Abstract
Background
A potential source of patellofemoral pain, one of the most common problems of the knee, is believed to be altered patellofemoral kinematics due to a force imbalance around the knee. Although no definitive etiology for this imbalance has been found, a weak vastus medialis is considered a primary factor. Therefore, this study’s purpose was to determine how the loss of vastus medialis obliquus force alters three-dimensional in vivo knee joint kinematics during a volitional extension task.
Methods
Eighteen asymptomatic female subjects with no history of knee pain or pathology participated in this IRB approved study. During the first visit, the patellofemoral and tibiofemoral kinematics were derived from velocity data acquired using dynamic cine-phase contrast MRI. The same kinematics were then acquired immediately after administering a motor branch block to the vastus medialis obliquus using 3–5cc of 1% lidocaine. A repeated measures analysis of variance was used to test the null hypothesis that the post- and pre-injection kinematics were no different.
Findings
The null hypothesis was rejected for patellofemoral lateral shift (p=0.003, max change=1.8mm, standard deviation=1.7mm), tibiofemoral lateral shift (p<0.001, max change=2.1mm, standard deviation=2.9mm), and tibiofemoral external rotation (p<0.001, max change=3.7°, standard deviation=4.4°).
Interpretation
The loss of vastus medialis obliquus function produced kinematic changes that mirrored the axial plane kinematics seen in individuals with patellofemoral pain, but could not account for the full extent of these changes. Thus, vastus medialis weakness is likely a major factor in, but not the sole source of, altered patellofemoral kinematics in such individuals.
Keywords: MRI, patella, tibia, femur, correlation, muscle, function, dynamic, quadriceps
Introduction
Patellofemoral (PF) pain syndrome (PFPS) is one of the most common problems of the knee, constituting 14–17% of all injuries presenting to sports injury clinics (Iwamoto et al., 2008, Taunton et al., 2002) and having a prevalence of 23.7% among midshipman at the U.S. Naval Academy (Boling et al., 2009). It is characterized by idiopathic anterior knee pain that is aggravated by deep knee flexion, prolonged sitting and repetitive flexion/extension (Wilson, 2007). The most widely accepted theory in regards to the source of this pain is that a force imbalance around the knee leads to static PF malalignment (Grelsamer et al., 2008, Laprade and Culham, 2003, Schutzer et al., 1986) and dynamic PF maltracking (Brossmann et al., 1993, Sheehan et al., 2009a, Stanford et al., 1988, Wilson et al., 2009a). In turn, this causes elevated PF joint contact stresses, which ultimately leads to pain.
This potential force imbalance around the knee has been primarily attributed to weak or altered quadriceps activation (Lin et al., 2008, Makhsous et al., 2004) and greater joint laxity (Amis et al., 2003). Quadriceps strengthening exercises, emphasizing the vastus medialis (VM), consisting of the vastus medialis obliquus (VMO) and vastus medialis longus (VML), have been suggested for the initial management of PF pain (Powers, 1998, Wilk et al., 2001). However, the results of these studies are not consistent (Wilk et al., 2001) and necessitate the need for directly quantifying the in vivo, dynamic relationship between knee joint kinematics and VM force.
Previous cadaver and EMG work has shown that the VM plays an important role in medially stabilizing the patella (Scuderi, 1992, Hanten and Schulthies, 1990), even though it is phylogenetically the weakest of the quadriceps and is typically the first of the quadriceps to atrophy after an injury and the last to recover (Thomee et al., 1995). Unfortunately, in vivo muscle force cannot be measured directly, without highly invasive techniques. Thus, the previously reported force imbalances between the VM (VMO and VML) and vastus lateralis (VL) were based on electromyographic (EMG) recordings, from which muscle force was approximated. Such EMG studies have failed to reach a consensus on the role of VM in PFPS, with some studies reporting no difference between VM activity in individuals with PFPS, as compared to pain-free individuals (Bevilaqua-Grossi et al., 2008, Owings and Grabiner, 2002, Powers et al., 1996) and others finding altered VM activity in individuals with PFPS (Cowan et al., 2002, Santos et al., 2008). This controversy may stem from the fact that the assumptions required to estimate muscle force from EMG may not be valid in the PFPS population. Specifically, an earlier study demonstrated that in individuals with PFPS a muscle may not contribute to useful work even if it is active (Sheehan, 2000). The influence of the VM on PF mechanics has also been studied using computational models (Elias et al., 2010, Dhaher and Kahn, 2002). Although these models have the potential to enhance our insight into PF joint mechanics, they have yet to be validated and therefore, provide little clinical utility.
Thus, the purpose of this study was to determine how the loss of function in the VM alters the dynamic control of knee joint kinematics in healthy individuals. Specifically, it was hypothesized that a complete (or near complete) block of the VMO using a short-acting agent (lidocaine) would result in PF lateral shift, lateral tilt, and valgus rotation. Since the quadriceps acts through the patella to exert a force and torque on the tibia (Yamaguchi and Zajac, 1989), it was also hypothesized that the VMO block would result in tibiofemoral (TF) lateral shift, external rotation, and valgus rotation. In order to explore the relationship between kinematic changes after the loss of VM strength and the initial kinematic state of the joint, correlations between the pre-injection kinematics and the change in kinematics pre- to post-injection were quantified.
Methods
To date, 23 asymptomatic females with no prior history of knee pain, trauma, leg surgery, or joint pathology (ligament/meniscus tears, arthritis, etc) and/or contra-indications to magnetic resonance (MR) imaging have been enrolled in this IRB approved study, involving two visits. Recruitment targeted females, as this is the population in which PFPS is most prevalent (Boling et al., 2009). During the first visit, subjects provided signed consent, their medical history was documented, and a physical examination including a clinical evaluation of the knee joint (Q- angle, laxity, etc) was performed. An “X” marking the location of the mid-patella was placed on the skin covering the knee (with a gentian violet marking pen). This mark was later used for alignment within the MR imager. A single knee, chosen at random, was studied for each subject.
