Abstract
Background
Breast-conserving surgery (BCS) is the most common treatment for ductal carcinoma in situ (DCIS); however, how often women experience subsequent diagnostic evaluations over time is not known.
Methods
We identified 2948 women with DCIS who were treated with BCS from 1990 to 2001 and followed for up to 10 years at three integrated health-care delivery systems. We calculated the percentages of diagnostic mammograms and ipsilateral invasive procedures following the initial breast excision to treat DCIS, estimated the 10-year cumulative incidence of these procedures, and determined hazard ratios for both types of procedures with Cox regression modeling. All statistical tests were two-sided.
Results
Over 10 years, 907 women (30.8%) had 1422 diagnostic mammograms and 1813 (61.5%) had 2305 ipsilateral invasive procedures. Diagnostic mammograms occurred in 7.3% of women in the first 6 months and continued at a median annual rate of 4.3%. Ipsilateral invasive procedures occurred in 51.5% of women in the first 6 months and continued at a median annual rate of 3.1%. The estimated 10-year cumulative risk of having at least one diagnostic mammogram after initial DCIS excision was 41.0% (95% confidence interval [CI] = 38.5% to 43.5%); at least one invasive procedure, 65.7% (95% CI = 63.7% to 67.8%); and either event, 76.1% (95% CI = 74.1% to 78.1%). Excluding events in the first 6 months following initial DCIS excision, corresponding risks were 36.4% (95% CI = 33.8% to 39.0%) for diagnostic mammograms, 30.4% (95% CI = 26.9% to 33.8%) for invasive procedures, and 49.5% (95% CI = 45.6% to 53.5%) for either event.
Conclusions
Women with DCIS treated with BCS continue to have diagnostic and invasive breast procedures in the conserved breast over an extended period. The frequency of ongoing diagnostic breast evaluations should be included in discussions about treatment.
CONTEXTS AND CAVEATS
Prior knowledge
Breast-conserving surgery (BCS) has been shown to be an effective alternative to mastectomy in the treatment of ductal carcinoma in situ (DCIS). However, the likelihood of additional diagnostic mammograms and invasive procedures following BCS is unknown.
Study design
Women treated with BCS between 1990 and 2001 at three large health-care systems were followed for up to 10 years. The percentages and cumulative incidence of diagnostic mammograms and ipsilateral invasive procedures following first breast excision were calculated.
Contribution
Over 10 years, the cumulative risk of having at least one diagnostic mammogram was 41%, and the risk of at least one invasive procedure was 66% after initial treatment with BCS.
Implications
Although treatment with BCS is a reasonable option for women with DCIS, invasive procedures and diagnostic evaluation for possible recurrent breast cancer can extend over a long period following initial excision and treatment.
Limitations
Women who had mastectomy within 6 months of the first DCIS excision were excluded. Thus, the rate of imaging and invasive procedures in the first 6 months may have been underestimated. Other imaging procedures such as breast magnetic resonance imaging were not evaluated. The cohort was drawn from women enrolled in three integrated health-care delivery systems, and therefore the findings may not be applicable to other settings.
From the Editors
Breast-conserving surgery (BCS) is commonly used to treat women with ductal carcinoma in situ (DCIS) and has been shown to be efficacious in reducing breast cancer mortality (1–4). Because BCS spares most of the breast, it is often considered to be most consistent with women’s preferences and improved quality of life (5–8). Although BCS for DCIS is an effective and safe alternative to mastectomy for women wanting to preserve their breasts, women continue to be at risk for recurrences in the ipsilateral breast. Up to 20% of women with DCIS will have a recurrence within 5 years, and about half of the recurrences will be invasive (1–3). Because women treated with BCS continue to undergo clinical and radiographic surveillance, partly due to heightened patient and/or clinician concerns (9,10), more women are likely to be at risk for subsequent diagnostic evaluations than those who are ultimately diagnosed with a recurrence.
Previous studies have reported a substantial risk of re-excisions following initial attempts at BCS, often leading to mastectomy (11–13). However, to our knowledge, no studies have reported the rates of subsequent diagnostic evaluations for recurrent breast cancer years after BCS. The clinical consequences of both diagnostic mammograms and invasive procedures are important because they may lead to anxiety and potential overtreatment. Understanding the likelihood of additional diagnostic imaging and invasive procedures may contribute to women's decision making about treatment. We determined the proportions, predictors, and cumulative incidence of diagnostic mammograms and ipsilateral invasive procedures experienced by a large cohort of women with DCIS who were treated with BCS between 1990 and 2001 and followed for up to 10 years.
Methods
Setting and Participants
The study was conducted under the auspices of the National Cancer Institute–funded Cancer Research Network, a consortium of 11 (now 14) integrated health-care delivery systems with more than 11 million enrollees (14). Three sites contributed patients to this study including Kaiser Permanente Northern California, Kaiser Permanente Southern California, and Harvard Pilgrim Health Care; all three Institutional Review Boards approved the study.
The original cohort was assembled to study risk factors for DCIS recurrence after BCS, and methods have been previously described (15,16). Briefly, we identified women who were diagnosed with an index unilateral DCIS between 1990 and 2001, were 85 years or younger at diagnosis, and had no prior breast cancer or another invasive cancer (except non-melanoma skin cancer). Women were excluded if the index DCIS was bilateral, the majority of care for DCIS was obtained outside the participating sites, they were followed for less than 6 months after diagnosis, they were diagnosed with invasive breast cancer, or had mastectomy within 6 months of the index DCIS.
