Abstract
Objectives:
We sought to evaluate the regulatory influence of endothelial nitric oxide (NO) on the basal functional states of the NO and RhoA/Rho-kinase signaling pathways in the penis employing endothelial NO synthase (eNOS) mutant mice and eNOS gene transfer technology.
Methods:
Four groups of mice were utilized: 1) wild type (WT), 2) eNOS gene deleted (eNOS −/−), 3) eNOS and neuronal NOS gene deleted (dNOS −/−), and 4) eNOS −/− mutant mice transfected intracavernosally with eNOS. Cyclic guanosine monophosphate (cGMP) concentration, protein kinase G (PKG) activity, activated RhoA, and Rho-kinase activity were determined in penes of WT and both mutant mouse groups. Constitutive NOS and PKG activities, RhoA, Rho-kinase-α and-β isoforms, and phosphorylated myosin light chain phosphatase target subunit (p-MYPT-1) expressions as well as Rho-kinase activity were determined in penes of eNOS−/− mice after eNOS gene transfer.
Results:
When compared with results in the WT penis, eNOS−/− and dNOS−/− mutant mouse penes had significant reductions in NOS activity, cGMP concentration, PKG activity, Rho-kinase activity and p-MYPT-1 expression (p<0.05) with no significant changes in activated RhoA or in RhoA and Rho-kinase-α and-β protein expressions. After eNOS gene transfer to penes of eNOS−/− mice, Rho-kinase-β and p-MYPT-1 expressions and total Rho-kinase activity were significantly increased from baseline levels (p<0.05).
Conclusions:
These data suggest that endothelial NO serves a role in the penis as a regulator of the basal signaling functions of the NO and RhoA/Rho-kinase erection mediatory pathways. These data offer new insight into the homeostasis of erection regulatory biology.
Keywords: eNOS, nitric oxide, PKG, RhoA, Rho-kinase, erectile dysfunction, priapism
1. Introduction
Relaxation of corporal smooth muscle is essential for normal erectile function, and substantial evidence exists to implicate nitric oxide (NO) as the principal mediator of penile erection [1,2]. The formation of NO from its substrate L-arginine occurs via regulatory actions of the enzyme NO synthase (NOS). The constitutive forms of the enzyme, neuronal NOS (nNOS) and endothelial NOS (eNOS), coupled to Ca2+ and calmodulin, are the principal NOS isoforms involved in the induction of penile erection. NO produced by eNOS in response to shear stress activation and (positive regulatory) phosphorylation of the enzyme is involved in signaling events that mediate maximal and sustained penile erection [3]. After its production, NO activates the soluble form of guanylate cyclase thus elevating intracellular levels of corporal smooth muscle cyclic guanosine monophosphate (cGMP). Increases in cGMP activate cGMP-dependent protein kinases (PKG) to reduce intracellular levels of calcium and promote smooth muscle relaxation.
Endothelial cells actively regulate basal vascular tone and vascular reactivity in physiological and pathological conditions, by responding to mechanical forces and neurohumoral mediators with the release of a variety of relaxing and contracting factors [4]. The penile vascular endothelium is a source of vasorelaxing factors such as NO and vasoconstrictor factors such as Rho-kinase. The RhoA/Rho-kinase signal transduction pathway has been shown to influence erectile function in vivo through an array of mechanisms, including phosphorylation of the myosin binding subunit of myosin light chain (MLC) phosphatase which results in increased myosin phosphorylation [5,6]. RhoA, a member of the Ras low molecular weight of GTP-binding proteins, mediates agonist-induced activation of Rho-kinase [7]. The exchange of GDP for GTP on RhoA and translocation of RhoA from the cytosol to the membrane are markers of its activation and enable the downstream activation of various effectors such as Rho-kinase, protein kinase N, phosphatidylinositol 3-kinase and tyrosine phosphorylation [8]. Numerous studies have established an important role for RhoA and Rho-kinase in cellular responses, including the contraction of smooth muscle cells, regulation of eNOS, and control of penile vascular function [5-11].
The present study was designed to evaluate the regulatory influence of endothelial NO on the integrative erection regulatory basis of the penis with a focus on the performances of the NO and RhoA/Rho-kinase signaling pathways. We hypothesized that besides serving as an erection mediator eNOS exerts a regulatory role in the balance of biological expressions and activities of the foremost molecular effectors of penile erection. The concept that the regulatory actions of eNOS and RhoA/Rho-kinase are coordinated basally at the penile level differs from recent descriptions of their interactions in the penis involved in immediate erectogenic events [5] or occurring in relation to conditional erectile dysfunction (ED)-disease states [12-15]. The endeavor herein precisely was to gain further understanding into the operation of steady state erection regulatory biology of the penis. We strategically employed mice lacking eNOS (eNOS-deficient mutants) or lacking eNOS and nNOS together (double NOS-deficient mutants) at baseline and after gene transfer of eNOS to the penis to study the effects of withdrawing and promoting endothelial NO bioactivity, respectively.
2. Methods
2.1. Animal Model
Age-matched, adult male eNOS−/−, and double mutant nNOS−/−, eNOS−/− mice (dNOS−/−), and WT littermate mice were used [16,17]. All experiments were conducted in accordance with the Johns Hopkins University School of Medicine guidelines for the care and use of animals.
