Abstract
Concurrent with US Food and Drug Administration (FDA) approval of the first therapeutic cancer vaccine, a wide spectrum of other cancer vaccine platforms that target a diverse range of tumor-associated antigens is currently being evaluated in randomized phase II and phase III trials. The profound influence of the tumor microenvironment and other immunosuppressive entities, however, can limit the effectiveness of these vaccines. Numerous strategies are currently being evaluated both preclinically and clinically to counteract these immunosuppressive entities, including the combined use of vaccines with immune checkpoint inhibitors, certain chemotherapeutics, small-molecule targeted therapies, and radiation. The potential influence of the appropriate patient population and clinical trial endpoint in vaccine therapy studies is discussed, as well as the potential importance of biomarkers in future directions of this field.
This article reviews the current state of the design, development, and clinical applications of therapeutic cancer vaccines. The numerous previous failures of cancer vaccines may be attributed to the fact that not all vaccines are created equal (there has been a clear evolution in vaccine design) and/or to issues in clinical trial design and patient population. This article covers the diversity of therapeutic cancer vaccine platforms and the wide range of tumor-associated antigens being targeted; the current state of clinical trials that test therapeutic cancer vaccines, including the results of recent randomized multicenter phase II and phase III studies; issues to be considered in future clinical trials; the profound influence of the tumor microenvironment and immunosuppressive factors in inhibiting optimal vaccine efficacy and potential strategies to combat these phenomena; combinatorial therapies, including the use of cancer vaccines with immune stimulants, inhibitors of immune suppression, certain chemotherapeutic agents, small-molecule targeted therapies, hormones, and radiation; the potential use of biomarkers to select those patients who are most likely to benefit from vaccine therapy; and other future directions in this field. This article is not designed to comprehensively review all preclinical and clinical studies of therapeutic cancer vaccine, but instead, to delineate the factors and concepts that are currently being evaluated to develop vaccines as therapeutics for a wide range of human cancers and cancer stages, either as monotherapies or as combination therapies.
Spectrum of Current Therapeutic Cancer Vaccine Platforms
Many diverse vaccine platforms have now been evaluated in phase II and/or phase III clinical trials, including injection of peptides or proteins in adjuvant, injection of recombinant viruses or other recombinant microorganisms, delivery of killed tumor cells, or delivery of protein- or peptide-activated dendritic cells (DCs) to the patient (Table 1). Each of the 14 platforms in Table 1 has strengths and weaknesses that can be influenced by the particular tumor-associated antigen (TAA) that is targeted, the disease and disease stage that is targeted, the clinical trial endpoint, and whether the vaccine is evaluated in combination with an immune stimulant, an inhibitor of immune suppression, or another mode of cancer therapy.
Table 1.
Spectrum of current vaccine platforms in phase II/III clinical studies*
| Vaccine platform | Example | Cancer type | Selected references |
| Peptides/proteins | |||
| Peptide | gp100 (modified), MUC-1 (Stimuvax), HER2/neu | Melanoma, lung | (1–9) |
| Protein | MAGE-A3, NY-ESO | Melanoma | (10) |
| Antibody | Anti-idiotype | Lymphoma | (11–14) |
| Glycoproteins | sTn-KLH | Melanoma | (15,16) |
| Recombinant vectors | |||
| Poxvirus | rV, rF-PSA-TRICOM (Prostvac) | Prostate | (17–29) |
| Saccharomyces cerevisiae (yeast) | yeast-ras | Pancreatic | (30,31) |
| Listeria | Listeria-mesothelin | Pancreatic | (32) |
| Alpha- and adenoviruses | adeno-CEA, alpha-CEA | Carcinoma | (33) |
| Tumor Cells | |||
| Autologous | adeno-CD40L, colon (BCG) | CLL, colon, melanoma | (34–36) |
| Dendritic cell/autologous tumor cell fusions | Myeloma | (37) | |
| Allogeneic | GVAX (+GM-CSF) | Pancreatic | (38–40) |
| Dendritic cells/APCs | |||
| APC–protein | Sipuleucel-T (PAP-GM-CSF) | Prostate | (41,42) |
| Dendritic cell–peptide | Glioma peptides | Glioma, melanoma | (43–45) |
| Dendritic cells–vector infected | rV, rF-CEA-MUC1-TRICOM (Panvac-DC) | Colorectal | (46,47) |
APC = antigen-presenting cell; BCG = Bacillus Calmette–Guerin adjuvant; CD40L = CD40 ligand; CEA = carcinoembryonic antigen; CLL = chronic lymphocytic leukemia; gp100 = glycoprotein 100; GM-CSF = granulocyte macrophage colony-stimulating factor; MAGE-A3 = melanoma-associated antigen 3; MUC-1 = mucin 1; NY-ESO = New York esophageal carcinoma antigen 1; PAP = prostatic acid phosphatase; PSA = prostate-specific antigen; rF = recombinant fowlpox; rV = recombinant vaccinia; STn-KLH = sialyl-Tn–keyhole limpet hemocyanin.
DCs are the most potent antigen-presenting cells (APCs) (43). Numerous phase II studies have now evaluated the use of peptide- or protein-pulsed, or viral vector–infected, DCs to treat patients with prostate cancer, colorectal cancer, melanoma, glioma, and other cancers (Tables 1 and 2) [eg, references (44,53)]. The Sipuleucel-T vaccine (41), which was recently approved by the US Food and Drug Administration (FDA) for the therapy of asymptomatic metastatic castrate-resistant prostate cancer (mCRPC), consists of APCs from peripheral blood mononuclear cells (PBMCs) that have been incubated with prostatic acid phosphatase (PAP, a prostate antigen) fused to granulocyte macrophage colony-stimulating factor (GM-CSF). This vaccine regimen consists of leukaphereses to purify PBMCs from the patient and processing in a central facility where the PAP fusion protein is added to the APCs; these cells are then reinfused to the patient for the purpose of conferring immunity; this process is repeated three times at biweekly intervals. One drawback of DC and/or APC platforms is that they require leukapheresis(es) and cell culture processing of PBMCs, and thus a limited number of vaccinations can be used.
Table 2.
Spectrum of current and potential therapeutic cancer vaccine targets*
| Target type | Examples | Selected references |
| Oncoprotein | point mutated: ras, B-raf, frame shift mutations, undefined unique tumor mutations; HER2/neu, MUC-1 C-terminus, p53 | (1,7,8,48,49) |
| Oncofetal antigen | CEA, MUC-1 | (2–4,19,26) |
| Cancer–testes | MAGE-A3, BAGE, SEREX-defined, NY-ESO | (10,50–52) |
| Tissue lineage | PAP, PSA, gp100, tyrosinase, glioma antigen | (5,6,24,25,27,41,44,53) |
| Stem cell/EMT | Brachyury, SOX-2, OCT-4, TERT, CD44high/CD24lo, CD133+ | (54–62) |
| Viral | HPV, HCV | (63,64) |
| Glycopeptides | STn-KLH | (15,16) |
| Antiangiogenic | VEGF-R | (65,66,67) |
| B-cell lymphoma | Anti-id | (11–14) |
BAGE = B melanoma antigen; CEA = carcinoembryonic antigen; EMT = epithelial–mesenchymal transition; gp100 = glycoprotein 100; HCV = hepatitis C virus; HPV = human papillomavirus; MAGE-A3 = melanoma-associated antigen-A3; MUC-1 = mucin 1; NY-ESO = New York esophageal carcinoma antigen 1; OCT-4 = octamer-binding transcription factor 4; PAP = prostatic acid phosphatase; PSA = prostate-specific antigen; SOX-2 = (sex determining region Y)-box-2; STn-KLH = sialyl-Tn–keyhole limpet hemocyanin; TERT = telomerase reverse transcriptase; VEGF-R = vascular endothelial growth factor receptor.
Vaccines based on peptides from TAAs, which are usually administered in an adjuvant and/or with an immune modulator, are generally cost-effective and have the advantage that the investigator knows exactly which epitope to evaluate in terms of patients’ immune responses (1). However, they also have a potential drawback because they target only one epitope or a few epitopes of the TAA. It is generally believed that for a cancer vaccine to be optimally efficacious, it must induce antigen-specific CD8+ cytolytic T cells (CTLs), which are responsible for tumor cell lysis, and antigen-specific CD4+ “helper” T cells, which provide cytokines to enhance CTL activity. Some polypeptide vaccines potentially contain both CD4 and CD8 epitopes; for example, Stimuvax contains both kinds of epitopes for mucin 1 (MUC-1), which is found as a cell surface proteoglycan associated with several tumor types (2–4). Protein-based vaccines are more costly than peptide-based vaccines, but they usually also contain both CD4 and CD8 epitopes. Many peptide- and protein-based vaccines are used as part of a DC vaccine platform.
Anti-idiotype vaccines are directed against specific antibodies found on the surface of B-lymphoma cells (11–13). They have the advantage of targeting a unique tumor-specific antigen. A disadvantage is that their generation and production are quite labor intensive in that, to date, each anti-idiotype vaccine has been patient specific. However, some researchers have shown that these patient-specific vaccines can be produced in less than a month [reviewed in (12)].
The most evaluated viral-based vectors are from the poxviridae family. They include vaccinia, modified vaccinia strain Ankara (MVA), and the avipoxviruses (fowlpox and canarypox; ALVAC). Poxviruses have the ability to accept large inserts of foreign DNA, and thus can accommodate multiple genes. Intracellular expression of the transgene(s) allows for processing of the tumor antigen by both the class I and class II major histocompatibility complex (MHC) pathways (17). Because poxvirus replication and transcription are restricted to the cytoplasm, there is minimal risk to the patient (or host) of insertional mutagenesis. It has been shown in preclinical studies that when the transgene for a TAA is inserted in vaccinia or MVA, it becomes more immunogenic, most likely because of the Toll-like receptors (TLRs) and other properties of the virus that induce a local inflammatory response. This same property of the non-avian poxviruses, however, leads to virus neutralization by the host immune response and limits their use to one, or at most two, vaccination(s). Recombinant avipoxviruses can be used multiple times; they will induce antiviral immune responses, but they are not neutralizing because their “late” viral coat proteins are not produced in mammalian cells (18–20).
Other viruses can also be used as vectors for TAAs. Alphaviruses such as Venezuelan Equine Encephalitis (VEE) virus are attractive vectors because, once they have infected the host, they replicate RNA in the cytoplasm and express high levels of a transgene (33). Recombinant adenovirus vectors are easy to engineer and have shown utility as vaccines and gene therapy agents (34), but clinical evaluation has been hindered by high levels of preexisting antiviral immunity. Newer variants of adenoviruses are being developed and evaluated that may potentially be less immunogenic.
Bacteria and yeasts have shown some promise as vaccine vectors in preclinical studies and may also serve as vectors for immunizing cancer patients. Heat-killed recombinant Saccharomyces cerevisiae is inherently nonpathogenic, can be easily propagated and purified, and is very stable. Recombinant yeasts have been shown to activate maturation of human DCs and to present both class I and class II epitopes of transgenes (30). Surprisingly, it appears that these vectors can be administered multiple times without eliciting host-neutralizing activity (31). Attenuated recombinant Listeria monocytogenes (Lm) bacteria have also been shown to target DCs, and, like viral and yeast vectors, they stimulate both innate and adaptive immune responses (32).
Although DNA vaccine platforms have shown promise in preclinical studies (65,66), early clinical trials have been disappointing. Their exact mode of action is not known at this time. However, new constructs and methods of administration may enhance their utility.