Data Sources
As previously described (15,16), we used Surveillance, Epidemiology, and End Results (SEER)–affiliated cancer registries to identify women at Kaiser Permanente Northern California and Southern California. At Harvard Pilgrim Health Care, we initially identified women using claims data followed by review of electronic outpatient medical records. At all sites, trained abstractors reviewed the medical records of potentially eligible patients to confirm the diagnosis, laterality, and treatment of the index DCIS, and to obtain information on patient and clinical factors at diagnosis. Standard quality control measures were used, which included training of the medical record abstractors, the standardization of data collection and processing, ongoing monitoring to ensure timeliness and accuracy of study protocols, and an independent abstraction of 10% of the charts by a second abstractor.
Definitions of Diagnostic Mammograms and Invasive Procedures
Beginning with the date of the first breast excision to treat DCIS (hereafter called the index DCIS excision), all diagnostic mammograms, invasive breast procedures, and breast cancer events were recorded for up to 10 years for each woman. We defined diagnostic mammograms as mammograms obtained for new symptoms and/or new abnormalities on a prior breast examination or mammogram and routine surveillance mammograms as those done in the absence of any new symptoms, signs, or abnormalities. In this analysis, we include only diagnostic mammograms. The laterality of the diagnostic mammogram was not initially abstracted from the medical records; medical record review and automated codes were later used to classify mammograms obtained only on the contralateral breast at two sites (Kaiser Permanente Northern California and Harvard Pilgrim Health Care). Detailed information was collected on all invasive breast procedures including laterality, type (fine needle aspiration, core biopsy, excisional biopsy, and mastectomy), dates, and pathology results. Indications for invasive procedures were not abstracted.
Covariates
We included the following patient characteristics recorded at the time of DCIS diagnosis: age (25–49, 50–59, 60–69, and 70–84 years), race (white, Asian, black, Hispanic, other, unknown), body mass index (<25, 25–29, ≥30 kg/m2, unknown), menopausal status (postmenopausal, pre/perimenopausal, unknown), diabetes mellitus (type I or II) (yes, no, unknown), family history of breast cancer (yes, no, unknown), and use of menopausal hormone therapy (former, current, never, unknown). Body mass index was obtained using the weight and height recorded before and closest to the index DCIS excision. Postmenopausal status was defined as having the last menstrual period noted in the chart 12 or more months before diagnosis, having had surgical menopause, having documentation of postmenopausal status in the medical record, or age of at least 60 years at diagnosis. Family history was defined as having breast cancer in a first-degree relative noted at or within 6 months of DCIS diagnosis.
Statistical Analyses
First, we described the patient cohort, including characteristics at the time of DCIS diagnosis, year of diagnosis, and initial treatment. We then recorded all diagnostic mammograms and invasive ipsilateral procedures after the index DCIS excision until one of the following events: recurrent ipsilateral DCIS and/or invasive breast cancer, contralateral DCIS and/or invasive breast cancer, last follow-up date, date of death, 10 years after index DCIS excision, or the end of the study (February 24, 2006), whichever occurred first. When we excluded contralateral mammograms obtained by the women at the two sites (n = 105, 7.4%), the overall results were similar; therefore, the entire cohort was included in the analysis.
Second, we calculated the percentage of women who had at least one diagnostic mammogram and/or ipsilateral invasive procedure and mean number of procedures per woman for each of the 10 years of follow-up, beginning immediately after the index DCIS excision. Point and interval estimates of hazard ratios (HR) for diagnostic mammograms and invasive procedures in relation to patient and treatment factors were obtained with Cox proportional hazards regression models. Wald tests were conducted to calculate two-sided statistical significance. The proportional hazards assumption was tested by fitting models containing cross-product terms between time (log) and patient and treatment factors. In general, we found no evidence of non-proportionality in hazard ratios, although several variables did meet the 5% significance level criteria. Further examination of time-stratified analyses demonstrated that heterogeneity was relatively modest and not of clinical significance; we therefore present results without stratification on time.
We estimated the cumulative incidence of having at least one diagnostic mammogram, ipsilateral invasive procedure, or either event over the 10-year period after the index DCIS excision, taking into account the risk of experiencing the other events of interest. These models distinguish between patients who are still alive and those who have already failed from competing causes and allow direct inference regarding the effects of covariates on the cumulative incidence function (17). In this case, in estimating the cumulative incidence for diagnostic mammograms, the likelihood of experiencing an ipsilateral invasive procedure during this period is taken into account.
Because diagnostic evaluations and re-excisions are common immediately following an index DCIS excision, we also calculated the cumulative incidence of each of these procedures starting 6 months after the index DCIS excision. To assess the rates of recurrences compared with the rates of diagnostic evaluations, we used the same methodology to estimate the cumulative incidence of recurrent ipsilateral DCIS and/or invasive breast cancer over the 10-year period. All analyses were done using SAS v. 9.1 (SAS Institute Inc, Cary, NC). All statistical tests were two-sided.
Results
Patient Selection and Characteristics
A total of 3668 potentially eligible DCIS patients were identified for the cohort study. Of these, 520 were ineligible for one or more of the following reasons: miscoding of DCIS in the tumor registry (n = 97), prior breast or other cancer (n = 216), bilateral breast cancer at diagnosis (n = 29), 85 years of age or older at diagnosis (n = 15), or had less than 6 months of follow-up (mastectomy within 6 months [n = 96], death within 6 months [n = 6], or not a member at diagnosis or left the participating institution within 6 months [n = 92]). In addition, medical records were unavailable on 82 patients and 29 did not have complete information on adjuvant therapy. Of the 3037 women determined to be eligible by chart review, 42 had no pathology report confirming breast-conserving therapy, and an additional 47 had no pathology-confirmed DCIS, leaving 2948 patients for this analysis.
