Abstract
Synchrotron radiation (SR) X-ray has great potential for its applications in medical imaging and cancer treatment. In order to apply SR X-ray in clinical settings, it is necessary to elucidate the mechanisms underlying the damaging effects of SR X-ray on normal tissues, and to search for the strategies to reduce the detrimental effects of SR X-ray on normal tissues. However, so far there has been little information on these topics. In this study we used the testes of rats as a model to characterize SR X-ray-induced tissue damage, and to test our hypothesis that NAD+ administration can prevent SR X-ray-induced injury of the testes. We first determined the effects of SR X-ray at the doses of 0, 0.5, 1.3, 4 and 40 Gy on the biochemical and structural properties of the testes one day after SR X-ray exposures. We found that 40 Gy of SR X-ray induced a massive increase in double-strand DNA damage, as assessed by both immunostaining and Western blot of phosphorylated H2AX levels, which was significantly decreased by intraperitoneally (i.p.) administered NAD+ at doses of 125 and 625 mg/kg. Forty Gy of SR X-ray can also induce marked increases in abnormal cell nuclei as well as significant decreases in the cell layers of the seminiferous tubules one day after SR X-ray exposures, which were also ameliorated by the NAD+ administration. In summary, our study has shown that SR X-ray can produce both molecular and structural alterations of the testes, which can be significantly attenuated by NAD+ administration. These results have provided not only the first evidence that SR X-ray-induced tissue damage can be ameliorated by certain approaches, but also a valuable basis for elucidating the mechanisms underlying SR X-ray-induced tissue injury.
Keywords: Synchrotron radiation, X-ray, NAD+, testes, tissue injury
Introduction
Synchrotron radiation (SR) X-ray is coherent, collimated, monochromatic and intensely bright light, which has enabled the light to have excellent potential for medical applications [1-3]. For examples, a number of studies have suggested that SR X-ray-based microbeam radiation therapy (MRT) may become a novel approach for treating such cancers as gliomas [4, 5]; and SR-based medical imaging could produce images with exceptionally high resolutions [1, 6]. However, in order to apply SR X-ray in clinical settings, it is required to expose the mechanisms underlying the effects of SR X-ray on biological tissues, and to find approaches to prevent potential damaging effects of SR X-ray on normal tissues [3]. However, there has been little information on these two important topics [3].
NAD+ is a fundamental molecule in cells, which plays important roles in energy metabolism, mitochondrial functions and cell death [7]. Previous studies by our laboratory and other laboratories have shown that NAD+ administration can reduce both genotoxic agent-induced neural cell death and ischemic brain damage [8, 9]. Because DNA damage and oxidative stress play important roles in the tissue damage induced by normal X-ray [10, 11], we hypothesized that NAD+ administration may decrease SR X-ray-induced tissue injury. In this study we tested this hypothesis by using the male gonads of rats as a model. Our study has suggested that NAD+ administration can significantly attenuate SR X-ray-induced pathological changes of the biological tissues on both molecular and tissue levels.
Materials and methods
Materials
The chemicals and antibodies used in this study were purchased from Sigma Chemicals (St. Louis, MO, USA) except where noted.
Exposures of male gonads of rats to SR X-ray
All animal procedures were conducted following the protocol approved by the institutional Animal Care and Use Committee, School of Bio-medical Engineering, Shanghai Jiao Tong University, Shanghai, China. Male Sprague-Dawley rats weighing 190 g - 220 g were used in this study, which were subjected to different doses of SR X-rays at beam line station BL13W1 of Shanghai Synchrotron Radiation Facility (SSRF) (Figure 1A and 1B). Rats were randomly divided into groups, with 5 dosage categories including control (0 Gy, receiving all procedures except radiation), 0.5 Gy, 1.3 Gy, 4 Gy, and 40 Gy. Before the SR X-ray exposures, rats were anesthetized with chloral hydrate. The gonads of the anesthetized rats were exposed to SR X-ray with other parts of the body protected by lead sheets. After the SR X-ray irradiation, rats were housed in animal room at 22–24°C with 12-hour light/dark circle and free access to food and water.
