Abstract
Background
Presence of a few renal cysts are considered of little relevance in healthy adults, though acquired renal cystic disease occurs in advanced kidney failure. The objective of this study was to detail renal cystic and solid lesions and to identify any association with clinical characteristics.
Study Design
Clinical-pathological correlation
Setting & Participants
Potential kidney donors undergoing a standardized evaluation at the Mayo Clinic from 2000 to 2008.
Predictors
Age, kidney function, and chronic kidney disease risk factors
Measurements
Renal cystic and solid lesions by contrast-enhanced computed tomographic images
Outcomes
Cyst number, diameter, and location
Results
After excluding 8 with cystic disease, 7 of whom had ADPKD, there were 1948 potential kidney donors (42% men; mean age, 43 years). A cortical, medullary or parapelvic cyst ≥5 mm was present in 12, 14 or 2.8%. For ages 19 to 49 y, 39%, 22%, 7.9%, and 1.6% had a cortical or medullary cyst ≥ 2, 5, 10, and 20 mm in diameter. For ages 50 to 75 y, 63%, 43%, 22%, and 7.8% had a cortical or medullary cyst ≥ 2, 5, 10, and 20 mm in diameter. The 97.5th percentile for number of cortical and medullary cysts ≥5 mm increased with age (10 for men and 4 for women in the 60–69 y group). After age-sex-adjustment, cortical and medullary cysts ≥5 mm associated with higher 24-h urine albumin excretion, as well as increased body surface area, hypertension, and higher GFR in some analyses. Angiomyolipomas, hyperdense cysts, and enhancing masses or cysts with concerning features for malignancy occurred in 2.2%, 1.2%, and 0.6% and associated with older age (p≤0.05 for each).
Limitations
Persons with known chronic kidney disease were excluded
Conclusions
Renal cysts are common, particularly in older men, and may be a marker of early kidney injury since they associate with albuminuria, hypertension, and hyperfiltration.
Keywords: renal cysts, renal tumors, albuminuria, GFR, blood pressure, clinical pathological correlation
Simple renal cysts are common, typically asymptomatic, and usually considered to be of little clinical relevance. The primary clinical concern centers on the diagnosis of various forms of polycystic kidney disease or cystic renal cell cancer. Renal cysts are common in the kidneys of persons with advanced chronic kidney disease,(1) but whether they associate with early forms of kidney disease is uncertain. The systematic study of simple renal cysts and their comorbid associations has been limited to clinical populations of small sample size and most have relied on sonographic imaging.(2–4) Contrast-enhanced computed tomographic (CT) imaging is superior to ultrasound since it has higher resolution to detect small cysts, can distinguish cortical, medullary and parapelvic cysts, and can detect vascular enhancement in malignant cysts. To our knowledge, there has been no systematic study of renal cysts by contrast-enhanced CT scan in a population not selected on the presence of disease.
Due to concerns regarding radiation, intravenous contrast toxicity, and expense, general population studies of the kidneys by contrast enhanced CT scan are not available. However, potential kidney donors offer an opportunity to systematically study renal cysts by CT scan in a relatively healthy population. Potential kidney donors undergo a contrast enhanced CT scan specifically to characterize the kidney and renal vascular anatomy as well as to detect occult pathology that may preclude donation. We have previously reported that 25% of potential kidney donors have a kidney or renal artery abnormality reported by a dedicated kidney radiologist.(5) However, renal cysts are generally not characterized in detail on a standard radiology report. Therefore, we re-examined a large series of contrast enhanced CT scans in order to 1) characterize renal cystic and solid lesions with regard to size, location, internal characteristics, and malignant features, and 2) assess for an association of renal cysts with kidney function, blood pressure, and other clinical characteristics.
METHODS
Population
A series of 1957 consecutive potential kidney donors scanned between 2000 and 2008 at the Mayo Clinic in Rochester, Minnesota with research authorization in accordance with Minnesota law were studied.(5) CT exams (pre-scheduled as part of the donor evaluation) were re-examined specifically for presence and characteristics of renal cysts and tumors. Electronic charts were abstracted for clinical and laboratory findings at the time of the donor evaluation.
Multidetector CT (MDCT) examination
From 2000 to 2005, all CT exams were acquired on the same 4-channel MDCT scanner (Qxi, GE Medical Systems, Milwaukee, WI) with a modified table top, which permitted the acquisition of both axial CT images and traditional film-screen urograms (6). From 2005 to 2008, all kidney donor CT evaluations were obtained on a 64-channel MDCT scanner (Sensation 64, Siemens Medical Solutions). Item S1 (available as online supplementary material) gives details on the imaging protocol.
