The BRCA1 tumor suppressor associates with both the DROSHA microRNA maturation complex and primary miRNA transcripts, and promotes transcript processing.
Abstract
MicroRNAs (miRNAs) are noncoding RNAs that function as key posttranscriptional regulators of gene expression. miRNA maturation is controlled by the DROSHA microprocessor complex. However, the detailed mechanism of miRNA biogenesis remains unclear. We show that the tumor suppressor breast cancer 1 (BRCA1) accelerates the processing of miRNA primary transcripts. BRCA1 increased the expressions of both precursor and mature forms of let-7a-1, miR-16-1, miR-145, and miR-34a. In addition, this tumor suppressor was shown to be directly associated with DROSHA and DDX5 of the DROSHA microprocessor complex, and it interacted with Smad3, p53, and DHX9 RNA helicase. We also found that BRCA1 recognizes the RNA secondary structure and directly binds with primary transcripts of miRNAs via a DNA-binding domain. Together, these results suggest that BRCA1 regulates miRNA biogenesis via the DROSHA microprocessor complex and Smad3/p53/DHX9. Our findings also indicate novel functions of BRCA1 in miRNA biogenesis, which may be linked to its tumor suppressor mechanism and maintenance of genomic stability.
Introduction
MicroRNAs (miRNAs) regulate gene expression by suppression of translation and cleavage of target mRNAs, and are involved in diverse physiological and pathological processes. The primary transcripts of miRNA (pri-miRNA) are cleaved into precursor miRNA (pre-miRNA) by nuclear DROSHA (RNASEN) ribonuclease type III (RNase III), and further processed to mature miRNAs by cytoplasmic DICER1 RNase III in mammalian miRNA biogenesis. The DROSHA complex consists of DROSHA, DGCR8 (DiGeorge syndrome critical region gene 8), DDX5 (RNA helicase p68), and DDX17 (RNA helicase p72; Lee et al., 2003; Fukuda et al., 2007). Recently, additional molecules were shown to be involved in miRNA maturation; e.g., the TGF-β signal transducer Smads promotes miR-21 maturation, and the tumor suppressor p53 enhances the processing of miR-16, miR-143, miR-145, and miR-206 (Davis et al., 2008; Suzuki et al., 2009), whereas estrogen receptor α attenuates maturation of pri-miRNAs into pre-miRNAs (Yamagata et al., 2009). However, only a limited picture is currently known regarding miRNA biogenesis.
Breast cancer 1 (BRCA1) is a human tumor suppressor gene that plays critical roles in maintenance of genomic stability (Huen et al., 2010). This gene is expressed in breast and other tissues, where it helps to repair damaged genomes and disrupts cells when the genome cannot be repaired (Shen et al., 1998). BRCA1 forms a large multiprotein complex known as the BRCA1-associated genome surveillance complex with DNA damage sensors and other tumor suppressors (Wang et al., 2000), whereas it also interacts with RNA polymerase II and histone deacetylase complexes, and is involved in transcriptional control through modulation of the chromatin structure (Jhanwar-Uniyal, 2003). Inherited mutations in BRCA1 or BRCA2 predispose to breast, ovarian, and other cancers (Venkitaraman, 2002). DHX9, also known as RHA, has been identified as a putative RNA helicase with homology to the other RNA helicases, which function to catalyze ATP-dependent unwinding of double-stranded nucleotides in transcription and splicing (Fuller-Pace, 2006). DHX9 has been found to interact with BRCA1 (Anderson et al., 1998), DDX5 (p68), and DDX17 (p72; Wilson and Giguère, 2007). Abnormalities in miRNAs contribute to the pathogenesis of human tumors (Lu et al., 2005), and the miRNA biogenesis machinery is related to tumor suppressor networks (Suzuki et al., 2009). Also, the expressions of several miRNAs are dysregulated in BRCA1/2 mutation (Lee et al., 2009). These findings led us to examine the participation of tumor suppressor BRCA1 in miRNA processing.
Results and discussion
BRCA1 regulates miRNA processing
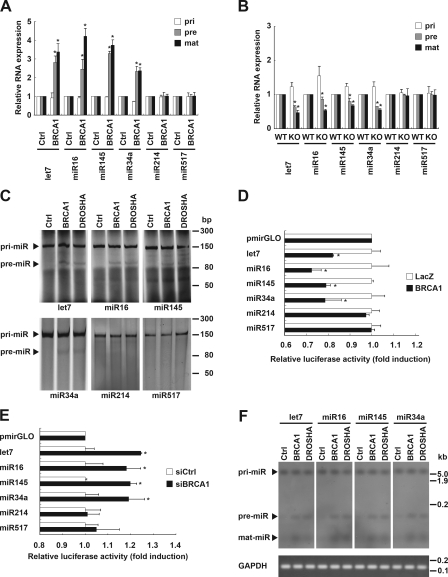
To determine whether BRCA1 regulates miRNA processing, we first examined the expression levels of primary, precursor, and mature forms of selected miRNAs using quantitative RT-PCR (qRT-PCR) after overexpression of a BRCA1 adenovirus in the human cervical cancer cell line HeLa. BRCA1 increased a set of precursor and mature miRNAs, including let-7a-1, miR-16-1, miR-145, and miR-34a, though their primary transcripts were found to be slightly decreased (Fig. 1 A and Fig. S1 A). Similar results were obtained with HeLa (Fig. S1 B), human renal cancer HEK293 (Fig. S1 C), and human osteosarcoma MG63 (Fig. S1 D) cell lines transfected with the BRCA1 plasmid vector. Furthermore, genetic knockout of BRCA1 attenuated the expressions of precursor and mature forms of the miRNAs, whereas their primary transcripts were slightly increased (Fig. 1 B). Similar results were acquired with siRNA-mediated BRCA1 knockdown (Fig. S1, E and F). The miRNAs analyzed above have also been shown to be decreased in various types of human cancer (Lu et al., 2005; Osaki et al., 2008). However, miR-17, miR-20, miR-106, and miR-214 were found to be increased in human cancers, whereas miR-23, miR-93, miR-182, and miR-517b were not changed (Osaki et al., 2008). BRCA1 did not significantly affect miRNA processing in the cancer–up-regulated (Fig. 1, A and B; and Fig. S1 G) or unchanged (Fig. 1 A and B; and Fig. S1 H) miRNAs.
