Abstract
Context
The social environment may influence cognitive function in aging. However, to our knowledge, no studies have investigated whether specific genes modify this association.
Objective
To examine whether the apolipoprotein E (APOE) epsilon-4 allele modified the associations of neighborhood psychosocial hazards and cognitive function.
Design
A cross-sectional analysis.
Setting
The Baltimore Memory Study, a population-based sample of older urban residents. The 65 study neighborhoods in Baltimore city were characterized using the Neighborhood Psychosocial Hazards scale, designed to assess social disorganization, physical disorder, public safety, and economic deprivation.
Participants
One thousand one hundred forty urban residents aged 50 to 70 years at baseline.
Main Outcome Measures
Cognitive performance on 20 standard tests was measured and combined to form 7 summary domain scores (language, processing speed, eye-hand coordination, executive functioning, verbal memory and learning, visual memory, and visuoconstruction).
Results
In analyses fully adjusted for individual covariates, we found that high (i.e., worse) neighborhood psychosocial hazards were not consistently associated with worse cognitive performance. However, the interaction of high neighborhood psychosocial hazards and APOE ε4 genotype was found to be associated with worse cognitive domain scores, with evidence of associations in the domains of processing speed (p = 0.02) and executive functioning (p < 0.001). Suggestive evidence was also found for eye-hand coordination (p = 0.05).
Conclusion
Living in a psychosocially hazardous neighborhood was associated with worse cognitive function in persons with the APOE ε4 allele, evidence of a novel gene x environment interaction.
Keywords: residential characteristics, psychosocial factors, apolipoprotein E, cognitive function, gene-environment interaction
INTRODUCTION
Cognitive decline in aging has a multifactorial etiology with both environmental and genetic components. A prominent genetic factor of relevance to cognitive decline is the ε4 variant of the apolipoprotein E ( APOE) gene, a strong predictor of increased risk and earlier onset of Alzheimer’s disease.1, 2 Apolipoprotein E (apoE) is critical for basic neurological processes relevant to non-demented neurological health including neuronal proliferation, repair, and remyelination.3 However, the impact of APOE genotype on non-demented cognitive function remains unclear, with worse cognitive performance for ε4 allele carriers not consistently reported.4 A meta-analysis found that in adults without dementia, the APOE ε4 genotype was associated with small decrements in global cognition, episodic memory, and executive functioning.5 The mixed findings and modest effects suggest that APOE may be important to non-demented cognitive function, but more complicated causal pathways may be involved, including interactions with other neurological insults that may modify the effects of APOE.
Substantial animal evidence supports that psychosocial stress profoundly influences neurological health.6, 7 Although the term psychosocial stress is common in scientific and popular literature, semantic confusion surrounds the term8: it may refer to both an exposure (a psychosocially challenging situation) or an outcome (the psychological or physiological state resulting from exposure). We prefer the term psychosocial hazard to describe relevant exposures-stable and perceptible features of environments that give rise to a heightened state of vigilance, alarm, or fear in residents that may lead to a biological stress response.9 In a variety of animal species, long-term exposure to psychosocial hazards is associated with structural and functional changes in the brain. For example, rats chronically exposed to rat intruders exhibit worse learning and memory and long-term potentiation deficits.10, 11 Similarly, hippocampal atrophy due to prolonged exposure to hierarchically dominant animals has been observed in rats12 and primates.13, 14 Notably, APOE genotype may interact with psychosocial hazards to influence cognition. Mice repeatedly subjected to rats as cagemates exhibit persistent learning deficits that are dependent on APOE genotype.15 In humans, APOE genotype modified the associations of self-reported stress levels and memory performance.16 Previously, we reported synergistic interactions of salivary cortisol levels and APOE genotype on cognitive function.17 In the present article, we tested the hypothesis that living in psychosocially hazardous neighborhood environments may interact with APOE genotype to influence cognitive function.
METHODS
Participant selection and recruitment
The Baltimore Memory Study is a population-based cohort study of cognitive decline in older urban residents. Sampling and recruitment have been described elsewhere.18 Participants were drawn from 65 neighborhoods in central and north Baltimore city, Maryland, targeted to ensure heterogeneity of demographic and place-level characteristics. In total, 1,140 participants were enrolled from the eligible population of 50-to 70-year-old residents who had lived in the Baltimore area for at least the 5 previous years. The Committee for Human Research of the Johns Hopkins Bloomberg School of Public Health approved the study. Participants provided written, informed consent prior to study entrance and were paid $50 for each visit completion.
Data collection
Data collection and cognitive testing were performed by trained research assistants on-site at the study clinic in Baltimore city.18 The present data is from each participant’s baseline visit between May 30, 2001 and September 20, 2002. A cognitive battery was administered to each participant along with a structured interview, which captured the following self-reported information: demographics, residential history, medical history, and medication, alcohol, and tobacco use. Instrumental activities of daily living were assessed using the Older Americans Resource Scale for Instrumental Activities of Daily Living 7-item scale.19 Self-reported race/ethnicity was assessed according to the 2000 U.S. Census method.20 Laboratory methods for APOE genotyping are published elsewhere.21
Cognitive measures
The testing procedure, cognitive battery, and creation of cognitive domain scores have been described in detail.22 Research assistants read a standard statement orienting participants to the tests and avoided any reference to testing as a means of evaluating individual ability. Tests did not require complex verbal responses in order to minimize potential information bias by socioeconomic status or race/ethnicity. Tests were administered by female and male technicians of white and African American race/ethnicity after random assignment. Subjects completed 20 tests in 7 cognitive domains: language (Boston Naming Test; letter fluency; and category fluency), processing speed (simple reaction time), eye-hand coordination (Purdue Pegboard Test dominant hand, non-dominant hand, and both hands; and Trail-Making Test A), executive functioning ( consisting of the mean of 3 z-transformed difference scores: Purdue Pegboard Test assembly minus both hands; Stroop C form minus A form; Trail-Making test B minus A), verbal memory and learning (Rey Auditory Verbal Learning Test immediate recall, delayed recall, and recognition), visual memory (Rey-Osterrieth Complex Figure Test delayed recall and symbol digit), and visuoconstruction (Rey-Osterrieth Complex Figure Test copy). The 7 domain scores, expressed in standard deviation units, were the primary study outcomes.
