Abstract
Numerous studies have explored the potential of different stem and progenitor cells to replace at-risk neuronal populations in a variety of neurodegenerative disease models. This study presents data from a side by side approach of engrafting two different stem/progenitor cell populations within the postnatal cerebellum of the weaver neurological mutant mouse: cerebellar-derived multipotent astrocytic stem cells (MASCs) and embryonic stem cell-derived neural precursor (ESNPs), for comparative analysis. We show here that both donor populations survive, migrate, and appear to initiate differentiation into neurons within the granuloprival host environment. Neither of these disparate stem/progenitor cell populations adopted significant region-specific identities despite earlier studies that suggested the potential of these cells to respond to in vivo cues when placed in a permissive/instructive milieu. However, data presented here suggest that molecular and cellular deficits present within weaver homozygous or heterozygous brains may promote a slightly more positive donor cell response towards acquisition of neuronal phenotype. Hence it is likely that a fine balance exists between a compromised host environment that is amenable to cell replacement and that of a degenerating cellular milieu where it is perhaps too deleterious to support extensive neuronal differentiation and functional cellular integration. These findings join a growing list of studies that show successful cell replacement depends largely on the interplay between the potentiality of the donor cells and the specific pathological conditions of the recipient environment, and that emergent therapies for neurological disorders involving the use of neural stem cells still require refinement.
Keywords: Neural stem cells, Embryonic stem cells, Adult stem cells, Stem cell transplantation
Introduction
Restoring tissue integrity and function following injury or neurological disease remains challenging due to the limited regenerative potential of the central nervous system (CNS). Recent advances in therapeutic strategies include enhancing recruitment of newly generated endogenous neurons to a lesioned or degenerating area and transplantation of exogenously generated stem/progenitor cells to replace at-risk or lost cells1–3. Neural stem/progenitor cell transplantation has been studied extensively with cells isolated and expanded from neurogenic regions within the CNS: the subependymal zone4 (SEZ) or the dentate gyrus of the hippocampus5–8. Another region that has not been widely viewed as a source of multipotent cells, but nonetheless retains transiently active cell proliferation, is the postnatal cerebellum. The cerebellar cortex continues to generate granule cells and forms the transient external granule cell layer up to postnatal day 159, and we and other groups have shown that cells derived from this area are capable of forming multipotent proliferative clones, or neurospheres, with the ability to form all three primary CNS cell types: neurons, astrocytes, and oligodendrocytes10–13. These cerebellar-derived neurogenic cells are glial fibrillary acidic protein (GFAP)-expressing astrocytes similar to the stem cells derived from the SEZ areas surrounding the lateral ventricle, and we refer to both the forebrain and cerebellar clonogenic cells as multipotent astrocytic stem/progenitor cells (MASCs). MASCs represent potential therapeutic candidates for replacement and repair following cell loss in the CNS resulting from injury or disease as they have the ability to respond to intrinsic environmental cues by anatomically integrating into a host brain and differentiating into neurons and astrocytes when transplanted into the lateral ventricles of normal adult mice 14. In these previous studies, engraftment in the hindbrain was less robust following intraventricular transplantation, possibly due to the extensive distance and other factors that could hinder the migration and integration of the MASCs.
Enhanced green fluorescent protein (EGFP)-expressing ESNPs also have been well characterized by our lab and others, with previous studies showing the ability of these cells to acquire multiple neuronal phenotypes and to functionally integrate into the developing brain both in vitro and in vivo15–20. Following transplantation into the lateral ventricle, a large percentage of the ESNPs were found to differentiate into glutamatergic neurons despite the failure to acquire region-specific identities, and they might be predisposed to respond to intrinsic cues when placed in an optimal environment.
The present study was designed as a comparative analysis to examine the potential of somatic tissue-derived MASCs versus embryonic stem cell-derived neurons to potentially thrive within a CNS environment, that might be conducive for such integration, using the neurological mutant mouse model, weaver (gene symbol wv). Weaver mice have been well characterized for cerebellar development studies due to the histopathological hallmark of severe granule cell loss, leading to a “granuloprival” cerebellum that results in reduced brain size21–23 as well as deficits within the Purkinje cell population24–27, dopaminergic neurons in the substantia nigra28–29, and the deep cerebellar nuclei27, 30–31. Clinical and pathological features are similar to patients suffering from cerebellar ataxia, including instability of gait and tremor of the extremities. These symptoms can be attributed to a single base pair mutation in the gene coding for a G-protein-coupled, inward rectifying potassium channel of the GIRK2 family32 that is expressed in all cell groups suffering defects in weaver but also expressed in regions where no damage has occurred or been detected to date33. The severe depletion of the granule interneurons makes weaver mouse an attractive model for studying and providing comparison of these two different donor populations because it has been shown that the MASC default neuron generation program is interneurons10, 14, 34 while the ESNPs have been shown to differentiate largely into glutamatergic neurons16.
There have been many previous transplantation studies in which fetal or embryonic cerebellar cells were transplanted into other neurological mutant mouse models such as the Purkinje cell degeneration (pcd) mutant35–40, including the use of an immortalized neural progenitor cell line41 for functional recovery or restoration of molecular homeostasis42–44, as well as many other studies3 attempting to replace at-risk neuronal populations, but there has been a paucity of successful cell integration findings leading up to the in vivo bioassay tested here. That is, we use a well-characterized cerebellar neurological mutant mouse with defined cell loss that occurs gradually beginning in early postnatal life, as a host for two completely different neural stem/progenitor cell populations. One of the cells studies here, MASCs, represents a potential indigenous source of cerebellar granule interneurons10–13, and the other, ESNPs, is believed to be amongst the most potent of stem cells capable of generating numerous types of neural cells15–20,45. Thus the current study provides insights into the developmental potential, cell fate choice and differentiation of both MASCs and ESNPs within an injured host CNS environment, and for attempting cell replacement following intra-parenchymal transplantation during a peak period of cell loss.
Materials and Methods
Generation of astrocyte monolayers and τESNP-GFP cells
MASCs
Astrocyte monolayers were derived from cerebella of neonatal (P1–P8) transgenic mice constitutively expressing GFP (strain #003116, Jackson Laboratory). Following decapitation, cerebella were removed for dissociation into single-cell suspensions as previously described11. Briefly, cerebellar tissue was isolated and minced with a razor blade before incubation in trypsin for 5 minutes in 37° water bath. Cells were triturated serially beginning with 5ml pipettes followed by glass Pasteur pipettes 3 times or until single-cell suspension is achieved. After being pelleted and washed several times in medium, cells were cultured in standard T75 tissue culture flasks with growth medium consisting of Dulbecco’s Modified Eagle Medium with F12 supplements (DMEM/F12, Invitrogen) containing N-2 supplement (Invitrogen) and 20μg/mL bovine pituitary extract (BPE, Invitrogen), 5% fetal bovine serum (FBS, Atlanta Biologicals), 20ng/mL epidermal growth factor (EGF, Sigma), and 10ng/mL basic fibroblast growth factor (bFGF, Sigma). Astrocyte monolayers within the culture flasks were passaged 1–4 times before being collected for transplantation.
