Abstract
Among the most common parasites of Drosophila in nature are parasitoid wasps, which lay their eggs in fly larvae and pupae. D. melanogaster larvae can mount a cellular immune response against wasp eggs, but female wasps inject venom along with their eggs to block this immune response. Genetic variation in flies for immune resistance against wasps and genetic variation in wasps for virulence against flies largely determines the outcome of any fly-wasp interaction. Interestingly, up to 90% of the variation in fly resistance against wasp parasitism has been linked to a very simple mechanism: flies with increased constitutive blood cell (hemocyte) production are more resistant. However, this relationship has not been tested for Drosophila hosts outside of the melanogaster subgroup, nor has it been tested across a diversity of parasitoid wasp species and strains. We compared hemocyte levels in two fly species from different subgroups, D. melanogaster and D. suzukii, and found that D. suzukii constitutively produces up to five times more hemocytes than D. melanogaster. Using a panel of 24 parasitoid wasp strains representing fifteen species, four families, and multiple virulence strategies, we found that D. suzukii was significantly more resistant to wasp parasitism than D. melanogaster. Thus, our data suggest that the relationship between hemocyte production and wasp resistance is general. However, at least one sympatric wasp species was a highly successful infector of D. suzukii, suggesting specialists can overcome the general resistance afforded to hosts by excessive hemocyte production. Given that D. suzukii is an emerging agricultural pest, identification of the few parasitoid wasps that successfully infect D. suzukii may have value for biocontrol.
Introduction
Fruitflies of the genus Drosophila are regularly attacked by parasitoid wasps. In natural D. melanogaster populations, upwards of 50% of fly larvae are found to be infected by wasps, suggesting they exert extremely strong selection pressures on Drosophila populations in nature [1], [2], [3]. Once infected, fruitfly larvae mount an immune response against wasp eggs, termed melanotic encapsulation, that is thought to involve several steps [4], [5]: The response begins when circulating, constitutively produced plasmatocytes recognize the wasp egg as foreign and signal to induce the differentiation of larger lamellocytes from pro-hemocytes in the lymph gland (the fly hematopoietic organ) and from other circulating plasmatocytes (via the intermediate podocyte form) [6], [7]. These newly derived lamellocytes migrate towards, and attach and spread around the wasp egg in a multi-layered capsule. In the final step, the inner cells of the capsule surrounding the wasp egg lyse and release reactive oxygen species and an impermeable layer of melanin, resulting in death of the wasp egg. However, parasitoid wasps can potentially evade host immune responses by using a non-reactive coating on their eggs, or suppress host immunity by injecting venom into hosts along with their eggs. There is both between and within species genetic variation in flies for resistance against wasps and among wasps for virulence against flies [8], [9], [10], [11], [12], [13], [14], [15], [16].
In previous work, Drosophila species from the melanogaster subgroup were found to have significantly different numbers of constitutively produced plasmatocytes, and there was a significant correlation (r2 = 0.90) between plasmatocyte counts and ability to melanotically encapsulate the eggs of the immune-evasive parasitoid wasp Asobara tabida [12]. It was also found that D. melanogaster strains artificially selected for resistance against A. tabida showed a significant increase in plasmatocyte numbers [17]. Furthermore, D. simulans, which makes significantly more plasmatocytes than its sister species D. melanogaster, was significantly more resistant against the more immune-suppressive wasp A. citri [18]. Finally, D. melanogaster mutants producing a wide range of hemocyte counts showed a significant correlation (r2 = 0.45) between constitutive plasmatocyte numbers and encapsulation ability against the wasp Leptopilina boulardi [19]. Altogether, this work suggests that high constitutive production of hemocytes is an effective and relatively simple mechanism by which hosts can evolve resistance to one of their most common groups of parasites.
We were interested in whether the relationship between Drosophila standing immune defense (hemocyte production) and immune resistance against wasps is general across a large panel of diverse wasp lineages with unique infection strategies, and whether the relationship extends beyond the melanogaster subgroup of the genus Drosophila. Pilot data from a study aimed at characterizing hemocyte lineages across the genus Drosophila (unpublished) suggested D. suzukii, a member of the melanogaster group but not the melanogaster subgroup, constitutively produces an extremely large number of hemocytes compared to other Drosophila. Thus, the goal of this study was to confirm whether D. suzukii constitutively produces higher numbers of hemocytes than D. melanogaster, and if so, to determine whether D. suzukii was also more resistant against a large panel of parasitoid wasp species and strains.
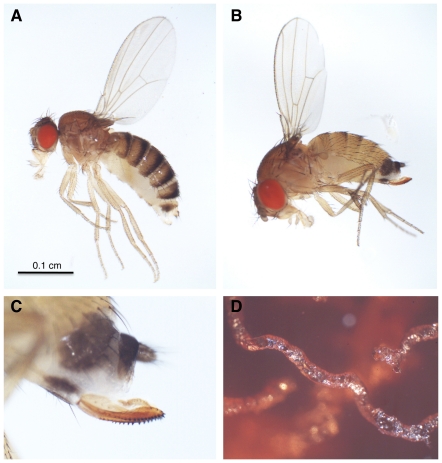
D. suzukii is native to east Asia but has recently gained widespread attention due to its spread as an agricultural pest in Europe and North America (Figure 1) [20], [21], [22], [23]. Although most of the ∼1,500 described Drosophila species lay their eggs and feed on decaying plant and fungal tissues, including rotting fruits (like D. melanogaster), D. suzukii is one of a handful of species that live on ripe fruits, using its serrated ovipositor to lay eggs in the flesh of soft-skinned fruits (Figure 1C). Its larvae subsequently burrow through the body of the fruit as they eat (Figure 1D), allowing bacteria and other microorganisms access to the inside of the fruit, which results in premature rotting. Because parasitoid wasps have been successfully used as biocontrol agents against a wide range of insect agricultural pests, including Coleopterans (e.g., weevils, bean beetles), Hemipterans (e.g., scale insects, whiteflies, aphids, leafhoppers, stinkbugs), Lepidopterans (e.g., various moth and butterfly larvae), and Dipterans (e.g., Tephritid fruitflies, blackflies) [24], [25], [26], [27], [28], study of D. suzukii resistance and susceptibility to parasitoid wasps may have added applied value.
Figure 1. Fly morphology and behavior.
(A) Female D. melanogaster; (B) female D. suzukii; (C) serrated ovipositor from female D. suzukii; (D) tunnel excavated by D. suzukii larva through agar food plate.
At least four families of parasitoid wasps are known to attack Drosophila in nature [29]. These wasps use a variety of infection strategies to defeat the fly immune response, including immune suppressive and evasive tactics, and vary in their host ranges from specialists of particular Drosophila species to generalist of the genus. Members of the families Braconidae and Figitidae are larval parasites – they lay single eggs in Drosophila larvae and, if not killed, the hatched wasp larva begins to consume internal fly tissues before eventually killing the fly and eclosing from the fly pupal case. Members of the families Diapriidae and Pteromalidae are pupal parasites - they lay single eggs inside Drosophila pupae, and the hatched wasp larva consumes the fly pupal tissues, also eventually killing the fly and eclosing from the fly pupal case. It is unclear whether fly pupae can mount an immune response or otherwise defend themselves once infected by pupal parasites. Pupal parasites of the genus Trichopria (Family Diapriidae) lay their eggs in the Drosophila hemocoel, like larval parasites, but those of the genus Pachycrepoideus (Family Pteromalidae) lay their eggs in the space between the Drosophila pupal case and the pupa, and act as ectoparasites in the early stages of their life by sucking fluids from the pupa externally [29]. A lack of pupal immunity against wasps may explain in part why pupal parasitoid wasps are thought to have more generalist host ranges than larval parasitoid wasps [30], [31].
The Drosophila-wasp system is ripe for study as a model for the co-evolution of pathogen infection strategies and host immune responses across lineages and communities of pathogens and hosts [32]. We attempted to answer the following questions: Is the melanotic encapsulation response observed in D. melanogaster conserved in D. suzukii? Does D. suzukii have higher constitutive hemocyte production than D. melanogaster? Is increased hemocyte production by D. suzukii associated with stronger resistance against a panel of parasitoid wasps with diverse life histories and infection strategies? Do wasps make different oviposition choices depending on host species? Do wasp phylogeny and biogeography play any role in fly-wasp interactions? From an applied point of view, which parasitoid wasp species show the most potential for use in D. suzukii biocontrol in the field?
Materials and Methods
Insect Species
The D. melanogaster genome strain 14021-0231.36 was acquired from the Drosophila Species Stock Center and was grown on standard cornmeal/yeast/molasses Drosophila medium. The two additional D. melanogaster strains originated from single wild-caught females collected in Atlanta, GA in the summer of 2010. The primary D. suzukii strain tested originated from four wild-caught females collected in Atlanta, GA in the summer of 2010, while two additional isofemale strains were collected in Atlanta, GA in the summer of 2011. D. suzukii were maintained on standard Drosophila medium supplemented with (thawed) frozen raspberries, which were found to enhance egg-laying but were otherwise unnecessary for fly development.