Figure 1.
BRCA1 facilitates DROSHA-mediated pri-miRNA processing. (A) Expression levels of the primary (pri), precursor (pre), and mature (mat) forms of the indicated miRNAs were examined in mouse BRCA1 adenovirus-infected HeLa cells using qRT-PCR analysis. Pri- and pre-miRNAs were normalized by GAPDH, and mature miRNA was normalized by U6 snRNA (*, P < 0.05 as compared with mock control; n = 3). (B) miRNA processing in mouse BRCA1 genetic knockout ES cells. Expression levels of the primary, precursor, and mature miRNAs were compared using qRT-PCR analyses of wild-type (WT) and knockout (KO) ES cells. (C) In vitro pri-miRNA processing assay of pri-let-7a-1/miR-16-1/miR-145/miR-34a/miR-214/miR-517b with immunoprecipitated BRCA1 or DROSHA complex from HeLa cells after BRCA1 or DROSHA transfection. (D) In vivo monitoring assay of pri-miRNA processing in HeLa cells carrying pri-let-7a-1/miR-16-1/miR-145/miR-214/miR-517b at the 3′ untranslated region of the luciferase gene. BRCA1 overexpression decreased luciferase signaling. The intensities were normalized by renilla luciferase and are shown as fold induction as compared with an empty pmirGLO vector (*, P < 0.05; n = 3). (E) BRCA1 knockdown increased luciferase signaling in an in vivo monitoring assay of pri-miRNA processing. (F) Northern blot analyses of miRNAs in BRCA1- and DROSHA-transfected HeLa cells. Total RNAs were purified and each miRNA was detected by its specific probe. Error bars represent standard deviation.
Next, to determine whether BRCA1 processes a pri-miRNA substrate, we performed an in vitro pri-miRNA processing assay by incubating an FITC-labeled pri-let-7a-1, miR-16-1, miR-145, miR-34a, miR-214, or miR-517b substrate with immunoprecipitated BRCA1 or DROSHA from HeLa cells. Immunoprecipitated BRCA1 and DROSHA each potentiated let-7a-1/miR-16-1/miR-145/miR-34a pri-miRNA processing (Fig. 1 C), indicating that immunoprecipitated BRCA1 contains a complex that possesses pri-miRNA processing activity. We also conducted an in vivo cellular monitoring assay of BRCA1 function. HeLa cells were transfected with a luciferase vector construct carrying a segment of pri-let-7a-1, miR-16-1, miR-145, miR-34a, miR-214, or miR-517b between the luciferase gene and polyadenylation signal. We speculated that the luciferase transcripts would lose their polyadenylation tail after DROSHA RNase III–mediated cleavage of pri-miRNAs, resulting in poor stability and decreased translation (Fig. S2 A). As expected, DROSHA activity was reflected by luciferase activity (Fig. S2 B). Using this monitoring system, we also observed that BRCA1 overexpression caused a decrease in luciferase activity of let-7a-1/miR-16-1/miR-145/miR-34a (Fig. 1 D and Fig. S1 A), whereas that activity was increased by knockdown of BRCA1 (Fig. 1 E and Fig. S1 E). The same results were also observed in Northern blot analysis (Fig. 1 F). Collectively, these results demonstrate that BRCA1 enhances DROSHA-mediated processing of cancer-associated specific miRNAs in vitro and in vivo.
Interaction between BRCA1 and the DROSHA microprocessor complex
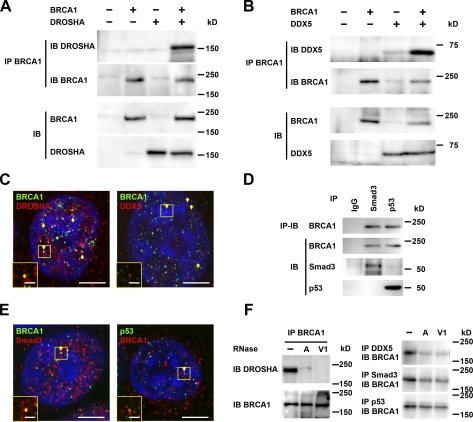
Evidence of an interaction between BRCA1 and the DROSHA microprocessor complex has not been previously presented. We speculated that the requirement of BRCA1 for miRNA processing could be shown by direct involvement of BRCA1 in the DROSHA microprocessor complex. To determine whether BRCA1 is involved with the DROSHA microprocessor complex, we examined the molecular interaction between BRCA1 and the DROSHA complex, and found that ectopically expressed DROSHA RNase III and BRCA1 had an association in HEK293 cells (Fig. 2 A). We also noted interaction between exogenous BRCA1 and DDX5 RNA helicase (Fig. 2 B), which was clarified by evidence of colocalization in the nuclei, where pri-miRNAs are processed. Some punctate structures of BRCA1/DROSHA or BRCA1/DDX5 were also colocalized in the nuclei, though the majority of BRCA1, DROSHA, and DDX5 proteins were individually localized (Fig. 2 C). These interactions were dose dependent (Fig. S2 C), and endogenous molecules also showed interactions (Fig. S2 D). Smad3 and p53 are involved in miRNA maturation (Davis et al., 2008; Suzuki et al., 2009), and interact with BRCA1 (Dubrovska et al., 2005; Zhang et al., 1998); our findings confirmed the association and colocalization of BRCA1 with Smad3 and p53. Indeed, BRCA1 might coimmunoprecipitate with Smad3 or p53 (Fig. 2 D), and colocalize with Smad3 or p53 (Fig. 2 E). The associations of BRCA1 with DROSHA and DDX5 were markedly decreased by treatment with RNase A (single-stranded RNA nuclease) and RNase V1 (double-stranded RNA nuclease), whereas the interactions with Smad3 and p53 were weakened by these RNases, indicating that BRCA1 interacts with the DROSHA complex via RNA molecules (Fig. 2 F). These results suggest that BRCA1 is involved in the DROSHA microprocessor complex and participates in its assembly, as well as that of Smad3 and p53.