Psychosocial hazards
Study neighborhood boundaries were based on community definitions of existing named neighborhoods developed by the Baltimore City Department of Planning, that have a rich history and meaning to residents and are therefore likely to be relevant spatial areas. We characterized the psychosocial environment for each study neighborhood using the Neighborhood Psychosocial Hazards (NPH) Scale,9 a 12-item scale based on theory and factor analysis. Data on neighborhood characteristics came from the 2000 U.S. Census and various city agencies, and all indicators were measured at the place level.9 To reduce dependent measurement error, no information from study subjects was used. Block-level census data were recombined into pre-established neighborhood boundaries by the U.S. Census Bureau via special tabulation. The final NPH Scale included items indicating social disorganization (percentage of single families, percentage of adults without a high school degree or general education diploma, and percentage of adults divorced, separated or widowed), public safety (911 calls per person per year, violent crimes), physical disorder (percentage of vacant houses, complaints about street conditions, number of liquor stores), and economic deprivation (per capita income, index of working class, percentage of unemployment, percentage of families living in poverty). The 12 variables were standardized and the resulting z-scores summed to yield the NPH Scale score which was normally distributed from −19.0 to 19.2 with a mean (SD) of 0.1 (9.6). A higher NPH score indicates a more psychosocially hazardous neighborhood.
Statistical analysis
Because of social stratification, persons in psychosocially hazardous neighborhoods may not be similar to persons in better neighborhoods. For example, persons in economically disadvantaged neighborhoods tend to be poor while persons in economically advantaged neighborhoods are likely better-off. As a result, associations of NPH with health outcomes may be due to differences in the types of persons residing in these neighborhoods, rather than a neighborhood causal effect.23 In such situations, propensity score methods can be implemented24 as has been done in a variety of clinical and epidemiological studies.25
We estimated the propensity score for each participant-the probability of exposure to high NPH-from a set of observed variables. Because propensity score methods are generally used with binary exposures, we dichotomized NPH exposure to create 2 groups (quartile 4 of NPH score [7.65 to 19.20] = “high NPH group” vs. Quartiles 1–3 of NPH score [−19.00 to 7.25] = “comparison group”). We non-parametrically estimated the log-odds of exposure to high NPH using generalized boosted modeling.26, 27 Variables in the propensity score model were factors that might plausibly determine assignment to neighborhood “treatments” including: age, sex, race/ethnicity, number of siblings, number of children alive, marital status, household wealth, educational attainment (respondent and spouse), public/private high school graduated from (respondent and spouse), retirement status, job control during primary lifetime occupation, smoking, and housing variables including type of housing, ownership, and length of tenure.28–30 Propensity score weights were then applied to the sample such that persons in the comparison group who were more similar to the high NPH group were given greater weight and those who were dissimilar were down weighted.27
To provide additional protection against confounding, we also applied “doubly robust” regression adjustments for the individual-level covariates of age, sex, race/ethnicity (African American, African American-mixed, and other, with white as the reference), education (9 levels), household wealth (natural log-transformed sum of household income plus household assets), and testing technician.31 Regression adjustment was implemented using generalized estimating equations with robust variances to account for within-neighborhood correlations in outcomes. To assess the interaction of NPH and APOE, effect modification models included an indicator for APOE ε4 status (one or two ε4 alleles versus none) and a high NPH-APOE ε4 cross-product term. Statistical interaction was evaluated through Waldtests. For analyses regarding the outcome of processing speed, 14 persons with extreme low values (scores < −4 SD units) were removed and the analyses were repeated.
Because the length of exposure to NPH (i.e., residential duration) may be conceptually important,32 all analyses were repeated using a subsample of participants who had lived in their current neighborhood for greater than 10 years. In addition, because African American individuals are more likely to reside in more psychosocially hazardous neighborhoods, we repeated analyses separately for white and African American individuals. Analyses were performed with R version 2.8.1.33
RESULTS
Sample characteristics
In total, a maximum of 1,124 persons from 63 neighborhoods who had complete NPH, APOE, and cognitive data were included in analyses (Table 1). The sample comprised primarily of white (53.8%) and African American individuals (41.5%). APOE ε4 prevalence differed by race/ethnicity: while 30.4% of the sample possessed at least one ε4 allele, 37.3% of African American individuals were ε4+ compared with only 24.7% of non-African American individuals. The different APOE genotype groups did not significantly differ on any other observed covariate, including health characteristics. Compared with the lower 3 quartiles, the highest quartile of NPH was substantially different in characteristics such as race/ethnicity, education, and wealth. Propensity score weighting reduced these differences so that the NPH exposure groups became more comparable (Table 1). For example, the high NPH group was 88.1% African American or mixed African American race/ethnicity, compared with 33.5% in the comparison group before propensity score weighting; after propensity score weighting, the comparison group was mathematically re-weighted to a group with 79.3% African American/mixed race/ethnicity.