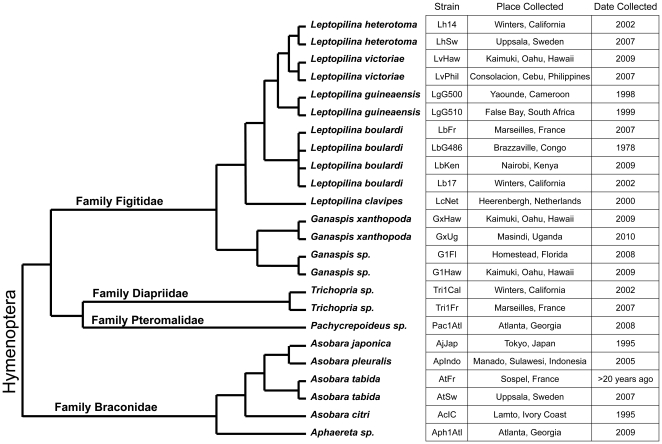
A total of 24 Drosophila parasitoid wasp strains collected from around the world were used for infection trials on D. melanogaster and D. suzukii (Figure 2). Strains LgG500 and LgG510 were provided by R. Allemand, strain LbG486 was provided by D. Hultmark, strains LcNet, AjJap, ApIndo, and AcIC were provided by J. van Alphen, strain GxUg was provided by J. Pool, and strain AtFr was provided by B. Wertheim. All other strains were collected by the Schlenke lab. These wasp strains represent: (1) at least 14 species, (2) representatives of all four Hymenopteran families known to infect Drosophila, (3) larval and pupal parasites, and (4) a worldwide range of collection localities (Figure 2). Morphology and cytochrome oxidase I (COI) sequences from the two Trichopria sp.1 strains suggested they were representatives of the same species, perhaps Trichopria drosophila (Ashmead). Furthermore, morphology and COI sequences from the two Ganaspis sp.1 strains suggest they are representatives of a single undescribed species. All wasp species were maintained in the lab on D. melanogaster strain Canton S, with the exception of L. clavipes, A. tabida, Aphaereta sp.1, and Pachycrepoideus sp.1, which were maintained on D. virilis. To grow wasps, adult flies were allowed to lay eggs in standard Drosophila medium for several days before they were replaced by adult wasps, which then attacked the developing fly larvae or pupae. Wasp vials were supplemented with approximately 500 uL of a 50% honey/water solution applied to the inside of the cotton vial plugs. COI sequences for all wasp strains as well as ITS2 sequences for Figitid wasps have been deposited in Genbank under accession numbers JQ808406–JQ808451. Wasp strains are available upon request.
Figure 2. Phylogenetic relationships and provenance of wasps used in this study.
Tree topology is derived from previous phylogenetic studies of Hymenopteran families [68], the family Figitidae [69], [70], and the family Braconidae [71]. Branch lengths are approximated.
Hemocyte Counts
Fly-wasp development for all experiments took place in a 25°C incubator on a 12∶12 light∶dark cycle. For hemocyte count experiments, adult female D. melanogaster and D. suzukii were allowed to lay eggs into fly food supplemented with yeast paste (50∶50 mix of baker's yeast and water) or raspberries, respectively, in 60 mm Petri dishes. After 72 hours, adult flies were removed and developmental stage and size-matched second instar fly larvae were collected for two independent experiments.
For hemocyte count experiments, D. melanogaster and D. suzukii larvae were either uninfected or were infected by the wasp strain LbG486, with three replicates per treatment. For parasitoid infections, 50 fly larvae were moved into 35 mm diameter Petri dishes filled with 1 mL of Drosophila medium. Ten female wasps were immediately allowed to attack these fly larvae for 3 hours, and five larvae per dish were later dissected to determine the number of wasp eggs laid per fly larva. Fourteen of fifteen D. melanogaster larvae across the three replicates were found to be infected by single wasp eggs, as well as fourteen of fifteen D. suzukii larvae, so we assumed the wasp infection rate was very similar across the two host fly species. Hemocytes were counted at two time-points, 12 and 24 hours post-infection, in which the induced cellular immune response was expected to be highly activated. Crystal cells, a distinct hemocyte type described below, were counted independently 33 hours post-infection.
In an experiment to test hemocyte induction absent wasp venom effects, D. melanogaster and D. suzukii larvae were either untreated or were pierced with a sterile needle to simulate the wounding associated with wasp oviposition. Such wounding has been shown to induce the production of lamellocytes [33]. For each of four replicates, 15 fly larvae were rinsed in 1× PBS, dried on Kimwipes, and immobilized on double sided tape. Their posterior cuticles were then pierced with flame-sterilized 0.1 mm diameter stainless steel dissecting pins (Fine Science Tools 26002-10), with care taken to avoid harming internal organs. Fly larvae were then removed from the tape with a wet paintbrush, and allowed to recover in a moist chamber for one hour before being moved to 35 mm diameter Petri dishes filled with 1 mL of Drosophila medium. Control larvae were treated identically except without piercing. Hemocytes were then counted 24 hours post-infection, while crystal cells were counted independently 33 hours post-infection.
To count hemocytes, 5 third instar larvae from each treatment replicate (including controls) were washed in Drosophila Ringer's solution, dried on a Kimwipe, and bled together into 20 µL of 1× PBS solution containing 0.01% phenylthiourea on a glass slide. Dissection into buffer limits evaporation, and phenylthiourea prevents the hemolymph from melanizing [34]. The buffer-hemolymph mixture was applied to a disposable hemocytometer (Incyto C-Chip DHC-N01) and allowed to sit for 30 minutes to allow hemocytes to settle. Hemocytes from each sample were counted from sixteen 0.25×0.25×0.1 mm squares (e.g., Figure 3A, 3B), which make up a total volume of 0.1 µL. Thus, the number of hemocytes from the whole 20 µL sample is expected to be ∼200 times the number counted, or a per larva value of 40 times the number counted.
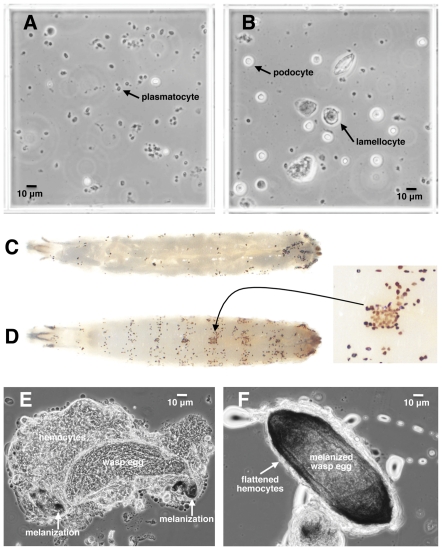
Figure 3. D. suzukii hemocytes and encapsulation of wasp eggs.
(A) A 0.25×0.25×0.1 mm hemocytometer field from normal D. suzukii larvae showing abundant plasmatocytes; (B) hemocytometer field from D. suzukii larvae 12 hours after infection by wasp strain LbG486 showing increased podocyte and lamellocyte numbers; (C) control D. melanogaster larva with melanized crystal cells; (D) control D. suzukii larva with melanized crystal cells, showing color variation in inset; (E) initiation of encapsulation of LbG486 egg by D. suzukii showing loose hemocyte aggregation and melanization at anterior and posterior tips of egg; (F) LbG486 egg melanotically encapsulated by D. suzukii, showing surrounding layer of tightly spread hemocytes.
The addition of hemolymph to the 20 µL of buffer is expected to increase the total buffer-hemolymph volume to greater than 20 µL, leading to a downward bias in our absolute hemocyte counts. However, the amount of hemolymph from five third instar larvae is only approximately 2.5 µL, and in practice about this much liquid evaporates before 20 µL of the buffer-hemolymph mixture can be pipetted onto the hemocytometer. Our hemocyte counts may also underestimate true hemocyte loads because a large fraction of plasmatocytes are sessile (i.e., docked on host tissues) [35], and may not detach from the larval tissues upon dissection. D. melanogaster and D. suzukii adults and larvae are similar in size, (Figure 1, 3), so we did not expect differences in species hemocyte counts to result from fly size differences, but we were careful to use larvae of the same size and developmental stage from both species for all experiments. Hemocytes were classified as plasmatocytes (small round cells with obvious nuclei), podocytes (activated plasmatocytes that are larger and refract more light than plasmatocytes), and lamellocytes (large, clear flattened cells) [7].
The fourth hemocyte cell type, crystal cells, are medium sized cells containing cytoplasmic crystals made up of the substrate that the phenoloxidase enzymatic cascade converts into melanin [36]. The crystals are rapidly lost upon dissection and the cells become difficult to recognize, so a separate method was used to count them. Crystal cells self-melanize when larvae are incubated at 60°C for 10 minutes [37]. Therefore, crystal cells were quantified separately by counting dark spots from the dorsal side of incubated whole larvae (e.g., Figure 3C, 3D) at 33 hours post-infection. Crystal cells were counted and averaged from three larvae per replicate. It is not yet known whether crystal cells play a role in the melanotic encapsulation response [4].
Multivariable regression models assuming Poisson distributions were specified to model hemocyte counts by fly species and immune challenge (wasp infection, piercing). When hemocyte counts were overdispersed, negative binomial distributions were specified instead of Poisson distributions.
Resistance Trials
Each fly-wasp infection combination was replicated three times. Adult female D. melanogaster and D. suzukii were allowed to lay eggs into fly food supplemented with yeast paste (50∶50 mix of baker's yeast and water) or raspberries, respectively, in 60 mm Petri dishes. After 72 hours, adult flies were removed and size-matched second instar fly larvae were collected for infections. For larval parasitoid infections, 50 fly larvae were moved into 35 mm diameter Petri dishes filled with 1 mL of Drosophila medium. Three female wasps were immediately allowed to attack these fly larvae for 72 hours. After attack, 10 of the 50 fly larvae were dissected to determine the percent of larvae infected, the number of wasp eggs laid per fly larva, and the proportion of fly larvae bearing encapsulated wasp eggs in each sample. 30 of the 40 remaining larvae were then moved into Drosophila vials to complete development. For pupal parasitoid infections, 40 fly larvae were moved into vials containing Drosophila medium, and were allowed to develop another 72 hours to the wandering third instar stage, just before they began pupating on top of the medium or on the sides of the vials. Three female wasps were then allowed to attack the fly pupae for 72 hours, at which time the wasps were removed and the fly pupae were left to complete development. The infection conditions were chosen to be optimal for wasp success. Control uninfected flies from both species were reared under identical conditions and showed nearly 100% survival (data not shown).
