Abstract
Background
We devised an open-label, randomized trial to evaluate whether therapeutic drug monitoring (TDM) of protease inhibitors (PIs) and dose escalation based upon a normalized inhibitory quotient (NIQ), which integrates PI trough concentration and drug resistance, could improve virologic outcome in PI-experienced patients with treatment failure. Secondary analyses through 48 weeks are presented.
Methods
Eligible HIV-infected subjects with a screening viral load of ≥1000 copies/mL initiated a new PI-based regimen at entry and had NIQ performed at week 2. Subjects with an NIQ ≤1 were randomized at week 4 to a standard-of-care (SOC) arm or TDM arm featuring PI dose escalation.
Results
One hundred and eighty-three subjects were randomized. There was no significant treatment difference in change from randomization to week 48 in HIV-1 RNA [P = .13, median (25th, 75th percentile log10 copies/mL change): −0.03 (−0.74, 0.62) with TDM and 0.11 (−2.3, 0.82) with SOC]. In subgroup analysis, patients with ≥0.69 active PIs benefited from TDM compared to those with <0.69 active PIs (P = .05).
Conclusions
While the TDM strategy of PI dose escalation did not improve virologic response at week 48 overall, in subgroup analysis, TDM favorably impacted virologic outcome in subjects taking PI-based regimens with moderate antiviral activity.
Keywords: antiretroviral therapy, clinical trials, HIV drug resistance, pharmacokinetics, protease inhibitors, therapeutic drug monitoring
The optimal management of antiretroviral-experienced patients experiencing virologic failure1–4 remains complex despite the advent of novel classes5–7 of antiretroviral agents with enhanced potency against drug-resistant viral isolates. Protease inhibitor (PI)–based therapy1,2,8–10 remains a critical component of antiretroviral therapy when alternative regimens are devised in the setting of virologic failure.
While therapeutic drug monitoring (TDM) has been utilized in HIV-infected individuals to guide individualized dosing of PI and non-nucleoside reverse transcriptase inhibitor (NNRTI) agents to confer improved adherence and virologic outcome,11–13 to identify potential antiretroviral drug-drug interactions,14 and to limit selected drug toxicities,15 the role of TDM in the setting of treatment failure to improve virologic efficacy has not been uniformly established.
The application of TDM based upon inhibitory quotient (IQ) defined as plasma trough concentration divided by 50% inhibitor concentration (IC50) for selected PI agents previously demonstrated an association between plasma trough concentrations achieved and virologic outcome in several retrospective studies.16–29
We conducted a randomized, multicenter trial to determine whether PI dose escalation incorporating a normalized inhibitory quotient (NIQ) as a component of a TDM strategy could enhance virologic efficacy and confer improved therapeutic outcome in antiretroviral-experienced patients. The primary week 24 study results were previously published.30 The week 48 secondary analysis results of the study are presented in this report.
METHODS
Participants
Eligible subjects were HIV-infected adults experiencing virologic failure within 90 days of study entry while on at least one PI-based regimen with plasma HIV-1 RNA level of ≥1000 copies/mL and a virtual phenotype resistance test demonstrating resistance to at least one drug on the failing regimen. Subjects initiated a new PI-containing regimen at study entry. Participants were recruited from 45 AIDS Clinical Trials Units in the United States and Puerto Rico. The study was approved by the institutional review boards at the participating sites, and all subjects provided written informed consent.
Normalized Inhibitory Quotient
Optimal PI drug concentrations were calculated by determining an NIQ for each PI in the subject’s antiretroviral regimen that was initiated at study entry. NIQ was defined as IQsubject divided by IQreference in which IQsubject was computed as subject’s PI trough concentration divided by fold change in IC50 of subject’s viral isolate; and IQreference was calculated as reference population trough PI drug concentration divided by fold-change IC50 of the resistance threshold to that PI. The reference IQs for the specific PIs used were derived from patient populations evaluated in prior studies18,19,31–33 and incorporated the ratio of patient trough concentrations to the fold change in IC50 of their virus isolate that was correlated with virologic success for that given PI.34 An NIQ ≤1 indicated that the subject’s IQ was below the threshold associated with virologic suppression for that given PI. The study identified subjects with NIQ ≤1 as having low likelihood of virologic response who potentially could benefit from PI dose escalation.
Study Design
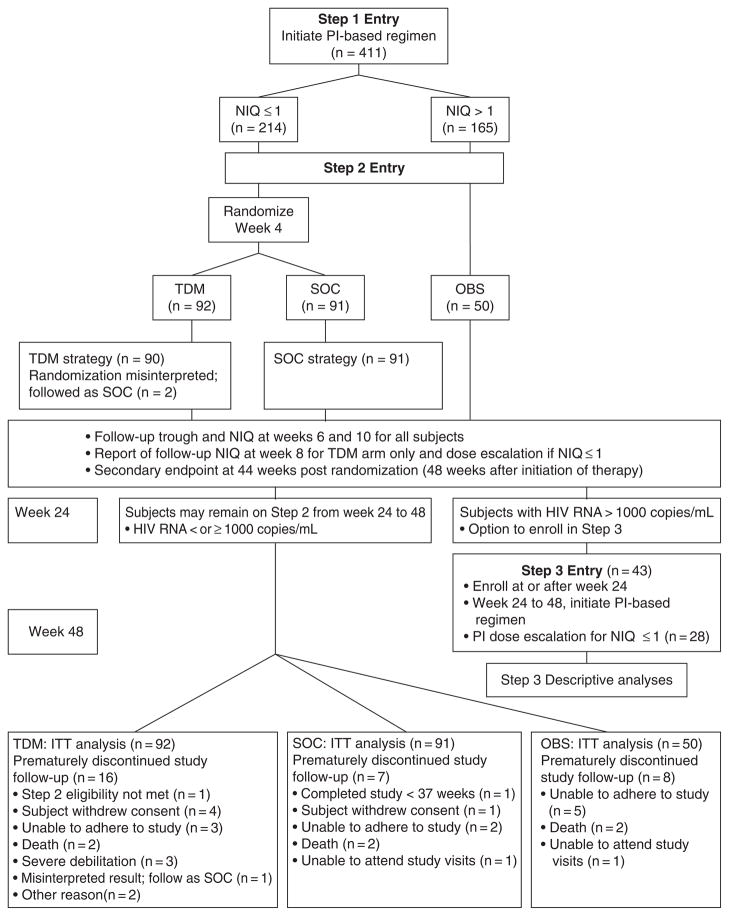
The study featured 3 steps (Figure 1). At study entry (Step 1), subjects initiated a new PI-containing regimen that was selected based upon virtual phenotype obtained at screening. At week 2, a PI trough concentration was obtained and an NIQ was calculated and used to determine eligibility for randomization at week 4 after study entry.
Figure 1.
Study design and disposition of patients. TDM = therapeutic drug monitoring and protease inhibitor (PI) dose escalation arm; SOC = standard of care arm; OBS = observational arm. NIQ = normalized inhibitory quotient; ITT = intent -to-treat.
At Step 2 entry (week 4), subjects with NIQ ≤1 were randomized to the standard of care (SOC) arm or the TDM arm. Randomization was performed using permuted blocks and was stratified by use of a new antiretroviral class of drugs versus none at study entry. Subjects randomized to the TDM arm received PI dose escalation recommendations from the study team based upon protocol-specified doses, and dose escalations were to be implemented within 72 hours of randomization. Subjects randomized to the SOC arm did not undergo PI dose escalation and remained on the same PI dose regimen initiated at study entry. PI trough concentrations were measured at 2 and 6 weeks after Step 2 entry (randomization) in both study arms; only subjects in the TDM arm received NIQ results based upon real-time PI trough concentrations and were eligible to undertake a second PI dose escalation at week 8 if their NIQ was ≤1. Subjects with NIQ >1 were assigned to the observational (OBS) arm that was capped at 50.