Table 1.
Characteristics of selected baseline covariates for participants in the Baltimore Memory Study.
| Overall | APOEε4+ | APOEε4− | NPH Quartile 4 | NPH Quartiles 1–3 | ||
|---|---|---|---|---|---|---|
| (unweighted) | (propensity score weighted) | |||||
| N=1124 | N=342 | N=782 | N=243 | N=881 | ||
| Age, years | 59.3 (6.0) | 58.8 (5.9) | 59.6 (6.0) | 59.6 (6.1) | 59.3 (5.9) | 59.4 (6.0) |
| Female, % | 65.6 | 63.2 | 66.6 | 68.3 | 64.8 | 70.4 |
| African American/mixed, % | 45.3 | 55.6 | 40.8 | 88.1 | 33.5 | 79.3 |
| Log household wealth, $ | 11.6 (1.9) | 11.5 (1.9) | 11.7 (1.8) | 10.2 (2.1) | 11.9 (1.7) | 10.7 (2.2) |
| Education, years | 14.7 (3.9) | 14.5 (3.8) | 14.7 (3.9) | 12.8 (3.5) | 15.2 (3.8) | 13.3 (3.4) |
| Lived in current home >10 yrs, % | 74.0 | 72.9 | 74.5 | 72.3 | 74.5 | 73.3 |
| History of chronic disease, % | ||||||
| Cerebrovascular accident | 5.4 | 4.7 | 5.8 | 9.1 | 4.4 | 5.7 |
| Myocardial infarction | 10.2 | 11.4 | 9.7 | 15.2 | 8.9 | 9.3 |
| Hypertension | 59.6 | 57.5 | 60.5 | 68.2 | 57.2 | 70.5 |
| Diabetes | 22.5 | 23.7 | 22.0 | 32.5 | 19.8 | 25.5 |
| CES-D Scale score ≥ 16, % | 13.7 | 14.0 | 13.6 | 15.2 | 13.3 | 19.1 |
| Any OARS IADL difficulty, % | 11.0 | 13.2 | 10.0 | 17.4 | 9.2 | 12.1 |
| NPH score | −1.0 (8.7) | −0.9 (8.5) | −1.0 (8.7) | 11.7 (3.6) | −4.5 (5.9) | −2.5 (5.0) |
| APOE ε4+, % | 30.4 | 100 | 0 | 32.9 | 29.7 | 38.6 |
Mean (SD) is provided for continuous covariates
APOE = apolipoprotein E gene
CES-D = Center for Epidemiologic Study - Depression
NPH = Neighborhood Psychosocial Hazards. A higher NPH score indicates a more psychosocially hazardous neighborhood.
OARS IADL = Older Americans Resource Scale for Instrumental Activities of Daily Living.
Propensity score weights are estimated from probability of residing in the most psychosocially hazardous quartile of neighborhoods. Weights are used to establish groups that are distributionally similar on the covariates that determined the propensity score. Variables used to estimate the propensity score include APOE genotype, age, sex, race/ethnicity, number of siblings, number of children alive, marital status, household wealth, educational attainment (respondent and spouse), public/private high school graduated from (respondent and spouse), retirement status, job control during primary lifetime occupation, smoking, and housing variables including type of housing, ownership, and length of tenure.
NPH and APOE main effects
In unadjusted analyses, persons in the most hazardous quartile of NPH performed substantially worse in all 7 cognitive domains (Table 2); the average decrement across all domains was −0.45 SD units (all p-values < 0.001). In adjusted models with both NPH and APOE terms (model 1, Table 3), persons in the most hazardous quartile of NPH scored lower than persons in the other 3 quartiles only for eye-hand coordination (−0.14 SD units; p = 0.020) (Table 3). APOE-ε4 was associated with worse performance in executive function (−0.12 SD units; p = 0.04) and visuoconstruction (−0.20 SD units; p = 0.006).
Table 2.
Unadjusted associations of neighborhood psychosocial hazards and APOE genotype with cognitive domain scores.
| Cognitive domain | NPH quartile 4 vs. quartiles 1–3 β (95% CI) |
APOE-ε4 genotype β (95% CI) |
|---|---|---|
| Language | −0.57 (−0.80, −0.35) | −0.09 (−0.21, 0.04) |
| Processing speed | −0.38 (−0.52, −0.24) | −0.07 (−.20, 0.06) |
| Eye-hand coordination | −0.43 (−0.56, −0.30) | −0.11 (−0.22, 0.00) |
| Executive function | −0.41 (−0.55, −0.28) | −0.08 (−0.18, 0.02) |
| Verbal memory & learning | −0.36 (−0.48, −0.23) | −0.10 (−0.25, 0.06) |
| Visual memory | −0.39 (−0.54, −0.23) | −0.05 (−0.20, 0.10) |
| Visuoconstruction | −0.64 (−0.85, −0.44) | −0.19 (−0.35, −0.04) |
Abbreviations: NPH, Neighborhood Psychosocial Hazards scale; APOE ε4, apolipoprotein E epsilon-4; CI, confidence interval
Table 3.