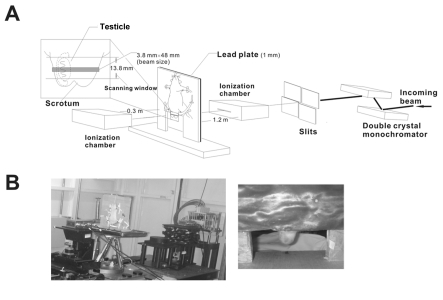
Figure 1.
Diagrammatic presentation (A) and photographs (B) of the experimental settings for the studies regarding the effects of SR X-ray exposures on the testes of rats.
Calculations of radiation doses
We calculated the radiation doses based on the air kerma of the SR X-ray at the entrance of the tissues. Air kerma is defined as Equation 1:
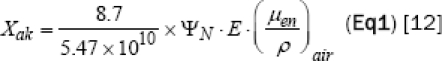
| Eq1 |
Where (ΨN) = Incident photon flux (in unit of photons/cm2), E = Photon energy (keV), (μen/ρ) air = Mass Energy-absorption coefficient of air (cm2/g) for photons of energy E.
We calculated the photon flux into a sample (ΨN) by using ionization chamber that measures the ionized electron currents as Equation 2:
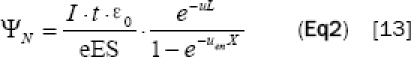
| Eq2 |
Where I = Current measured by ionization chamber (A), t = Irradiation time (s), μen= Air linear absorption coefficient (m-1), μ= Air linear attenuation coefficient (m-1), e = Electric charge of electron (C), ε0 = Ionization potential of air (eV), E = Photon energy (eV), x = Length of electrode length (m), L = Distance between ionization chamber and sample (m).
The dose (D) delivered to a sample is defined as Equation 3 [12];
![]()
| Eq3 |
Where D = Dose (mGy), (Ψ/ρ)tissue= Energy absorption coefficient of the sample (cm2/g) at energy E. Considering the factor of the sample thickness, average dose is expressed as Equation 4:
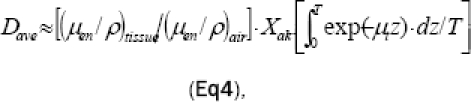
| Eq4 |
Where T = Sample thickness (m), μt= Tissue linear attenuation coefficient (m-1).
Sample collections
One day after SR X-ray exposures, the rats were sacrificed and the testes were removed, weighed and snap frozen in liquid nitrogen until further analysis.
Phosphorylated H2AX (γ-H2AX) Immunostaining
Cryosections of testes (8-10 μm in thickness) were obtained by a Leica Cryostat and mounted onto poly-L-lysine coated slides. Cryosections were fixed in 4% paraformaldehyde in PBS (pH 7.4) for 15 minutes at room temperature (RT). After washed in PBS, the slides were blocked in 10% BSA for 1 hr at RT. Then the slides were incubated in 2 μg/ml monoclonal anti-phosphohistone H2AX (Millipore, Billerica, MA, USA) containing with 1% BSA at 4°C over night. After washed in PBS, the slides were incubated with Alexa Fluor 568 goat anti-mouse (1:500 dilution) (Molecular Probes, Eugene, Oregon, USA) for 1 hr in darkness. Then the slides were stained in 0.2% DAPI solution (Beyotime, Hai-men, Jiangshu Province, China) for 5 mins. After washes in PBS, the slides were mounted in Fluorescence Mounting Medium (Beyotime, Haimen, Jiangshu Province, China) and viewed under a Leica SP5 confocal fluorescence microscope.