For the dynamic scanning all participants were placed supine in an MR imager (3.0 T, Philips Medical Systems, Best, NL), with the knee bent and supported on a wooden block. A customized coil holder held a pair of large flex-coils medial-lateral and a pair of medium flex-coils anterior to the knee. The “X” denoting the mid-patella was aligned with the imager’s superior-inferior reference plane and the location of this reference plane was marked on the coil holder. Subjects were then asked to cyclically flex and extend their knee while a dynamic cine-phase contrast MR image set (x, y, z velocity and anatomic images frames) was acquired (Sheehan et al., 1999). In order to establish anatomical coordinate systems dynamic, cine images (anatomic images only) were acquired in three axial planes (Seisler and Sheehan, 2007, Sheehan and Mitiguy, 1999).
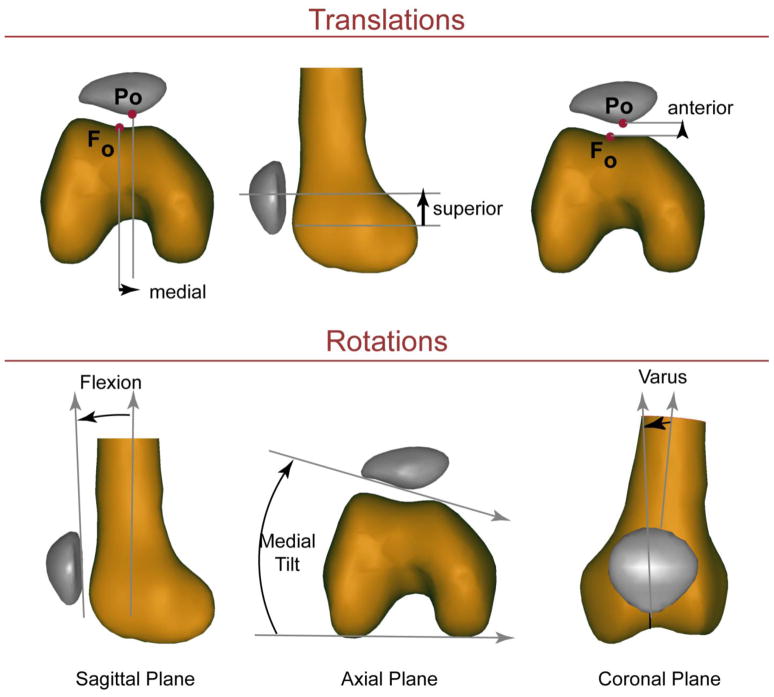
Three-dimensional PF (Figure 1) and TF kinematics were quantified by integrating the velocity data throughout the motion cycle, accuracy <0.3mm (Behnam et al., 2011). In addition, a set of high resolution 3D static images were acquired and then read by a radiologist to rule out knee pathology. Identical to a previous study (Harbaugh 2010), the lateral trochlear inclination angle (LTI) was measured from these 3D static images.
Figure 1. Three-dimensional PF kinematics.
The kinematics reported were based on a 3D anatomical coordinate system established in the patella and femur in the full extension time frame (Seisler and Sheehan, 2007), with medial, superior and anterior shift along with flexion, medial tilt and varus being positive. The kinematics were similarly defined for the tibiofemoral joint with internal rotation being positive. All rotations were calculated based on an xyz-body fixed coordinate system such that the rotation sequence was flexion, tilt, varus rotation.
If the first scan revealed any abnormal knee joint kinematics, defined as any single PF or TF kinematic parameter being more than one standard deviations (SD) from a previously defined control average (Seisler and Sheehan, 2007), the subject was removed from the study. This occurred in five instances; resulting in a final cohort of eighteen subjects (age=26.6 years, SD=8.1years; height=164.7cm, SD=7.3 cm; mass=57.4kg, SD=8.0 kg; 8 right and 10 left knees). The subject that remained in the study were asked to return within a week for the second segment of the study. The scanning protocol was saved on the MR scanner so that the identical dynamic scanning parameters could be used for the second visit, removing potential kinematic errors associated with variations in the scan plane locations relative to the joint (Shibanuma et al., 2005).