The women had a mean age of 58.2 years (SD = 11.4 years) and a median follow-up of 4.8 years (range = 0.5–15.7 years). Approximately 42% (n = 1247) were treated with BCS alone, 42% (n = 1243) with adjuvant radiation, 11% (n = 328) with both adjuvant radiation and tamoxifen, and 4% (n = 130) with tamoxifen alone (Table 1). Eleven percent (n = 325) of the women had a local recurrence, 173 ipsilateral DCIS and 152 ipsilateral invasive breast cancer.
Table 1.
Characteristics of the 2948 women with DCIS treated with breast-conserving surgery*
| Characteristic | No. (%) |
| Age at diagnosis, y | |
| 25–49 | 753 (25.5) |
| 50–59 | 842 (28.6) |
| 60–69 | 790 (26.8) |
| 70–84 | 563 (19.1) |
| Diagnosis year | |
| 1990–1991 | 247 (8.4) |
| 1992–1993 | 347 (11.8) |
| 1994–1995 | 441 (15.0) |
| 1996–1997 | 553 (18.8) |
| 1998–1999 | 655 (22.2) |
| 2000–2001 | 705 (23.9) |
| Race | |
| White | 2015 (68.4) |
| Asian | 353 (12.0) |
| Black | 285 (9.7) |
| Hispanic | 252 (8.5) |
| Other | 7 (0.2) |
| Unknown | 36 (1.2) |
| BMI at diagnosis, kg/m2 | |
| <25 (normal) | 1107 (37.6) |
| 25–29 (overweight) | 900 (30.5) |
| ≥30 (obese) | 647 (21.9) |
| Unknown | 294 (10.0) |
| Menopausal status at diagnosis | |
| Postmenopausal | 2033 (69.0) |
| Pre/perimenopausal | 849 (28.8) |
| Unknown | 66 (2.2) |
| Diabetes at diagnosis | |
| Yes | 213 (7.2) |
| No | 2729 (92.6) |
| Unknown | 6 (0.2) |
| Family history of breast cancer at diagnosis | |
| Yes | 518 (17.6) |
| No | 2362 (80.1) |
| Unknown | 68 (2.3) |
| Menopausal hormone therapy use at diagnosis | |
| Former | 1230 (41.7) |
| Current | 635 (21.5) |
| Never | 839 (28.5) |
| Unknown | 244 (8.3) |
| Treatment of DCIS | |
| Breast-conserving surgery alone | 1247 (42.3) |
| Breast-conserving surgery/radiation | 1243 (42.2) |
| Breast-conserving surgery/radiation/tamoxifen | 328 (11.1) |
| Breast-conserving surgery/tamoxifen | 130 (4.4) |
N = 2948; BMI = body mass index; DCIS = ductal carcinoma in situ.
Diagnostic Mammograms and Ipsilateral Invasive Procedures
Over 10 years of follow-up, 907 (30.8%) of the women had 1422 diagnostic mammograms (Table 2) to evaluate new abnormalities on breast examination or surveillance mammogram (n = 1213 events, 85.3%) or new symptoms (n = 209 events, 14.7%). Most women (61.5%, n = 1813) had at least one ipsilateral invasive procedure over the study period (Table 2). Of the 2305 procedures, excisional biopsies were the most common (84.0%), whereas core biopsies accounted for 8.4% and fine needle aspirations for 3.6%. Rates of diagnostic mammograms varied by treatment, with highest rates observed among women treated with adjuvant radiation alone (34.9%) and lowest among those treated with adjuvant tamoxifen alone (22.3%) (Table 2). Similar results were found for invasive procedures, with 64.7% occurring among women treated with adjuvant radiation alone and 49.2% among those treated with adjuvant tamoxifen alone (Table 2).
Table 2.
Diagnostic mammograms and ipsilateral invasive procedures after index DCIS excision in 2948 women over 10 years of follow-up*
| Treatment | No. (%) |
||||||
| Diagnostic mammograms | Any ipsilateral invasive procedures | Excisional biopsy | Core biopsy | FNA | Mastectomy | Other/Unknown | |
| All women (N = 2948) | |||||||
| Patients† | 907 (30.8) | 1813 (61.5) | 1641 (55.7) | 175 (5.9) | 70 (2.4) | 34 (1.2) | 59 (2.0) |
| Events‡ | 1422 (100) | 2305 (100) | 1936 (84.0) | 193 (8.4) | 82 (3.6) | 34 (1.5) | 60 (2.6) |
| BCS only (N = 1247) | |||||||
| Patients | 337 (27.0) | 760 (60.9) | 671 (53.8) | 90 (7.2) | 37 (3.0) | 23 (1.8) | 21 (1.7) |
| Events | 527 (100) | 996 (100) | 807 (81.0) | 101 (10.1) | 43 (4.3) | 23 (2.3) | 22 (2.2) |
| BCS with radiation (N = 1243) | |||||||
| Patients | 434 (34.9) | 804 (64.7) | 739 (59.5) | 66 (5.3) | 29 (2.3) | 8 (0.6) | 27 (2.2) |
| Events | 679 (100) | 1007 (100) | 864 (85.8) | 73 (7.2) | 35 (3.5) | 8 (0.8) | 27 (2.7) |
| BCS with XRT + TAM (N = 328) | |||||||
| Patients | 107 (32.6) | 185 (56.4) | 174 (53.0) | 12 (3.7) | 4 (1.2) | 1 (0.3) | 6 (1.8) |
| Events | 167 (100) | 226 (100) | 203 (89.8) | 12 (5.3) | 4 (1.8) | 1 (0.4) | 6 (2.7) |
| BCS with TAM (N = 130) | |||||||
| Patients | 29 (22.3) | 64 (49.2) | 57 (43.8) | 7 (5.4) | 0 (0) | 2 (1.5) | 5 (3.8) |
| Events | 49 (100) | 76 (100) | 62 (81.6) | 7 (9.2) | 0 (0) | 2 (2.6) | 5 (6.6) |
BCS = breast-conserving surgery; FNA = fine needle aspiration; TAM = adjuvant tamoxifen; XRT = adjuvant radiation treatment.