Associations of neighborhood psychosocial hazards (NPH), APOE ε4 and NPH-APOE ε4 interaction with cognitive domain scores.
| Model 1* |
Model 2* |
|||||
|---|---|---|---|---|---|---|
| Cognitive domain | NPH quartile 4 vs. quartiles 1–3 β (95% CI) |
APOE ε4 β (95% CI) |
NPH quartile 4 vs. quartiles 1–3 β (95% CI) |
APOE ε4 β (95% CI) |
Interaction β (95% CI) |
p-value for interaction |
| Language | −0.05 (−0.19, 0.08) | 0.00 (−0.12, 0.11) | −0.04 (−0.16, 0.08) | 0.01 (−0.16, 0.19) | −0.03 (−0.28, 0.22) | 0.80 |
| Processing speed | −0.16 (−0.32, 0.01) | −0.12 (−0.34, 0.09) | −0.02 (−0.21, 0.17) | 0.09 (−0.13, 0.32) | −0.39 (−0.71, −0.06) | 0.02 |
| Eye-hand coordination | −0.14 (−0.27, −0.02) | −0.09 (−0.24, 0.05) | −0.04 (−0.18, 0.10) | 0.07 (−0.16, 0.29) | −0.29 (−0.58, 0.00) | 0.05 |
| Executive function | −0.02 (−0.12, 0.08) | −0.12 (−0.24, −0.01) | 0.13 (0.03, 0.23) | 0.10 (−0.06, 0.27) | −0.41 (−0.65, −0.18) | < 0.001 |
| Verbal memory & learning | −0.05 (−0.22, 0.12) | −0.12 (−0.31, 0.07) | −0.04 (−0.21, 0.13) | −0.11 (−0.38, 0.17) | −0.02 (−0.40, 0.36) | 0.91 |
| Visual memory | −0.05 (−0.18, 0.08) | −0.10 (−0.30, 0.09) | −0.03 (−0.22, 0.15) | −0.08 (−0.24, 0.08) | −0.04 (−0.38, 0.30) | 0.81 |
| Visuoconstruction | −0.15 (−0.32, 0.03) | −0.20 (−0.35, −0.06) | −0.13 (−0.32, 0.07) | −0.17 (−0.40, 0.05) | −0.05 (−0.33, 0.22) | 0.70 |
Abbreviations: NPH, Neighborhood Psychosocial Hazards scale; APOE ε4, apolipoprotein E epsilon-4; CI, confidence interval
All models are generalized estimating equations regression adjusted for age, sex, race/ethnicity, education, household wealth, and testing technician. In addition, these models implement propensity score weights, which adjust for age, sex, race/ethnicity, number of siblings, number of children alive, marital status, household wealth, educational attainment (respondent and spouse), public/private high school graduated from (respondent and spouse), retirement status, job control during primary lifetime occupation, smoking, and housing variables including type of housing, ownership, and length of tenure.
NPH x APOE interaction
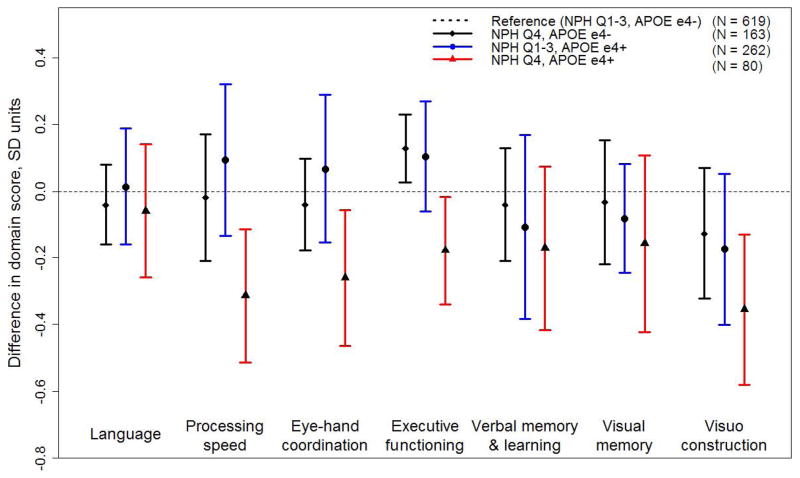
In adjusted models with a high NPH x APOE ε4 cross-product term (model 2, Table 3), interactions were observed for processing speed (−0.39 SD units; p = 0.02) and executive functioning (−0.41 SD units; p < 0.001). Additionally, there was evidence of a borderline association with eye-hand coordination (−0.29 SD units; p = 0.05). The Figure displays adjusted mean differences by NPH and APOE groups. Compared with APOE ε4− persons in the less hazardous neighborhoods (reference group), ε4− persons in the most hazardous neighborhoods did not score worse in any of the domains. APOEε4+ persons in less hazardous neighborhoods also did not perform worse than the reference group in any domain. However, APOE ε4+ persons living in the most hazardous neighborhoods performed significantly worse than the reference group in processing speed (−0.31 SD units; 95% confidence interval: −0.51, −0.11), eye-hand coordination (−0.26 SD units;95% confidence interval: −0.47, −0.06), executive functioning (−0.18 SD units; 95% confidence interval: −0.34, −0.02) and visuoconstruction (−0.36 SD units; 95% confidence interval: −0.58, −0.13).