2.2. Collection of Tissue Specimens
Penile specimens were obtained following a lethal dose of sodium pentobarbital (80 mg/kg intraperitoneally). The mouse penis was removed by cutting the crura of the corpus cavernosum at the point of adhesion to the lower pubic bone, snap frozen in liquid nitrogen, and stored at −70° C until processing for molecular analyses. All molecular analyses were determined under basal conditions.
2.3. Adenovirus Vectors
Replication-deficient recombinant adenoviruses encoding nuclear-targeted β-galactosidase (AdCMVβgal) and endothelial nitric oxide synthase (AdCMVeNOS), both driven by a cytomegalovirus (CMV) promoter, were generated by the University of Iowa Gene Transfer Vector Core Laboratory [17]. The virus was suspended in phosphate buffer physiologic saline solution (PBS) with 3% sucrose and kept at −70°C until use.
2.4. In Vivo Gene Delivery to the Corpora Cavernosa
Mice were anesthetized initially by being placed in a jar containing isoflurane-soaked gauze and then intubated and ventilated in supine position with 95% O2/5% CO2 and 2% isoflurane using a custom-designed, constant-flow mouse ventilator with tidal volume set to 6.7 μl/g at 140 breaths/min. Ten μl of AdCMVeNOS (1×1012 parts/ml) was injected into the corpus cavernosum with a 30-gauge needle attached to a 50 microliter syringe [17]. Mice were allowed to recover and then studied two days later. This time point was initially determined in preliminary studies to result in maximum transgene expression.
2.5. Western Blot Analysis
Penes were excised and homogenized in a buffer containing 50 mM Tris-HCl (pH 7.5), 2 mM EDTA, 2 mM EGTA, 150 mM NaCl, 50 mM NaF, 10% glycerol, 10 μg/ml leupeptin, 2 μg/ml aprotinin, 10 μg/ml trypsin inhibitor, 1 mM phenylmethylsulfornyl fluoride (PMSF) and 1 mM Na3VO4. The supernatant was collected after centrifugation at 10,000 g for 30 min. Cytosolic and membrane fractions were isolated for RhoA, Rho-kinase-α and-β isoforms, and phosphorylated MLC phosphatase target subunit (p-MYPT-1) Western blot analysis. The membrane fraction was used for RhoA protein expression because it contains activated RhoA [7]. Protein concentration was determined by the BCA kit (Pierce), and equal amounts of protein were loaded to 4-20% Tris-HCl gel (Bio-Rad). After their separation by SDS-polyacrylamide gel electrophoresis, the proteins were transferred to polyvinylidene fluoride membranes and incubated with primary antibodies (RhoA, p-MYPT1 at Thr696 from Santa Cruz Biotechnology; Rho-kinase-α and -β from BD Bioscience) overnight at 4 °C. Finally, the membranes were incubated with a horseradish peroxidase-linked secondary antibody and visualized using an enhanced chemiluminescence kit (Amersham). The densitometry results were normalized by β-actin expression.
2.6. NOS Activity Assay
For the determination of constitutive NOS activity (Calbiochem-Novabiochem Corporation, La Jolla, CA), radiolabelled L-arginine to L-citrulline conversion was assayed in penile extracts [17]. Enzyme activity was expressed as L-citrulline production in picomoles per min per mg of protein.
2.7. cGMP Levels
Penile tissue was rinsed with PBS, quick frozen in liquid nitrogen, and stored at −70° C until determination of cGMP levels. The samples were assayed for cGMP using a commercial enzyme immunoassay kit (Amersham Pharmacia Biotech, Piscataway, NJ) [17]. Penile cGMP levels were expressed as ficomoles per mg of protein.
2.8. PKG Activity Assay
Penes was homogenized in extraction buffer (50 mM potassium phosphate buffer, pH 7.0, 1 mM EDTA, 1 mM EGTA, 0.2 mM PMSF, 1 μg/ml pepstatin, 0.5 μg/ml leupeptin, 10 mM NaF, 2 mM Na3VO4, 10 mM β-mercaptoethanol), and PKG activity was determined by colorimetric analysis (CycLex, Nagano, Japan) according to manufacturer's instructions. Protein concentration was determined by the BCA kit (Pierce), and 30 μg of penile lysates from each sample was used for the measurement of PKG activity. PKG activity was expressed as a percentage of WT control PKG activity [18].
2.9. RhoA Activation Assay
Penes were snap frozen in liquid nitrogen after excision and homogenized in a cold homogenization buffer containing 10 mM Tris-HCl (pH 7.4), 1 mM EGTA, 1 mM EDTA, 1 mM PMSF, 1 mM Na3VO4, 10 μg/ml leupeptin, and 2 μg/ml aprotinin. The homogenates were centrifuged at 500 × g at 4 °C for 5 min. The supernatant was collected and centrifuged at 100,000 × g at 4°C for 20 min. This resulted in a supernatant (cytosolic) and pellet (membrane), which was re-suspended in homogenization buffer containing 1% Triton X-100. RhoA activation was assessed using a precipitation assay for RhoA-GTP in the membrane fraction, according to the manufacturer's instructions (Upstate Biotechnology, Lake Placid, NY). RhoA activation was expressed as a percentage of WT control RhoA activation [19].