CT scan review
All of the CT images were initially reviewed by board-certified radiologists with expertise in genitourinary imaging and the reported findings have been previously described.(5) Since renal cysts were not reported, or when reported lacked sufficient detail, all CT scan images were re-examined by a radiologist (K.S.) to count and characterize all cystic renal lesions. A cystic lesion was defined as a sharply defined non-enhancing hypodense circular or nearly circular region.(7) The smallest detectable cystic lesion was defined as 2 mm based on the acquisition parameters of the CT exams. For each patient with cystic lesions we determined: maximum transverse diameter, side (left or right), polarity (upper, middle, or lower) and parenchymal location (cortical, medulla, or parapelvic/renal sinus), wall outline (smooth or irregular) and thickness (in mm), presence and number of septa, calcification, and soft tissue enhancement. Any thickened smooth, thickened irregular, or enhancement of septa were identified. Any fine, slightly thickened, or thick nodular calcifications were identified. Hyperdense cysts without enhancement, fat containing angiomyolipomas (attenuation of −10 HU or less), and enhancing masses were also characterized by number, diameter, and location.
A second radiologist (T.J.V.) re-examined all CT scan images where 4 or more cysts were documented by the first radiologist. This included a recount of the number of cysts and remeasurement of the diameter of largest cyst. The number of cortical and medullary cysts with a diameter ≥2 mm, ≥ 5 mm, ≥10 mm, and ≥20 mm were counted in each kidney. The angiographic phase images distinguished cortical from medullary cysts. The number of parapelvic cysts (cysts located in the renal sinus of lymphatic origin) in each kidney and the diameter of the largest parapelvic cyst were also assessed. Complex cysts were identified using the Bosniak classification assessing for risk of malignancy of the cystic lesion based on enhancement, septa, and calcification.(8) The highest Bosniak category for the cysts among donors with any cysts was identified. Among 167 donors with 4 or more cysts (excluding those with innumerable cysts), the interobserver correlation between the two radiologists was 0.89 for the total number of cysts and 0.92 for the diameter of the largest cyst.
Clinical characteristics
A diagnosis of autosomal dominant polycystic kidney disease (ADPKD) was identified from the CT scan reports and confirmed to meet the following criteria: family history of ADPKD with CT-detected cysts which in the radiologist’s opinion would be detectable by ultrasound meeting Ravine’s criteria (9) or cystic disease with bilateral renal enlargement typical for ADPKD in the absence of findings suggesting another cystic disease. The electronic medical record was reviewed to capture age, sex, race, height, weight, blood pressure, 24-h urinary albumin excretion, measured glomerular filtration rate (GFR) by iothalamate clearance, and serum creatinine. Body mass index (BMI) and body surface area (BSA) were calculated from height and weight.(10) Obesity was defined by a BMI >30 kg/m2. Estimated GFR was not used since serum creatinine based equations are inaccurate in donor populations,(11) though analyses with serum creatinine were adjusted for age and sex. Hypertension was defined by use of antihypertensive therapy for high blood pressure or a systolic blood pressure >140 mmHg or a diastolic blood pressure >90 mmHg at the initial evaluation. A familial relationship between the potential donor and a recipient with ADPKD was identified.
Statistical analysis
The number, size, and location of renal cystic lesions were compared by demographics, body size, blood pressure, and kidney function. To assess these associations with age-sex-adjustment, logistic regression was used to predict the presence of any cyst and linear regression was used to predict the diameter of the largest cyst among donors with any cyst (cyst diameter was log-transformed for homoscedasticity). Multivariable models assessed for independence among characteristics that associated with cysts after age-sex-adjustment. The 97.5th percentile for number of cysts by each age group was calculated by determining the rank ordered value at rank = 0.975 (n + 1) where n is the sample size of the age group. If this calculated rank was not an integer, the value was linearly interpolated from the rank ordered values above and below. All statistical analyses were performed using JMP, version 8.02 (SAS Institute, www.jmp.com).
