Abstract
BACKGROUND
Moderate global cooling of myocardial tissue was shown to destabilize 2-dimensional (2-D) reentry and facilitate its termination.
OBJECTIVE
This study sought to test the hypothesis that regional cooling destabilizes rotors and facilitates termination of spontaneous and DC shock-induced subepicardial reentry in isolated, endocardially ablated rabbit hearts.
METHODS
Fluorescent action potential signals were recorded from 2-D subepicardial ventricular myocardium of Langendorff-perfused rabbit hearts. Regional cooling (by 5.9°C ± 1.3°C) was applied to the left ventricular anterior wall using a transparent cooling device (10 mm in diameter).
RESULTS
Regional cooling during constant stimulation (2.5 Hz) prolonged the action potential duration (by 36% ± 9%) and slightly reduced conduction velocity (by 4% ± 4%) in the cooled region. Ventricular tachycardias (VTs) induced during regional cooling terminated earlier than those without cooling (control): VTs lasting >30 seconds were reduced from 17 of 39 to 1 of 61. When regional cooling was applied during sustained VTs (>120 seconds), 16 of 33 (48%) sustained VTs self-terminated in 12.5 ± 5.1 seconds. VT termination was the result of rotor destabilization, which was characterized by unpinning, drift toward the periphery of the cooled region, and subsequent collision with boundaries. The DC shock intensity required for cardioversion of the sustained VTs decreased significantly by regional cooling (22.8 ± 4.1 V, n = 16, vs 40.5 ± 17.6 V, n = 21). The major mode of reentry termination by DC shocks was phase resetting in the absence of cooling, whereas it was unpinning in the presence of cooling.
CONCLUSION
Regional cooling facilitates termination of 2-D reentry through unpinning of rotors.
Keywords: Spiral-wave reentry, Regional myocardial cooling, Unpinning, Optical mapping, Ventricular tachyarrhythmia
Introduction
High-energy DC shock application by implantable cardioverter-defibrillator (ICD) is the most effective procedure for preventing sudden cardiac death resulting from ventricular tachycardia/ventricular fibrillation (VT/VF). Large-scale clinical trials have demonstrated that ICD therapy is superior over any pharmacological therapy to prevent cardiac death.1,2 The usefulness of ICD therapy currently available is, however, limited by a number of adverse effects of high-energy shocks, such as myocardial damages causing arrhythmias, increased pacing threshold,3,4 and mechanical dysfunction giving rise to hemodynamic deterioration.5 In addition, painful DC shocks by ICD often cause serious psychological disorders.6,7 Theoretical and experimental studies have revealed that spiral-wave (SW) reentry rotating around a functional obstacle is the major mechanism of VT/VF.8,9 Arguably, should SW reentry be regulatable by procedures other than DC shocks or those combined with low-energy shocks, it could lead to innovative therapeutic modalities for prevention of arrhythmic death. Although several conceptual approaches have been proposed to terminate SW reentry by low-energy DC application, e.g., resonant drift,10,11 controlling chaos,12 synchronized pacing,13,14 and unpinning of SWs,15,16 feasibility of these approaches has not yet been validated. In isolated rabbit hearts, we have previously shown that moderate hypothermia facilitates termination of VT through destabilization (unpinning) of SW reentry.17 Using high-density electrode mapping in rabbit hearts, Boersma et al18 demonstrated that regional cooling (RC) of the ventricle during programmed electrical stimulation prevented stabilization of functional reentry and resulted in only brief episodes of polymorphic VT that terminated spontaneously. Here we hypothesized that moderate RC of the ventricular myocardium could be a novel procedure to destabilize already-established and sustained VT and lead to its termination. To test this hypothesis, we carried out high-resolution optical mapping experiments in 2-dimensional (2-D) ventricular myocardium.
Methods
Experimental model and optical mapping
The protocol was approved by the Institutional Animal Care and Use Committee at Nagoya University. The experimental model and procedures of optical mapping are essentially the same as reported previously.17,19,20 Briefly, optical membrane potential signals were recorded from a 2-D ventricular muscle layer of Langendorff-perfused rabbit hearts subjected to endocardial cryoablation; 2,3-butandione monoxime (BDM) was applied to minimize motion artifacts. Action potential duration (APD) and conduction velocity (CV) were measured during constant pacing (basic cycle length [BCL] 180 to 400 ms) from the apex. The details of experimental procedures and data analysis are described in the Online Supplemental Methods.