Hematoxylin staining
Tissue cryosections were fixed with 4% paraformaldehyde in PBS (pH 7.4) for 10 mins at RT, washed in PBS, followed by staining with hematoxylin dye (Beyotime, Haimen, Jiangshu Province, China) for approximately 10 min at RT. After the slides were washed in water for 30 min, the slides were mounted with an aqueous mounting medium and viewed under a Leica microscope, which is interfaced with a digital camera. To count the cell layers of seminiferous tubules, the slides were examined at 200X magnification. Cell layers of all of the seminiferous tubules in each testicular cryosection were counted in a blinded fashion, the average of which is defined as the ‘number of cell layer’ of the seminiferous tubules of a testis. To quantify the nuclear area of the spermatogenic cells in seminiferous tubules, the slides were examined under a microscope at 200X magnification. Approximately 100 to 200 nuclei of each slide were randomly chosen and quantified by using Image Pro Plus (version 6.0.0.260; Media Cyberneticsm Inc.).
Western blots
As described previously [14], tissue lysates were centrifuged at 12,000 g for 20 min at 4°C. After quantifications of the protein samples using BCA Protein Assay Kit (Pierce Biotechonology, Rockford, IL, USA), 120 μg of total protein was electrophoresed through a 14% SDS-polyacrylamide gel, and then wet electrotransferred to 0.45 μm nitrocellulose membranes (Millipore, CA, USA). The blots were incubated overnight at 4°C with a monoclonal anti-phospho-histone H2AX (Millipore, Billerica, MA, USA) (1:200 dilution), then incubated with a rabbit anti-goat polyclonal HRP-conjugated secondary antibody (EPITOMICS, Hangzhou, Zhejiang Province, China). Protein signals were detected using an ECL detection system (Pierce Biotechonology, Rockford, IL, USA). An anti-β-actin antibody with 1/1000 dilution (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used to normalize sample loading and transfer. The intensities of the bands were quantified by den-sitometry using Gel-Pro Analyzer.
Statistical analyses
All data are presented as mean ± SE. Data were assessed by one-way ANOVA, followed by Student-Newman-Keuls post hoc test. P values less than 0.05 were considered statistically significant.
Results
NAD+ administration can attenuate SR X-ray-induced increases in double-strand DNA damage
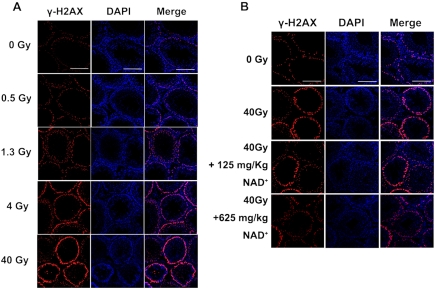
One day after irradiation by 0, 0.5, 1.3, 4 and 40 Gy SR X-ray, the testes of the rats were collected for various histological and biochemical assays. Double-strand DNA (dsDNA) damage was assessed by immunostaining of γ-H2AX - a marker of dsDNA damage [15], which shows that 40 Gy SR X-ray induced a remarkable increases in γ-H2AX (Figure 2A). We also determined the effects of NAD+ administration on the SR X-ray-induced dsDNA damage. We found that both 125 and 625 mg/kg NAD+ can markedly attenuate the SR X-ray-induced increases in the signals of γ-H2AX (Figure 2B).
Figure 2.
(A) Immunostaining shows that SR X-ray dose-dependently induced double-strand DNA damage, as assessed by γ-H2AX immnustaining. N= 5 rats for each experimental condition. (B) Effects of NAD+ administration on the SR X-ray-induced double-strand DNA damage of the testes, as assessed by γ-H2AX immunostaining. N= 8 - 11 rats for each experimental condition. The testes of rats were exposed to 0.5, 1.3, 4 or 40 Gy SR X-ray. For the rats exposed to 40 Gy of SR X-ray, a subset of the rats was pre-administered intraperitoneally with normal saline solution, 125 mg/kg NAD+ or 625 mg/kg NAD+. One day after the SR X-ray exposures, γ-H2AX immnustaining was applied to determine the effects of NAD+ on the SR X-ray-induced dsDNA damage.
