011) such as diabetes mellitus and Alzheimer’s disease, in which affected tissues are devoted to extracellular protein synthesis (Katayama et al., 2004). RA shares a common feature with these disease conditions, in that synoviocytes and other inflammatory cells produce large amount of proteins like cytochemokines and matrix metalloproteinases that perpetuate arthritic conditions.
GRP78/BiP is a molecular chaperone with antiapoptotic properties and a central regulator of ER homeostasis (Lee, 2007; Pfaffenbach and Lee, 2011). GRP78 promotes tumor proliferation, survival, metastasis, and resistance to a wide variety of therapies (Lee, 2007; Dong et al., 2008; Pfaffenbach and Lee, 2011). The discovery of GRP78 expression on the cell surfaces of cancer cells has led further to the development of new therapeutic approaches to cancer (Lee, 2007; Pfaffenbach and Lee, 2011). Rheumatoid synovium can be viewed as a local tumor because synoviocytes, the principal components of the pannus, proliferate abnormally in RA joints in a manner reminiscent of tumors; for example, they resist apoptosis and also exhibit other features of metastatic cancer cells (Firestein, 1996; Müller-Ladner et al., 1996; Okamoto et al., 2000). Therefore, it can be postulated that abnormal GRP78 response to ER stress contributes to synoviocyte hyperplasia and leads to bone and cartilage destruction in RA, although the exact role of ER stress in the pathophysiology of RA remains to be determined.
We demonstrate herein that ER stress–associated gene signatures are highly expressed in RA synovia and synovial macrophages. In cultured synoviocytes, proinflammatory cytokines, such as TNF and IL-1β, increased GRP78 expression. Down-regulation of Grp78 transcripts using small interfering RNA (siRNA) increased the apoptosis of RA FLSs while abolishing TNF- or TGF-β–induced FLS proliferation and cyclin D1 up-regulation. Moreover, Grp78 siRNA blocked VEGF165-stimulated angiogenesis in vitro and impeded synoviocyte proliferation and angiogenesis in Matrigel implants that were engrafted into immunodeficient mice. On the contrary, BiP-inducible factor X (BIX), a selective GRP78 inducer, prevented the apoptosis of FLSs and increased the progression of experimental osteoarthritis (OA) in mice, mimicking RA pathology. Moreover, heterozygous deficiency of the Grp78 gene resulted in a limited degree of inflammatory cell infiltration, angiogenesis, and synovial hyperplasia and prevented the progression of experimental arthritis in mice. Collectively, our results suggest that GRP78 is crucial for the survival and proliferation of RA synoviocytes and angiogenesis, which suggests that the ER chaperone GRP78 response contributes to the pathogenesis of RA.
RESULTS
Expression of ER stress–associated genes in RA synovia and synovial cells
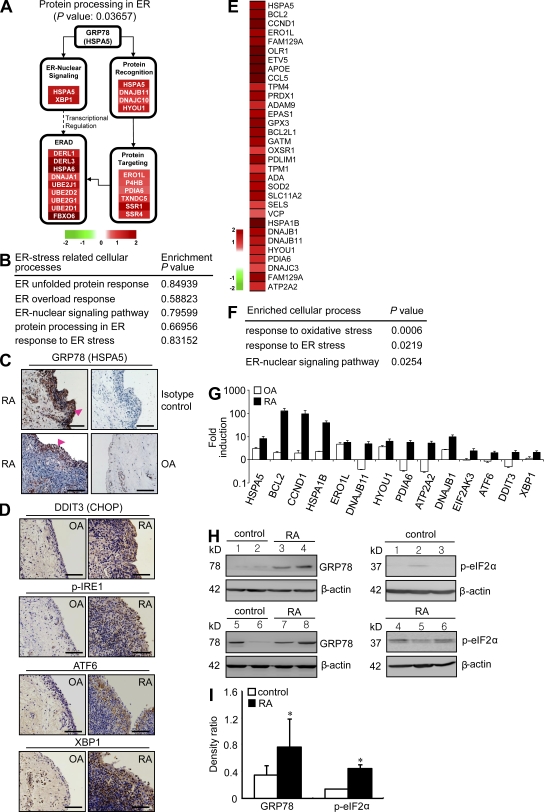
To demonstrate that ER stress response is activated in RA synovium, we first investigated global gene expression profiles in the synovial tissues of RA patients. We analyzed three synovial tissue–derived microarray datasets published previously in the GEO DataSets database (available under accession nos. GSE1919, GSE7307, and GSE12021). As shown in Fig. 1 A, differentially expressed genes (DEGs) between RA and normal subjects were identified using integrative statistical hypothesis testing (Bolstad et al., 2003; Hwang et al., 2005; see Materials and methods). From the KEGG pathway database, protein processing in ER pathways, including ER nuclear signaling, ER protein recognition (e.g., GRP78/HSPA5), ERAD, and ER protein targeting pathway, was significantly enriched by DEGs up-regulated in RA synoviums (Fig. 1 A). However, unlike RA synoviums, there was no statistical difference in above ER stress–associated pathways between OA and normal synoviums (Fig. 1 B). Immunohistochemical analysis was also performed to examine the expressions and localizations of ER stress marker proteins in the synovial tissues of three RA patients and three OA patients. The expression of GRP78, a well-established ER stress marker, was observed in all three RA synovial tissue sections at high levels. Positive staining was predominantly seen in the lining layer of the hyperplastic synovium (Fig. 1 C). GRP78 was also expressed in the lining layer of OA synovium (Fig. 1 C), but its expression level was relatively weak compared with that in RA synovium. Moreover, RA synoviums exhibited higher expression levels of other ER stress–associated molecules, including DDIT3 (CHOP), IRE1, ATF6, and XBP-1 (spliced and total) in the lining layer and/or sublining leukocytes as compared with OA synoviums (Fig. 1 D).
Figure 1.
ER stress response is increased in RA synovia and synovial cells. (A) Illustration of protein processing in the ER pathway with up-regulated genes in RA synovial tissue. Node colors represent fold change in RA synovial tissues as compared with normal synovial tissues (red, up-regulated; and green, down-regulated). (B) Functional enrichment analysis of up-regulated genes in OA tissues compared with normal. (C and D) Representative immunohistochemical stainings for ER stress–associated molecules in synovium sections obtained from RA and OA patients. (C) Tissues stained with anti-GRP78 antibody are representative of three RA and three OA patients. Intense staining in the synovium was observed in the lining layer (arrowheads). (D) Immunohistochemical staining of RA and OA synovia using anti-DDIT3 (CHOP), anti–p-IRE1, anti-ATF6, and anti-XBP1 antibody. Bars, 120 µm. (E) Heat map displaying 32 ER stress–associated up-regulated genes in RA macrophages. (F) Cellular processes enriched by DEGs in RA macrophages. Functional enrichment analysis of up-regulated DEGs was performed using DAVID software. (G) Quantitative real-time PCR assays of a subset of DEGs up-regulated in RA macrophages (n = 5) and proximal UPR genes. Four independent macrophage samples of healthy subjects and nine OA macrophages isolated from OA synovial tissues were used as controls. Fold inductions were calculated using the 2−ΔΔCt method. (H) Basal expression levels of ER stress–associated molecules in macrophages of healthy controls and RA patients, as determined by Western blot analysis using anti-GRP78 (left) and p-eIF2α (right) antibody. (I) Comparison of the optical density ratio ([GRP78 or p-eIF2α]/β-actin) between RA macrophages and normal (control) macrophages. *, P < 0.05 versus normal macrophages. (G and I) Data show mean ± SD.
We also analyzed ER-associated gene expression profiles in synovial cells isolated from RA joint fluids. Fig. 1 E shows the DEGs between RA synovial macrophages (n = 6) and normal macrophages derived from peripheral blood monocytes (n = 2). Of the 616 up-regulated DEGs found in RA synovial macrophages, the heat map shows 32 DEGs associated with ER stress processes. Three cellular processes, including response to oxidative stress, response to ER stress, and the ER-nuclear signaling pathway, were significantly enriched by 32 DEGs using DAVID software (Fig. 1, E and F). Using real-time PCR analysis, we also confirmed that independent RA macrophages (n = 5) exhibited higher messenger RNA (mRNA) expression levels of 10 selected DEGs and proximal UPR genes, including CHOP, ATF6, and the spliced form of XBP1 (sXBP1), than independent normal (n = 4) and OA macrophages (n = 9; Fig. 1 G). Moreover, basal levels of GRP78 and p-eIF2α protein were also higher in RA macrophages than normal macrophages (Fig. 1, H and I). Collectively, ER stress–associated gene signatures were highly expressed in synoviums and synovial cells of RA patients.