The study protocol stipulated the following criteria for study follow-up discontinuation after Step 2 entry: malignancy requiring systemic chemotherapy, requirement for prohibited medications, inability by subject to adhere to study requirements, request by the subject to withdraw, request by the provider to withdraw the subject, the subject reaches a defined study endpoint, or pregnancy.
The primary endpoint of the study was change in log10 HIV-1 RNA 20 weeks after randomization at Step 2 (week 24 after study entry). The secondary endpoint was change in log10 HIV-1 RNA 44 weeks after randomization (week 48 after study entry).
Antiretroviral Regimens
All US Food and Drug Administration (FDA)–approved antiretroviral drugs available during enrollment (June 2002 to May 2006) were allowed. The PI-based regimens were selected by the subject’s primary care provider and were based upon the virtual phenotype resistance test obtained at screening.
Subjects initiated protocol-specified doses of PIs in combination with low-dose ritonavir for pharmacokinetic enhancement. Dual-PI regimens were allowed provided that there were no known adverse pharmacokinetic interactions between the PIs.
Prespecified PI dosing escalation algorithms were devised for each PI-based regimen.34 Implementation of PI dose escalation was not allowed if nonadherence or dose-related toxicity was identified.
Study Evaluations
Clinical assessments and laboratory tests were performed at screening; study entry; week 2; Step 2 entry (randomization) at week 4; and at weeks 2, 6, 12, 16, 20, 28, 36, and 44 weeks following randomization. Timed plasma PI trough concentrations were obtained 2 weeks after study entry and at 2 and 6 weeks post randomization in Step 2.30
Monitoring
The Division of AIDS Data and Safety Monitoring Board reviewed the study’s efficacy and safety results annually on 3 occasions without modification to the study. The O’Brien-Fleming stopping rule with Lan and DeMets spending function was used to evaluate interim efficacy primary endpoint results.35 No early stopping boundary was reached for the duration of the study.
Signs and symptoms and laboratory values were graded according to the Division of AIDS grading scale.36 Protocol-specified criteria were formulated to guide treatment interruption and/ or delayed PI dose escalation in response to selected toxicities.
Outcomes
The primary comparison evaluating the difference between TDM and SOC in the distribution of change in log10 plasma HIV-1 RNA level from randomization (week 4) to week 24 was previously published.30
Secondary analyses evaluated virologic and safety outcomes that included change in log10 HIV-1 RNA level from randomization to week 48 after study entry (44 weeks post randomization at Step 2); time to virologic failure, defined as a confirmed HIV-1 RNA level ≥1000 copies/ mL at or after week 24 (week 20 after step 2 randomization); and the combined endpoint of time to first grade 3 or 4 sign/symptom or laboratory abnormality.
Statistical Analysis
Analyses were intent-to-treat unless otherwise specified. The analysis evaluating change in plasma HIV-1 RNA level required censoring data methods and the primary comparison approach specified a Gehan-Wilcoxon test. If no viral load result was available at either week 4 or week 48, the subject was excluded from the analysis. If both values were below the assay quantification limit (<50 copies/mL), the change in viral load was defined to be zero and subjects were deemed to be virologic successes and included in the analysis.
Sensitivity analyses for this endpoint included (a) nonparametric approach where premature study discontinuations that resulted in missing viral load data were ranked as the worst HIV-1 RNA outcome in both arms, and (b) last observation carried forward for missing HIV-1 RNA data. Censored regression models were used to explore the associations of baseline characteristics with the week 48 change in HIV-1 RNA and the possible interaction with TDM effect. For categorical variables, univariate censored regression model testing the TDM effect was conducted. For continuous variables, such as the number of active PIs at baseline, subjects were classified into 2 categories using the sample median.
Time to event distributions were summarized using Kaplan-Meier (KM) curves and the log-rank test was used to compare the event time distributions. In evaluating time from Step 2 randomization to study discontinuation, subjects who prematurely discontinued study follow-up were considered an event at their last study evaluation date. Subjects who completed the study at 37 or more weeks of follow-up after Step 2 randomization/entry were censored at their last study visit. In determining time from randomization at Step 2 entry to first permanent discontinuation of a PI in the initial regimen, subjects who prematurely discontinued a PI for reasons other than completion of study/step were considered events at the time of treatment modification.
Wilcoxon rank sum tests and Kruskal-Wallis tests were used to compare continuous endpoints.
RESULTS
Baseline Characteristics
The baseline characteristics for 411 subjects enrolled at study entry (Step 1) have been previously reported.30 Table 1 presents the baseline characteristics for the 233 subjects who registered to Step 2 and were either randomized to the SOC or TDM arms or assigned to the OBS arm. The baseline characteristics in the 2 randomized arms were well balanced.
Table 1.
Baseline characteristics at Step 2 randomization
| Subjects in Step 2 (n = 233)
|
|||
|---|---|---|---|
| Characteristics at Step 2 entry | SOC arm (n = 91) | TDM arm (n = 92) | Observational arm (n = 50) |
| Sex | |||
| Male | 83 (91%) | 81 (88 %) | 43 (86%) |
| Female | 8 (9%) | 11 (12%) | 7 (14%) |
| Race/Ethnicity | |||
| White non-Hispanic | 44 (48%) | 46 (50%) | 19 (38%) |
| Black non-Hispanic | 24 (26%) | 21 (23%) | 21 (42%) |
| Hispanic | 21 (23%) | 23 (25%) | 9 (18%) |
| Asian, Pacific Island | 1 (1%) | 2 (2%) | 1 (2%) |
| American Indian, Alaskan | 1 (1%) | 0 (0%) | 0 (0%) |
| Median age, years | 46 | 44 | 44 |
| IV drug use | |||
| Never | 81 (89%) | 75 (82%) | 34 (68%) |
| Current/previous | 10 (11%) | 17 (18%) | 16 (32%) |
| PI regimens | |||
| APV | 4 (4.4%) | 5 (5.4%) | 4 (8%) |
| ATV | 6 (6.6%) | 2 (2.2%) | 0 (0%) |
| Fos-APV | 12 (13.2%) | 13 (14.1%) | 11 (22%) |
| IDV | 2 (2.2%) | 4 (4.4%) | 2 (4%) |
| LPV/r | 12 (13.2%) | 10 (10.9%) | 17 (34%) |
| SQV | 11 (12.1%) | 8 (8.7%) | 6 (12%) |
| TPV | 2 (2.2%) | 5 (5.4%) | 0 (0%) |
| NFVa | 1 (1.1%) | 0 (0%) | 0 (0%) |
| Dual-PI regimens | |||
| APV+LPV/r | 3 (3.3%) | 1 (1.1%) | 3 (6%) |
| IDV+LPV/r | 3 (3.3%) | 4 (4.4%) | 0 (0%) |
| SQV+fos-APV | 15 (16.5%) | 18 (19.6%) | 1 (2%) |
| SQV+APV | 5 (5.5%) | 6 (6.5%) | 3 (6%) |
| SQV+LPV/r | 15 (16.5%) | 16 (17.4%) | 3 (6%) |
| New class of ARV drugs | 9 (10%) | 14 (15%) | 7 (14%) |
| Median HIV-1 RNA copies/mL | 4606 | 3850 | 310 |
| Median change in RNA from Step 1 (study entry) to 2 (randomization), log10 copies/mL | −1.0 | −1.0 | −2.0 |
| Median CD4 cell count, cells/mm3 | 226 | 220 | 270 |
| Change in CD4 cell count from Step 1 to Step 2, cells/mm3 | +22 | +9 | +38 |
Note: Values given as n (%) unless otherwise indicated. SOC = standard of care; TDM = therapeutic drug monitoring; IV = intravenous; ARV = antiretroviral. Protease inhibitors (PIs) used in the study included amprenavir (APV), atazanavir (ATV), fos-amprenavir (fos-APV), indinavir (IDV), lopinavir/ritonavir (LPV/r); saquinavir (SQV), tipranavir (TPV), and nelfinavir (NFV).