Sensitivity analyses
We conducted stratified analyses for white and African American individuals. The magnitudes of coefficients were approximately similar for each race/ethnicity and to the original results, although power reductions due to smaller sample sizes altered our significance testing. Similarly, subset analyses for the 74% of study participants who had remained in their homes for more than 10 years did not produce noticeably different results from the overall sample. We redefined exposure to high NPH at the 60th, 70th, and 80th percentiles and found that results were not sensitive to choice of cut point. Finally, we performed the same analyses without propensity score adjustment and found results in congruence with our propensity score-derived results (sensitivity analysis results not shown).
DISCUSSION
Our findings provide evidence that among persons with the APOE ε4 allele, cognitive performance in processing speed and executive function was significantly worse for persons residing in neighborhoods with higher levels of psychosocial hazards (p < 0.05), with additional suggestive evidence for eye-hand coordination (p = 0.05). This is consistent with studies suggesting that psychosocial hazards in the environment may create conditions of differential vulnerability to the impact of individual-level risk factors.34–38 To judge the importance of the reported interactions, it may be useful to consider that a meta-analysis of APOE ε4 main effects showed small associations of less than −0.10 SD units.5 Our estimates for APOE main effects averaged-0.11 SD units across all domains (the largest association being in visuoconstruction at −0.20 SD units), while estimates for an increase of 1 year in age ranged from −0.01 to −0.04 SD units. In comparison, analysis of NPH and APOE group differences indicated that persons with both high NPH and APOE ε4 performed from −0.18 SD units worse in executive functioning to −0.36 SD units worse in visuoconstruction, compared with those without either. While clinical relevance is difficult to establish for cognitive assessments in overall healthy populations, prior studies indicate that even small decrements in performance predict subsequent dementia risk.39
A substantial problem in observational studies of residential contexts and health outcomes is that social stratification along class and socioeconomic lines places different types of persons in different neighborhoods thereby confounding observed associations. In this complex causal scenario, propensity score techniques are, in principle, methodologically more sound than standard regression alone. We attempted to address social stratification using propensity score methods that adjusted for sociodemographic and housing variables for both the respondent and spouse, as suggested by research on residential mobility and housing decisions. The propensity score methods weighted comparison groups such that they were distributionally similar in regard to the covariates that determined the propensity score. In addition, propensity score weighting allowed for the adjustment of a large number of covariates that otherwise may have been inappropriate for regression adjustment.40 While propensity score adjustment is excellent for handling observed confounders, the only way to protect against unobserved confounding is to conduct a randomized study. However, given the ethical and logistical problems involved with randomly assigning participants to neighborhoods, observational studies are the only reasonable way to study neighborhood effects.
Our results have several limitations. First, participants were not clinically assessed for dementia. The prevalence of dementia is less than 1% at ages 60–64 and less than 2% at ages 65–69.41 Because the sample comprised community-dwelling participants (mean age 59.3 years) randomly sampled from the general population, the prevalence of dementia is unlikely to be high enough to influence our results. Second, the age range of the sample was 50 to 70 years and thus results may not generalize to other ages. However, we observed significant decrements in cognitive performance even in a relatively younger community cohort for which cognitive deficits may not be as readily evident. In an older sample (e.g., 70 to 90 years of age) that is expected to have a higher proportion of non-cognitively intact persons, cognitive differences between NPH and APOE genotype groups may be even larger. Third, it is possible that the NPH Scale does not adequately capture the intensity of psychosocial hazards that participants experience. Since the NPH scale is a continuous measure of multiple neighborhood aspects that are distinctly perceptible and of relevance to neighborhood residents, a higher NPH score should reflect greater intensity of exposure. In addition, because the NPH scale is based on objective measurement at the place level, independent of study participants, the potential for same-source bias is reduced. Finally, because our analysis was cross-sectional, we cannot be certain about the temporal relations of NPH and cognitive function. However, in the subset of study participants who had resided in their homes for greater than 10 years (74%), results did not substantively differ. This suggests that our results were not due to persons with pre-existing cognitive impairment moving into psychosocially hazardous neighborhoods.
One interesting finding of our study is that unadjusted associations between NPH and cognition were strong and in the anticipated direction but were severely attenuated on adjustment. This indicates the possibility of substantial confounding by individual covariates or by selection processes that result in non-random assignment to neighborhood “treatments.” Another possibility is that we have introduced more bias through adjustment of mediators on the causal pathway. If, for example, current residence in psychosocially hazardous neighborhoods is a marker for lifelong occupancy in such neighborhoods, then it is plausible that neighborhood conditions are antecedent to, and play a formative role in determining an individual’s sociodemographic (income, education and wealth), behavioral (e.g., diet, physical activity), and health (e.g., obesity) risk profiles. Also, substantial race/ethnic differences in cognitive performance are seen throughout the life course. To the extent race/ethnicity is a proxy for unmeasured factors in the environment (such as higher exposure to neurotoxicants, long-term exposure to racial discrimination), adjustment for race/ethnicity would lead to downward bias in the true associations. Observational data are inadequate to determine whether the large magnitude of attenuation in our associations is the result of confounding bias or over adjustment for variables on the causal pathway between social context and cognitive outcomes.