Figure 2.
Interaction of BRCA1 with DROSHA complex. (A) HEK293 cells were transfected with BRCA1 and/or DROSHA, and subjected to immunoprecipitation (IP) with anti-BRCA1, followed by immunostaining with the anti-DROSHA or anti-BRCA1 antibody. IB, immunoblot. (B) Immunoprecipitation assays were performed to detect the interaction between BRCA1 and DDX5. (C) Colocalization of BRCA1 and DROSHA/DDX5 was analyzed by immunocytochemistry. Green, BRCA1; red, DROSHA or DDX5; yellow, colocalization (arrows). Bars, 5 µm (inset, 1 µm). (D) Interaction between BRCA1 and Smad3/p53 was detected in HEK293 cells by immunoprecipitation. (E) Colocalization of BRCA1 and Smad3/p53 was detected by immunocytochemistry. Left: green, BRCA1; red, Smad3; yellow, colocalization (arrows). Right: red, BRCA1; green, and p53; yellow, colocalization. White boxes denote the regions enlarged in the insets. Bars, 5 µm (inset, 1 µm). (F) RNA dependence of interactions of BRCA1 with DROSHA, DDX5, Smad3, and p53. Immunoprecipitates were treated with RNase A (single-stranded RNA nuclease) or RNase V1 (double-stranded RNA nuclease), and subjected to immunoblot analysis.
BRCA1 is directly associated with a pri-miRNA structure
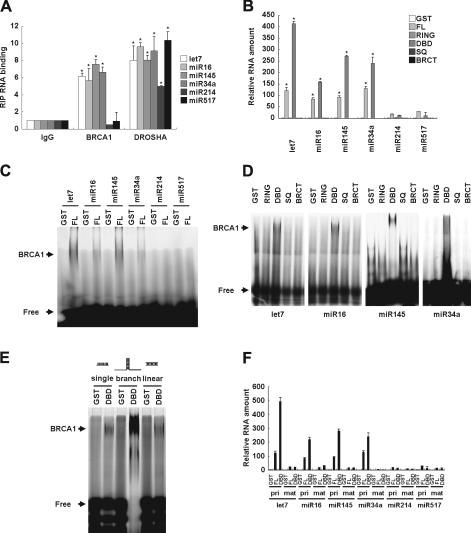
Because BRCA1 directly binds to DNA with a higher affinity for branched DNA structures via its DNA-binding domain (DBD), but has no sequence specificity (Paull et al., 2001), we speculated that BRCA1 directly associates with a similar branched pri-miRNA structure (root of stem-loop of pri-miRNA). To examine this possibility, we investigated whether BRCA1 engages with pri-miRNAs in vivo. RNA immunoprecipitation (RIP) analysis showed the in vivo associations of overexpressed BRCA1 with pri-let-7a-1, pri-miR-16-1, pri-miR-145, and pri-miR-34a, but not with pri-miR-214 and pri-miR-517b (Fig. 3 A), which were attenuated by BRCA1 siRNA (Fig. S2 E). However, the endogenous expression level of BRCA1 was not adequate to perform a RIP assay (Fig. S2 F). To reveal miRNA binding via the DBD domain, we constructed GST fusion proteins from various domains of BRCA1 (Fig. S3 A) and purified recombinant GST-BRCA1 domain proteins (Fig. S3 B), then conjugated them to glutathione-Sepharose beads for a pull-down assay with in vitro transcribed 150-nt pri-miRNAs. The pri-miRNA transcripts pulled down with GST-BRCA1 fusion proteins were quantitated by qRT-PCR analysis. BRCA1 full-length (FL) and DBD were shown to pull down pri-let-7a-1, miR-16-1, miR-145, and miR-34a, as compared with the other domains or miR-214 and miR-517b (Fig. 3 B), which indicates direct binding of the DBD of BRCA1 with pri-miRNAs.
Figure 3.
Direct association of BRCA1 DBD with miRNAs. (A) RIP analysis of association between pri-let-7a-1/miR-16-1/miR-145/miR-214/miR-517b and BRCA1 or DROSHA in HeLa cells. Cells were transfected with BRCA1 or DROSHA, then immunoprecipitated with the anti-BRCA1 or anti-DROSHA antibody, and subjected to qRT-PCR analysis with pri-let-7a-1, miR-16-1, miR-143, miR34a, miR-214, or miR-517b primers. As a control, an RNA sample from nontransfected cells was immunoprecipitated with nonspecific IgG and subjected to qPCR (*, P < 0.05 compared with IgG control; n = 3). (B) In vitro transcribed pri-let-7a-1/miR-16-1/miR-145/miR-214/miR-517b was mixed with bead-immobilized GST-BRCA1 (FL, RING, DBD, SQ, or BRCT) proteins. Associated RNA was eluted and subjected to qRT-PCR analysis to detect pri-let-7a-1/miR-16-1/miR-145. Relative amounts normalized to GST alone are presented (*, P < 0.05; n = 3). (C) EMSA was performed with an FITC-labeled pri-let-7a-1/miR-16-1/miR-145/miR-214/miR-517b probe and recombinant GST-BRCA1 FL proteins. Shifted bands as a result of specific binding to BRCA1 are shown. “Free” indicates an unbound probe. The experiment was performed three times and a representative blot is shown. (D) EMSA was performed with an FITC-labeled pri-let-7a-1/miR-16-1/miR-145 probe and recombinant GST-BRCA1 (RING, DBD, SQ, or BRCT) proteins. (E) EMSAs were performed with a synthetic FAM-labeled single-stranded RNA (20-mer), linear RNA duplex (matched 20-mer), or branched RNA (matched 18-mer and nonmatched 7-mer), as well as recombinant GST-BRCA1 DBD protein or GST alone. Shifted bands as a result of specific binding to BRCA1 are indicated. (F) In vitro transcribed pri-let-7a-1/miR-16-1/miR-145/miR-214/miR-517b or synthesized mature-let-7a1/miR-16-1/miR-145/miR-214/miR-517b RNA duplexes were mixed with recombinant GST-BRCA1 DBD or GST alone. Shown is the relative amount of miRNA pulled down with GST-BRCA1 DBD fusion protein normalized to the amount pulled down with GST alone. Error bars represent standard deviation.