All PI-based regimens were pharmacokinetically boosted with ritonavir except for nelfinavir.
PI-Based Regimens
There were 13 different PI regimens used in the study (Table 1). All regimens were ritonavir (RTV)-boosted with the exception of nelfinavir. The PI combination regimens were comparable between the SOC and TDM arms with saquinavir+ fosam-prenavir (18%), saquinavir + lopinavir/ritonavir (17%), fosamprenavir (14%), and lopinavir/ritonavir (12%) being the most frequently used regimens.
PI Dose Escalations in the TDM Arm
Sixty-two of 85 patients (73%) in the intent-to treat group undertook all the recommended PI dose escalations stipulated at Step 2 entry (week 4) and at week 8 for NIQ ≤1. Eight of 23 patients did not comply with PI dose escalations because of protocol-mandated toxicity management. The remaining 15 deviations with no PI dose adjustment in the TDM arm occurred due to site error and patient or physician preference. No SOC patient underwent PI dose escalation.
Study Follow-up Disposition and PI Treatment Discontinuation
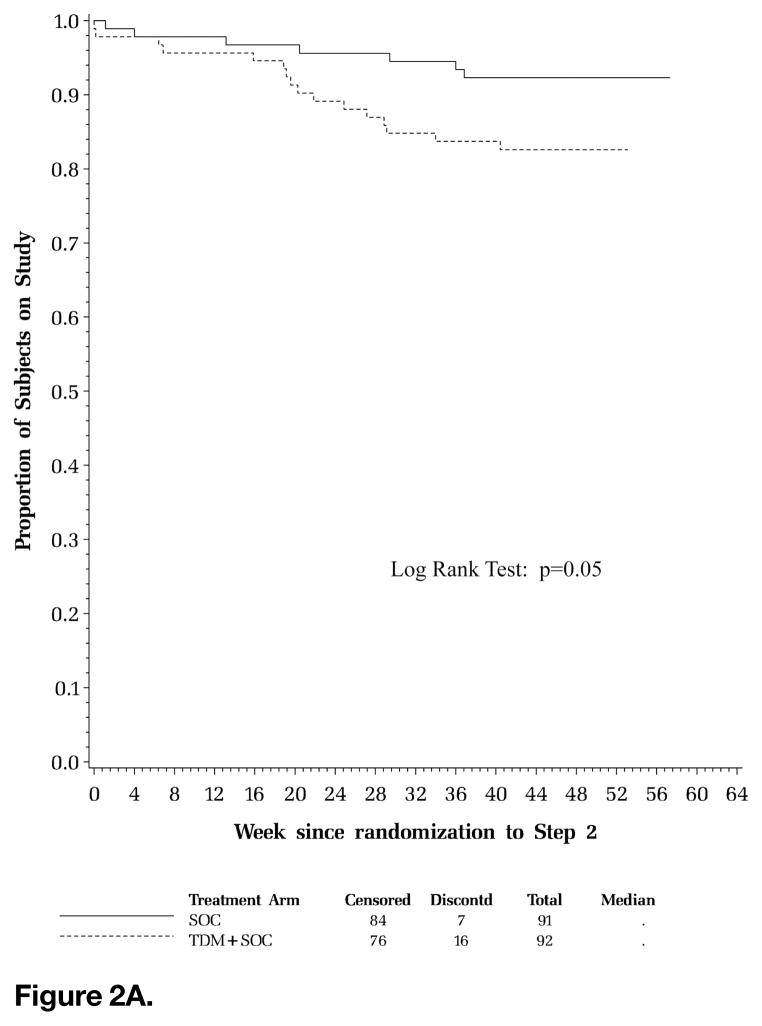
Sixteen (17%) subjects in the TDM arm prematurely discontinued the study prior to completing 37 weeks of follow-up post randomization at Step 2 compared to 7 (8%) subjects in the SOC arm. More subjects in the TDM arm discontinued the study for reasons of severe debilitation compared to the SOC arm (3 vs 0) and withdrawal of consent (4 vs 1). There was a total of 6 deaths in Step 2 with 2 deaths each in the randomized (SOC, n = 2; TDM, n = 2) and observational (OBS, n = 2) arms. Four subjects in the SOC (n = 2) and TDM (n = 2) arms discontinued the study in Step 2 for inability to adhere to study requirements. The time to premature study discontinuation (Figure 2A) was significantly shorter for subjects in the TDM arm compared to those in the SOC arm (P = .05, log-rank test).
Figure 2.
Figure 2A. Time to premature discontinuation of scheduled clinic evaluations. Kaplan-Meier curve of time to premature study discontinuation. Solid line represents standard of care (SOC) arm; hatched line represents therapeutic drug monitoring (TDM) arm; horizontal axis displays week since randomization to Step 2; vertical axis displays proportion of subjects on study.
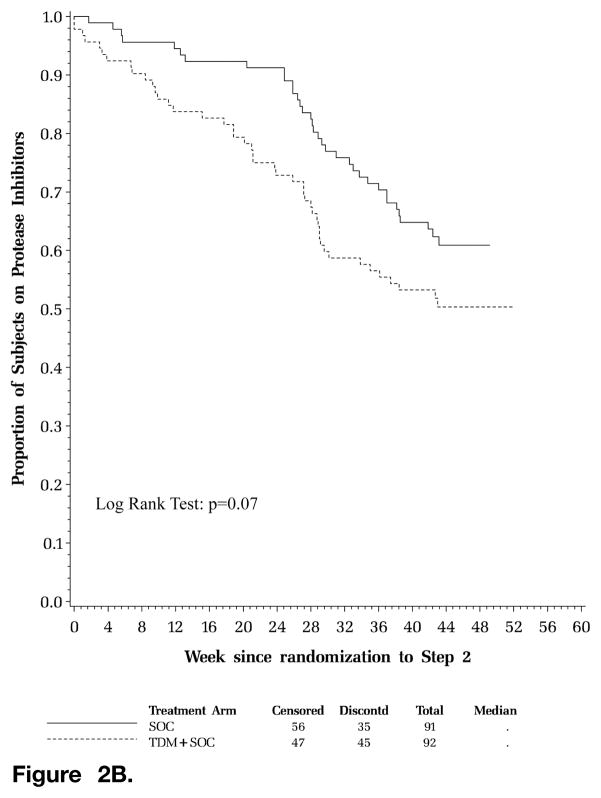
Figure 2B. Time to first permanent discontinuation of protease inhibitor (PI) in the initial regimen. Kaplan-Meier curve of time to permanent discontinuation of first PI. Solid line represents standard of care (SOC) arm; hatched line represents therapeutic drug monitoring (TDM) arm; horizontal axis displays week since randomization to Step 2; vertical axis displays proportion of subjects on PIs.