Percent calculated from total number of women in cohort and in treatment group.
Percent calculated from total number of events for entire cohort and for each treatment group.
Diagnostic mammograms were common immediately after the index DCIS excision (Table 3), occurring in 7.3% of women during the first 6 months and 5.3% of women in the second 6 months. However, diagnostic mammograms were most commonly used during year 2, occurring in 11.4% of women (2.5% of women had more than one diagnostic mammogram). Following the first 6 months, diagnostic mammograms occurred at an annual median of 4.3% (range = 3.5%–11.4%).
Table 3.
Diagnostic mammography after DCIS excision by year of follow-up*
| Year of follow-up | Total No. of women | 0 diagnostic mammograms, % | At least one diagnostic mammogram, No. (%) | 1 diagnostic mammogram, % | 2-4 diagnostic mammograms, % | Diagnostic mammograms, total (mean per patient) |
| 1 (0–6 mo) | 2948 | 92.7 | 214 (7.3) | 6.5 | 0.8 | 241 (0.08) |
| 1 (7–12 mo) | 2937 | 94.7 | 157 (5.3) | 4.9 | 0.5 | 171 (0.06) |
| 2 | 2821 | 88.6 | 321 (11.4) | 8.9 | 2.5 | 398 (0.14) |
| 3 | 2593 | 93.8 | 162 (6.2) | 5.3 | 0.9 | 186 (0.07) |
| 4 | 2296 | 95.8 | 97 (4.2) | 3.6 | 0.6 | 112 (0.05) |
| 5 | 1813 | 95.1 | 89 (4.9) | 4.2 | 0.7 | 105 (0.06) |
| 6 | 1378 | 95.6 | 60 (4.4) | 3.8 | 0.6 | 69 (0.05) |
| 7 | 1051 | 96.4 | 38 (3.6) | 3.3 | 0.3 | 42 (0.04) |
| 8 | 879 | 95.7 | 38 (4.3) | 4.0 | 0.3 | 41 (0.05) |
| 9 | 752 | 96.1 | 29 (3.9) | 3.3 | 0.5 | 34 (0.05) |
| 10 | 571 | 96.5 | 20 (3.5) | 3.0 | 0.5 | 23 (0.04) |
N = 2948. DCIS = ductal carcinoma in situ.
In contrast to diagnostic mammograms, ipsilateral invasive breast procedures were most common in the first 6 months of follow-up, with 51.5% of women undergoing at least one procedure (48.6% undergoing one procedure and 2.9% undergoing more than one procedure; Table 4). Invasive procedures were much less common during the rest of the 10-year follow-up but continued to occur with an annual median rate of 3.1% (range = 1.8%–4.8%).
Table 4.
Ipsilateral invasive procedures after DCIS excision by year of follow-up*
| Year of follow-up | Total No. of women | 0 invasive procedures, % | At least one invasive procedure, No. (%) | 1 invasive procedure, % | 2–4 invasive procedures, % | Invasive procedures, total (mean per patient) |
| 1 (0–6 mo) | 2948 | 48.5 | 1518 (51.5) | 48.6 | 2.9 | 1611 (0.55) |
| 1 (7–12 mo) | 2937 | 95.3 | 137 (4.7) | 4.3 | 0.4 | 149 (0.05) |
| 2 | 2821 | 95.2 | 134 (4.8) | 4.2 | 0.6 | 151 (0.05) |
| 3 | 2593 | 95.5 | 116 (4.5) | 4.0 | 0.4 | 130 (0.05) |
| 4 | 2296 | 96.9 | 71 (3.1) | 2.8 | 0.3 | 77 (0.03) |
| 5 | 1813 | 97.0 | 55 (3.0) | 2.6 | 0.4 | 63 (0.03) |
| 6 | 1378 | 97.8 | 31 (2.2) | 2.1 | 0.1 | 34 (0.02) |
| 7 | 1051 | 96.8 | 34 (3.2) | 2.9 | 0.3 | 37 (0.04) |
| 8 | 879 | 97.2 | 25 (2.8) | 2.7 | 0.1 | 26 (0.03) |
| 9 | 752 | 97.9 | 16 (2.1) | 2.0 | 0.1 | 17 (0.02) |
| 10 | 571 | 98.2 | 10 (1.8) | 1.8 | 0.0 | 10 (0.02) |
N = 2948. Includes fine needle aspiration, needle/core biopsy, excisional biopsy, and mastectomy. DCIS = ductal carcinoma in situ.