Diverse pathophysiological mechanisms may under lie APOE main effects on cognitive function, including glucose hypometabolism,42 promotion of amyloid plaque formation, 43 and impairment of synaptic plasticity.44 The adverse cognitive effects of APOE interaction with psychosocial hazards may be due to apoE isoform-specific reparative activity. Brain injury stimulates neuronal apoE production 45, 46 and apoE, because of its roles in lipid uptake and redistribution, facilitates cell proliferation, membrane repair, and remyelination.3 However, of the apoE isoforms, ε4 appears to possess a diminished reparative capacity, interferes with the beneficial function of the other isoforms,47 and promotes cell death after excitotoxic injury when neuronally expressed.48 By impairing neuronal repair mechanisms, the ε4 isoform can synergistically enhance damage due to neurological insults.49 Therefore, persons with the ε4 allele may be more vulnerable to deleterious effects of non-specific risk factors. The biological plausibility of this notion is supported by studies showing that the ε4 allele interacts with a range of diverse neurological insults, including lead toxicity, cardiovascular disease, and head injury, to adversely impact cognitive function.50–53
Animal studies have shown adverse neuroanatomical and neurophysiological effects attributable to psychosocial hazards.10–15 Grootendorst et al. reported that the effects of environmental stressors and corticosterone on cognitive performance in mice were dependent on APOE genotype.15, 54 In humans, markers of exposure to psychosocial hazards such as self-reported stress levels and cortisol levels, have also been shown to interact with APOE.16, 17 Savitz et al. found that childhood sexual abuse and APOE ε4 genotype interacted to reduce memory performance in a sample of subjects with mood disorder, supporting the idea that a harmful psychosocial environment can interact with genotype to influence cognition.55
Evidence of decreased performance in persons with the ε4 allele living in psychosocially hazardous neighborhoods was found in multiple domains, including processing speed, eye-hand coordination, executive functioning, and visuoconstruction. Domain-specific associations are difficult to interpret since neuropsychological tests may tap into more than 1 domain. For example, while executive functioning involves complex tasks such as decision-making and abstract reasoning, lower scores on the timed executive function tests might be due to slower processing speed. Compounding the difficulty of interpretation is that the mapping of tests to specific brain structures cannot be achieved with high reliability. It is noteworthy but unsurprising that differences in non-memory domains were observed. A meta-analysis found that APOE genotype is associated with decrements in global cognition, episodic memory, and executive functioning.5 While the associations of psychosocial hazards with hippocampally-dependent functions are well-known, associations with other regional functions, particularly those of the prefrontal cortex such as executive function, have also been reported.56–58 In addition, glucocorticoid receptors are well-distributed throughout the brain, including the hippocampus, neocortex, and cerebellum.59 Therefore, it is not surprising we observed decreased cognitive performance in a variety of domains.
In summary, using a measure of NPH based on theory and assessed independently from study participants, we found evidence that APOE genotype modified relations of NPH with cognitive function in older adults. Our findings suggest that for genetically vulnerable persons, a psychosocially hazardous neighborhood environment may be detrimental for cognitive function in aging.
Figure 1.
Adjusted mean differences with 95% confidence interval (CI) in cognitive domain scores by level of neighborhood psychosocial hazards (NPH) and apolipoprotein ε4 (APOE) genotype in the Baltimore Memory Study (n=1124). Generalized estimating equations are adjusted for age, sex, race/ethnicity, education, wealth, testing technician, with propensity score weights that adjust for adjust for age, sex, race/ethnicity, number of siblings, number of children alive, marital status, household wealth, educational attainment (respondent and spouse), public/private high school graduated from (respondent and spouse), retirement status, job control during primary lifetime occupation, smoking, and housing variables including type of housing, ownership, and length of tenure.
Acknowledgments
Grant Support: This work was supported by research grants from the National Institutes of Health (R01 AG19604) and the Johns Hopkins Bayview Medical Center General Clinical Research Center (MO1 RR02719).
We wish to thank Anne Jedlicka for assistance with genotyping. Brian Lee had full access to all of the data in the study and takes responsibility for the integrity of thedata and the accuracy of the data analysis.
List of abbreviations
- APOE
apolipoprotein E
- NPH
neighborhood psychosocial hazards
- SD
standard deviation
- CI
confidence interval
Footnotes
All work was performed at the Johns Hopkins Bloomberg School of Public Health, 615 N. Wolfe St., Baltimore, MD 21205.
Disclosure: The authors have reported no conflicts of interest.
Presented in part at the 2008 annual meeting of the Society for Epidemiologic Research, Chicago, IL, 25 June 2008.
