Abstract
Objective
Low vitamin D levels have been implicated in the development of and increased morbidity from asthma. The prevalence of asthma among urban African American (AA) youth is high. The goal of this study was to examine the prevalence of vitamin D insufficiency and deficiency among urban AA youth with asthma compared with non-asthmatic controls.
Study Design
A cross-sectional case-control study was conducted at an urban pediatric medical center. Total 25-hydroxyvitamin D insufficiency (< 30 ng/mL) and deficiency (< 20 ng/mL) were assessed in urban self-reported AA patients, aged 6 to 20 years, with (n = 92) and without (n = 21) physician-diagnosed asthma.
Results
Blood samples were available for 85 (92%) cases. After adjusting for age, gender, body mass index percentile, and season of sampling, the median vitamin D level of cases [18.5 (interquartile range (IQR): 11.3, 25.1)] was significantly lower than that of controls [40.4 (IQR: 34.6, 49.5), P = 0.002]. The prevalences of vitamin D insufficiency and deficiency were significantly greater among cases than controls [73/85 (86%) vs. 4/21 (19%), adjusted odds ratio (OR) = 41.7 (95% confidence interval (95%CI): 4.4 to 398.5) for insufficiency and 46/85 (54%) vs. 1/21 (5%), adjusted OR = 19.5 (95%CI: 1.4 to 272.0) for deficiency].
Conclusions
A majority of this sample of urban AA youth with persistent asthma were vitamin D deficient and/or insufficient. Given the emerging associations between low vitamin D levels and asthma, strong consideration should be given to routine vitamin D testing in urban AA youth, particularly those with asthma. Clinical trials of vitamin D supplementation among urban AA youth with asthma are warranted.
Introduction
Asthma has become considerably more prevalent and severe in the United States during the last 40 years for reasons that are not clear.(1, 2) Epidemiologic data show an association between vitamin D deficiency and asthma incidence.(3–6) In fact, increased prenatal vitamin D intake may reduce childhood asthma risk by as much as 40%.(7) Increased asthma severity in older children has been linked to low vitamin D levels.(8)
Vitamin D deficiency is more common among African American (AA) individuals,(9) especially those from urban environments(10) or with obesity.(11) Similar epidemiological patterns exist among youth with asthma. For example, asthma affects 83 per 1,000 U.S. children overall, however AA youth are disproportionately affected with a rate of 105 per 1,000.(12) This population also has the highest asthma-related morbidity and mortality of any U.S. racial/ethnic group.(13)
Herein, we examine the prevalences of vitamin D insufficiency and deficiency among urban AA youth with asthma compared with non-asthmatic controls. We hypothesized that those with asthma would have significantly lower levels of vitamin D than those without asthma.
Methods
Study Design
We conducted a cross-sectional case-control study between April, 2008 and June, 2009 at Children's National Medical Center (CNMC), an urban pediatric medical center in Washington, DC. All cases and controls were studied in the CNMC General Clinical Research Center. This study was approved by the CNMC Institutional Review Board. All participants and/or their guardians provided informed consent and assent.
Study Cohorts
The Asthma Severity Modifying Polymorphisms (AsthMaP) Project based at CNMC provided the case data and samples for this study. AsthMaP is a single-center cross-sectional study designed to find associations among urban environments, genetics, and asthma. AsthMaP consists of a predominantly AA convenience sample of otherwise healthy children aged 6 to 20 years, inclusive, with physician-diagnosed asthma for greater than one year.
The control cohort was made up of a convenience sample of healthy urban AA children between the ages of 6 and 9 years, inclusive, with no history of asthma enrolled in a local bone health study.
25-Hydroxyvitamin D Measurement
Circulating levels of 25-hydroxyvitamin D are considered the most reliable measure of overall vitamin D status.(14) A direct enzyme immunoassay was performed on plasma (from cases) or serum (from controls) using the IDS 25-Hydroxy Vitamin D Direct EIA kit (Immunodiagnostic Systems Ltd., Fountain Hills, AZ). This assay has been shown to reliably measure both 25-hydroxyvitamin D isoforms (i.e. D2 and D3).(15)
Statistical Analyses
Total 25-hydroxyvitamin D insufficiency and deficiency were defined as levels < 30 ng/mL (<75 nmol/L)(16, 17) and < 20 ng/mL (<50 nmol/L)(18), respectively. All means are reported with standard errors of the mean (SE) and medians with an interquartile range (IQR). Differences in descriptive statistics and frequencies were tested with Student's T-tests and Chi-squares. Two-tailed P Values ≤ 0.05 were considered significant.