RESULTS
Among 1957 potential kidney donors with CT scan reports, 1956 had images that could be identified and re-examined. There were 7 donors diagnosed with ADPKD based on renal cyst characteristics (number of cysts ranging from 26 to innumerable). Six of the seven donors with ADPKD were related to a recipient with ADPKD and met the Ravine’s criteria, while the seventh patient had more than 50 cysts in each kidney with an appearance consistent with ADPKD despite no family history of ADPKD. There was one other donor with innumerable tiny cysts likely from lithium-induced microcystic disease.(12)
After excluding these 8 donors with cystic diseases, 1948 were left for further analysis. Table 1 describes the clinical characteristics and Table 2 describes the cortical and medullary cysts both tables stratifed by age group (18–49 y and 50–75 y). The older age group was more likely to have cysts and their cysts were more numerous and wider in diameter. The increase in cyst prevalence from the younger to older age groups was stronger for cortical cysts ≥ 5 mm (12% vs 33%, p<0.001) than medullary cysts ≥ 5 mm (12% vs 17%, p=0.007). Men compared to women were more likely to have cortical cysts ≥ 5 mm (23% vs 15%, p<0.001), but a gender difference in medullary cysts ≥ 5 mm was less clear (15% vs 13%, p=0.09) Cortical or medullary cysts ≥ 5 mm were equally likely in the left versus right kidney (17% vs 16%, p=0.5 by McNemar’s Test). The prevalence of a cortical or medullary cyst ≥ 5 mm did not differ between the 5% of donors related to a recipient with ADPKD versus those who were not (33% vs 28%, p=0.4). Further, findings were not substantively different after excluding these donors with a family history of ADPKD (data not shown).
Table 1.
Characteristics of potential kidney donors.
| fCharacteristic | Ages 18 to 49 y (n=1345) | Ages 50 to 75 y (n=603) | p-value* |
|---|---|---|---|
| Age, y | 38 ± 8 | 58 ± 6 | <0.001 |
| Male | 579 (43%) | 243 (40%) | 0.3 |
| White | 1157 (90%) | 543 (95%) | 0.02 |
| Height, cm | 172 + 10 | 170 + 9 | <0.001 |
| Body Surface Area, m2 | 1.99 + 0.25 | 1.96 + 0.23 | 0.002 |
| BMI (kg/m2) | 28.5 + 5.7 | 28.2 + 4.7 | 0.9 |
| BMI > 30 kg/m2 | 466 (35%) | 201 (33%) | 0.6 |
| Systolic BP, mmHg | 123 + 14 | 130 + 18 | <0.001 |
| Diastolic BP, mmHg | 74 + 10 | 76 + 10 | <0.001 |
| Hypertension | 109 (8.1%) | 176 (29%) | <0.001 |
| 24-h urine albumin excretion, mg | 8.1 + 13.6 | 8.2 + 25.5 | 0.006 |
| 24-h urine albumin >30 mg | 46 (3.7%) | 22 (3.8%) | 0.9 |
| mGFR (ml/min/1.73 m2) | 106 + 18 | 92 + 18 | <0.001 |
| SCr (mg/dl) | 0.87 + 0.17 | 0.87 + 0.17 | 0.9 |
N = 1948. Continuous variables are given as number (percentage); categorical variables as mean ± SD.
Abbreviations: BMI, body mass index; BP, blood pressure; mGFR, measured glomerular filtration rate; SCr, serum creatinine.
Wilcoxon test or Fisher’s exact test
Conversion factors for units: SCr in mg/dL to μmol/L, ×88.4; GFR in mL/min/1.73 m2 to mL/s/1.73 m2, ×0.01667.
Table 2.
Cortical and medullary cyst and mass characteristics among potential kidney donors.