The total numbers of flies and wasps that eclosed from all wasp treatments were determined 15 days and 30 days post-infection, respectively, times by which all viable flies and wasps should have emerged. Fly-wasp interactions may yield one of three outcomes, which were compared between D. melanogaster and D. suzukii infections: (1) a successful immune response by the fly, (2) a successful parasitism by the wasp, or (3) death of the fly and the wasp within it. Furthermore, for larval parasitoid infections, the numbers of wasp eggs counted from dissected fly larvae were assessed for evidence of under-dispersion, as wasps are known to preferentially choose un-infected hosts for oviposition [38], [39], [40], [41]. If wasps layed eggs in fly larvae randomly, without regard to host infection status, the number of wasp eggs per larva would have been expected to follow a Poisson distribution, where the average number of wasp eggs per fly larva and the variance in the number of wasp eggs per fly larva should have been equal. Thus, for each fly-wasp pair, we compared the average number of wasp eggs laid per 10 dissected fly larvae to the variance in the number wasp eggs laid per 10 dissected fly larvae across the three replicates of each treatment, using one-tailed paired t-tests. Although some figures show data for each wasp strain separately, values for wasp strains of the same species were averaged into single species values for all statistical analyses unless otherwise noted.
Results
Hemocytes
D. suzukii hemocytes were morphologically similar to those of D. melanogaster (Figure 3). In normal D. suzukii larvae, there were an abundance of small round cells in the hemolymph that were presumably homologous to plasmatocytes. In D. suzukii infected by wasps, medium-sized round cells resembling podocytes became much more numerous, as well as large irregular shaped cells that resembled D. melanogaster lamellocytes. Heating D. suzukii larvae resulted in the formation of darkened cells throughout the hemocoel. In D. melanogaster, this phenomenon has been attributed to the self-melanization of crystal cells, and suggested that D. suzukii also possesses hemocytes responsible for carrying melanization factors. Interestingly, while all self-melanized crystal cells in D. melanogaster were dark black (Figure 3C), D. suzukii showed both brown and black cells (Figure 3D, inset). Finally, D. suzukii larvae encapsulated and melanized wasp eggs with hundreds of hemocytes that flattened and spread over the wasp eggs to form a tight capsule (Figure 3E, 3F). Thus, the stereotypic melanotic encapsulation response used by D. melanogaster against parasitoid wasps appears to be conserved in its relative, D. suzukii.
Though hemocyte morphology was similar in the two fly species, we found significant differences in constitutive and induced hemocyte counts between D. melanogaster and D. suzukii. We used two methods for inducing immune responses in these flies. First, we infected flies with wasp strain LbG486, which is relatively avirulent in D. melanogaster and has been shown to induce production of hemocytes, and especially lamellocytes, in particular infected D. melanogaster strains [42], [43]. Second, in order to stimulate lamellocyte production in the absence of any possible immune inhibitory effects of wasp venoms, we pierced D. melanogaster and D. suzukii larvae with sterile needles [33].
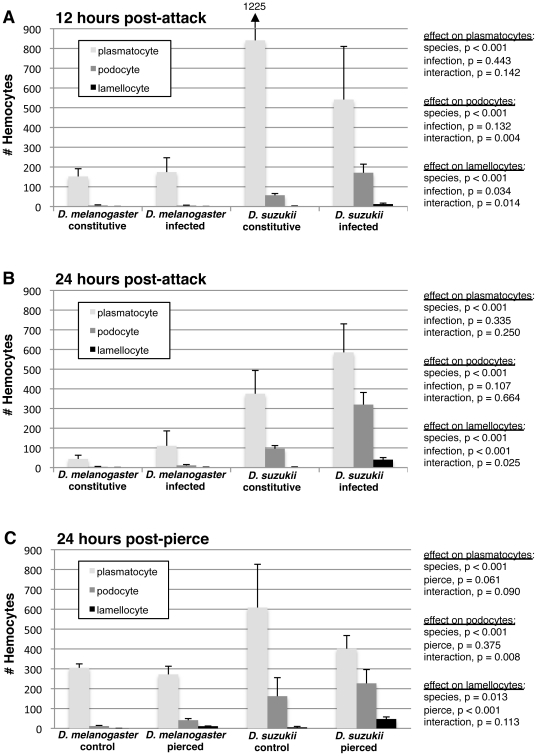
We tested the effects of fly species and immune challenge on fly hemocyte counts using standard regression methods (Figure 4). We found consistent, significant species effects on plasmatocyte, podocyte, and lamellocyte numbers. Across time-points and immune treatments, D. suzukii had significantly more plasmatocytes, producing up to five times more plasmatocytes than D. melanogaster. D. suzukii larvae also produced significantly more podocytes than D. melanogaster, including constitutively produced podocytes, which are not normally found in D. melanogaster larvae. Furthermore, D. suzukii larvae produced significantly more lamellocytes than D. melanogaster larvae. We found no effect of immune challenge on plasmatocyte or podocyte numbers, although as expected there were significantly more lamellocytes in immune-challenged flies. Interestingly, the D. melanogaster genome strain used in the present study was not resistant to LbG486, unlike D. suzukii, (see below), and also showed no significant increase in lamellocyte numbers at two time-points post-infection when infected by LbG486 (Figure 4A, 4B). Finally, there were significant species-by-immune challenge interaction effects on podocyte and lamellocyte numbers in some experiments, usually due to significantly greater induction of these cell types after an immune challenge in D. suzukii. Thus, like D. melanogaster, D. suzukii induces hematopoiesis and/or hemocyte differentiation during a cellular immune response, although this induction is often stronger than that observed in D. melanogaster.
Figure 4. Hemocyte count comparison between D. melanogaster and D. suzukii.
(A) 12 hours after infection by wasp strain LbG486; (B) 24 hours after infection by wasp strain LbG486; (C) 24 hours after piercing with a sterile needle. Average (+) standard deviation shown. Numbers are approximately one fortieth of the number of cells per one fly larva (Methods).
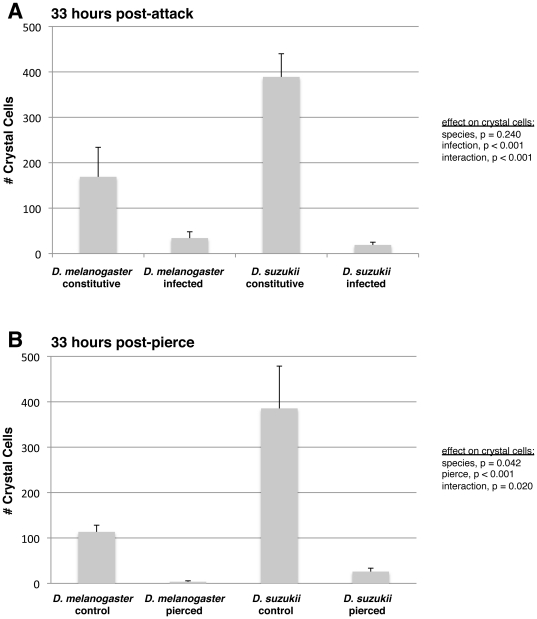
We next tested the effects of fly species and immune challenge on fly crystal cell counts using standard regression methods (Figure 5). There was a significant effect of species on crystal cell numbers in the piercing experiment, whereby D. suzukii had more than three times the number of constitutively produced crystal cells compared to D. melanogaster (Figure 5B). There was a similar, albeit non-significant trend in the wasp-attack experiment (Figure 5A). There were consistent, significant immune challenge effects of crystal cell counts, whereby both species showed significant reductions in crystal cell numbers following wasp infection or piercing, suggesting either crystal cells or their crystals (which are thought to contain the melanization precursors [36]) were spent during the wound healing or immune responses. Significant melanization was observed around the wound site in both species.
Figure 5. Crystal cell count comparison between D. melanogaster and D. suzukii.
(A) 33 hours after infection by wasp strain LbG486; (B) 33 hours after piercing with a sterile needle. Average (+) standard deviation shown.
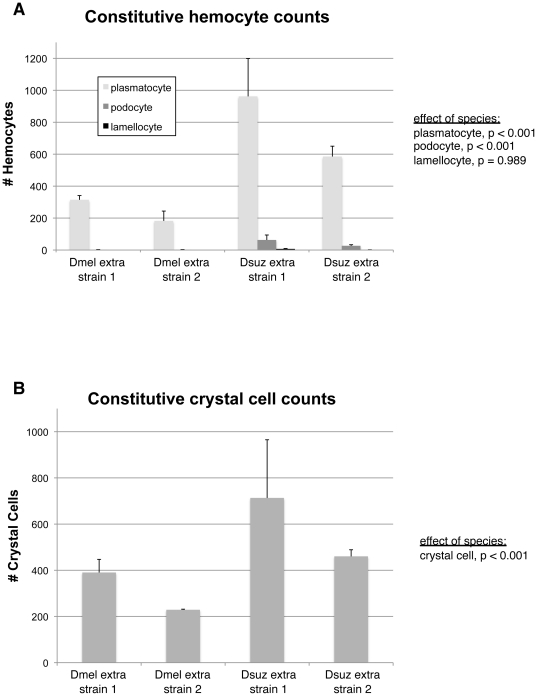
In order to confirm that hemocyte count differences between D. melanogaster and D. suzukii are general, we conducted further hemocyte counts experiments using two more strains of both fly species (Figure 6). Once again, we found a significant effect of species on constitutive numbers of plasmatocytes, podocoytes, and crystal cells, with the D. suzukii strains having greater numbers of these cell types in every case.