GRP78 expression is induced in synoviocytes by proinflammatory cytokines
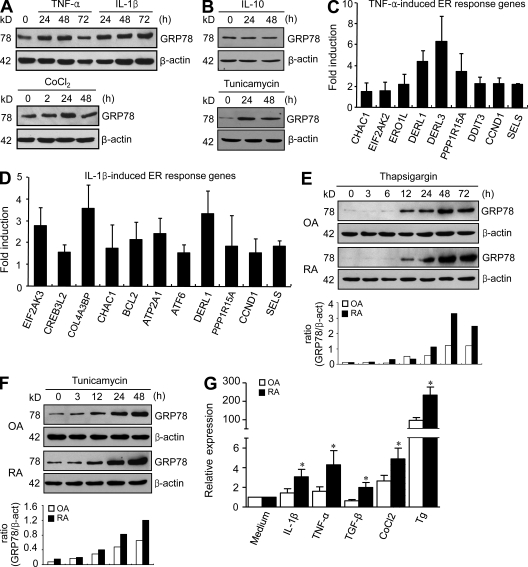
In addition to synovial macrophages, FLSs are a major component of the lining layer and invasive pannus. To determine whether FLSs are also involved in ER stress responses, we analyzed the mRNA expression profiles of primary RA (n = 3) and OA (n = 3) FLSs. Interestingly, unlike in freshly isolated RA macrophages, the aforementioned ER processes were not enriched by DEGs up-regulated in RA FLSs (not depicted). Western blot analysis revealed that GRP78, IRE1, XBP1, and eIF2α expression levels were similar in RA (n = 8) and OA (n = 8; not depicted) FLSs, which contrasts with the results in synovial tissues and macrophages (Fig. 1). We postulated that favorable culture conditions from passage 3 to 8, during which the medium contained high growth factors and culture was performed under normoxic conditions, might hamper abnormal ER response in RA FLSs. Therefore, we sought to identify stimuli that mimic or trigger ER responses in FLSs. In inflamed joints, rheumatoid synoviocytes are exposed to various proinflammatory cytokines, such as IL-1β and TNF, and hypoxia (Feldmann et al., 1996). Thus, an experiment was conducted to determine the effect of the proinflammatory cytokines on the GRP78 expression in FLSs because GRP78 is known to be the central regulator of ER stress (Lee, 2007; Pfaffenbach and Lee, 2011). GRP78 expression in the RA FLSs increased up to 48 h after stimulation with 1 ng/ml IL-1β or 10 ng/ml TNF (Fig. 2 A). Furthermore, GRP78 was also found to be induced by 100 µM CoCl2 treatment, which simulates hypoxic conditions (Fig. 2 A). In contrast, 10 ng/ml IL-10, an antiinflammatory cytokine, failed to increase GRP78 expression, whereas 20 µg/ml tunicamycin instigated it in the same cells (Fig. 2 B). Both IL-1β and TNF induced other ER stress response genes, including CHAC1, EIF2AK2, ERO1L, DERL1, DERL3, PPP1R15A, DDIT3, CCND1, SELS, EIF2AK3, CREB3L2, COL4A3BP, BCL2, ATP2A1, and ATF6, in RA FLSs (Fig. 2, C and D). In addition, thapsigargin or tunicamycin time-dependently increased GRP78 protein expression in both RA and OA synoviocytes, but these increases occurred to a greater extent in RA cells than in OA cells (Fig. 2, E and F). Moreover, Grp78 mRNA expression levels in RA FLSs, stimulated with IL-1β, TNF, TGF-β, CoCl2, or thapsigargin, were significantly higher than those in stimulated OA FLSs, indicating that RA FLSs hyperrespond to these cytokines and other ER stresses (Fig. 2 G). Collectively, these results suggest that RA synoviocytes exhibit increased GRP78 response to proinflammatory cytokines and hypoxia, which induces hyper-UPR of RA FLSs.
Figure 2.
Induction of GRP78 in synoviocytes by proinflammatory cytokines. (A and B) Regulation of GRP78 expression in RA FLSs by proinflammatory cytokines. Cells were stimulated with 10 ng/ml TNF, 1 ng/ml IL-1β, 10 ng/ml IL-10, or 100 µM CoCl2 for the indicated times. 20 µg/ml tunicamycin was used as a positive control. GRP78 levels in FLSs were determined by Western blot analysis. The data shown are representative of more than four experiments with similar results. (C and D) Quantitative real-time PCR assays of ER stress response genes induced by TNF (C) or IL-1β (D) in RA FLSs. Cells were stimulated with 10 ng/ml TNF or 1 ng/ml IL-1β for 48 h. Fold inductions were calculated using the 2−ΔΔCt method. Data show mean ± SD. (E and F) RA (n = 4) and OA (n = 4) FLSs were treated with 10 µM thapsigargin (E) or 20 µg/ml tunicamycin (F) for the indicated times. GRP78 expression was determined by Western blot analysis. In the lower panel, the optical density ratio of GRP78/β-actin (β-act) expression is presented as the mean values of four separate experiments. (G) Comparison of Grp78 mRNA expression in OA (n = 6) versus RA (n = 6) FLSs, as determined by real-time PCR. Cells were stimulated with 1 ng/ml IL-1β, 10 ng/ml TNF, 10 ng/ml TGF-β, 100 µM CoCl2, or 5 µM thapsigargin (Tg) for 8 h. Bars show mean and SEM. *, P < 0.05 versus OA FLSs.
GRP78 is a regulator of synoviocyte survival
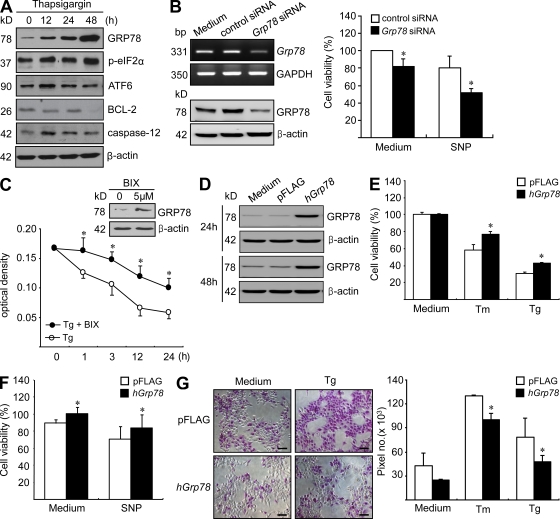
ER chaperones like GRP78 serve as a class of regulators of cell survival. GRP78 protects against ER stress–induced apoptosis in a wide variety of tissues and organs, as well as in cancer cells (Lee, 2007; Dong et al., 2008; Pfaffenbach and Lee, 2011). However, the role of GRP78 in FLS death under conditions of ER stress remains to be defined. Therefore, we investigated whether GRP78 regulates cell survival in RA FLSs. Several ER stress–associated proteins, including GRP78, ATF-6, BCL-2, and caspase-12, are involved in cell survival or death (Kaufman, 2002; Rutkowski and Kaufman, 2004; Schröder and Kaufman, 2005). As shown in Fig. 3 A, thapsigargin treatment increased the expressions of GRP78, p-eIF2α, ATF-6, and caspase-12 in rheumatoid synoviocytes but decreased BCL-2 expression. To examine the role of GRP78 in the survival of RA FLSs, we introduced Grp78 siRNA into the RA FLSs. As shown in Fig. 3 B, Grp78 knockdown rendered FLSs more susceptible to apoptosis in the presence or absence of sodium nitroprusside (SNP). Recently, by screening chemical libraries, Kudo et al. (2008) identified a novel molecule BiP/GRP78 inducer X (BIX) that selectively induces GRP78. It has been reported that BIX pretreatment reduces ER stress–induced cell death by preferentially increasing GRP78 expression in neuroblastoma cells (Kudo et al., 2008; Oida et al., 2008). As shown in Fig. 3 C, BIX increased GRP78 protein expression in RA FLSs and protected RA FLSs from the cell death induced by thapsigargin. Additionally, overexpression of the Grp78 gene blocked tunicamycin-, thapsigargin-, or SNP-induced apoptotic death of RA FLSs (Fig. 3, D–G), indicating that GRP78 inhibits FLS death. Moreover, when treated with thapsigargin or tunicamycin, RA FLSs showed less apoptosis than OA FLSs, as assessed by MTT assay, DNA fragmentation ELISA, and the APOPercentage apoptosis assay (not depicted), which is in accord with our results on inducible GRP78 expression (Fig. 2, E–G) and with a previous study (Yamasaki et al., 2006). The aforementioned findings suggest that intracellular GRP78 plays a critical role in maintaining synoviocyte survival against proapoptotic ER stress conditions in joints.
Figure 3.
Role of GRP78 in synoviocyte survival. (A) Changes in ER sensor proteins in RA synoviocytes treated with 10 µM thapsigargin. The expressions of GRP78, p-eIF2α, ATF-6, BCL-2, and caspase-12 were determined by Western blot analysis. (B) The effect of Grp78 knockdown on synoviocyte survival. 2 d after transfection with siRNA for Grp78, the mRNA and protein expression levels of GRP78 in RA FLSs were determined by RT-PCR and Western blot analysis, respectively (left). The apoptosis of RA FLSs was induced by treating cells with 1 mM SNP for 12 h 2 d after Grp78 siRNA transfection. Degree of cell death was assessed by MTT assay (right). Results are the mean ± SD of more than four independent experiments performed in duplicate. *, P < 0.05 versus control siRNA–transfected cells. (C) RA FLSs were treated with BIX for 12 h, and then GRP78 expression was determined by Western blot analysis. Synoviocyte apoptosis was induced by treating RA FLSs with 10 µM thapsigargin in the presence or absence of BIX. *, P < 0.05 versus thapsigargin-treated cells in the absence of BIX. (D) SV40-immortalized RA FLSs were transfected with either the pFLAG-hGrp78 gene or pFLAG vector only. The protein expression levels for GRP78 were determined by Western blotting. (E and F) RA FLSs transfected with either the pFLAG-hGrp78 gene or pFLAG vector were treated with 5 µM thapsigargin (Tg) for 1 h, 10 µg/ml tunicamycin (Tm) for 12 h, or 5 mM SNP for 24 h. Cell viability was determined by MTT assay. *, P < 0.05 versus vector-transfected cells. (G) The apoptosis of RA FLSs harboring the pFLAG-hGrp78 gene or pFLAG vector was induced by treating cells with 5 µM thapsigargin or 10 µg/ml tunicamycin for 3 h. Degrees of apoptosis were assessed by APOPercentage apoptosis assay, a colorimetric method. Apoptotic cells appeared bright pink. Fold increase in apoptosis levels was expressed as pixel numbers. *, P < 0.05 versus vector-transfected cells. Bars, 100 µm. (C and E–G) Data show mean ± SD.