| Characteristic | Ages 18 to 49 y (n=1345) | Ages 50 to 75 y (n=603) | p-value* |
|---|---|---|---|
| Simple cysts
| |||
| Largest cyst 2 to 4 mm | 223 (17%) | 120 (20%) | 0.08 |
| Any cyst ≥ 2 mm | 521 (39%) | 377 (63%) | <0.001 |
| Any cyst ≥ 5 mm | 298 (22%) | 257 (43%) | <0.001 |
| Any cyst ≥ 10 mm | 106 (7.9%) | 134 (22%) | <0.001 |
| Any cyst ≥ 20 mm | 21 (1.6%) | 47 (7.8%) | <0.001 |
| Number of cysts ≥ 5 mm | <0.001† | ||
| 0 | 1047 (78%) | 346 (57%) | |
| 1 | 240 (18%) | 155 (26%) | |
| 2 | 41 (3.0%) | 59 (9.8%) | |
| 3 | 13 (0.9%) | 26 (4.3%) | |
| 4 | 3 (0.2%) | 7 (1.2%) | |
| 5 or more | 1 (0.1%) | 10 (1.7%) | |
| Diameter of largest cyst ≥ 5 mm | 10 ± 7.5 | 15 ± 13 | <0.001 |
| Any cortical cysts ≥ 5 mm | 156 (12%) | 198 (33%) | <0.001 |
| Any medullary cyst ≥ 5 mm | 163 (12%) | 101 (17%) | 0.007 |
| Unilateral cyst or cysts ≥ 5 mm | 267 (20%) | 189 (31%) | <0.001 |
| Bilateral cysts ≥ 5mm | 31 (2.3%) | 68 (11%) | <0.001 |
| Any left kidney cyst ≥ 5mm | 167 (12%) | 168 (28%) | <0.001 |
| Any right kidney cyst ≥ 5mm | 162 (12%) | 157 (26%) | <0.001 |
| Largest cyst ≥ 5 mm in upper pole | 66 (4.9%) | 96 (16%) | 0.001 |
| Largest cyst ≥ 5 mm in lower pole | 78 (5.8%) | 69 (11%) | <0.001 |
| Ravine Criteria‡ for ADPKD 1 (≥5 mm cysts) | 4 (0.3%) | 5 (0.8%) | 0.1 |
| Angiomyolipomas and hyperdense cysts
| |||
| Any angiomyolipoma | 25 (1.9%) | 18 (3.0%) | 0.1 |
| Largest diameter among angiomyolipomas, mm | 5.4 ± 4.4 | 4.5 ± 2.0 | 0.8 |
| Any hyperdense cyst without contrast enhancement | 12 (0.9%) | 12 (2.0%) | 0.04 |
| Largest diameter among hyperdense cysts, mm | 9.6 ± 5.0 | 9.6 ± 3.2 | 0.9 |
| Cysts or masses with concerning features for malignancy | |||
| Septa in any of the cysts | 149 (11%) | 129 (21%) | <0.001 |
| Any thickened irregular septa | 0 (0.0%) | 1 (0.2%) | 0.3 |
| Any thickened smooth septa | 0 (0.0%) | 0 (0.0%) | 0.9 |
| Any enhancement of septa | 0 (0.0%) | 3 (0.5%) | 0.03 |
| Any irregular cyst walls | 0 (0.0%) | 1 (0.2%) | 0.3 |
| Any thickened cyst walls (>1mm) | 3 (0.2%) | 4 (0.7%) | 0.1 |
| Any fine cyst calcification | 2 (0.15%) | 7 (1.2%) | 0.005 |
| Any slightly thickened cyst calcification | 0 (0.0%) | 0 (0.0%) | 0.9 |
| Any thick nodular cyst calcification | 0 (0.0%) | 0 (0.0%) | 0.9 |
| Any cyst soft tissue enhancement | 0 (0.0%) | 1 (0.2%) | 0.3 |
| Any enhancing masses | 4 (0.3%) | 5 (0.8%) | 0.2 |
| Bosniak Classification** | <0.001† | ||
| No cysts | 817 (61%) | 207 (34%) | |
| Category I | 372 (28%) | 257 (43%) | |
| Category II | 156 (12% | 136 (23%) | |
| Category IIF | 0 (0.0%) | 0 (0.0%) | |
| Category III | 0 (0.0%) | 3 (0.5%) | |
| Category IV | 0 (0.0%) | 0 (0.0%) | |
| Enhancing mass or Category III cyst diameter, mm | 19.3 ± 8.0 | 14.0 + 8.6 | 0.4 |
N = 1948. Continuous variables are given as number (percentage); categorical variables as mean ± SD.
Abbreviation: ADPKD, autosomal dominant polycystic kidney disease.
Wilcoxon test or Fisher’s exact test
highest if multiple cysts
Likelihood Ratio test
Ravine Criteria for ADPKD are intended for assessing ultrasound cysts in 1st degree relatives with ADPKD.
The cyst thresholds are ages 15–29 years: at least 2 cysts, 30–59 years: at least 2 cysts in each kidney, and ages 60 and older: at least 4 cysts in each kidney.(9)
Table 2 also characterizes angiomyolipomas, hyperdense cysts, and cysts or masses with concerning features for malignancy. These were all more prevalent in the older age group. Among persons with any cortical cyst ≥ 5 mm, 41% had a septa in a cortical cyst and among persons with any medullary cyst ≥ 5 mm, 36% had a septa in a medullary cyst. By the Bosniak criteria,(8) only 3 of donors had a concerning cyst (Category IIF, III, or IV); two underwent surgical resection that confirmed renal cell cancer and the third was managed elsewhere. Enhancing masses were found in 9 donors, and after surgical resection 3 were confirmed renal cell cancers, one was a papillary adenoma, and one was an angiomyolipoma (the remaining 4 were managed elsewhere). Older age associated with enhancing masses or concerning cysts (OR per decade, 1.9; p=0.01), hyperdense cysts (OR per decade, 1.4; p=0.05), and angiomyolipomas (OR per decade, 1.4; p=0.01).