The interactions of BRCA1 with pri-miRNAs were also examined using an electrophoretic mobility shift assay (EMSA) with FITC-labeled pri-let-7a-1, miR-16-1, miR-145, and miR-34a as probes (Fig. 3, C and D). Consistent with the pull-down assay results, bands for pri-let-7a-1, miR-16-1, miR-145, and miR-34a were shifted with the BRCA1 FL and DBD domains (Fig. 3, C and D). In addition to the interaction of BRCA1 with the stem-loop structure of pri-miRNA (Fig. 3 D), we explored the preferred RNA structure of BRCA1. A previous study showed that BRCA1 had a preference for a branched DNA structure (Paull et al., 2001). We also examined whether BRCA1 binds with branched RNA by EMSA and found that BRCA1 DBD preferentially interacts with branched FITC-labeled RNA, whereas it was only weakly associated with single-stranded RNA and the nonbranched linear RNA duplex (Fig. 3 E). Next, we clarified BRCA1 binding with branched miRNA using a pull-down assay. BRCA1 FL and DBD preferentially pulled down branched stem-loop pri-let-7a-1, miR-16-1, miR-145, and miR-34a, whereas nonbranched mature let-7a-1, miR-16-1, and miR-145 were only slightly affected (Fig. 3 F). Although our results demonstrate the ability of BRCA1 to bind to specific RNAs, the mechanism related to processing of only specific pri-miRNAs by BRCA1 remains to be determined. These results indicate that BRCA1 binds to miRNA and that the BRCA1 DBD is responsible for that binding; they also suggest that BRCA1 recognizes the root of the stem-loop on the secondary structure of specific pri-miRNAs.
DHX9 RNA helicase on miRNA maturation via BRCA1-induced miRNA processing
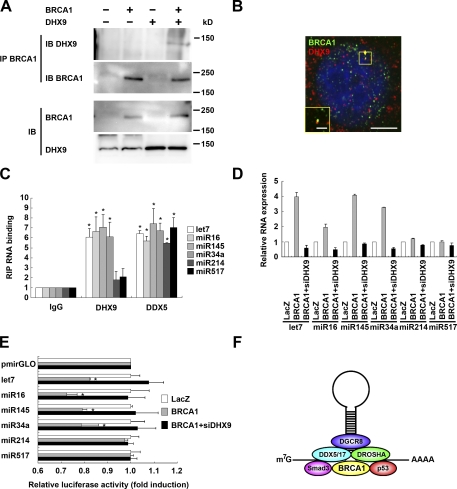
DHX9, an Asp-Glu-Ala-His box motif (DEAH) domain/RNA helicase (Fig. S3 C), interacts with BRCA1 (Anderson et al., 1998), and dominant-negative truncated DHX9 inhibits BRCA1 functions (Schlegel et al., 2003). Thus, we examined the effects of DHX9 RNA helicase on miRNA maturation via BRCA1-induced miRNA processing. We confirmed that BRCA1 can be coimmunoprecipitated with DHX9 (Fig. 4 A) and colocalized with DHX9 (Fig. 4 B). DHX9 catalyzes the unwinding of DNA and RNA (Fuller-Pace, 2006), and may have an ability to bind DNA or RNA. To investigate this possibility, we performed RIP analyses of the relationships between DHX9 and miRNAs, which showed that overexpressed DHX9 associates with pri-let-7a-1, miR-16-1, miR-145, and miR-34a, and that it weakly associated with pri-miR-214 and miR-517b (Fig. 4 C and Fig. S3 D). DHX9 siRNA (siDHX9) suppressed the processing of the pri-miRNAs let-7a-1, miR-16-1, miR-145, and miR-34a, in comparison with BRCA1-induced processing of pri-miRNAs (Fig. 4 D and Fig. S3, E and F). Furthermore, siDHX9 also attenuated the decrease in luciferase by BRCA1 in a monitoring assay of the microprocessors in let-7a-1, miR-16-1, miR-145, and miR-34a (Fig. 4 E), which suggests that DHX9 functions in miRNA processing with BRCA1. Therefore, DHX9 RNA helicase may endow some properties on miRNA biogenesis with the BRCA1-DROSHA microprocessor components.
Figure 4.
Regulation of miRNA processing by BRCA1 and DHX9. (A) Immunoprecipitation (IP) assays were performed after transfection with BRCA1 and DHX9 in HEK293 cells. IB, immunoblot. (B) Confocal microscopy indicated colocalization (yellow) of BRCA1 (green) and DHX9 (red) in the cellular nuclei. Bars, 5 µm (inset, 1 µm). (C) RIP analysis. After transfection of DHX9 or DDX5, overexpressed proteins were immunoprecipitated by the anti-DHX9 or anti-DDX5 antibody and subjected to qRT-PCR analysis with pri-let-7a-1, miR-16-1, miR-143, miR34a, miR-214, and miR-517b primers. (D) HeLa cells were transfected with BRCA1 or BRCA1 with siDHX9. The amounts of primary and precursor forms of let-7a-1, miR-16-1, miR-143, miR34a, miR-214, and miR-517b were examined. (E) In vivo monitoring analysis of pmirGLO-let-7a-1, miR-16, and miR-145. Mean luciferase intensities were determined after transfection with BRCA1 or BRCA1 with siDHX9. (F) BRCA1 regulates miRNA biogenesis. BRCA1 recognizes pri-miRNA, associates with the DROSHA microprocessor and Smads/p53, and enhances miRNA processing. Error bars represent standard deviation.