GRP78 promotes synoviocyte proliferation
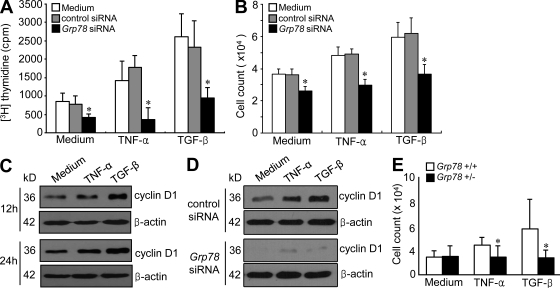
Several studies have shown that RA FLSs proliferate more rapidly than synoviocytes obtained from normal or OA joints (Mohr et al., 1975; Firestein, 1996). Therefore, we investigated whether GRP78 mediates the proliferation of RA FLSs using [3H]thymidine incorporation assays. When FLSs were stimulated with TGF-β or TNF for 1–5 d, the DNA synthesis activities of RA FLSs peaked at 3 d (not depicted). When we added these cytokines for 3 d to RA FLSs, TNF- or TGF-β–induced increases in [3H]thymidine incorporation were completely prevented by Grp78 siRNA but not by control (scrambled) siRNA (Fig. 4 A). The number of RA FLSs stimulated with TGF-β or TNF was also markedly reduced by Grp78 siRNA transfection (Fig. 4 B). The regulation of cyclin D1 expression represents an important step for the control of cellular proliferation in response to growth factors (Terada et al., 1999). As shown in Fig. 4 C, cyclin D1 levels in RA FLSs were time-dependently increased by TNF or TGF-β treatment. The down-regulation of Grp78 by siRNA resulted in a marked decrease in both the basal and cytokine-stimulated expressions of cyclin D1 in RA FLSs (Fig. 4 D). Moreover, when stimulated with TGF-β or TNF for 5 d, FLSs of Grp78+/− mice showed lower proliferative responses than those of wild-type littermates (Grp78+/+; Fig. 4 E). These results suggest that GRP78 is an important mediator of FLS proliferation.
Figure 4.
Effects of GRP78 on TGF-β– or TNF-induced synoviocyte proliferation. (A) Synoviocyte proliferative responses to TGF-β and TNF. 24 h after transfection with Grp78 siRNA, RA FLSs (n = 4; 1 × 104 cells) were treated with 10 ng/ml TGF-β or 10 ng/ml TNF for 72 h. FLS proliferation rate was determined by [3H]thymidine incorporation assay. Results are the means ± SD of four independent experiments performed in triplicate. *, P < 0.05 versus nontransfected cells. (B) 4 d after stimulating RA FLSs with 10 ng/ml TGF-β or 10 ng/ml TNF, manual cell counts were performed by trypan blue exclusion to identify viable cells. The results shown are the mean ± SD of three independent experiments performed in triplicate. *, P < 0.05 versus nontransfected cells. (C) Cyclin D1 expression in RA FLSs stimulated with 10 ng/ml TNF or 10 ng/ml TGF-β, as determined by Western blot analysis. A representative of three independent experiments is shown. (D) Effect of Grp78 knockdown on TNF- or TGF-β–induced increases in cyclin D1 expression in RA synoviocytes. Data are representative of three independent experiments. (E) Proliferation of FLSs obtained from Grp78+/− and Grp78+/+ mice. Cell number was determined by trypan blue exclusion 5 d after stimulating mouse FLSs with 10 ng/ml TNF or 10 ng/ml TGF-β. Data are presented as the mean ± SD of four mice per group. *, P < 0.05 versus FLSs from Grp78+/+ littermates.
Effect of GRP78 on VEGF-induced angiogenesis
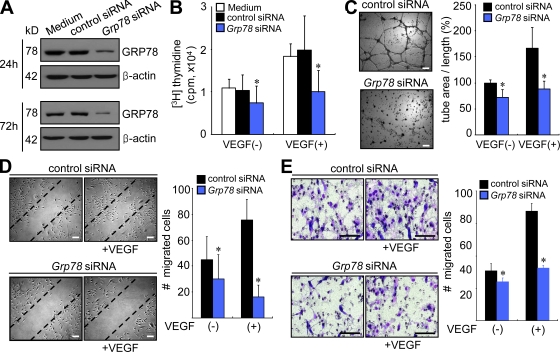
In addition to the abnormal proliferation of synoviocytes, increased angiogenesis is a critical step in the establishment and progression of RA (Koch, 1998; Firestein, 1999). In a previous study, partial reduction of GRP78 in Grp78+/− heterozygous mice and in mice with conditional Grp78 knockout in endothelial cells substantially reduced tumor microvessel densities (Dong et al., 2011). Furthermore, knockdown of Grp78 using siRNA suppressed human microvascular endothelial cell proliferation and increased apoptosis (Dong et al., 2011). In agreement, we found that Grp78 siRNA nearly completely inhibited VEGF165-stimulated human umbilical vein endothelial cell (HUVEC) proliferation (Fig. 5, A and B) but did not affect cell viability until 48 h after transfection, as determined by the MTT assay (not depicted). Under nonlethal time conditions, we then tested whether Grp78 siRNA regulates tube formation by endothelial cells. As was expected, VEGF165-induced tube formation was markedly reduced in the presence of the Grp78 siRNA (Fig. 5 C). During angiogenesis, endothelial cells migrate in response to several chemotactic factors. Therefore, we attempted to determine whether GRP78 affects VEGF165-induced HUVEC migration. As shown in Fig. 5 D, Grp78 siRNA suppressed VEGF165-induced wound migration of HUVECs without decreasing cell viability, as determined 24 h after transfection. Furthermore, VEGF165-induced HUVEC chemotaxis in a Boyden chamber was also blocked by transfection with Grp78 siRNA for 24 h but was not affected by control siRNA (Fig. 5 E). Collectively, these results indicate that GRP78 directly mediates VEGF165-induced migration/chemotaxis and endothelial cell proliferation.
Figure 5.
Grp78 siRNA inhibits proliferation, tube formation, migration, and chemotaxis of endothelial cells. (A) GRP78 expression levels in HUVECs were determined by Western blot analysis. (B) Effect of Grp78 siRNA on HUVEC proliferation. HUVECs were incubated for 24 h in DME supplemented with 1% FCS and then treated with Grp78 siRNA for 72 h in the presence of 20 ng/ml VEGF165. HUVEC proliferation was determined by [3H]thymidine incorporation assay. Results are mean ± SD and are representative of three independent experiments. *, P < 0.05. (C) HUVEC tube formation with Grp78 siRNA. 12 h after transfection with Grp78 siRNA or control siRNA, HUVECs were plated on Matrigel matrices with 20 ng/ml VEGF165 for 12 h. The total length of the tube network was calculated using Image-Pro Plus software. Results are mean ± SD and are representative of three independent experiments. *, P < 0.05. (D) Grp78 siRNA effect on HUVEC wound migration induced by VEGF165. After 12 h of the transfection with Grp78 siRNA or control siRNA, confluent HUVECs were incubated in M199 containing 1% FCS and 20 ng/ml VEGF165 for 12 h. Cells migrating beyond the reference line were photographed and counted. Data show mean ± SD. *, P < 0.05. (E) HUVEC chemotaxis in a Boyden chamber, as determined at 24 h after Grp78 siRNA transfection. Migrated HUVECs were stained violet using Diff-Quik kit. The mean ± SD of three independent experiments is presented on the right. *, P < 0.05. Bars, 100 µm.
Reduction of synoviocyte proliferation by the down-regulation of Grp78 transcripts in vivo
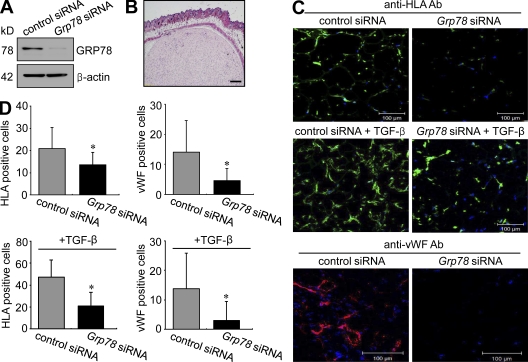
To confirm the effect of GRP78 on synoviocyte proliferation in vivo, we subcutaneously implanted Matrigel plugs containing RA FLSs transfected with Grp78 siRNA into immunodeficient mice in the presence or absence of TGF-β (Fig. 6, A and B). Before Matrigel implantation, we first confirmed that the Grp78 down-regulatory effect persisted at 7 d after Grp78 siRNA transfection into RA FLSs (Fig. 6 A). We then counted the number of RA FLSs implanted and endothelial cells recruited in Matrigels placed in mice for 7 d in the presence or absence of TGF-β. Without TGF-β, the number of cells positive for human HLA class I, indicating RA FLSs, was significantly lower in Matrigel implants containing Grp78 siRNA than in those with control siRNA (Fig. 6, C and D). When 50 ng/ml TGF-β was added to implants (Fig. 6, C and D), the number of HLA+ cells increased in both Grp78 siRNA– and control siRNA–transfected gels, but it was greater in control gels (2.4-fold for control siRNA vs. 1.5-fold for Grp78 siRNA), indicating that Grp78 siRNA mitigates synoviocyte proliferation in vivo. It has been shown that human RA FLSs induce angiogenesis through a VEGF-mediated pathway in immunodeficient mice (del Rey et al., 2009). In parallel with this, we found that Grp78 down-regulation in FLSs resulted in a decrease in the extent of angiogenesis in Matrigel implants (Fig. 6, C and D), as determined by immunofluorescent staining for mouse von Willebrand factor (vWF), suggesting that suppression of synoviocyte proliferation by Grp78 siRNA reduces endothelial cell recruitment to the surroundings of FLSs.
Figure 6.
Inhibition of RA synoviocyte proliferation and angiogenesis by Grp78 siRNA in immunodeficient mice. Matrigel containing RA FLSs was subcutaneously injected into the back skin of immunodeficient mice. (A) 7 d after transfecting with the Grp78 siRNA, GRP78 expression levels in RA FLSs were determined by Western blot analysis. (B) Hematoxylin and eosin staining of Matrigels containing RA FLSs from immunodeficient mice. (C) Effect of Grp78 siRNA on RA FLS proliferation and HUVEC infiltration. RA FLSs were implanted in Matrigels for 7 d in the absence or presence of 50 ng/gel TGF-β. RA FLSs in Matrigel were identified by immunofluorescence labeling for HLA class I antigen. Infiltrating mouse endothelial cells were stained using mouse anti-vWF antibody. The cells positive for HLA class I are shown in green in the top and the middle panels. Representative photographs of endothelial cells are shown in red in the bottom panel. Bars: (B) 300 µm; (C) 100 µm. (D) Numbers of RA FLSs and endothelial cells in Matrigels treated with vehicle or TGF-β. Cells were manually counted under a magnification of ×200. Values are the mean ± SD of eight mice per group. *, P < 0.05 versus control siRNA–transfected cells.