ESNPs
Cells were derived from the J1 ES cell line carrying the EGFP cDNA knock-in at the tau gene and collected at stage IV of a four-step culture protocol as previously described15. Briefly, cells were expanded in mitomysin C-inhibited embryonic fibroblasts and gelatin in step I, followed by induction of embryoid bodies (EBs) in step II, and attachment of the EBs to laminin-, poly-L-ornithine (LPO)-coated surface to derive neural precursor cells in step III. Cells were transferred to another LPO-coated surface in stage IV and cultured with bFGF before dissociation into single-cell suspension for transplantation.
Qualitative and quantitative analysis of MASCs and ESNPs used for transplantation
Both populations of donor cells were characterized extensively as previously described11,15, including the ability of MASCs to give rise to neurospheres that can differentiate into neurons, astrocytes, and oligodendrocytes, and the potential of ESNPs to give rise to various neuronal populations based on substrate specificity. In this study, MASCs cultured as astrocyte monolayers containing mostly astrocytes, and ESNPs cultured on LPO-coated surface containing mostly neural precursors were used for transplantation. To confirm the identity of our donor populations, MASCs and ESNPs were plated onto LPO-coated glass coverslips and processed for immunofluorescence with antibodies against the following antigens: chicken GFP (1:1000, Aves), mouse β-III tubulin (1:1000, Promega), mouse NeuN (1:1000, Chemicon), rabbit GFAP (1:10, Thermo Fisher), rat CD11b (1:100, PharMingen), mouse S-100β (1:500, Sigma), mouse O4 (1:150, Chemicon), and mouse CNPase (1:250, Chemicon). For quantification of both cell types, 16 random fields on the coverslips were counted at 40X magnification for the number of cells immunopositive for the specific antibody applied and for the total number of cells observed with DAPI nuclear staining.
Cell transplantation and tissue analysis
For transplants, GFP+ MASCs or ESNPs were collected, triturated into single cell suspension and resuspended at a concentration of 5 × 104 or 1 × 105 cells/μl in serum-free DMEM/N-2 medium. Postnatal day 1– 8 weaver (strain B6CBACaAw-J/A-Kcnj6wv/J, Jackson Laboratory) mouse pups, including homozygous (wv/wv), heterozygous (wv/+), or control wildtype (+/+) littermates, were first cryo-anesthetized before unilaterally injected with 1μl of the cell suspension into the right hemisphere of the cerebellum using a Hamilton syringe with 25s gauge needle. A total of 23 mice received MASCs and 28 received ESNPs grafted within the cerebellar cortex. Animals were sacrificed at one, two, three, four, or five weeks post transplantation and transcardially perfused with 4% paraformaldehyde in 0.1M phosphate buffered saline (PBS, pH=7.4). Brains were then removed, postfixed overnight in perfusate, and sectioned through the coronal plane at a thickness of 30μm using a vibratome. Immunohistochemical analysis was carried out with the following antibodies using standard immunostaining procedures: rabbit GFP (1:1000, Invitrogen), chicken GFP (1:1000, Aves), mouse β-III tubulin (1:1000, Promega), mouse NeuN (1:1000, Chemicon), rabbit GFAP (1:10, Thermo Fisher), rabbit glutamate (1:500, Sigma), rabbit RU49/ZFP-38 (1:1000, CeMines), rabbit gamma-aminobutyric acid(A) Receptor alpha 6 subunit (GABAAα6) (1:500, Chemicon), rabbit MATH-1 (mouse atonal homolog 1) (1:200, Chemicon), mouse S100β (1:1000, Sigma), guinea pig glial glutamate transporter (GLAST; 1:1000, Chemicon) and mouse stage-specific embryonic antigen-1 (SSEA-1) (1:200, Abcam).
Quantification of Cell Survival and Migration Distance
Each 30um coronal cerebellar section containing GFP+, donor-derived cells was counted manually under fluorescent microscope, and there should not be an overlap of cells during the counting process since the size of the donor derived cells is estimated to be less than 20um in diameter. Due to the tendency of the donor populations, both MASCs and ESNPs, to re-aggregate in vivo following transplantation, cell numbers within clusters were estimated based on the number of nuclei visible in DAPI staining and thus quantification may be lower than actual number of cells present. For migration distance along the rostral/caudal orientation, an injection point was first determined based on either the presence of a needle tract or on the location containing the largest number of cells and then serial sections were followed until donor-derived cells were no longer visible. The distance was therefore estimated based on the number of sections containing GFP+ donor cells from the injection point to the last visible location and the thickness of the tissue sections. A two way ANOVA analysis was performed to determine whether there was any statistical significance in cell migration and survival when the two different donor populations were transplanted into wildtype and weaver (wv/−, wv/wv) mice.
Results
Cerebellar-derived MASCs show extensive migration but limited differentiation following intracerebellar transplantation in postnatal weaver mice
Astrocytes derived from postnatal mouse cerebella have been shown to harbor stem-like characteristics through the expression of stem cell markers such as nestin, and their ability to give rise to neurons, astrocytes, and oligodendrocytes when cultured in neurosphere-like conditions (Fig 1, B and C). When cultured as monolayers, MASCs are highly purified astrocyte populations that are greater than 90% immunopositive for markers including GFAP and S100β with a few CD11b positive microglia mixed in (Fig 1, A and D). Collected cells were directly injected into the right hemisphere of the cerebella of wv/+, wv/wv, and +/+ littermates between postnatal day 1–8, with 23 mice injected with MASCs and 28 with ESNPs. Donor cells are distinguished from the host tissue through their expression of GFP, and analysis of cell survival, migration, and integration were done following survival periods ranging from one week to five weeks post transplantation. As early as one week post transplantation, cells can be seen to migrate away from the site of injection and to settle in all three primary cerebellar layers -molecular, Purkinje, and granule cell layers (Fig 2, A and B) -with predilection towards the white matter. No specific migration pattern was observed and the age at which the mice received the donor cells did not seem to have any major impact based on the range chosen for this study. The majority of cells that survived and showed active migration seemed to adopt an astrocyte-like morphology with numerous, fine processes, but only some were found to be immunopositive for the glial marker GFAP (Fig 2, D) as well as S100β (Fig 2, E). Most of the cells remained immature in morphology with some expression of radial glial marker such as the glutamate transporter GLAST (Fig 2, F), but a small population of MASCs did express the neuronal marker β-III tubulin (Fig 2, C). A majority of the cells also did not possess antigenic expression for mature neuronal cell markers such as NeuN or for cerebellar specific markers such as MATH-1, RU49, and GABAAα6. Overall, transplanted MASCs survived and migrated in all three host environments consisting of +/+, wv/+, and wv/wv cerebella without significant differences between the experiment groups, but cells found to express the antigenic profiles mentioned above, including β-III tubulin, GFAP, S100β, and GLAST, seemed to be more prevalent (15 out of 23 animals) in the wv/+ and wv/wv transplants as opposed to the wildtype littermates (8 out of 23). The number of surviving cells found within the transplanted cerebella compared to the number of cells initially grafted ranged from 0.3% to 9% and averaged 3% for survival (Fig 5, A). For migration distance, an average of 691um was found for MASCs post transplantation (Fig 5, B).