The use of whole tumor–cell vaccines has the advantage of presenting the patient's immune system with a range of both known and undefined TAAs as immunogens. However, this same property also potentially diminishes the relative level of expression of a particular TAA or group of TAAs and its presentation and processing by APCs. The use of a killed whole tumor–cell vaccine is usually accompanied by an immune stimulant such as GM-CSF, Bacillus Calmette–Guerin adjuvant (BCG), or CD40 ligand (CD40L).
Autologous tumor cell vaccines have a great advantage because they present the unique set of TAAs, such as particular point mutations or fusion gene products, from a given patient's own tumor (35). Because this technology depends on the availability of tumor biopsies, it is feasible for only some tumor types and stages. In one variation of this technique, DCs and autologous tumor cells are fused together before immunization of the patient (37). DC–tumor cell fusions combine the unique properties of whole tumor–cell vaccines with the enhanced antigen-presenting power of DCs.
Alternatively, allogeneic whole tumor–cell vaccines, which typically contain two or three established and characterized human tumor cell lines of a given tumor type, may be used to overcome many logistical limitations of autologous tumor–cell vaccines. The GVAX vaccine platforms (38,39,40), which contain allogeneic pancreatic, prostate, or breast tumor cells, are a testament to the ability to provide such a vaccine for multicenter evaluation.
Spectrum of Vaccine Targets
The validity of a vaccine target will depend on the ability of a tumor cell to process the TAA in the context of MHC for T-cell or for B-cell recognition, the level of expression, the relative specificity of the TAA for tumor vs normal adult tissue, and the degree of inherent “tolerance” to the given TAA (68,69). Common targets include oncoproteins, oncofetal antigens, differentiation-associated proteins, and viral proteins, among others (Table 2). The ideal target is a somatic point mutation that helps to drive the neoplastic process. Clinical trials are underway to test vaccines that target the relatively few RAS mutations found in colorectal and pancreatic cancer. However, large numbers of tumor-associated mutations among the various exons of TP53 make generating the large number of possible mutant p53 vaccines somewhat prohibitive. Similarly, it is difficult to target the wide array of frameshift mutations and unique mutations that occur in individual tumors. Non-mutated oncoproteins that have served as vaccine targets include overexpressed HER2/neu (ERBB2), p53, and the carboxy terminus of MUC-1 (48,49).
Numerous cancer vaccine trials have targeted a class of antigens categorized as oncofetal antigens, such as carcinoembryonic antigen (CEA), underglycosylated MUC-1, tumor-associated glycopeptides (15,16,19,68), and “cancer–testes” antigens defined by serological expression cloning (SEREX) immunodetection such as melanoma-associated antigen (MAGE-A3) and B melanoma antigen (BAGE) (10,50–52,70). These antigens are overexpressed in many tumor types and to a lesser extent in some normal adult tissues. Numerous trials have targeted “tissue lineage” antigens that are overexpressed in tumors and normally expressed in a non-vital organ, such as PAP, prostate-specific antigen (PSA), and the melanoma-associated antigens glycoprotein 100 (gp100) and tyrosinase. The recent FDA approval of the Gardasil vaccine targeting the human papillomavirus (HPV) (63,64,67) for the prevention of cervical cancer renders HPV an attractive target for cervical cancer therapy, as does targeting the hepatitis C virus for liver cancer therapy.
The most provocative potential targets for vaccine therapy are molecules that are associated with cancer “stem cells” and/or the epithelial–mesenchymal transition (EMT) process, both of which are associated with drug resistance. Drivers of EMT are also associated with tumor cell extravasation and intravasation to the metastatic site. Recent studies have described the plasticity of so-called “cancer stem cells” and the similarities between cells undergoing EMT and the acquisition of “stem-like” characteristics (54).
EMT and cancer “stemness” are associated with the proteins (sex determining region Y)-box-2 (SOX-2) and octamer-binding transcription factor 4 (OCT-4) and with carcinoma cells that are CD44high and CD24low and/or are CD133+ (55–59). Each of these gene products is currently being evaluated for immunogenicity in terms of generating human T-cell responses in vitro, but some of them also have a relatively broad range of expression in some normal adult tissues. The T-box transcription factor Brachyury has recently been identified as a major driver of EMT (60). It has been shown to be selectively expressed on both primary and metastatic lesions of several carcinoma types. T-cell epitopes have been identified on the Brachyury protein that have the ability to generate human T cells capable of lysing a range of human carcinoma cells (61). Vaccines are currently being developed to target gene products associated with EMT and cancer cells with stem-like characteristics. Preclinical studies have also shown the potential of vaccines that target molecules involved in tumor angiogenesis, such as the vascular endothelial growth factor receptor (VEGF-R) (65,66).
A potent means to enhance immunogenicity of a self-antigen is by altering specific amino acids of TTAs to develop enhancer agonist epitopes, which are designed to enhance binding either to the MHC or to the T-cell receptor, resulting in higher levels of T-cell responses and/or higher avidity T cells (71–73). For example, the gp100 melanoma vaccine contains an enhancer agonist epitope, the PROSTVAC vaccine contains a PSA enhancer agonist epitope, and the PANVAC vaccine contains enhancer agonist epitopes for both CEA and MUC-1.
Current State of Cancer Vaccine Clinical Trials
Many vaccines were initially tested in patients with metastatic disease who had undergone multiple previous therapies. Clinical studies have now shown that patients will generally respond better to vaccines when they have been treated with fewer previous chemotherapeutic regimens and when a longer time has elapsed since their last chemotherapy (21,22). In many other forms of cancer therapy that kill tumor cells when ample doses of the therapeutic agent are delivered, the limiting factor is usually host toxicity. By contrast, cancer vaccines have demonstrated minimal toxicity, but their success is limited by the number of host-induced effector cells induced by the vaccine vs immunosuppressive entities. Consequently, patients with large tumor burdens would be even less likely to respond to vaccines than to chemotherapy.
Prostate Cancer as a Prototype for Vaccine Therapy
Although the vast majority of previous and ongoing vaccine trials have been conducted in patients with metastatic melanoma, several characteristics render prostate cancer a prototype disease for the evaluation of therapeutic cancer vaccines (23). First, time is often required to generate a sufficient immune response to curtail tumor growth, and prostate cancer is generally an indolent disease that may not lead to metastatic disease or death for over a decade or more. Second, prostate cancer cells express a variety of well-characterized TAAs. Third, the serum marker PSA can be used to identify patients with minimal tumor burden and those responding to therapy. Last, a well-defined nomogram, the Halabi nomogram (74), can be used at presentation of metastatic disease to predict a patient's probable response to standard of care chemotherapy and/or hormone therapy.
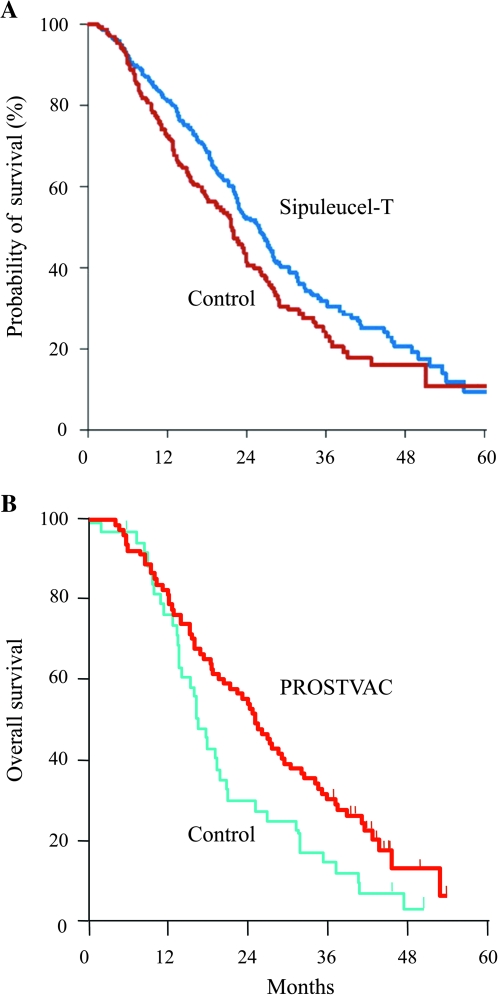
After early clinical trials of the Sipuleucel-T vaccine demonstrated its safety, larger trials were conducted in patients with minimally symptomatic mCRPC. Although a pair of small phase III trials failed to meet their primary endpoint of improved disease progression, there was evidence that Sipuleucel-T prolonged survival in mCRPC (42). A larger phase III trial (41) was then conducted in which more than 500 patients were enrolled and overall survival (OS) was set as the primary endpoint. In this trial, again, no change in time to cancer progression was seen; however, OS was improved in the vaccine arm (25.8 vs 21.7 months; P = .032; Figure 1, A). In April 2010, the FDA approved Sipuleucel-T for the treatment of minimal or non-symptomatic mCRPC.
Figure 1.
Overall survival (OS) in patients with metastatic castrate-resistant prostate cancer using cancer vaccines. A) Results of the primary efficacy analysis of treatment with Spiluleucel-T as compared with placebo control. Sipuleucel-T did improve patients’ OS (hazard ratio for death = 0.78; 95% confidence interval = 0.61 to 0.98; P = .03) [adapted with permission from reference (41)]. The placebo control consisted of cultured antigen-presenting cells (APCs) from leukapheresis, without prostatic acid phosphatase–granulocyte macrophage colony-stimulating factor (PAP–GM-CSF) antigen. Per the trial protocol, the control group could receive cryopreserved APCs with antigen upon disease progression. B) OS of a randomized, placebo-controlled 43-center trial of PROSTVAC vaccine consisting of recombinant vaccinia and fowlpox vectors containing transgenes for prostate-specific antigen, B7.1, intercellular adhesion molecule 1, and lymphocyte function-associated antigen-3 (PSA-TRICOM) in patients with metastatic castrate-resistant prostate cancer; the trial compared PROSTVAC vaccine vs empty vector. There was an OS advantage of 8.5 months (OS = 25.1 vs 16.6 months; P = .006) and a 44% reduction in death in the vaccine arm [adapted with permission from reference (24)].
A second prostate cancer vaccine has also been evaluated in the same population of men with mCRPC. This “off-the-shelf” platform (PROSTVAC) consists of a recombinant vaccinia virus priming vaccination and multiple fowlpox booster vaccinations. Each vector contains transgenes for PSA and three costimulatory molecules (CD80, intercellular adhesion molecule 1, and lymphocyte function–associated antigen-3 that are collectively designated TRICOM) (72,75). A 43-center randomized placebo-controlled phase II trial enrolled 125 minimally symptomatic mCRPC patients (24). Similar to the experience with Sipuleucel-T, treatment with PROSTVAC did not alter time to progression; however, it improved median OS relative to a placebo, the control vector (OS = 25.1 vs 16.6 months, P = .006; Figure 1, B). Over the course of follow-up, 44% fewer patients who received PROSTVAC died compared with the control cohort (hazard ratio [HR] = 0.56) (24). The median OS in a second PROSTVAC single-arm phase II study (25) was 26.6 months, which was similar to that observed in the PROSTVAC arm of the larger PROSTVAC randomized trial.
Another point to consider in the treatment of mCRPC and other cancers is the patients’ quality of life. The Sipleucel-T and PROSTVAC vaccines compared favorably with the chemotherapeutic agents and hormonal therapies that are FDA approved for mCRPC, especially because there were fewer serious adverse events seen with these vaccines than with the other agents (Table 3).
Table 3.