BIX increases synovial hyperplasia and angiogenesis in mice with collagenase-induced OA
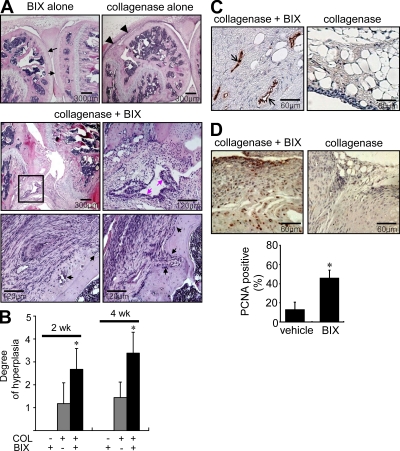
We then examined the pathological role of GRP78 in vivo. To this end, BIX was injected periarticularly into the joints of mice with collagenase-induced OA every other day for 2 or 4 wk. Both synovial proliferation and bone erosion were rarely detected in joints of mice injected periarticularly with BIX alone (Fig. 7 A), indicating that the injection procedure itself did not cause joint pathology nonspecifically. Synoviocyte proliferation was only modest in vehicle-treated OA mice as well (Fig. 7 A). However, BIX plus collagenase–treated mice showed a marked increase in synoviocyte proliferation in joints and exhibited cartilage and bone damage mimicking rheumatoid synovitis (Fig. 7 A). As a whole, BIX plus collagenase–treated mice showed significantly higher degrees of synovial hyperplasia and pannus formation than vehicle plus collagenase–treated mice (n = 6 per each group; Fig. 7 B). In addition, vWF staining of joints revealed new vessel formation with synoviocyte hyperplasia in BIX plus collagenase–treated mice but not in control mice (Fig. 7 C). Moreover, immunohistochemical staining for proliferating cell nuclear antigen (PCNA) revealed that the number of positive synoviocytes was much higher in BIX plus collagenase–treated mice (n = 6) than in control mice (n = 6; Fig. 7 D), demonstrating that BIX increased synoviocyte proliferation. These results show that repeated injections of BIX cause synoviocyte proliferation and angiogenesis in mice with collagenase-induced OA.
Figure 7.
Increase in synovial hyperplasia and bone erosion in mice treated with BIX, a selective BiP/GRP78 inducer. (A) Hematoxylin and eosin staining of the joints of mice administered periarticularly on alternate days for 2 or 4 wk with BIX. Black arrows and arrowheads in the top panel indicate intact cartilages and minimal synovial proliferation, respectively. Pink arrows in the middle panel indicate the enhanced proliferation of synoviocytes, and black arrows in the bottom panel represent bone erosion. The rectangular area in the middle left image is magnified in the middle right image. (B) The histological scores for degrees of synovial hyperplasia in mice injected with BIX alone, collagenase (COL) alone, and collagenase plus BIX (n = 6 per group). *, P < 0.05 versus collagenase (only)-treated mice without injecting BIX. (C) vWF staining of the synovia of mice treated with collagenase plus BIX versus collagenase alone. Positive cells are shown in brown (black arrows). (D) Evaluation of synovial proliferation by PCNA staining. Positive staining in the synoviums is indicated by brown nuclei. Ratios of positive cells (the number of positive synoviocytes/total synoviocytes counted) are presented in the bottom panel. *, P < 0.001. (B and D) Data show mean ± SD. Bars: (A, top and middle left) 300 µm; (A, middle right and bottom) 120 µm; (C and D) 60 µm.
Decreased arthritis severity in Grp78 heterozygous mice
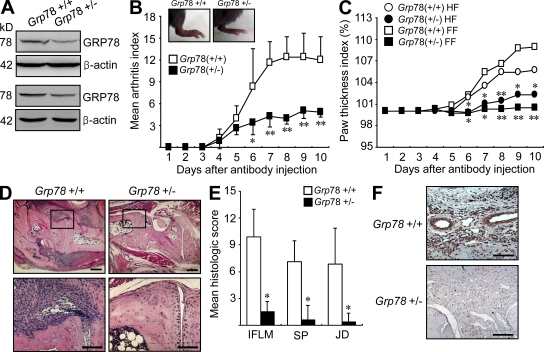
Finally, we attempted to determine the role played by GRP78 in an in vivo animal model of RA. To this end, experimental arthritis was induced in mice with reduced GRP78 by injection of anti–type II collagen antibody. In this passive model of arthritis, the innate immune system, including macrophages, FLSs, and endothelial cells, plays a central role in the progression of joint disease independent of adaptive immunity (Ji et al., 2002). Because complete Grp78 deficiency proved lethal, we used a line of heterozygous (Grp78+/−) mice (Luo et al., 2006). In agreement with the previous report that GRP78 protein level was reduced by about half in the Grp78+/− mice (Luo et al., 2006), the GRP78 protein level in the joint tissues was ∼50% compared with that of the wild-type (Grp78+/+) littermates (Fig. 8 A). As shown in Fig. 8 B, the severity of arthritis was significantly lower in Grp78+/− mice than in Grp78+/+ littermates. Paw swelling, which was assessed according to the diameter of arthritic ankles, was also much lower in Grp78+/− mice than in Grp78+/+ mice (Fig. 8 C). Compared with the Grp78+/+ mice, the Grp78+/− mice also developed a limited degree of inflammatory cell infiltration, joint destruction, and synovial hyperplasia, as seen upon histological analysis (Fig. 8, D and E). Moreover, Grp78 haploinsufficiency mitigated the extent of angiogenesis in the synovium of mice with antibody-induced arthritis, as determined by immunohistochemical staining for vWF (Fig. 8 F), suggesting that GRP78 is essential for synovial hyperplasia and pathological angiogenesis in vivo. Collectively, these findings indicate that partial ablation of the gene for Grp78 sufficiently blocks the progression of experimental arthritis, suppressing synovial proliferation and angiogenesis in the arthritic joints.
Figure 8.
Arthritis induction in Grp78+/+ and Grp78+/− mice. (A) Western blot analysis of GRP78 in the joint tissues. Lysates obtained from the joints of Grp78+/− (n = 2) and wild-type littermates (Grp78+/+; n = 2) were subjected to Western blot analysis using anti-GRP78 and anti–β-actin antibodies. (B) The severity of anti–type II collagen antibody–induced arthritis in Grp78+/− (n = 7) and wild-type littermates (Grp78+/+; n = 7). Values are mean and SD. *, P < 0.05; **, P < 0.005 versus Grp78+/+ mice. (C) Effect of Grp78 deficiency on paw edema in mice with antibody-induced arthritis, as determined by the paw thickness index calculated at the indicated time points. Results are paw thickness index of forefoot (FF) and hind foot (HF). Values are mean. *, P < 0.05; **, P < 0.005 versus Grp78+/+ mice. (D) Hematoxylin and eosin staining of ankle joint sections obtained from Grp78+/− and Grp78+/+ mice 10 d after arthritis induction. The rectangular areas in the top images are magnified in the bottom images. (E) Mean histological scores of inflammatory cell infiltration (IFLM), synovial proliferation (SP), and joint destruction (JD) in Grp78+/− (n = 4) versus Grp78+/+ mice (n = 4) as determined on day 10 after antibody administration. Values are the mean and SD of four mice per group. *, P < 0.001 versus Grp78+/+ mice. (F) Immunohistochemical staining for vWF. Positive cells are shown in brown. Representative photographs are shown. Bars: (D, top) 300 µm; (D, bottom) 120 µm; (F) 60 µm.
DISCUSSION
GRP78/BiP is a stress protein of the hsp70 family and is involved in the regulation of ER homeostasis. GRP78 level is widely used as a sentinel marker of ER stress under pathological conditions (Lee, 2007; Pfaffenbach and Lee, 2011). GRP78 has also been suggested to be a putative autoantigen in RA (Bläss et al., 2001; Corrigall et al., 2001). GRP78-specific antibodies were found to be present in 63% of RA patients, in 7% of patients with other rheumatic diseases, and in no healthy subjects (Bläss et al., 2001). Mice with collagen-induced arthritis have been shown to produce high levels of anti-GRP78 antibodies (Corrigall et al., 2001). T cell responses to GRP78 have been reported to be elevated in RA patients, particularly in joints (Corrigall et al., 2001). In one study (Corrigall et al., 2001), although GRP78 injected into mice failed to induce experimental arthritis, it completely inhibited the development of arthritis when given before injecting type II collagen, which suggests that exogenous GRP78 has immune tolerogenic potential that could be useful for treating RA (Panayi and Corrigall, 2008). In addition, there may be a link between the enhanced activation of the GRP78 in plasma cells and the local production of anti–citrullinated protein (anti-CCP) antibodies in RA (Dong et al., 2009).
GRP78 can be induced by a variety of conditions, including amino acid deprivation, glucose starvation, perturbation of ER Ca2+ homeostasis, and inhibition of vesicle trafficking from the ER to the Golgi apparatus (Gomer et al., 1991; Yu et al., 1999). RA joints are exposed to hypoxia and low glucose, and both have been suspected to be ER stressors in RA. Our microarray and real-time PCR data revealed that RA synovium and synovial macrophages contained elevated levels of ER-associated gene expressions as compared with OA and normal synovium and macrophages. In particular, response to hypoxic stress was most significantly enriched by DEGs up-regulated in freshly isolated RA macrophages as compared with normal macrophages. In parallel, hypoxic stimuli cobalt chloride induced GRP78 expression in cultured RA FLSs, suggesting that hypoxia triggers ER response in RA joints.
During monocyte to macrophage differentiation, GRP78 expression can be increased (Dickhout et al., 2011). Because RA synoviums contain high numbers of inflammatory cells (e.g., macrophages) compared with OA synoviums, higher levels of ER signature in RA synoviums could be just a result of higher numbers of macrophages expressing UPR genes. To address this issue, we freshly isolated OA macrophages from OA synovial tissues (n = 9), immediately obtained mRNA from the cells, and then determined the expression of ER stress–related genes by real-time PCR analysis. We provide the first evidence that expression levels of ER stress–associated genes, including GRP78, BCL2, CCND1, HSPA1B, DNAJB11, HYOU1, PDIA6, ATP2A2, DNAJB1, EIF2AK3, DIT3, and sXBP1, were higher in RA macrophages (n = 5) than in OA tissue macrophages (Fig. 1 G); only EROL1 mRNA expression was comparable between RA and OA macrophages. These data clearly show that RA macrophages contain a high level of UPR genes, which may contribute to elevated ER signatures in RA synoviums.