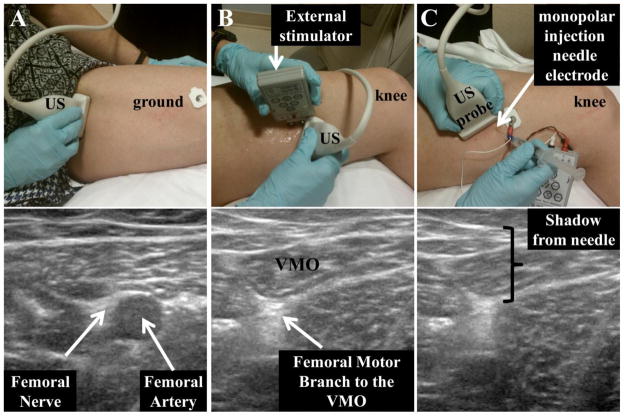
For the second visit, scanning began immediately after completion of a femoral nerve motor branch block to the VMO/distal VM. Using direct ultrasound (US) visualization, along with surface and percutaneous electrical stimulation, a stimulating needle electrode was inserted and advanced to near nerve location (Figure 2). Percutanous stimulation was performed to confirm an isolated contraction in the VMO/distal VM and then 3 cc of 1% lidocaine were injected using a 25–27 g sterile Teflon coated monopolar injection needle electrode (Barrack et al., 1983, Khin Myo et al., 1999). A single physician (author KEA, board certified by the American Board of Physical Medicine and Rehabilitation and the American Board of Electrodiagnostic Medicine) performed all nerve blocks. Evaluation of the effectiveness of the motor block was assessed by the absence of visible twitch (visual surface inspection and B-mode US) in response to percutaneus electrical stimulation of the motor nerve (Figure 3 and supplemental material). An absence of a twitch response upon stimulation indicated a complete or near complete block of the VMO. In addition to the twitch response, the absence of a visible VMO contraction was confirmed in a similar manner while the subject performed a maximal isometric contraction of the quadriceps. If the twitch response was not ablated the procedure was repeated at a second site with up to 2 cc of 1% lidocaine. This second injection was necessary for five subjects. Confirmation of the effectiveness of the block was performed as noted above. Force measurements were not used to define the effectiveness of the muscle block, as there are no in vivo data, to date, in regards to the isolated force capability of the VMO. Similar to previous reports (Smith et al., 2009), a distinct fascial plane separating the VMO from the VML could not be found in approximately one-third of the subjects. In such cases it was confirmed that the distal third of the VM was blocked. Immediately following the muscle block, the subject was transported to the MR scanner by wheelchair. The “X” marked on the subjects skin during the first visit was then aligned with the reference mark on the coil holder and both marks were jointly aligned with the MR’s reference plane, resulting in the subject being placed in the same location within the scanner for both visits. All dynamic scanning was completed within 20 minutes of completing the muscle block (lidocaine has a minimum effective period of 1 hour) using the same series of dynamic exams as were acquired in the first visit. Identical to the first exam, 3D PF and TF kinematics were quantified by integrating the velocity data throughout the motion cycle.
Figure 2. Localization of the Femoral Motor Branch to the VMO.
A) Using ultrasound (US) the femoral nerve was located next to the femoral artery on the superior medial aspect of the thigh. The artery and nerve were followed distally using US until the distal femoral motor branch to the VMO was found. B) The US probe was held in position while surface electrical stimulation was used to confirm that the femoral motor nerve branch to the VMO had been found. C) Next, 25–27 g Teflon coated hypodermic needle was inserted into the skin/muscle after appropriate skin preparation. The same stimulator was used for both the surface and the percutaneous stimulation using manufacturer supplied attachments. Stimulation together with US was used to guide the needle to the correct location. When the location of the motor branch was confirmed 3cc of 1% lidocaine was injected. For additional visualization see supplementary video.
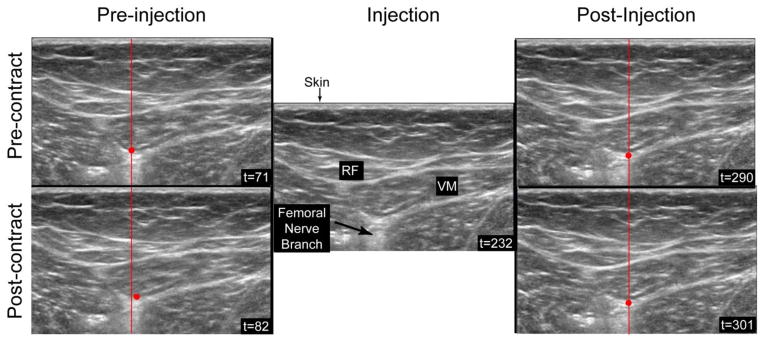
Figure 3. Lidocaine Block of the Femoral Nerve Branch to the VMO.
All the US images are from a single B-mode US capture (see supplementary video) that occurred after the needle was injected at the site of the femoral nerve branch to the VMO. A constant stimulation (0–50mA, 2K ohm load, pulse width 0.22 milliseconds, rise time < 10 microsecond) was provided throughout the capture. The left images demonstrate the shift in the VMO caused by the contraction (t=71 to t=82). The red dot indicates the same point in the pre- and post-injection images. Pre-injection, this point can be seen to move between time frames. After stimulation confirmed the correct location of the needle, a bolus of 1% lidocaine was injected (center image, t=232), after which not further contraction of the muscle could be seen, such that the red dot remains stationary between time = 290 to time=301).
Statistical Analyses
An a priori power analysis determined that 13 subjects would be needed to find significant kinematic differences pre- to post-injection, assuming that the loss of VM force would result in kinematics alterations equal to half the difference seen between a previous control and PFPS cohort {50% of the 2.9mm and 3.1° of lateral shift and tilt, respectively (Sheehan et al., 2009a)} and that the variation within the data would be as large as the differences found (α=0.05, β= 0.90). Since secondary hypotheses and correlations were included within the study, the target population was increased to ensure adequate power. All data were interpolated to single knee angle (KA) increments for averaging across subjects. Data were collected from −1° to 55° of knee flexion, yet not all subjects reached these ranges due to their limb length, flexibility, and the restrictions of the closed bore environment. In the knee angle range of 10° to 35° all subjects were represented.
A repeated measures analysis of variance (ANOVA) was used to test the null hypothesis that the post- and pre-injection kinematics were no different across the knee angles of extension. In order to minimize the statistical testing, the null hypothesis was evaluated at every 5° of knee angle from 10° to 40°, as the minimum number of subjects for adequate power (13) was represented at each of these angles. A Kolmogorov-Smirnov test for normality was run. If the data were normally distributed a repeated measures ANOVA was run using Hotellings T2 test statistic; if not, the non-parametric Friedman’s test statistic was used. Upon rejection of the null hypothesis a post-hoc analysis (Wilcoxon signed rank test) was completed to determine at which knee angles the null hypothesis could be rejected. Pearson’s correlations between the change in kinematics post-injection and the pre-injection kinematics were quantified at these same knee angles. This was followed by a step-wise linear regression. Significance was set at p≤0.05.