WORKS CITED
- 1.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993 Aug 13;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 2.Tang MX, Stern Y, Marder K, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, white, and Hispanics. Jama. 1998 Mar 11;279(10):751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y. Apolipoprotein E and Alzheimer disease. Neurology. 2006 Jan 24;66(2 Suppl 1):S79–85. doi: 10.1212/01.wnl.0000192102.41141.9e. [DOI] [PubMed] [Google Scholar]
- 4.Savitz J, Solms M, Ramesar R. Apolipoprotein E variants and cognition in healthy individuals: a critical opinion. Brain Res Rev. 2006 Jun;51(1):125–135. doi: 10.1016/j.brainresrev.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Small BJ, Rosnick CB, Fratiglioni L, Backman L. Apolipoprotein E and cognitive performance: a meta-analysis. Psychol Aging. 2004 Dec;19(4):592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- 6.McEwen BS. Effects of adverse experiences for brain structure and function. Biol Psychiatry. 2000 Oct 15;48(8):721–731. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- 7.Sapolsky RM. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp Gerontol. 1999 Sep;34(6):721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- 8.McEwen BS. From molecules to mind. Stress, individual differences, and the social environment. Ann N Y Acad Sci. 2001 May;935:42–49. [PubMed] [Google Scholar]
- 9.Glass TA, Rasmussen MD, Schwartz BS. Neighborhoods and obesity in older adults: The Baltimore Memory Study. Am J Prev Med. 2006;31(6):455–463. doi: 10.1016/j.amepre.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran TT, Srivareerat M, Alkadhi KA. Chronic psychosocial stress triggers cognitive impairment in a novel at-risk model of Alzheimer’s disease. Neurobiol Dis. 2010 Mar;37(3):756–763. doi: 10.1016/j.nbd.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Srivareerat M, Tran TT, Alzoubi KH, Alkadhi KA. Chronic Psychosocial Stress Exacerbates Impairment of Cognition and Long-Term Potentiation in beta-Amyloid Rat Model of Alzheimer’s Disease. Biol Psychiatry. 2008 Oct 10; doi: 10.1016/j.biopsych.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 12.McKittrick CR, Magarinos AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000 May;36(2):85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 13.Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989 May;9(5):1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magarinos AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996 May 15;16(10):3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grootendorst J, de Kloet ER, Vossen C, Dalm S, Oitzl MS. Repeated exposure to rats has persistent genotype-dependent effects on learning and locomotor activity of apolipoprotein E knockout and C57Bl/6 mice. Behav Brain Res. 2001 Nov 1;125(1–2):249–259. doi: 10.1016/s0166-4328(01)00294-7. [DOI] [PubMed] [Google Scholar]
- 16.Peavy GM, Lange KL, Salmon DP, et al. The Effects of Prolonged Stress and APOE Genotype on Memory and Cortisol in Older Adults. Biol Psychiatry. 2007 Jun 1; doi: 10.1016/j.biopsych.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee BK, Glass TA, Wand GS, et al. Apolipoprotein E Genotype, Cortisol, and Cognitive Function in Community-Dwelling Older Adults. Am J Psychiatry. 2008 Jul 1; doi: 10.1176/appi.ajp.2008.07091532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz BS, Glass TA, Bolla KI, et al. Disparities in cognitive functioning by race/ethnicity in the Baltimore Memory Study. Environ Health Perspect. 2004 Mar;112(3):314–320. doi: 10.1289/ehp.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDowell I, Newell C. Measuring Health: A Guide to Rating Scales and Questionnaires. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 20.Grieco E, Cassidy R. Census 2000 Brief. Washington, DC: US Census Bureau; 2001. Overview of race and Hispanic origin. [Google Scholar]
- 21.Schafer JH, Glass TA, Bolla KI, Mintz M, Jedlicka AE, Schwartz BS. Homocysteine and cognitive function in a population-based study of older adults. J Am Geriatr Soc. 2005 Mar;53(3):381–388. doi: 10.1111/j.1532-5415.2005.53153.x. [DOI] [PubMed] [Google Scholar]
- 22.Shih RA, Glass TA, Bandeen-Roche K, et al. Environmental lead exposure and cognitive function in community-dwelling older adults. Neurology. 2006 Nov 14;67(9):1556–1562. doi: 10.1212/01.wnl.0000239836.26142.c5. [DOI] [PubMed] [Google Scholar]
- 23.Oakes JM. The (mis)estimation of neighborhood effects: causal inference for a practicable social epidemiology. Soc Sci Med. 2004 May;58(10):1929–1952. doi: 10.1016/j.socscimed.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 25.Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006 Aug;63(8):898–906. doi: 10.1001/archpsyc.63.8.898. [DOI] [PubMed] [Google Scholar]
- 26.Lee BK, Lessler J, Stuart EA. Improving propensity score weighting using machine learning. Stat Med. 2009 Dec 3; doi: 10.1002/sim.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCaffrey DF, Ridgeway G, Morral AR. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods. 2004 Dec;9(4):403–425. doi: 10.1037/1082-989X.9.4.403. [DOI] [PubMed] [Google Scholar]
- 28.Harding DJ. Counterfactual Models of Neighborhood Effects: The Effect of Neighborhood Poverty on Dropping Out and Teenage Pregnancy. American Journal of Sociology. 2003 Nov;109(3):676–719. [Google Scholar]
- 29.South SJ, Crowder KD. Escaping distressed neighborhoods: individual, community, and metropolitan influences. American Journal of Sociology. 1997;(102):1040–1084. [Google Scholar]
- 30.South SJ, Deane GD. Race and residential mobility: individual determinants and structural constraints. Social Forces. 1993;(72):147–167. [Google Scholar]