Case-control associations were determined for vitamin D (insufficiency, deficiency, and absolute levels) by multinomial logistic regression analysis. Where noted, odds ratios (OR) and P Values were corrected for age, gender, body mass index (BMI) percentile, and season of sampling. All statistical tests were performed with SPSS Statistics 17.0 (SPSS Inc., Chicago, IL).
Results
Cases
At the time of these analyses, there were 92 AA youth studied in AsthMaP. Of those, 58 (63%) were male and the mean (SE) age was 11.1 (0.4) years. The mean BMI percentile for age was 69 (3). A total of 83 out of 92 (90%) had persistent asthma by National Institutes of Health, National Asthma Education and Prevention Program (NAEPP) criteria.(19) A detailed description of measured asthma phenotypes in these cases can be found in Table 1. Notable phenotypes included a mean fractional excretion of nitric oxide (FENO) = 41.5 (4.7), mean serum immunoglobulin E (IgE) = 543 (98.6) IU/mL, and blood eosinophilia = 5.3 (0.4) %.
TABLE 1.
CHARACTERISTICS OF CASES AND CONTROLS
| Variable | Cases (n=92) | Controls (n=21) | P Value |
|---|---|---|---|
| Sex, % male | 63 | 38.1 | 0.05 |
| Age, yr (SE) | 11.1 (0.4) | 7 (0.3) | <0.001 |
| Body mass index percentile (SE) | 69 (3) | 60 (8) | 0.22 |
| NAEPP* severity level, % | |||
| 1 - Intermittent | 9 | ||
| 2 - Mild Persistent | 23 | ||
| 3 - Moderate Persistent | 35 | ||
| 4 - Severe Persistent | 33 | ||
| FENo†, ppb (SE) | 41.5 (4.7) | ||
| FEV-1‡ change with bronchodilator, mean (SE) | 9.2 (1.1) | ||
| Post-bronchodilator FEV1, mean (SE) | 91.8 (1.7) | ||
| Serum IgE, IU/mL (SE) | 543 (98.6) | ||
| Blood eosinophils, % | 5.3 (0.4) | ||
| One or more positive allergen skin prick tests, % | 38 |
National Asthma Education and Prevention Program(19)
Fractional Excretion of Nitric Oxide
Forced Expiratory Volume in 1 second
Controls
When compared to the cases, the control cohort (n = 21) had fewer males (38%; P = 0.05) and a lower mean age of 7 (0.3) years (P < 0.001). The mean BMI percentile of 60 (8) was not significantly different from that of the cases. (Table 1)
Vitamin D Insufficiency and Deficiency
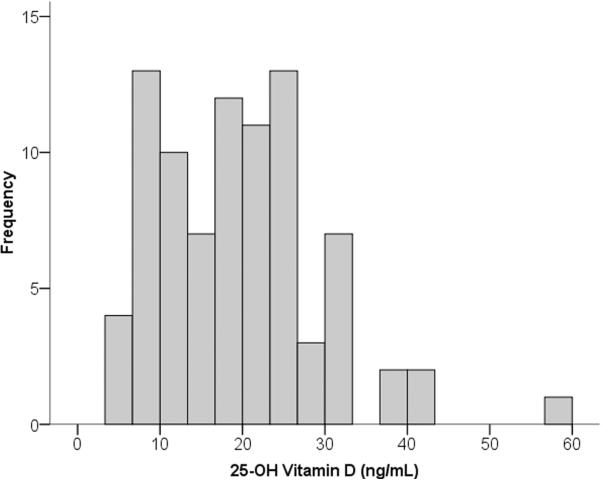
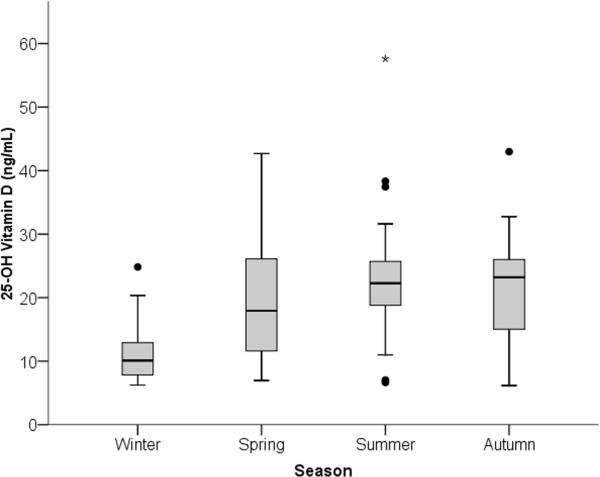
Of the 85 (92%) cases with blood samples available for vitamin D testing, 73 out of 85 (86%) had insufficient vitamin D levels (< 30 ng/mL), and 46 out of 85 (54%) had deficient levels (< 20 ng/mL). (Figure 1) A typical pattern of seasonal variation in vitamin D levels can be seen in Figure 2 where levels are lowest among the samples collected during the Washington, DC winter (i.e. December through February) and highest during the summer (i.e. June through August). Notably, all of the samples collected during the winter (13 out of 13) were in the insufficient range with 11 (85%) in the deficient range.