Results
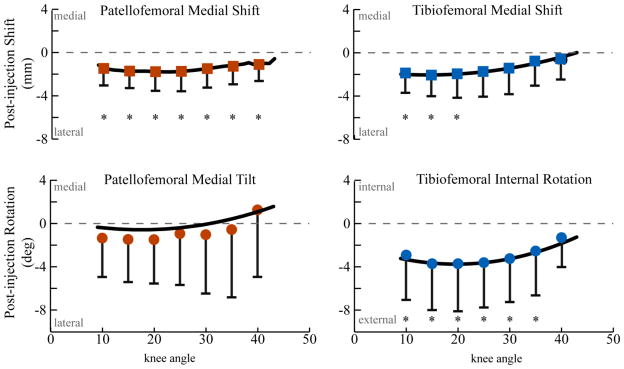
The null hypothesis (that the pre- and post-injection kinematics were no different) was rejected for PF lateral shift (p=0.003), TF lateral shift (p<0.001), and TF external rotation (p<0.001). The Wilcoxon post-hoc tests revealed that the rejection of the null hypothesis was valid over a range of knee angles (Figure 4). Post-injection, the maximum lateral shift of the patellar and tibial origins (1.8mm, SD=1.7mm, p=0.003 KA=20° and 2.1mm, SD=2.9mm, p=0.02, KA = 15°) and the maximum external tibial rotation (3.7°, SD=4.4°, p=0.006, KA=20°) occurred at similar points in the extension arc. These changes were 3.2 to 4.8 times greater than the subject repeatability (Behnam et al., 2011). The value of PF tilt trended laterally post-injection (max=1.6, KA =15°), but significant differences pre- to post-injection were not found. PF and TF varus-valgus rotation demonstrated insignificant post-injection changes that were less than the subject repeatability. No individual reported pain, based on a Visual Analog Scale, following the kinematic trials during visit 1 and visit 2.
Figure 4. Difference in patellofemoral and tibiofemoral kinematics between the pre- and post-injection states.
A second order polynomial was fit to each curve and then the actual data was plotted every 5 degrees with one standard deviation bars. The repeated measures ANOVA rejected the null hypothesis (that there was no difference in kinematics pre- to post-injection) for PF medial shift (p=0.03), TF lateral shift (p<0.001) and TF external rotation (p<0.001). A “*” indicates a p-value of less than 0.05 for the Wilcoxson post-hoc test.
The lateral shift of the PF origin post-injection was significantly correlated with the initial value of PF origin’s superior displacement in terminal extension (Table 1, Pearson correlation coefficients (r-values) ranged from 0.47 to 0.48 for knee angles ranging from 10°–20°, p<0.05). The lateral shift of the TF origin demonstrated a similar correlation, but also demonstrated correlations with TF origin’s posterior displacement and LTI. In general, it was most likely that the change in TF origin’s lateral position post-injection was due to the change in TF external rotation, as a moderate correlation existed between these two variables for all knee angles (r-values ranged from 0.66–0.71, p<0.05). TF external rotation was not correlated with any initial kinematic variable. Using a combination of variables (LTI, pre-injection PF medial and superior displacement) enabled 42 – 72% of the variability in post-injection change in PF lateral shift to be explained (Table 2). Similar regressions were found for the change in post-injection TF lateral shift, but not for PF medial tilt of TF internal rotation.
Table 1.
Correlations between the differences in kinematics pre- and post-injection and the pre-injection kinematics. Correlations with the change in kinematics are listed only for those variables (and the corresponding knee angles) where p>0.05
| Post-injection Change in PF medial translation
| ||
|---|---|---|
| Knee Angle (deg) | Pre-injection PF superior displacement |
|
| r | p | |
| 10 | −0.47 | 0.049 |
| 15 | −0.57 | 0.014 |
| 20 | −0.48 | 0.043 |
| 25–40 | no significant correlations | |
| Post-injection Change in TF medial translation
| ||||||
|---|---|---|---|---|---|---|
| Knee Angle (deg) | LTI | Pre-injection PF superior displacement |
Pre-injection TF anterior displacement |
|||
| r | p | r | p | r | p | |
| 10 | −0.57 | 0.013 | ||||
| 15 | −0.48 | 0.044 | −0.47 | 0.049 | −0.50 | 0.035 |
| 20 | −0.54 | 0.021 | −0.47 | 0.049 | ||
| 25–40 | no significant correlations | |||||
| Post-injection Change in TF internal rotation
| ||
|---|---|---|
| Knee Angle (deg) | LTI | |
| r | p | |
| 20 | −0.48 | 0.046 |
| 10–15 | no significant correlations | |
| 25–40 | no significant correlations | |
Table 2.
Regression coefficients for the differences in kinematics pre- and post-injection and the pre-injection kinematics. Regressions with the change in kinematics are listed only for those variables (and the corresponding knee angles) where p>0.05. The full equations are not provided, but the variables contributing to the regression equations are listed with and “X”.