A total of 45 subjects (49%) in the TDM arm compared to 35 subjects (38%) in the SOC arm prematurely discontinued their first PI on the initial regimen. There were 9 and 8 virologic failures reported in the SOC and TDM arms, respectively, as the reason for permanent discontinuation of the first PI. There were more clinician requests (TDM 8; SOC 5) and unmanageable intolerance issues (TDM 2; SOC 0) in the TDM arm compared to the SOC arm cited as reasons for discontinuation of the first PI. There was a nonsignificant trend for a shorter time to first permanent discontinuation of the PI in the initial regimen for subjects in the TDM arm versus those in the SOC arm (P = .07, log-rank test; Figure 2B).
HIV-1 Viral Load Response
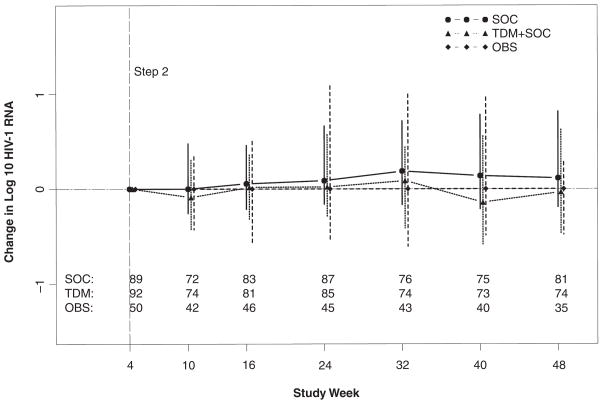
The 2 randomized arms had comparable HIV-1 RNA level distributions at all study weeks. At week 48, the change endpoint was designated as missing data for subjects without RNA result available for Step 2 entry (SOC 1) or at week 48 (SOC 8; TDM 18) or missing HIV-1 RNA levels at both study Step 2 entry and week 48 (SOC 1). Among subjects with data, there was no significant difference in the change in plasma HIV-1 RNA level from randomization to week 48 post entry (week 44 post Step 2 randomization) between the 2 randomized arms (P = .13, Gehan-Wilcoxon test). The median (interquartile range) change in HIV-1 RNA level from Step 2 entry to this time point was estimated to be 0.11 (−0.23, 0.82) in the SOC arm and −0.03 (−0.74, 0.62) log10 copies/mL in the TDM arm (Figure 3).
Figure 3.
Median change in plasma HIV-1 RNA from Step 2 randomization. Symbols represent median log10 viral load; vertical lines represent interquartile range. Solid line represents standard of care (SOC) arm; dotted line represents therapeutic drug monitoring (TDM) arm; dashed line represents observational (OBS) arm; horizontal axis displays study week; vertical axis displays median change in log10 HIV-1 RNA.
Sensitivity analyses were conducted to further evaluate viral load response given the higher number of premature study discontinuations in the TDM arm (n=18) versus the SOC arm (n=8) with subsequent missing viral load data at week 48.
When worst rank (ie, the worst HIV-1 RNA outcome) was assigned for missing HIV-1 RNA data in both arms, there was no significant difference in the change in plasma HIV-1 RNA level from randomization to week 44 post randomization between the 2 arms (P = .86, Gehan-Wilcoxon test).
No significant difference in the change in plasma HIV-1 RNA level from randomization to week 44 post randomization between the 2 arms was found (P = .28, Gehan-Wilcoxon test) when using the last observation carried forward for missing HIV-1 RNA data in both arms.
There was no apparent treatment effect modification (P = .86; results not shown) conferred by the randomization stratification factor (whether a subject started a new antiretroviral class of drug), which was similar to the primary analysis findings.30
Subgroup Analyses: Response to TDM
In subgroup analyses, the difference in change from Step 2 entry to week 48 in plasma HIV-1 RNA level between the TDM and SOC arms according to the number of active PIs in the subject’s regimen and race/ethnicity was assessed.
The treatment effect differed by the number of active PIs (P = .05). Subjects with at least 0.69 active PIs in their study regimen benefited significantly from receiving the TDM strategy, whereas no significant difference was observed for those with fewer than 0.69 active PIs (Table 2). This finding was concordant with the primary analysis.30
Table 2.
Therapeutic drug monitoring (TDM) effect on change in HIV-1 viral load (log 10 c/mL) by active protease inhibitor (PI) score and race/ethnicity
| Subgroup definition | Category | N | Estimated mean change in SOC | Estimated mean change in TDM | Estimated mean (standard error) difference between arms | 95% CI | P |
|---|---|---|---|---|---|---|---|
| Active PI score | Active PI < 0.69 | 78 | 0.11 | 0.22 | 0.11 (0.28) | (−0.44, 0.67) | .05 |
| Active PI ≥0.69 | 77 | 0.26 | −0.41 | −0.67 (0.28) | (−1.22,−0.11) | ||
| Race categories | White non-Hispanic | 74 | 0.06 | 0.00 | −0.06 (0.27) | (−0.60, 0.47) | .63 |
| Black non-Hispanic | 39 | 0.26 | −0.28 | −0.54 (0.46) | (−1.44, 0.36) | ||
| Hispanic | 39 | 0.43 | 0.10 | −0.34 (0.36) | (−1.05, 0.37) |
Note: SOC = standard of care.
No significant differential effect of TDM by race/ethnicity on viral load response at week 48 was observed (P = .63). This was in contrast to the primary endpoint subgroup analysis that demonstrated that black and Hispanic patients appeared more likely than whites to benefit from TDM.30 The reasons for this discordant result may relate to fewer observations being available at week 48 in the white non-Hispanic subgroup (n = 74) compared to week 24 (n = 84).
Virologic Failure
There was a nonsignificant trend for shorter time to virologic failure for subjects randomized to the SOC arm compared to the TDM arm (P = .08, log-rank test). There were 71 virologic failures in the SOC arm, 56 in the TDM arm, and 18 in the OBS arm. A total of 134 subjects (67 in SOC arm, 49 in TDM arm, and 18 in OBS arm) had confirmed virologic failures before their first PI discontinuation date. When excluding the 11 subjects (4 in SOC arm, 7 in TDM arm) who had virologic failure after their first PI discontinuation date, there was a significantly shorter time to virologic failure for subjects randomized to the SOC arm compared to the TDM arm (P = .05, log-rank test).
CD4 Cell Count Responses
The median (interquartile range) change in CD4 count from Step 2 entry (week 4) to week 48 was estimated to be +4.2 (−39.5, 56.5) and + 19.4 (−48.0, +76.0) cells/mm3 in the SOC and TDM arms, respectively. There was no significant difference between the randomized study arms in change in CD4 cell count from randomization to week 48 (P = .65, Wilcoxon rank-sum test).
Safety and Adverse Events
There were comparable numbers of grade 3 or 4 sign/symptoms in the 2 randomized arms for all categories except for neurologic events with 6 reported in the SOC arm and 1 in the TDM arm. The total number of subjects with grade 3 and 4 signs/symptoms (n=16) and, separately, of laboratory abnormalities (n=38) reported was the same in both randomized arms. The most frequent grade 3 or 4 signs/symptoms reported were ache/ pain/discomfort (SOC 6; TDM 2); fatigue/malaise (SOC 1; TDM 3); respiratory symptoms including cough and dyspnea (SOC 2; TDM 4); and gastrointestinal symptoms including diarrhea (SOC 3; TDM 2), nausea (SOC 0; TDM 2), and vomiting (SOC 1; TDM 3).