Based on our findings, we propose a novel BRCA1 function in the regulation of miRNA processing. The expressions of miRNA are dysregulated in several human cancers, and functional miRNA defects are known to be related to carcinogenesis (Calin et al., 2004; Lee et al., 2007; Szafranska et al., 2007), while global disruption of miRNA processing promotes cellular transformation and tumorigenesis (Kumar et al., 2007). The expression of let-7a-1 is low in lung tumors, and let-7a-1 miRNA down-regulates RAS expression (Johnson et al., 2005); miR-16-1 decreases expression of the apoptosis regulator protein BCL2 in B cell lymphomas (Cimmino et al., 2005); and miR-145 is down-regulated in colorectal carcinomas (Slaby et al., 2007). Adequate gene regulation by miRNAs may intrinsically involve the tumor suppressor mechanism with BRCA1 and p53. Embryonic stem (ES) cells are characterized by low levels of let-7a-1 and miR-145 expressions, which are induced by ES cell differentiation and inhibit the expressions of OCT4, SOX2, and KLF4 (Marson et al., 2008; Xu et al., 2009). Pluripotent stem cells (iPS) can be generated from fibroblast cultures by the addition of OCT4, SOX2, and KLF4 (Takahashi and Yamanaka, 2006). miRNA biogenesis may contribute to the maintenance of self-renewing, pluripotent, and differentiated states of ES, iPS, and cancer stem cells.
Furthermore, let-7a-1 and miR-145 may be useful as selection markers for cancer stem cells. Our results provide an intriguing molecular basis for a novel function of BRCA1 in miRNA processing and its role as an accessory factor for the DROSHA microprocessor (Fig. 4 F). BRCA1 may recognize the root of the stem-loop structure on specific pri-miRNAs. Other recent findings demonstrated a similar regulatory pathway of miRNA maturation mediated by Smad proteins and p53, which indicates a close relationship between the miRNA processing machinery and well-characterized nuclear factors. Further investigations are needed to provide insight into the relationships between BRCA1-mutated cancer risk and miRNA biosynthesis, as well as between BRCA1-maintained genomic stability and miRNA biogenesis. In summary, our observations offer new information regarding BRCA1 and miRNA biogenesis for cancer diagnosis and therapeutic strategies.
Materials and methods
Cell cultures
The human uterocervical cancer cell line HeLa, renal cancer cell line HEK293, and osteosarcoma cell line MG63 were purchased from the Institute of Physical and Chemical Research (RIKEN). Cells were maintained in DME (Wako Chemicals USA) supplemented with 10% FBS (Invitrogen). Mouse BRCA1 genetic knockout ES cells were gifts from K. Komatsu (Kyoto University, Kyoto, Japan), M. Jasin (Sloan-Kettering Institute, New York, NY), and B.H. Koller (University of North Carolina, Chapel Hill, NC).
Antibodies
The following antibodies were used: BRCA1 monoclonal MAB22101 (R&D Systems) or polyclonal JM3364 (MBL), DROSHA polyclonal no. 3364 (Cell Signaling Technology), DDX5 polyclonal no. 4387 (Cell Signaling Technology), Smad3 polyclonal GTX108638 (GeneTex), p53 monoclonal no. 610183 (BD), and DHX9 polyclonal ARP36343 (Aviva Systems Biology). HRP-linked anti–mouse IgG, HRP-linked anti–rabbit IgG (Cell Signaling Technology), Alexa Fluor 488 anti–mouse IgG, and Alexa Fluor 568 anti–rabbit IgG (Invitrogen) were used as secondary antibodies.
Plasmid vectors and expression
cDNA of mouse Brca1, mouse Drosha, human DDX5, and human DHX9 were purchased from Thermo Fisher Scientific and subcloned into an expression vector (mBrca1/pAd/CMV/V5-DEST, mBrca1/pCMV-SPORT6, mDrosha/pcDNA3, hDDX5/pCMV-Tag5A, DHX9/pcDNA3). Mouse Smad3 and mouse p53 were PCR amplified and cloned into pcDNA6 (Invitrogen). Mouse Brca1 was transfected into HeLa cells using FuGENE 6 transfection reagent (Roche). Mouse Brca1 Ring (1–448), DBD (449–1048), SQ (1049–1493), and BRCT (1494–1811) domains were fused into the GST expression vector pGEX6P1 and expressed in Escherichia coli BL21. Fusion proteins were purified using glutathione–Sepharose 4B (GE Healthcare). The primer sequences used are shown in Table S1.
siRNAs
siRNA for BRCA1 and a universal negative control siRNA were synthesized by Sigma-Aldrich. Stealth RNAi for DHX9 (three sets) were purchased from Invitrogen. siRNAs were introduced into cultured cells using Lipofectamine RNAiMax transfection reagent (Invitrogen). The transfected cells were used for subsequent experiments after 48 h. siDHX9-3 was most effective and used for DHX9 knockdown analysis. The target sequences of these siRNA duplexes are shown in Table S1.