RC
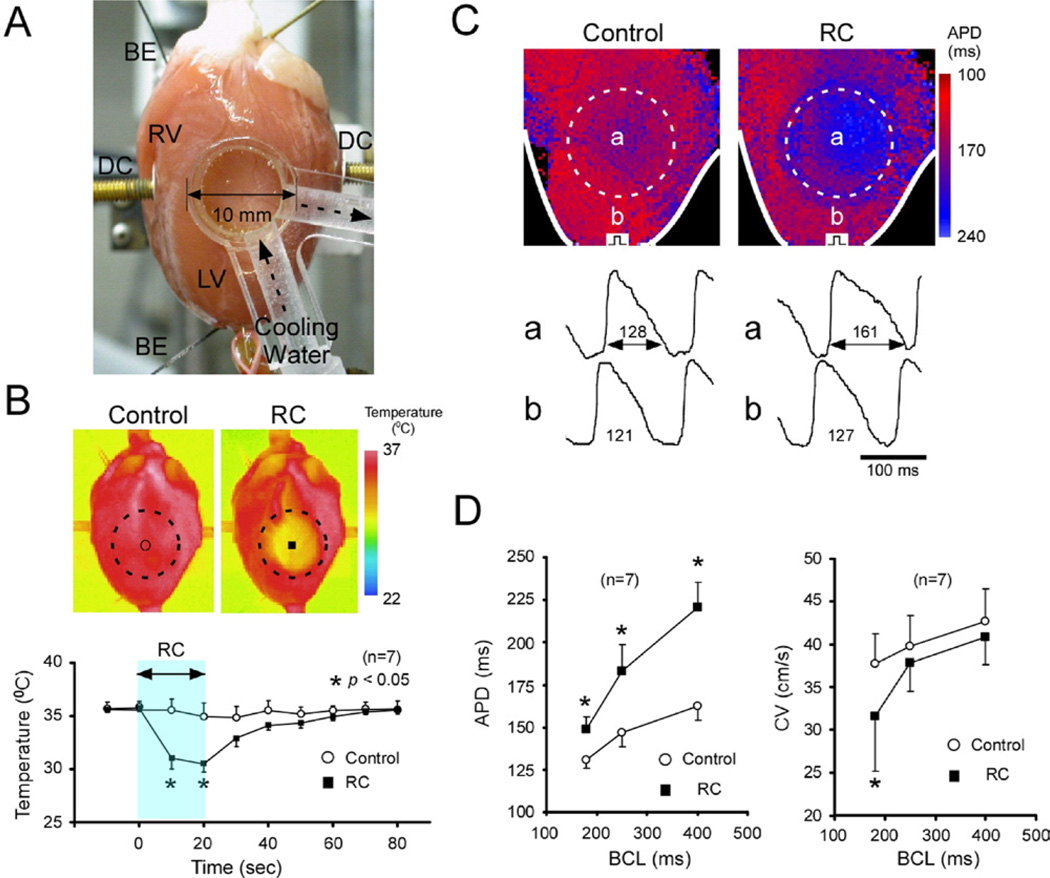
The temperature of the central region of the left ventricular (LV) free wall was temporarily reduced by applying a transparent cooling device (diameter, 10 mm) perfused with cold water and in abutting contact with the epicardial surface (Figure 1A). In pilot experiments using thermography (TVS-200, Nippon Avionics, Tokyo, Japan) (Figure 1B), we confirmed that the temperature in the target area was decreased by 5.9°C ± 1.3°C (n = 7, P <.05) from baseline (36.0°C). The temperature change was reversed completely after removal of the device. Temperature outside the cooled region remained unchanged.
Figure 1.
RC of 2-D rabbit hearts. A: A transparent cooling device (diameter, 10 mm) was in contact with the LV subepicardial surface, and water (20°C) was circulated inside the device. B: Thermography images (top) and changes of temperature (bottom) in response to RC. Those without RC served as control. *P <.05 vs control. C: Changes of APD (BCL, 400 ms) in response to RC. Top, APD color gradient maps with and without RC (control); bottom, optical action potential signals inside (a) and outside (b) the RC region. Numerals are APD (in ms). D: Effects of RC on APD (left) and CV (right) in the RC region at BCLs 180 to 400 ms. *P <.05 vs control (without RC). 2-D = two-dimension; APD = action potential duration; BCL = basic cycle length; BE = electrodes for recording distant bipolar electrograms; CV = conduction velocity; DC = paddle electrodes for DC-application; LV = left ventricle; RC = regional cooling.
Experimental protocols
Reentrant VTs (lasting ≥3 beats) were induced by modified cross-field stimulation using 1 of 2 protocols. First, in 8 hearts, VTs were induced before and 20 seconds after application of RC to compare their duration and dynamics. Second, in 15 additional hearts, sustained VTs (>120 seconds) were induced and RC was applied to observe its effects on VT duration and dynamics. If the sustained VTs did not terminate during the 30-second observation period of RC, 10-ms monophasic DC shocks were applied at increasing or decreasing voltage (by 5 to 10 V in steps from 25 V) to determine the threshold intensity for cardioversion. Sustained VTs without RC served as control subjects.
In 6 rabbits, effects of RC on VT/VF were examined in 3-dimensional (3-D) ventricles without cryoablation. Sustained VT/VFs (>120 seconds) were induced, and cardioversion by RC alone or in combination with DC shocks (8/2-ms biphasic, 25 to 100 V) was attempted.
Statistical analysis
Data are expressed as mean ± SD. Statistical comparisons were performed by 2-way analysis of variance with Bonferroni post hoc test or Welch 2-sample t test when appropriate. Differences were considered significant when P <.05.
Results
APD and CV during constant pacing
Effects of RC on APD and CV were examined in 7 hearts. Representative changes in APD (BCL, 400 ms) are shown in Figure 1C. Cooling (20 seconds) increased APD in the RC region, whereas APD outside the RC region was unchanged. Figure 1D summarizes the changes of APD and CV in the RC region. RC caused a significant increase of APD (BCLs, 180 to 400 ms); the longer the BCL, the greater the APD prolongation. RC decreased CV, although the changes remained statistically insignificant at BCLs 250 and 400 ms.
In 3 hearts, all of the RC-induced changes of APD and CV were reversed completely within 60 seconds after removal of the cooling device (data not shown).
VT induced during RC application
In 8 hearts, VTs were induced before (control) and 20 seconds after RC. In control subjects, 18 of 39 VTs (46%) terminated within 5 seconds, 4 VTs (10%) terminated in 5 to 30 seconds, and 17 VTs (44%) persisted for >30 seconds. During RC, in contrast, 58 of 61 VTs (95%) terminated within 5 seconds, 2 TVs (3%) terminated in 5 to 30 seconds, and 1 VT (2%) persisted >30 seconds. Thus, most of VTs induced during RC terminated earlier than in control subjects. The VT cycle length during RC (178 ± 20 ms, n = 61) was significantly longer compared with control subjects (143 ± 23 ms, n = 39, P <.05). Reversibility of the RC effects on the VT persistence was tested in 3 hearts. The incidence of persisted (>30 seconds)/all VTs was 11 of 23 (48%) in control subjects, 1 of 26 (4%) during RC, and 8 of 13 (62%) 5 to 20 minutes after removal of the cooling device.