Figure 6. Hemocyte counts in other D. melanogaster and D. suzukii strains.
(A) Constitutive plasmatocyte, podocyte, lamellocyte counts; (B) constitutive crystal cell counts. Average (+) standard deviation shown.
Fly Resistance
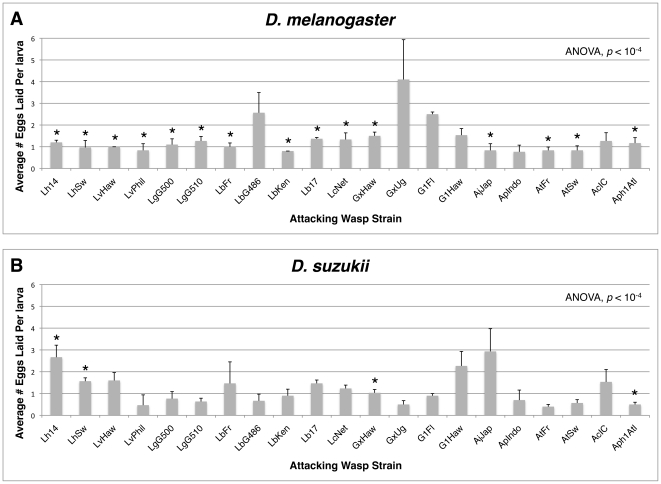
In the next experiment, both host species were infected with a panel of parasitoid wasps. Since we did not observe the flies and wasps for the duration of the infection period, it was important to know whether wasp infection rates were similar across the two fly species, so that any difference in fly eclosion could be attributed to a successful encapsulation response rather than a lack of infection. We compared the average number of eggs laid in the larvae of both fly species by the panel of parasitoid wasps. Although significant differences existed in the number of eggs laid by different wasp strains within a fly species (D. melanogaster ANOVA p<10−4, D. suzukii ANOVA p<10−4) (Figure 7), there was no overall difference between fly species in the number of eggs laid by the different wasp species (Figure 8), which averaged close to 1.25 eggs per fly larva in both fly hosts. Thus there was no evidence of an overall infection preference by wasps for one fly species over the other, and no evidence of differences in alternative mechanisms of host defense, such as behavioral or physical immunity (e.g., a thickened cuticle) by the flies.
Figure 7. Numbers of eggs laid by each wasp strain in D. melanogaster (A) and D. suzukii (B).
Average number of eggs per larva (+) standard deviation shown. ANOVA results compare egglay numbers within fly species across wasp treatments. * = significant under-dispersion of wasp eggs in fly larvae at p<0.05 using a one-tailed paired t-test (Methods).
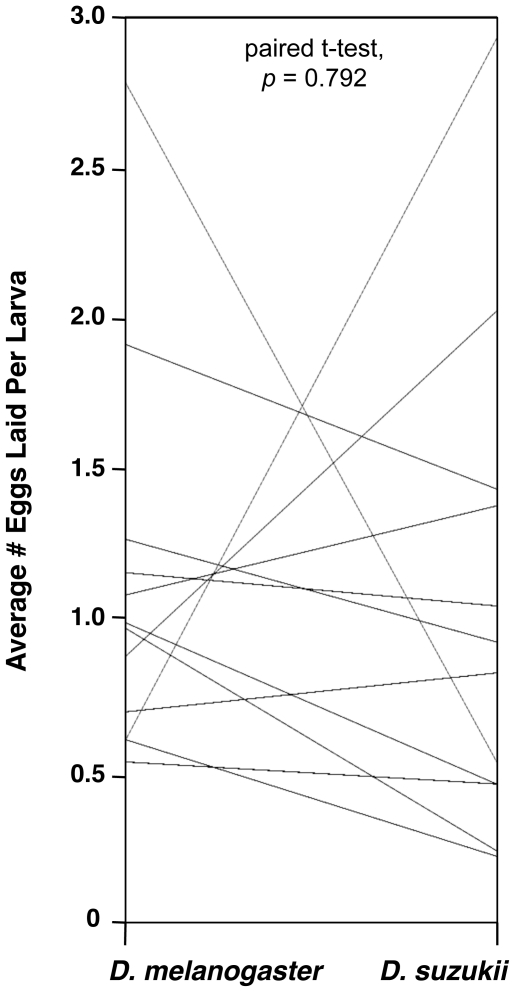
Figure 8. Parallel plot comparing average egglay numbers for each wasp species between hosts.
There was no overall difference between fly species in numbers of eggs laid by wasps, nor was there a correlation between the number of eggs laid in D. melanogaster and the number of eggs laid in D. suzukii across the panel of wasp species (as indicated by the non-parallel connecting lines).
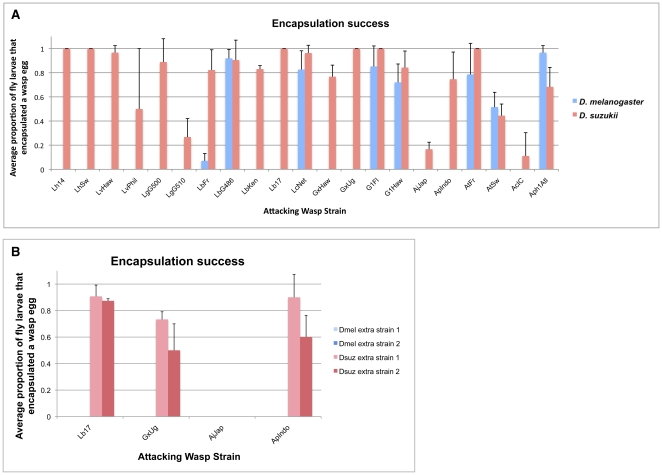
D. suzukii was able to melanotically encapsulate at least a small proportion of eggs from all 21 larval parasitoid wasp strains tested, whereas D. melanogaster was able to encapsulate some proportion of eggs from only 8 of 21 wasp strains (LbFr, LbG486, LcNet, G1Fl, G1Haw, AtFr, AtSw, and Aph1Atl) and only 5 of 12 wasp species (Figure 9A). The difference in the proportion of wasp species that the flies could melanotically encapsulate was statistically significant (Fisher's exact test p = 0.005). Qualitative melanotic encapsulation differences between D. melanogaster and D. suzukii held across additional strains tested of both species (Figure 9B). As expected, the D. suzukii strains were able to encapsulate 3 of the 4 larval parasites tested (Lb17, GxUg, ApIndo, but not AjJap), while D. melanogaster was not able to encapsulate any of the parasites.
Figure 9. Encapsulation success of wasp-infected fly larvae.
(A) Average proportion of fly larvae that encapsulated a wasp egg; (B) average proportion of fly larvae from additional fly strains that encapsulated a wasp egg.
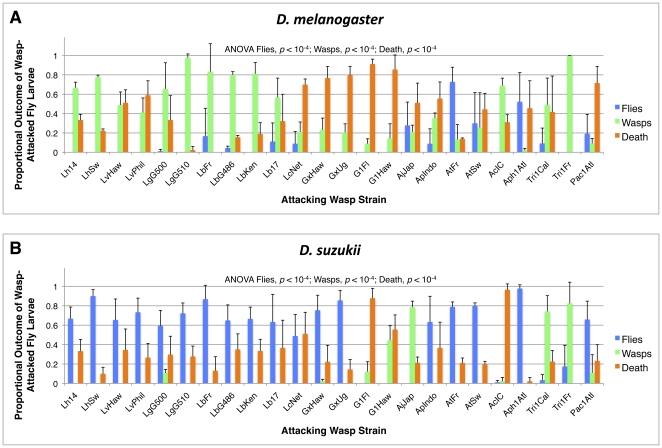
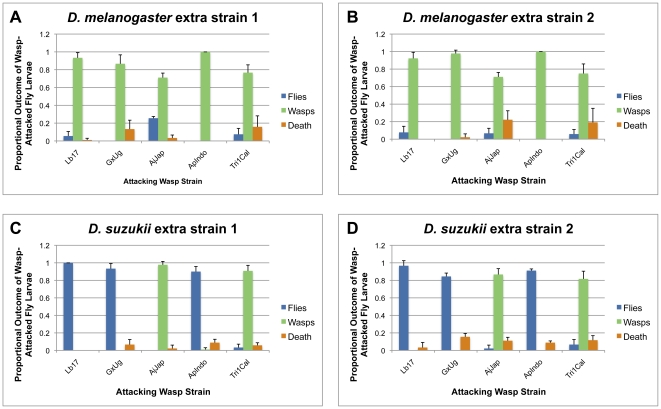
D. suzukii was also consistently more resistant to our panel of parasitoid wasp species than D. melanogaster (Figure 10, 11). A greater proportion of D. suzukii eclosed after wasp infection compared to D. melanogaster for 20 of the 24 wasp strains tested, the exceptions being D. suzukii infected by wasp strains G1Fl and G1Haw (for which no flies of either species eclosed), AjJap, and Tri1Cal. This corresponded to a significantly higher fly eclosion rate for D. suzukii compared to D. melanogaster across wasp species (Figure 11A). Furthermore, a lesser proportion of wasps eclosed from infected D. suzukii larvae compared to D. melanogaster for 19 of the 24 wasp strains tested, the exceptions being D. suzukii infected by wasp strains G1Fl, G1Haw, AjJap, Tri1Cal, and Pac1Atl. This corresponded to a significantly lower wasp eclosion rate in D. suzukii compared to D. melanogaster across wasp species (Figure 11B). The proportion of attacks that led to death of both the fly and the wasp growing within the fly was also lower in D. suzukii, with D. suzukii showing a lower proportion of death than D. melanogaster for 17 of the 24 wasp strains tested. However, this difference was not significant across wasp species (Figure 11C). When we tested additional strains of both D. suzukii and D. melanogaster, we found qualitatively similar eclosion results (Figure 12). As expected, a greater proportion of D. suzukii eclosed following infection compared to D. melanogaster for 3 wasp strains (Lb17, GxUg, ApIndo) D. suzukii was previously successful against, but not for two wasp strains D. suzukii previously did poorly against (AjJap, Tri1Cal).