Some evidence demonstrates cross talk between the inflammatory response and ER stress response (Xue et al., 2005; Zhang and Kaufman, 2008). For example, in murine fibrosarcoma cells, the proinflammatory cytokine TNF was found to trigger UPR, increasing the expressions of XBP1 and GRP78 and the phosphorylation of eIF2 (Xue et al., 2005). IL-1 and TNF also have been reported to induce ER stress in hepatocytes, leading to the activation of CREBH, a transcription factor which mediates acute phase response in liver (Zhang et al., 2006). In the present study, we demonstrated first that IL-1β and TNF up-regulated GRP78 expression in RA synoviocytes, which indicates that proinflammatory cytokines act as ER stressors in RA joints. Moreover, RA FLSs showed a greater GRP78 response to proinflammatory cytokines (IL-1β, TNF, or TGF-β), CoCl2, or thapsigargin than OA FLSs. Considering that RA synovium is exposed to various proinflammatory cytokines and hypoxia, these findings may explain how inflammatory RA synovium exhibits higher levels of GRP78 expression than noninflammatory OA synovium (Fig. 1, A and C).
If a cell fails to maintain ER homeostasis, the UPR will initiate apoptosis to protect the organism by removing stressed cells that produce misfolded or malfunctioning proteins (Zhang and Kaufman, 2008). Several pathways have been implicated in ER stress–induced apoptosis, and the full induction of apoptosis seems to require the concomitant activations of several death signals, including CHOP, eIF2α, ATF6, and caspase-12 (Kaufman, 2002; Rutkowski and Kaufman, 2004; Schröder and Kaufman, 2005). In this study, we showed that thapsigargin treatment increased the expression of GRP78, p-eIF2α, ATF-6, and caspase-12 while decreasing BCL-2 expression. Moreover, the down-regulation of Grp78 transcripts using siRNA was found to increase apoptosis of RA FLSs. On the contrary, GRP78 overexpression prevented FLSs from apoptotic death induced by an ER stressor or SNP. Given a higher GRP78 response to ER stress in RA FLSs than in OA FLSs, our data suggest that ER hyperresponsiveness may confer apoptotic resistance on rheumatoid synoviocytes under ER stress conditions, leading to synovial hyperplasia.
GRP78 promotes survival in both proliferating and dormant tumors (Reddy et al., 2003; Li and Lee, 2006; Lee, 2007) and induces the proliferation and invasion of cancer cells (Reddy et al., 2003; Misra et al., 2005, 2006; Pyrko et al., 2007). Evidence is also emerging that GRP78 protects many tissues and organs under ER stress (Wang et al., 2010; Pfaffenbach and Lee, 2011). The present study shows that GRP78 mediates TNF- or TGF-β–induced synoviocyte proliferation, regulating cyclin D1 expression in vitro. In a xenotransplantation model, we also found that GRP78 plays a role in synoviocyte proliferation in vivo. It is well known that IL-1β, TNF, and TGF-β levels are greater in the sera, synovial fluid, and inflamed synovial tissues of RA patients than in those of OA patients (Feldmann et al., 1996). Therefore, the synoviocytes of RA patients may have a greater opportunity to be stimulated by these cytokines than the OA synoviocytes. Under these circumstances, proinflammatory cytokines, such as TNF and IL-1β, induce GRP78 expression in RA FLSs (Fig. 2, A–D), and this increased level of GRP78, in turn, could lead to synovial hyperplasia, which facilitates the further secretion of cytokines and thus establishes a vicious cycle. The suggestion of cross talk between ER stress and chronic inflammation was bolstered by the recent finding that IRE1 activates the NF-κB and JNK pathway, leading to the production of TNF (Hu et al., 2006).
Evidence is emerging that a subfraction of GRP78 can be expressed on the cell surface, particularly under pathophysiologic conditions (Ni et al., 2011). Cell surface GRP78 has been implicated in cell signaling regulating proliferation and viability distinct from its chaperone function in the ER. Indeed, binding of cell surface GRP78 with various ligands triggers multiple biological processes in different cell types. Furthermore, surface GRP78 is a potential receptor for targeted cancer therapy in cancer and chronic vascular disease (Sato et al., 2010). For example, binding of activated α2-macroglobulin to GRP78 induces the UPR and cancer cell proliferation (Misra et al., 2006). Interaction of GRP78 with cell surface T-cadherin promotes endothelial cell survival (Philippova et al., 2008). In a recent study, surface-expressed citrullinated GRP78 on monocytes/macrophages was found to bind anti-CCP antibodies and stimulate TNF production (Lu et al., 2010). We also found that GRP78 was expressed in the surface of FLSs as well as in the ER (unpublished data). Although binding partners of GRP78 on FLSs have not been identified, surface GRP78 could play proinflammatory, prosurvival, and proliferative roles by association with its functional ligands that might exist in the RA joints, as it does in cancer and endothelial cells (Misra et al., 2006; Philippova et al., 2008; Ni et al., 2011).
Angiogenesis is a critical event in the formation and maintenance of invasive pannus in RA, especially during the early disease stages (Koch, 1998; Firestein, 1999). The initiation of angiogenesis is known to be associated with the up-regulation of many angiogenic factors, and in particular, VEGF165 plays a crucial role in neovascularization during pannus formation (Koch, 1998; Firestein, 1999). VEGF165 levels are elevated in the serum, synovial fluid, and inflamed synovium of RA patients (Lee et al., 2001), and these levels have been shown to be highly correlated with RA disease activity (Lee et al., 2001). In this study, we demonstrate for the first time that VEGF165-induced angiogenesis is dependent on GRP78. Under nonapoptotic conditions, Grp78 siRNA was found to repress VEGF165-induced proliferation, tube formation, wound migration, and the chemotaxis of endothelial cells, indicating that GRP78 directly controls VEGF165-induced angiogenesis. In contrast, RA FLSs are the major source of several angiogenic factors, including VEGF, in joints (Koch, 1998; Firestein, 1999). We found that knockdown of the Grp78 transcript in RA FLSs significantly impeded the recruitment of endothelial cells to Matrigel implants engrafted into immunodeficient mice. These data, together with previous studies (Koch, 1998; Firestein, 1999), suggest that GRP78-mediated FLS proliferation increases angiogenic factor production and that this indirectly contributes to neovascularization and pannus formation.
We also found that repeated injections of BIX, a selective inducer of GRP78, into the joints of mice with experimental OA promoted synovial hyperplasia, bone erosion, and new vessel formation in synovia. Furthermore, PCNA+ proliferating cells were also found to be markedly increased by BIX. Conversely, mice with Grp78 haploinsufficiency (Grp78+/−) showed only limited synovial proliferation histologically, decreased angiogenesis, and lower levels of experimentally induced arthritis. These observations demonstrate that GRP78 is crucial to the development of chronic arthritis, involving synoviocyte proliferation and angiogenesis in vivo. Interestingly, repeated injections of BIX alone did not affect the joint pathology. This suggests that increased GRP78 expression alone is not sufficient to establish an RA pathology, which might require additional stimuli to sustain abnormal UPR, such as proinflammatory cytokines and hypoxia. Nevertheless, it is remarkable to observe that a 50% reduction in the GRP78 activity resulted in a significant suppression of arthritis in vivo. Collectively, our work provides the first evidence that normal synovium may be converted to a rheumatoid pathology when it is repeatedly exposed to ER stress to instigate GRP78 expression and that, conversely, genetic ablation of Grp78 prevents the development of arthritis in mice.
In addition to UPR, ERAD is also required to avoid ER stress in the cells (Yamasaki et al., 2005). The UPR relieves ER stress by inducing genes such as ER chaperones to increase the protein-folding capacity of the ER as well as by inhibiting general protein translation. In contrast, ERAD eliminates misfolded or unassembled proteins that accumulate in the ER through the ubiquitin–proteasome system (Yamasaki et al., 2005). Recently, synoviolin, an E3 ubiquitin ligase for ERAD, was identified in RA FLSs and synovial lining tissue (Amano et al., 2003). Heterozygous synoviolin knockout mice are protected against collagen-induced arthritis development associated with reduced synovial hypercellularity (Amano et al., 2003). The aforementioned studies on synoviolin (Amano et al., 2003; Yamasaki et al., 2005) are common to our results in that dysregulated ER response critically contributes to synovial hyperplasia and the development of chronic arthritis. Thus, it would be interesting to investigate whether two biological processes, UPR and ERAD, affect each other to induce RA.
In summary, RA joints were found to contain the genetic signatures of ER stress. GRP78, a central regulator of ER response, was highly expressed in RA synoviums and synoviocytes and conferred apoptotic resistance on RA synoviocytes. GRP78 controlled synoviocyte proliferation and angiogenesis in vitro. The critical roles played by GRP78 in synoviocyte proliferation and angiogenesis were confirmed in Matrigel implants and in an animal model of arthritis, which suggests that ER chaperone GRP78 contributes to the pathogenesis of RA by inducing synovial hyperplasia and angiogenesis. These findings provide new insights of the role of GRP78 in the pathogenesis of RA and explain how normal synoviocytes develop an aggressive phenotype in RA joints. Thus, we suggest that the regulation of ER stress response offers a new treatment target for chronic autoimmune arthritis.
MATERIALS AND METHODS
Patients.