| Post-injection Change in PF medial translation
| ||||||
|---|---|---|---|---|---|---|
| Knee Angle (deg) | LTI | Pre-injection PF medial disp. | Pre-injection PF superior disp. | Pre-injection PF medial tilt | R2 | p-value |
| 10 | X | X | X | 0.72 | <0.001 | |
| 15 | X | X | X | X | 0.62 | 0.009 |
| 20 | X | X | X | 0.50 | 0.019 | |
| 25 | X | X | X | 0.44 | 0.01 | |
| 30 | X | X | X | 0.41 | 0.05 | |
| 35 | X | X | X | 0.49 | 0.02 | |
| 40 | No significant correlations | |||||
| Post-injection Change in PF medial tilt
| |||||
|---|---|---|---|---|---|
| Knee Angle (deg) | Pre-injection anterior disp. | PF Pre-injection TF anterior disp. | Pre-injection TF internal rotation | R2 | p-value |
| 10–25 | No significant correlations | ||||
| 25 | X | X | X | 0.59 | 0.002 |
| 30–40 | No significant correlations | ||||
| Post-injection Change in TF medial translation
| |||||||
|---|---|---|---|---|---|---|---|
| Knee Angle (deg) | LTI | Pre-injection medial | PF disp. superior | Pre-injection superior | TF disp. anterior | R2 | p-value |
| 10 | No significant correlations | ||||||
| 15 | X | X | X | 0.66 | 0.001 | ||
| 20 | X | X | X | 0.70 | .001 | ||
| 25 | X | X | X | 0.50 | .001 | ||
| 30 | X | X | 0.41 | 0.05 | |||
| 35 | X | X | X | 0.70 | 0.001 | ||
| 40 | X | X | X | 0.75 | <0.001 | ||
Discussion
This study advances our clinical understanding of the role of the VMO in PFPS by providing the first in vivo data pertaining to the contributions of the isolated VMO to PF and TF kinematics. The kinematic changes mirrored the difference in axial plane kinematics seen between individuals diagnosed with PFPS and controls, measured previously using the identical pre-injection paradigm (Sheehan et al., 2009a). Even though the muscle block likely produced a greater loss in VM strength than that experienced by individuals with PFPS, the post-injection changes in PF lateral shift and tilt were only 51% and 46% of the differences between the PFPS and controls cohorts (Sheehan et al., 2009a). Both the change in PF tilt (pre- to post-injection) and the difference in this variable between individuals with PFPS and controls trended laterally, but were not significant. The functional loss of the VMO did not produce changes in the other planes of motion. Specifically, the previous PFPS cohort demonstrated increased PF superior displacement, flexion, valgus, and TF external rotation (Sheehan et al., 2009a). Thus, the loss in VM function cannot explain all the kinematics changes in PFPS cohort and it is most likely that VM weakness is a major factor in, but not the sole source of, PF maltracking.
To relate the kinematic changes seen following a VM block to those seen in PFPS, it is important to understand that there are likely subgroups within the PFPS population (Lin et al., 2008, Schutzer et al., 1986, Sheehan et al., 2009b), each with unique kinematic alterations of varying etiologies. In a previous study the PFPS cohort was divided into two groups, lateral and non-lateral maltrackers (Sheehan et al., 2009b). The lateral maltrackers demonstrated increased PF lateral and superior shift, lateral tilt, flexion, and valgus rotation. The change in kinematics following the nerve block could account for a portion of the lateral shift and tilt seen in the lateral maltrackers. Increased ligament laxity likely would have increased this shift and tilt, as well as increased the patellar ligament length, resulting in the observed PF superior shift (patella alta) in the lateral maltrackers. Patella alta reduces the influence of the femoral groove on PF kinematics in terminal extension, resulting in increased PF lateral shift (as supported by the correlations within this study), lateral tilt, and valgus rotation. Therefore, a combination of VM weakness and generalized ligament laxity could account for the kinematic variations in the lateral maltracking group. These changes would result in higher contact stresses when the patella is forced to re-engage with the femoral sulcus in early flexion and would reduce the overall contact area (Ward et al., 2007), both of which would likely leading to PF pain.
The non-lateral maltrackers (Sheehan et al., 2009b) demonstrated increased PF flexion and increased TF internal rotation only, with a trend towards PF medial translation and medial tilt. A larger LTI combined with a normative PF superior location in the non-lateral maltrackers limited lateral PF shift and influenced patellar tilt in this subgroup (Harbaugh et al., 2010). Thus, in the non-lateral maltrackers a loss of VM strength likely would have resulted in increased contact force between the lateral femoral sulcus and the patella, resulting in PF pain, without excessive lateral shift and tilt being present. The non-lateral maltrackers demonstrated increased TF internal, not external rotation, as was found post-injection. This discrepancy could be explained by the fact that the controls did not have time to adapt to the loss of VM strength, whereas the individuals with PFPS did. With a long-term loss of VM strength, the tibia could be medially stabilized using the medial hamstrings, which may have lead to the observed internal tibal rotation in the non-lateral maltrackers.
The high LTI and the trend towards medial tilt in the non-lateral maltracking group may help explain the variability in the post-injection change in PF lateral tilt. In the presence of a high LTI, a loss of strength in the VMO strength could result in the lateral patellar edge riding up the lateral femoral sulcus, resulting in the observed trend towards medial tilt in the non-lateral maltrackers and the post-injection medial tilt observed in some subjects.
The correlations and regressions found within this study demonstrated that other factors can alter how much VMO weakness influences PF kinematics. Specifically, the correlation between the change in PF lateral shift post-injection and the pre-injection PF superior position demonstrates that patella alta can couple with VMO weakness, resulting in excessive PF lateral shift. The regressions add to this by demonstrating that multiple factors (LTI, pre-injection PF medial and superior shift) coupled together explain 41–72% of the post-injection changes in PF lateral shift with the loss of VMO strength. Whereas, the lack of correlations/regressions with the change in TF external rotation demonstrates that VMO weakness does not couple with any of the current variables in the control of this parameter.