Associations between clinical characteristics and presence of cortical or medullary cysts at different diameter thresholds (2, 5, 10, and 20 mm) are shown in Table 3. The presence and number of cysts ≥ 5 mm was strongly associated with older age (Figure 1) and male gender. After age-sex-adjustment, urine albumin excretion was associated with the presence of any cyst ≥ 2 mm, ≥ 5 mm, ≥ 10 mm, or ≥20 mm. There was evidence that higher blood pressure, hypertension, higher GFR, and lower serum creatinine levels associated with cyst presence, but this was not consistent across all analyses. The association of any cyst ≥ 10 mm with age (OR per decade, 1.8; p<0.001), male gender (OR, 2.4; p<0.001), BSA (OR per 1-SD, 1.2; p=0.04), hypertension (OR, 1.5; p=0.06), 24-h urine albumin excretion (OR per doubling, 1.1; p=0.007), and serum creatinine (OR per 1-SD, 0.8; p=0.009) all persisted in a multivariable model. Conversely, the presence of very small cysts alone (2–4 mm) was not statistically significantly associated with age (OR per decade, 1.1; p=0.1), male gender (OR, 1.0; p=0.9), or 24-h urine albumin excretion (OR per doubling, 1.0; p=0.9) and had the opposite association with BSA (OR per 1-SD, 0.7; p<0.001, hypertension (OR, 0.7; p=0.06) and serum creatinine (OR per 1-SD, 1.2; p=0.008) in a multivariable model.
Table 3.
Association of characteristics of potential donors with cortical/medullary cyst number and size*
| Presence of only cysts 2–4 mm | Presence of any cyst ≥2 mm | Presence of any cyst ≥5 mm | Presence of any cyst ≥ 10 mm | Presence of any cyst ≥ 20 mm | |
|---|---|---|---|---|---|
| Age (/10-y increase) | 1.1 (0.06) | 1.6 (<0.001) | 1.6 (<0.001) | 1.8 (<0.001) | 2.4 (<0.001) |
| Male sex | 0.9 (0.3) | 1.3 (0.002) | 1.6 (<0.001) | 2.5 (<0.001) | 3.3 (<0.001) |
| Body surface area (/1-SD) | 0.7 (<0.001) | 0.8 (<0.001) | 1.0 (0.7) | 1.3 (0.001) | 1.5 (0.006) |
| BMI (/1-SD) | 0.8 (<0.001) | 0.9 (0.003) | 1.0 (0.8) | 1.2 (0.003) | 1.2 (0.1) |
| BMI > 30 kg/m2 | 0.6 (<0.001) | 0.8 (0.01) | 1.0 (0.8) | 1.4 (0.02) | 1.3 (0.4) |
| Systolic BP (/1-SD) | 0.9 (0.04) | 0.9 (0.07) | 1.0 (0.8) | 1.1 (0.6) | 1.1 (0.3) |
| Diastolic BP (/1-SD) | 1.0 (0.5) | 0.9 (0.1) | 1.0 (0.3) | 1.1 (0.6) | 1.2 (0.2) |
| Hypertension | 0.7 (0.04) | 0.8 (0.1) | 1.0 (0.9) | 1.4 (0.04) | 1.1 (0.7) |
| 24-h urine albumin excretion (per doubling) | 1.0 (0.2) | 1.1 (0.04) | 1.1 (<0.001) | 1.2 (<0.001) | 1.2 (0.05) |
| Urine albumin excretion >30 mg | 0.8 (0.5) | 1.5 (0.1) | 1.8 (0.03) | 2.4 (0.02) | 1.4 (0.6) |
| mGFR (/1-SD) | 0.9 (0.1) | 1.0 (0.6) | 1.1 (0.04) | 1.1 (0.3) | 1.1 (0.6) |
| SCr (/1-SD) | 1.2 (0.02) | 1.0 (0.8) | 0.9 (0.1) | 0.8 (0.009) | 1.2 (0.3) |
Values shown are odds ratio (P).
Abbreviations: BMI, body mass index; BP, blood pressure; mGFR, measured glomerular filtration rate; SCr, serum creatinine; SD, standard deviation.
Based on 1948 donors. Adjusted for age and gender, except for age (adjusted for gender only) and gender (adjusted for age only).