38 patients who fulfilled the 1987 revised criteria of the American Rheumatism Association for RA (Arnett et al., 1988) were studied. The mean age of the RA patients (8 males and 30 females) was 53.4 ± 10.2 yr. The mean disease duration was 63.4 mo (range 6–145 mo). 26 (68.4%) of 38 RA patients were positive for rheumatoid factor, and 29 patients (76.3%) were positive for anti-CCP antibody. All RA patients were treated with disease-modifying antirheumatic drugs (DMARDs), including methotrexate (52.6%), antimalarial drugs (65.7%), sulfasalazine (31.5%), bucillamine (13.2%), and anti-TNF antibody (7.9%). Comparisons were made with 35 patients with OA (7 males and 28 females) and 12 healthy controls (3 males and 9 females) who had no rheumatic diseases. The mean ages of the OA patients and healthy controls were 54.4 yr and 51.6 yr, respectively. No difference was found in age and sex between RA and OA or healthy controls. The study protocol was approved by the Institutional Review Board of the Catholic Medical Center (VCMC08BR067). All patients gave written informed consent to the study protocol.
Isolation of FLSs, endothelial cells, and synovial fluid macrophages.
FLSs were obtained from the synovial tissues of RA or OA patients as described previously (Yoo et al., 2006) and incubated in DME supplemented with FCS (Invitrogen) or with insulin-transferrin-selenium A (ITSA; Life Technologies). In some experiments, mouse FLSs were isolated from the joints of mice with arthritis, which was induced by anti–type II collagen antibodies, in the same way as performed for human FLSs. HUVECs were isolated from normal-term umbilical cord veins and maintained in M199 medium containing 20% FCS as described previously (Lee et al., 2006). Monocytes/macrophages were obtained from mononuclear cells of healthy controls or from the synovial fluid of RA patients using anti-CD14 magnetic beads (Miltenyi Biotec) according to the manufacturer’s instructions. To induce differentiation into macrophages, monocytes of healthy controls were cultured for 24 h in RPMI 1640 medium supplemented with 10% FCS in the presence of 20 ng/ml of human M-CSF. OA macrophages were freshly isolated from synovial tissues of OA patients using anti-CD14 magnetic beads.
Identification of DEGs.
Microarray data derived from synovial tissues can be obtained at the GEO DataSets database. Normal synovial tissues were used as controls. The intensities of arrays were normalized using the quantile normalization procedure (Bolstad et al., 2003). Using normalized intensities, DEGs in RA or OA samples compared with normal were determined by the following integrated statistical hypothesis testing: (a) two independent tests, the Student’s t test and the log2 median ratio test, were performed; (b) p-values of each test were computed using an empirical distribution of the null hypothesis that the means of the genes are not different, which was obtained from random permutations of the samples; (c) individual p-values were combined to compute the false discovery rate using Stouffer’s method (Hwang et al., 2005); and (d) DEGs were selected as the genes with a false discovery rate of <0.05 and a fold change >1.5. Finally, functional enrichment analysis of DEGs was performed using DAVID software to identify cellular processes overrepresented by the DEGs.
Real-time PCR.
Total RNA was subjected to cDNA synthesis using a High Capacity cDNA RT kit (Applied Biosystems) according to the manufacturer’s instructions. Quantitative real-time PCR was performed in the StepOnePlus Real-Time PCR System (Applied Biosystems) using SYBR Premix (Applied Biosystems) according to the manufacturer’s instructions. GAPDH was used as an internal control for PCR amplification. Transcript levels were calculated relative to controls and are expressed as −ΔΔCt. The gene-specific primers used are listed in Table S1.
Western blot analysis.
FLSs were lysed in lysis buffer, and insoluble material was removed by centrifugation at 12,000 g for 20 min at 4°C. Final protein concentrations were determined using the Bradford protein assay (Bio-Rad Laboratories). Electrophoresis was performed using SDS-PAGE, and blots were transferred to nitrocellulose membranes. Membranes were incubated with antibodies to GRP78 (Abcam), BCL-2 (Santa Cruz Biotechnology, Inc.), cyclin D1 (Cell Signaling Technology), p-eIF2α (Cell Signaling Technology), ATF-6 (Santa Cruz Biotechnology, Inc.), caspase-12 (Cell Signaling Technology), and β-actin (Sigma-Aldrich). Membranes were then visualized using an enhanced chemiluminescent technique. Resulting films were scanned, and optical densities were quantified using Quantity On 1-D software (Bio-Rad Laboratories). Experiments were repeated three to four times for separate samples.
Immunohistochemical staining.
5-µm sections of paraffin-embedded synoviums of RA and OA patients were treated with pepsin for 30 min and blocked with BSA for 30 min at room temperature. Tissue sections were then incubated with antibodies to GRP78 (1:100; Stressgen Bioreagents), CHOP (1:100; Santa Cruz Biotechnology, Inc.), p-IRE1 (1:100; Abcam), ATF-6 (1:100; Santa Cruz Biotechnology, Inc.), and XBP-1 (1:100; Abcam) overnight at 4°C. Isotype control antibody (Sigma-Aldrich) was used as a negative control. Each slide was washed three times in TBS and incubated with biotinylated anti–mouse IgG (Vector Laboratories) in a humid chamber for 30 min. GRP78-positive cells were detected using peroxidase-conjugated streptavidin (Vector Laboratories) followed by 3′3-diaminobenzidine tetrahydrochloride (DAB; Vector Laboratories). The slides were counterstained with hematoxylin.
Immunocytochemistry of synoviocytes.
For the detection of GRP78 antigen in synoviocytes, immunofluorescence staining of synoviocytes was performed on chamber slides. In brief, cells were fixed with methanol for 10 min at 20°C and permeabilized with 0.25% Triton X-100 in PBS for 10 min at room temperature. Cells were incubated with anti-GRP78 antibody (1:100 dilution) and CellLight ER-RFP (Invitrogen) for 2 h at room temperature and then incubated with Alexa Fluor 488–conjugated secondary antibody (Invitrogen). After washing in PBS, the coverslips were mounted on glass slides with ProLong Antifade kit (Invitrogen), and the cells were examined using a confocal microscope (LSM 510; Carl Zeiss).
FACS analysis of GRP78.
Surface expressions of GRP78 on FLSs of RA or OA patients were detected by flow cytometry. In brief, cells were detached with 1 mM EDTA in PBS and stained with DyLight 488–conjugated anti-GRP78 antibody (Abcam) for 1 h at 4°C in the dark. DyLight 488–conjugated IgG2a isotype antibody (Abcam) was used as a control. Fluorescence was measured by the FACSCanto II system (BD), and the data were analyzed using FlowJo 8.7.3 software (Tree Star).
Cloning and overexpression of the human Grp78 gene.
The human Grp78 gene was amplified by PCR from cDNA obtained from rheumatoid synoviocytes using two pairs of primers encompassing the Grp78 coding region (262–2226). The forward primers encoded an XhoI site, and the reverse primers contained a HindIII restriction site: Grp78 forward primer, 5′-CTCGAGATGAAGCTCTCCCTG-3′; and Grp78 reverse primer, 3′-AAGCTTCTACAACTCATCTTTTTC-5′. PCR was performed over 35 cycles of 94°C for 30 s, 55°C for 60 s, and 72°C for 60 s. The resulting PCR product was cloned into pFLAG/CMV2 vector (Sigma-Aldrich) to generate the pFLAG-hGrp78 construct, which was verified by sequencing (ABI PRISM 310; Applied Biosystems). The plasmid DNA transfection of the hGrp78 gene into SV40-immortalized rheumatoid synoviocytes was conducted in 6-well plates using Lipofectamine 2000 reagent (Yoo et al., 2006).
Cell viability: MTT assay.
Synoviocyte viability was determined by an MTT assay as described previously (Lee et al., 2006; Kong et al., 2010).
Detection of apoptosis.
FLS apoptosis levels were determined using the APOPercentage Apoptosis Assay kit (Biocolor Ltd; Kong et al., 2010). Digital images of APOPercentage dye–labeled cells, which appear bright pink against a white background under a light microscope, were used to quantify apoptotic cell numbers. Fold increases in apoptosis level were expressed as pixel numbers. Degrees of apoptosis were also determined using cellular DNA fragmentation ELISA (Roche) as described previously (Lee et al., 2006).
Down-regulation of Grp78 transcripts.
For the down-regulation of Grp78 transcripts, RA FLSs were transfected with Grp78 siRNA (Santa Cruz Biotechnology, Inc.) using Lipofectamine 2000 (Invitrogen). Scrambled RNAs were provided by Santa Cruz Biotechnology, Inc. Grp78 mRNA and protein expression levels in FLSs were determined by RT-PCR and by Western blot analysis using anti-GRP78 monoclonal antibody (Abcam), respectively. To detect Grp78 mRNA, RT-PCR was performed using the following Grp78-specific primers: sense, 5′-GCTCGACTCGAATTCCAAAG-3′; and antisense, 5′-TTTGTCAGGGGTCTTTCACC-3′. PCR amplification was conducted over 25 cycles of denaturation at 94°C for 30 s, annealing at 56°C for 60 s, and elongation at 72°C for 30 s. GAPDH mRNA expression was used as an internal control.
Synoviocyte proliferation assay.
After knocking down of Grp78 mRNA, RA FLSs were subjected to a proliferation assay based on [3H]thymidine incorporation as described previously (Lee et al., 2006; Yoo et al., 2006). Proliferative responses were expressed as counts per minute in stimulated FLSs as compared with unstimulated cells. Analysis was performed in triplicate, and results are expressed as the mean ± SD.
Wound migration and capillary tube formation assays.
Wound migration of HUVECs was measured as described previously (Lee et al., 2006; Yoo et al., 2006). In brief, HUVECs plated to confluence on 60-mm culture dishes were wounded with pipette tips and then treated with 20 ng/ml VEGF165 in M199 medium supplemented with 0.5% FCS for 12 h. Migration was quantified by counting cells that had moved beyond a reference line. To determine capillary tube-forming ability, HUVECs were seeded on a layer of previously polymerized Matrigel (BD) containing 20 ng/ml VEGF165 and incubated for 12 h. Tube-forming ability was quantified by measuring the lengths of tubes in five randomly chosen low-power fields (×50) per well using Image-Pro Plus version 4.5 software (Media Cybernetics).
Assay of endothelial cell chemotaxis.