Optical images of excitation were analyzed in 5 hearts (9 VTs before RC and 13 VTs during RC) exhibiting visible rotor(s). In control, the rotors were, in most cases (7 of 9) stable with small meandering. The 13 VTs induced during RC, in contrast, were all unstable with remarkable meandering of rotors along the periphery of the RC region, and they terminated shortly. Action potential traces revealed frequent intermittent conduction block in the RC region with longer APD, giving rise to drift of the reentry circuit (Online Supplementary Figure 1). The rotors terminated by collision with anatomical boundaries in 7 VTs (Online Supplementary Figure 1), whereas by mutual annihilation in the RC region in 2 cases (Online Supplementary Figure 2). The mode of rotor termination was unable to be analyzed in the remaining 4 cases.
Termination of sustained VT by RC
We next examined the effects of RC applied during sustained VTs (lasting >120 seconds). We induced 76 sustained VTs in 15 hearts and observed them for 30 seconds with and without RC (33 VTs with RC, and 43 VTs without RC as control subjects). None of the 43 sustained VTs terminated in control subjects, whereas 16 of 33 (48%) sustained VTs terminated during the observation period with RC. Average time to termination was 12.5 ± 5.1 seconds (n = 16).
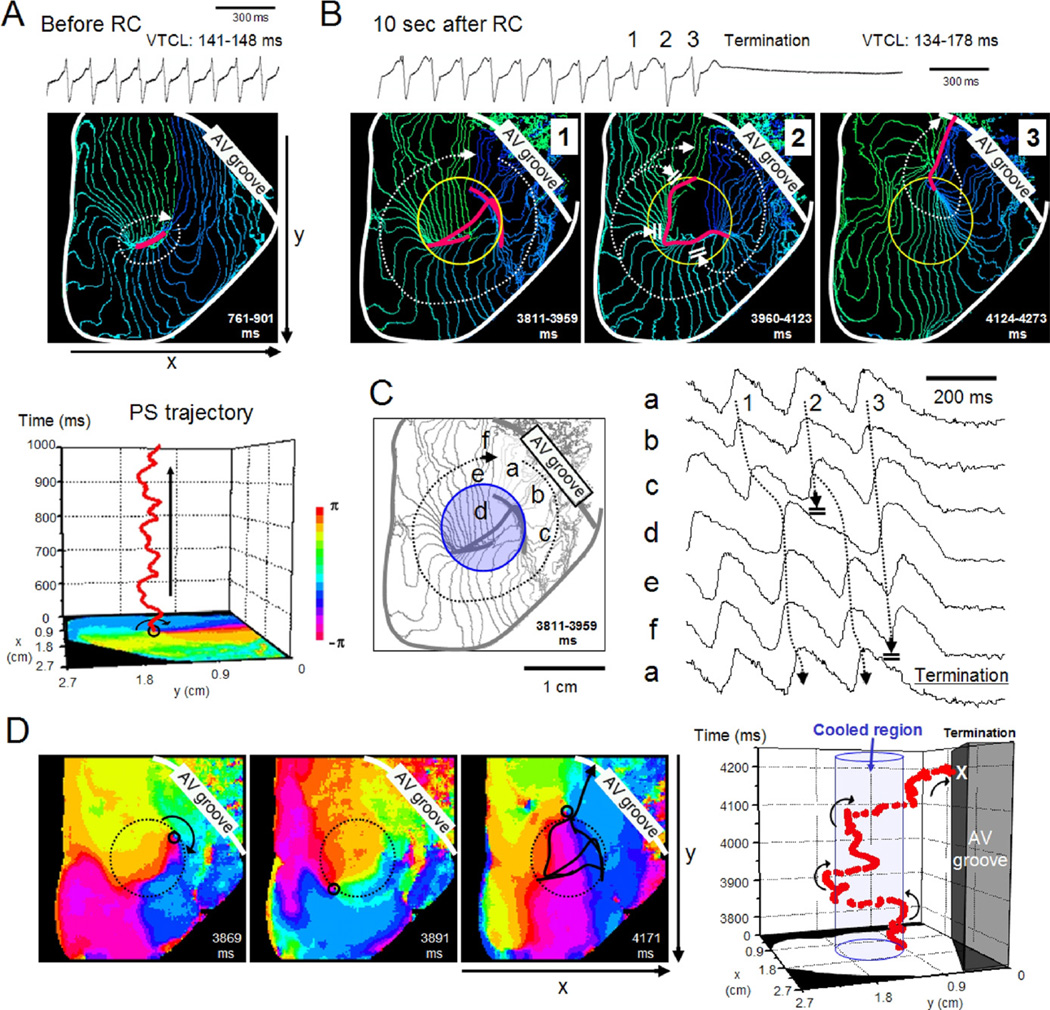
Optical images of excitation were analyzed in 16 sustained VTs that terminated during the RC application in 8 hearts. Figure 2 shows a representative experiment. Before the RC application, a stable clockwise rotor circulated around a functional block line (FBL) (approximately 5.1 mm); the bipolar electrogram showed a monomorphic pattern (Figure 2A, and Online Supplementary Video 1). A 3-D plot of the phase singularity (PS) trajectory obtained after phase mapping confirmed the stationarity of the rotor activity. Application of RC resulted in a dramatic change of the rotor dynamics and the VT terminated after approximately 10 seconds. Figure 2B shows isochrone maps during the last 3 beats prior to VT termination. A clockwise rotor circulated around a very long and curved FBL in the RC region in beat 1 and 2. The FBL configuration changed beat to beat in such a way that during beat 3 the FBL extended from the RC region to the atrioventricular groove, resulting in termination of reentry. The bipolar electrogram showed a polymorphic pattern before termination. Action potentials (Figure 2C) from the RC region (d) were longer compared with those outside (a– c, e, f), and this provided a substrate for conduction block. In Figure 2D, phase maps (left) and a 3-D plot of the PS trajectory (right) demonstrated that a single PS moved along the periphery of the RC region and collided with the atrioventricular groove (Online Supplementary Video 2). The mode of rotor termination by RC could be analyzed in 6 sustained VTs. In 4 sustained VTs, rotor terminated by drift and subsequent collision of PSs with boundaries, whereas in the remaining 2 cases, by mutual annihilation of PSs with opposite chiralities in the RC region.