2.10. Rho-kinase Activity Assay
Penes were homogenized in a buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and 1 μg/ml leupeptin. Rho-kinase activity was analyzed in the presence of 0.1 mM ATP by a Rho-kinase activity assay kit according to manufacturer's instructions, using a peroxidase coupled anti-phospho-MYPT1 Thr696 monoclonal antibody (MBL International Corporation). Rho-kinase activity was expressed as a percentage of WT control Rho-kinase activity [13].
2.11. Statistics
The data are expressed as means ± S.E.M. The unpaired Student's t-test or One way ANOVA analysis with Newman-Keuls multiple comparison post test was performed to determine the significance of differences between mean values. P<0.05 was considered to be statistically significant.
3. Results
3.1. NO/cGMP/PKG Measurements
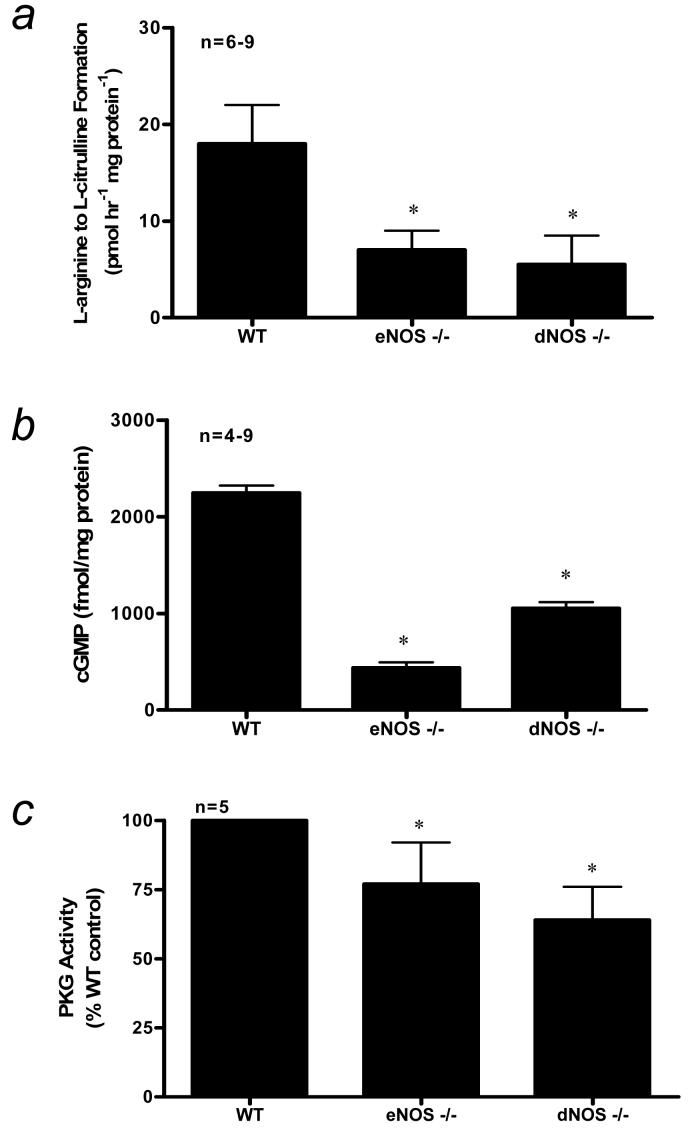
Previous studies from this laboratory have shown significant reductions in eNOS protein expression in penes of eNOS-deficient mice [16,17]. However, there has not been a full characterization of the NO/cGMP/PKG pathway in penes of these mice. Herein, we found a significant reduction (p<0.05) in constitutive NOS activities, cGMP levels, and PKG activities in eNOS−/− and dNOS−/− mouse penes when compared to that of the WT control mouse penis (Fig. 1A-1C).
Figure 1.
(a) NOS activity as measured by calcium-dependent conversion of L-arginine to L-citrulline (b) cGMP level, and (c) PKG activity in WT, eNOS−/−, and dNOS−/− mouse penes. n indicates number of tissue samples; * (p<0.05) when compared to WT.