Figure 10. Infection outcomes for host larvae infected by each wasp strain.
Average (+) standard deviation shown for D. melanogaster (A) and D. suzukii (B). ANOVA results compare fly eclosion, wasp eclosion, or death proportions within fly species across wasp treatments.
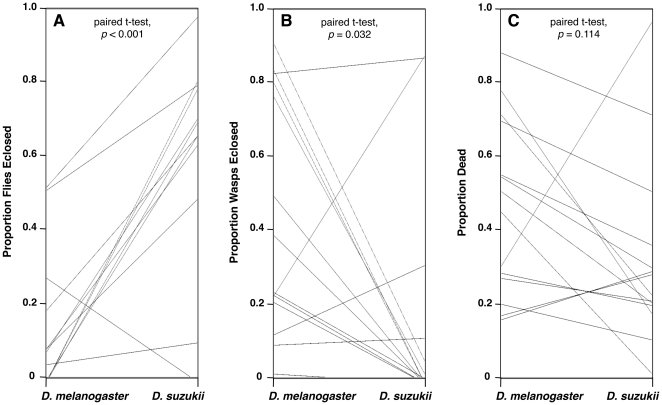
Figure 11. Parallel plot comparing outcomes between host larvae infected by each wasp species.
(A) fly eclosion; (B) wasp eclosion; (C) death. There were significant overall differences between fly species in fly eclosion and wasp eclosion proportions, but not in proportion dead. There is no correlation between fly eclosion, wasp eclosion, or death proportions between D. melanogaster and D. suzukii across the panel of wasp species (as indicated by the non-parallel connecting lines).
Figure 12. Infection outcomes for host larvae of other strains.
(A, B) D. melanogaster extra strain 1 and 2; (C, D) D. suzukii extra strain 1 and 2. Average (+) standard deviation shown.
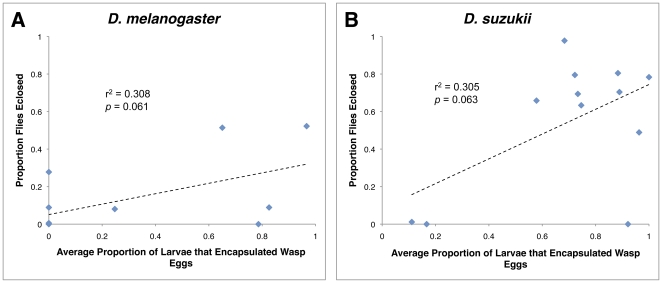
Given our understanding of the Drosophila immune response against wasp parasitism, we expect that flies that successfully encapsulate particular wasp species will also have greater eclosion success against those same wasp species. To test this expectation, we assayed for correlations between encapsulation success and fly eclosion for both flies species infected by the panel of wasp species. Although we found a trend in the expected direction for both fly species, there was no significant correlation in either fly species (Figure 13).
Figure 13. Relationship between encapsulation rate and fly eclosion.
Average proportion of fly larvae that encapsulated a wasp egg for D. melanogaster (A) and D. suzukii (B).
Wasp Choice
Previous work using D. melanogaster has shown that wasps can differentiate between infected and un-infected flies, and that they preferentially lay eggs in fly hosts that have not already been infected [38], [39], [40], [41], [44], [45]. This preference is presumably adaptive because it limits competition between juvenile wasps that require the resources from an entire fly to complete development. Such preference should lead to an under-dispersion of wasp eggs in any group of infected fly larvae, i.e., a more even distribution of eggs per larvae than expected by chance. We found significant under-dispersion of wasp eggs in D. melanogaster larvae for 15 of the 21 larval parasite wasp strains (Figure 7). The wasp strains that laid the most eggs in D. melanogaster tended to show the least under-dispersion, suggesting that the wasps could not differentiate between infected flies once they were infected with more than one wasp egg [38], [39]. Only 4 of 21 wasp strains showed a significant under-dispersion of eggs across D. suzukii larvae. This suggests that whatever cue the wasps use to identify infected D. melanogaster larvae, whether it is a tag left by the previous wasp or some aspect of the D. melanogaster response to infection [40], is generally missing in D. suzukii larvae. In no fly-wasp interaction was there a significant over-dispersion of wasp eggs.
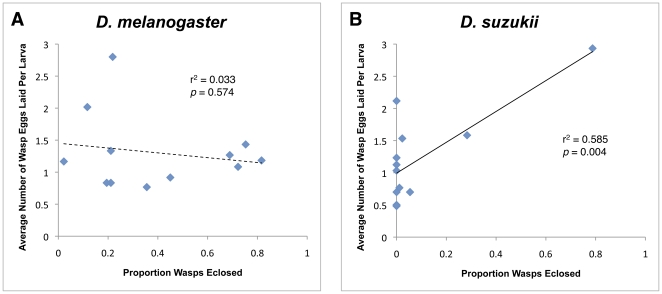
Drosophila parasitoid wasps can also distinguish between fly host species, and preferentially lay eggs in host species in which their offspring have a higher chance of survival [29], [46], [47]. We tested whether larval parasitoid wasps tended to lay more eggs in the fly hosts that their offspring more successfully eclosed from in our trials (note that in our trials the wasps did not have a choice between host species, only whether or not to lay eggs in a single given host) (Figure 14). There was no relationship between wasp species success and the number of eggs laid per larva with D. melanogaster as host (r2 = 0.033, ANOVA p = 0.574). For D. suzukii, however, there was a highly significant relationship (r2 = 0.585, ANOVA p = 0.004) that was due in large part to the wasp species A. japonica (strain AjJap) and Ganaspis sp.1 (combined strains G1Fl and G1Haw). AjJap in particular laid the highest number of eggs in D. suzukii in our infection trials, and also had the highest eclosion success.
Figure 14. Relationship between wasp eclosion success and number of eggs wasps choose to lay in a host.
There was no significant relationship for the panel of wasp species attacking D. melanogaster (A), but there was a significant relationship for the panel of wasp species attacking D. suzukii (B).
Specificity In Fly-Wasp Interactions
As described above, we found significant differences in the number of eggs laid by different wasp strains within fly species but not between fly species. This could mean that wasps that lay higher numbers of eggs in D. melanogaster also lay higher numbers of eggs in D. suzukii, i.e., some wasps could have generally higher egglay rates than others. However, there was no correlation between the number of eggs laid in D. melanogaster and the number of eggs laid in D. suzukii for the panel of wasp species (r2 = 0.016, ANOVA p = 0.696), suggesting that egglay rate is a plastic wasp trait that wasps tailor to the host species they encounter.
There were significant differences across the panel of wasp strains in the infection outcomes within fly species (ANOVA p<10−4 for all six comparisons: fly survival, wasp survival, death in D. melanogaster, D. suzukii) (Figure 10). Although these differences in infection outcomes were due to significant variation both between wasp species and within wasp species (variation amongst strains), the largest differences in infection outcomes are seen between wasp species rather than wasp strains. For each fly host, some wasp species were very successful infectors, some were very susceptible to the fly immune responses, and some induced a large amount of death. As described above there were also significant differences in the infection outcomes between fly species. Despite the superior wasp resistance of D. suzukii, it is possible that wasps that were more successful in D. melanogaster were also more successful in D. suzukii, i.e., some wasps are generally more virulent than others. However, there was no correlation in the proportions of any of the three infection outcomes between D. melanogaster and D. suzukii (fly success r2 = 0.102, ANOVA p = 0.265; wasp success r2 = 0.001, ANOVA p = 0.908; death r2 = 0.041, ANOVA p = 0.489). This indicates there was specificity in the outcome of wasp infections depending on the particular host fly species, despite D. melanogaster and D. suzukii being part of the same Drosophila species group.
There is a strong influence of wasp phylogeny on D. melanogaster infection outcomes. Members of the Leptopilina clade that includes L. boulardi and L. heterotoma are very successful against D. melanogaster, showing an average of 69% wasp eclosion. Infections by L. clavipes and members of the genus Ganaspis, which are likewise members of the family Figitidae, did not result in high eclosion rates in D. melanogaster, but instead caused an average of 79% death of D. melanogaster larvae (Figure 8). Thus, D. melanogaster appears to lack an immune mechanism to counter shared virulence strategies of Figitid parasitoids. There appeared to be little influence of wasp phylogeny on the ultimate outcome of D. suzukii–wasp interactions, as D. suzukii was resistant to the majority of wasps tested. However, the larval parasitoid that eclosed from D. suzukii at the greatest rate (79%), A. japonica, is endemic to Japan where it is sympatric with D. suzukii.