Cumulative Incidence of Diagnostic Mammograms, Ipsilateral Invasive Procedures, and Recurrent Breast Cancer
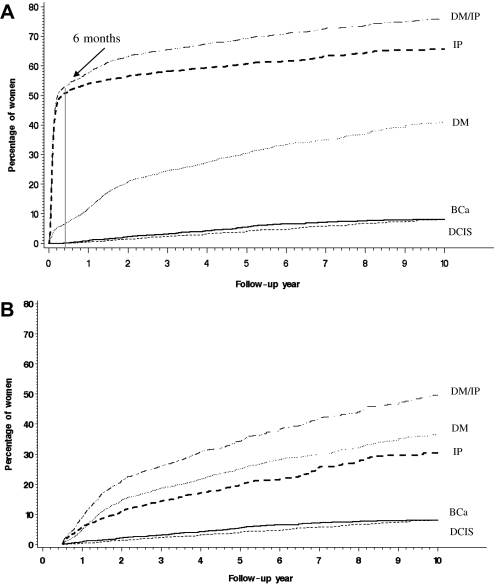
Using cumulative incidence curves, we estimated that following index DCIS excision, 41.0% (95% CI = 38.5% to 43.5%) of the women had at least one diagnostic mammogram, 65.7% (95% CI = 63.7% to 67.8%) had at least one invasive procedure, and 76.1% (95% CI = 74.1% to 78.1%) had either event (Figure 1, A). Ipsilateral invasive procedures were very common in the first 6 months after the index DCIS excision, when 51.3% of the women (95% CI = 49.5% to 53.1%) had at least one or more. Whereas diagnostic mammograms were also common during the first 6 months, the cumulative incidence of 7.3% (95% CI = 6.3% to 8.2%) was much lower than invasive procedures. When events occurring during the first 6 months following index DCIS excision were excluded, 36.4% (95% CI = 33.8% to 39.0%) of the women had at least one diagnostic mammogram over the following 10 years; 30.4% (95% CI = 26.9% to 33.8%) had at least one invasive procedure, and 49.5% (95% CI = 45.6% to 53.5%) had either a diagnostic mammogram or an invasive procedure (Figure 1, B). Over the 10-year follow-up, an estimated 8.0% (95% CI = 6.8% to 9.3%) and 8.1% (95% CI = 6.7% to 9.5%) had recurrent DCIS or invasive cancer, respectively.
Figure 1.
Cumulative incidence of at least one diagnostic mammogram, ipsilateral invasive procedure, recurrent DCIS, or invasive breast cancer in women over 10 years following DCIS excision. This approach estimates the risk of experiencing an event while taking into account the risk of experiencing the other events of interest during this period (17). A) Follow-up begins at the time of index DCIS excision. B) Follow-up begins 6 months after index DCIS excision. BCa = recurrent ipsilateral invasive breast cancer; DCIS = recurrent ipsilateral ductal carcinoma in situ; DM = diagnostic mammogram; IP = invasive procedure. DM/IP, dotted and dashed line; IP, bold dashed line; DM, dotted line; BCa, bold solid line; DCIS, light dashed line. All statistical tests were two-sided.
Factors Associated with Diagnostic Mammograms and Invasive Procedures
Users of menopausal hormones were more likely to undergo diagnostic mammograms than nonusers (HR of diagnostic mammogram: former users, 1.24, 95% CI = 1.03 to 1.48, P = .022; current users, 1.32, 95% CI = 1.08 to 1.60, P = .005) (Table 5). Women who received adjuvant radiation treatment alone were more likely to undergo diagnostic mammograms (HR of diagnostic mammogram = 1.19, 95% CI = 1.02 to 1.38, P = .023) and invasive procedures (HR of invasive procedure = 1.15, 95% CI = 1.04 to 1.28, P = .006) following index DCIS excision than women who had BCS alone. Women aged 70 years and older were less likely to have invasive procedures (HR of invasive procedure = 0.73, 95% CI = 0.60 to 0.90, P = .002) than women under age 50. Although obesity was not related to diagnostic mammograms, obese women were less likely to undergo invasive procedures (HR of invasive procedure = 0.88, 95% CI = 0.78 to 0.99, P = .036). Family history and race were not related to either diagnostic procedure.
Table 5.