Figure 2.
Termination of sustained VT by RC. A: Bipolar electrogram (top) and 4-ms isochrone map (middle) of sustained VT (>120 seconds) before RC application. Bottom, trajectory of a PS plotted on space-time axes. Stable reentrant activity was maintained. B: Bipolar electrogram (top) and isochrone maps (bottom) of 3 consecutive beats prior to VT termination approximately 10 seconds after RC. A clockwise rotor rotating around a long and curved FBL (pink) changed circuits in each excitation. Yellow circle, RC region. C: Optical action potential signals (a–f in the isochrone map) prior to VT termination. Wave propagation was frequently blocked at the periphery of the RC region. D: Left, phase maps of the last 2 beats. Black circle, PS of clockwise rotation; dotted circle, RC region. Right, PS trajectory plotted on space-time axes. Blue column, RC region. PS = phase singularity; VT = ventricular tachycardia; other abbreviations as in Figure 1.
RC failed to terminate 17 of 33 (52%) of the sustained VTs. The failure was attributable in part to the topological relationship between the rotor and the RC region. In other words, the success rate of RC cardioversion was relatively high (12 of 19) when the rotation center was located within or in the vicinity of the RC region. However, success was low (4 of 14) when the rotation center was far from the RC region.
Cardioversion of sustained VT by DC shock
When the sustained VTs did not terminate during the 30-second observation period, DC cardioversion was attempted. DC shocks of 15 to 80 V were applied to 21 sustained VTs in the absence of RC (in 12 hearts) and 16 sustained VTs in presence of RC (in 9 hearts) to evaluate the threshold DC shock intensity for cardioversion. The threshold DC shock intensity required for VT termination was significantly less with RC (22.8 ± 4.1 V, n = 16) than that without RC (40.5 ± 17.6 V, n = 21, P <.05).
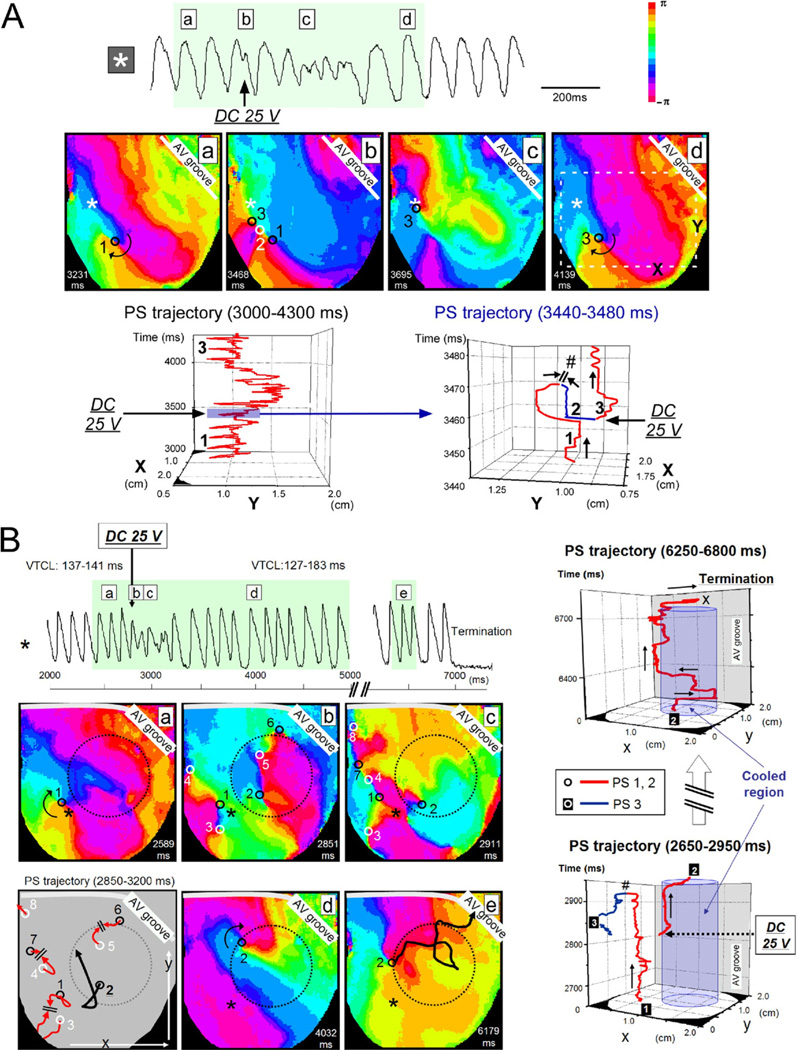
The mode of rotor modification and termination by DC shocks with and without RC was also different. Representative experiments are shown in Figure 3. Figure 3A is the consequence of a 25-V shock that failed to terminate the reentrant activity in the absence of RC. A single clockwise rotor (PS1) was present before the DC shock; shock application generated a pair of PSs (counterclockwise PS2 and clockwise PS3). Then, PS1 and PS2 collided with each other and disappeared, whereas PS3 survived by anchoring and subsequently maintained the reentrant activity. Thus, as confirmed by the 3-D PS trajectory plots, the rotor dynamics were destabilized transiently by the DC shock, but restabilized after PS repining. In this heart, application of a 30-V DC shock resulted in generation of multiple PSs exhibiting irregular meandering, and the activation pattern was transformed from VT to VF (Online Supplementary Figure 3A), and application of a high-voltage (50 V) DC shock resulted in a prompt disappearance of reentrant activities by shock-induced phase resetting (Online Supplementary Figure 3B).