Discussion
Previous studies have shown that fly species and strains with a greater constitutive production of hemocytes are more resistant against and/or are better able to encapsulate parasitoid wasp eggs [12], [18], [19], [48]. Although a correlation does not necessarily imply causation, these data suggest that evolution of higher constitutive production of hemocytes is a relatively simple way for hosts to defeat one of their most common classes of parasites. However, the previous studies were limited to flies in the melanogaster subgroup and to a few wasp species/strains that represent only a small fraction of the diverse virulence strategies used by Drosophila parasitoid wasps. To determine if increased hemocyte production by flies is a panacea against wasp infection, we first compared hemocyte numbers between D. melanogaster and D. suzukii, a relative of D. melanogaster outside the melanogaster subgroup.
We found that third instar D. suzukii larvae made constitutively greater numbers of plasmatocytes, podocytes, and crystal cells than D. melanogaster larvae, and also induce greater production of podocytes and lamellocytes (Figure 4, 5, 6). Compared to our recently wild-derived D. suzukii strains, the D. melanogaster genome strain we used may have had relatively poor genetic immune ability for its species due to its homozyosity and its long-term selection in a lab environment. However, hemocyte counts from the two additional wild-caught D. melanogaster strains we assayed were very similar to those from the genome strain. The hemocyte numbers we observed in our D. melanogaster strains were also similar to those seen in a variety of other studies where the unit of measurement was cells per larva [33], [35], [42], and also appeared similar to numbers found in studies that counted cells per volume of hemolymph (using a rough conversion factor of approximately 0.5 uL hemolymph per third instar larva) [12], [19], [43], [49], [50]. Thus, we have no reason to believe that differences we observe between our D. melanogaster and D. suzukii strains were due to a biased sampling of strains rather than actual species differences. In comparison with hemocyte numbers from other studies, D. suzukii appears to have somewhat greater constitutive hemocyte counts than D. simulans, which has the highest counts of any member of the melanogaster subgroup [12].
Using a diverse panel of parasitoid wasp strains and species, we found that infection rates in D. melanogaster and D. suzukii were similar (Figure 7, 8), but that D. suzukii was significantly better at melanotically encapsulating, and surviving infection by, the wasps (Figure 9, 10, 11,12). The panel of wasps included relatively specialist and generalist wasp species, such as L. boulardi and L. heterotoma, respectively [15], as well as relatively immune evasive versus immune suppressive wasp species, such as A. tabida and G. xanthopoda, respectively [51], [52]. Our data suggest that a general protection against parasitoid wasps is afforded to fly species that have higher constitutive hemocyte loads. The association between hemocyte load and encapsulation ability reported previously [12] also appears to extend beyond the melanogaster subgroup of fly hosts, as D. suzukii is part of the melanogaster group but not the melanogaster subgroup. Future infection trials using the same panel of parasitoid wasps, but a much wider range of fly species, will be needed for determining the true extent of the relationship between hemocyte load and resistance against parasitoid wasps.
The current model for the melanotic encapsulation process is that plasmatocytes act as sentinels of wasp infection and signal to activate other circulating plasmatocytes as well as the lymph gland once infection is recognized [4], [5]. The activated plasmatocytes develop cytoskeletal projections and become known as podocytes, which may be an intermediate form between the smaller plasmatocytes and larger lamellocytes [6], [7]. Lamellocytes are also induced via differentiation of pro-hemocytes in the lymph gland. The lamellocytes then migrate towards and surround the wasp egg, forming a tight capsule. The capsule becomes melanized, but it is not yet known whether melanin precursors stored in crystal cells are used in this process. Thus, any or all of the hemocyte cell types that D. suzukii produced in excess may have been responsible for the relatively high resistance of D. suzukii against wasp eggs.
Flies with more hemocytes may suffer fewer effects of wasp venom for a variety of reasons, enabling them to mount a quicker and/or stronger encapsulation reaction against wasps. For example, venoms often alter hemocyte structure and function [50], [53], and thus an increased number of hemocytes could potentially dilute the effects of a standard dose of venom. Alternatively, hemocytes may be responsible for destroying venom components found in the hemolymph, via endocytosis or some other mechanism, preventing the venom from exerting its effects on other tissues. It is unclear whether an excess of constitutively produced hemocytes (plasmatocytes, crystal cells) or the increased induced production of podocytes and lamellocytes drives the relationship between hemocyte counts and wasp resistance, but the distinction may be unimportant given that constitutively produced cells can differentiate into induced cell types [6], [7]. However, in support of the idea that constitutive production of hemocytes alone is not sufficient for wasp resistance, Drosophila species of the obscura group that make relatively high numbers of plasmatocytes, but apparently do not produce a lamellocyte class of cells, are unable to encapsulate foreign objects and are highly susceptible to wasp infection [54], [55].
Unlike for D. melanogaster, larval parasitoid wasps rarely under-dispersed their eggs across D. suzukii larvae. Wasps are thought to discriminate naïve host larvae from previously infected larvae either by recognizing a mark left by the previous wasp, or by recognizing the host response to infection [40]. Given that D. suzukii has a significantly more robust immune response against wasp infection than D. melanogaster, it seems unlikely that these wasps use host immune cues to avoid superparasitism. If fly hemocytes are responsible for clearing wasp venom components from the hemolymph, wasp “possession marks” might also be lost in fly hosts that make abundant hemocytes, leading to more random dispersal of wasp eggs across host larvae.
We expected to find a correlation between encapsulation ability and fly success in both D. melanogaster and D. suzukii, but although there was a trend in this direction, the correlations were not significant (Figure 13). Three factors likely contribute to this lack of correlation. First, we counted fly larvae as having successful encapsulations if any encapsulation was seen, even if flies were super-parasitized and hadn't encapsulated all wasp eggs they were infected by. Thus, flies scored as showing encapsulation could still succumb to infection. Second, some fly-wasp combinations that yielded encapsulations culminated in neither fly nor wasp eclosion, but high rates of death of by both fly and wasp. Third, wasp parasites sometimes die inside their fly hosts even if the fly has not encapsulated them by the time-point we assayed.
Interestingly, D. suzukii does not have a clear survival advantage over D. melanogaster when infected by the two pupal parasite species (three strains) in our panel of wasps. Very little is known about the determinants of infection outcomes with regards to pupal parasites of flies, or even whether venom plays an important role. Although Trichopria acts as a pupal endoparasitoid, the Drosophila pupal stage does not appear able to mount melanotic encapsulation responses against them. Furthermore, Pachycrepoideus lays its eggs in the space between the pupal case and the pupa, and acts as an ectoparasite for most of its development [29], which could negate any ability the flies have to mount an internal, physiological immune response. In other systems, pupal parasitoid wasps are known to have more generalist host ranges than larval parasites [30], [31], but they do not have unlimited host ranges either, so some specificity in their utilization of host resources is inherent. Although our data suggests increased hemocyte load has little effect on fly resistance against pupal parasites, a definitive statement will require data from a greater range of pupal parasite species.
Still, if increased hemocyte load provides general protection against larval parasitoids, why do some fly species, such as D. melanogaster, produce such low numbers compared to their close relatives? Hosts face an evolutionary tradeoff between investing in immune responses against parasites versus investing in other aspects of fitness [56], [57], [58], [59]. The constitutive production and maintenance of hemocytes must obviously impart an energetic cost on the host, diverting resources from other aspects of host fitness. Thus, if hosts are rarely infected by wasps in nature, or are commonly infected by specialist wasps that can overcome hemocyte-based immunity, it may make evolutionary sense to invest in fecundity rather than immunity, or in other aspects of immunity, such as behavioral immunity. On the other hand, investment in high constitutive hemocyte levels might be selected in host species that are commonly infected by non-specialist parasites.
Although D. suzukii is generally more resistant against larval wasp parasites than D. melanogaster, there were a small number of obvious exceptions. A. japonica is sympatric with D. suzukii in its native east Asian range, and was significantly more successful at infecting D. suzukii than D. melanogaster. Previous studies showed A. japonica successfully parasitizes D. suzukii both in the field and in the lab [60], [61]. A japonica also laid approximately three times more eggs in D. suzukii than in D. melanogaster, and laid the highest number of eggs in D. suzukii of any larval parasitoid wasp. Altogether, these data suggest A. japonica may have co-evolved a specialized virulence strategy able to overcome the high hemocyte load of D. suzukii, and may have evolved an infection preference for D. suzukii as well. The only other larval parasite able to eclose from D. suzukii hosts at any appreciable rate is Ganaspis sp.1, an undescribed species collected in Florida and Hawaii. Although G. xanthopoda was found to emerge from D. suzukii pupae collected in the field in Japan [61], the two G. xanthopoda strains used in this study, from Hawaii and Uganda, were very poor infectors of D. suzukii, suggesting populations of this wasp species may have locally adapted to D. suzukii host use in Japan.
D. suzukii has recently spread into Europe and North America as a pest species [20], [21], [22], [23]. It was first documented in the United States in California in 2008, from where it quickly spread to Oregon and Washington. In these west coast states, D. suzukii was responsible for up to 80% yield losses in berry and cherry crops depending on location, and is estimated to be causing yearly monetary losses in the range of 500 million dollars [62], [63]. In 2009, D. suzukii became established in Florida, and in 2010 reports of collections were made from a handful of new states [23]. However, experimental studies testing the efficacy of various management strategies for D. suzukii are as yet lacking [64], [65].