To quantify the effects of NAD+ on the SR X-ray-induced changes of dsDNA damage, we conducted Western Blot assays using γ-H2AX antibody, showing that 40 Gy SR X-ray induced a significant increase in γ-H2AX signals, which was attenuated by administration of either 125 or 625 mg/kg NAD+ (Figure 3A). Quantifications of the Western blot results indicate that both 125 and 625 mg/kg NAD+ significantly attenuated SR X-ray-induced increases in the levels of γ-H2AX (Figure 3B).
Figure 3.
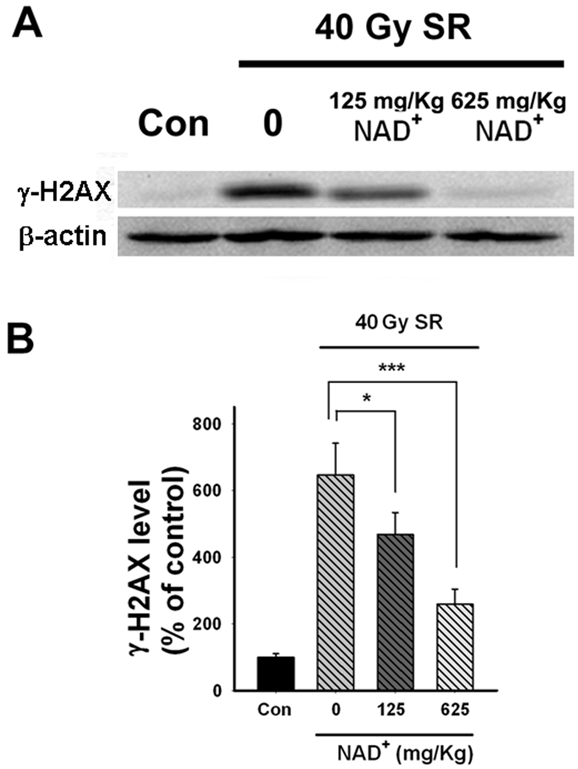
(A) Effects of NAD+ administration on the SR X-ray-induced dsDNA damage of the testes, as assessed by Western blot. (B) Quantifications of the results of Western blot showed that 40 Gy SR X-ray significantly increased double-strand DNA damage, which was significantly attenuated by administration of 125 or 625 mg/kg NAD+. The testes of rats were exposed to 0.5, 1.3, 4 or 40 Gy SR X-ray. For the rats exposed to 40 Gy of SR X-ray, a subset of the rats was pre-administered intraperitoneally with normal saline solution, 125 mg/kg NAD+ or 625 mg/kg NAD+. One day after the SR X-ray exposures, the effects of NAD+ administration on SR X-ray-induced dsDNA damage was assessed by Western blot using anti-γ-H2AX antibody. N= 8 - 11 rats for each experimental condition. *p < 0.05; **p < 0.01; ***p < 0.001.
NAD+ administration can attenuate SR X-ray-induced increases in abnormal cell nuclei in the testes
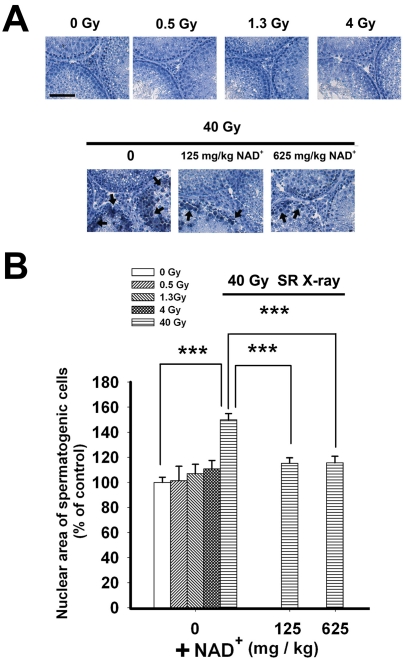
One day after exposures to SR X-ray, hematoxylin staining was applied to determine the effects of SR X-ray on the structural properties of the cells of the testes. We found that 40 Gy SR X-ray increased abnormal nuclei of the testes one day after the irradiation, which appeared to be decreased by administration of either 125 mg/kg or 625 mg/kg NAD+ (Figure 4A). Quantifications of the nuclear area of the spermatogenic cells in the seminiferous tubules of the testes showed that 40 Gy SR X-ray significantly increased the nuclear area, which was significantly attenuated by administration of either 125 mg/kg or 625 mg/kg NAD+ (Figure 4B).