Relationship of patient and treatment factors to the risk of diagnostic mammograms and ipsilateral invasive procedures after index DCIS excision in multivariable analyses*
| Factor | Risk of diagnostic mammograms |
Risk of ipsilateral invasive procedures |
||
| HR (95% CI) | Overall P | HR (95% CI) | Overall P | |
| Age at diagnosis, y | .49 | .004 | ||
| 25–49 | 1.00 (referent) | 1.00 (referent) | ||
| 50–59 | 0.92 (0.74 to 1.14) | 0.94 (0.81 to 1.10) | ||
| 60–69 | 0.87 (0.67 to 1.13) | 0.92 (0.76 to 1.10) | ||
| 70–84 | 0.80 (0.60 to 1.07) | 0.73 (0.60 to 0.90)† | ||
| Diagnosis year | .005 | .006 | ||
| 1990–1991 | 1.00 (referent) | 1.00 (referent) | ||
| 1992–1993 | 0.84 (0.64 to 1.11) | 1.09 (0.89 to 1.34) | ||
| 1994–1995 | 0.75 (0.58 to 0.98)‡ | 1.22 (1.01 to 1.48)‡ | ||
| 1996–1997 | 0.83 (0.64 to 1.07) | 1.28 (1.06 to 1.55)† | ||
| 1998–1999 | 1.15 (0.88 to 1.49) | 1.12 (0.92 to 1.36) | ||
| 2000–2001 | 0.95 (0.72 to 1.25) | 0.97 (0.79 to 1.18) | ||
| Race | .72 | .20 | ||
| White | 1.00 (referent) | 1.00 (referent) | ||
| Asian | 0.96 (0.77 to 1.18) | 1.02 (0.88 to 1.18) | ||
| Black | 0.98 (0.78 to 1.24) | 1.16 (1.00 to 1.36) | ||
| Hispanic | 1.06 (0.84 to 1.34) | 0.92 (0.77 to1.10) | ||
| Other | 0.61 (0.28 to 1.31) | 0.84 (0.55 to 1.26) | ||
| BMI at diagnosis, kg/m2 | .43 | .10 | ||
| <25 (normal) | 1.00 (referent) | 1.00 (referent) | ||
| 25–29 (overweight) | 1.01 (0.86 to 1.19) | 0.96 (0.86 to 1.08) | ||
| ≥30 (obese) | 1.11 (0.94 to 1.30) | 0.88 (0.78 to 0.99)‡ | ||
| Menopausal status at diagnosis | .89 | .49 | ||
| Postmenopausal | 1.00 (referent) | 1.00 (referent) | ||
| Pre/perimenopausal | 1.05 (0.84 to 1.32) | 1.05(0.90 to 1.23) | ||
| Diabetes at diagnosis | .65 | .24 | ||
| No | 1.00 (referent) | 1.00 (referent) | ||
| Yes | 1.02 (0.79 to 1.32) | 0.90 (0.74 to 1.09) | ||
| Family history of breast cancer at diagnosis | .59 | .08 | ||
| No | 1.00 (referent) | 1.00 (referent) | ||
| Yes | 1.07 (0.91 to 1.27) | 1.13 (1.00 to 1.27) | ||
| Menopausal hormone therapy use at diagnosis | .03 | .33 | ||
| None | 1.00 (referent) | 1.00 (referent) | ||
| Former Use | 1.24 (1.03 to 1.48)‡ | 1.03 (0.91 to 1.17) | ||
| Current Use | 1.32 (1.08 to 1.60)† | 1.13 (0.99 to 1.30) | ||
| Treatment of DCIS | .08 | .04 | ||
| BCS alone | 1.00 (referent) | 1.00 (referent) | ||
| BCS/tamoxifen | 0.87 (0.59 to 1.29) | 0.96 (0.74 to 1.25) | ||
| BCS/radiation | 1.19 (1.02 to 1.38)‡ | 1.15 (1.04 to 1.28)† | ||
| BCS/radiation/tamoxifen | 1.16 (0.91 to 1.47) | 1.05 (0.88 to 1.26) | ||
Analyses adjusted for diagnosis year; age, race, body mass index, menopausal status, diabetes, family history, menopausal hormone therapy use (all at the time of diagnosis); treatment of DCIS, year of follow-up and site. BCS = breast-conserving surgery; BMI = body mass index; CI = confidence interval; DCIS = ductal carcinoma in situ; HR = hazard ratio. All statistical tests were two-sided.
P < .01.
P < .05.
Discussion
We found that diagnostic mammograms and invasive breast procedures were common in the ipsilateral breast after BCS and continued for as long as 10 years. Overall, we estimated that about three quarters of the women were at risk for at least one of these diagnostic procedures during the first decade after BCS, with one-third of the women experiencing a diagnostic mammogram and two-thirds, an invasive breast procedure. Because approximately half of the women had at least one invasive procedure (usually a re-excision) shortly after the index DCIS excision, we also estimated the cumulative incidence of diagnostic procedures starting 6 months later. We found that when using this cut point, about 50% of the women were at risk for at least one subsequent procedure, 36% for at least one diagnostic mammogram, and 30% for at least one invasive procedure.
Re-excisions to obtain clear margins present a unique burden to women undergoing BCS because women who initially choose to undergo mastectomy following diagnosis are not usually subject to these repeat procedures. Our findings are consistent with those of others (11) who found that between 20% and 70% of women undergoing BCS have repeat excisions to attain clear margins. A recent study (12) that included 714 women with in situ and invasive breast cancer found that 51% of the women had one additional excision, 42% had two excisions, and approximately 7% had three. Notably, 11% of the women ultimately had a mastectomy. Likewise, Morrow et al. (13) found that approximately 38% of 1459 women who initially received BCS had repeat excisions, and 12% of all women who received BCS subsequently had mastectomy. Women with DCIS were most likely to undergo re-excisions compared with women with invasive cancers. Based on these data showing that women often undergo mastectomies following repeat excisions, our study may have underestimated the true frequency of early invasive procedures because we excluded women who had mastectomy within 6 months of diagnosis. Various intraoperative approaches, such as positron emission tomography, radioguided occult lesion localization, and near-infrared fluorescence optical imaging, aimed at reducing re-excisions have been tested (11,18) but at this time are unlikely to be used in most clinical settings.
Diagnostic mammograms shortly following DCIS excision are commonly performed to check for remaining microcalcifications associated with DCIS. Later, diagnostic mammograms are used in follow-up of clinical and/or radiographic findings associated with breast tissue abnormalities that have been reported after surgery and/or breast radiation therapy (19). The latter is consistent with our finding that diagnostic mammograms were more common among those treated with adjuvant radiation therapy. Likewise, invasive procedures were also more common among women treated with adjuvant therapy than BCS alone. Similar to previous randomized controlled trials, we reported lower rates of DCIS recurrences in our cohort with the use of adjuvant therapy (16); however, it appears that adjuvant treatment may in fact be associated with increased rates of subsequent diagnostic imaging. Because radiation therapy use continues to increase, unless methods to differentiate between surgical and/or radiation-induced changes in the breast are developed, the incidence of invasive procedures may also increase.