3.2. RhoA Activation and Rho-kinase Activity
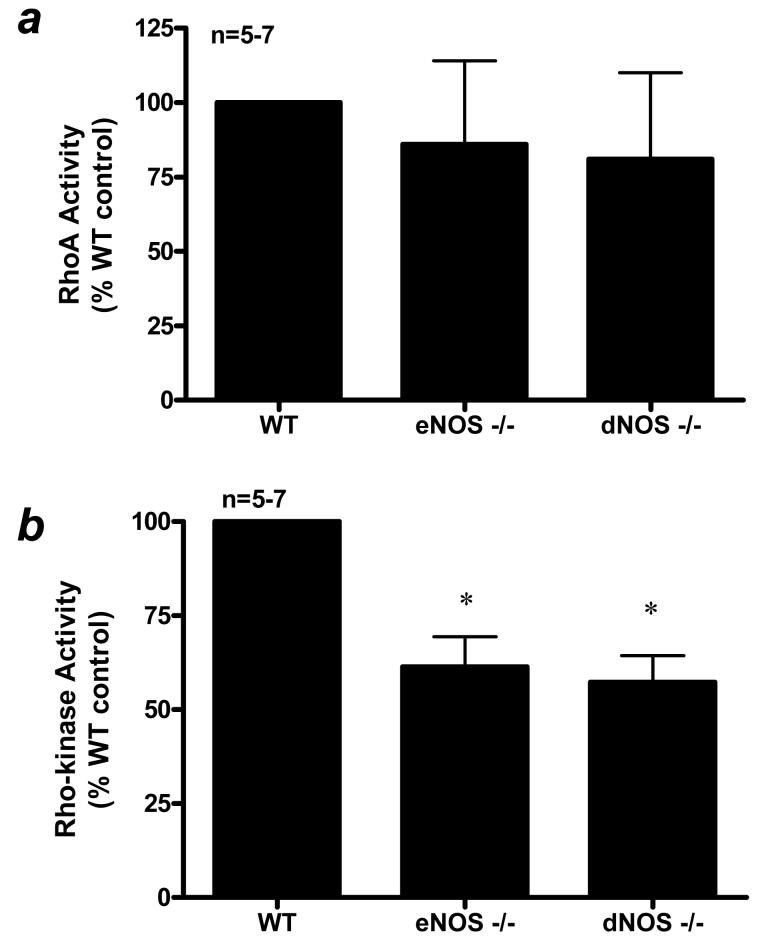
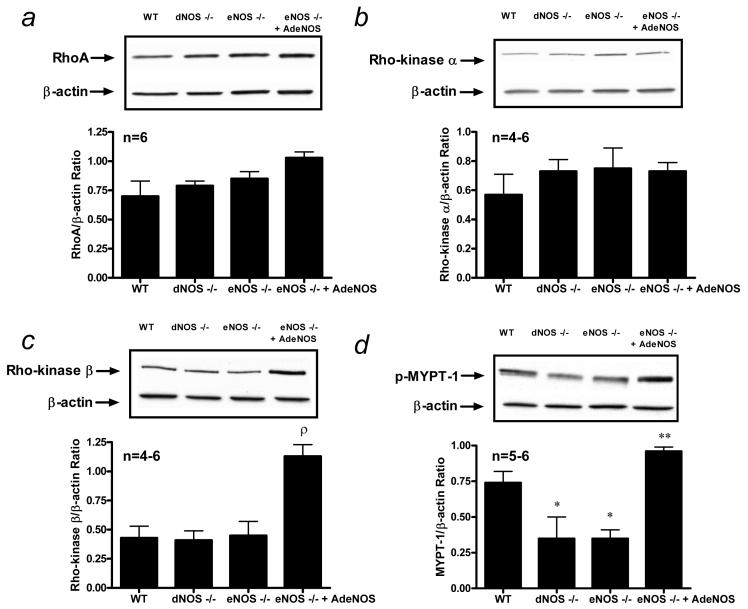
A major goal of this study was to determine the influence of endothelial NO production on the function of the RhoA/Rho-kinase pathway. Penile RhoA activation was unchanged in eNOS−/− and dNOS−/− mouse penes when compared to that of the WT mouse penis (Fig. 2A). Consistent with the RhoA activation assay data (Fig. 2), RhoA protein expression was unchanged in WT, eNOS−/−, and dNOS−/− mutant mouse penes (Fig. 3A). There was no change in Rho-kinase-α and -βprotein expressions in the WT, eNOS−/−, and dNOS−/− mouse penes at baseline (p>0.05; Fig. 3B and 3C). However, Rho-kinase activity, as measured by MYPT-1 phosphorylation, was significantly decreased (p<0.05) in eNOS−/− and dNOS−/− mouse penes relative to that of the WT control mouse penis (Fig. 2B and 3D).
Figure 2.
(a) RhoA activation and (b) Rho-kinase activity in WT, eNOS−/−, and dNOS−/− mouse penes. n indicates number of tissue samples; * (p<0.05) when compared to WT.
Figure 3.
Western blot analysis demonstrating penile expression of (a) RhoA (membrane), (b) Rho-kinase-α (cytosol), (c) Rho-kinase-β (cytosol), and (d) p-MYPT-1 (cytosol) in WT (lane 1), dNOS−/− (lane 2), eNOS−/− (lane 3) mouse penes and eNOS−/− mouse penes after intracavernous administration of AdCMVeNOS (lane 4). Quantitative densitometry of the protein expression for RhoA, Rho-kinase-α, Rho-kinase-β, and p-MYPT-1 (ratio of targeted protein expressed per β-actin) is shown in each panel. n indicates number of tissue samples; ρ (p<0.05) when compared to WT, eNOS−/−, and dNOS−/−; * (p<0.05) when compared to WT; ** (p<0.05) when compared to eNOS−/− and dNOS−/−.