Figure 4.
(A) Hematoxylin staining shows that SR X-ray induced an increase in enlarged nuclei of the spermatogenic cells in the testes, which appeared to be decreased by administration of either 125 mg/kg or 625 mg/kg NAD+. (B) Quantifications of the nuclear area of the spermatogenic cells showed that 40 Gy SR X-ray significantly increased the area of the nuclei, which was significantly attenuated by administration of either 125 mg/kg or 625 mg/kg NAD+. The testes of rats were exposed to 0.5, 1.3, 4 or 40 Gy SR X-ray. For the rats exposed to 40 Gy of SR X-ray, a subset of the rats was pre-administered intraperitoneally with normal saline solution, 125 mg/kg NAD+ or 625 mg/kg NAD+. One day after the SR X-ray exposures, hematoxylin staining was applied to determine the effects of NAD+ on the SR Xray-induced changes of the nuclear area of the spermatogenic cells of the testes. N= 5 - 13 rats for each experimental condition. Scale bar = 100 μM.
NAD+ administration can attenuate SR X-ray-induced decreases in the cell layers of seminiferous tubules
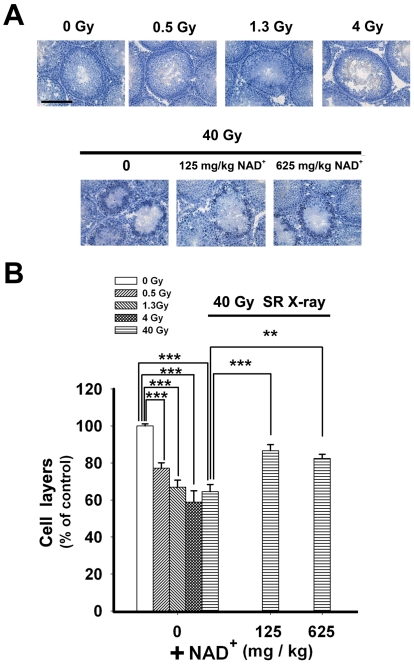
Our hematoxylin staining study indicated that SR X-ray dose-dependently decreased the number of the cell layers of the seminiferous tubules, which appeared to be attenuated by administration of either 125 or 625 mg/kg NAD+ (Figure 5A). Quantifications of the number of the cell layers showed that 40 Gy SR X-ray significantly reduced the the number of the cell layers, which was significantly ameliorated by administration of either 125 mg/kg or 625 mg/kg NAD+ (Figure 5B).
Figure 5.
Effects of NAD+ administration on the SR X-ray-induced changes of the cell layers of the seminiferous tubules of rat testes, assessed by hematoxylin staining. (A) Hematoxylin staining shows that SR X-ray induced a decrease in the cell layers of the seminiferous tubules of rat testes, which appeared to be ameliorated by administration of either 125 mg/kg or 625 mg/kg NAD+. (B) Quantifications of the cell layers of the seminiferous tubules of the testes showed that 40 Gy SR X-ray significantly decreased the number of the cell layers, which was significantly ameliorated by administration of either 125 mg/kg or 625 mg/kg NAD+. The testes of rats were exposed to 0.5, 1.3, 4 or 40 Gy SR X-ray. For the rats exposed to 40 Gy of SR X-ray, a subset of the rats was pre-administered intraperitoneally with normal saline solution, 125 mg/kg NAD+ or 625 mg/kg NAD+. One day after the SR X-ray exposures, hematoxylin staining was applied to determine the effects of NAD+ on the SR X-ray-induced changes of the cell layers of the seminiferous tubules. N= 5 - 13 rats for each experimental condition. Scale bar = 200 μM.