The most frequent laboratory abnormalities included creatine phosphokinase (CPK) elevations (SOC 4; TDM 2); hematology abnormalities, including neutropenia (SOC 5; TDM 7); metabolic derangements with fasting hyperglycemia (SOC 2; TDM 2); elevated fasting cholesterol (SOC 0; TDM 5); elevated fasting low-density lipoprotein (SOC 1; TDM 5); elevated triglyceride levels (SOC 5; TDM 10); abnormal liver function tests with elevated total bilirubin (SOC 2; TDM 3); elevated gamma-glutamyl transpeptidase (GGT) (SOC 3; TDM 3); elevated serum glutamic pyruvic transaminase (SGPT) (SOC 2; TDM 0); elevated serum glutamic oxaloacetic transaminase (SGOT) (SOC 4; TDM 3); and abnormal pancreatic function with serum lipase elevation (SOC 7; TDM 7).
There was no significant difference between the 2 randomized arms (P = .68, log-rank test, primary safety endpoint) in time to first grade 3 or 4 sign/symptom or laboratory abnormality that was at least one grade higher than at Step 2 entry (randomization).
DISCUSSION
This interventional study was the first randomized trial that evaluated the impact of TDM using IQ to guide PI dose escalation on virologic outcome in antiretroviral-experienced subjects.
The study patient population was heavily treatment-experienced with extensive protease resistance. The SOC and TDM arms had comparable distributions of the number of active PI drugs in the antiretroviral regimens at study entry with 63% of subjects having less than one active PI in the regimen and 24% with no active PI. Since PI trough concentrations achieved at week 2 were generally similar in all 3 study arms, extensive protease resistance rather than low PI trough concentrations emerged as the major determinant of low NIQs. This study primarily addressed whether selectively increasing PI drug exposure in individuals with NIQ ≤1 might overcome drug resistance and enhance virologic suppression.
A potential limitation of the A5146 study was not evaluating the impact of the TDM strategy with darunavir in this extensively treated population. Darunavir was not included because it was FDA approved immediately prior to completion of accrual.
The TDM strategy of PI dose escalation based on NIQ did not provide any improved long-term virologic outcome at week 48. This finding paralleled that in the primary analysis evaluating viral load change from randomization to week 24.30
The heterogeneity of the diverse number of PI-containing regimens initiated at study entry and the different PI dose escalation algorithms stipulated for each PI regimen30,34 may have negatively impacted the ability to detect a long-term viral load difference between the randomized arms. In subgroup analysis, there was evidence of a differential treatment effect by number of active PIs in the regimen. Subjects with ARV regimens containing at least 0.69 active PIs were shown to significantly benefit from TDM with an improved virologic outcome at week 48. This finding was also observed in the week 24 primary endpoint.30 The week 48 results suggest that the TDM strategy with PI dose escalation if NIQ ≤1 could favorably impact longer term viral load response in subjects taking combination regimens with intermediate protease resistance compared to regimens with extensive protease resistance.
The TDM strategy employing serial PI dose escalations was safe and generally well tolerated. There was no significant difference in the number of adverse signs/symptoms or laboratory abnormalities reported between the randomized arms.
The time to permanent discontinuation of scheduled clinic evaluations was significantly shorter for subjects in the TDM arm than those in the SOC arm. The differential higher rate of discontinuation of clinic evaluations seen in the TDM arm is not readily explained as there were no excess deaths, increased study treatment dose-dependent toxicities following PI dose escalation, or greater numbers of confirmed virologic failures in this arm compared to the SOC arm. Similar numbers of patients in each arm discontinued the study because of inability to adhere to required study procedures.
There was also a trend of shorter time to first PI discontinuation for subjects in the TDM arm than in the SOC arm. The higher rate of premature PI discontinuations observed in the TDM arm is not readily attributable to higher rates of virologic failure or selected dose-dependent toxicities. Of note, the TDM arm had more frequent subject- and clinician-initiated requests to discontinue the PI-based regimen compared to the SOC arm. There were similar numbers of subjects in the randomized arms who discontinued the first PI for reasons of noncompliance with study medications and/or study visits.
These week 48 secondary analysis results need to be cautiously interpreted due to the observed differential treatment arm effect noted in time to discontinuation of study follow-up and discontinuation of the first PI in the initial regimen.
Although there were no significant differences reported in grade 3 or 4 clinical or lab-related toxicities between the 2 randomized arms, we were unable to determine whether PI dose escalation in the TDM arm may have been associated with a potentially lower grade of intolerance due to higher pill burden, increased daily ritonavir dosing for pharmacokinetic enhancement, or increased PI daily dosing. Because as many as 62 of 85 (73%) subjects in the TDM arm undertook all the recommended PI dose escalations,30 substantial intolerance to the increased ritonavir and/or PI daily dosing seems unlikely to account for the higher number of premature study treatment discontinuations seen in the TDM arm.
TDM has been used in an array of HIV settings to limit antiretroviral drug exposure within therapeutic range and prevent specific toxicities such as efavirenz-associated neurotoxicity15; to identify factors influencing antiretroviral drug metabolism such as weight and ethnicity37; to delineate potential drug-drug interactions of antiretroviral agents when used in novel combinations or with other drugs that affect metabolism of cytochrome P450 system14,38; to monitor maternal pharmacokinetics of PI and NNRTI agents in pregnancy to assess individual plasma drug concentrations achieved during stages of gestation39; and to individualize antiretroviral dosing of selected PI and NNRTI drugs12,13,15,40,41 to optimize virologic efficacy in treatment-experienced patients with virologic failure.16–29
Current clinical application of TDM varies with greater use in Canada and European HIV treatment centers and less use in the United States.2,42–46 However, most centers that provide clinical criteria for TDM often include patients with pre-existing resistance as demonstrated by phenotype (eg, IC50) based on the underlying premise that increasing drug exposure in relation to the IC50 will provide improved antiviral activity.
The TDM strategy in A5146 utilized an NIQ that integrates both drug exposure and viral drug resistance. The NIQ was based upon a predicted IC50 fold change from a virtual phenotype report34 and was shown to correlate with virologic outcome in treatment-experienced patients in retrospective studies.25,29 The A5146 study design could have incorporated an alternative modality with the use of a genotypic inhibitory quotient (GIQ) in which the number of resistance mutations determines the extent of drug resistance, rather than the IC50 fold change.30,47,48 To date, it has not been established which of these approaches in determining IQ could best validate predicting virologic outcomes.
Patients having an NIQ ≤1 indicated that the IQ was below the threshold associated with virologic success for the given PI. An NIQ ≤1 was therefore selected on an empiric basis to target those patients with reduced likelihood of virologic success who might selectively benefit from PI dose escalation.30,34 Given that the subjects with NIQ >1 in the OBS arm achieved better virologic outcomes than the 2 randomized arms, the choice of an NIQ ≤1 cutoff appears clinically justified.
The extent of drug resistance was very high in the A5146 study population; this resulted in low NIQs that could not be overcome despite achieving increased concentrations of PIs following serial dose escalation.
Despite the inherent limitations in conducting a randomized, interventional trial incorporating a time-sensitive TDM strategy, the study demonstrated that in PI-experienced subjects with virologic failure, TDM may be more beneficial if undertaken at earlier stages of resistance when the current PI retains at least partial activity.