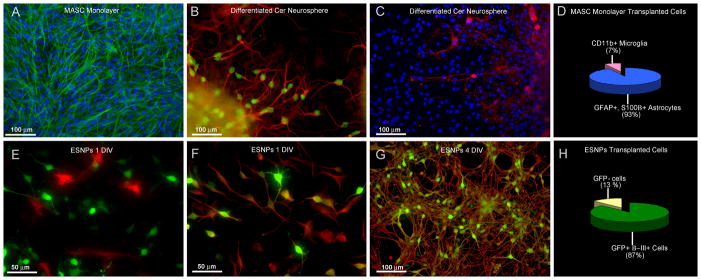
Figure 1. In vitro characterization of both donor populations shows contrasting cell types.
A) Cerebellum-derived MASCs derived from p0–p8 postnatal mouse cerebellum and cultured as GFAP+ (green) astrocyte monolayers were used for in vivo transplantation. (blue=DAPI) B) When cultured as neurospheres, MASCs are capable of differentiating into neurons expressing neuronal markers including β-III tubulin (red) and NeuN (green). C) MASC-derived neurospheres are also found to contain CNPase (red)-expressing oligodendrocytes. (blue=DAPI) D) Quantitative analysis shows that MASC monolayers used for transplantation contain mostly astrocytes (93% as shown in blue) and a few microglia (7% as shown in pink). τEGFP-ESNP cells, on the other hand, express mostly a neuronal fate in culture. E) Following one day in culture, GFP+ ESNPs (green) are not immunopositive for GFAP (red), however, F) shows that they do co-localize with the neuronal marker β-III tubulin (red). G) ESNPs used for in vivo transplantation are mostly immunolabeled for both GFP (green) and β-III tubulin (red) after four days in culture. H) Quantitative analysis shows that ESNPs contain mostly GFP+ immature neurons (87% as shown in green). (DIV=days in vitro)
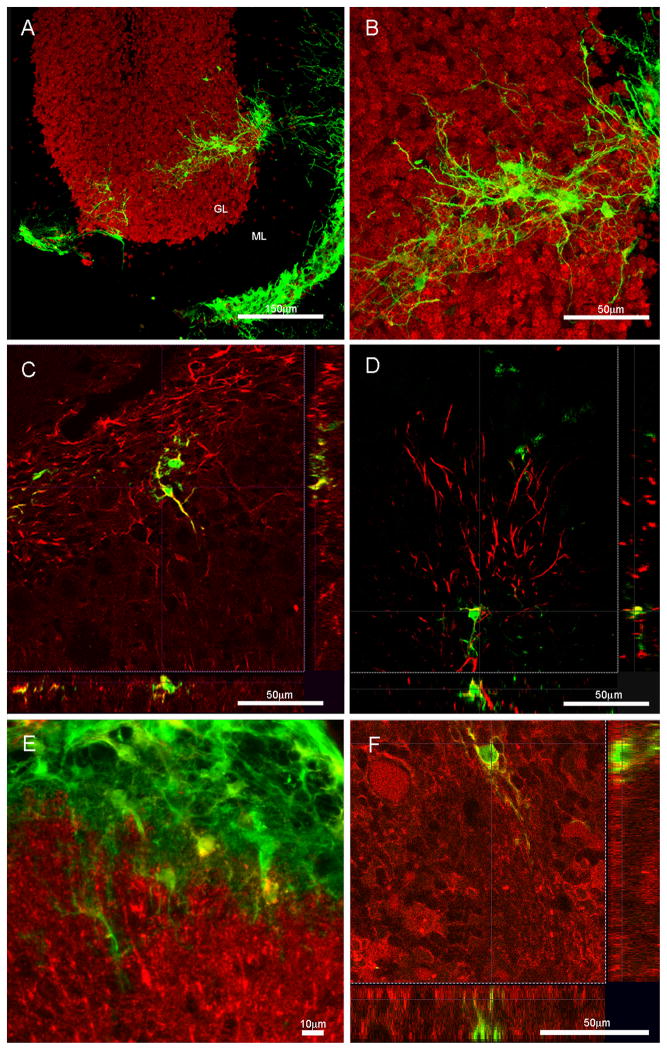
Figure 2. MASCs are able to survive, migrate, and differentiate upon transplantation into the weaver cerebellum.
A) Confocal image shows donor-derived GFP+ MASCs (green) have survived and migrated away from the injection site on top of the cerebellar pial surface, and moved into the granule and molecular layers of the cerebellum. B) Higher magnification micrograph of the same field as in A) shows extended arborizations of the grafted cells with astrocyte-like morphologies. C) A small number of the grafted cells do display neuronal phenotypes through expression of the neuronal marker β-III tubulin (red). D) and E) show that some of the cells maintain glial cell identities and are immuopositive for GFAP (red) in D and S100β in E. F) A small population of the GFP+ donor cells also express the radial glia cell marker GLAST (red). (GL=granule layer, ML=molecular layer)
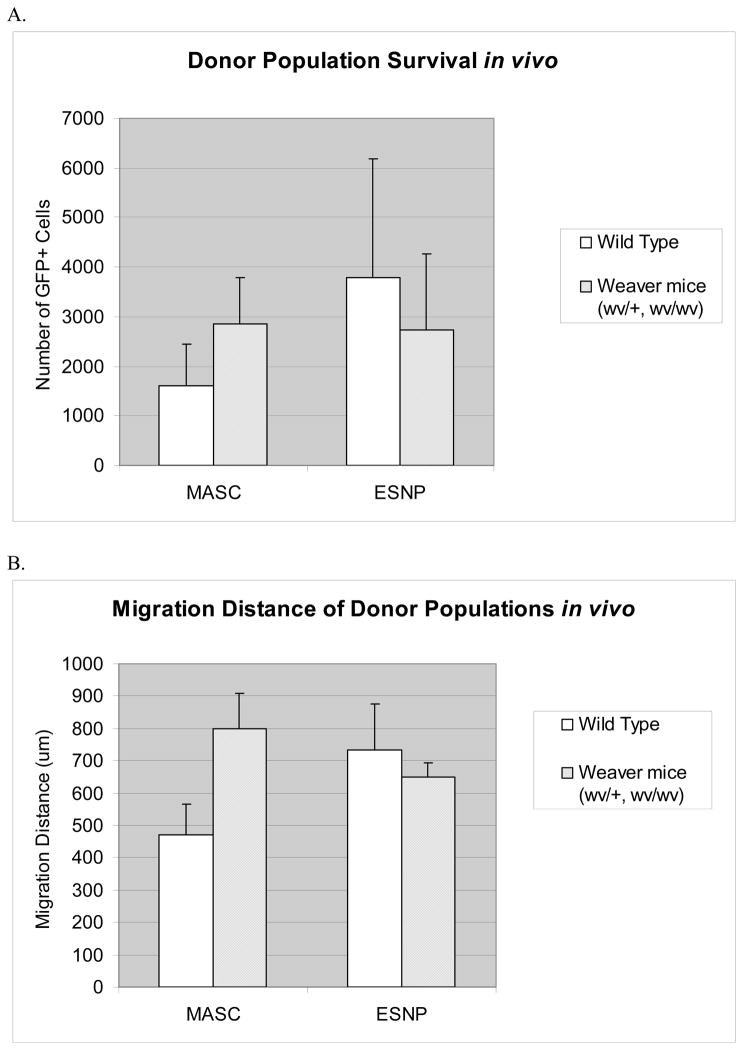
Figure 5. Following cerebellar transplantation, both ESNP and MASC donor populations show a similar trend in cell survival and migration distance.