One common pest management strategy is the use of biocontrol agents such as natural enemies (parasites, predators) [66], and parasitoid wasps have successfully controlled numerous other arthropod pests in the past [24], [25], [26], [27], [28]. Furthermore, Drosophila parasitoid wasps often infect a large proportion of fly larvae in natural populations [1], [2], [3], and the potential for an endemic Figitid species (L. boulardi) to control native Drosophila populations in California was previously considered [67]. It appears the wasp species with the highest potential for use in biocontrol of D. suzukii are the larval parasites A. japonica and Ganaspis sp.1, and the pupal parasite Trichopria sp.1. A. citri might also be considered a potential biocontrol agent for D. suzukii because of the high death rates it caused in D. suzukii, but this wasp had much higher eclosion rates using D. melanogaster as host than D. suzukii. Because infection trial conditions in this study were designed to be ideal for success of the wasps, and such conditions (easy access to hosts, no competition with other parasites, controlled temperature, abundant resources, etc) are unlikely to be replicated in the field, extensive field experiments will be required to assess the efficacy of the use of parasitoid wasps in D. suzukii biocontrol in practice.
Acknowledgments
We thank R. Allemand, D. Hultmark, J. van Alphen, J. Pool, and B. Wertheim for providing wasp strains, Kate Ellingson for help with statistical analyses, and members of the Schlenke lab for helpful comments on the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by National Institutes of Health (NIH) grant AI081879 to TAS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Driessen G, Hemerik L, van Alphen JJM. Drosophila species breeding in the stinkhorn (Phallus impudicus Pers.) and their larval parasitoids. Neth J Zool. 1990;40:409–427. [Google Scholar]
- 2.Fleury F, Ris N, Allemand R, Fouillet P, Carton Y, et al. Ecological and genetic interactions in Drosophila-parasitoids communities: A case study with D. melanogaster, D. simulans and their common Leptopilina parasitoids in southeastern France. Genetica. 2004;120:181–194. doi: 10.1023/b:gene.0000017640.78087.9e. [DOI] [PubMed] [Google Scholar]
- 3.Janssen A, Driessen G, De Haan M, Roodboi N. The impact of parasitoids on natural populations of temperate woodland Drosophila. Neth J Zool. 1988;38:61–73. [Google Scholar]
- 4.Carton Y, Poirie M, Nappi AJ. Insect immune resistance to parasitoids. Insect Sci. 2008;15:67–87. [Google Scholar]
- 5.Eslin P, Prevost G, Havard S, Doury G. Immune resistance of Drosophila hosts against Asobara parasitoids: Cellular aspects. Adv Parasitol. 2009;70:189–215. doi: 10.1016/S0065-308X(09)70007-7. [DOI] [PubMed] [Google Scholar]
- 6.Honti V, Csordas G, Markus R, Kurucz E, Jankovics F, et al. Cell lineage tracing reveals the plasticity of the hemocyte lineages and of the hematopoietic compartments in Drosophila melanogaster. Mol Immunol. 2010;47:1997–2004. doi: 10.1016/j.molimm.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Rizki MTM. Alterations in the haemocyte population of Drosophila melanogaster. J Morphol. 1957;100:437–458. [Google Scholar]
- 8.Carton Y, Kitano H. Evolutionary relationships to parasitism by seven species of the Drosophila melanogaster subgroup. Biol J Linn Soc. 1981;16:227–241. [Google Scholar]
- 9.Carton Y, Nappi AJ. Immunogenetic aspects of the cellular immune response of Drosophilia against parasitoids. Immunogenetics. 2001;52:157–164. doi: 10.1007/s002510000272. [DOI] [PubMed] [Google Scholar]
- 10.Dubuffet A, Colinet D, Anselme C, Dupas S, Carton Y, et al. Variation of Leptopilina boulardi success in Drosophila hosts: what is inside the black box? Adv Parasitol. 2009;70:147–188. doi: 10.1016/S0065-308X(09)70006-5. [DOI] [PubMed] [Google Scholar]
- 11.Dupas S, Carton Y. Two non-linked genes for specific virulence of Leptopilina boulardi against Drosophila melanogaster and D. yakuba. Evol Ecol. 1999;13:211–220. [Google Scholar]
- 12.Eslin P, Prevost G. Hemocyte load and immune resistance to Asobara tabida are correlated in species of the Drosophila melanogaster subgroup. J Insect Physiol. 1998;44:807–816. doi: 10.1016/s0022-1910(98)00013-4. [DOI] [PubMed] [Google Scholar]
- 13.Moreau SJ, Eslin P, Giordanengo P, Doury G. Comparative study of the strategies evolved by two parasitoids of the genus Asobara to avoid the immune response of the host, Drosophila melanogaster. Dev Comp Immunol. 2003;27:273–282. doi: 10.1016/s0145-305x(02)00101-5. [DOI] [PubMed] [Google Scholar]
- 14.Poirie M, Frey F, Hita M, Huguet E, Lemeunier F, et al. Drosophila resistance genes to parasitoids: Chromosomal location and linkage analysis. Proc R Soc Lond B. 2000;267:1417–1421. doi: 10.1098/rspb.2000.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlenke TA, Morales J, Govind S, Clark AG. Contrasting infection strategies in generalist and specialist wasp parasitoids of Drosophila melanogaster. PLoS Pathog. 2007;3:1486–1501. doi: 10.1371/journal.ppat.0030158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wajnberg E, Prevost G, Bouletreau M. Genetic and epigenetic variation in Drosophila larvae suitability to a Hymenopterous endoparasitoid. Entomophaga. 1985;30:187–191. [Google Scholar]
- 17.Kraaijeveld AR, Limentani EC, Godfray HCJ. Basis of the trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Proc R Soc Lond B. 2001;268:259–261. doi: 10.1098/rspb.2000.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreau SJM, Guillot S, Populaire C, Doury G, Prevost G, et al. Conversely to its sibling Drosophila melanogaster, D. simulans overcomes the immunosuppressive effects of the parasitoid Asobara citri. Dev Comp Immunol. 2005;29:205–209. doi: 10.1016/j.dci.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Sorrentino RP, Melk JP, Govind S. Genetic analysis of contributions of dorsal group and JAK-Stat92E pathway genes to larval hemocyte concentration and the egg encapsulation response in Drosophila. Genetics. 2004;166:1343–1356. doi: 10.1534/genetics.166.3.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calabria G, Maca J, Bachli G, Serra L, Pascual M. First records of the potential pest species Drosophila suzukii (Diptera: Drosophilidae) in Europe. J Appl Entomol. 2011;136:139–147. [Google Scholar]
- 21.Hauser M. A historic account of the invasion of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the continental United States, with remarks on their identification. Pest Manag Sci. 2011;67:1352–1357. doi: 10.1002/ps.2265. [DOI] [PubMed] [Google Scholar]
- 22.Lee JC, Bruck DJ, Dreves AJ, Ioriatti C, Vogt H, et al. In Focus: Spotted wing drosophila, Drosophila suzukii, across perspectives. Pest Manag Sci. 2011;67:1349–1351. doi: 10.1002/ps.2271. [DOI] [PubMed] [Google Scholar]
- 23.Walsh DB, Bolda MP, Goodhue RE, Dreves AJ, Lee J, et al. Drosophila suzukii (Diptera: Drosophilidae): Invasive pest of ripening soft fruit expanding its geographic range and damage potential. J Integ Pest Management. 2011;2:G1–G7. [Google Scholar]
- 24.Clausen CP. Washington, editor. Introduced parasites and predators of arthropod pests and weeds: A world review. D.C.: USDA. 1978. 545
- 25.Debach P. London: Cambridge University Press; 1974. Biological control by natural enemies.323 [Google Scholar]
- 26.Greathead D. Parasitoids in classical biological control. In: Waage J, Greathead D, editors. Insect parasitoids. London: Academic Press; 1986. pp. 289–318. [Google Scholar]
- 27.Huffaker CB, editor. New York: Plenum Press; 1971. Biological control.511 [Google Scholar]
- 28.LaSalle J, Gauld ID. Parasitic Hymenoptera, biological control and biodiversity. In: LaSalle J, Gauld ID, editors. Hymenoptera and biodiversity. Wallingford, UK: CAB International; 1993. pp. 197–215. [Google Scholar]
- 29.Carton Y, Bouletreau M, van Alphen JJM, van Lenteren JC. The Drosophila parasitic wasps. In: Ashburner M, Carson L, Thompson JN, editors. The Genetics and Biology of Drosophila. London: Academic Press; 1986. pp. 347–394. [Google Scholar]
- 30.Askew RR, Shaw MR. Parasitoid communities: Their size, structure and development. In: Waage J, Greathead D, editors. Insect parasitoids. London: Aacademic Press; 1986. pp. 225–264. [Google Scholar]
- 31.Godfray HCJ. Princeton, editor. Parasitoids: Behavioral and evolutionary ecology. N.J.: Princeton University Press. 1994. 473
- 32.Fleury F, Gibert P, Ris N, Allemand R. Ecology and life history evolution of frugivorous Drosophila parasitoids. Adv Parasitol. 2009;70:3–44. doi: 10.1016/S0065-308X(09)70001-6. [DOI] [PubMed] [Google Scholar]