The current approach to virologic failure in the United States does not routinely incorporate TDM strategies within clinical practice guidelines. This is in large measure due to the recently expanded armamentarium featuring inherently more potent PI agents, including darunavir,9,10 with proven efficacy in patients with extensive protease resistance; the addition of second-generation NNRTI agents etravirine49,50 and the recently FDA-approved rilpivirine51,52; the availability of selected agents from new antiretroviral drug classes including the integrase inhibitor raltegravir5,53 and the CCR5 inhibitor maraviroc6,7 that can be used in novel combinations to devise individualized regimens based upon prior antiretroviral treatment history; and resistance testing with the goal of providing at least 3 active drugs in combination.
While the PIs featured in A5416 are no longer routinely used as the first-line agents for virologic failure, A5146 was designed as a strategy trial in which the results may prove clinically applicable and relevant to newer antiretroviral agents. First, dose escalations of PIs and dual-PI regimens are feasible, can increase the NIQ, and are generally well tolerated. Second, resistance is the primary driving force influencing TDM, rather than variations in PI trough levels. Third, TDM may potentially benefit those patients with intermediate level of resistance.
The concept of using TDM to maximize drug exposure in relation to virus susceptibility remains feasible and should be applicable across the PI class, including newer intrinsically more potent agents such as darunavir9,10 that would be selectively used in virologic failure. The use of TDM as a global strategy for managing virologic failure in resource-limited regions that lack ready access to newer PI agents remains a consideration.
TDM with PI dose adjustment may also serve to optimize viral load response and limit selected toxicities in an aging HIV population that may incur higher plasma concentrations of PIs on standard doses due to decreased clearance based upon age-related changes in the pharmacokinetics of PI drugs.54
Further research is needed to identify additional patient covariates that might determine whether implementing a TDM interventional strategy in patients experiencing virologic failure is likely to enhance virologic response.
Acknowledgments
The project described was supported by award U01AI068636 from the National Institute of Allergy and Infectious Diseases (AI-25859, AI-25915, AI-27658, AI-27661, AI-32782, AI-34853, AI-38855, AI-38858-09S1, AI-46370, AI-04637, AI-68634, AI-69415, Al-69418, AI-69419, AI-69423, AI-69424, AI-69428, AI-69432, AI-69434, AI-69439, AI-69447, AI-69450, AI-69452, AI-69467, AI-69470, AI -69471, AI-69472, A1-69474, AI-69477, AI-69484, AI-69494, AI-69495, AI-69501, AI-69502, AI-69511, AI-69513, AI-69532, AI-69556); P30-AI-050409, P30-AI-045008, P30-AI-050410), and supported in part by the General Clinical Research Center Units funded by the National Center for Research Resources (RR-00032, RR-024156, RR 024160, RR-024992, RR-024996, RR-025747, RR-025780). Virco performed drug resistance testing and provided partial financial support for drug concentration testing which was performed in Dr. Morse’s laboratory at SUNY Buffalo.
Participating Site Staff Members: Frances Canchola, RN, and Luis Mendez: University of Southern California (Site 1201); Charles Hicks, MD, and Joan Riddle, RN: Duke University Medical Center CRS (Site 1601); Sharon Riddler, MD, MPH, and Carol Oriss, BSN, RN: University of Pittsburgh (Site 1001); Charles E. Davis, MD: IHV Baltimore Treatment (Site 4651); Patricia Walton, BSN, RN, and Barbara Philpotts, BSN, RN: Case CRS (Site 2501); Susan L. Koletar, MD, and Mark D. Hite, RN: The Ohio State University (Site 2301); Kim Scarsi, PharmD, MS: Northwestern University (Site 2701); Jorge L. Santana Bagur, MD, and Olga Mendez, MD: Puerto Rico-AIDS Clinical Trials Unit (Site 5401); Margarita Vasquez, RN, and Judith A Aberg, MD: New York University/NYC HHC at Bellevue Hospital Center (Site 401); Philip Keiser, MD, and Jesse Tarbutton, BS: UT Southwestern Medical Center at Dallas (Site 3751); Lorna Nagamine, RN, and Scott Scouza, PharmD: University of Hawaii (Site 5201); Judith Feinberg, MD, and Michelle Saemann, RN: University of Cincinnati CRS (Site 2401); Donna Mildvan, MD, and Manuel Revuelta, MD: Beth Israel Medical Center (Site 2851); Jane Reid, RNC, MS, and Mary Adams, RN, MPh: University of Rochester (Site 1101); Beverly Putnam, RN, ANP, and John R. Koeppe, MD: University of Colorado Hospital (Site 6101); Valerie Hughes, FNP, and Todd Stroberg, RN: Cornell University (Site 7804); MetroHealth (Site 2503); Neah Kim, ANP, and Amanda Youmans, ANP: Beth Israel Deaconess (Partners/Harvard) (Site 103); Linda Meixner, RN, and Richard Haubrich, MD: UCSD (Site 701); Jody Lawrence, MD, and Mary Payne, RN-UCSF: San Francisco General Hospital (Site 801); Karen Tashima, MD, and Deborah Perez RN: The Miriam Hospital (Site 2951); Michael Morgan, FNP, and Husamettin Erdem, MD: Vanderbilt University (Site 3652); Molly Eaton, MD, and Fifi Derso, RN: Emory University HIV/AIDS Clinical Trials Unit (Site 5802); Pablo Tebas, MD, and Wayne Wagner, RN: University of Pennsylvania (Site 6201); Sadia Shaik and Mario Guerrero, MD: Harbor-UCLA Medical Center (Site 603); Mark Rodriguez, RN, BSN, and Debra Demarco, RN, BSN: Washington University (Site 2101); Mitchell Goldman, MD, and Deborah O’Connor, RN, MSN: Indiana University School of Medicine-Infectious Disease Research (Site 2601); Margaret A. Fischl, MD, and Hector Bolivar, MD: University of Miami AIDS Clinical Research Unit (Site 901); University of Minnesota ACTU (Site 1501); Kerry Upton, RN, and Dana Green, BS: University of Alabama Therapeutics CRS (Site 5801); Paul Edward Sax, MD, and Jon Gothing, RN, BSN, ACRN: Brigham and Women’s Hospital (Site 107); Sue Richard, MSN, ANP, and C. Susan Pedersen, BS, BSN: University of North Carolina (Site 3201); Helene Hardy, PharmD, and Paul R. Skolnik, MD: Boston Medical Center (Site 104); SUNY - Buffalo, Erie County Medical Center (Site 1102); Shelia Dunaway, MD, and Ann C. Collier, MD: University of Washington (Site 1401); Columbia University–HIV Prevention and Treatment CRS (Site 30329); Sandra Valle, PA-C, and Deborah Slamowitz, RN: Stanford University AIDS (Site 501); Mitchell Goldman, MD, and Deborah O’Connor, RN, MSN: Indiana University School of Medicine, Wishard Memorial (Site 2603); Richard B. Pollard, MD: UC Davis Medical Center (Site 3851,3852); William A. O’Brien, MD, and Gerianne Casey: University of Texas Medical Branch (Site 6301); Christine Hurley, RN, and Roberto Corales, DO: AIDS Care (Site 1108); Cook County Hospital Core Center (Site 2705).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Trial registration: ClinicalTrials.govNCT0041769 We would like to acknowledge the invaluable support provided by Barbara Bastow, RN, BSN; data managers David Rusin and Bernadette Jarocki; Jennifer Nowak; Robin DiFrancesco; and Nancy Reynolds, PhD, RN.
References
- 1.US Department of Health and Human Services. [Accessed November 3, 2008];Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. http://aidsinfo.nih.gov/Guidelines/aspx.