Overall survival in patients with metastatic castrate-resistant prostate cancer who were treated with vaccines or other therapeutic modalities*
| Agent | Type of therapy | Stop treatment AE, % | Improvement in median OS, mo | Hazard ratio | Reduction in death rate, % |
| Docetaxel | Chemotherapy | 11 | 2.4 | 0.76 | 24 |
| Cabazitaxel | Chemotherapy | 18 | 2.4 | 0.70 | 30 |
| Abiraterone | Hormone | 19 | 3.9 | 0.66 | 34 |
| Sipuleucel-T | Vaccine | 1.5 | 4.1 | 0.78 | 22 |
| PROSTVAC† | Vaccine | ∼2 | 8.5 | 0.56 | 44 |
AE = adverse event; OS = overall survival; PROSTVAC = a recombinant vaccinia virus priming vaccination and multiple fowlpox booster vaccinations. Each vector contains transgenes for prostate-specific antigen and three costimulatory molecules (CD80, intercellular adhesion molecule 1, and lymphocyte function–associated antigen 3) that are collectively designated TRICOM.
Results of a 43-center randomized placebo-controlled phase II trial.
Phase III Studies
In addition to Sipuleucel-T, two different vaccine platforms have also recently demonstrated positive results in phase III trials. One phase III trial (5) compared patients with locally advanced stage III or IV melanoma who were treated with a modified gp100 peptide vaccine in Montanide ISA-51 adjuvant plus standard high-dose interleukin 2 (IL-2) vs such patients who were treated with high-dose IL-2 alone (5). The serious adverse events in both groups were consistent with IL-2 therapy. The group that was treated with the gp100 peptide vaccine plus IL-2 demonstrated a statistically significant improvement in objective clinical response rate compared with the group that was treated with IL-2 alone (16% vs 6%, P = .03). Patients treated with the peptide vaccine plus IL-2 had a slightly longer progression-free survival (2.2 vs 1.6 months; P = .008). The median OS was also longer in the vaccine plus IL-2 group than in the IL-2-only group (OS = 17.8 vs 11.1 months; P = .06). This was the first phase III trial to demonstrate a clinical benefit for a peptide vaccine in melanoma. The findings in this trial were not observed in three previous independent phase II clinical trials that were reported together elsewhere (6). Those studies did not detect a benefit for the combination treatment, although the clinical trials were not powered to assess a progression-free endpoint, unlike the larger phase III trial.
A phase III study that used an anti-idiotype vaccine in patients with follicular lymphoma has also shown evidence of patient benefit (11,12). In this study, patients were required to have previously untreated advanced-stage follicular lymphoma; they were treated on-trial with standard chemotherapy before vaccination. Those patients who achieved a complete remission were randomly assigned to receive Id–keyhole limpet hemocyanin (KLH) plus GM-CSF vs unconjugated KLH plus GM-CSF as a control. After a median follow-up of 56.6 months, the median time to relapse for the Id-KLH plus GM-CSF arm was 44.2 months vs 30.6 months for the KLH plus GM-CSF control arm, suggesting a benefit associated with use of this vaccine (P = .045; HR = 1.6). These results were in contrast to the negative results observed in two other phase III studies that have used recombinant idiotype vaccine for follicular lymphoma (12,14). One of several key differences in the positive study compared with the two negative phase III trials of related idiotype vaccine products was the low tumor burden required for all vaccinated patients on the positive study, although other issues may have contributed to survival outcomes (11–13).
Other vaccines have shown mixed results in phase III trials. For example, a phase III trial (35) using autologous tumor cells plus BCG showed a survival advantage in patients with Dukes stage B2-C3 colon cancer (33.3% deaths in the vaccine group compared with 56.5% in the control group; P = .039) and was ineffective in Dukes C and D patients.
There are several ongoing phase III trials employing different vaccine platforms in a range of human cancers. These include the use of Stimuvax, a vaccine targeting liposomal MUC-1 peptide, in non–small cell lung cancer (NSCLC) (2,76); Lucanix, an allogeneic whole tumor–cell vaccine containing a transforming growth factor-beta 2 (TGF-β2) antisense transgene, in NSCLC (77); and vaccination with the MAGE-A3 protein, in stage III melanoma and in NSCLC (78). The PROSTVAC phase III trial was initiated in 2011 in patients with mCRPC. There are also numerous ongoing randomized phase II studies that employ most of the vaccine platforms noted in Table 1.
It is emphasized that in virtually all of the therapeutic cancer vaccine trials reported to date, there have been extremely low levels of toxicity—mostly limited to grade I and II levels. Moreover, although many of the target TAAs are also expressed at some levels in selected normal adult tissues, virtually no evidence of autoimmunity has been observed, with the exception of vitiligo resulting from the administration of some melanoma vaccines (36,45,79).
Issues in Vaccine Clinical Trial Design
Recent randomized phase II and III vaccine trials have revealed two major issues to be considered in clinical trial design: appropriate patient population and clinical trial endpoint. As described above, patients who have received fewer regimens of chemotherapy and who have had a longer time since their last chemotherapy appear to generally respond better to vaccines. There are now several examples of greater vaccine efficacy in patients with low grade or more indolent disease.
A clinical trial employing the PANVAC vaccine platform (ie, sequential administration of recombinant vaccinia and recombinant fowlpox vectors delivering the TAAs CEA, MUC-1, and TRICOM) in colorectal cancer patients showed some evidence of patient benefit (26), but no evidence of clinical benefit in patients with large volume liver metastases. These findings are in contrast to recent results from a trial to evaluate the same vaccine in colorectal cancer patients following metastasectomy (surgical removal) of liver or lung metastases. In this multicenter trial (46,47), 74 patients who had no evidence of disease after resection and completion of their physician-determined perioperative chemotherapy were vaccinated with PANVAC (ie, with vaccine alone or with vaccine-modified DCs). Data from a prospectively registered, comparable contemporary control group of colorectal cancer patients who had undergone metastasectomy were also available (46,47). The 2-year relapse-free survival was similar in all groups: 50% for the DC-PANVAC group, 56% for the PANVAC group, and 55% for the contemporary control group. However, the 2-year OS was 95% for the vaccinated group and 75% for the contemporary control group; after approximately 40 months of follow-up, 67 of 74 (90%) of the vaccinated patients survived vs approximately 47% OS in the contemporary control group; the data for 3- to 5-year survival data of colorectal cancer patients after metastasectomy in five other trials ranges from 28% to 58% (80–85). A randomized trial is necessary to confirm these results. It is of interest, however, that this is yet another example of a vaccine trial that shows little or no evidence of an improvement in relapse-free survival, yet has an apparent benefit in OS (69).
Another example of the advantage of the use of vaccines in patients with more indolent disease is seen in the use of the Halabi nomogram in patients presenting with mCRPC (74). This nomogram was developed to predict survival using data from more than 1100 mCRPC patients who were treated with chemotherapy and hormone therapy. When this patient population was treated on trial with docetaxel, the standard of care, the nomogram accurately predicted survival both for patients predicted to live less than 18 months after therapy was initiated and for those who were predicted to live longer than 18 months (Table 4) (25). In a contemporary trial at the same institution in which the same patient population was treated with the PROSTVAC vaccine, the Halabi nomogram accurately predicted survival for those patients with a Halabi-predicted survival (HPS) of less than 18 months. However, the actual survival for patients with more indolent disease far exceeded that predicted [HPS of 20.9 months vs actual survival >37.3 months; see reference (25) and Table 4].
Table 4.
Predicted survival of patients with metastatic castrate-resistant prostate cancer using the Halabi nomogram vs actual survival*
| Docetaxel therapy (n = 22) |
PROSTVAC (n = 32) |
|||||
| All patients | Patients with HPS <18 mo | Patients with HPS ≥18 mo | All patients | Patients with HPS <18 mo | Patients with HPS ≥18 mo | |
| Predicted survival by Halabi score, mo | 16.5 | 13.0 | 21.0 | 17.4 | 12.3 | 20.9 |
| Actual median overall survival, mo | 15.5 | 15.4 | 16.9 | 26.6 | 14.6 | Not reached (8 of 15 patients alive at 37.3 mo) |
| Difference, mo | −1.0 | 2.4 | −4.1 | 9.2 | 2.3 | ≥16.4 |
| Patients surviving longer than predicted by Halabi nomogram | 11 (50%) of 22 | 8 (62%) of 13 | 3 (33%) of 9 | 22 (69%) of 32 | 10 (59%) of 17 | 12 (80%) of 15† |
HPS = Halabi nomogram–predicted survival; PROSTVAC (also known as PSA-TRICOM) = prostate-specific antigen, CD80, intercellular adhesion molecule 1 and lymphocyte function-associated antigen 3. Adapted from (25) with permission.
P = .035.
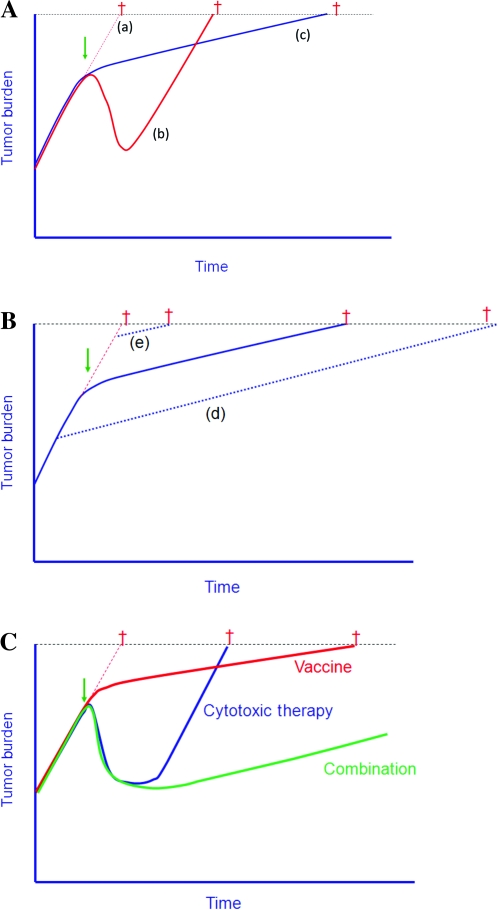
In patients who are treated with traditional cytotoxic agents, it is widely believed that improved time to progression is a prerequisite for improved OS. A recent study (86) evaluated tumor regression and growth rates in four chemotherapy trials and one vaccine trial in patients with mCRPC. Figure 2 illustrates the growth rate constants defined in that study. Cytotoxic agents affect the tumor only during the period of administration; soon after the drug is discontinued, because of drug resistance or toxicity, antitumor activity ceases and the growth rate of the tumor increases (Figure 2, A, line b). With vaccine therapy, the mechanism of action and kinetics of clinical response appear to be very different (86). Therapeutic vaccines do not directly target the tumor; rather, they target the immune system. Immune responses often take time to develop and can potentially be enhanced by continued booster vaccinations; any resulting tumor–cell lysis can lead to cross-priming of additional TAAs, thus broadening the immune repertoire (a phenomenon known as antigen cascade or epitope spreading). This broader, and perhaps more relevant, immune response may also take some time to develop. Although a vaccine may not induce any substantial reduction in tumor burden, vaccines as monotherapy have the potential to apply antitumor activity over a long period, resulting in a slower tumor growth rate (Figure 2, A, line c). This deceleration in growth rate may continue for months or years and, more importantly, through subsequent therapies. This process can thus lead to clinically significant improved OS, often with little or no difference in time to progression and a low rate of, or lack of, objective response [Figure 2, A, line c; reference (69)]. Thus, treating patients with a vaccine when they have a lower tumor burden, as compared with a greater tumor burden, may result in far better outcomes (Figure 2, B, line d vs line e). It is hypothesized that the combined use of vaccine and cytotoxic therapy (Figure 2, C) may result in both tumor regression (via the cytotoxic therapy) and reduced tumor growth rate (via vaccine therapy) (86,87,88). These concepts will be discussed below. Thus, early clinical trials with vaccine may have been terminated prematurely with the observance of tumor progression before sufficient vaccine boosts could be administered. This phenomenon has actually led to modifications in how vaccine clinical trials are now designed and to “new immune response criteria” for immunotherapy (89).