Figure 3.
Rotor modification by DC shock applied to sustained VT in the absence and presence of RC. A: Failure of cardioversion by low-intensity shock in the absence of RC through repining of PS. Top, action potential trace; middle, phase maps before and after 25-V DC shock application. Black and white circles, PSs of clockwise and counterclockwise rotation, respectively. *Site of action potential recording. Bottom, trajectory of PSs plotted on space-time axes. A part of the trajectory in the left panel (immediately before and after DC shock application) is expanded in the right panel to show generation, mutual annihilation (#), and repining of PSs. B: Success of cardioversion by low-intensity DC shock in the presence of RC through unpinning of PS. Left, action potential trace (top) and phase maps (bottom) before (a) and after application of a 25-V DC shock (b– e). The shock application generated new PSs (black and white circles, clockwise and counterclockwise rotation, respectively). Trajectory of the PSs is illustrated in the right bottom panel. PS8 was pushed out of the observation area after meandering. PS1–PS3, PS4–PS7, and PS5–PS6 dissipated by mutual annihilation within 100 ms. PS2 survived and drifted in the periphery of the RC region (unpinning), and collided with the atrioventricular groove. *Site of action potential recording. Right, trajectory of PSs 1–3 plotted on space-time axes. Blue columns indicate the RC region. Abbreviations as in Figure 2.
Figure 3B is the consequence of a 25-V DC shock, which terminated the reentrant activity in the presence of RC. Before the shock, a single clockwise rotor (PS1) anchored to a site close to the RC region (left, a). DC shock at 25 V created 7 new PSs (PSs2 to 8) of either chirality that meandered following complex trajectories (b and c). Then, PS1–PS3, PS4–PS7, and PS5–PS6 disappeared by mutual annihilation. PS8 moved out of the left margin (toward the posterior surface). PS2 survived and drifted along the periphery of the RC region (d and e). The VT terminated by collision of PS2 with the atrioventricular groove approximately 4 seconds after the DC shock application (top). Figure 3B, right, illustrates the trajectory of PSs plotted on space-time axes.
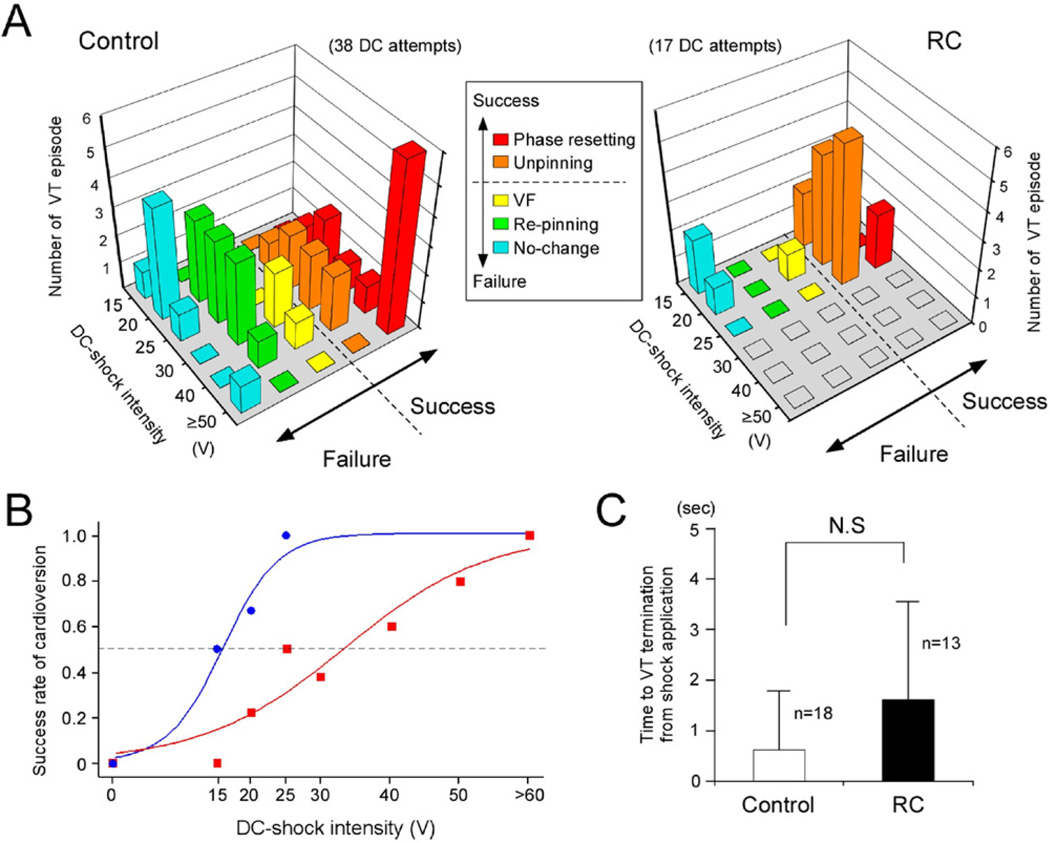
Figure 4 summarizes the data obtained from 18 sustained VTs (in 12 hearts) without RC and 13 sustained VTs (in 9 hearts) with RC exhibiting visible rotors; 38 and 17 DC shocks were applied without and with RC, respectively. In the cases of cardioversion failure, the mode of SW modification was classified into 3 types: no substantial change, repining of rotors (see Figure 3A), and transformation from VT to VF (see Online Supplementary Figure 3A). In the case of cardioversion success, the mode of SW modification was classified into 2 forms; unpinning of rotors followed by collision and extinction (see Figure 3B), and immediate disappearance of rotors by phase resetting (see Online Supplementary Figure 3B). As shown in Figure 4A, for DC shocks without RC (control), the major mode of success was phase resetting at high DC shock intensities (≥50 V); transformation from VT to VF often occurred at intermediate intensities (30 to 40 V), and repining was the major mode of failure at relatively low intensities (20 to 30 V). For DC shocks with RC, in contrast, the major mode of success was unpinning with relatively low intensities (15 to 25 V), and the major mode of failure was no change. Figure 4B compares the success rate of DC cardioversion with and without RC. The intensity-response curve with RC was shifted to the left from that without RC (control) by 17.7 V. Average time to VT termination tended to be longer in the presence of RC (1.6 ± 1.9 seconds, n = 13) compared with that in the absence of RC (0.6 ± 1.2 seconds, n = 18), although the difference remained statistically insignificant (Figure 4C).