Although the current literature suggests that the VM plays a central role in medially stabilizing the patella, data demonstrating a direct link between VM force and in vivo PF kinematics during a volitional activity have not been available. Makhsous and colleagues (2004) quantified the individual contributions to the TF extension torque from the VMO, VML, and VL using surface electrical stimulation. They concluded that the VM in individuals with PFPS (n = 10) contributes significantly less to knee extension as compared to healthy controls (n = 11). Wilson and colleagues (2009b) echoed these results, reporting that individuals with PFPS had significantly less VMO tendon strain (defined as the change in length of the tendon from rest to contracted state), but no difference in the VL tendon strain, as compared to a control cohort. The PF joint kinematics were not quantified in either study. Sakai and co-authors (2000) found a significant change in patellar lateral shift (0.5mm) at 15° knee flexion when a knee extensor torque was produced without a contribution from the VMO. Despite giving insights into the VMO’s potential role in controlling PF kinematics, this study was limited by its use of cadaveric specimen (n = 7), as the simulated quadriceps force likely did not represent the true physiological state. Lin and colleagues (2010) focused on maximally stimulating the VMO, VML, and the VL using surface stimulation at two static knee angles (n=7) and reported that the VMO primarily translates and tilts the patella medially. The reported change in PF lateral shift (~0.75mm) was less than the technique accuracy (1.1mm) reported in a previous study by the same group (Wilson et al., 2009a), placing the validity of the results into question. The smaller shift seen in this past study is likely due to the fact that direct electrical stimulation using surface stimulation may not have activated the VMO to its full strength. The motor branch block directly affects the nerve, essentially eliminating the function of the muscle innervated by that branch.
The current study provides crucial data for validation of musculoskeletal modeling. Specifically, the estimation of the quadriceps ability to produce a torque on the tibia is complicated by the fact that the patella serves as an intermediary (a dynamic fulcrum) between the quadriceps and tibia. Therefore, the term effective quadriceps moment arm was coined to define the ability of the quadriceps to generate a torque on the tibia (Yamaguchi and Zajac, 1989). Yet, this property has only been calculated two-dimensionally in the sagittal plane. The post-injection external tibial rotation clearly demonstrates that acting through the patella, the VM effective moment arm has a component that results in internal tibial rotation. Further, the current data provide a unique validation criterion for simulating the weak VM or no-VMO conditions using computational models (Farrokhi et al., 2011, Elias et al., 2010, Besier et al., 2009) that rely on muscle forces either estimated through optimization or assumed from literature values.
A limitation of this study was the fact that a medial-lateral and a superior-inferior vector was used to establish both the patellar and femoral coordinate systems, effectively zeroing out the initial offset in varus rotation. This likely resulted in the minimal change in varus post-injection, even though it was expected that the loss of the VMO would result in a PF valgus rotation, (Wilson and Sheehan, 2010). Work is ongoing to determine if an initial offset for varus can be established. The data were collected during a loaded (weight of the tibia), open chain extension exercise and the results might vary slightly during a closed chain or more heavily loaded activity. Yet, measuring PF kinematics in weight-bearing conditions does not always demonstrate an unstable joint condition, even if one is present (Powers et al., 2003). In an open-chain exercise the quadriceps force increases as the knee extends into terminal extension, whereas the opposite occurs for closed-chain exercise (Hungerford and Barry, 1979). Thus, the open-chain exercise used in the current study would be more likely to demonstrate potential joint instability resulting from a loss of VM strength by requiring high quadriceps forces during terminal extension.
Conclusions
This study has advanced the clinical understanding of PFPS by providing a direct in vivo link between VMO weakness in isolation and knee joint kinematic alterations. In doing so it has demonstrated that VMO weakness is most likely a major factor in, but not the sole source of, PF maltracking. Thus, isolated VM strengthening will likely not fully correct PF maltracking in most individuals. In addition, it demonstrated that even with kinematic alterations in the PF and TF joint, pain was not experienced during the post-injection trial, indicating that pain may be a factor that develops over time. Combining these findings with past results pertaining to the kinematics and bone shape alterations in individuals with PFPS supports two paths to PFPS in two kinematically unique subgroups. Such knowledge will likely help foster the next generation of treatments for PFPS that focus on first elucidating subject-specific factors leading to PF pain and then devising an intervention plan that specifically targets these factors in order to optimize treatment (Derasari et al., 2010). Future work is required to provide further evidence on the validity of these paths and extend the current methodology to create a clinical diagnostic tool that will help guide therapeutic or surgical treatments for patients with PF pain.