Figure 1.
Histogram of plasma vitamin D levels among urban African American youth with asthma. Of the 85 children represented in this graph, 73 (86%) had insufficient vitamin D levels (<30 ng/mL) including 46 (54% of the cohort) with deficient levels (<20 ng/mL).
Figure 2.
Seasonal variation in plasma vitamin D levels among urban African American youth with asthma. Seasons in Washington, DC are denoted as winter (December through February), spring (March through May), summer (June through August), and autumn (September through November). Data are presented as medians (black bars) and interquartile ranges (gray boxes).
After adjusting for age, gender, BMI percentile, and season of sampling, the median vitamin D level of those with asthma [18.5 (IQR: 11.3, 25.1)] was significantly lower than that of the non-asthmatic controls [40.4 (IQR: 34.6, 49.5), P = 0.002]. Further, the prevalence of vitamin D insufficiency was significantly greater among cases than controls [73 out of 85 (86%) vs. 4 out of 21 (19%), adjusted odds ratio (OR) = 41.7 (95% confidence interval (95%CI): 4.4 to 398.5)]. A similar association was observed for vitamin D deficiency: 46 out of 85 (54%) vs. 1 out of 21 (5%), adjusted OR = 19.5 (95%CI: 1.4 to 272.0). Table 2 summarizes these results.
TABLE 2.
VITAMIN D LEVELS IN THE CASES AND CONTROLS
| Variable | Cases (n=85) | Controls (n=21) | Adjusted† Odds Ratio (95% CI) | Adjusted† P Value |
|---|---|---|---|---|
| 25-OH Vitamin D, ng/mL (IQR) | 18.5 (11.3, 25.1) | 40.4 (36.6, 49.5) | *1.21 (1.07 to 1.37) | 0.002 |
| Vitamin D Insufficiency (<30 ng/mL), n (%) | 73 (86) | 4 (19) | 42 (4.4 to 399) | 0.001 |
| Vitamin D Deficiency (<20 ng/mL), n (%) | 46 (54) | 1 (5) | 20 (1.4 to 272) | 0.027 |
Odds ratio for asthma is for each unit increase in vitamin D level
Adiusted for age, gender, BMI percentile, and season of sampling
Subgroup analysis was undertaken for all children 9 years old and younger to explore potential confounding due to the age difference between the cases and controls. There were 29 cases which met this age criterion in addition to all 21 controls. Using identical methods as in the overall analyses, the subgroup analysis results were similar to those for the overall cohort: Median vitamin D level Cases vs. Controls = 23.1 (IQR: 18.0, 26.3) vs. 40.4 (IQR: 34.6, 49.5), P = 0.026; prevalence of vitamin D insufficiency Cases vs. Controls = 24 out of 29 (83%) vs. 4 out of 21 (19%), adjusted OR = 65.2 (95%CI: 3.9 to 1089.5); vitamin D deficiency Cases vs. Controls = 10 out of 29 (35%) vs. 1 out of 21 (5%), adjusted OR = 40.9 (95%CI: 1.6 to 1058.9).
Discussion
A majority of this sample of urban AA youth with persistent asthma in Washington, DC were vitamin D deficient and/or insufficient. Although vitamin D deficiency has been documented in many populations around the world regardless of sun exposure,(20, 21) we believe this is the first report of vitamin D levels in urban AA youth with asthma and the first to compare levels between groups with and without asthma.