Figure 4.
Modification of rotor dynamics by DC shocks applied to sustained VT. A: Intensity-dependent modification of rotor dynamics by DC shocks in the absence (control) and presence of RC. In the case of cardioversion success, the rotor modification was either phase resetting (red) or unpinning of PSs followed by collision and extinction (orange). When the cardioversion was failed, the rotor modification was either no substantial change (blue), repining of PSs (green) or transformation from VT to VF (yellow). B: Intensity-response of the success rate of DC cardioversion without (control, red) and with RC (blue). The shock intensity for 50% success was 33.3 V without RC (38 VTs) and 15.5 V with RC (18 VTs). C: Time required for VT termination from the instant of shock application without RC (control, n = 18) and with RC (n = 13, not significant). Abbreviations as in Figure 2.
Effects of RC on VT/VF induced in 3-D hearts
We examined the effects of RC on the sustained VT/VFs (>120 seconds) in 6 intact hearts without cryoablation. A total of 17 sustained VT/VFs were induced. In 8 control VT/VFs, in which RC was not imposed, all (8 of 8) continued during the 30-second observation period. Subsequent application of biphasic DC shocks terminated 3 of 8 VT/VFs. In 9 VT/VFs, in which RC cardioversion was attempted, 1 of 9 terminated within 30 seconds by RC alone; subsequent application of DC shocks in the presence of RC terminated 7 of 8 VT/VFs. The threshold amplitude for the DC cardioversion was 56.7 ± 5.8 V (n = 3) in the absence of RC (control), and 31.4 ± 8.5 V (n = 7) in the presence of RC (P <.05). Time for cardioversion after DC application was 1.3 ± 1.7 seconds (n = 3) in the absence of RC and 12.2 ± 11.2 seconds (n = 7) in the presence of RC (P <.05). DC shocks at the maximum voltage (100 V) failed to terminate 5 of 8 VT/VFs in the absence of RC, but 1 of 8 in the presence of RC. Thus, RC facilitated cardioversion in 3-D hearts when combined with DC shocks in association with a certain modification of the mode of reentry termination.
Discussion
The major findings in the present study are as follows. First, rotors induced during RC were initially confined to the RC region, but were unstable and terminated early by collision following drift. Second, rotors underlying sustained VTs were transformed by RC from stationary to nonstationary; RC terminated approximately 50% of sustained VTs. Third, the threshold intensity of DC shocks for cardioversion of sustained VTs was reduced in the presence of RC; the major mode of rotor termination by DC shocks was changed from phase resetting to unpinning.
RC destabilizes rotors in favor of their termination
In the experiments in which VTs were induced during RC (the first protocol), 98% of the VTs self-terminated within 5 seconds because the rotors were unstable and drifted around the RC region. This finding, which is essentially concordant with a previous report by Boersma et al,18 can be explained by creation of a region of long refractoriness and reduced conductivity by RC. During constant stimulation, RC prolonged the APD and decreased the CV in the RC region, which explained why the reentrant activity was impaired in the RC region, and was accompanied by intermittent conduction block and long tortuous PS meandering trajectories in the periphery of the RC region. We have previously demonstrated that global myocardial cooling (30°C to 33°C) caused an increase of the maximum APD restitution slope and a broadening of CV restitution curves compared with control subjects.17 These changes of the restitution properties would increase destabilization of the reentry in the RC region through an enhancement of wavefront-tail interactions.17 Temperature-dependent alterations of electrotonic effects, short-term memory, and perhaps intracellular Ca2+ dynamics might also contribute to rotor destabilization,9 but such factors remain to be elucidated.
Mathematical model analysis of the rotor dynamics has demonstrated that spatial gradients in refractoriness play important roles in the stability of the rotation center in the cardiac muscle with normal excitability.21–23 In a medium with stepwise heterogeneity, rotors move along the border separating regions with different refractoriness.21 Our observation showing enormous PS drift in the periphery of the RC region is consistent with theoretical prediction.
To assess the potential usefulness of RC for cardioversion, we investigated the effects of RC on sustained VTs and found that RC terminated approximately 50% of sustained VTs. In the cases of successful cardioversion, the rotor dynamics changed dramatically from stationary to nonstationary. The nonstationary rotors shared common features with those induced after RC application in terms of long tortuous FBLs confined to the RC region and tremendous drift of rotors leading to their collision and extinction. The effects of RC on VT perpetuation were reversible upon removal of RC, suggesting a potential advantage of RC as a therapeutic procedure. The failure of RC cardioversion was attributable partly to the topological relationship between the pre-existing rotor and the RC region; when the rotation center was outside the RC region, the success rate of cardioversion was low. The RC cardioversion also depends on the size of the cooling area. In our pilot experiments, we tested the effects of RC to reduce the temperature by approximately 6°C from the baseline (36°C) in a circular area of 3 different size (5, 8, and 10 mm in diameter). Sustained VTs were terminated efficiently (16 of 33 VTs, 48%) by RC alone only with the largest size tested.