qRT-PCR assays and Northern blot analysis
qRT-PCR assays were performed for determining the expression levels of primary, precursor, and mature miRNAs, as described previously (Davis et al., 2008; Suzuki et al., 2009). In brief, total RNA from the cells was prepared using TRIsure reagent (Bioline) or an RNeasy Plus mini kit (QIAGEN), then reverse transcribed with a TaqMan microRNA reverse transcription kit (Applied Biosystems) or SuperScript III First-Strand Synthesis System (Invitrogen). A qPCR assay was performed using a StepOnePlus system (Applied Biosystems), according to the manufacturer’s instructions. The qPCR reaction was performed using SYBR Green PCR Master Mix (Applied Biosystems) for pri- and pre-miRNAs. For detection of mature miRNAs, a TaqMan MicroRNA assay kit (Applied Biosystems) was used, according to the manufacturer’s protocol. The TaqMan reaction was performed with TaqMan Fast Universal PCR Master Mix (Applied Biosystems). Data analysis was performed using the comparative Ct method. Results were normalized to human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) for pri- and pre-miRNAs, or human U6 small nuclear RNA (snRNA; hRNU6-1) for mature miRNAs. The primer sequences used are shown in Table S1. Northern blotting assays were performed using a DIG Northern Starter kit (Roche), according to the manufacturer’s instructions. Probes for detection of miRNAs were transcribed and internally labeled with DIG (DIG RNA labeling kit; Roche) using linearized pSPT18 vector (Roche) containing pre-let-7a-1, miR-16-1, miR-145, or miR-34a.
In vitro pri-miRNA processing analysis
An in vitro pri-miRNA processing assay was performed as described previously (Davis et al., 2008; Suzuki et al., 2009). A linearized pSPT18 vector (Roche) containing 150 bp of pri-let-7a-1, pri-miR-16-1, pri-miR-145, or pri-miR-34a was transcribed in vitro and internally labeled with FITC (DIG RNA labeling kit; Roche). pri-miRNA was denatured for 5 min by heating at 65°C and renatured by gradually decreasing the temperature. The processing reaction was contained using an immunoprecipitated DROSHA complex from HeLa cells or immunoprecipitated BRCA1 complex from HeLa cells, with MgCl2, ATP, RNase inhibitor, and an in vitro transcribed internally FITC-labeled pri-miRNA. The reaction mixture was incubated at 37°C for 90 min, and RNA was loaded on 7.5% urea denaturing polyacrylamide gels, then analyzed using a Typhoon 9400 (GE Healthcare). The primer sequences used for cloning are shown in Table S1.
In vivo monitoring of pri-miRNA processing
Plasmid constructs with pri-miRNA at the 3′ untranslated region of firefly luciferase cDNA (pmirGLO-let-7a-1, miR-16-1, miR-145, miR-34a, miR-214, and miR-517b) and expression vectors or siRNAs were transfected into HeLa cells. Cell extracts were prepared at 48 h after transfection, and the ratio of firefly and renilla luciferase was measured using a Dual-Luciferase Reporter Assay system (Promega). Values were further normalized by that of an empty pmirGLO vector and are indicated with standard deviation. The primer sequences used for cloning are shown in Table S1.
Immunoprecipitation and immunoblot assays
HEK293 cells were transfected with mouse Brca1 and/or Drosha/Ddx5/Smad3/p53/DHX9 using FuGENE 6 transfection reagent (Roche). Mouse Brca1 was used to distinguish endogenous human BRCA1. Approximately 48 h after transfection, cells were lysed with NP-40 lysis buffer containing 1% Nonidet P40, 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, and Halt protease inhibitor (Thermo Fisher Scientific). For immunoprecipitation, cleared lysates were incubated with the anti-BRCA1, anti-Smad3, anti-p53, anti-DDX5, or control IgG antibodies. Total cell lysates or proteins in immunoprecipitates were subjected to SDS-PAGE and transferred to PVDF membranes (Bio-Rad Laboratories). Immunoblotting was performed with the indicated antibodies.
Immunocytochemistry
1 d before transfection, HeLa cells were plated on round cover glasses in 24-well culture plates. Mouse Brca1 and Drosha/Ddx5/Smad3/p53/DHX9 vectors were transiently transfected using FuGENE 6 transfection reagent (Roche). Cells were fixed with 3.7% formaldehyde, then permeated with 0.1% Triton X-100 and 5% bovine serum albumin to block nonspecific staining. Fixed, permeated, and blocked cells were incubated for 1 h at room temperature with anti-BRCA1 and anti-DROSHA/DDX5/Smad3/p53/DHX9 antibodies, then incubated with Alexa Fluor 488/568–conjugated anti-IgG. Nuclei were visualized using DAPI (Wako Chemicals USA). Digital photographs of fluorescence were acquired with an inverted microscope (Axiovert 200M; Carl Zeiss) and an LSM510 laser scanning confocal imaging system (α Plan-Fluar 100×/1.45 NA oil M27 objective lens) used in conjunction with LSM 510 AIM acquisition software (Carl Zeiss), and processed with Photoshop (Adobe).
RIP and qRT-PCR
RIP (RNA-ChIP) was performed as described previously (Davis et al., 2008; Suzuki et al., 2009). All buffers used in this study contained 0.5 U/µL RNase inhibitor (Toyobo). First, nuclei from HeLa cells were isolated from 1% formaldehyde-fixed cells and used for chromatin fragmentation. After immunoprecipitation with anti-BRCA1, anti-DROSHA, anti-DHX9, or anti-DDX5 antibodies, the beads were washed, then RNA was eluted and precipitated by ethanol. The precipitated RNA pellets were resuspended in nuclease-free water containing RNase inhibitor, 50 mM Tris-HCl, pH 7.5, 10 mM MgCl2, and DNase I. The mixture was incubated for 30 min at 37°C and extracted once with phenol/chloroform. RNA was then precipitated with ethanol and dissolved in nuclease-free water. An aliquot of RNA was used for a cDNA synthesis reaction and qPCR analysis. The primer sequences used are shown in Table S1.