- 33.Markus R, Kurucz E, Rus F, Ando I. Sterile wounding is a minimal and sufficient trigger for a cellular immune response in Drosophila melanogaster. Immunol Lett. 2005;101:108–111. doi: 10.1016/j.imlet.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 34.Lerner AB, Fitzpatrick TB. Biochemistry of melanin formation. Physiol Rev. 1950;30:91–126. doi: 10.1152/physrev.1950.30.1.91. [DOI] [PubMed] [Google Scholar]
- 35.Lanot R, Zachary D, Holder F, Meister M. Postembryonic hematopoiesis in Drosophila. Dev Biol. 2001;230:243–257. doi: 10.1006/dbio.2000.0123. [DOI] [PubMed] [Google Scholar]
- 36.Rizki MT, Rizki RM. Functional significance of the crystal cells in the larva of Drosophila melanogaster. J Biophys Biochem Cytol. 1959;5:235–240. doi: 10.1083/jcb.5.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams MJ, Ando I, Hultmark D. Drosophila melanogaster Rac2 is necessary for a proper cellular immune response. Genes to Cells. 2005;10:813–823. doi: 10.1111/j.1365-2443.2005.00883.x. [DOI] [PubMed] [Google Scholar]
- 38.van Alphen JJM, Nell HW. Superparasitism and host discrimination by Asobara tabida Nees (Braconidae: Alysiinae), a larval parasitoid of Drosophilidae. Neth J Zool. 1982;32:232–260. [Google Scholar]
- 39.van Lenteren JC. The development of host discrimination and the prevention of superparasitism in the parasite Pseudeucoila bochei Weld (Hym.: Cynipidae). Neth J Zool. 1976;26:1–83. [Google Scholar]
- 40.van Lenteren JC. Host discrimination by parasitoids. In: Nordlund DA, Jones RL, Lewis WJ, editors. Semiochemicals: Their role in pest control. New York: John Wiley and Sons; 1981. pp. 153–179. [Google Scholar]
- 41.Vet LEM, Meyer M, Bakker K, van Alphen JJM. Intra- and interspecific host discrimination in Asobara (Hymenoptera), larval endoparasitoids of Drosophilidae: Comparison between closely related and less closely related species. Anim Behav. 1984;32:871–874. [Google Scholar]
- 42.Rizki TM, Rizki RM. Lamellocyte differentiation in Drosophila larvae parasitized by Leptopilina. Dev Comp Immunol. 1992;16:103–110. doi: 10.1016/0145-305x(92)90011-z. [DOI] [PubMed] [Google Scholar]
- 43.Russo J, Brehelin M, Carton Y. Haemocyte changes in resistant and susceptible strains of D. melanogaster caused by virulent and avirulent strains of the parasitic wasp Leptopilina boulardi. J Insect Physiol. 2001;47:167–172. doi: 10.1016/s0022-1910(00)00102-5. [DOI] [PubMed] [Google Scholar]
- 44.Bakker K, Bagchee SN, Vanzwet WR, Meelis E. Entomol Exp Appl 10: 295–&; 1967. Host discrimination in Pseudeucoila bochei (Hymenoptera: Cynipidae). [Google Scholar]
- 45.Bakker K, Eijsackers HJP, van Lenteren JC, Meelis E. Some models describing distribution of eggs of the parasite Pseudeucoila bochei (Hym., Cynip.) over its hosts, larvae of Drosophila melanogaster. Oecologia. 1972;10:29–57. doi: 10.1007/BF00822760. [DOI] [PubMed] [Google Scholar]
- 46.Rouault J. Influence of entomophagous parasites in the competition between Drosophila sibling species: An experimental approach. Comptes Rendus Hebdomadaires Des Seances De L'Academie Des Sciences Serie D. 1979;289:643–646. [Google Scholar]
- 47.van Alphen JJM, Janssen ARM. Host selection by Asobara tabida Nees (Braconidae: Alysiinae), a larval parasitoid of fruit inhabiting Drosophila species. Neth J Zool. 1982;32:194–214. [Google Scholar]
- 48.Kraaijeveld AR, Hutcheson KA, Limentani EC, Godfray HCJ. Costs of counterdefenses to host resistance in a parasitoid of Drosophila. Evolution. 2001;55:1815–1821. doi: 10.1111/j.0014-3820.2001.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 49.Brehelin M. Comparative study of structure and function of blood cells from two Drosophila species. Cell Tiss Res. 1982;221:607–615. doi: 10.1007/BF00215704. [DOI] [PubMed] [Google Scholar]
- 50.Labrosse C, Eslin P, Doury G, Drezen JM, Poirie M. Haemocyte changes in D. melanogaster in response to long gland components of the parasitoid wasp Leptopilina boulardi: A Rho-GAP protein as an important factor. J Insect Physiol. 2005;51:161–170. doi: 10.1016/j.jinsphys.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Chiu H, Sorrentino RP, Govind S. Suppression of the Drosophila cellular immune response by Ganaspis xanthopoda. In: Beck G, Sugumaran M, Cooper EL, editors. Phylogenetic perspectives on the vertebrate immune system. New York: Kluwer Academic; 2001. pp. 161–167. [DOI] [PubMed] [Google Scholar]
- 52.Eslin P, Giordanengo P, Fourdrain Y, Prevost G. Avoidance of encapsulation in the absence of VLP by a braconid parasitoid of Drosophila larvae: An ultrastructural study. Can J Zool. 1996;74:2193–2198. [Google Scholar]
- 53.Rizki RM, Rizki TM. Parasitoid virus-like particles destroy Drosophila cellular immunity. Proc Natl Acad Sci U S A. 1990;87:8388–8392. doi: 10.1073/pnas.87.21.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eslin P, Doury G. The fly Drosophila subobscura: A natural case of innate immunity deficiency. Dev Comp Immunol. 2006;30:977–983. doi: 10.1016/j.dci.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Havard S, Eslin P, Prevost G, Doury G. Encapsulation ability: Are all Drosophila species equally armed? An investigation in the obscura group. Can J Zool. 2009;87:635–641. [Google Scholar]
- 56.Fellowes MDE, Godfray HCJ. The evolutionary ecology of resistance to parasitoids by Drosophila. Heredity. 2000;84:1–8. doi: 10.1046/j.1365-2540.2000.00685.x. [DOI] [PubMed] [Google Scholar]
- 57.Schmid-Hempel P. Evolutionary ecology of insect immune defenses. Ann Rev Entomol. 2005;50:529–551. doi: 10.1146/annurev.ento.50.071803.130420. [DOI] [PubMed] [Google Scholar]
- 58.Sheldon BC, Verhulst S. Ecological immunology: Costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- 59.Siva-Jothy MT, Moret Y, Rolff J. Insect immunity: An evolutionary ecology perspective. Adv Insect Physiol. 2005;32:1–48. [Google Scholar]
- 60.Ideo S, Watada M, Mitsui H, Kimura MT. Host range of Asobara japonica (Hymenoptera: Braconidae), a larval parasitoid of Drosophilid flies. Entomol Science. 2008;11:1–6. [Google Scholar]
- 61.Mitsui H, Van Achterberg K, Nordlander G, Kimura MT. Geographical distributions and host associations of larval parasitoids of frugivorous Drosophilidae in Japan. J Nat Hist. 2007;41:1731–1738. [Google Scholar]
- 62.Bolda MP, Goodhue RE, Zalom FG. Spotted wing Drosophila: Potential economic impact of a newly established pest. Giannini Foundation of Agricultural Economics, University of California. 2010;13:5–8. [Google Scholar]
- 63.Goodhue RE, Bolda M, Farnsworth D, Williams JC, Zalom FG. Spotted wing drosophila infestation of California strawberries and raspberries: economic analysis of potential revenue losses and control costs. Pest Manag Sci. 2011;67:1396–1402. doi: 10.1002/ps.2259. [DOI] [PubMed] [Google Scholar]
- 64.Beers EH, Van Steenwyk RA, Shearer PW, Coates WW, Grant JA. Developing Drosophila suzukii management programs for sweet cherry in the western United States. Pest Manag Sci. 2011;67:1386–1395. doi: 10.1002/ps.2279. [DOI] [PubMed] [Google Scholar]
- 65.Bruck DJ, Bolda M, Tanigoshi L, Klick J, Kleiber J, et al. Laboratory and field comparisons of insecticides to reduce infestation of Drosophila suzukii in berry crops. Pest Manag Sci. 2011;67:1375–1385. doi: 10.1002/ps.2242. [DOI] [PubMed] [Google Scholar]
- 66.Dent D. London: Chapman & Hall; 1995. Integrated pest management.356 [Google Scholar]
- 67.Hertlein MB. Seasonal development of Leptopilina boulardi (Hymenoptera: Eucoilidae) and its hosts, Drosophila melanogaster and Drosophila simulans (Diptera: Drosophilidae), in California. Env Entomol. 1986;15:859–866. [Google Scholar]
- 68.Dowton M. Simultaneous analysis of 16S, 28S, COI and morphology in the Hymenoptera: Apocrita - evolutionary transitions among parasitic wasps. Biol J Linn Soc. 2001;74:87–111. [Google Scholar]
- 69.Allemand R, Lemaitre C, Frey F, Bouletreau M, Vavre F, et al. Phylogeny of six African Leptopilina species (Hymenoptera: Cynipoidea: Figitidae), parasitoids of Drosophila, with description of three new species. Ann Soc Entomol Fr. 2002;39:319–332. [Google Scholar]
- 70.Schilthuizen M, Nordlander G, Stouthamer R, Van Alphen JJM. Morphological and molecular phylogenetics in the genus Leptopilina (Hymenoptera: Cynipoidea: Eucoilidae). Syst Entomol. 1998;23:253–264. [Google Scholar]
- 71.Seyahooei MA, Van Alphen JJM, Kraaijeveld K. Metabolic rate affects adult life span independently of developmental rate in parasitoid wasps. Biol J Linn Soc. 2011;103:45–56. [Google Scholar]