3.3. Influence of In Vivo Gene Transfer of eNOS
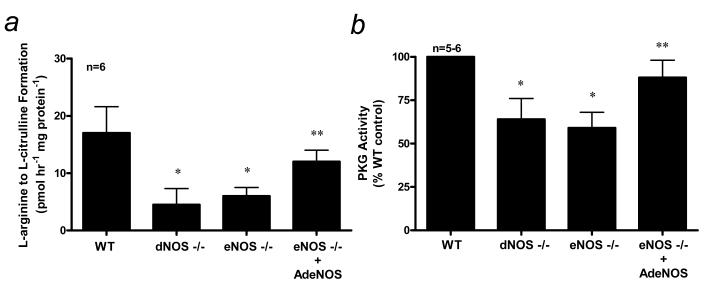
In order to determine the effect of restoration of endothelial NO production in eNOS mutant mouse penes, we directly injected into the eNOS−/− mutant mouse corpus cavernosum an adenoviral vector encoding the gene for eNOS. In the first series of experiments, constitutive NOS activity and PKG activity were measured in WT, eNOS−/− and dNOS−/− mouse penes at baseline and two days after intracavernous administration of AdCMVeNOS in eNOS−/− mutant mice (Fig. 4). Constitutive NOS and PKG activities were significantly increased (p<0.05) in the eNOS−/− mouse penis transfected with AdCMVeNOS (Fig. 4A and 4B), consistent with restored endothelial NO production in the penes of these mice. Importantly, constitutive NOS and PKG activities were elevated to levels similar to that of the WT mouse penis (Fig. 4A and 4B). Gene transfer of the reporter gene (AdCMVβgal) had no effect on penile constitutive NOS and PKG activities (data not shown). Our laboratory previously showed elevations in basal cGMP concentration after in vivo gene transfer of eNOS to the eNOS−/− mutant mouse penis, which importantly confirms existent endothelial NO bioactivity for purposes of this study [17].
Figure 4.
(a) NOS activity as measured by calcium-dependent conversion of L-arginine to L-citrulline and (b) PKG activity in WT, eNOS−/−, and dNOS−/− mouse penes and eNOS−/− mouse penes after intracavernous administration of AdCMVeNOS. n indicates number of tissue samples; * (p<0.05) when compared to WT; ** (p<0.05) when compared to eNOS−/− and dNOS−/−mice.
In the second series of experiments, we sought to determine the effect of restored expression of eNOS on RhoA and Rho-kinase expressions and activities in NOS mutant mouse penes (Figs. 3 and 5). Adenoviral gene transfer of eNOS to the eNOS−/− mouse penis did not influence RhoA protein expression (p>0.05; Fig. 3A) or Rho-kinase-α protein expression (p>0.05; Fig. 3B), although it did significantly upregulate Rho-kinase-β protein expression (p<0.05; Fig. 3C). In measuring Rho-kinase activity after adenoviral gene transfer of eNOS to the eNOS−/− mouse penis, we found a significant increase (p<0.05) in phosphorylation of MYPT-1 (Fig. 3D).
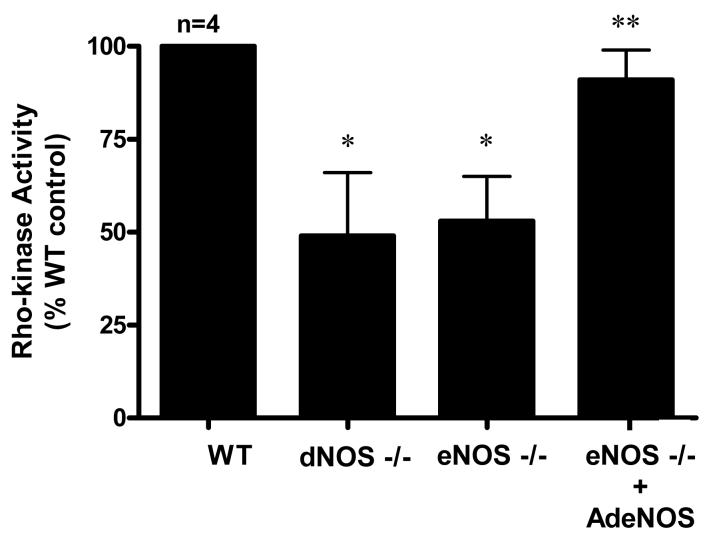
Figure 5.
Rho-kinase activity in WT, eNOS−/−, and dNOS−/− mouse penes and eNOS−/− mouse penes after intracavernous administration of AdCMVeNOS. n indicates number of tissue samples; * (p<0.05) when compared to WT; ** (p<0.05) when compared to eNOS−/− and dNOS−/−mice.
Since there was no change in membrane bound RhoA as measured by Western blot analysis, we did not perform RhoA activation assay after eNOS gene transfer. However, we did further validate the change in Rho-kinase activity as measured by MYPT-1 phosphorylation by performing a Rho-kinase activity assay (Fig. 5). We found a significant reduction (p<0.05) in Rho-kinase activity in eNOS−/− and dNOS−/− mutant mouse penes, and after adenoviral gene transfer of eNOS to the eNOS−/− mouse penis we found an upregulation (p<0.05) in Rho-kinase activity (Fig. 5). Gene transfer of the reporter gene (AdCMVβgal) had no effect on penile Rho-kinase activity (data not shown).