Figure 2.
Tumor growth rates following chemotherapy vs vaccine therapy [adapted from data in references (86–88)]. A) Average tumor growth rates and time to death in patients with metastatic prostate cancer, from five clinical trials [four with chemotherapy and one with PROSTVAC vaccine (also known as PSA-TRICOM)]. Growth rate of tumor if no therapy is initiated (line a). With the use of chemotherapy, there was an initial tumor reduction, but the growth rate of tumors at relapse was similar to the initial tumor growth rate before therapy (line b). With PROSTVAC, there was a reduction in tumor growth rate following vaccine therapy (line c). Thus, for patients who received vaccine therapy with little if any tumor reduction (among whom there was virtually no increase in time to progression), an increase in overall survival was observed. Dagger denotes time to death. B) This phenomenon could potentially be enhanced if vaccine therapy is initiated earlier in disease progression or in patients with low tumor burden metastatic disease (line d) but would have minimal effect in patients with large tumor burden (line e). C) Predictions of enhanced overall survival if patients are treated with both vaccination and chemotherapy.
In some ways, our progress has been paralyzed by the insistence by some on the paradigm that only after tumor lesions are reduced using classic Response Evaluation Criteria in Solid Tumors (RECIST criteria) (90) can a therapeutic modality be considered beneficial. Indeed, randomized clinical studies with small-molecule targeted therapeutics, as well as with different immunotherapeutic agents, have sometimes demonstrated increased survival with minimal, if any, reduction in tumor burden or time to progression. In addition to the Sipuleucel-T and PROSTVAC vaccine trials described above, a phase III trial leading to FDA approval, in which patients with metastatic melanoma were treated with ipilimumab (an anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody), demonstrated a statistically significant advantage in survival without a statistically significant difference in time to progression (91). Patients with unresectable stage III or IV melanoma were randomly assigned to receive ipilimumab plus gp100 peptide, ipilimumab alone, or gp100 alone. The median OS was 10.0 months among patients receiving ipilimumab plus gp100, as compared with 6.4 months among patients receiving gp100 alone (HR for death = 0.68; P < .001). The median progression-free survival was 2.76 months in the ipilimumab plus gp100 group, 2.86 months in the ipilimumab-alone group, and 2.76 months in the gp100-alone group.
Influence of the Tumor Microenvironment and Immunosuppressive Factors
One of the major reasons for the limited success of therapeutic cancer vaccines to date is likely to be the negative influence of the tumor microenvironment and other immunosuppressive factors (92–95). Preclinical studies have shown that the interstitial pressure within a large tumor mass diminishes diffusion of macromolecules, such as antibodies, and effector cells, such as T cells (96,97). Most solid tumors also lack T-cell costimulatory molecules. Thus, when activated T cells, especially those of relatively low avidity directed against self-antigens, bind to tumors lacking costimulatory molecules, they are anergized and lose lytic capacity. Similarly, it has been shown in preclinical models of chronic viral infection that T cells chronically exposed to viral antigen can become exhausted (98,99). The inhibitory co-receptor programmed death 1 (PD-1) has been shown to be present on such exhausted T cells (100).
The tumor microenvironment has been shown to contain a range of immunosuppressive immune cell types including CD4+ regulatory T cells (Tregs), myeloid derived suppressor cells (MDSCs), suppressor CD8+ T cells, tumor-associated macrophages (TAMs), and regulatory natural killer (NK)/NKT cells (101–109). Analysis of PBMCs from patients with several types of cancer has also shown increased levels of MDSCs and Tregs, as well as an increased suppressive function of Tregs on effector T cells (101,110–112). These suppressive cells, tumor cells, and other cells in the tumor microenvironment can also release into the microenvironment a number of soluble immunosuppressive factors, including TGF-β, IL-10, indoleamine-pyrrole 2,3 dioxygenase (IDO), and VEGF (106,113,114). Strategies to combat these immunosuppressive entities with combination therapies are described below. The tumor microenvironment can also influence the phenotype of tumor cells. For example, it has been shown (62) that IL-8 can drive carcinoma cells to the EMT phenotype and toward more stem-like characteristics.
As with any phenotypic analysis of cell types within a tumor mass, many tumors display antigenic heterogeneity, which is reflected both at the level of antigen expression and at the level of the antigen–peptide MHC complex. Tumor cells within masses have also been shown to have defects in MHC–peptide complexing machinery (115–119). Analysis of cloned populations of carcinoma cells has shown that this antigenic heterogeneity is an extremely “plastic” property, in which cloned cells will often revert to express a more heterogeneous antigenic population upon passage. In preclinical models, a tumor expressing CEA in only some of its cells can be cured with a CEA-based vaccine (20). CEA must be present in the vaccine and in some tumor cells for cures; however, analysis of the tumor masses in regressing tumors showed the presence of higher levels and greater avidity of T cells to other TAAs (120). When cured animals were subsequently challenged with CEA-negative tumor cells, they would also reject these tumors (120). This antigen cascade phenomenon has also been seen in clinical trials in which vaccinated patients develop higher levels of T-cell responses to TAAs compared with responses seen to the antigen found in the vaccine (1,7,121–124). This phenomenon is reported in some trials to be associated with patient benefit (7,121,124).
Immune Combination Therapies
Preclinical studies have clearly demonstrated that vaccine combined with a wide range of immune stimulants, or inhibitors of immune suppression, will greatly enhance antitumor responses. A major problem in the field of vaccine therapy, however, is the availability of many of these other agents. A National Cancer Institute workshop in July 2007 listed 12 such agents (125,126), and most are still not available for use in vaccine combination. Many of these agents were evaluated as monotherapies to maximally tolerated dose in phase I trials and were found to have little or no activity and subsequently abandoned. Preclinical studies have demonstrated that when some of these agents are used far below the maximally tolerated dose with vaccine, and/or at lower doses locally with vaccine, they can greatly enhance vaccine efficacy (127,128).
Potential immune stimulants include cytokines such as IL-2, IL-15, IL-7, GM-CSF, and interferon (IFN) (129–140). IL-2 has the disadvantage of enhancing Tregs along with effector cells (131,141). IL-15, now in phase I, has the advantage of enhancing effector T-cell response and not Tregs (130,135,136). GM-CSF has been used successfully in the GVAX and other vaccine platforms (39,40,142). However, the use of GM-CSF is a dual-edged sword in which higher levels may be immunosuppressive (25). IFNs have been shown to enhance immune responses and to enhance the expression of TAAs and MHC on tumor cells (143–145). Antibody–cytokine fusions, such as hu14.18-IL2, have shown promise in patients with refractory neuroblastoma (146) and other similar immunocytokines are currently in development. A range of TLRs has been shown to enhance vaccine efficacy in preclinical studies, but most are being evaluated clinically as monotherapies. Classic adjuvants such as incomplete Freund’s, ISCOMs, Detox, and chitosan provide both a depot effect for the protein or peptide vaccine (or cytokine) and a level of immune stimulation in regional lymph nodes (147–150). Clinical trials will need to carefully evaluate both the doses and scheduling of all of these immune stimulants with vaccine.
Perhaps, the most promising area of immune vaccine combination therapy is that of immune checkpoint inhibitors and inhibitors of immunosuppressive entities. Ipilimumab has been shown in several preclinical models to enhance the avidity of T cells and to enhance antitumor effects in combination with vaccines such as CEA-TRICOM recombinant poxviruses (91,151–153). A recent clinical trial of the PROSTVAC vaccine in combination with ipilimumab showed evidence of enhanced survival in patients with mCRPC compared with what had been seen in previous trials with PSA-TRICOM as monotherapy (154); a randomized trial is thus warranted. The recent FDA approval of ipilimumab for metastatic melanoma (91) will undoubtedly make vaccine combination therapies with this agent more feasible with a range of vaccine platforms. Ongoing clinical studies with anti-PD-1 and anti-PD-1 ligand 1 monoclonal antibodies as monotherapies are also yielding promising results (155–159). These agents should also enhance clinical vaccine efficacy.
Other agents that will reduce or eliminate immune suppressive factors on cells are also being evaluated. Monoclonal anti-CD25-diptherial toxin (Ontak) has been shown to reduce Tregs and to enhance vaccine efficacy (160,161). Both monoclonal and small-molecule inhibitors to TGF-β are also currently in clinical evaluation as monotherapies, but their full potential may be realized in vaccine combination therapy (162–165).
Very few cancer chemotherapy or small-molecule targeted therapy regimens employ one agent—and at times, they may use three or more agents. Each of the diverse vaccine platforms described in Table 1 can potentially activate the host adaptive and innate immune response differentially, and the same or different TAAs may be targeted (see Table 2), making the use of combination vaccine therapy quite an attractive possibility. A recent preclinical study (166) that used two diverse recombinant CEA vaccine platforms (poxviral and yeast) in mice demonstrated that each vector processed TAA epitopes differentially and activated a different T-cell repertoire and cytokine profile, resulting in enhanced antitumor activity. Evidence for the potential of combination vaccine therapies is also seen in the preclinical and clinical results using diversified prime-boost strategies with vaccinia and fowlpox vectors (18,27), and the only acquired immune deficiency syndrome (AIDS) prevention vaccine to show some evidence of promising results used a diversified recombinant avipox prime–protein boost regimen (167).
Vaccines in Combination with Other Therapeutic Modalities
It was commonly assumed that chemotherapy and vaccines do not mix. However, preclinical studies have demonstrated, and early clinical evidence is emerging, that multiple forms of therapy can be used concurrently with cancer vaccines or after vaccine therapy, with additive or synergistic effects. Some of these phenomena are summarized in Table 5.
Table 5.
Vaccines in combination with other therapeutic modalities*
| Modality | Mechanism of action to enhance vaccine efficacy |
| Chemotherapy | “Immunologic” tumor cell death |
| Alterations in tumor cell phenotype | |
| Enriched effector:regulator cell ratios | |
| Radiation | Alteration in tumor cell phenotype |
| Small-molecule targeted therapeutics | Alterations in tumor cell phenotype |
| Enriched effector:regulator cell ratios | |
| Hormonal therapy | Thymic regeneration and induction of naive T cells |
| Monoclonal antibodies | Enhanced ADCC |
| Imids† (lenalidomide) | Stimulate T-cell proliferation |
ADCC = antibody-dependent cell-mediated cytotoxicity.
Imids are a novel class of immunomodulators.
Certain chemotherapeutic agents can enhance vaccine-mediated T-cell killing by several distinct mechanisms. Oxaliplatin and anthracyclines such as doxorubicin will induce what has been termed “immunogenic tumor cell death,” which results in enhanced cross-priming of TAAs by DCs and subsequent activation of T cells (168–171). Agents such as docetaxel have been shown to suppress MDSCs and to increase the expression of TAAs, peptide-MHC complexes, adhesion molecules, and death receptors such as FAS on the surface of tumor cells and thus render them more susceptible to vaccine-induced T-cell killing (172,173); this same phenomenon has been observed when tumor cells have been exposed to external beam radiation, radiolabeled monoclonal antibodies, and bone-seeking radionuclides (174–176). Clinical trials that have used PROSTVAC vaccine plus the taxane docetaxel in patients with prostate cancer (28) have been completed, and others are in progress that are testing PANVAC vaccine in combination with docetaxel in patients with metastatic breast cancer (177). A trial that uses PROSTVAC vaccine in combination with a bone-seeking chelated radionuclide in patients with prostate cancer metastatic to the bone is also in progress (178). Low-dose paclitaxel (another taxane) has also been shown to enhance DC function in preclinical studies (179).