Discussion
Our study has shown that SR X-ray can dose-dependently induce acute damage of the testes on both molecular and tissue levels, which includes dsDNA damage, abnormal nuclei, and decreased cell layers of the seminiferous tubules of rat testes. More importantly, our study has provided first evidence that SR X-ray-induced injury of normal tissues can be attenuated by certain approaches: The damage can be significantly attenuated by NAD+ administration. This finding has also provided a valuable basis for elucidating the molecular and cellular mechanisms underlying SR X-ray-induced tissue damage.
Due to the characteristic properties of SR X-ray, such as high intensity and coherence, SR X-ray has great potential for applications in medical imaging and medical treatment of such diseases as cancer [1-3]. However, in order to apply SR X-ray in medical settings, it is required to elucidate the mechanisms underlying SR X-ray-produced tissue damage, and to search for the strategies that can prevent SR X-ray-induced injury of normal tissues [3]. Our current study belongs to one of the few studies that investigate SR X-ray-induced damage of normal tissues. The study is also the first one that searches for the approaches that can prevent SR X-ray-induced damage of normal tissues.
Three lines of our experiments have indicated that NAD+ administration can significantly attenuate SR X-ray-induced damage of the testes on both molecular and tissue levels: First, both Western blot and immunostaining assay have indicated that NAD+ administration can significantly attenuate SR X-ray-induced dsDNA damage; second, hematoxylin staining has indicated that NAD+ administration can attenuate SR X-ray-induced increase in abnormal cell nuclei; and third, our cell layer counting has shown that NAD+ administration can significantly attenuate SR X-ray-induced reductions in the cell layers of seminiferous tubules of rat testes.
DNA damage belongs to the pivotal damage produced by X-ray irradiation [11]. Both single-strand DNA damage and dsDNA damage can initiate cell death pathways [7, 16, 17]. It has been reported that dsDNA damage can induce apoptosis by activating such pathways as p53-dependent pathway [17-19]. Our observation that NAD+ can prevent SR-induced increases in dsDNA damage suggests that the drug may prevent the dsDNA-initiated cell death pathway, which may partially underlie our observations that NAD+ administration can attenuate the SR X-ray-induced reduction in the cell layers.
The protective effect of NAD+ on the SR X-ray-induced damage has also shed light for elucidating the mechanisms underlying the SR X-ray-induced tissue injury. Previous studies of our lab and other labs have indicated that NAD+ may decrease tissue injury by preventing mito-chondrial alteration [20] and glycolytic inhibition [8, 20]. Our current findings have implicated that mitochondrial impairments and compromise of energy metabolism may contribute to the SR X-ray-induced tissue injury. Future studies that elucidate the protective mechanisms of NAD+ on SR X-ray-induced tissue damage may indicate key components in the SR X-ray-initiated cell death pathways.
In this study we used male gonads of rats as the model to study the mechanisms underlying the SR X-ray-induced injury of biological tissues. Because gonads are one of the most radiosensitive organs, the study on the mechanisms of irradiation injury of gonads would provide valuable information for establishing the safety standard of SR X-ray in medical settings. To our knowledge, our current study is the first that studies the damaging effects of SR X-ray on gonads. However, since it remains possible that the mechanisms underlying the SR X-ray-induced injury of different organs may be differential, it is warranted to use other organs to study the mechanisms underlying the SR X-ray-induced injury of biological tissues in the future. Increasing evidence has indicated that NAD+, NADH and multiple NAD+-dependent enzymes, such as a PARP-1 and sirtuins, play important roles in not only numerous biological functions, but also in the pathology of multiple major diseases [21-25]. Future studies are also needed to investigate the roles of not only NAD+, but also NAD+-dependent enzymes and NADH in SR X-ray-induced damage of both normal and tumor tissues.