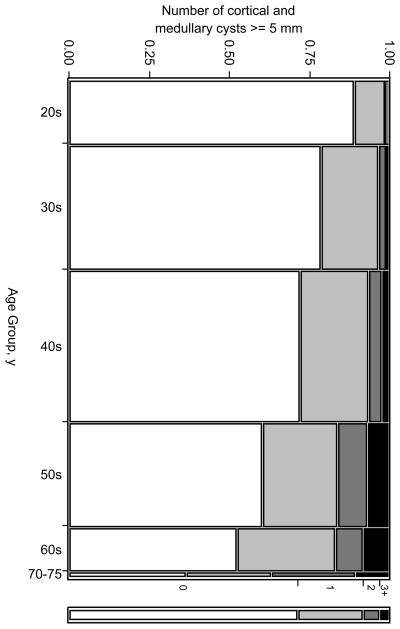
Figure 1.
Mosaic plot of the number of cortical and medullary simple cysts ≥ 5 mm by age group among 1948 potential kidney donors. The width of each column is proportional to the sample size of each age group. Black represents the fraction with 3 or more cysts and white represents the fraction with no cysts.
Table 4 compares these same clinical characteristics with the presence of any cyst in the cortex and in the medulla, as well as with the diameter of the largest cyst in each location. After age-sex-adjustment, higher urine albumin excretion was associated with both cortical cysts and medullary cysts. Medullary cysts associated with higher measured GFR and lower serum creatinine. Higher BSA or BMI was associated with larger cysts in both the cortex and the medulla. Gender associations with cortical cyst diameter (14% larger in men, p=0.1) and medullary cyst diameter (2.5% larger in men, p=0.7) were substantially attenuated after adjusting for BSA in multivariable models. However, the higher likelihood of a cortical or medullary cyst in men did not change substantively with adjustment for BSA. The magnitude of association between largest cortical cyst diameter with diastolic BP per SD (6.1% difference, p=0.08) and with 24-h urine albumin excretion (4.0% difference, p=0.07) were not substantially changed in a multivariable model.
Table 4.
Association of characteristics of potential donors with cortical or medullary cyst types*
| OR (p-value)a for any cortical cyst ≥5 mm | % Difference (p-value)b for largest cortical cyst diameter ≥5 mm | OR (p-value) for any medullary cyst cyst ≥5 mma | % Difference (p-value) for largest medullary cyst diameter ≥5 mmc | |
|---|---|---|---|---|
| Age (/10-y increase) | 1.9 (<0.001) | 11% (<0.001) | 1.4 (<0.001) | 0.5% (0.9) |
| Male sex | 1.9 (<0.001) | 30% (<0.001) | 1.3 (0.05) | 15% (0.008) |
| Body surface area (/1-SD) | 1.1 (0.3) | 12% (0.004) | 1.0 (0.9) | 11% (0.002) |
| BMI (/1-SD) | 1.0 (0.7) | 9.1% (0.01) | 1.0 (0.8) | 9.5% (0.001) |
| BMI > 30 kg/m2 | 1.1 (0.7) | 15% (0.04) | 1.0 (0.8) | 14% (0.02) |
| Systolic BP (/1-SD) | 1.0 (0.7) | 6.7% (0.05) | 1.0 (0.6) | −2.0% (0.5) |
| Diastolic BP (/1-SD) | 1.0 (0.9) | 7.4% (0.03) | 1.0 (0.5) | 2.0% (0.5) |
| Hypertension | 1.1 (0.8) | 15% (0.1) | 1.1 (0.5) | −1.4% (0.9) |
| 24-h urine albumin excretion (per doubling) | 1.1 (0.03) | 4.4% (0.04) | 1.1 (0.003) | 2.9% (0.1) |
| Urine albumin excretion >30 mg | 2.4 (0.003) | 0.0% (0.9) | 1.3 (0.5) | 5.5% (0.7) |
| mGFR (/1-SD) | 1.1 (0.09) | −0.4% (0.9) | 1.1 (0.1) | 6.4% (0.03) |
| SCr (/1-SD) | 0.9 (0.4) | −0.2% (0.9) | 0.8 (0.01) | −10% (0.001) |
Based on 1948 potential kidney donors. Adjusted for age and gender, except for age (adjusted for gender only) and gender (adjusted for age only).
n=1948
n=357
n=264
Abbreviations: BMI, body mass index; BP, blood pressure; mGFR, measured glomerular filtration rate; OR, odds ratio; SCr, serum creatinine.