As a consequence of the homeostatic proliferation of immune cells following certain chemotherapeutic regimens such as cisplatin with vinorelbine, different immune subsets have been shown to differentially recover, leading to enhanced effector to regulatory T-cell ratios (180). Clinical studies with cyclophosphamide (used at relatively low doses) have been shown to diminish the number of Tregs (40). Certain chemotherapeutic agents, such as lenalidomide, have been termed “immunostimulatory,” and have been shown to stimulate T-cell proliferation and to enhance IL-2 and IFN-γ (181,182).
Preclinical studies have now demonstrated that certain small-molecule targeted therapeutics have the ability to enhance vaccine-mediated T-cell lysis of tumors. Both a BCL-2 inhibitor (183) and the tyrosine kinase inhibitor sunitinib (184–186) have been shown to enhance the ratio of TAA-specific T cells to regulatory cells, resulting in enhanced vaccine efficacy. A B-raf inhibitor has been shown to enhance the expression of TAAs on melanoma cells containing a BRAF mutation, resulting in enhanced T-cell lysis of tumor in vitro (187). The mTOR inhibitor, rapamycin, has been shown to enhance IL-12 production and the generation of memory CD8+ T cells (188,189). Vaccines are also now being used with monoclonal antibodies such as the HER2/neu inhibitor trastuzumab, resulting in enhanced HER2/neu-specific immune responses in breast cancer patients (1).
Hormonal therapy that is used in the treatment of several different stages of prostate cancer has been shown to induce thymic regeneration and the induction of naive T cells (190–192); it is at this interval that vaccine therapy may be most effective (29,193). A clinical study has demonstrated increased levels of infiltrating T cells in prostate cancer biopsies post- (vs pre-) hormonal therapy (194). Randomized clinical trials that employ PROSTVAC vaccine in combination with either nilutamide or flutamide hormone therapy in patients with non-metastatic prostate cancer have been completed and are ongoing (195,196).
In all of the above vaccine combination therapies, the dose and scheduling of the combining agent, the appropriate patient population, and the clinical endpoint are very important. There are many anecdotal reports and several publications that imply that patients who have received vaccine therapy, and whose tumors have nevertheless progressed, often undergo unexpected clinical responses when subsequent therapies are administered (28,197,198). This phenomenon, however, has not been validated prospectively or in a randomized trial. The Eastern Cooperative Oncology Group has recently initiated a multicenter randomized clinical trial in patients with metastatic prostate cancer to prospectively evaluate this phenomenon (199). Patients will receive either docetaxel or 2 months treatment with the PROSTVAC vaccine followed by docetaxel; OS will be the primary endpoint.
Biomarkers
The most common biomarker used in vaccine therapy trials has been the immune response of patients to TAAs post- (vs pre-) vaccination. Most trials have analyzed antibody responses to TAAs and/or analyzed PBMCs for CD8+ and/or CD4+ responses to the TAA in the vaccine using enzyme-linked immunosorbent spot– or fluorescence-activated cell sorting–based assays for cytokine production, or by binding of a peptide tetramer complex to the surface of T cells. Preclinical studies have shown, however, that the level of cytokine production by a T cell is not always associated with its lytic capacity. Because of the limited availability of samples, however, few studies have actually been able to measure the lytic capacity of T cells. Unfortunately, tumor biopsy specimens, the more appropriate site to obtain TAA-specific T cells, are unavailable in many trials.
More recently, a more comprehensive analysis of immune cell subsets from PBMCs has occurred in some studies including, in addition to T-cell responses, analyses of Tregs, MDSCs, NK, DCs, and antibodies to TAAs (9,200). Ratios of effector to regulatory cells have also been analyzed (25). Numerous studies have used analyses of multiple serum cytokines and chemokines.
What have emerged from these bioassay studies to date, at times, are associations between clinical outcome and an immune assay(s); however, these results are far from having identified any one assay as a “surrogate” for clinical responses. Potential reasons for this may be that PBMC analyses may not reflect which immune cells are actually at the tumor site; few studies have actually analyzed the “antigen cascade” phenomenon, where the true biomarker may be a T-cell population directed against a TAA not in the vaccine but generated via cross-priming; the diversity of the immune responses among individuals may not allow any one marker or set of markers as a surrogate for clinical response; and only recently has survival benefit emerged as a prominent endpoint in many vaccine studies, and adequate samples may not yet be available for comprehensive correlative analyses with survival.
Future Directions
Ongoing and future studies with therapeutic cancer vaccines will involve combination therapies with a range of therapeutic modalities as detailed here, including combinations of vaccines. Clinical trial designs will involve patients with more indolent metastatic disease and treatment in the adjuvant or neoadjuvant settings. There will also be less reliance on strict RECIST criteria, with more trials employing the new “immune response criteria” (89) and survival as endpoints when vaccine is used as a monotherapy.
As the long-term safety profiles of therapeutic vaccines are established, they most probably will be used in patients with a high risk of cancer, such as in patients with high-grade prostatic intraepithelial neoplasia who are at risk for the development of prostate cancer and familial adenomatous polyposis patients who are at risk for the development of colorectal cancer. Celecoxib has been used for the treatment of familial adenomatous polyposis (201), and preclinical studies have demonstrated that antitumor effects are greatly enhanced when CEA-TRICOM vaccine is combined with celecoxib (202).
Numerous studies have demonstrated that analysis of the immune infiltrate in colorectal and other cancer biopsies before chemotherapy can serve as a very strong independent prognostic indicator for survival (203–209). This phenomenon has yet to be exploited to define which patients may respond best to vaccine therapy. Analyses of PBMC immune cell subsets for detailed HLA typing may also vbetter reveal which patients are most likely to respond. Finally, more detailed analyses of mutations and unique tumor gene products, along with a further delineation of cancer cell stemness and the EMT phenotype, may also reveal new targets for vaccine therapy.
Funding
This study was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
The funders did not have any involvement in the preparation of this review article; the collection, analysis, and interpretation of the data; the writing of the article; and the decision to submit the article for publication. The author thanks Debra Weingarten for her editorial assistance in the preparation of this article.
References
- 1.Disis ML, Wallace DR, Gooley TA, et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol. 2009;27(28):4685–4692. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butts C, Murray N, Maksymiuk A, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol. 2005;23(27):6674–6681. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 3.Pejawar-Gaddy S, Rajawat Y, Hilioti Z, et al. Generation of a tumor vaccine candidate based on conjugation of a MUC1 peptide to polyionic papillomavirus virus-like particles. Cancer Immunol Immunother. 2010;59(11):1685–1696. doi: 10.1007/s00262-010-0895-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finn OJ, Gantt KR, Lepisto AJ, et al. Importance of MUC1 and spontaneous mouse tumor models for understanding the immunobiology of human adenocarcinomas. Immunol Res. 2011;50(2–3):261–268. doi: 10.1007/s12026-011-8214-1. [DOI] [PubMed] [Google Scholar]
- 5.Schwartzentruber DJ, Lawson DH, Richards JM, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364(22):2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sosman JA, Carrillo C, Urba WJ, et al. Three phase II cytokine working group trials of gp100 (210M) peptide plus high-dose interleukin-2 in patients with HLA-A2-positive advanced melanoma. J Clin Oncol. 2008;26(14):2292–2298. doi: 10.1200/JCO.2007.13.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salazar LG, Wallace D, Mukherjee P, et al. HER-2/neu (HER2) specific T-cell immunity in patients with HER2+ inflammatory breast cancer (IBC) and prognosis. American Society of Clinical Oncology 2009 Annual Meeting. J Clin Oncol. 2009;27(suppl):15s. Abstract 3057. [Google Scholar]
- 8.Brichard VG, Lejeune D. Cancer immunotherapy targeting tumour-specific antigens: towards a new therapy for minimal residual disease. Expert Opin Biol Ther. 2008;8(7):951–968. doi: 10.1517/14712598.8.7.951. [DOI] [PubMed] [Google Scholar]
- 9.Disis ML. Immunologic biomarkers as correlates of clinical response to cancer immunotherapy. Cancer Immunol Immunother. 2011;60(3):433–442. doi: 10.1007/s00262-010-0960-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karbach J, Neumann A, Atmaca A, et al. Efficient in vivo priming by vaccination with recombinant NY-ESO-1 protein and CpG in antigen naive prostate cancer patients. Clin Cancer Res. 2011;17(4):861–870. doi: 10.1158/1078-0432.CCR-10-1811. [DOI] [PubMed] [Google Scholar]
- 11.Schuster SJ, Neelapu SS, Gause BL, et al. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J Clin Oncol. 2011;29(20):2787–2794. doi: 10.1200/JCO.2010.33.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendandi M. Idiotype vaccines for lymphoma: proof-of-principles and clinical trial failures. Nat Rev Cancer. 2009;9(9):675–681. doi: 10.1038/nrc2717. [DOI] [PubMed] [Google Scholar]
- 13.Inoges S, Rodriguez-Calvillo M, Zabalegui N, et al. Clinical benefit associated with idiotypic vaccination in patients with follicular lymphoma. J Natl Cancer Inst. 2006;98(18):1292–1301. doi: 10.1093/jnci/djj358. [DOI] [PubMed] [Google Scholar]
- 14.Freedman A, Neelapu SS, Nichols C, et al. Placebo-controlled phase III trial of patient-specific immunotherapy with mitumprotimut-T and granulocyte-macrophage colony-stimulating factor after rituximab in patients with follicular lymphoma. J Clin Oncol. 2009;27(18):3036–3043. doi: 10.1200/JCO.2008.19.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilewski TA, Ragupathi G, Dickler M, et al. Immunization of high-risk breast cancer patients with clustered sTn-KLH conjugate plus the immunologic adjuvant QS-21. Clin Cancer Res. 2007;13(10):2977–2985. doi: 10.1158/1078-0432.CCR-06-2189. [DOI] [PubMed] [Google Scholar]
- 16.Ragupathi G, Damani P, Srivastava G, et al. Synthesis of sialyl Lewis(a) (sLe (a), CA19-9) and construction of an immunogenic sLe(a) vaccine. Cancer Immunol Immunother. 2009;58(9):1397–1405. doi: 10.1007/s00262-008-0654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moss B. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc Natl Acad Sci U S A. 1996;93(21):11341–11348. doi: 10.1073/pnas.93.21.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodge JW, Higgins J, Schlom J. Harnessing the unique local immunostimulatory properties of modified vaccinia Ankara (MVA) virus to generate superior tumor-specific immune responses and antitumor activity in a diversified prime and boost vaccine regimen. Vaccine. 2009;27(33):4475–4482. doi: 10.1016/j.vaccine.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall JL, Hoyer RJ, Toomey MA, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18(23):3964–3973. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- 20.Hodge JW, Grosenbach DW, Aarts WM, Poole DJ, Schlom J. Vaccine therapy of established tumors in the absence of autoimmunity. Clin Cancer Res. 2003;9(5):1837–1849. [PubMed] [Google Scholar]
- 21.von Mehren M, Arlen P, Gulley J, et al. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7.1) in patients with metastatic carcinoma. Clin Cancer Res. 2001;7(5):1181–1191. [PubMed] [Google Scholar]
- 22.von Mehren M, Arlen P, Tsang KY, et al. Pilot study of a dual gene recombinant avipox vaccine containing both carcinoembryonic antigen (CEA) and B7.1 transgenes in patients with recurrent CEA-expressing adenocarcinomas. Clin Cancer Res. 2000;6(6):2219–2228. [PubMed] [Google Scholar]