RC reduces the DC shock intensity required for cardioversion
When RC failed to terminate sustained VTs, we attempted DC cardioversion in the presence of RC. Those VTs were likely to be maintained by stationary rotors with PSs anchored at structural discontinuities. Our experiments showed that application of relatively low-intensity DC shocks always created new multiple PSs, resulting from shock-induced virtual electrode polarization.24 SW reentry can be induced by a combination of depolarization and hyperpolarization at a close proximity.24,25
In control subjects (without RC), relatively weak DC shocks caused displacement of preexisting PSs (unpinning) and generated new PSs. Eventually such new PSs disappeared, but organized stationary SW reentry was resumed when the survived PSs were anchored again. Another mode of cardioversion failure was transformation from VT to VF, which was the result of irregular meandering of multiple, widely dispersed PSs. The major mode of cardioversion success in control subjects was immediate PS disappearance by phase resetting at intensities larger than the upper limit of vulnerability.26 In contrast, when DC shocks of weak intensities were applied in the presence of RC, unpinning of PSs was not followed by repining. Instead, PSs drifted along the periphery of the RC region, eventually colliding and disappearing. Thus, the major mode of cardioversion success in the presence of RC was unpinning. The threshold shock intensity was reduced significantly (by approximately 50%) from control subjects. Transformation from VT to VF rarely occurred in the presence of RC. These results suggest that RC facilitates DC cardioversion by confining and destabilizing rotors in the RC region.
Ripplinger et al16 demonstrated in isolated rabbit right ventricular preparations that unpinning and destabilization of rotors leading to VT termination can be induced by weak DC shocks, provided that shocks are applied at a certain phase of the VT cycle.16,22 A greater reduction of the DC shock intensity might be possible for cardioversion in combination with RC if the shocks were applied at a restricted phase. Further experimental studies are required to address the issue.
Study limitations
We showed facilitation of VT termination by RC in 2-D ventricular myocardium of rabbit hearts through unpinning and collision of rotors. We used BDM, which is known to affect ion channels and intracellular Ca2+ dynamics and to reduce muscle contraction. However, this does not seem to invalidate our results, because characteristic modification of rotor dynamics by RC was preserved in the absence of BDM (Online Supplementary Figure 5). Extrapolation of our observations in 2-D tissue preparations to 3-D and larger hearts is not straightforward. The chance of collision of rotors with boundaries would be reduced, and wave breakup would be enhanced in a larger 3-D tissue mass. In our experiments using intact 3-D rabbit hearts, in fact, RC alone was not effective for self-termination of sustained VT/VFs. However, the threshold intensity of DC shocks for cardioversion was significantly reduced in the presence of RC, and this was associated with an increase in the time required for cardioversion after the shock, suggesting alterations in the mode of reentry termination. Accordingly, a certain benefit of RC favoring low-energy defibrillation is considered to be preserved in 3-D hearts. Structural discontinuities and functional heterogeneities would alter the requirements for rotor termination. In addition, focal activities may also play roles in VT/VF.27 To the best of our knowledge, there are no efficient RC devices applicable to clinical practice, and this is a critical issue to be solved in the future. Despite these limitations, the present study provides a new perspective toward the development of low-energy cardioversion/defibrillation.
Supplementary Material
Acknowledgments
This study was supported by Grant-in-Aid for Scientific Research (B) 19390210 and (C) 20590860 from the Japanese Society for Promotion of Sciences and Grant-in-Aid for Scientific Research on Innovative Area 22136010 from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
ABBREVIATIONS
- 2-D
two-dimension
- 3-D
three-dimension
- APD
action potential duration
- BCL
basic cycle length
- BDM
2,3-butandione monoxime
- CV
conduction velocity
- FBL
functional block line
- ICD
implantable cardioverter-defibrillator
- LV
left ventricle
- PS
phase singularity
- RC
regional cooling
- SW
spiral wave
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Footnotes
Appendix
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.hrthm.2011.08.013.