Vitamin D deficiency is more common in AA individuals than in other races.(11, 20, 22) In fact, one study of preadolescent AA children in the Pittsburgh area found that 71% had Vitamin D levels below 30 ng/mL during the winter and early spring, including almost 50% with levels below 20 ng/mL.(9) There are multiple factors that contribute to this, including dark skin,(23) low vitamin D intake,(24, 25) urban environments,(10) and high rates of poverty(26) and obesity.(11) In addition, latitude is an important consideration. The effect of the relatively northern latitude of Washington, DC can be seen in the seasonal variation in vitamin D levels among our cases (i.e. levels lowest during winter months). As expected, the overall distribution of vitamin D levels among our asthma cases was considerably lower than that of a recently reported Costa Rican (i.e. equatorial) cohort of children with asthma in which only 28% of levels were < 30 ng/mL.(27)
Traditionally, the principal role of vitamin D was thought to be regulation of calcium and phosphorus homeostasis and bone formation and resorption. More recently, vitamin D has been implicated in wound healing and immunomodulation.(28, 29) In fact, vitamin D deficiency has been linked to a variety of non-bone related diseases, including, cancer, depression, and autoimmune inflammatory states.(30–33) More recently, epidemiologic data show an association between vitamin D levels and lung function, allergy, and asthma.(3–6) In fact, increased prenatal vitamin D intake may reduce childhood asthma risk by as much as 40%.(7) However, the role of vitamin D in airway inflammatory diseases such as asthma remains unclear,(3–5) although an inverse relationship with susceptibility to respiratory viruses is strongly suspected.(34)
The normal circulating vitamin D levels used by clinical laboratories were derived from healthy population distributions with the purpose of sustaining bone health.(35) These levels are likely lower than optimal levels due to sun deprivation in most northern populations.(36) Authoritative groups disagree on blood levels that define insufficiency with respect to linear growth and bone mass. Data to determine sufficient levels for lung health and other outcomes are lacking. As a result we have analyzed our vitamin D levels both using definitions of vitamin D deficiency and insufficiency(16, 17, 37) as well as a continuous variable.
Our study may be limited by the comparability of case and control vitamin D levels in two ways. First, as only plasma or serum was available for cases and controls, respectively, we could not compare identical sample types. However, simultaneous plasma and serum 25-hydroxyvitamin D levels measured by a similar chemiluminescent assay were reported to be equivalent.(38) Second, there was the possibility of confounding due to the age difference between the case and control cohorts. This was not supported by a subgroup analysis in the youngest children (≤ 9 years) that showed similar results to the findings in the complete cohorts.
The public health implications of very low vitamin D levels among urban AA youth with asthma extend beyond traditional bone health associations and the possible indication of poor overall nutrition. Rather, emerging associations between low vitamin D levels and asthma, obesity, and immunodeficiency necessitate strong consideration for routine vitamin D testing in urban AA youth, particularly those with asthma. Clinical trials of vitamin D supplementation among urban AA youth with asthma that control confounding factors such as adiposity, physical activity, ultraviolet exposure, and baseline vitamin D status are warranted.
Acknowledgment Section
The authors would like to acknowledge Jennifer Lerner and Matthew Foerster for their efforts collecting data and samples from the AsthMaP Project participants.
Funding/Support: This study was funded by grants P20MD000198 from the National Center on Minority Health and Health Disparities and M01RR020359 from the National Center for Research Resources, National Institutes of Health, Bethesda, Maryland, USA. Additional funding was provided by the Children's Research Institute of Children's National Medical Center, Washington, DC, USA.
Role of the Sponsor: The National Institutes of Health and the Children's Research Institute did not participate in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
List of Abbreviations
- AA
African American
- OR
Odds ratio
- SE
Standard error of the mean
- NAEPP
National Asthma Education and Prevention Program
- IQR
Interquartile range
- FENO
Fractional excretion of nitric oxide
- IgE
Immunoglobulin E
Footnotes
Financial Disclosures: None reported.