Supplementary Material
Acknowledgments
The authors wish to thank Sara Sadeghi for her help in enrolling subjects; Cris Zampieri, PhD for her help in data collection; Aaron Heuser, PhD, for his statistical support; Beth Rasch, PhD for her help in protocol development; Steven Stanhope, PhD, for the conversations that planted the seeds for this work; and Bonnie Damaska and the Diagnostic Radiology Department at the National Institutes of Health for providing access and support for the MR imaging. This research was supported by the NIH Clinical Center Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10:215–20. doi: 10.1016/s0968-0160(03)00006-1. [DOI] [PubMed] [Google Scholar]
- Barrack RL, Skinner HB, Brunet ME, Haddad RJ., Jr Functional performance of the knee after intraarticular anesthesia. Am J Sports Med. 1983;11:258–61. doi: 10.1177/036354658301100414. [DOI] [PubMed] [Google Scholar]
- Behnam AJ, Herzka DA, Sheehan FT. Assessing the accuracy and precision of musculoskeletal motion tracking using cine-PC MRI on a 3.0T platform. J Biomech. 2011;44:193–197. doi: 10.1016/j.jbiomech.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besier TF, Fredericson M, Gold GE, Beaupre GS, Delp SL. Knee muscle forces during walking and running in patellofemoral pain patients and pain-free controls. J Biomech. 2009;42:898–905. doi: 10.1016/j.jbiomech.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilaqua-Grossi D, Felicio LR, Leocadio LP. Analysis of the reflex response time of the patellar stabilizer muscles in individuals with patellofemoral pain syndrome. Revista Brasileira De Fisioterapia. 2008;12:26–30. [Google Scholar]
- Boling M, Padua D, Marshall S, Guskiewicz K, Pyne S, Beutler A. Gender differences in the incidence and prevalence of patellofemoral pain syndrome. Scand J Med Sci Sports. 2009;20:725–730. doi: 10.1111/j.1600-0838.2009.00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossmann J, Muhle C, Schroder C, Melchert UH, Bull CC, Spielmann RP, Heller M. Patellar tracking patterns during active and passive knee extension: evaluation with motion-triggered cine MR imaging. Radiology. 1993;187:205–12. doi: 10.1148/radiology.187.1.8451415. [DOI] [PubMed] [Google Scholar]
- Cowan SM, Hodges PW, Bennell KL, Crossley KM. Altered vastii recruitment when people with patellofemoral pain syndrome complete a postural task. Arch Phys Med Rehabil. 2002;83:989–95. doi: 10.1053/apmr.2002.33234. [DOI] [PubMed] [Google Scholar]
- Derasari A, Brindle TJ, Alter KE, Sheehan FT. McConnell taping shifts the patella inferiorly in patients with patellofemoral pain: a dynamic magnetic resonance imaging study. Phys Ther. 2010;90:411–9. doi: 10.2522/ptj.20080365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaher YY, Kahn LE. The effect of vastus medialis forces on patello-femoral contact: a model-based study. J Biomech Eng. 2002;124:758–67. doi: 10.1115/1.1516196. [DOI] [PubMed] [Google Scholar]
- Elias JJ, Kilambi S, Cosgarea AJ. Computational assessment of the influence of vastus medialis obliquus function on patellofemoral pressures: model evaluation. J Biomech. 2010;43:612–7. doi: 10.1016/j.jbiomech.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrokhi S, Keyak JH, Powers CM. Individuals with patellofemoral pain exhibit greater patellofemoral joint stress: a finite element analysis study. Osteoarthritis Cartilage. 2011;19:287–94. doi: 10.1016/j.joca.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grelsamer RP, Weinstein CH, Gould J, Dubey A. Patellar tilt: the physical examination correlates with MR imaging. Knee. 2008;15:3–8. doi: 10.1016/j.knee.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Hanten WP, Schulthies SS. Exercise effect on electromyographic activity of the vastus medialis oblique and vastus lateralis muscles. Phys Ther. 1990;70:561–5. doi: 10.1093/ptj/70.9.561. [DOI] [PubMed] [Google Scholar]
- Harbaugh CM, Wilson NA, Sheehan FT. Correlating femoral shape with patellar kinematics in patients with patellofemoral pain. J Orthop Res. 2010;28:865–72. doi: 10.1002/jor.21101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungerford DS, Barry M. Biomechanics of the patellofemoral joint. Clin Orthop Relat Res. 1979:9–15. [PubMed] [Google Scholar]
- Iwamoto J, Takeda T, Sato Y, Matsumoto H. Retrospective case evaluation of gender differences in sports injuries in a Japanese sports medicine clinic. Gend Med. 2008;5:405–14. doi: 10.1016/j.genm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Khin Myo H, Ishii T, Sakane M, Hayashi K. Effect of anesthesia of the sinus tarsi on peroneal reaction time in patients with functional instability of the ankle. Foot Ankle Int. 1999;20:554–9. doi: 10.1177/107110079902000903. [DOI] [PubMed] [Google Scholar]
- Laprade J, Culham E. Radiographic measures in subjects who are asymptomatic and subjects with patellofemoral pain syndrome. Clin Orthop Relat Res. 2003:172–82. doi: 10.1097/01.blo.0000079269.91782.f5. [DOI] [PubMed] [Google Scholar]
- Lin F, Wilson NA, Makhsous M, Press JM, Koh JL, Nuber GW, Zhang LQ. In vivo patellar tracking induced by individual quadriceps components in individuals with patellofemoral pain. J Biomech. 2010;43:235–41. doi: 10.1016/j.jbiomech.2009.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YF, Lin JJ, Jan MH, Wei TC, Shih HY, Cheng CK. Role of the vastus medialis obliquus in repositioning the patella: a dynamic computed tomography study. Am J Sports Med. 2008;36:741–6. doi: 10.1177/0363546507312171. [DOI] [PubMed] [Google Scholar]
- Makhsous M, Lin F, Koh JL, Nuber GW, Zhang LQ. In vivo and noninvasive load sharing among the vasti in patellar malalignment. Med Sci Sports Exerc. 2004;36:1768–75. doi: 10.1249/01.mss.0000142302.54730.7f. [DOI] [PubMed] [Google Scholar]