4. Discussion
In this study, we examined the influence of endothelial NO bioactivity in the penis on the functions of molecular mediators of the NO signaling pathway (cGMP and PKG) and the molecular cascade of the Rho-kinase signaling pathway finding that these pathways preserve an erection regulatory balance basally (distinct from erection stimulatory or pathophysiologic ED conditions) based on this influence. In particular, we found a significant reduction in constitutive NOS activity, cGMP concentration, and PKG activity in eNOS−/− and dNOS−/− (combined eNOS−/−, nNOS−/−) mutant mouse penes. Additionally, we showed that penes of these NOS mutant mice exhibit a downregulation of Rho-kinase activity without a change in Rho-kinase protein expression. Adenoviral gene transfer of eNOS to the eNOS−/− mouse penis restored constitutive NOS and PKG activities. Restored expression of eNOS in the eNOS−/− mouse penis selectively upregulated Rho-kinase-β protein expression with a concurrent increase in total Rho-kinase activity. These data suggest that the set point of Rho-kinase activity in the penis corresponds with endothelial NO synthesis in this organ providing new insight into the regulatory basis for functional penile vascular homeostasis.
Endothelial and neuronal NO plays a prominent role in the regulation of erectile physiology in healthy and diseased penile vasculature. Recent investigations in a number of experimental models of ED-disease states have revealed elevations in Rho-kinase expression/activity in association with impairments in erectile function [12-15]. The interaction of NO/cGMP signaling with RhoA/Rho-kinase has only recently been studied in the vasculature, focusing on disease states or applying in vitro techniques where changes in experimental conditions occur rather rapidly. Extensive crosstalk is evident between endothelial NO and Rho signaling pathways. Inhibition of RhoA or Rho-kinase upregulates eNOS in vivo via mechanisms that involve, but are not limited to, inhibition of actin cytoskeletal changes, leading to the stabilization of eNOS mRNA and increases in endothelial NO synthesis [10,20]. Inhibition of RhoA in penes of streptozotocin-induced diabetic rats increased eNOS expression and activity thus restoring erectile function [12]. These observations suggest that Rho signaling capably limits NO synthesis.
In addition to evidence supporting the suppressive effects of Rho signaling on NOS, there is growing evidence that NO signaling can affect Rho pathway function, in particular the membrane association of RhoA, Rho-mediated contractile responses, and RhoA activation [21]. In vascular smooth muscle cells, NO donors or stable analogs of cGMP induce a rise in RhoA gene transcription and protein expression, which can be inhibited by selective PKG inhibitors [21]. Conversely, after chronic inhibition of NO synthesis in vivo, RhoA mRNA and protein expressions are markedly reduced in aorta and pulmonary artery in association with inhibition of RhoA-mediated Ca2+ sensitization suggesting that long-term NO depletion downregulates RhoA/Rho-kinase [21]. The reduced contractile response to Rho-kinase inhibition in vitro in eNOS−/− mouse aortas additionally suggests the absence of endothelial NO yields reduced Rho-kinase activity [22]. Consistently, aortas of eNOS-overexpressing mice have less vascular reactivity to vasodilators suggesting an adaptive elevation in Rho-kinase activity as a result of increased endothelial NO content [23]. Our data showing that decreased tonic NO signaling leads to a significant reduction in Rho-kinase activity in the penis and also that eNOS gene transfer to the penis resets a higher level of Rho-kinse activity fit with these previous observations. From this, we infer that eNOS gauges Rho-kinase activity in a concordant manner in the penis, which preserves homeostatic regulatory balance of penile vascular function.
Two key findings of this study are 1) the significant reduction in Rho-kinase activity in the absence of any measurable change in Rho-kinase protein expression, and 2) the selective increase in Rho-kinase-β protein expression after eNOS gene transfer. The measurable changes in the enzyme activity of Rho-kinase, different from its protein expression results, may be due to post-translational modification and subcellular localization of the protein and would have particular relevance for the functional state of this pathway. Another potential mechanism may be reduced agonist-induced phosphorylation of Rho-kinase by norepinephrine and endothelin-1 in the penis under conditions of chronic NO-biounavailability with subsequent downregulation of the enzyme. Both isoforms of Rho-kinase have been identified in the penis in studies of both experimental animals and humans [24,25]. However, it has remained unclear as to the predominant isoform involved in regulation of Ca2+-sensitization and increased vascular tone in the penis. Our data suggest that Rho-kinase-β may be the predominant isoform and, more importantly, the isoform regulated by NO biosynthesis in the penis thus representing an ideal molecular target for new pharmacological and gene therapies for ED.