- 23.Madan RA, Mohebtash M, Schlom J, Gulley JL. Therapeutic vaccines in metastatic castration-resistant prostate cancer: principles in clinical trial design. Expert Opin Biol Ther. 2010;10(1):19–28. doi: 10.1517/14712590903321421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(7):1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59(5):663–674. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall JL, Gulley JL, Arlen PM, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23(4):720–731. doi: 10.1200/JCO.2005.10.206. [DOI] [PubMed] [Google Scholar]
- 27.Kaufman HL, Wang W, Manola J, et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22(11):2122–2132. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 28.Arlen PM, Gulley JL, Parker C, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12(4):1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanda MG, Smith DC, Charles LG, et al. Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology. 1999;53(2):260–266. doi: 10.1016/s0090-4295(98)00539-1. [DOI] [PubMed] [Google Scholar]
- 30.Remondo C, Cereda V, Mostbock S, et al. Human dendritic cell maturation and activation by a heat-killed recombinant yeast (Saccharomyces cerevisiae) vector encoding carcinoembryonic antigen. Vaccine. 2009;27(7):987–994. doi: 10.1016/j.vaccine.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wansley EK, Chakraborty M, Hance KW, et al. Vaccination with a recombinant Saccharomyces cerevisiae expressing a tumor antigen breaks immune tolerance and elicits therapeutic antitumor responses. Clin Cancer Res. 2008;14(13):4316–4325. doi: 10.1158/1078-0432.CCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh R, Paterson Y. Listeria monocytogenes as a vector for tumor-associated antigens for cancer immunotherapy. Expert Rev Vaccines. 2006;5(4):541–552. doi: 10.1586/14760584.5.4.541. [DOI] [PubMed] [Google Scholar]
- 33.MacDonald GH, Johnston RE. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J Virol. 2000;74(2):914–922. doi: 10.1128/jvi.74.2.914-922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okur FV, Yvon E, Biagi E, et al. Comparison of two CD40-ligand/interleukin-2 vaccines in patients with chronic lymphocytic leukemia. Cytotherapy. 2011;13(9):1128–1139. doi: 10.3109/14653249.2011.592523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoover HC, Jr, Brandhorst JS, Peters LC, et al. Adjuvant active specific immunotherapy for human colorectal cancer: 6.5-year median follow-up of a phase III prospectively randomized trial. J Clin Oncol. 1993;11(3):390–399. doi: 10.1200/JCO.1993.11.3.390. [DOI] [PubMed] [Google Scholar]
- 36.Luiten RM, Kueter EW, Mooi W, et al. Immunogenicity, including vitiligo, and feasibility of vaccination with autologous GM-CSF-transduced tumor cells in metastatic melanoma patients. J Clin Oncol. 2005;23(35):8978–8991. doi: 10.1200/JCO.2005.01.6816. [DOI] [PubMed] [Google Scholar]
- 37.Avigan D, Vasir B, Gong J, et al. Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses. Clin Cancer Res. 2004;10(14):4699–4708. doi: 10.1158/1078-0432.CCR-04-0347. [DOI] [PubMed] [Google Scholar]
- 38.Laheru D, Lutz E, Burke J, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14(5):1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lutz E, Yeo CJ, Lillemoe KD, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253(2):328–335. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emens LA, Asquith JM, Leatherman JM, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27(35):5911–5918. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Eng J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 42.Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115(16):3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 43.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 44.Okada H, Kalinski P, Ueda R, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29(3):330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banchereau J, Palucka AK, Dhodapkar M, et al. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61(17):6451–6458. [PubMed] [Google Scholar]
- 46.Lyerly HK, Hobeika A, Niedzwiecki D, et al. A dendritic cell-based vaccine effects on T-cell responses compared with a viral vector vaccine when administered to patients following resection of colorectal metastases in a randomized Phase II study. American Society of Clinical Oncology 2011 Annual Meeting. J Clin Oncol. 2011;29(suppl) (Abstract 2533) [Google Scholar]
- 47.Morse M, Niedzwiecki D, Marshall J, et al. Survival rates among patients vaccinated following resection of colorectal cancer metastases in a Phase II randomized study compared with contemporary controls. American Society of Clinical Oncology 2011 Annual Meeting. J Clin Oncol. 2011;29(suppl) (Abstract 3557) [Google Scholar]
- 48.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9(12):874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raina D, Kosugi M, Ahmad R, et al. Dependence on the MUC1-C oncoprotein in non-small cell lung cancer cells. Mol Cancer Ther. 2011;10(5):806–816. doi: 10.1158/1535-7163.MCT-10-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gnjatic S, Ritter E, Buchler MW, et al. Seromic profiling of ovarian and pancreatic cancer. Proc Natl Acad Sci U S A. 2010;107(11):5088–5093. doi: 10.1073/pnas.0914213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gnjatic S, Wheeler C, Ebner M, et al. Seromic analysis of antibody responses in non-small cell lung cancer patients and healthy donors using conformational protein arrays. J Immunol Methods. 2009;341(1–2):50–58. doi: 10.1016/j.jim.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 52.Hofmann O, Caballero OL, Stevenson BJ, et al. Genome-wide analysis of cancer/testis gene expression. Proc Natl Acad Sci U S A. 2008;105(51):20422–20427. doi: 10.1073/pnas.0810777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wheeler CJ, Black KL. Vaccines for glioblastoma and high-grade glioma. Expert Rev Vaccines. 2011;10(6):875–886. doi: 10.1586/erv.11.71. [DOI] [PubMed] [Google Scholar]
- 54.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 55.Dhodapkar KM, Feldman D, Matthews P, et al. Natural immunity to pluripotency antigen OCT4 in humans. Proc Natl Acad Sci U S A. 2010;107(19):8718–8723. doi: 10.1073/pnas.0915086107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dhodapkar MV, Dhodapkar KM. Spontaneous and therapy-induced immunity to pluripotency genes in humans: clinical implications, opportunities and challenges. Cancer Immunol Immunother. 2011;60(3):413–418. doi: 10.1007/s00262-010-0944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hua W, Yao Y, Chu Y, et al. The CD133+ tumor stem-like cell-associated antigen may elicit highly intense immune responses against human malignant glioma. J Neurooncol. 2011;105(2):149–157. doi: 10.1007/s11060-011-0572-y. [DOI] [PubMed] [Google Scholar]
- 58.Mine T, Matsueda S, Li Y, et al. Breast cancer cells expressing stem cell markers CD44+ CD24 lo are eliminated by Numb-1 peptide-activated T cells. Cancer Immunol Immunother. 2009;58(8):1185–1194. doi: 10.1007/s00262-008-0623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spisek R, Kukreja A, Chen LC, et al. Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy. J Exp Med. 2007;204(4):831–840. doi: 10.1084/jem.20062387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernando RI, Litzinger M, Trono P, et al. The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J Clin Invest. 2010;120(2):533–544. doi: 10.1172/JCI38379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palena C, Polev DE, Tsang KY, et al. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin Cancer Res. 2007;13(8):2471–2478. doi: 10.1158/1078-0432.CCR-06-2353. [DOI] [PubMed] [Google Scholar]
- 62.Fernando RI, Castillo MD, Litzinger M, Hamilton DH, Palena C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 2011;71(15):5296–5306. doi: 10.1158/0008-5472.CAN-11-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kemp TJ, Garcia-Pineres A, Falk RT, et al. Evaluation of systemic and mucosal anti-HPV16 and anti-HPV18 antibody responses from vaccinated women. Vaccine. 2008;26(29–30):3608–3616. doi: 10.1016/j.vaccine.2008.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kemp TJ, Hildesheim A, Safaeian M, et al. HPV16/18 L1 VLP vaccine induces cross-neutralizing antibodies that may mediate cross-protection. Vaccine. 2011;29(11):2011–2014. doi: 10.1016/j.vaccine.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaplan CD, Kruger JA, Zhou H, et al. A novel DNA vaccine encoding PDGFRbeta suppresses growth and dissemination of murine colon, lung and breast carcinoma. Vaccine. 2006;24(47–48):6994–7002. doi: 10.1016/j.vaccine.2006.04.071. [DOI] [PubMed] [Google Scholar]
- 66.Xiang R, Luo Y, Niethammer AG, Reisfeld RA. Oral DNA vaccines target the tumor vasculature and microenvironment and suppress tumor growth and metastasis. Immunol Rev. 2008;222(1):117–128. doi: 10.1111/j.1600-065X.2008.00613.x. [DOI] [PubMed] [Google Scholar]
- 67.Frazer IH, Lowy DR, Schiller JT. Prevention of cancer through immunization: prospects and challenges for the 21st century. Eur J Immunol. 2007;37(suppl 1):S148–S155. doi: 10.1002/eji.200737820. [DOI] [PubMed] [Google Scholar]
- 68.Cheever MA, Allison JP, Ferris AS, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15(17):5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gulley JL, Arlen PM, Hodge JW, Schlom J. Vaccines and immunostimulants. In: Hong W, editor. Cancer Medicine 8. Shelton, CT: People's Medical Publishing House-USA; 2010. pp. 725–736. [Google Scholar]
- 70.Gnjatic S, Old LJ, Chen YT. Autoantibodies against cancer antigens. Methods Mol Biol. 2009;520:11–19. doi: 10.1007/978-1-60327-811-9_2. [DOI] [PubMed] [Google Scholar]
- 71.Dzutsev AH, Belyakov IM, Isakov DV, Margulies DH, Berzofsky JA. Avidity of CD8 T cells sharpens immunodominance. Int Immunol. 2007;19(4):497–507. doi: 10.1093/intimm/dxm016. [DOI] [PubMed] [Google Scholar]
- 72.Hodge JW, Chakraborty M, Kudo-Saito C, Garnett CT, Schlom J. Multiple costimulatory modalities enhance CTL avidity. J Immunol. 2005;174(10):5994–6004. doi: 10.4049/jimmunol.174.10.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oh S, Hodge JW, Ahlers JD, et al. Selective induction of high avidity CTL by altering the balance of signals from APC. J Immunol. 2003;170(5):2523–2530. doi: 10.4049/jimmunol.170.5.2523. [DOI] [PubMed] [Google Scholar]
- 74.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21(7):1232–1237. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 75.Hodge JW, Sabzevari H, Yafal AG, et al. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59(22):5800–5807. [PubMed] [Google Scholar]
- 76.Mellstedt H, Vansteenkiste J, Thatcher N. Vaccines for the treatment of non-small cell lung cancer: investigational approaches and clinical experience. Lung Cancer. 2011;73(1):11–17. doi: 10.1016/j.lungcan.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 77.Nemunaitis J, Nemunaitis M, Senzer N, et al. Phase II trial of Belagenpumatucel-L, a TGF-beta2 antisense gene modified allogeneic tumor vaccine in advanced non small cell lung cancer (NSCLC) patients. Cancer Gene Ther. 2009;16(8):620–624. doi: 10.1038/cgt.2009.15. [DOI] [PubMed] [Google Scholar]
- 78.Vansteenkiste J, Zielinski H, Linder A, et al. Final results of a muilti-center, double-blind, randomized, placebo-controlled phase II study to assess the efficacy of MAGE-A3 immunotherapeutic as adjuvant therapy in stage Ib/II non-small cell lung cancer (NCSLC) J Clin Oncol. 2007;25(18S) (Abstract 7554) [Google Scholar]
- 79.Lane C, Leitch J, Tan X, et al. Vaccination-induced autoimmune vitiligo is a consequence of secondary trauma to the skin. Cancer Res. 2004;64(4):1509–1514. doi: 10.1158/0008-5472.can-03-3227. [DOI] [PubMed] [Google Scholar]
- 80.Andres A, Majno PE, Morel P, et al. Improved long-term outcome of surgery for advanced colorectal liver metastases: reasons and implications for management on the basis of a severity score. Ann Surg Oncol. 2008;15(1):134–143. doi: 10.1245/s10434-007-9607-1. [DOI] [PubMed] [Google Scholar]
- 81.Arru M, Aldrighetti L, Castoldi R, et al. Analysis of prognostic factors influencing long-term survival after hepatic resection for metastatic colorectal cancer. World J Surg. 2008;32(1):93–103. doi: 10.1007/s00268-007-9285-y. [DOI] [PubMed] [Google Scholar]