A) No statistically significant differences were found in the number of cells surviving, for MASCs between the wildtype mice (mean=1602) and weaver mice (wv/+, wv/wv) (mean=2857), or for ESNPs between wildtype mice (mean=3794) and weaver mice (wv/+, wv/wv) (mean=2722), nor between the two donor populations.
B) The migration distance of the transplanted cells also showed no statistically significant differences between the experimental conditions or between the donor populations. For MASCs, an average migration distance of 472um was found in wildtype mice, and an average of 800um was found in weaver mice. For ESNPs, an average of 733um was found in wt animals versus an average of 651um in weaver mice.
ESNPs exhibit multiple neuronal morphologies and phenotypes upon transplantation within the postnatal weaver cerebellum
ESNPs carry the EGFP reporter gene behind the tau promoter and GFP fluorescence was shown to be restricted to neuronal progeny through in vitro characterization15. Specifically, after one day in culture, GFP positive cells were found to be immunopositive for β-III tubulin (Fig 1, F) but did not colocalize with GFAP-expressing cells (Fig 1, E). The same trend was observed after 4 days in culture where majority of the cells became both GFP and β-III tubulin immunopositive (Fig 1, G). As seen with the MASCs, ESNPs exhibited the ability to survive, migrate, and differentiate post-transplantation in the weaver mouse model. However, as compared to MASCs, ESNPs seemed to show a greater affinity towards each other following injection into the host environment and had the tendency to re-aggregate into clusters and remained at the site of injection. However, ESNPs remained viable and appeared to exhibit the same survival and migration trends as the MASCs without any significant differences between the average number of cells surviving or the average migration distance between the two donor populations. Small groups of cells did have the ability to migrate and were capable of moving away from the injection site into all three cerebellar layers once out of the cell clusters (Fig 3, A). The appearance of these cells also was vastly different from that of MASCs, with more varied cell morphologies having neuronal characteristics, including long processes and small cell bodies (Fig 3, B). For example, bipolar cells resembling young migratory neurons, and cells with more complex, somatic-neuritic morphologies including ramified processes that gave rise to thin varicose axons were observed. A small number of cells were also found to acquire processes that bifurcate into T shapes (Fig 3, C), resembling the parallel fibers that characterize cerebellar granule neurons, but the processes were much shorter than typical granule neuron parallel fibers and did not extend into the molecular layer. Immunohistochemical analysis showed that the majority of ESNPs expressed both neuronal cell markers β-III tubulin (Fig 3, D) and NeuN (Fig 3, E), but still seemed to lack region-specific gene/marker expression when tested for granule cell-specific antibodies MATH-1, GABAAα6, and RU49. However, a small population of ESNPs was immunolabeled for the excitatory neurotransmitter glutamate (Fig 3, F) which is expressed exclusively by granule cells within the cerebellum. Donor cells exhibiting the antigenic profile mentioned above, including β-III tubulin, NeuN, and glutamate were found in 20 wv/+ and wv/wv animals and in only eight +/+ littermates out of 28 successful transplants. As observed with the MASCs donor populations, there was no significant statistical differences in the number of survived cells or in migration distances between the three (+/+, wv/+, and wv/wv) experimental groups. The number of surviving cells post-transplantation ranged from 0.3% to 12% and averaged 3% (Fig 5, A), and the average migration distance in ESNPs post-transplantation was found to be 620um (Fig 5, B).
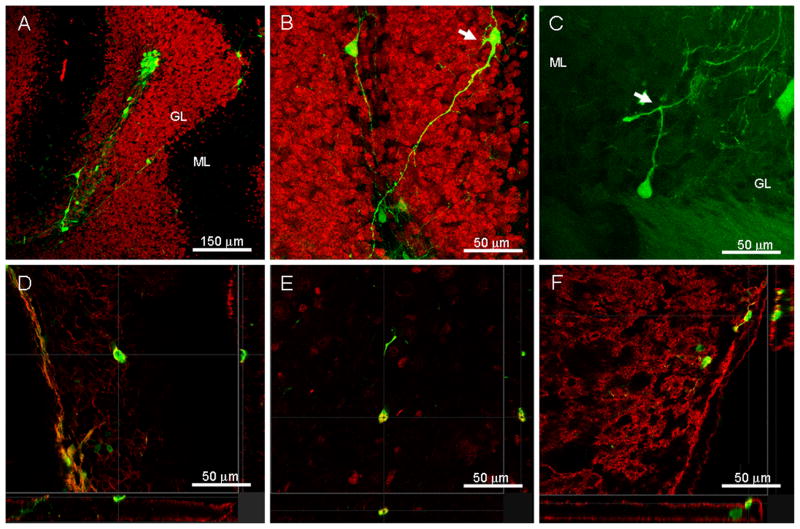
Figure 3. Grafted ESNPs have the ability to survive, migrate, and differentiate into mature cellular phenotypes following transplantation into the weaver mouse cerebellum.