GST-BRCA1 RNA pull-down assay
RNA synthesis was performed using an in vitro transcription reaction with a DIG RNA labeling kit (Roche) and linearized pSPT18 vector (Roche) containing 150 bp of pri-let-7a-1, pri-miR-16-1, pri-miR-145, pri-miR-34a, pri-miR-214, or pri-miR-517b. pri-miRNA was denatured and renatured. Different domains of GST-Brca1 fusion proteins (RING, DBD, SQ, and BRCT) were expressed in E. coli and bound to glutathione-Sepharose beads. Brca1 bound beads were washed and resuspended in binding buffer (20 mM Tris-HCl, pH 7.6, 100 mM KCl, 0.1% Tween-20, and 0.1% Triton X-100), then incubated with in vitro transcribed 150 bp of pri-let-7a-1, pri-miR-16-1, pri-miR-145, pri-miR-34a, pri-miR-214, pri-miR-517b, synthetic mature-let-7a-1, mature-miR-16-1, mature miR-145, pri-miR-34a, mature-miR-214, and mature-miR-517b (20 pmol) for 1 h at 4°C, and washed four times with binding buffer. Bound RNA was eluted by addition of elution buffer (1% SDS and 150 mM NaCl) for 15 min at room temperature. TRIsure reagent (Bioline) was added and RNA was purified using the usual method. RNA was resuspended in water and used in a reverse transcription reaction, followed by qPCR to detect relative levels of RNA binding to GST-Brca1 fusion proteins. The primer sequences used are shown in Table S1.
EMSA
EMSA was performed using in vitro transcribed FITC-labeled pri-let-7a-1/pri-miR-16-1/pri-miR-145, or synthetic FAM-labeled miR-16-1–based RNA (20 pmol). Denatured and renatured pri-miRNA or synthetic RNA was mixed with recombinant GST-Brca1 (RING, DBD, SQ, BRCT) protein at 4°C for 45 min in EMSA buffer (10 mM Tris-HCl, pH 7.5, 150 mM KCl, 10% glycerol, 50 µg/ml poly[dI-dC], and RNase inhibitor). Bound complexes were resolved on 6% native polyacrylamide gels in 0.5× Tris/borate/EDTA (TBE) buffer and then analyzed using a Typhoon 9400 (GE Healthcare).
Statistical analysis
Values are presented as the mean ± SD of results of separate experiments and were compared using a Student’s t test. Values at P < 0.05 were considered to indicate significant differences. All analyses were conducted using JMP IN software, version 5 (SAS).
Online supplemental material
Fig. S1 shows confirmation of BRCA1 overexpression, expression of miRNA by BRCA1 transfection in HeLa/HEK293/MG63, confirmation of BRCA1 expression in siBRCA1 knockdown/knockout, expression of miRNA by siBRCA1 knockdown, and expression of cancer–up-regulated/non–cancer-regulated miRNA by BRCA1 overexpression. Fig. S2 shows system and validation for in vivo monitoring of pri-miRNA processing, association of BRCA1 with DROSHA in a dose-dependent manner, endogenous associations of BRCA1/DROSHA/DDX5, and RIP analysis of BRCA1 knockdown cells and endogenous expression. Fig. S3 shows GST fusion proteins with different Brca1 domains, expression of GST-fusion Brca1 proteins, comparison of RNA helicases DDX5/DDX17/DHX9, relative mRNA expression of overexpressed DHX9 and siDHX9 knockdown, and expression of miRNA by siDHX9 knockdown. Table S1 shows oligonucleotide sequences. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201110008/DC1.
Acknowledgments
We thank all members of the Department of Oral Frontier Biology, Osaka University Graduate School of Dentistry, for their assistance and encouragement.
This work was supported in part by Grants-in-Aid for Scientific Research from the Japanese Society for the Promotion of Science (KAKENHI no. C21592356 and B20390528).
Footnotes
Abbreviations used in this paper:
- BRCA1
- breast cancer 1
- DBD
- DNA-binding domain
- EMSA
- electrophoretic mobility shift assay
- ES
- embryonic stem
- FL
- full length
- miRNA
- microRNA
- pre-miRNA
- precursor miRNA
- pri-miRNA
- primary miRNA
- qRT-PCR
- quantitative RT-PCR
- RIP
- RNA immunoprecipitation
References
- Anderson S.F., Schlegel B.P., Nakajima T., Wolpin E.S., Parvin J.D. 1998. BRCA1 protein is linked to the RNA polymerase II holoenzyme complex via RNA helicase A. Nat. Genet. 19:254–256 10.1038/930 [DOI] [PubMed] [Google Scholar]
- Calin G.A., Sevignani C., Dumitru C.D., Hyslop T., Noch E., Yendamuri S., Shimizu M., Rattan S., Bullrich F., Negrini M., Croce C.M. 2004. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA. 101:2999–3004 10.1073/pnas.0307323101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino A., Calin G.A., Fabbri M., Iorio M.V., Ferracin M., Shimizu M., Wojcik S.E., Aqeilan R.I., Zupo S., Dono M., et al. 2005. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA. 102:13944–13949 10.1073/pnas.0506654102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B.N., Hilyard A.C., Lagna G., Hata A. 2008. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 454:56–61 10.1038/nature07086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovska A., Kanamoto T., Lomnytska M., Heldin C.H., Volodko N., Souchelnytskyi S. 2005. TGFbeta1/Smad3 counteracts BRCA1-dependent repair of DNA damage. Oncogene. 24:2289–2297 10.1038/sj.onc.1208443 [DOI] [PubMed] [Google Scholar]
- Fukuda T., Yamagata K., Fujiyama S., Matsumoto T., Koshida I., Yoshimura K., Mihara M., Naitou M., Endoh H., Nakamura T., et al. 2007. DEAD-box RNA helicase subunits of the Drosha complex are required for processing of rRNA and a subset of microRNAs. Nat. Cell Biol. 9:604–611 10.1038/ncb1577 [DOI] [PubMed] [Google Scholar]