References
- 1.Beasley R. The burden of asthma with specific reference to the United States. J Allergy Clin Immunol. 2002;109(5 Suppl):S482–9. doi: 10.1067/mai.2002.122716. [DOI] [PubMed] [Google Scholar]
- 2.Burr ML, Butland BK, King S, Vaughan-Williams E. Changes in asthma prevalence: two surveys 15 years apart. Arch Dis Child. 1989;64(10):1452–6. doi: 10.1136/adc.64.10.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128(6):3792–8. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 4.Wjst M. The vitamin D slant on allergy. Pediatr Allergy Immunol. 2006;17(7):477–83. doi: 10.1111/j.1399-3038.2006.00456.x. [DOI] [PubMed] [Google Scholar]
- 5.Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85(3):853–9. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- 6.Saglani S, Bush A. Asthma in preschool children: the next challenge. Curr Opin Allergy Clin Immunol. 2009;9(2):141–5. doi: 10.1097/ACI.0b013e3283292230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120(5):1031–5. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Ginde AA, Mansbach JM, Camargo CA., Jr. Vitamin D, respiratory infections, and asthma. Curr Allergy Asthma Rep. 2009;9(1):81–7. doi: 10.1007/s11882-009-0012-7. [DOI] [PubMed] [Google Scholar]
- 9.Rajakumar K, Fernstrom JD, Janosky JE, Greenspan SL. Vitamin D insufficiency in preadolescent African-American children. Clin Pediatr (Phila) 2005;44(8):683–92. doi: 10.1177/000992280504400806. [DOI] [PubMed] [Google Scholar]
- 10.Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin Pediatr (Phila) 2007;46(1):42–4. doi: 10.1177/0009922806289311. [DOI] [PubMed] [Google Scholar]
- 11.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531–7. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 12.Asthma Prevalence, Health Care Use and Mortality. 2002 [Google Scholar]
- 13.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, et al. National surveillance for asthma--United States, 1980–2004. MMWR Surveill Summ. 2007;56(8):1–54. [PubMed] [Google Scholar]
- 14.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122(2):398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 15.Carter GD, Carter R, Jones J, Berry J. How accurate are assays for 25-hydroxyvitamin D? Data from the international vitamin D external quality assessment scheme. Clin Chem. 2004;50(11):2195–7. doi: 10.1373/clinchem.2004.040683. [DOI] [PubMed] [Google Scholar]
- 16.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 17.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 18.Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88(2):558S–64S. doi: 10.1093/ajcn/88.2.558S. [DOI] [PubMed] [Google Scholar]
- 19.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 20.Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76(1):187–92. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 21.Binkley N, Novotny R, Krueger D, Kawahara T, Daida YG, Lensmeyer G, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab. 2007;92(6):2130–5. doi: 10.1210/jc.2006-2250. [DOI] [PubMed] [Google Scholar]
- 22.Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr. 1998;67(6):1232–6. doi: 10.1093/ajcn/67.6.1232. [DOI] [PubMed] [Google Scholar]
- 23.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74–6. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 24.Moore CE, Murphy MM, Holick MF. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr. 2005;135(10):2478–85. doi: 10.1093/jn/135.10.2478. [DOI] [PubMed] [Google Scholar]
- 25.Pinto JM, Schneider J, Perez R, DeTineo M, Baroody FM, Naclerio RM. Serum 25-hydroxyvitamin D levels are lower in urban African American subjects with chronic rhinosinusitis. J Allergy Clin Immunol. 2008;122(2):415–7. doi: 10.1016/j.jaci.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 26.Saintonge S, Bang H, Gerber LM. Implications of a new definition of vitamin D deficiency in a multiracial us adolescent population: the National Health and Nutrition Examination Survey III. Pediatrics. 2009;123(3):797–803. doi: 10.1542/peds.2008-1195. [DOI] [PubMed] [Google Scholar]
- 27.Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179(9):765–71. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97(1–2):93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92(1):4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Mathieu C, Badenhoop K. Vitamin D and type 1 diabetes mellitus: state of the art. Trends Endocrinol Metab. 2005;16(6):261–6. doi: 10.1016/j.tem.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. Jama. 2006;296(23):2832–8. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 32.Mullin GE, Dobs A. Vitamin d and its role in cancer and immunity: a prescription for sunlight. Nutr Clin Pract. 2007;22(3):305–22. doi: 10.1177/0115426507022003305. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz GG, Skinner HG. Vitamin D status and cancer: new insights. Curr Opin Clin Nutr Metab Care. 2007;10(1):6–11. doi: 10.1097/MCO.0b013e328011aa60. [DOI] [PubMed] [Google Scholar]
- 34.Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, et al. Epidemic influenza and vitamin D. Epidemiology and Infection. 2006;134(06):1129–40. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haddad JG, Chyu KJ. Competitive protein-binding radioassay for 25-hydroxycholecalciferol. J Clin Endocrinol Metab. 1971;33(6):992–5. doi: 10.1210/jcem-33-6-992. [DOI] [PubMed] [Google Scholar]
- 36.Hollis BW, Wagner CL. Normal serum vitamin D levels. N Engl J Med. 2005;352(5):515–6. doi: 10.1056/NEJM200502033520521. author reply -6. [DOI] [PubMed] [Google Scholar]
- 37.Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85(3):649–50. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 38.Ersfeld DL, Rao DS, Body J-J, Sackrison JL, Miller AB, Parikh N, et al. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON® automated analyzer. Clinical Biochemistry. 2004;37(10):867–74. doi: 10.1016/j.clinbiochem.2004.06.006. [DOI] [PubMed] [Google Scholar]