References
- 1.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 2.Lee DS, Green LD, Liu PP, et al. Effectiveness of implantable defibrillators for preventing arrhythmic events and death: a meta-analysis. J Am Coll Cardiol. 2003;41:1573–1582. doi: 10.1016/s0735-1097(03)00253-5. [DOI] [PubMed] [Google Scholar]
- 3.Weaver WD, Cobb LA, Copass MK, Hallstrom AP. Ventricular defibrillation: a comparative trial using 175-J and 320-J shocks. N Engl J Med. 1982;307:1101–1106. doi: 10.1056/NEJM198210283071801. [DOI] [PubMed] [Google Scholar]
- 4.Waldecker B, Brugada P, Zehender M, Stevenson W, Wellens HJ. Dysrhythmias after direct-current cardioversion. Am J Cardiol. 1986;57:120–123. doi: 10.1016/0002-9149(86)90963-x. [DOI] [PubMed] [Google Scholar]
- 5.Runsiö M, Kallner A, Källner G, Rosenqvist M, Bergfeldt L. Myocardial injury after electrical therapy for cardiac arrhythmias assessed by troponin-T release. Am J Cardiol. 1997;79:1241–1245. doi: 10.1016/s0002-9149(97)00090-8. [DOI] [PubMed] [Google Scholar]
- 6.Godemann F, Butter C, Lampe F, et al. Panic disorders and agoraphobia: side effects of treatment with an implantable cardioverter/defibrillator. Clin Cardiol. 2004;27:321–326. doi: 10.1002/clc.4960270604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamphuis HC, Verhoeven NW, Leeuw R, Derksen R, Hauer RN, Winnubst JA. ICD: a qualitative study of patient experience the first year after implantation. J Clin Nurs. 2004;13:1008–1016. doi: 10.1111/j.1365-2702.2004.01021.x. [DOI] [PubMed] [Google Scholar]
- 8.Jalife J. Ventricular fibrillation: mechanisms of initiation and maintenance. Annu Rev Physiol. 2000;62:25–50. doi: 10.1146/annurev.physiol.62.1.25. [DOI] [PubMed] [Google Scholar]
- 9.Weiss JN, Qu Z, Chen PS, et al. The dynamics of cardiac fibrillation. Circulation. 2005;112:1232–1240. doi: 10.1161/CIRCULATIONAHA.104.529545. [DOI] [PubMed] [Google Scholar]
- 10.Biktashev VN, Holden AV. Design principles of a low voltage cardiac defibrillator based on the effect of feedback resonant drift. J Theor Biol. 1994;169:101–112. doi: 10.1006/jtbi.1994.1132. [DOI] [PubMed] [Google Scholar]
- 11.Morgan SW, Plank G, Biktasheva IV, Biktashev VN. Low energy defibrillation in human cardiac tissue: a simulation study. Biophys J. 2009;96:1364–1373. doi: 10.1016/j.bpj.2008.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garfinkel A, Spano ML, Ditto WL, Weiss JN. Controlling cardiac chaos. Science. 1992;257:1230–1235. doi: 10.1126/science.1519060. [DOI] [PubMed] [Google Scholar]
- 13.Pak HN, Liu YB, Hayashi H, et al. Synchronization of ventricular fibrillation with real-time feedback pacing: implication to low-energy defibrillation. Am J Physiol Heart Circ Physiol. 2003;285:H2704–H2711. doi: 10.1152/ajpheart.00366.2003. [DOI] [PubMed] [Google Scholar]
- 14.Pak HN, Okuyama Y, Oh YS, et al. Improvement of defibrillation efficacy with preshock synchronized pacing. J Cardiovasc Electrophysiol. 2004;15:581–587. doi: 10.1046/j.1540-8167.2004.03573.x. [DOI] [PubMed] [Google Scholar]
- 15.Ripplinger CM, Krinsky VI, Nikolski VP, Efimov IR. Mechanisms of unpinning and termination of ventricular tachycardia. Am J Physiol Heart Circ Physiol. 2006;291:H184–H192. doi: 10.1152/ajpheart.01300.2005. [DOI] [PubMed] [Google Scholar]
- 16.Li W, Ripplinger CM, Lou Q, Efimov IR. Multiple monophasic shocks improve electrotherapy of ventricular tachycardia in a rabbit model of chronic infarction. Heart Rhythm. 2009;6:1020–1027. doi: 10.1016/j.hrthm.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harada M, Honjo H, Yamazaki M, et al. Moderate hypothermia increases the chance of spiral wave collision in favor of self-termination of ventricular tachycardia/fibrillation. Am J Physiol Heart Circ Physiol. 2008;294:H1896–H1905. doi: 10.1152/ajpheart.00986.2007. [DOI] [PubMed] [Google Scholar]
- 18.Boersma L, Zetelaki Z, Brugada J, Allessie M. Polymorphic reentrant ventricular tachycardia in the isolated rabbit heart studied by high-density mapping. Circulation. 2002;105:3053–3061. doi: 10.1161/01.cir.0000019407.35848.af. [DOI] [PubMed] [Google Scholar]
- 19.Yamazaki M, Honjo H, Nakagawa H, et al. Mechanisms of destabilization and early termination of spiral wave reentry in the ventricle by a class III antiarrhythmic agent, nifekalant. Am J Physiol Heart Circ Physiol. 2007;292:H539–H548. doi: 10.1152/ajpheart.00640.2006. [DOI] [PubMed] [Google Scholar]
- 20.Ishiguro YS, Honjo H, Opthof T, et al. Early termination of spiral wave reentry by combined blockade of Na+ and L-type Ca2+ currents in a perfused two-dimensional epicardial layer of rabbit ventricular myocardium. Heart Rhythm. 2009;6:684–692. doi: 10.1016/j.hrthm.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Fast VG, Kléber AG. Role of wavefront curvature in propagation of cardiac impulse. Cardiovasc Res. 1997;33:258–271. doi: 10.1016/s0008-6363(96)00216-7. [DOI] [PubMed] [Google Scholar]
- 22.Fast VG, Rohr S, Gillis AM, Kléber AG. Activation of cardiac tissue by extracellular electrical shocks: formation of ’secondary sources’ at intercellular clefts in monolayers of cultured myocytes. Circ Res. 1998;82:375–385. doi: 10.1161/01.res.82.3.375. [DOI] [PubMed] [Google Scholar]
- 23.Kléber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–488. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 24.Efimov IR, Cheng Y, Van Wagoner DR, Mazgalev T, Tchou PJ. Virtual electrode-induced phase singularity: a basic mechanism of defibrillation failure. Circ Res. 1998;82:918–925. doi: 10.1161/01.res.82.8.918. [DOI] [PubMed] [Google Scholar]
- 25.Trayanova NA, Gray RA, Bourn DW, Eason JC. Virtual electrode-induced positive and negative graded responses: new insights into fibrillation induction and defibrillation. J Cardiovasc Electrophysiol. 2003;14:756–763. doi: 10.1046/j.1540-8167.2003.03042.x. [DOI] [PubMed] [Google Scholar]
- 26.Gray RA, Chattipakorn N. Termination of spiral waves during cardiac fibrillation via shock-induced phase resetting. Proc Natl Acad Sci USA. 2005;102:4672–4677. doi: 10.1073/pnas.0407860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabereaux PB, Dosdall DJ, Ideker RE. Mechanisms of VF maintenance: wandering wavelets, mother rotors, or foci. Heart Rhythm. 2009;6:405–415. doi: 10.1016/j.hrthm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.