In summary, our study has characterized certain SR X-ray-induced damage of rodent testes, which can be significantly attenuated by NAD+ administration. This result suggests that NAD+ may be used to decrease SR X-ray-produced damage of normal tissues, which would further enhance the potential of using SR X-ray in medical settings. So far there has been little information regarding the mechanisms underlying the effects of SR X-ray on normal tissues. Our finding regarding the protective effect of NAD+ provides a valuable basis for future investigation on these mechanisms.
Acknowledgments
This study was supported by a National Key Basic Research ‘973 Program’ Grant #2010CB834306 (to WY and WX), a Pujiang Scholar Program Award 09PJ1405900 (to WY), Chinese National Science Foundation Grants #81171098 (to WY) and #30900756grants (to WX), and a Key Shanghai Jiao Tong University Grant for Interdisciplinary Research on Engineering and Physical Sciences (to WY). We would like to express our gratitude to Prof. Lisa Xu (SJTU) and Prof. Xizeng Wu (University of Alabama at Birmingham, USA) for valuable advice.
References
- 1.Suortti P, Thomlinson W. Medical applications of synchrotron radiation. Phys Med Biol. 2003;48:R1–35. doi: 10.1088/0031-9155/48/13/201. [DOI] [PubMed] [Google Scholar]
- 2.Bouchet A, Lemasson B, Le Duc G, Maisin C, Brauer-Krisch E, Siegbahn EA, Renaud L, Khalil E, Remy C, Poillot C, Bravin A, Laissue JA, Barbier EL, Serduc R. Preferential effect of synchrotron microbeam radiation therapy on intracerebral 9L gliosarcoma vascular networks. Int J Radiat Oncol Biol Phys. 2010;78:1503–1512. doi: 10.1016/j.ijrobp.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, He X, Sheng C, Ma Y, Nie H, Xia W, Ying W. Interactions between synchrotron radiation X-ray and biological tissues - theoretical and clinical significance. Int J Physiol Pathophysiol Pharmacol. 2011;3:243–248. [PMC free article] [PubMed] [Google Scholar]
- 4.Smilowitz HM, Blattmann H, Brauer-Krisch E, Bravin A, Di Michiel M, Gebbers JO, Hanson AL, Lyubimova N, Slatkin DN, Stepanek J, Laissue JA. Synergy of gene-mediated immunoprophylaxis and microbeam radiation therapy for advanced intracerebral rat 9L gliosarcomas. J Neurooncol. 2006;78:135–143. doi: 10.1007/s11060-005-9094-9. [DOI] [PubMed] [Google Scholar]
- 5.Serduc R, Bouchet A, Brauer-Krisch E, Laissue JA, Spiga J, Sarun S, Bravin A, Fonta C, Renaud L, Boutonnat J, Siegbahn EA, Esteve F, Le Duc G. Synchrotron microbeam radiation therapy for rat brain tumor palliation-influence of the microbeam width at constant valley dose. Phys Med Biol. 2009;54:6711–6724. doi: 10.1088/0031-9155/54/21/017. [DOI] [PubMed] [Google Scholar]
- 6.Kidoguchi K, Tamaki M, Mizobe T, Koyama J, Kondoh T, Kohmura E, Sakurai T, Yokono K, Umetani K. In vivo X-ray angiography in the mouse brain using synchrotron radiation. Stroke. 2006;37:1856–1861. doi: 10.1161/01.STR.0000226904.96059.a6. [DOI] [PubMed] [Google Scholar]
- 7.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 8.Ying W, Garnier P, Swanson RA. NAD+ repletion prevents PARP-1-induced glycolytic blockade and cell death in cultured mouse astrocytes. Biochem Biophys Res Commun. 2003;308:809–813. doi: 10.1016/s0006-291x(03)01483-9. [DOI] [PubMed] [Google Scholar]