- Owings TM, Grabiner MD. Motor control of the vastus medialis oblique and vastus lateralis muscles is disrupted during eccentric contractions in subjects with patellofemoral pain. Am J Sports Med. 2002;30:483–7. doi: 10.1177/03635465020300040601. [DOI] [PubMed] [Google Scholar]
- Powers CM. Rehabilitation of patellofemoral joint disorders: a critical review. J Orthop Sports Phys Ther. 1998;28:345–54. doi: 10.2519/jospt.1998.28.5.345. [DOI] [PubMed] [Google Scholar]
- Powers CM, Landel R, Perry J. Timing and intensity of vastus muscle activity during functional activities in subjects with and without patellofemoral pain. Phys Ther. 1996;76:946–55. doi: 10.1093/ptj/76.9.946. discussion 956–67. [DOI] [PubMed] [Google Scholar]
- Powers CM, Ward SR, Fredericson M, Guillet M, Shellock FG. Patellofemoral kinematics during weight-bearing and non-weight-bearing knee extension in persons with lateral subluxation of the patella: a preliminary study. J Orthop Sports Phys Ther. 2003;33:677–85. doi: 10.2519/jospt.2003.33.11.677. [DOI] [PubMed] [Google Scholar]
- Sakai N, Luo ZP, Rand JA, An KN. The influence of weakness in the vastus medialis oblique muscle on the patellofemoral joint: an in vitro biomechanical study. Clin Biomech (Bristol, Avon) 2000;15:335–9. doi: 10.1016/s0268-0033(99)00089-3. [DOI] [PubMed] [Google Scholar]
- Santos EP, Bessa SNF, Lins CAA, Marinho AMF, Silva KMP, Brasileiro JS. Electromyographic activity of vastus medialis obliquus and vastus lateralis muscles during functional activities in subjects with patellofemoral pain syndrome. Revista Brasileira De Fisioterapia. 2008;12:304–310. [Google Scholar]
- Schutzer SF, Ramsby GR, Fulkerson JP. Computed tomographic classification of patellofemoral pain patients. Orthop Clin North Am. 1986;17:235–48. [PubMed] [Google Scholar]
- Scuderi GR. Surgical treatment for patellar instability. Orthop Clin North Am. 1992;23:619–30. [PubMed] [Google Scholar]
- Seisler AR, Sheehan FT. Normative three-dimensional patellofemoral and tibiofemoral kinematics: a dynamic, in vivo study. IEEE Trans Biomed Eng. 2007;54:1333–41. doi: 10.1109/TBME.2007.890735. [DOI] [PubMed] [Google Scholar]
- Sheehan FT, Derasari A, Brindle TJ, Alter KE. Understanding patellofemoral pain with maltracking in the presence of joint laxity: complete 3D in vivo patellofemoral and tibiofemoral kinematics. J Orthop Res. 2009a;27:561–70. doi: 10.1002/jor.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan FT, Derasari A, Fine KM, Brindle TJ, Alter KE. Q-angle and J-sign: indicative of maltracking subgroups in patellofemoral pain. Clin Orthop Relat Res. 2009b;468:266–75. doi: 10.1007/s11999-009-0880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan FT, Mitiguy P. In regards to the “ISB recommendations for standardization in the reporting of kinematic data”. J Biomech. 1999;32:1135–6. doi: 10.1016/s0021-9290(99)00077-9. [DOI] [PubMed] [Google Scholar]
- Sheehan FT, Pappas G, Drace JE. In vivo measures of musculoskeletal dynamics using cine phase contrast magnetic resonance imaging. In: HERZOG W, editor. Skeletal Muscle Mechanics: From Mechanism to Function. John Wiley & Sons, Ltd; 2000. [Google Scholar]
- Sheehan FT, Zajac FE, Drace JE. In vivo tracking of the human patella using cine phase contrast magnetic resonance imaging. J Biomech Eng. 1999;121:650–6. doi: 10.1115/1.2800868. [DOI] [PubMed] [Google Scholar]
- Shibanuma N, Sheehan FT, Stanhope SJ. Limb positioning is critical for defining patellofemoral alignment and femoral shape. Clin Orthop Relat Res. 2005;434:198–206. doi: 10.1097/01.blo.0000155078.52475.63. [DOI] [PubMed] [Google Scholar]
- Smith TO, Nichols R, Harle D, Donell ST. Do the vastus medialis obliquus and vastus medialis longus really exist? A systematic review. Clin Anat. 2009;22:183–99. doi: 10.1002/ca.20737. [DOI] [PubMed] [Google Scholar]
- Stanford W, Phelan J, Kathol MH, Rooholamini SA, el-Khoury GY, Palutsis GR, Albright JP. Patellofemoral joint motion: evaluation by ultrafast computed tomography. Skeletal Radiol. 1988;17:487–92. doi: 10.1007/BF00364042. [DOI] [PubMed] [Google Scholar]
- Taunton JE, Ryan MB, Clement DB, McKenzie DC, Lloyd-Smith DR, Zumbo BD. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med. 2002;36:95–101. doi: 10.1136/bjsm.36.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomee R, Renstrom P, Karlsson J, Grimby G. Patellofemoral pain syndrome in young women. II. Muscle function in patients and healthy controls. Scand J Med Sci Sports. 1995;5:245–51. [PubMed] [Google Scholar]
- Ward SR, Terk MR, Powers CM. Patella alta: association with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Joint Surg Am. 2007;89:1749–55. doi: 10.2106/JBJS.F.00508. [DOI] [PubMed] [Google Scholar]
- Wilk KE, Reinold MM, Andrews JR. Postoperative treatment principles in the throwing athlete. Sports Medicine and Arthroscopy Review. 2001;9:69–95. [Google Scholar]
- Wilson NA, Press JM, Koh JL, Hendrix RW, Zhang LQ. In vivo noninvasive evaluation of abnormal patellar tracking during squatting in patients with patellofemoral pain. J Bone Joint Surg Am. 2009a;91:558–66. doi: 10.2106/JBJS.G.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NA, Press JM, Zhang LQ. In vivo strain of the medial vs. lateral quadriceps tendon in patellofemoral pain syndrome. J Appl Physiol. 2009b;107:422–8. doi: 10.1152/japplphysiol.00024.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NA, Sheehan FT. Dynamic in vivo quadriceps lines-of-action. J Biomech. 2010;43:2106–13. doi: 10.1016/j.jbiomech.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T. The measurement of patellar alignment in patellofemoral pain syndrome: are we confusing assumptions with evidence? J Orthop Sports Phys Ther. 2007;37:330–41. doi: 10.2519/jospt.2007.2281. [DOI] [PubMed] [Google Scholar]
- Yamaguchi GT, Zajac FE. A planar model of the knee joint to characterize the knee extensor mechanism. Journal of Biomechanics. 1989;22:1–10. doi: 10.1016/0021-9290(89)90179-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.