A) Confocal microscopy shows that grafted GFP+ ESNPs (green) can re-aggregate into cell clusters, but they retain the ability to migrate away from the injection site. B) Higher magnification image shows that some of these cells display mature neuronal phenotypes (arrow) with ramified processes and thin, varicose axons. C) An example of a small number of the donor-derived cells (green) found to possess axon-like processes that bifurcate into T shapes (arrow), resembling parallel fibers of the granule neurons. D) A small population of the donor cells also express the pan neuronal marker β-III tubulin (red), the mature neuronal marker NeuN (red) in E), and in F) the neurotransmitter glutamate (red) which is expressed exclusively by granule cells within the cerebellum. (GL=granule layer, ML=molecular layer)
ESNP transplantation can give rise to neoplasia
ESNPs were dissociated into single cell suspensions at the time of transplantation but retained the ability to re-aggregate into small to medium sized cell clusters within the host environment (Fig 3, A) as mentioned above. More often than not, these clusters remained in place and did not seem to invade the host tissue. However, in four out of 51 animals with cells successfully transplanted within the cerebellum, neoplastic-like formations were found four weeks post-transplantation within the injected hemisphere while the contralateral hemisphere was unaffected (Fig 4, A). This was observed only in animals injected with ESNPs but not with the MASCs transplants, and the EGFP positive cells could be found bordering the transformed host tissue or in the center of the cell mass within the cerebellum (Fig 4, D and E). In one case, a solid tumor-like sphere was formed and believed to have its own source of blood and nutrient supply, being that it was separated from the rest of the underlying parenchyma. In other cases, the host tissue seemed to undergo the process of transformation which caused the injected hemisphere to swell and appear enlarged when compared to the contralateral control side (arrow, Fig 4, A). Cells within the tumor-like structures were immunopositive for β-III tubulin (Fig 4, D and E) and SSEA-1 (inset, Fig 4, D) during the early stages of transformation. Hematoxylin and eosin (H & E) staining showed at least one tumor to be a teratoma at its end stage, with the presence of cells containing lineages outside of the CNS, such as skeletal muscle fibers (Fig 4, B) and hair follicles (Fig 4, C). Overall, three wv/+ and one +/+ mice out of 28 transplants had neoplastic formations that appeared to be teratomas. It is noteworthy that these neoplasias occurred despite what appeared to be homogeneous ESNP starting cultures that served as sources for these wv cerebellar transplants.
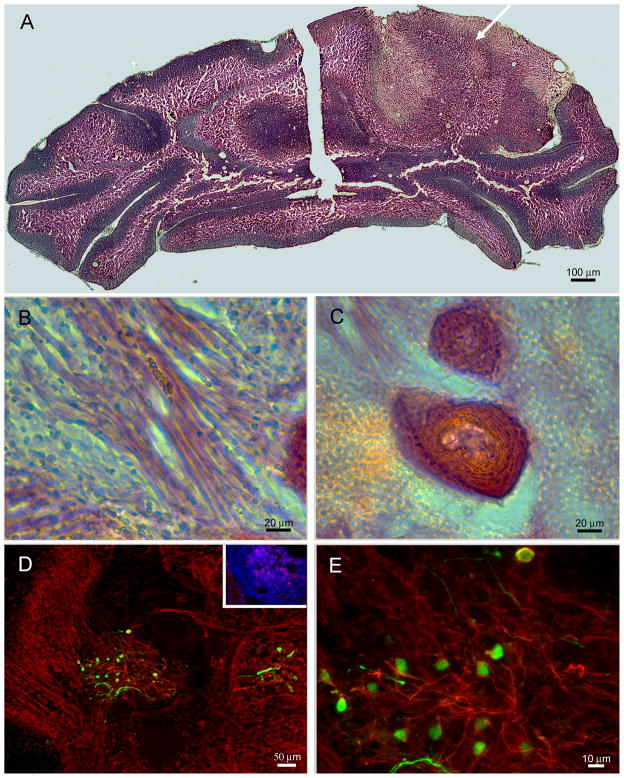
Figure 4. Grafted ESNPs are capable of transforming host tissue and give rise to tumor-like spheres.
A) Montage of images from H& E staining shows the transplanted hemisphere being disrupted by a protruding cell mass (arrow) within the parenchyma but not on the control contralateral side. B) Non-neural lineages such as skeletal muscle fibers or in C) hair follicles can be seen with the teratoma-like neoplasia within the cerebellum based on morphological analysis from H&E staining of the tissue. D) GFP+ ESNPs (green) can be seen within the transformed tissue, and they are also immunopositive for SSEA-1 (red, inset), and β-III tubulin (red) in D and E. Presence of GFP+, β-III tubulin+ cells, same as the grafted population, within the transformed tissue suggests the ESNPs are the most probable cells of origin for the tumor-like neoplasias.
Discussion
The current study shows that two donor stem/progenitor cell populations, derived from either a postnatal neurogenic zone or an embryonic cell line, have the ability to survive, migrate, and initiate differentiation into neuronal phenotypes within the granuloprival weaver mouse cerebellum. No significant differences were found between the two donor populations or within the experimental groups for cell survival and migration patterns/distance. However, one major disparity between MASCs and ESNPs seems to be the morphological phenotypes adopted by the grafted cells. MASCs appear to maintain more immature, glial-like morphologies while the ESNPs seem to acquire more mature, neuronal morphologies, suggesting that inherent differences within differentiation potential do exist between these two neural stem/progenitor cell populations. Regardless, neither of these donor populations gave rise to cells with significant region-specific identities, particularly neurons of the granule, Purkinje, or molecular layers, despite earlier studies that suggested the potential of these stem/progenitor cells to respond to in vivo cues when placed in a permissive/instructive environment. Specifically, cerebellar- or lateral ventricle SEZ-derived MASCs were previously observed to enter into the rostral migratory stream after transplantation into the SEZ, and found to migrate into the olfactory bulb where a small population differentiated into olfactory interneurons14. This suggests that the spatially and temporally restricted population of neurogenic astrocytes, MASCs, retain the ability to respond to in vivo cues provided by the host system. The low percentage of transplants that generated olfactory interneurons reported by Zheng et al.14 was explained by the possibility that cerebellar MASCs were not optimally primed to respond to cues related to olfactory neurogenesis, hence it was expected that homotypic transplantation into the mutant cerebella would prompt more impressive neural differentiation and engraftment, should the MASCs retain a transcriptional profile capable of responding to specific cerebellar neurogenesis cues. Klein et al.13 showed that cerebellar-derived neurospheres gave rise to both GABA-expressing and glutamate-expressing interneurons resembling Lugaro cells and granule cells respectively following transplantation into wildtype P4 cerebellum, but differentiated into glial cells when transplanted into the forebrain, demonstrating those cerebellar-derived cells retained intrinsic regional character in an uninjured CNS environment. Furthermore, it has been shown that in vivo neurogenesis can increase in response to acute injury46–48 in the SEZ and hippocampus, as well as in long-term pathological conditions such as Huntington’s Disease49–50 and stroke51–52, which would suggest that the weaver mouse should be a suitable model for inducing cellular replacement with its discrete and localized pattern of granule neuron degeneration. However, in the present study, the majority of the MASCs retained glial-like or undifferentiated stem/progenitor cell morphologies and lacked expression of region specific transcription factors, with only a small number of cells differentiating into immature neurons or astrocytes.