Table 5 presents the upper limit (97.5 percentile) by age and gender groups for the number of cortical and medullary cysts ≥ 5 mm in both kidneys and in the kidney with the most cysts. There were 20 donors whose number of cysts exceeded these thresholds; one was related to a recipient with ADPKD, but did not meet the Ravine criteria. In 7 of these potential donors, the presence of these cysts specifically prevented donation, though a definitive diagnosis ADPKD was not made. Urine albumin excretion was higher in these 20 donors compared to the other donors (median values of 11 vs 5 mg/24 h; p<0.001 by the Rank-Sums Test). There were no statistically significant differences in the age, gender, BSA, BMI, systolic or diastolic blood pressure, treated hypertension, GFR, or serum creatinine between these two groups (p>0.1 for all).
Table 5.
Upper limit for number of cortical and medullary cysts ≥ 5mm by age group
| Age group | No. of cysts in both kidneys | No. of cysts in the kidney with the most cysts | ||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| 18–29 years (n=256) | 1 | 1 | 1 | 1 |
| 30–39 years (n=491) | 2 | 2 | 2 | 2 |
| 40–49 years (n=598) | 3 | 2 | 2 | 2 |
| 50–59 years (n=409) | 5 | 3 | 5 | 2 |
| 60–69 years (n=171) | 10 | 4 | 7 | 4 |
Value shown in the 97.5th percentile among 1925 donors ages 18 to 69 years
Parapelvic cysts (3.0%) were less common than cortical and medullary cysts, but the largest parapelvic cyst had a mean ± SD diameter of 26 ± 13 mm. This was wider than seen with the mean diameter of the largest cortical or medullary cyst (Table 2). Among the 58 donors with parapelvic cysts, 41% had 1 cyst, 26% had 2 to 5 cysts, 21% had 6 to 10 cysts, and 12% had 11 or more parapelvic cysts. Bilateral parapelvic cysts were present in 15 (21%). Parapelvic cysts were less likely to contain internal septa (16% of donors with parapelvic cysts) than was seen with cortical or medullary cysts. Table 6 describes the clinical characteristics that associate with parapelvic cysts, including age, male gender, and BSA. Unlike cortical and medullary cysts, the presence of parapelvic cysts did not associate with urine albumin excretion.
Table 6.
Association of characteristics of potential donors with parapelvic cysts.*
| OR (p-value) for any parapelvic cyst ≥5 mma | % Difference (p- value) for largest parapelvic cyst diameterb | |
|---|---|---|
| Age (/10-y increase) | 2.5 (<0.001) | 11% (0.2) |
| Male sex | 3.0 (<0.001) | 13% (0.3) |
| Body surface area (/1-SD) | 1.4 (0.04) | −6.6% (0.4) |
| BMI (/1-SD) | 1.3 (0.1) | −5.2% (0.5) |
| BMI > 30 kg/m2 | 1.8 (0.03) | −20% (0.1) |
| Systolic BP (/1-SD) | 1.3 (0.06) | −1.6% (0.8) |
| Diastolic BP (/1-SD) | 1.2 (0.2) | 6.6% (0.4) |
| Hypertension | 1.3 (0.4) | −5.8% (0.7) |
| 24-h urine albumin excretion (per doubling) | 1.0 (0.7) | −1.2% (0.8) |
| Urine albumin excretion >30 mg | 1.0 (0.9) | −17% (0.6) |
| mGFR (/1-SD) | 0.8 (0.10) | −0.9% (0.9) |
| SCr (/1-SD) | 1.2 (0.3) | 1.0% (0.9) |
Based on 1948 potential kidney donors. Adjusted for age and gender, except for age (adjusted for gender only) and gender (adjusted for age only).