In the penis, vascular smooth muscle cells are continuously subjected to the action of basally released NO from the vascular endothelium. Endothelial NO can regulate the vascular tone in the penis by controlling downstream targets of NO [cGMP, PKG, or phosphodiesterase type 5 (PDE5)] as well as other signaling pathways (RhoA/Rho-kinase) [4]. Recently, we have shown that eNOS−/− mutant mice have an exaggerated erectile response to cavernous nerve stimulation and have phenotypic changes in erectile function consistent with priapism [16,17]. Episodes of priapism pertain to decreased NO bioavailability which downregulates penile PDE5 expression/activity [17]. When PDE5 is reduced, cGMP accumulates in the corporal smooth muscle in response to an erectogenic stimulus in vivo rendering the penile vasculature uncontrollably dilated. Similar findings were found in vitro using aorta and corpus cavernosum from eNOS−/− mutant mice, where electrical field stimulation, acetylcholine, and sodium nitroprusside caused constant corporal vasodilation [26]. In the present study, reduced total Rho-kinase activity may contribute to the susceptibility of corporal tissue to excessive relaxation manifest as priapism. These data provide additional support for aberrant signaling mechanisms involved in priapism by which the penis has decreased responsiveness to contractile mechanisms (Rho-kinase) as a result of long-term decreased NO bioactivity.
Given the importance of the NO/cGMP/PDE5 pathway in corporal smooth muscle function, chronic administration of PDE5 inhibitors may offer long-term beneficial effects on erectile tissue function in ED patients. Recent pre-clinical evidence suggests that PDE5 inhibitor therapy can improve endothelial cell function in the penis via phosphorylation of eNOS [27,28]. Importantly, daily PDE5 inhibitor therapy improves erectile function in a select group of ED patients potentially through an endothelial-dependent manner [29-32]. Taken together with the present experimental data, endothelial cell function in the penis is paramount in restoring and preserving penile vascular function.
Conclusions
The results of the present study provide evidence, at the molecular biologic level, for a homeostatic regulatory role of endothelial NO influencing basal molecular signaling of the NO and Rho-kinase erection mediatory pathways in the penis. Our data suggest that tonic release of NO by eNOS influences erection regulatory biology. The influence apparently is such that the functional state of Rho-kinase in the penile vasculature adjusts concordantly. These data provide important insights into the additional role of endothelial NO in the penis, besides being an erection effector, as a homeostatic regulator of penile vascular function and health.
Acknowledgments
This study was supported by grants from National Institute of Health (DK 02568 and DK 67223) to A.L. Burnett and from the National Kidney Foundation of Maryland to T.J. Bivalacqua. A.L. Burnett and H.C. Champion labs contributed equally to this study.
Source of funding: National Institute of Health (DK 02568 and DK 67223) to A.L. Burnett and from the National Kidney Foundation of Maryland to T.J. Bivalacqua.
Footnotes
Take Home Message: Endothelial nitric oxide influences basal molecular signaling of the nitric oxide and RhoA/Rho-kinase erection mediatory pathways in the penis with implications for steady state erection regulatory biology.
References
- 1.Rajfer J, Aronson WJ, Bush PA, Dorey FJ, Ignarro LJ. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med. 1992;326:90–4. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- 2.Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science. 1992;257:401–3. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- 3.Hurt KJ, Musicki B, Palese MA, et al. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc Natl Acad Sci U S A. 2002;99:4061–6. doi: 10.1073/pnas.052712499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bivalacqua TJ, Usta MF, Champion HC, Kadowitz PJ, Hellstrom WJG. Endothelial dysfunction in erectile dysfunction: role of the endothelium in erectile physiology and disease. J Androl. 2003;24:S17–37. doi: 10.1002/j.1939-4640.2003.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 5.Chitaley K, Wingard CJ, Webb CR, et al. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nat Med. 2001;7:119–22. doi: 10.1038/83258. [DOI] [PubMed] [Google Scholar]
- 6.Kimura K, Ito M, Amano M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–8. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 7.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–58. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz M. J Cell Sci. 2004;117:5457–8. doi: 10.1242/jcs.01582. [DOI] [PubMed] [Google Scholar]
- 9.Ming XF, Viswambharan H, Barandier C, et al. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002;22:8467–77. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266–71. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi R, Nishimura J, Hirano K, Naito S, Kanaide H. Modulation of Ca2+ sensitivity regulates contractility of rabbit corpus cavernosum smooth muscle. J Urol. 2003;169:2412–6. doi: 10.1097/01.ju.0000065808.45445.a1. [DOI] [PubMed] [Google Scholar]