ESNPs, on the other hand, have been shown to acquire complex morphologies and adopted excitatory neurotransmitter phenotypes both in vivo and in vitro15–20, and therefore were expected to generate more neuronal phenotypes following induction into a dominant neuronal lineage prior to transplantation. Morphologically, these donor cells did appear to be more mature than those derived from cerebellar MASCs, exhibiting extensive numbers of varicose processes and round somata, and they also express mature neuronal markers including NeuN and the neurotransmitter glutamate not seen with the MASC population. Despite the more mature antigenic and morphological profiles, ESNPs still did not appear to significantly commit to regional differentiation identities as indicated by the lack of cerebellar-specific transcriptional factor expressions for MATH-1, GABAAα6, and RU49. This is in keeping with many previous studies that showed engraftment of neural progenitor cells into metabolic disease models42,53 or neurological mutants43–44 seem to exert corrective, therapeutic effects on the at-risk neuronal population, but did not show the acquisition of region specific phenotype through appropriate expression of transcriptional factors or morphological profiles, e.g. including parallel fiber axonal arbors typical for granule cells or the dense and elaborate dendritic trees characteristic of Purkinje neurons. More specifically, some grafted cells within the granule cell layer in this study were found to express the neurotransmitter glutamate that is expressed solely by the granule neurons within the cerebellum but we do not believe that was enough to pronounce a de novo genesis of cerebellar granule neurons because they lack evidence of the characteristic parallel fibers associated with granule neurons. On the other hand, we also observed donor-derived cells differentiated into neuronal phenotypes with axon-like processes that bifurcated into T-shapes resembling that of parallel fibers (Fig 3, C) but again, we hesitate to label those cells as granule neurons due to lack of expression for regional-specific markers such as GABAAα6, MATH-1, or RU49.
One possibility for this lack of impressive regional specification for cerebellar-derived MASCs studied here is that they did not undergo appropriate, terminal phenotypic differentiation due to a downregulation of receptors or transcription or other morphogenetic factors during the in vitro culturing process prior to transplantation; an alternative explanation could be there is a lack of requisite fate choice and differentiation signals within the abnormal weaver cerebellum which may result from a severe homeostatic imbalance within the injured host environment that could be non-conducive for complete functional integration. The weaver mutation is shown to be intrinsic32 to the affected granule neurons, but earlier reports54–55 have shown that some mutant granule cells can be rescued phenotypically and functionally when co-cultured with wildtype cells in vitro or after transplantation into a wildtype cerebellum. This suggests that the host environment plays a critical role in the proper differentiation of the granule cells and that the mutant gene actions may indirectly hinder host-donor cell interactions through effects brought on by the severe loss of granule neurons that would then trigger a cascade of downstream events affecting the rest of the host environment. However, data presented here also seem to suggest that the cellular deficits present within some of the wv/+ or wv/wv brains may promote a slightly enhanced donor cell response towards acquisition of neuronal phenotypy with more cells acquiring neuronal/glial marker expression in heterozygous and homozygous animals as compared to the wildtype littermates. Hence it is likely that a fine balance exists between an impaired host environment that is amenable to cell replacement versus one that may be too toxic for immediate (and within the timeframes, albeit rather protracted here) and desired repair. This is also supported by the observations on the embryonic cell line-derived donor population, ESNPs, studied here. This is a donor population primed for the generation of neuronal lineage diversity and expected to be more responsive to neurogenic cues within the host environment, so a lack of regional commitment suggests, again, that successful engraftment is highly dependent upon the interplay between the host environment and the donor populations, and that the context of the injury conditions can be specific and crucial for the promotion of preferential cell replacement. An approach to get around the potentially suppressive environment would be to induce the donor populations into cerebellar neuronal lineages prior to transplantation. Growth factors such as the bone morphogenetic protein (BMP) have been shown to specify granule cell identities56 and it was added to the ESNP cultures in vitro here in an attempt to guide differentiation of ESNPs toward a cerebellar cell fate. MASCs were also cultured in conditioned media derived from primary cultures of wildtype cerebellar granule neurons, but no apparent changes were observed with either donor population based on in vitro and in vivo characterization of the cells following priming conditions. Many of the culture conditions for fate determination seem to be specific towards the particular population of cells under study so further optimization of the priming conditions for MASCs and ESNPs would be needed in the future.
Another phenomenon observed here was the aggressive nature of the neoplastic formations within a small number of ESNP transplants. Previous studies reported that donor cell clusters of varied sizes with teratoma appearances were found when ESNPs were transplanted into the SEZ, but they did not seem to disrupt the parenchyma nor was there evidence of infiltration by non-neural donor cells16. In contrast, our study shows that host tissue surrounding the GFP+ neoplastic formation was also transformed and adjoined with the neoplasm as confirmed by H & E staining. The difference could be attributed to particular locations of the injection sites, but it does not preclude the possibility of ESNPs giving rise to neoplastic formations. In comparison, previous studies have shown similar invasive sphere-like structures forming within ventricular walls of animals intraventricularly transplanted with cerebellar MASCs, but in these experiments no changes were detected in the surrounding tissues57. These same donor cells did not give rise to neoplastic formations in any of the current transplants when injected directly into the cerebellum. It is possible that the neoplastic formations arose due to excessive cell proliferation in culture, but not every animal within the same experimental group developed tumor-like structures despite receiving the same starting donor population. While excessive Ki67 expression was found within the transformed tissue as expected, a similar degree of Ki67 expression was found between the MASCs and the ESNPs cultures in vitro. This suggests, once again, that differences exist between the in vivo proliferation and differentiation potential of the two different stem/progenitor cell populations following transplantation. Another possibility for these neoplastic formations is that undifferentiated, GFP− ES cells were among the ESNP donor population at the time of engraftment and were responsible for the tumor-like formations. One strategy to circumvent such undesired tissue growth is to pre-sort for the GFP+ ESNPs using fluorescence-activated cell sorting (FACS) to ensure transplantation of lineage-restricted cells only.
More studies are clearly needed to uncover ways to coax stem/progenitor cells into appropriate patterns of cellular differentiation before neural stem/progenitor cells can be considered for therapeutic applications within neurologically compromised patients. The present study presents an important in vivo bioassay, within a presumptive cell-replacement-supportive neurological mutant mouse brain, that utilizes two of the most disparate and plastic stem/progenitor cell populations that have always been assumed to be promising candidates for neuro-cellular replacement therapies.
Acknowledgments
We thank Dr. E. D. Laywell for helpful discussion and critical review of the manuscript, and Dr. J.Y. Zhang for help on the statistical analysis of the data. We also thank K. Andrasik and S. Suek for excellent technical support on the genotyping of the weaver mice, Dr. B. Scheffler, Dr. A.K. Goetz, and Dr. S. Wang for providing ESNPs, M. Jorgensen of the UF McKnight Brain Institute CTAC for immunostaining of the tumors, and D. Smith of the UF McKnight Brain Institute CTAC for the confocal images. This work was supported by NIH/NINDS grants NS37556 and NS055165, and the McKnight Brain Institute of the University of Florida.