The chemotactic migration of HUVECs was assayed using a transwell chamber equipped with a 6.5-mm-diameter polycarbonate filter of pore size 8 µm. In brief, 50 ng/ml of recombinant VEGF in DME containing 1% FCS was placed in lower wells, and HUVECs suspended in DME containing 1% FCS at a final concentration of 5 × 104 cells/ml were placed in upper wells. The chamber was then incubated at 37°C for 12 h. Nonmigrating cells on the upper surfaces of filters were removed by wiping with a cotton swab, and migrated cells were fixed and stained with a Diff-Quik kit (Baxter Diagnostics). Chemotaxis was quantified by counting cells that migrated to the lower side of the filters at ×200. Eight random fields were counted for each assay.
Matrigel plug assay in immunodeficient mice.
500 µl Matrigel containing 5 × 104 RA FLSs was injected subcutaneously into the abdomen of athymic nude mice (The Jackson Laboratory) as described previously (del Rey et al., 2009). After 7 d, skins were pulled back to expose the Matrigel plugs, which remained intact. Plugs were embedded in paraffin and stained using hematoxylin and eosin or used for immunolabeling experiments. Sections were stained with anti–mouse vWF antibody (Abcam) and anti–human HLA (class I) antibody (Abcam). Sections were counterstained with hematoxylin. Quantitative data were obtained by counting the number of positively staining cells per area in digitalized images covering the whole Matrigel area under confocal microscopy.
Effect of BIX on synoviocyte proliferation in mice with experimental OA.
Male C57BL/6 mice (Orient), aged 8–12 wk, were used in all experiments. BIX, a selective GRP78 inducer (Kudo et al., 2008; Oida et al., 2008), was synthesized and purified at the Korea Research Institute of Chemical Technology. Experimental OA was induced as previously described (Yoo et al., 2007). In brief, 1 U of type VII collagenase from Clostridium histolyticum (Sigma-Aldrich) was injected twice on days 0 and 2 into the right knee joints of mice. The right knee joints of negative control mice were injected twice with the same volume (5 µl) of PBS. From day 2, 20 µg BIX was administered to experimental mice periarticularly every other day for 2 or 4 wk. Control mice received vehicle alone. Histological assessment of joints was performed at 2 or 4 wk after the primary collagenase injection. Immunohistochemical staining for vWF was also performed in knee joints using the anti-vWF antibody (Abcam). Degrees of synovial hyperplasia, pannus formation, and joint destruction were determined using a scoring protocol, whereby severity was scored on a scale of 0–4, where 0 = absent, 1 = minimal, 2 = mild, 3 = moderate, and 4 = severe.
Induction of anticollagen antibody-induced arthritis.
8-wk-old heterozygous Grp78+/− mice, backcrossed for eight generations onto the C57BL/6 background, and their wild-type littermates (Grp78+/−) were used for this study. 5 mg anti–type II collagen antibody (Chondrex) was injected intravenously into the two groups of mice, and 3 d later, 50 µg LPS was administered intraperitoneally (Kong et al., 2010). The clinical severity of arthritis was graded as described previously (Kong et al., 2010). During the course of arthritis, forefoot and hind foot swelling were also measured daily using a microcaliper. The paw thickness index was defined as the diameter of an arthritic ankle divided by its diameter on day 0 and was expressed as a percentage (Kong et al., 2010). On day 10, paws and ankles were harvested. Degrees of inflammation, synovial hyperplasia, and joint destruction were determined using a standard scoring protocol as previously described (Kong et al., 2010).
Statistical analysis.
Data are expressed as the mean ± SD. Comparisons of the numerical data between groups were performed by the paired or unpaired Mann-Whitney U test. P-values <0.05 were considered statistically significant.
Online supplemental material.
Table S1 shows the gene-specific primers used for real-time PCR. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20111783/DC1.
Acknowledgments
We are thankful to Drs. Jong Dae Ji (Korea University College of Medicine, Seoul, South Korea) and Tae-Hwan Kim (Hanyang University Hospital for Rheumatic Diseases, Seoul, South Korea) for providing us with mononuclear cells from the synovial fluids of RA patients.
This work was supported by grants from the Korea Healthcare Technology R&D Project, the Ministry for Health, Welfare and Family Affairs (No. A092258), the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (R33-2008-000-10064-0 and 2009-0080087), and the National Institutes of Health of the USA (grant CA27607).
The authors have no conflicting financial interests.
Footnotes
Abbreviation used:
- BIX
- BiP-inducible factor X
- DEG
- differentially expressed gene
- ERAD
- ER-associated degradation
- FLS
- fibroblast-like synoviocyte
- HUVEC
- human umbilical vein endothelial cell
- mRNA
- messenger RNA
- OA
- osteoarthritis
- PCNA
- proliferating cell nuclear antigen
- RA
- rheumatoid arthritis
- siRNA
- small interfering RNA
- SNP
- sodium nitroprusside
- UPR
- unfolded protein response
- VEGF
- vascular endothelial growth factor
- vWF
- von Willebrand factor
References
- Amano T., Yamasaki S., Yagishita N., Tsuchimochi K., Shin H., Kawahara K., Aratani S., Fujita H., Zhang L., Ikeda R., et al. 2003. Synoviolin/Hrd1, an E3 ubiquitin ligase, as a novel pathogenic factor for arthropathy. Genes Dev. 17:2436–2449 10.1101/gad.1096603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett F.C., Edworthy S.M., Bloch D.A., McShane D.J., Fries J.F., Cooper N.S., Healey L.A., Kaplan S.R., Liang M.H., Luthra H.S., et al. 1988. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 31:315–324 10.1002/art.1780310302 [DOI] [PubMed] [Google Scholar]
- Bläss S., Union A., Raymackers J., Schumann F., Ungethüm U., Müller-Steinbach S., De Keyser F., Engel J.M., Burmester G.R. 2001. The stress protein BiP is overexpressed and is a major B and T cell target in rheumatoid arthritis. Arthritis Rheum. 44:761–771 [DOI] [PubMed] [Google Scholar]
- Bolstad B.M., Irizarry R.A., Astrand M., Speed T.P. 2003. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 19:185–193 10.1093/bioinformatics/19.2.185 [DOI] [PubMed] [Google Scholar]
- Corrigall V.M., Bodman-Smith M.D., Fife M.S., Canas B., Myers L.K., Wooley P., Soh C., Staines N.A., Pappin D.J., Berlo S.E., et al. 2001. The human endoplasmic reticulum molecular chaperone BiP is an autoantigen for rheumatoid arthritis and prevents the induction of experimental arthritis. J. Immunol. 166:1492–1498 [DOI] [PubMed] [Google Scholar]
- del Rey M.J., Izquierdo E., Caja S., Usategui A., Santiago B., Galindo M., Pablos J.L. 2009. Human inflammatory synovial fibroblasts induce enhanced myeloid cell recruitment and angiogenesis through a hypoxia-inducible transcription factor 1alpha/vascular endothelial growth factor-mediated pathway in immunodeficient mice. Arthritis Rheum. 60:2926–2934 10.1002/art.24844 [DOI] [PubMed] [Google Scholar]
- Dickhout J.G., Lhoták S., Hilditch B.A., Basseri S., Colgan S.M., Lynn E.G., Carlisle R.E., Zhou J., Sood S.K., Ingram A.J., Austin R.C. 2011. Induction of the unfolded protein response after monocyte to macrophage differentiation augments cell survival in early atherosclerotic lesions. FASEB J. 25:576–589 10.1096/fj.10-159319 [DOI] [PubMed] [Google Scholar]
- Dong D., Ni M., Li J., Xiong S., Ye W., Virrey J.J., Mao C., Ye R., Wang M., Pen L., et al. 2008. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res. 68:498–505 10.1158/0008-5472.CAN-07-2950 [DOI] [PubMed] [Google Scholar]
- Dong D., Stapleton C., Luo B., Xiong S., Ye W., Zhang Y., Jhaveri N., Zhu G., Ye R., Liu Z., et al. 2011. A critical role for GRP78/BiP in the tumor microenvironment for neovascularization during tumor growth and metastasis. Cancer Res. 71:2848–2857 10.1158/0008-5472.CAN-10-3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W., Li X., Feng Y., Fan C., Chen Z., Zhu P. 2009. The differential expressions of 78-kDa glucose-regulated protein of infiltrating plasma cells in peripheral joints with the histopathological variants of rheumatoid synovitis. Arthritis Res. Ther. 11:R4 10.1186/ar2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava R.A., Olsen N.J., Spencer-Green G., Yeo K.T., Yeo T.K., Berse B., Jackman R.W., Senger D.R., Dvorak H.F., Brown L.F. 1994. Vascular permeability factor/endothelial growth factor (VPF/VEGF): accumulation and expression in human synovial fluids and rheumatoid synovial tissue. J. Exp. Med. 180:341–346 10.1084/jem.180.1.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Brennan F.M., Maini R.N. 1996. Rheumatoid arthritis. Cell. 85:307–310 10.1016/S0092-8674(00)81109-5 [DOI] [PubMed] [Google Scholar]
- Firestein G.S. 1996. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 39:1781–1790 10.1002/art.1780391103 [DOI] [PubMed] [Google Scholar]
- Firestein G.S. 1999. Starving the synovium: angiogenesis and inflammation in rheumatoid arthritis. J. Clin. Invest. 103:3–4 10.1172/JCI5929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomer C.J., Ferrario A., Rucker N., Wong S., Lee A.S. 1991. Glucose regulated protein induction and cellular resistance to oxidative stress mediated by porphyrin photosensitization. Cancer Res. 51:6574–6579 [PubMed] [Google Scholar]