- 2.Hammer SM, Eron JJ, Reiss P, et al. Antiretroviral treatment of adult HIV infection. 2008 recommendations of the International AIDS Society-USA Panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett JA, Buda JJ, von Scheele B, Mauskopf JA, Davis A, King MS, Lanier ER. Minimizing resistance consequences after virologic failure on initial combination therapy. J Acquir Defic Syndr. 2006;41:323–331. doi: 10.1097/01.qai.0000197070.69859.f3. [DOI] [PubMed] [Google Scholar]
- 4.Montaner J, Guimaraes D, Chung J, Gafoor Z, Salgo M, De Masi R. Prognostic staging of extensively pretreated patients with advanced HIV-1 disease. HIV Clin Trials. 2005;6:281–290. doi: 10.1310/0XH7-F2V2-1K0R-NU2W. [DOI] [PubMed] [Google Scholar]
- 5.Steigbigel RT, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359:339–354. doi: 10.1056/NEJMoa0708975. [DOI] [PubMed] [Google Scholar]
- 6.Gulick RM, Lalezari J, Goodrich J, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med. 2008;359:1429–1441. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fatkenheuer G, Nelson M, Lazzarin A, et al. Subgroup analyses of maraviroc in previously treated R5 HIV-1 infection. N Engl J Med. 2008;359:1442–1455. doi: 10.1056/NEJMoa0803154. [DOI] [PubMed] [Google Scholar]
- 8.Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337(11):725–33. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 9.Madruga JV, Berger D, Mcmurchie M, et al. the TITAN Study Group. Efficacy and safety of darunavir-ritonavir compared with lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomized, controlled phase III study. Lancet. 2007;370:49–58. doi: 10.1016/S0140-6736(07)61049-6. [DOI] [PubMed] [Google Scholar]
- 10.Clotet B, Bellos N, Molina JM, et al. POWER 1 and 2 Study Groups. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patient with HIV-1infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomized trials. Lancet. 2007;369:1169–1178. doi: 10.1016/S0140-6736(07)60497-8. [DOI] [PubMed] [Google Scholar]
- 11. [Accessed March 4, 2011];Guidelines: Clinical management and treatment of HIV infected adults in Europe. www.europeanaidsclinicalsociety.org.
- 12.Aarnoutse RE, Schapiro JM, Boucher CA, Hekster YA, Burger DM. Therapeutic drug monitoring: an aid to optimizing response to antiretroviral drugs? Drugs. 2003;63:741–753. doi: 10.2165/00003495-200363080-00002. [DOI] [PubMed] [Google Scholar]
- 13.Burger D, Hugen P, Reiss P, et al. ATHENA Cohort Study Group. Therapeutic drug monitoring of nelfinavir and indinavir in treatment-naïve HIV-1 infected individuals. AIDS. 2003;17:1157–1165. doi: 10.1097/00002030-200305230-00007. [DOI] [PubMed] [Google Scholar]
- 14.Boffito M, Acosta E, Burger D, et al. Therapeutic drug monitoring and drug-drug interactions involving antiretroviral drugs. Antivir Ther. 2005;10:469–477. [PubMed] [Google Scholar]
- 15.Mello AF, Buclin T, Decosterd LA, et al. Successful efavirenz dose reduction guided by therapeutic drug monitoring. Antivir Ther. 2011;16:189–197. doi: 10.3851/IMP1742. [DOI] [PubMed] [Google Scholar]
- 16.Colombo S, Buclin T, Cavassini M, et al. Population pharmacokinetics of atazanavir in patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 2006;50(11):3801–3808. doi: 10.1128/AAC.00098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durant J, Clevenbergh P, Garraffo R, et al. Importance of protease inhibitor plasma levels in HIV-infected patients treated with genotypic-guided therapy: pharmacological data from the Viradapt Study. AIDS (Lond) 2000;14(10):1333–1339. doi: 10.1097/00002030-200007070-00005. [DOI] [PubMed] [Google Scholar]
- 18.Shulman N, Zolopa A, Havlir D, et al. Virtual inhibitory quotient predicts response to ritonavir boosting of indinavir-based therapy in human immunodeficiency virus-infected patients with ongoing viremia. Antimicrob Agents Chemother. 2002;46(12):3907–3916. doi: 10.1128/AAC.46.12.3907-3916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu A, Isaacson J, Brun S, et al. Pharmacokinetic-pharmacodynamic analysis of lopinavir-ritonavir in combination with efavirenz and two nucleoside reverse transcriptase inhibitors in extensively pretreated human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2003;47(1):350–359. doi: 10.1128/AAC.47.1.350-359.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcelin AG, Lamotte C, Delaugerre C, et al. Genotypic inhibitory quotient as predictor of virological response to ritonavir-amprenavir in human immunodeficiency virus type 1 protease inhibitor-experienced patients. Antimicrob Agents Chemother. 2003;47(2):594–600. doi: 10.1128/AAC.47.2.594-600.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casado JL, Moreno A, Sabido R, et al. Individualizing salvage regimens: the inhibitory quotient (Ctrough/IC50) as predictor of virological response. AIDS (Lond) 2003;17(2):262–264. doi: 10.1097/01.aids.0000050800.28043.4d. [DOI] [PubMed] [Google Scholar]
- 22.Taburet AM, Raguin G, Le Tiec C, et al. Interactions between amprenavir and the lopinavir-ritonavir combination in heavily pretreated patients infected with human immunodeficiency virus. Clin Pharmacol Ther. 2004;75(4):310–323. doi: 10.1016/j.clpt.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 23.De Luca A, Baldini F, Cingolani A, Di Giambenedetto S, Hoetelmans RM, Cauda R. Deep salvage with amprenavir and lopinavir/ritonavir: correlation of pharmacokinetics and drug resistance with pharmacodynamics. J Acquir Immune Defic Syndr. 2004;35(4):359–366. doi: 10.1097/00126334-200404010-00005. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez de Requena D, Gallego O, Valer L, Jimenez-Nacher I, Soriano V. Prediction of virological response to lopinavir/ritonavir using the genotypic inhibitory quotient. AIDS Res Human Retroviruses. 2004;20(3):275–278. doi: 10.1089/088922204322996509. [DOI] [PubMed] [Google Scholar]
- 25.Castagna A, Gianotti N, Galli L, et al. The NIQ of lopinavir is predictive of a 48-week virological response in highly treatment-experienced HIV-1-infected subjects treated with a lopinavir/ritonavir-containing regimen. Antivir Ther. 2004;9(4):537–543. [PubMed] [Google Scholar]
- 26.Barrios A, Rendon AL, Gallego O, et al. Predictors of virological response to atazanavir in protease inhibitor-experienced patients. HIV Clin Trials. 2004;5(4):201–205. doi: 10.1310/3HL3-HHBD-WKLR-XELL. [DOI] [PubMed] [Google Scholar]
- 27.Marcelin AG, Dalban C, Peytavin G, et al. Clinically relevant interpretation of genotype and relationship to plasma drug concentrations for resistance to saquinavir-ritonavir in human immunodeficiency virus type 1 protease inhibitor-experienced patients. Antimicrob Agents Chemother. 2004;48(12):4687–4692. doi: 10.1128/AAC.48.12.4687-4692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcelin AG, Cohen-Codar I, King MS, et al. Virological and pharmacological parameters predicting the response to lopinavir-ritonavir in heavily protease inhibitor-experienced patients. Antimicrob Agents Chemother. 2005;49(5):1720–1726. doi: 10.1128/AAC.49.5.1720-1726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winston A, Hales G, Amin J, van Schaick E, Cooper DA, Emery S. The normalized inhibitory quotient of boosted protease inhibitors is predictive of viral load response in treatment-experienced HIV-1-infected individuals. AIDS (Lond) 2005;19(13):1393–1399. doi: 10.1097/01.aids.0000181009.77632.36. [DOI] [PubMed] [Google Scholar]
- 30.Demeter LM, Jiang H, Mukherjee AL, et al. Therapeutic drug monitoring of protease inhibitors using a normalized inhibitory quotient in antiretroviral-experienced HIV-1-infected patients (AIDS Clinical Trials Group A5146) AIDS. 2009;23:357–368. doi: 10.1097/QAD.0b013e32831f9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. [Accessed August 4, 2007]; www.fda.gov/cder/foi/label/2005/021814lbl.pdf.