Footnotes
Author Contribution:
K. Amy Chen: Conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing.
Tong Zheng: Conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing
Derek Lanuto: Collection of data.
Dennis A. Steindler: Conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
References
- 1.Daniela F, Vescovi AL, Bottai D. The stem cells as a potential treatment for neurodegeneration. Methods Mol Biol. 2007;399:199–213. doi: 10.1007/978-1-59745-504-6_14. [DOI] [PubMed] [Google Scholar]
- 2.Steindler DA, Pincus DW. Stem cells and neuropoiesis in the adult human brain. Lancet. 2002;359(9311):1047–1054. doi: 10.1016/S0140-6736(02)08096-0. [DOI] [PubMed] [Google Scholar]
- 3.Rossi F, Cattaneo E. Opinion: neural stem cell therapy for neurological diseases: dreams and reality. Nat Rev Neurosci. 2002;3(5):401–409. doi: 10.1038/nrn809. [DOI] [PubMed] [Google Scholar]
- 4.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264(5162):1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197(4308):1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 6.Kirschenbaum B, Nedergaard M, Preuss A, et al. In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain. Cereb Cortex. 1994;4(6):576–589. doi: 10.1093/cercor/4.6.576. [DOI] [PubMed] [Google Scholar]
- 7.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A. 1999;96(10):5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatten ME, Alder J, Zimmerman K, et al. Genes involved in cerebellar cell specification and differentiation. Curr Opin Neurobiol. 1997;7(1):40–47. doi: 10.1016/s0959-4388(97)80118-3. [DOI] [PubMed] [Google Scholar]
- 10.Laywell ED, Rakic P, Kukekov VG, et al. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci U S A. 2000;97(25):13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laywell ED, Kearns SM, Zheng T, et al. Neuron-to-astrocyte transition: phenotypic fluidity and the formation of hybrid asterons in differentiating neurospheres. J Comp Neurol. 2005;493(3):321–333. doi: 10.1002/cne.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee A, Kessler JD, Read TA, et al. Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci. 2005;8(6):723–729. doi: 10.1038/nn1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein C, Butt SJ, Machold RP, et al. Cerebellum- and forebrain-derived stem cells possess intrinsic regional character. Development. 2005;132(20):4497–4508. doi: 10.1242/dev.02037. [DOI] [PubMed] [Google Scholar]
- 14.Zheng T, Marshall GP, 2nd, Laywell ED, et al. Neurogenic astrocytes transplanted into the adult mouse lateral ventricle contribute to olfactory neurogenesis, and reveal a novel intrinsic subependymal neuron. Neuroscience. 2006;142(1):175–185. doi: 10.1016/j.neuroscience.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 15.Goetz AK, Scheffler B, Chen HX, et al. Temporally restricted substrate interactions direct fate and specification of neural precursors derived from embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103(29):11063–11068. doi: 10.1073/pnas.0510926103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wernig M, Benninger F, Schmandt T, et al. Functional integration of embryonic stem cell-derived neurons in vivo. J Neurosci. 2004;24(22):5258–5268. doi: 10.1523/JNEUROSCI.0428-04.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benninger F, Beck H, Wernig M, et al. Functional integration of embryonic stem cell-derived neurons in hippocampal slice cultures. J Neurosci. 2003;23(18):7075–7083. doi: 10.1523/JNEUROSCI.23-18-07075.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brüstle O, Spiro AC, Karram K, et al. In vitro-generated neural precursors participate in mammalian brain development. Proc Natl Acad Sci U S A. 1997;94(26):14809–14814. doi: 10.1073/pnas.94.26.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brüstle O, Jones KN, Learish RD, et al. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285(5428):754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 20.Okabe S, Forsberg-Nilsson K, Spiro AC, et al. Development of neuronal precursor cells and functional postmitotic neurons from embryonic stem cells in vitro. Mech Dev. 1996;59(1):89–102. doi: 10.1016/0925-4773(96)00572-2. [DOI] [PubMed] [Google Scholar]
- 21.Sidman RL, Appel SH, Fullier JF. Neurological Mutants of the Mouse. Science. 1965;150(3695):513–516. doi: 10.1126/science.150.3695.513. [DOI] [PubMed] [Google Scholar]
- 22.Rakic P, Sidman RL. Sequence of developmental abnormalities leading to granule cell deficit in cerebellar cortex of weaver mutant mice. J Comp Neurol. 1973;152(2):103–132. doi: 10.1002/cne.901520202. [DOI] [PubMed] [Google Scholar]
- 23.Smeyne RJ, Goldowitz D. Development and death of external granular layer cells in the weaver mouse cerebellum: a quantitative study. J Neurosci. 1989;9(5):1608–1620. doi: 10.1523/JNEUROSCI.09-05-01608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenman LM, Gallagher E, Hawkes R. Regionalization defects in the weaver mouse cerebellum. J Comp Neurol. 1998;394(4):431–444. [PubMed] [Google Scholar]
- 25.Herrup K, Trenkner E. Regional differences in cytoarchitecture of the weaver cerebellum suggest a new model for weaver gene action. Neuroscience. 1987;23(3):871–85. doi: 10.1016/0306-4522(87)90164-3. [DOI] [PubMed] [Google Scholar]
- 26.Smeyne RJ, Goldowitz D. Purkinje cell loss is due to a direct action of the weaver gene in Purkinje cells: evidence from chimeric mice. Brain Res Dev Brain Res. 1990;52(1–2):211–218. doi: 10.1016/0165-3806(90)90237-s. [DOI] [PubMed] [Google Scholar]
- 27.Bayer SA, Wills KV, Wei J, et al. Phenotypic effects of the weaver gene are evident in the embryonic cerebellum but not in the ventral midbrain. Brain Res Dev Brain Res. 1996;96(1–2):130–137. doi: 10.1016/0165-3806(96)00107-1. [DOI] [PubMed] [Google Scholar]
- 28.Triarhou LC, Low WC, Norton J, et al. Reinstatement of synaptic connectivity in the striatum of weaver mutant mice following transplantation of ventral mesencephalic anlagen. J Neurocytol. 1988;17(2):233–243. doi: 10.1007/BF01674210. [DOI] [PubMed] [Google Scholar]