- Hu P., Han Z., Couvillon A.D., Kaufman R.J., Exton J.H. 2006. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol. Cell. Biol. 26:3071–3084 10.1128/MCB.26.8.3071-3084.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D., Rust A.G., Ramsey S., Smith J.J., Leslie D.M., Weston A.D., de Atauri P., Aitchison J.D., Hood L., Siegel A.F., Bolouri H. 2005. A data integration methodology for systems biology. Proc. Natl. Acad. Sci. USA. 102:17296–17301 10.1073/pnas.0508647102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H., Ohmura K., Mahmood U., Lee D.M., Hofhuis F.M., Boackle S.A., Takahashi K., Holers V.M., Walport M., Gerard C., et al. 2002. Arthritis critically dependent on innate immune system players. Immunity. 16:157–168 10.1016/S1074-7613(02)00275-3 [DOI] [PubMed] [Google Scholar]
- Katayama T., Imaizumi K., Manabe T., Hitomi J., Kudo T., Tohyama M. 2004. Induction of neuronal death by ER stress in Alzheimer’s disease. J. Chem. Neuroanat. 28:67–78 10.1016/j.jchemneu.2003.12.004 [DOI] [PubMed] [Google Scholar]
- Kaufman R.J. 2002. Orchestrating the unfolded protein response in health and disease. J. Clin. Invest. 110:1389–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A.E. 1998. Review: angiogenesis: implications for rheumatoid arthritis. Arthritis Rheum. 41:951–962 [DOI] [PubMed] [Google Scholar]
- Kong J.S., Yoo S.A., Kim H.S., Kim H.A., Yea K., Ryu S.H., Chung Y.J., Cho C.S., Kim W.U. 2010. Inhibition of synovial hyperplasia, rheumatoid T cell activation, and experimental arthritis in mice by sulforaphane, a naturally occurring isothiocyanate. Arthritis Rheum. 62:159–170 10.1002/art.25017 [DOI] [PubMed] [Google Scholar]
- Kudo T., Kanemoto S., Hara H., Morimoto N., Morihara T., Kimura R., Tabira T., Imaizumi K., Takeda M. 2008. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 15:364–375 10.1038/sj.cdd.4402276 [DOI] [PubMed] [Google Scholar]
- Lee A.S. 2007. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 67:3496–3499 10.1158/0008-5472.CAN-07-0325 [DOI] [PubMed] [Google Scholar]
- Lee M.S., Yoo S.A., Cho C.S., Suh P.G., Kim W.U., Ryu S.H. 2006. Serum amyloid A binding to formyl peptide receptor-like 1 induces synovial hyperplasia and angiogenesis. J. Immunol. 177:5585–5594 [DOI] [PubMed] [Google Scholar]
- Lee S.S., Joo Y.S., Kim W.U., Min D.J., Min J.K., Park S.H., Cho C.S., Kim H.Y. 2001. Vascular endothelial growth factor levels in the serum and synovial fluid of patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 19:321–324 [PubMed] [Google Scholar]
- Li J., Lee A.S. 2006. Stress induction of GRP78/BiP and its role in cancer. Curr. Mol. Med. 6:45–54 10.2174/156652406775574523 [DOI] [PubMed] [Google Scholar]
- Lu M.C., Lai N.S., Yu H.C., Huang H.B., Hsieh S.C., Yu C.L. 2010. Anti-citrullinated protein antibodies bind surface-expressed citrullinated Grp78 on monocyte/macrophages and stimulate tumor necrosis factor alpha production. Arthritis Rheum. 62:1213–1223 10.1002/art.27386 [DOI] [PubMed] [Google Scholar]
- Luo S., Mao C., Lee B., Lee A.S. 2006. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol. Cell. Biol. 26:5688–5697 10.1128/MCB.00779-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra U.K., Deedwania R., Pizzo S.V. 2005. Binding of activated alpha2-macroglobulin to its cell surface receptor GRP78 in 1-LN prostate cancer cells regulates PAK-2-dependent activation of LIMK. J. Biol. Chem. 280:26278–26286 10.1074/jbc.M414467200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra U.K., Deedwania R., Pizzo S.V. 2006. Activation and cross-talk between Akt, NF-kappaB, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78. J. Biol. Chem. 281:13694–13707 10.1074/jbc.M511694200 [DOI] [PubMed] [Google Scholar]
- Mohr W., Beneke G., Mohing W. 1975. Proliferation of synovial lining cells and fibroblasts. Ann. Rheum. Dis. 34:219–224 10.1136/ard.34.3.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Ladner U., Kriegsmann J., Franklin B.N., Matsumoto S., Geiler T., Gay R.E., Gay S. 1996. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am. J. Pathol. 149:1607–1615 [PMC free article] [PubMed] [Google Scholar]
- Ni M., Zhang Y., Lee A.S. 2011. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem. J. 434:181–188 10.1042/BJ20101569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oida Y., Izuta H., Oyagi A., Shimazawa M., Kudo T., Imaizumi K., Hara H. 2008. Induction of BiP, an ER-resident protein, prevents the neuronal death induced by transient forebrain ischemia in gerbil. Brain Res. 1208:217–224 10.1016/j.brainres.2008.02.068 [DOI] [PubMed] [Google Scholar]
- Okamoto K., Kobayashi T., Kobata T., Hasunuma T., Kato T., Sumida T., Nishioka K. 2000. Fas-associated death domain protein is a Fas-mediated apoptosis modulator in synoviocytes. Rheumatology (Oxford). 39:471–480 10.1093/rheumatology/39.5.471 [DOI] [PubMed] [Google Scholar]
- Panayi G.S., Corrigall V.M. 2008. BiP, an anti-inflammatory ER protein, is a potential new therapy for the treatment of rheumatoid arthritis. Novartis Found. Symp. 291:212–216 10.1002/9780470754030.ch16 [DOI] [PubMed] [Google Scholar]
- Pfaffenbach K.T., Lee A.S. 2011. The critical role of GRP78 in physiologic and pathologic stress. Curr. Opin. Cell Biol. 23:150–156 10.1016/j.ceb.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippova M., Ivanov D., Joshi M.B., Kyriakakis E., Rupp K., Afonyushkin T., Bochkov V., Erne P., Resink T.J. 2008. Identification of proteins associating with glycosylphosphatidylinositol- anchored T-cadherin on the surface of vascular endothelial cells: role for Grp78/BiP in T-cadherin-dependent cell survival. Mol. Cell. Biol. 28:4004–4017 10.1128/MCB.00157-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrko P., Schönthal A.H., Hofman F.M., Chen T.C., Lee A.S. 2007. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 67:9809–9816 10.1158/0008-5472.CAN-07-0625 [DOI] [PubMed] [Google Scholar]
- Reddy R.K., Mao C., Baumeister P., Austin R.C., Kaufman R.J., Lee A.S. 2003. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J. Biol. Chem. 278:20915–20924 10.1074/jbc.M212328200 [DOI] [PubMed] [Google Scholar]
- Rutkowski D.T., Kaufman R.J. 2004. A trip to the ER: coping with stress. Trends Cell Biol. 14:20–28 10.1016/j.tcb.2003.11.001 [DOI] [PubMed] [Google Scholar]
- Sato M., Yao V.J., Arap W., Pasqualini R. 2010. GRP78 signaling hub a receptor for targeted tumor therapy. Adv. Genet. 69:97–114 10.1016/S0065-2660(10)69006-2 [DOI] [PubMed] [Google Scholar]
- Schröder M., Kaufman R.J. 2005. ER stress and the unfolded protein response. Mutat. Res. 569:29–63 10.1016/j.mrfmmm.2004.06.056 [DOI] [PubMed] [Google Scholar]
- Stevens C.R., Williams R.B., Farrell A.J., Blake D.R. 1991. Hypoxia and inflammatory synovitis: observations and speculation. Ann. Rheum. Dis. 50:124–132 10.1136/ard.50.2.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada Y., Inoshita S., Nakashima O., Kuwahara M., Sasaki S., Marumo F. 1999. Regulation of cyclin D1 expression and cell cycle progression by mitogen-activated protein kinase cascade. Kidney Int. 56:1258–1261 10.1046/j.1523-1755.1999.00704.x [DOI] [PubMed] [Google Scholar]
- Wang M., Ye R., Barron E., Baumeister P., Mao C., Luo S., Fu Y., Luo B., Dubeau L., Hinton D.R., Lee A.S. 2010. Essential role of the unfolded protein response regulator GRP78/BiP in protection from neuronal apoptosis. Cell Death Differ. 17:488–498 10.1038/cdd.2009.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X., Piao J.H., Nakajima A., Sakon-Komazawa S., Kojima Y., Mori K., Yagita H., Okumura K., Harding H., Nakano H. 2005. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J. Biol. Chem. 280:33917–33925 10.1074/jbc.M505818200 [DOI] [PubMed] [Google Scholar]
- Yamasaki S., Yagishita N., Tsuchimochi K., Nishioka K., Nakajima T. 2005. Rheumatoid arthritis as a hyper-endoplasmic-reticulum-associated degradation disease. Arthritis Res. Ther. 7:181–186 10.1186/ar1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S., Yagishita N., Tsuchimochi K., Kato Y., Sasaki T., Amano T., Beppu M., Aoki H., Nakamura H., Nishioka K., Nakajima T. 2006. Resistance to endoplasmic reticulum stress is an acquired cellular characteristic of rheumatoid synovial cells. Int. J. Mol. Med. 18:113–117 [PubMed] [Google Scholar]
- Yoo S.A., Park B.H., Park G.S., Koh H.S., Lee M.S., Ryu S.H., Miyazawa K., Park S.H., Cho C.S., Kim W.U. 2006. Calcineurin is expressed and plays a critical role in inflammatory arthritis. J. Immunol. 177:2681–2690 [DOI] [PubMed] [Google Scholar]
- Yoo S.A., Park B.H., Yoon H.J., Lee J.Y., Song J.H., Kim H.A., Cho C.S., Kim W.U. 2007. Calcineurin modulates the catabolic and anabolic activity of chondrocytes and participates in the progression of experimental osteoarthritis. Arthritis Rheum. 56:2299–2311 10.1002/art.22731 [DOI] [PubMed] [Google Scholar]
- Yu Z., Luo H., Fu W., Mattson M.P. 1999. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp. Neurol. 155:302–314 10.1006/exnr.1998.7002 [DOI] [PubMed] [Google Scholar]
- Zhang K., Kaufman R.J. 2008. From endoplasmic-reticulum stress to the inflammatory response. Nature. 454:455–462 10.1038/nature07203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Shen X., Wu J., Sakaki K., Saunders T., Rutkowski D.T., Back S.H., Kaufman R.J. 2006. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 124:587–599 10.1016/j.cell.2005.11.040 [DOI] [PubMed] [Google Scholar]