- 32. [Accessed August 4, 2007]; www.fda.gov/cder/foi/nda/2003/021567_reyataz_toc.htm.
- 33. [Accessed May 19, 2005]; www.fda.gov/ohrms/dockets/ac/05/slides/2005-4139S1_09_FDA-Zheng.ppt.
- 34.Demeter LM, Mukherjee AL, DiFrancesco R, et al. The design and implementation of A5146, a prospective trial assessing the utility of therapeutic drug monitoring using an inhibitory quotient in antiretroviral-experienced HIV-infected patients. HIV Clin Trials. 2008;9:61–72. doi: 10.1310/hct0901-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reboussin DM, DeMets DL, Kim KM, Lan KK. Computations for group seqeuntial boundaries using the Lan-DeMets spending function method. Control Clin Trials. 2000;21:190–207. doi: 10.1016/s0197-2456(00)00057-x. [DOI] [PubMed] [Google Scholar]
- 36. [Accessed December 28, 2004]; http://rcc.tech-res.com/tox_tables.htm.
- 37.Stohr W, Back D, Dunn D, et al. for the UK CHIC Study. Factors influencing efavirenz and nevirapine plasma concentration: effect of ethnicity, weight, and co-medication. Antivir Ther. 2008;13:675–685. [PubMed] [Google Scholar]
- 38.Lakhman SS, Ma Q, Morse GD. Pharmacogenomics of CYP3A: considerations for HIV treatment. Pharmacogenomics. 2009;10:1323–1339. doi: 10.2217/pgs.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roustit M, Jlaiel M, Leclercq P, Stanke-Labesque F. Pharmacokinetics and therapeutic drug monitoring of antiretrovirals in pregnant women. Br J Clin Pharmacol. 2008;66:179–195. doi: 10.1111/j.1365-2125.2008.03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fabbiani M, Di Giambenedetto S, Bracciale L, et al. Pharamcokinetic variability of antiretroviral drugs and correlation with virological outcome: 2 years of experience in routine clinical practice. J Antimicrob Chemother. 2009;64:109–117. doi: 10.1093/jac/dkp132. [DOI] [PubMed] [Google Scholar]
- 41.Molto J, Santos JR, Valle M, Miranda J, Blanco A, Negredo E, Clotet B. Monitoring atazanavir concentrations with boosted or unboosted regimens in HIV-infected patients in routine clinical practice. Ther Drug Monitoring. 2007;29:648–651. doi: 10.1097/FTD.0b013e31815704c1. [DOI] [PubMed] [Google Scholar]
- 42.Khoo SH, Lloyd J, Dalton M, et al. Pharmacologic optimization of protease inhibitors and nonnucleoside reverse transcriptase inhibitors (POPIN)-a randomized controlled trial of therapeutic drug monitoring and adherence support. J Acquir Immune Defic Syndr. 2006;41(4):461–467. doi: 10.1097/01.qai.0000218345.65434.21. [DOI] [PubMed] [Google Scholar]
- 43.del Rio C. Current concepts in antiretroviral therapy failure. Top HIV Med. 2006;14:102–106. [PubMed] [Google Scholar]
- 44.Park-Wyllie LY, Levine MA, Holbrook A, et al. Outcomes of dosage adjustments used to manage antiretroviral drug interactions. Clin Infect Dis. 2007;45(7):933–936. doi: 10.1086/521252. [DOI] [PubMed] [Google Scholar]
- 45.van Luin MK, Kuks PF, Burger DM. Use of therapeutic drug monitoring in HIV disease. Curr Opin HIVAIDS. 2008;3(3):266–271. doi: 10.1097/COH.0b013e3282f82c1b. [DOI] [PubMed] [Google Scholar]
- 46.Cleijsen RM, Van de Ende ME, Kroon FP, et al. Therapeutic drug monitoring of the HIV protease inhibitor atazanavir in clinical practice. J Antimicrob Chemother. 2007;60(4):897–900. doi: 10.1093/jac/dkm298. [DOI] [PubMed] [Google Scholar]
- 47.Hoefnagel JG, van der Lee MJ, Koopmans PP, et al. The genotypic inhibitory quotient and the cumulative number of mutations predict the response to lopinavir therapy. AIDS. 2006;20:1069–1071. doi: 10.1097/01.aids.0000222083.44411.02. [DOI] [PubMed] [Google Scholar]
- 48.Barrail-Tran A, Morand-Joubert L, Poizat G, et al. Predictive values of the human immunodeficiency virus phenotype and genotype and of amprenavir and lopinavir inhibitory quotients in heavily pretreated patients on a ritonavir-boosted dual protease inhibitor regimen. Antimicrob Agents Chemother. 2008;52:1642–1646. doi: 10.1128/AAC.01314-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Picchio G, Vingerhoets J, Tambuyzer L, Coakley E, Had-dad M, Witek J. Prevalence of susceptibility to etravirine by genotype and phenotype in samples received for routine HIV-1 resistance testing in the USA [published online ahead of print May 10, 2011] AIDS Res Hum Retroviruses. doi: 10.1089/AID.2011.0049. [DOI] [PubMed] [Google Scholar]
- 50.Schiller DS, Youssef-Bessler M. Etravirine: a second generation nonnucleoside reverse transcriptase inhibitor (NNRTI) active against NNRTI-resistant strains of HIV. Clin Ther. 2009;31:692–704. doi: 10.1016/j.clinthera.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 51.Azlin H, Tirry I, Vingerhoets J, et al. TMC 278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV- 1. Antimicrob Agents Chemother. 2010;54:718–727. doi: 10.1128/AAC.00986-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goebel F, Yakovlev A, Pozniak AL, et al. Short-term antiviral activity of TMC 278- a novel NNRTI in treatment-naïve HIV-1 infected subjects. AIDS. 2006;20:1721–1726. doi: 10.1097/01.aids.0000242818.65215.bd. [DOI] [PubMed] [Google Scholar]
- 53.Scherrer AU, von Wyl V, Boni J, et al. and the Swiss Study Cohort. Viral suppression rates in salvage treatment with raltegravir improved with the administration of genotypic partially active or inactive nucleoside/tide reverse transcriptase inhibitors. J Acquir Immune Defic Syndr. 2011;57:24–31. doi: 10.1097/QAI.0b013e318211925e. [DOI] [PubMed] [Google Scholar]
- 54.Crawford KW, Spritzler J, Kalayjian RC, et al. Age-related changes on plasma concentrations of the HIV protease inhibitor lopinavir. AIDS Res Hum Retroviruses. 2010;26:635–643. doi: 10.1089/aid.2009.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]