- 12.Bivalacqua TJ, Champion HC, Usta MF, et al. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2004;101:9121–6. doi: 10.1073/pnas.0400520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin L, Liu T, Lagoda GA, Champion HC, Bivalacqua TJ, Burnett AL. Elevated RhoA/Rho-kinase activity in the aged rat penis: mechanism for age-associated erectile dysfunction. FASEB J. 2006;20:536–8. doi: 10.1096/fj.05-4232fje. [DOI] [PubMed] [Google Scholar]
- 14.Wilkes N, White S, Stein P, Bernie J, Rajasekaran M. Phosphodiesterase-5 inhibition synergizes rho-kinase antagonism and enhances erectile response in male hypertensive rats. Int J Impot Res. 2004;16:187–94. doi: 10.1038/sj.ijir.3901149. [DOI] [PubMed] [Google Scholar]
- 15.Wingard CJ, Johnson JA, Holmes A, Prikosh A. Improved erectile function after Rho-kinase inhibition in a rat castrate model of erectile dysfunction. Am J Physiol REgul Integr Comp Physiol. 2003;284:R1572–9. doi: 10.1152/ajpregu.00041.2003. [DOI] [PubMed] [Google Scholar]
- 16.Burnett AL, Chang AG, Crone JK, Huang PL, Sezen SE. Noncholinergic penile erection in mice lacking the gene for endothelial nitric oxide synthase. J Androl. 2002;23:92–7. doi: 10.1002/j.1939-4640.2002.tb02601.x. [DOI] [PubMed] [Google Scholar]
- 17.Champion HC, Bivalacqua TJ, Takimoto E, Kass DA, Burnett AL. Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc Natl Acad Sci U S A. 2005;102:1661–6. doi: 10.1073/pnas.0407183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takimoto E, Champion HC, Belardi D, et al. cGMP catabolism by phosphodiesterase 5A regulates cardiac adrenergic stimulation by NOS3-dependent mechanism. Circ Res. 2005;96:100–9. doi: 10.1161/01.RES.0000152262.22968.72. [DOI] [PubMed] [Google Scholar]
- 19.Nakakuki T, Ito M, Iwasaki H, et al. Rho/Rho-kinase pathway contributes to C-reactive protein-induced plasminogen activator inhibitor-1 expression in endothelial cells. Arterioscler Thromb Vasc Biol. 2005;25:2088–93. doi: 10.1161/01.ATV.0000183607.50230.9f. [DOI] [PubMed] [Google Scholar]
- 20.Fagan KA, Oka M, Bauer NR, et al. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rhokinase. Am J Physiol Lung Cell Mol Physiol. 2004;287:L656–64. doi: 10.1152/ajplung.00090.2003. [DOI] [PubMed] [Google Scholar]
- 21.Sauzeau V, Rolli-Derkinderen M, Marionneau C, Loirand G, Pacaud P. RhoA expression is controlled by nitric oxide through cGMP-dependent protein kinase activation. J Biol Chem. 2003;278:9472–80. doi: 10.1074/jbc.M212776200. [DOI] [PubMed] [Google Scholar]
- 22.Budzyn K, Marley PD, Sobey CG. Chronic mevastatin modulates receptor-dependent vascular contraction in eNOS-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2004;287:R342–8. doi: 10.1152/ajpregu.00156.2004. [DOI] [PubMed] [Google Scholar]
- 23.Yamashita T, Kawashima S, Ohashi Y, et al. Mechanisms of reduced nitric oxide/cGMP-mediated vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. Hypertension. 2000;36:97–102. doi: 10.1161/01.hyp.36.1.97. [DOI] [PubMed] [Google Scholar]
- 24.Chang S, Hypolite JA, Changolkar A, Wein AJ, Chacko S, DiSanto ME. Increased contractility of diabetic rabbit corpora smooth muscle in response to endothelin is mediated via Rho-kinase beta. Int J Impot Res. 2003;15:53–62. doi: 10.1038/sj.ijir.3900947. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Eto M, Steers WD, Somlyo AP, Somlyo AV. RhoA-mediated Ca2+ sensitization in erectile function. J Biol Chem. 2002;277:30614–21. doi: 10.1074/jbc.M204262200. [DOI] [PubMed] [Google Scholar]
- 26.Nangle MR, Cotter MA, Cameron NE. An in vitro investigation of aorta and corpus cavernosum from eNOS and nNOS gene-deficient mice. Pflugers Arch. 2004;448:139–45. doi: 10.1007/s00424-003-1232-7. [DOI] [PubMed] [Google Scholar]
- 27.Musicki B, Champion HC, Becker RE, Liu T, Kramer MF, Burnett AL. Erection capability is potentiated by long-term sildenafil treatment: role of blood flow-induced endothelial nitric-oxide synthase phosphorylation. Mol Pharmacol. 2005;68:226–32. doi: 10.1124/mol.104.010678. [DOI] [PubMed] [Google Scholar]
- 28.Behr-Roussel D, Gorny D, Mevel K, Caisey S, Bernabe J, Burgess G, Wayman C, Alexandre L, Giuliano F. Chronic sildenafil improves erectile function and endothelium-dependent cavernosal relaxations in rats: lack of tachyphylaxis. Eur Urol. 2005;47:87–91. doi: 10.1016/j.eururo.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Montorsi F, Briganti A, Salonia A, Rigatti P, Burnett AL. Can phosphodiesterase type 5 inhibitors cure erectile dysfunction? Eur Urol. 2006;49:979–86. doi: 10.1016/j.eururo.2005.12.055. [DOI] [PubMed] [Google Scholar]
- 30.Hellstrom WJ, Kendirci M. Type 5 phosphodiesterase inhibitors: curing erectile dysfunction. Eur Urol. 2006;49:942–5. doi: 10.1016/j.eururo.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 31.McMahon CG. Treatment of erectile dysfunction with chronic dosing of tadalafil. Eur Urol. 2006;50:215–7. doi: 10.1016/j.eururo.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 32.Porst H, Giuliano F, Glina S, et al. Evaluation of the Efficacy and Safety of Once-a-Day Dosing of Tadalafil 5mg and 10mg in the Treatment of Erectile Dysfunction: Results of a Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. Eur Urol. 2006;50:351–9. doi: 10.1016/j.eururo.2006.02.052. [DOI] [PubMed] [Google Scholar]