- 82.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235(6):759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.House MG, Ito H, Gonen M, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210(5):744, 752–752. doi: 10.1016/j.jamcollsurg.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 84.Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241(5):715–722. doi: 10.1097/01.sla.0000160703.75808.7d. discussion 722–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sasaki A, Iwashita Y, Shibata K, et al. Analysis of preoperative prognostic factors for long-term survival after hepatic resection of liver metastasis of colorectal carcinoma. J Gastrointest Surg. 2005;9(3):374–380. doi: 10.1016/j.gassur.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 86.Stein WD, Gulley JL, Schlom J, et al. Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res. 2011;17(4):907–917. doi: 10.1158/1078-0432.CCR-10-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gulley JL, Madan RA, Schlom J. The impact of tumour volume on potential efficacy of therapeutic vaccines [review] Curr Oncol. 2011;18(3):e150–e157. doi: 10.3747/co.v18i3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010;15(9):969–975. doi: 10.1634/theoncologist.2010-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoos A, Eggermont AM, Janetzki S, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102(18):1388–1397. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer. 2006;42(8):1031–1039. doi: 10.1016/j.ejca.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 91.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cham CM, Driessens G, O’Keefe JP, Gajewski TF. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur J Immunol. 2008;38(9):2438–2450. doi: 10.1002/eji.200838289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gajewski TF, Chesney J, Curriel TJ. Emerging strategies in regulatory T-cell immunotherapies. Clin Adv Hematol Oncol. 2009;7(1):1–10. ; quiz 11–12. [PubMed] [Google Scholar]
- 94.Gajewski TF, Meng Y, Blank C, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213(1):131–145. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 95.Gajewski TF, Meng Y, Harlin H. Immune suppression in the tumor microenvironment. J Immunother. 2006;29(3):233–240. doi: 10.1097/01.cji.0000199193.29048.56. [DOI] [PubMed] [Google Scholar]
- 96.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10(6):417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 97.Fukumura D, Duda DG, Munn LL, Jain RK. Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation. 2010;17(3):206–225. doi: 10.1111/j.1549-8719.2010.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22(2):223–230. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2009;106(21):8623–8628. doi: 10.1073/pnas.0809818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vezys V, Penaloza-Macmaster P, Barber DL, et al. 4-1BB signaling synergizes with programmed death ligand 1 blockade to augment CD8 T cell responses during chronic viral infection. J Immunol. 2011;187(4):1634–1642. doi: 10.4049/jimmunol.1100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cesana GC, DeRaffele G, Cohen S, et al. Characterization of CD4+CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol. 2006;24(7):1169–1177. doi: 10.1200/JCO.2005.03.6830. [DOI] [PubMed] [Google Scholar]
- 102.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32(1):19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13(18, pt 1):5256–5261. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- 104.Lindenberg JJ, Fehres CM, van Cruijsen H, Oosterhoff D, de Gruijl TD. Cross-talk between tumor and myeloid cells: how to tip the balance in favor of antitumor immunity. Immunotherapy. 2011;3(1):77–96. doi: 10.2217/imt.10.95. [DOI] [PubMed] [Google Scholar]
- 105.Lutsiak ME, Tagaya Y, Adams AJ, Schlom J, Sabzevari H. Tumor-induced impairment of TCR signaling results in compromised functionality of tumor-infiltrating regulatory T cells. J Immunol. 2008;180(9):5871–5881. doi: 10.4049/jimmunol.180.9.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mocellin S, Wang E, Marincola FM. Cytokines and immune response in the tumor microenvironment. J Immunother. 2001;24(5):392–407. [PubMed] [Google Scholar]
- 107.Nagaraj S, Gupta K, Pisarev V, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13(7):828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nagaraj S, Schrum AG, Cho HI, Celis E, Gabrilovich DI. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol. 2010;184(6):3106–3116. doi: 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shevach EM. The resurrection of T cell-mediated suppression. J Immunol. 2011;186(7):3805–3807. doi: 10.4049/jimmunol.1100364. [DOI] [PubMed] [Google Scholar]
- 110.Rasku MA, Clem AL, Telang S, et al. Transient T cell depletion causes regression of melanoma metastases. J Transl Med. 2008;6:12. doi: 10.1186/1479-5876-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vergati M, Cereda V, Madan RA, et al. Analysis of circulating regulatory T cells in patients with metastatic prostate cancer pre- versus post-vaccination. Cancer Immunol Immunother. 2011;60(2):197–206. doi: 10.1007/s00262-010-0927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yokokawa J, Cereda V, Remondo C, et al. Enhanced functionality of CD4+CD25(high)FoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin Cancer Res. 2008;14(4):1032–1040. doi: 10.1158/1078-0432.CCR-07-2056. [DOI] [PubMed] [Google Scholar]
- 113.di Bari MG, Lutsiak ME, Takai S, et al. TGF-beta modulates the functionality of tumor-infiltrating CD8(+) T cells through effects on TCR signaling and Spred1 expression. Cancer Immunol Immunother. 2009;58(11):1809–1818. doi: 10.1007/s00262-009-0692-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang L. TGFbeta and cancer metastasis: an inflammation link. Cancer Metastasis Rev. 2010;29(2):263–271. doi: 10.1007/s10555-010-9226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hsieh CH, Hsu YJ, Chang CC, et al. Total HLA class I loss in a sarcomatoid renal carcinoma cell line caused by the coexistence of distinct mutations in the two encoding beta2-microglobulin genes. Cancer Immunol Immunother. 2009;58(3):395–408. doi: 10.1007/s00262-008-0565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Michel S, Linnebacher M, Alcaniz J, et al. Lack of HLA class II antigen expression in microsatellite unstable colorectal carcinomas is caused by mutations in HLA class II regulatory genes. Int J Cancer. 2010;127(4):889–898. doi: 10.1002/ijc.25106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Racanelli V, Leone P, Frassanito MA, et al. Alterations in the antigen processing-presenting machinery of transformed plasma cells are associated with reduced recognition by CD8+ T cells and characterize the progression of MGUS to multiple myeloma. Blood. 2010;115(6):1185–1193. doi: 10.1182/blood-2009-06-228676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Respa A, Bukur J, Ferrone S, et al. Association of IFN-gamma signal transduction defects with impaired HLA class I antigen processing in melanoma cell lines. Clin Cancer Res. 2011;17(9):2668–2678. doi: 10.1158/1078-0432.CCR-10-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Seliger B, Stoehr R, Handke D, et al. Association of HLA class I antigen abnormalities with disease progression and early recurrence in prostate cancer. Cancer Immunol Immunother. 2010;59(4):529–540. doi: 10.1007/s00262-009-0769-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kudo-Saito C, Schlom J, Hodge JW. Induction of an antigen cascade by diversified subcutaneous/intratumoral vaccination is associated with antitumor responses. Clin Cancer Res. 2005;11(6):2416–2426. doi: 10.1158/1078-0432.CCR-04-1380. [DOI] [PubMed] [Google Scholar]
- 121.Butterfield LH, Ribas A, Dissette VB, et al. Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clin Cancer Res. 2003;9(3):998–1008. [PubMed] [Google Scholar]
- 122.Gulley JL, Arlen PM, Tsang KY, et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res. 2008;14(10):3060–3069. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lechleider RJ, Arlen PM, Tsang KY, et al. Safety and immunologic response of a viral vaccine to prostate-specific antigen in combination with radiation therapy when metronomic-dose interleukin 2 is used as an adjuvant. Clin Cancer Res. 2008;14(16):5284–5291. doi: 10.1158/1078-0432.CCR-07-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ribas A, Timmerman JM, Butterfield LH, Economou JS. Determinant spreading and tumor responses after peptide-based cancer immunotherapy. Trends Immunol. 2003;24(2):58–61. doi: 10.1016/s1471-4906(02)00029-7. [DOI] [PubMed] [Google Scholar]
- 125.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222(1):357–368. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 126.Cheever MA, Creekmore SP, editors. National Cancer Institute Agent Workshop Proceedings: July 12, 2007. http://web.ncifcrf.gov/research/brb/workshops/NCI%20Immunotherapy%20Workshop%207-12-07.pdf. Accessed April 11, 2011. [Google Scholar]
- 127.Grosenbach DW, Barrientos JC, Schlom J, Hodge JW. Synergy of vaccine strategies to amplify antigen-specific immune responses and antitumor effects. Cancer Res. 2001;61(11):4497–4505. [PubMed] [Google Scholar]
- 128.Tatari-Calderone Z, Semnani RT, Nutman TB, Schlom J, Sabzevari H. Acquisition of CD80 by human T cells at early stages of activation: functional involvement of CD80 acquisition in T cell to T cell interaction. J Immunol. 2002;169(11):6162–6169. doi: 10.4049/jimmunol.169.11.6162. [DOI] [PubMed] [Google Scholar]
- 129.Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci. 2012;33(1):35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Becker TC, Wherry EJ, Boone D, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195(12):1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Burton JD, Bamford RN, Peters C, et al. A lymphokine, provisionally designated interleukin T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci U S A. 1994;91(11):4935–4939. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3(8):630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 133.Fry TJ, Mackall CL. Interleukin-7: master regulator of peripheral T-cell homeostasis? Trends Immunol. 2001;22(10):564–571. doi: 10.1016/s1471-4906(01)02028-2. [DOI] [PubMed] [Google Scholar]
- 134.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174(11):6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 135.Mohamadzadeh M, Berard F, Essert G, et al. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J Exp Med. 2001;194(7):1013–1020. doi: 10.1084/jem.194.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oh S, Berzofsky JA, Burke DS, Waldmann TA, Perera LP. Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc Natl Acad Sci U S A. 2003;100(6):3392–3397. doi: 10.1073/pnas.0630592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1(5):426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 138.Tan JT, Ernst B, Kieper WC, et al. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195(12):1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol. 2011;11(5):330–342. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sportes C, Babb RR, Krumlauf MC, et al. Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clin Cancer Res. 2010;16(2):727–735. doi: 10.1158/1078-0432.CCR-09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Burchill MA, Yang J, Vang KB, Farrar MA. Interleukin-2 receptor signaling in regulatory T cell development and homeostasis. Immunol Lett. 2007;114(1):1–8. doi: 10.1016/j.imlet.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Borrello IM, Levitsky HI, Stock W, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting cellular immunotherapy in combination with autologous stem cell transplantation (ASCT) as postremission therapy for acute myeloid leukemia (AML) Blood. 2009;114(9):1736–1745. doi: 10.1182/blood-2009-02-205278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Greiner JW, Guadagni F, Goldstein D, et al. Intraperitoneal administration of interferon-gamma to carcinoma patients enhances expression of tumor-associated glycoprotein-72 and carcinoembryonic antigen on malignant ascites cells. J Clin Oncol. 1992;10(5):735–746. doi: 10.1200/JCO.1992.10.5.735. [DOI] [PubMed] [Google Scholar]
- 144.Greiner JW, Guadagni F, Noguchi P. Recombinant interferon enhances monoclonal antibody-targeting of carcinoma lesions in vivo. Science. 1987;235(4791):895–898. doi: 10.1126/science.3580039. [DOI] [PubMed] [Google Scholar]
- 145.Greiner JW, Hand PH, Noguchi P, et al. Enhanced expression of surface tumor-associated antigens on human breast and colon tumor cells after recombinant human leukocyte alpha-interferon treatment. Cancer Res. 1984;44(8):3208–3214. [PubMed] [Google Scholar]