Increased rates of diagnostic imaging and invasive procedures may be attributable to concerns about recurrences. Women with DCIS have high levels of anxiety about their diagnosis (10,20,21) and as a result, may be more vigilant about new breast symptoms and findings. We found that close to 15% of diagnostic mammograms were attributable to new symptoms. Clinician attention to breast abnormalities among these women at substantial increased risk of local recurrence may also be heightened, resulting in additional diagnostic evaluations.
BCS has become the most common treatment for women with DCIS (1–4,13). However, recent data (22) show that mastectomy rates have again begun to increase, and the rates of contralateral prophylactic mastectomy among women with DCIS almost tripled between 1998 and 2003 (23). The reasons for these trends are not clear but may be related to the growing use of breast magnetic resonance imaging (MRI) and increased awareness of genetic testing (24). Women's preferences also appear to have a role in the decision for BCS vs mastectomy (25–27); a recent study found that after women were informed about mortality, treatment, and recurrences after BCS and mastectomy, 35% chose mastectomy (28). We did not find any study reporting women's choices after being informed about diagnostic procedures that may occur following initial treatment.
Our study also had some limitations. As noted earlier, we may have underestimated imaging and invasive procedures in the first 6 months after DCIS excision because we excluded women who had mastectomy within 6 months of the index DCIS excision. We had incomplete information about the laterality of the diagnostic mammograms and may have overestimated their risk by including only mammograms of the contralateral breast, which may have occurred regardless of initial DCIS treatment. However, when we excluded contralateral mammograms at two sites (7.4%), the results were similar. Furthermore, we did not evaluate the use of other imaging procedures, such as breast mri, which was rare during the study period. Because breast mri use continues to increase, invasive procedures to investigate abnormal findings may become more common (29). Alternatively, it is also possible that additional imaging may reduce the use of surgical procedures. Finally, our cohort was drawn from women enrolled in three integrated health-care delivery systems; therefore, our study findings may not be generalizable to other settings. However, unlike academic or research settings, these settings may better represent “real-world” clinical practice. Because our systems are integrated and continue to provide care for patients following diagnosis with low disenrollment rates (30), we were able to obtain comprehensive information about our patients’ evaluations over time.
Breast-conserving treatment is a reasonable option for women with DCIS and results in similar long-term mortality outcomes as mastectomy; however, invasive procedures and diagnostic evaluation workup for possible recurrent breast cancer extends over a long period following DCIS excision and treatment. The frequent need for re-excision among women choosing BCS warrants more scientific attention, including efforts to decrease its occurrence. However, decreasing early re-excisions and long-term diagnostic evaluations to detect recurrences among women at risk for recurrences remains a challenge. Although our findings do not directly address the relative benefits and harms of mastectomy vs BCS, they can inform women and their clinicians about the frequency of diagnostic imaging and invasive procedures following BCS and assist them in making treatment decisions. The fact that women undergoing BCS are likely to have diagnostic and invasive breast procedures in the conserved breast over an extended period of time is important and needs to be included in discussions about treatment options.
Funding
Supported by Grant U19CA79689 (LN, LAH, NA, RH, LCC, SJS, CPQ, SWF) to the Cancer Research Network from the National Cancer Institute, Increasing Effectiveness of Cancer Control Interventions and by Public Health Service Grant R01CA81302 (LAH, CPQ).
Footnotes
The funders did not have any involvement in the design of the study; the collection, analysis, and interpretation of the data; the writing of the article; or the decision to submit the article for publication.
References
- 1.Fisher B, Costantino J, Redmond C, et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med. 1993;328(22):1581–1586. doi: 10.1056/NEJM199306033282201. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353(9169):1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from the National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998;16(2):441–452. doi: 10.1200/JCO.1998.16.2.441. [DOI] [PubMed] [Google Scholar]
- 4.Baxter NN, Virnig BA, Durham SB, Tuttle TM. Trends in the treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2004;96(6):443–448. doi: 10.1093/jnci/djh069. [DOI] [PubMed] [Google Scholar]
- 5.Curran D, van Dongen JP, Aaronson NK, et al. Quality of life of early-stage breast cancer patients treated with radical mastectomy or breast-conserving procedures: results of EORTC Trial 10801. The European Organization for Research and Treatment of Cancer (EORTC), Breast Cancer Co-operative Group (BCCG) Eur J Cancer. 1998;34(3):307–314. doi: 10.1016/s0959-8049(97)00312-2. [DOI] [PubMed] [Google Scholar]
- 6.de Haes JC, van Oostrom MA, Welvaart K. The effect of radical and conserving surgery on the quality of life of early breast cancer patients. Eur J Surg Oncol. 1986;12(4):337–342. [PubMed] [Google Scholar]