- Fuller-Pace F.V. 2006. DExD/H box RNA helicases: multifunctional proteins with important roles in transcriptional regulation. Nucleic Acids Res. 34:4206–4215 10.1093/nar/gkl460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen M.S., Sy S.M., Chen J. 2010. BRCA1 and its toolbox for the maintenance of genome integrity. Nat. Rev. Mol. Cell Biol. 11:138–148 10.1038/nrm2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhanwar-Uniyal M. 2003. BRCA1 in cancer, cell cycle and genomic stability. Front. Biosci. 8:s1107–s1117 10.2741/1131 [DOI] [PubMed] [Google Scholar]
- Johnson S.M., Grosshans H., Shingara J., Byrom M., Jarvis R., Cheng A., Labourier E., Reinert K.L., Brown D., Slack F.J. 2005. RAS is regulated by the let-7 microRNA family. Cell. 120:635–647 10.1016/j.cell.2005.01.014 [DOI] [PubMed] [Google Scholar]
- Kumar M.S., Lu J., Mercer K.L., Golub T.R., Jacks T. 2007. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat. Genet. 39:673–677 10.1038/ng2003 [DOI] [PubMed] [Google Scholar]
- Lee Y., Ahn C., Han J., Choi H., Kim J., Yim J., Lee J., Provost P., Rådmark O., Kim S., Kim V.N. 2003. The nuclear RNase III Drosha initiates microRNA processing. Nature. 425:415–419 10.1038/nature01957 [DOI] [PubMed] [Google Scholar]
- Lee E.J., Gusev Y., Jiang J., Nuovo G.J., Lerner M.R., Frankel W.L., Morgan D.L., Postier R.G., Brackett D.J., Schmittgen T.D. 2007. Expression profiling identifies microRNA signature in pancreatic cancer. Int. J. Cancer. 120:1046–1054 10.1002/ijc.22394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.H., Subramanian S., Beck A.H., Espinosa I., Senz J., Zhu S.X., Huntsman D., van de Rijn M., Gilks C.B. 2009. MicroRNA profiling of BRCA1/2 mutation-carrying and non-mutation-carrying high-grade serous carcinomas of ovary. PLoS ONE. 4:e7314 10.1371/journal.pone.0007314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Getz G., Miska E.A., Alvarez-Saavedra E., Lamb J., Peck D., Sweet-Cordero A., Ebert B.L., Mak R.H., Ferrando A.A., et al. 2005. MicroRNA expression profiles classify human cancers. Nature. 435:834–838 10.1038/nature03702 [DOI] [PubMed] [Google Scholar]
- Marson A., Levine S.S., Cole M.F., Frampton G.M., Brambrink T., Johnstone S., Guenther M.G., Johnston W.K., Wernig M., Newman J., et al. 2008. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 134:521–533 10.1016/j.cell.2008.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaki M., Takeshita F., Ochiya T. 2008. MicroRNAs as biomarkers and therapeutic drugs in human cancer. Biomarkers. 13:658–670 10.1080/13547500802646572 [DOI] [PubMed] [Google Scholar]
- Paull T.T., Cortez D., Bowers B., Elledge S.J., Gellert M. 2001. Direct DNA binding by Brca1. Proc. Natl. Acad. Sci. USA. 98:6086–6091 10.1073/pnas.111125998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel B.P., Starita L.M., Parvin J.D. 2003. Overexpression of a protein fragment of RNA helicase A causes inhibition of endogenous BRCA1 function and defects in ploidy and cytokinesis in mammary epithelial cells. Oncogene. 22:983–991 10.1038/sj.onc.1206195 [DOI] [PubMed] [Google Scholar]
- Shen S.X., Weaver Z., Xu X., Li C., Weinstein M., Chen L., Guan X.Y., Ried T., Deng C.X. 1998. A targeted disruption of the murine Brca1 gene causes gamma-irradiation hypersensitivity and genetic instability. Oncogene. 17:3115–3124 10.1038/sj.onc.1202243 [DOI] [PubMed] [Google Scholar]
- Slaby O., Svoboda M., Fabian P., Smerdova T., Knoflickova D., Bednarikova M., Nenutil R., Vyzula R. 2007. Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology. 72:397–402 10.1159/000113489 [DOI] [PubMed] [Google Scholar]
- Suzuki H.I., Yamagata K., Sugimoto K., Iwamoto T., Kato S., Miyazono K. 2009. Modulation of microRNA processing by p53. Nature. 460:529–533 10.1038/nature08199 [DOI] [PubMed] [Google Scholar]
- Szafranska A.E., Davison T.S., John J., Cannon T., Sipos B., Maghnouj A., Labourier E., Hahn S.A. 2007. MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene. 26:4442–4452 10.1038/sj.onc.1210228 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126:663–676 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Venkitaraman A.R. 2002. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 108:171–182 10.1016/S0092-8674(02)00615-3 [DOI] [PubMed] [Google Scholar]
- Wang Y., Cortez D., Yazdi P., Neff N., Elledge S.J., Qin J. 2000. BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes Dev. 14:927–939 [PMC free article] [PubMed] [Google Scholar]
- Wilson B.J., Giguère V. 2007. Identification of novel pathway partners of p68 and p72 RNA helicases through Oncomine meta-analysis. BMC Genomics. 8:419 10.1186/1471-2164-8-419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N., Papagiannakopoulos T., Pan G., Thomson J.A., Kosik K.S. 2009. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 137:647–658 10.1016/j.cell.2009.02.038 [DOI] [PubMed] [Google Scholar]
- Yamagata K., Fujiyama S., Ito S., Ueda T., Murata T., Naitou M., Takeyama K., Minami Y., O’Malley B.W., Kato S. 2009. Maturation of microRNA is hormonally regulated by a nuclear receptor. Mol. Cell. 36:340–347 10.1016/j.molcel.2009.08.017 [DOI] [PubMed] [Google Scholar]
- Zhang H., Somasundaram K., Peng Y., Tian H., Zhang H., Bi D., Weber B.L., El-Deiry W.S. 1998. BRCA1 physically associates with p53 and stimulates its transcriptional activity. Oncogene. 16:1713–1721 10.1038/sj.onc.1201932 [DOI] [PubMed] [Google Scholar]