- 146.Shusterman S, London WB, Gillies SD, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children's Oncology Group (COG) phase II study. J Clin Oncol. 2010;28(33):4969–4975. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Atanackovic D, Altorki NK, Stockert E, et al. Vaccine-induced CD4+ T cell responses to MAGE-3 protein in lung cancer patients. J Immunol. 2004;172(5):3289–3296. doi: 10.4049/jimmunol.172.5.3289. [DOI] [PubMed] [Google Scholar]
- 148.Mata-Haro V, Cekic C, Martin M, et al. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316(5831):1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 149.Zaharoff DA, Hoffman BS, Hooper HB, et al. Intravesical immunotherapy of superficial bladder cancer with chitosan/interleukin-12. Cancer Res. 2009;69(15):6192–6199. doi: 10.1158/0008-5472.CAN-09-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW. Chitosan solution enhances the immunoadjuvant properties of GM-CSF. Vaccine. 2007;25(52):8673–8686. doi: 10.1016/j.vaccine.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chakraborty M, Schlom J, Hodge JW. The combined activation of positive costimulatory signals with modulation of a negative costimulatory signal for the enhancement of vaccine-mediated T-cell responses. Cancer Immunol Immunother. 2007;56(9):1471–1484. doi: 10.1007/s00262-007-0291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190(3):355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100(8):4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Madan RA, Mohebtash M, Arlen PM, et al. Overall survival analysis of a phase l trial of a vector-based vaccine (PSA-TRICOM) and ipilimumab in the treatment of metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(suppl):15s. (Abstract 2550) [Google Scholar]
- 155.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li B, VanRoey M, Wang C, et al. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor–secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clin Cancer Res. 2009;15(5):1623–1634. doi: 10.1158/1078-0432.CCR-08-1825. [DOI] [PubMed] [Google Scholar]
- 157.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107(17):7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kline J, Gajewski TF. Clinical development of mAbs to block the PD1 pathway as an immunotherapy for cancer. Curr Opin Investig Drugs. 2010;11(12):1354–1359. [PubMed] [Google Scholar]
- 159.Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer–preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37(5):430–439. doi: 10.1053/j.seminoncol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 160.Litzinger MT, Fernando R, Curiel TJ, et al. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood. 2007;110(9):3192–3201. doi: 10.1182/blood-2007-06-094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ruter J, Barnett BG, Kryczek I, et al. Altering regulatory T cell function in cancer immunotherapy: a novel means to boost the efficacy of cancer vaccines. Front Biosci. 2009;14:1761–1770. doi: 10.2741/3338. [DOI] [PubMed] [Google Scholar]
- 162.Morris JC, Shapiro GI, Tan AR, et al. Phase I/II study of GC1008: a human anti-transforming growth factor-beta (TGFβ) monoclonal antibody (MAb) in patients with advanced malignant melanoma (MM) or renal cell carcinoma (RCC). American Society of Clinical Oncology 2008 Annual Meeting. J Clin Oncol. 2008;26(20 suppl) (Abstract 9028) [Google Scholar]
- 163.Bogdahn U, Hau P, Stockhammer G, et al. Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol. 2011;13(1):132–142. doi: 10.1093/neuonc/noq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bueno L, de Alwis DP, Pitou C, et al. Semi-mechanistic modelling of the tumour growth inhibitory effects of LY2157299, a new type I receptor TGF-beta kinase antagonist, in mice. Eur J Cancer. 2008;44(1):142–150. doi: 10.1016/j.ejca.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 165.Bogdahn U, Hau P, Olyushin V, et al. Targeted therapy with AP 12009 in recurrent or refractory glioblastoma patients: results of a phase IIb study. American Society of Clinical Oncology 2008 Annual Meeting. J Clin Oncol. 2008;26(20 suppl) (Abstract 2018) [Google Scholar]
- 166.Boehm AL, Higgins J, Franzusoff A, Schlom J, Hodge JW. Concurrent vaccination with two distinct vaccine platforms targeting the same antigen generates phenotypically and functionally distinct T-cell populations. Cancer Immunol Immunother. 2010;59(3):397–408. doi: 10.1007/s00262-009-0759-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 168.Kepp O, Galluzzi L, Martins I, et al. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev. 2011;30(1):61–69. doi: 10.1007/s10555-011-9273-4. [DOI] [PubMed] [Google Scholar]
- 169.Locher C, Conforti R, Aymeric L, et al. Desirable cell death during anticancer chemotherapy. Ann N Y Acad Sci. 2010;1209:99–108. doi: 10.1111/j.1749-6632.2010.05763.x. [DOI] [PubMed] [Google Scholar]
- 170.Tesniere A, Schlemmer F, Boige V, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 171.Zitvogel L, Kepp O, Senovilla L, et al. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res. 2010;16(12):3100–3104. doi: 10.1158/1078-0432.CCR-09-2891. [DOI] [PubMed] [Google Scholar]
- 172.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14(11):3536–3544. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kodumudi KN, Woan K, Gilvary DL, et al. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16(18):4583–4594. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chakraborty M, Gelbard A, Carrasquillo JA, et al. Use of radiolabeled monoclonal antibody to enhance vaccine-mediated antitumor effects. Cancer Immunol Immunother. 2008;57(8):1173–1183. doi: 10.1007/s00262-008-0449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chakraborty M, Wansley EK, Carrasquillo JA, et al. The use of chelated radionuclide (samarium-153-ethylenediaminetetramethylenephosphonate) to modulate phenotype of tumor cells and enhance T cell-mediated killing. Clin Cancer Res. 2008;14(13):4241–4249. doi: 10.1158/1078-0432.CCR-08-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gelbard A, Garnett CT, Abrams SI, et al. Combination chemotherapy and radiation of human squamous cell carcinoma of the head and neck augments CTL-mediated lysis. Clin Cancer Res. 2006;12(6):1897–1905. doi: 10.1158/1078-0432.CCR-05-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Docetaxel Alone or in Combination With Vaccine to Treat Breast Cancer. http://www.clinicaltrials.gov/ct2/show/NCT00179309?term=gulley&rank=9. Accessed April 11, 2011. [Google Scholar]
- 178.153Sm-EDTMP With or Without a PSA/TRICOM Vaccine To Treat Men With Androgen-Insensitive Prostate Cancer. http://www.clinicaltrials.gov/ct2/show/NCT00450619?term=Samarium+vaccine&rank=1. Accessed April 11, 2011. [Google Scholar]
- 179.Pfannenstiel LW, Lam SS, Emens LA, Jaffee EM, Armstrong TD. Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice. Cell Immunol. 2010;263(1):79–87. doi: 10.1016/j.cellimm.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gameiro SR, Caballero JA, Higgins JP, Apelian D, Hodge JW. Exploitation of differential homeostatic proliferation of T-cell subsets following chemotherapy to enhance the efficacy of vaccine-mediated antitumor responses. Cancer Immunol Immunother. 2011;60(9):1227–1242. doi: 10.1007/s00262-011-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Corral LG, Haslett PA, Muller GW, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163(1):380–386. [PubMed] [Google Scholar]
- 182.Noonan KA, Ferguson A, Emerling A, et al. Lenalidomide augments immune responses to prevnar vaccination in relapsed myeloma patients: implications for cancer and infectious vaccines. American Society of Hematology 2009 Annual Meeting. Blood. 2009;114 (Abstract 1864) [Google Scholar]
- 183.Farsaci B, Sabzevari H, Higgins JP, et al. Effect of a small molecule BCL-2 inhibitor on immune function and use with a recombinant vaccine. Int J Cancer. 2010;127(7):1603–1613. doi: 10.1002/ijc.25177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Finke JH, Rini B, Ireland J, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14(20):6674–6682. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 185.Ko JS, Zea AH, Rini BI, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15(6):2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 186.Farsaci B, Higgins JP, Hodge JW. Consequence of dose scheduling of sunitinib on host immune response elements and vaccine combination therapy [published online ahead of print June 1, 2011] Int J Cancer. doi: 10.1002/ijc.26219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Boni A, Cogdill AP, Dang P, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70(13):5213–5219. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 188.Araki K, Turner AP, Shaffer VO, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460(7251):108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ohtani M, Nagai S, Kondo S, et al. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112(3):635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lee DK, Hakim FT, Gress RE. The thymus and the immune system: layered levels of control. J Thorac Oncol. 2010;5(10) suppl 4:S273–S276. doi: 10.1097/JTO.0b013e3181f20474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sportes C, Hakim FT, Memon SA, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205(7):1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Williams KM, Gress RE. Immune reconstitution and implications for immunotherapy following haematopoietic stem cell transplantation. Best Pract Res Clin Haematol. 2008;21(3):579–596. doi: 10.1016/j.beha.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Arredouani MS, Tseng-Rogenski SS, Hollenbeck BK, et al. Androgen ablation augments human HLA2.1-restricted T cell responses to PSA self-antigen in transgenic mice. Prostate. 2010;70(9):1002–1011. doi: 10.1002/pros.21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98(25):14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Madan RA, Gulley JL, Schlom J, et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14(14):4526–4531. doi: 10.1158/1078-0432.CCR-07-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bilusic M, Gulley J, Heery C, et al. A randomized phase II study of flutamide with or without PSA-TRICOM in nonmetastatic castration-resistant prostate cancer. J Clin Oncol. 2011;29(suppl 7) (Abstract 163) [Google Scholar]
- 197.Antonia SJ, Mirza N, Fricke I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12(3, pt 1):878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 198.Gribben JG, Ryan DP, Boyajian R, et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin Cancer Res. 2005;11(12):4430–4436. doi: 10.1158/1078-0432.CCR-04-2111. [DOI] [PubMed] [Google Scholar]
- 199.Docetaxel and Prednisone With or Without Vaccine Therapy in Treating Patients With Metastatic Hormone-Resistant Prostate Cancer. http://www.clinicaltrials.gov/ct2/show/NCT01145508?term=McNeel&rank=5. Accessed March 29, 2011. [Google Scholar]
- 200.Butterfield LH, Palucka AK, Britten CM, et al. Recommendations from the iSBTc-SITC/FDA/NCI Workshop on Immunotherapy Biomarkers. Clin Cancer Res. 2011;17(10):3064–3076. doi: 10.1158/1078-0432.CCR-10-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jacoby RF, Seibert K, Cole CE, Kelloff G, Lubet RA. The cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the min mouse model of adenomatous polyposis. Cancer Res. 2000;60(18):5040–5044. [PubMed] [Google Scholar]
- 202.Zeytin HE, Patel AC, Rogers CJ, et al. Combination of a poxvirus-based vaccine with a cyclooxygenase-2 inhibitor (celecoxib) elicits antitumor immunity and long-term survival in CEA.Tg/MIN mice. Cancer Res. 2004;64(10):3668–3678. doi: 10.1158/0008-5472.CAN-03-3878. [DOI] [PubMed] [Google Scholar]
- 203.Camus M, Tosolini M, Mlecnik B, et al. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res. 2009;69(6):2685–2693. doi: 10.1158/0008-5472.CAN-08-2654. [DOI] [PubMed] [Google Scholar]
- 204.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 205.Jass JR, Love SB, Northover JM. A new prognostic classification of rectal cancer. Lancet. 1987;1(8545):1303–1306. doi: 10.1016/s0140-6736(87)90552-6. [DOI] [PubMed] [Google Scholar]
- 206.Jochems C, Schlom J. Tumor-infiltrating immune cells and prognosis: the potential link between conventional cancer therapy and immunity. Exp Biol Med (Maywood). 2011;236(5):567–579. doi: 10.1258/ebm.2011.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 208.Pages F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27(35):5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 209.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]