- 29.Roffler-Tarlov S, Martin B, Graybiel AM, et al. Cell death in the midbrain of the murine mutation weaver. J Neurosci. 1996;16(5):1819–1826. doi: 10.1523/JNEUROSCI.16-05-01819.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maricich SM, Soha J, Trenkner E, et al. Failed cell migration and death of purkinje cells and deep nuclear neurons in the weaver cerebellum. J Neurosci. 1997;17(10):3675–3683. doi: 10.1523/JNEUROSCI.17-10-03675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martí J, Wills KV, Ghetti B, et al. Evidence that the loss of Purkinje cells and deep cerebellar nuclei neurons in homozygous weaver is not related to neurogenetic patterns. Int J Dev Neurosci. 2001;19(6):599–610. doi: 10.1016/s0736-5748(01)00036-3. [DOI] [PubMed] [Google Scholar]
- 32.Patil N, Cox DR, Bhat D, et al. A potassium channel mutation in weaver mice implicates membrane excitability in granule cell differentiation. Nat Genet. 1995;11(2):126–129. doi: 10.1038/ng1095-126. [DOI] [PubMed] [Google Scholar]
- 33.Schein JC, Hunter DD, Roffler-Tarlov S. Girk2 expression in the ventral midbrain, cerebellum, and olfactory bulb and its relationship to the murine mutation weaver. Dev Biol. 1998;204(2):432–450. doi: 10.1006/dbio.1998.9076. [DOI] [PubMed] [Google Scholar]
- 34.Scheffler B, Walton NM, Lin DD, et al. Phenotypic and functional characterization of adult brain neuropoiesis. Proc Natl Acad Sci U S A. 2005;102(26):9353–9858. doi: 10.1073/pnas.0503965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Triarhou LC, Zhang W, Lee WH. Amelioration of the behavioral phenotype in genetically ataxic mice through bilateral intracerebellar grafting of fetal Purkinje cells. Cell Transplant. 1996;5(2):269–277. doi: 10.1177/096368979600500215. [DOI] [PubMed] [Google Scholar]
- 36.Triarhou LC, Low WC, Ghetti B. Transplantation of ventral mesencephalic anlagen to hosts with genetic nigrostriatal dopamine deficiency. Proc Natl Acad Sci U S A. 1986;83(22):8789–8793. doi: 10.1073/pnas.83.22.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Triarhou LC, Low WC, Ghetti B. Transplantation of cerebellar anlagen to hosts with genetic cerebellocortical atrophy. Anat Embryol (Berl) 1987;176(2):145–154. doi: 10.1007/BF00310047. [DOI] [PubMed] [Google Scholar]
- 38.Sotelo C, Alvarado-Mallart RM. Growth and differentiation of cerebellar suspensions transplanted into the adult cerebellum of mice with heredodegenerative ataxia. Proc Natl Acad Sci U S A. 1986;83(4):1135–1139. doi: 10.1073/pnas.83.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sotelo C, Alvarado-Mallart RM. Reconstruction of the defective cerebellar circuitry in adult Purkinje cell degeneration mutant mice by Purkinje cell replacement through transplantation of solid embryonic implants. Neuroscience. 1987;20(1):1–22. doi: 10.1016/0306-4522(87)90002-9. [DOI] [PubMed] [Google Scholar]
- 40.Sotelo C, Alvarado-Mallart RM. Integration of grafted Purkinje cell into the host cerebellar circuitry in Purkinje cell degeneration mutant mouse. Prog Brain Res. 1988;78:141–154. doi: 10.1016/s0079-6123(08)60277-0. [DOI] [PubMed] [Google Scholar]
- 41.Snyder EY, Deitcher DL, Walsh C, et al. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell. 1992;68(1):33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- 42.Snyder EY, Taylor RM, Wolfe JH. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature. 1995;374(6520):367–370. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- 43.Rosario CM, Yandava BD, Kosaras B, et al. Differentiation of engrafted multipotent neural progenitors towards replacement of missing granule neurons in meander tail cerebellum may help determine the locus of mutant gene action. Development. 1997;124(21):4213–4224. doi: 10.1242/dev.124.21.4213. [DOI] [PubMed] [Google Scholar]
- 44.Li J, Ma Y, Teng YD, et al. Purkinje neuron degeneration in nervous (nr) mutant mice is mediated by a metabolic pathway involving excess tissue plasminogen activator. Proc Natl Acad Sci U S A. 2006;103(20):7847–7852. doi: 10.1073/pnas.0602440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang SC, Wernig M, Duncan ID, et al. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19(12):1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 46.Jin K, Minami M, Lan JQ, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98(8):4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jankovski A, Garcia C, Soriano E, et al. Proliferation, migration and differentiation of neuronal progenitor cells in the adult mouse subventricular zone surgically separated from its olfactory bulb. Eur J Neurosci. 1998;10(12):3853–3868. doi: 10.1046/j.1460-9568.1998.00397.x. [DOI] [PubMed] [Google Scholar]
- 48.Gould E, Tanapat P. Lesion-induced proliferation of neuronal progenitors in the dentate gyrus of the adult rat. Neuroscience. 1997;80(2):427–436. doi: 10.1016/s0306-4522(97)00127-9. [DOI] [PubMed] [Google Scholar]
- 49.Curtis MA, Penney EB, Pearson AG, et al. Increased cell proliferation and neurogenesis in the adult human Huntington’s disease brain. Proc Natl Acad Sci U S A. 2003;100(15):9023–0027. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Curtis MA, Waldvogel HJ, Synek B, et al. A histochemical and immunohistochemical analysis of the subependymal layer in the normal and Huntington’s disease brain. J Chem Neuroanat. 2005;30(1):55–66. doi: 10.1016/j.jchemneu.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Jin K, Wang X, Xie L, et al. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103(35):13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 53.Lee JP, Jeyakumar M, Gonzalez R, et al. Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nat Med. 2007;13(4):439–447. doi: 10.1038/nm1548. [DOI] [PubMed] [Google Scholar]
- 54.Gao WQ, Liu XL, Hatten ME. The weaver gene encodes a nonautonomous signal for CNS neuronal differentiation. Cell. 1992;68(5):841–854. doi: 10.1016/0092-8674(92)90028-b. [DOI] [PubMed] [Google Scholar]
- 55.Gao WQ, Hatten ME. Neuronal differentiation rescued by implantation of Weaver granule cell precursors into wild-type cerebellar cortex. Science. 1993;260(5106):367–369. doi: 10.1126/science.8469990. [DOI] [PubMed] [Google Scholar]
- 56.Alder J, Lee KJ, Jessell TM, et al. Generation of cerebellar granule neurons in vivo by transplantation of BMP-treated neural progenitor cells. Nat Neurosci. 1999;2(6):535–540. doi: 10.1038/9189. [DOI] [PubMed] [Google Scholar]
- 57.Zheng T, Steindler DA, Laywell ED. Transplantation of an indigenous neural stem cell population leading to hyperplasia and atypical integration. Cloning Stem Cells. 2002;4(1):3–8. doi: 10.1089/153623002753631995. [DOI] [PubMed] [Google Scholar]