- 7.Nissen MJ, Swenson KK, Ritz LJ, Farrell JB, Sladek ML, Lally RM. Quality of life after breast carcinoma surgery: a comparison of three surgical procedures. Cancer. 2001;91(7):1238–1246. [PubMed] [Google Scholar]
- 8.Janz NK, Mujahid M, Lantz PM, et al. Population-based study of the relationship of treatment and sociodemographics on quality of life for early stage breast cancer. Qual Life Res. 2005;14(6):1467–1479. doi: 10.1007/s11136-005-0288-6. [DOI] [PubMed] [Google Scholar]
- 9.Partridge A, Winer JP, Golshan M, et al. Perceptions and management approaches of physicians who care for women with ductal carcinoma-in-situ (DCIS) Clin Breast Cancer. 2008;8(3):275–280. doi: 10.3816/CBC.2008.n.032. [DOI] [PubMed] [Google Scholar]
- 10.Partridge A, Adloff K, Blood E, et al. Risk perceptions and psychosocial outcomes of women with ductal carcinoma in situ: longitudinal results from a cohort study. J Natl Cancer Inst. 2008;100(4):243–251. doi: 10.1093/jnci/djn010. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs L. Positive margins: the challenge continues for breast surgeons. Ann Surg Oncol. 2008;15(5):1271–1272. doi: 10.1245/s10434-007-9766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waljee J, Hu E, Newman L, Alderman A. Predictors of re-excision among women undergoing breast-conserving surgery for cancer. Ann Surg Oncol. 2008;15(5):1297–1303. doi: 10.1245/s10434-007-9777-x. [DOI] [PubMed] [Google Scholar]
- 13.Morrow M, Jagsi R, Alderman AK, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA. 2009;302(14):1551–1556. doi: 10.1001/jama.2009.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner EH, Greene SM, Hart G, et al. Building a research consortium of large health systems: the Cancer Research Network. J Natl Cancer Inst Monogr. 2005;(35):3–11. doi: 10.1093/jncimonographs/lgi032. [DOI] [PubMed] [Google Scholar]
- 15.Nekhlyudov L, Habel LA, Achacoso NS, et al. Adherence to long-term surveillance mammography among women with ductal carcinoma in situ treated with breast-conserving surgery. J Clin Oncol. 2009;27(19):3211–3216. doi: 10.1200/JCO.2008.18.5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habel LA, Achacoso NS, Haque R, et al. Declining recurrence rates among DCIS patients treated with breast-conserving surgery in the community setting. Breast Cancer Res. 2009;11(6):R85. doi: 10.1186/bcr2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007;13(2, pt 1):559–565. doi: 10.1158/1078-0432.CCR-06-1210. [DOI] [PubMed] [Google Scholar]
- 18.Pleijhuis RG, Graafland M, de Vries J, Bart J, de Jong JS, van Dam GM. Obtaining adequate surgical margins in breast-conserving therapy for patients with early-state breast cancer: current modalities and future directions. Ann Surg Oncol. 2009;16(10):2717–2730. doi: 10.1245/s10434-009-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dershaw DD. Mammography in patients with breast cancer treated by breast conservation (lumpectomy with or without radiation) AJR Am J Roentgenol. 1995;164(2):309–316. doi: 10.2214/ajr.164.2.7839960. [DOI] [PubMed] [Google Scholar]
- 20.Bluman LG, Borstelmann NA, Rimer BK, Iglehart JD, Winer EP. Knowledge, satisfaction, and perceived cancer risk among women diagnosed with ductal carcinoma in situ. J Womens Health Gend Based Med. 2001;10(6):589–598. doi: 10.1089/15246090152543175. [DOI] [PubMed] [Google Scholar]
- 21.De Morgan S, Redman S, White KJ, Cakir B, Boyages J. “Well, have I got cancer or haven’t I?” The psycho-social issues for women diagnosed with ductal carcinoma in situ. Health Expect. 2002;5(4):310–318. doi: 10.1046/j.1369-6513.2002.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katipamula R, Degnim AC, Hoskin T, Boughey JC, Loprinzi C, Grant CS. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol. 2009;27(25):4082–4088. doi: 10.1200/JCO.2008.19.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009;27(9):1362–1367. doi: 10.1200/JCO.2008.20.1681. [DOI] [PubMed] [Google Scholar]
- 24.Morrow M, Harris JR. More mastectomies: is this what patients really want? J Clin Oncol. 2009;27(25):4038–4040. doi: 10.1200/JCO.2009.23.0078. [DOI] [PubMed] [Google Scholar]
- 25.Fagerlin A, Lakhani I, Lantz PM, et al. An informed decision? Breast cancer patients and their knowledge about treatment. Patient Educ Couns. 2006;64(1–3):302–312. doi: 10.1016/j.pec.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Goel V, Sawka CA, Thiel EC, Gort EH, O’Connor AM. Randomized trial of a patient decision aid for choice of surgical treatment for breast cancer. Med Decis Making. 2001;21(1):1–6. doi: 10.1177/0272989X0102100101. [DOI] [PubMed] [Google Scholar]
- 27.Katz SJ, Lantz PM, Janz NK, et al. Patterns and correlates of local therapy for women with ductal carcinoma-in-situ. J Clin Oncol. 2005;23(13):3001–3007. doi: 10.1200/JCO.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins ED, Moore CP, Clay KF, et al. Can women with early-stage breast cancer make an informed decision for mastectomy? J Clin Oncol. 2009;27(4):519–525. doi: 10.1200/JCO.2008.16.6215. [DOI] [PubMed] [Google Scholar]
- 29.Smith-Bindman R, Miglioretti DL, Larson EB. Rising use of diagnostic medical imaging in a large integrated health system. Health Aff (Milwood). 2008;27(6):1491–1502. doi: 10.1377/hlthaff.27.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Field TS, Cernieux J, Buist DS, et al. Retention of enrollees following a cancer diagnosis within health maintenance organizations in the Cancer Research Network. J Nat Cancer Inst. 2004;96(2):148–152. doi: 10.1093/jnci/djh010. [DOI] [PubMed] [Google Scholar]