- 31.Kang J, Schafer J. Demystifying double robustness:a comparison of alternative strategies for estimating a population mean from incomplete data. Statistical Science. 2007;22:523–580. doi: 10.1214/07-STS227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang IA, Llewellyn DJ, Langa KM, Wallace RB, Huppert FA, Melzer D. Neighborhood deprivation, individual socioeconomic status, and cognitive function in older people: analyses from the English Longitudinal Study of Ageing. J Am Geriatr Soc. 2008 Feb;56(2):191–198. doi: 10.1111/j.1532-5415.2007.01557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R Development Core Team. R. A language and environment for statistical computing. 2008. [Google Scholar]
- 34.Gee GC, Payne-Sturges DC. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environ Health Perspect. 2004 Dec;112(17):1645–1653. doi: 10.1289/ehp.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gravlee CC. How race becomes biology: embodiment of social inequality. Am J Phys Anthropol. 2009 May;139(1):47–57. doi: 10.1002/ajpa.20983. [DOI] [PubMed] [Google Scholar]
- 36.Rhodes T, Lilly R, Fernandez C, et al. Risk factors associated with drug use: the importance of ‘risk environment’. Drugs-Education Prevention and Policy. 2003 Nov;10(4):303–329. [Google Scholar]
- 37.Bellinger DC. Effect modification in epidemiologic studies of low-level neurotoxicant exposures and health outcomes. Neurotoxicol Teratol. 2000 Jan-Feb;22(1):133–140. doi: 10.1016/s0892-0362(99)00053-7. [DOI] [PubMed] [Google Scholar]
- 38.Weiss B, Bellinger DC. Social ecology of children’s vulnerability to environmental pollutants. Environ Health Perspect. 2006 Oct;114(10):1479–1485. doi: 10.1289/ehp.9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elias MF, Beiser A, Wolf PA, Au R, White RF, D’Agostino RB. The preclinical phase of alzheimer disease: A 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000 Jun;57(6):808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 40.Dehejia R, Wahba S. Causal effects in non-experimental studies: re-evaluating the evaluation of training programs. Journal of the American Statistical Association. 1999;94:1053–1062. [Google Scholar]
- 41.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005 Dec 17;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reiman EM, Chen K, Alexander GE, et al. Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci U S A. 2005 Jun 7;102(23):8299–8302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holtzman DM, Fagan AM, Mackey B, et al. Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer’s disease model. Ann Neurol. 2000 Jun;47(6):739–747. [PubMed] [Google Scholar]
- 44.Chen Y, Durakoglugil MS, Xian X, Herz J. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):12011–12016. doi: 10.1073/pnas.0914984107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris FM, Tesseur I, Brecht WJ, et al. Astroglial regulation of apolipoprotein E expression in neuronal cells. Implications for Alzheimer’s disease. J Biol Chem. 2004 Jan 30;279(5):3862–3868. doi: 10.1074/jbc.M309475200. [DOI] [PubMed] [Google Scholar]
- 46.Boschert U, Merlo-Pich E, Higgins G, Roses AD, Catsicas S. Apolipoprotein E expression by neurons surviving excitotoxic stress. Neurobiol Dis. 1999 Dec;6(6):508–514. doi: 10.1006/nbdi.1999.0251. [DOI] [PubMed] [Google Scholar]
- 47.Buttini M, Akeefe H, Lin C, et al. Dominant negative effects of apolipoprotein E4 revealed in transgenic models of neurodegenerative disease. Neuroscience. 2000;97(2):207–210. doi: 10.1016/s0306-4522(00)00069-5. [DOI] [PubMed] [Google Scholar]
- 48.Buttini M, Masliah E, Yu GQ, et al. Cellular source of apolipoprotein E4 determines neuronal susceptibility to excitotoxic injury in transgenic mice. Am J Pathol. 2010 Aug;177(2):563–569. doi: 10.2353/ajpath.2010.090973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horsburgh K, McColl B, White F, McCulloch J. Apolipoprotein E influences neuronal death and repair. International Congress Series. 2003;1252:171–178. [Google Scholar]
- 50.Blair CK, Folsom AR, Knopman DS, Bray MS, Mosley TH, Boerwinkle E. APOE genotype and cognitive decline in a middle-aged cohort. Neurology. 2005 Jan 25;64(2):268–276. doi: 10.1212/01.WNL.0000149643.91367.8A. [DOI] [PubMed] [Google Scholar]
- 51.Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of APOE epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. Jama. 1999 Jul 7;282(1):40–46. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- 52.Stewart WF, Schwartz BS, Simon D, Kelsey K, Todd AC. ApoE genotype, past adult lead exposure, and neurobehavioral function. Environ Health Perspect. 2002 May;110(5):501–505. doi: 10.1289/ehp.02110501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teasdale GM, Murray GD, Nicoll JA. The association between APOE epsilon4, age and outcome after head injury: a prospective cohort study. Brain. 2005 Nov;128(Pt 11):2556–2561. doi: 10.1093/brain/awh595. [DOI] [PubMed] [Google Scholar]
- 54.Grootendorst J, Kempes MM, Lucassen PJ, Dalm S, de Kloet ER, Oitzl MS. Differential effect of corticosterone on spatial learning abilities in apolipoprotein E knockout and C57BL/6J mice. Brain Res. 2002 Oct 25;953(1–2):281–285. doi: 10.1016/s0006-8993(02)03399-1. [DOI] [PubMed] [Google Scholar]
- 55.Savitz J, van der Merwe L, Stein DJ, Solms M, Ramesar R. Genotype and childhood sexual trauma moderate neurocognitive performance: a possible role for brain-derived neurotrophic factor and apolipoprotein E variants. Biol Psychiatry. 2007 Sep 1;62(5):391–399. doi: 10.1016/j.biopsych.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 56.Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci U S A. 2009 Jan 20;106(3):912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liston C, Miller MM, Goldwater DS, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006 Jul 26;26(30):7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orem DM, Petrac DC, Bedwell JS. Chronic self-perceived stress and set-shifting performance in undergraduate students. Stress. 2008;11(1):73–78. doi: 10.1080/10253890701535103. [DOI] [PubMed] [Google Scholar]
- 59.Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci. 2000 Jun 15;20(12):4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]