- 9.Ying W, Wei G, Wang D, Wang Q, Tang X, Shi J, Zhang P, Lu H. Intranasal administration with NAD+ profoundly decreases brain injury in a rat model of transient focal ischemia. Front Biosci. 2007;12:2728–2734. doi: 10.2741/2267. [DOI] [PubMed] [Google Scholar]
- 10.Bolus NE. Basic review of radiation biology and terminology. J Nucl Med Technol. 2001;29:67–73. [PubMed] [Google Scholar]
- 11.Ward JF. The complexity of DNA damage: relevance to biological consequences. Int J Radiat Biol. 1994;66:427–432. doi: 10.1080/09553009414551401. [DOI] [PubMed] [Google Scholar]
- 12.Attix FH. Introduction to Radiological Physics and Radiation Dosimetry. Wiley-VCH; 2007. [Google Scholar]
- 13.Knoll GF. Radiation Detection and Measurement. Wiley; 2000. [Google Scholar]
- 14.Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis. 2008;32:329–339. doi: 10.1016/j.nbd.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T, Halicka D, Traganos F, Darzynkiewicz Z. Cytometric analysis of DNA damage: phosphorylation of histone H2AX as a marker of DNA double-strand breaks (DSBs) Methods Mol Biol. 2009;523:161–168. doi: 10.1007/978-1-59745-190-1_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12:440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Basu S, Kolesnick R. Stress signals for apoptosis: ceramide and c-Jun kinase. Oncogene. 1998;17:3277–3285. doi: 10.1038/sj.onc.1202570. [DOI] [PubMed] [Google Scholar]
- 18.Shen Y, White E. p53-dependent apoptosis pathways. Adv Cancer Res. 2001;82:55–84. doi: 10.1016/s0065-230x(01)82002-9. [DOI] [PubMed] [Google Scholar]
- 19.Yamada Y, Coffman CR. DNA damage-induced programmed cell death: potential roles in germ cell development. Ann N Y Acad Sci. 2005;1049:9–16. doi: 10.1196/annals.1334.002. [DOI] [PubMed] [Google Scholar]
- 20.Alano CC, Ying W, Swanson RA. Poly(ADP-ribose) polymerase-1-mediated cell death in astrocytes requires NAD+ depletion and mito-chondrial permeability transition. J Biol Chem. 2004;279:18895–18902. doi: 10.1074/jbc.M313329200. [DOI] [PubMed] [Google Scholar]
- 21.Ma Y, Chen H, Xia W, Ying W. Oxidative stress and PARP activation mediate the NADH-induced decrease in glioma cell survival. Int J Physiol Pathophysiol Pharmacol. 2011;3:21–28. [PMC free article] [PubMed] [Google Scholar]
- 22.Nie H, Chen H, Han J, Hong Y, Ma Y, Xia W, Ying W. Silencing of SIRT2 induces cell death and a decrease in the intracellular ATP level of PC12 cells. Int J Physiol Pathophysiol Pharmacol. 2011;3:65–70. [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Y, Chen H, He X, Nie H, Sheng C, Wang Q, Xia W, YingW NAD+ Metabolism and NAD+-Dependent Enzymes: Promising Therapeutic Targets for Neurological Diseases. Curr Drug Targets. 2012;13:222–229. doi: 10.2174/138945012799201711. [DOI] [PubMed] [Google Scholar]
- 24.He X, Nie H, Hong Y, Sheng C, Xia W, Ying W. SIRT2 activity is required for the survival of C6 glioma cells. Biochem Biophys Res Commun. 2012;417:468–472. doi: 10.1016/j.bbrc.2011.11.141. [DOI] [PubMed] [Google Scholar]
- 25.Zheng C, Han J, Xia W, Shi S, Liu J, Ying W. NAD+ administration decreases ischemic brain damage partially by blocking autophagy in a mouse model of brain ischemia. Neurosci Lett. 2012 doi: 10.1016/j.neulet.2012.01.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]