n=1948
n=58
DISCUSSION
Renal cysts are common and rarely have concerning features for malignancy. Older age and male gender are associated with renal cysts, but more strongly with cortical cysts than with medullary cysts. A renal cyst in the cortex or medulla ≥5 mm may be a marker of early kidney injury given the association with albuminuria, hyperfiltration, and hypertension in relatively healthy adults. This is further supported by similar associations with cyst size among those with cysts. Kidney function may associate more with cyst growth than cyst development since these same associations are not evident for 2–4 mm cysts. Conversely, parapelvic cysts do not associate with kidney function and hence do not appear to be markers of kidney injury. This is consistent with their lymphatic origin(13) in contrast to the tubular origin of cortical and medullary cysts.(14)
The age- and gender-specific 97.5th percentile threshold for number of cortical and medullary cysts ≥ 5 mm may be a useful tool for future studies on the relevance of cysts. Donors whose number of cysts exceeded these thresholds had a median 24-h urine albumin excretion of 11 mg compared to 5 mg for other donors. Notably, a spot urine albumin excretion of 10–29 mg/g is associated with a higher risk of mortality than <10 mg/g.(15) Further work is needed to determine the clinical relevance of these cyst thresholds, their pathogenic origin, and whether they provide independent prognostic information. Since these cyst thresholds associate with albuminuria, they may identify patients at increased risk for developing CKD. They may also help identify patients that should be monitored more closely for ADPKD in the absence of a family history for ADPKD. It was recently shown that some PKD mutations do not inactivate the gene, but result in hypomorphic alleles with incomplete penetrance.(16, 17) Individuals with two hypomorphic alleles in trans have severe ADPKD, whereas individuals with only one hypomorphic allele have a much milder phenotype, with only a few cysts, which may be interpreted as benign simple cysts of limited clinical significance. We cannot rule out the possibility that some potential donors with an elevated number of cysts could indeed have a mild form of ADPKD.
The threshold for detection and the size of cysts was dependent on body size for cortical, medullary, and parapelvic cysts. Smaller BSA and BMI were associated with more detection of 2–4 mm cysts. This likely reflects decreased detection of small hypovascular lesions by MDCT when more tissue surrounds the kidneys.(18) Ironically, having only very small cysts may reflect a state of improved health because peri-renal obesity likely interferes with the detection of these cysts. Individuals with smaller BSA may also have less cyst growth than persons with higher BSA. The finding that a larger BSA was associated with detection of cortical and medullary cysts >10 mm as well as increased cyst diameter for both cortical and medullary cysts supports this hypothesis. Body surface area is the strongest correlate of kidney size,(19) and larger kidneys may form larger cysts. Men were both more likely to have cysts and to have larger cysts than women. The larger cyst size in men than women was mostly explained by gender differences in BSA, however the higher prevalence in men of cysts ≥5 mm was not entirely explained by gender differences in BSA, suggesting pathways associated with gender but independent of body size also contribute.
This study found the prevalence of cysts was strongly dependent on the threshold for cyst diameter ranging from 47% for ≥ 2 mm to 3.5% for ≥ 20 mm. This is consistent with previous prevalence estimates of 11% by ultrasound,(20) and 27 to 65% with higher resolution magnetic resonance imaging and CT scan studies.(21–23) Further, this study uniquely characterizes the prevalence of cysts in the cortex, medulla, and renal sinus (parapelvic) where the origin and relevance of the cysts may differ. This study also describes the prevalence of angiomyolipomas, hyperdense cysts, and enhancing masses or cysts with concerning features for malignancy (Bosniak Category IIF, III, or IV). Hyperdense cysts are likely due to hemorrhage, but occasionally are malignant.(24, 25) Some have advocated radiographic surveillance of Bosniak Category II cysts (cysts with thin septa or hyperdense cysts),(25) but given the overall prevalence of 15% in this healthy population a more granular classification would be needed.
Several other studies have explored the relationship of renal cysts with CKD and CKD risk factors. Among patients presenting for routine health-care, sonographic renal cysts associated with older age, male gender, hypertension, but not GFR or dipstick proteinuria.(2) A similar ultrasound study found older age, male gender, dipstick proteinuria, elevated serum creatinine, and smoking to associate with presence of cysts.(20) Among hospitalized patients, renal cysts by contrast computed tomography (CT) were associated with older age, male gender, reduced GFR, but not hypertension or dipstick proteinuria.(3) Similar to our study, other investigators have found large cysts to be more strongly associated with hypertension than are small cysts.(4) We further found renal cysts to associate with increased GFR (hyperfiltration) and with urine albumin excretion at levels that would have been missed by urine dipstick. The exclusion of overt CKD in the potential donor population may explain associations with increased GFR since hyperfiltration may precede a subsequent GFR decline.
Potential kidney donors are selected from the general population on apparent health not disease. Therefore, the associations revealed by this study may be even stronger in the general population where overt kidney disease was not excluded. A prospective study could better represent the general population, but concerns regarding radiation exposure and use of radiocontrast would be potential barriers.
In conclusion, this study details the characteristics of renal cysts in healthy adults. Older age, male gender, larger body size, hypertension, urine albumin excretion, and increased GFR associated with cysts ≥ 5 mm in the cortex and medulla. Smaller cysts (2–4 mm) did not have these same associations suggesting cysts growth is relevant to the pathophysiology behind these associations. Obesity may lead to both larger cysts and impair the detection of very small cysts. The upper limit for number of cortical and medullary cysts was defined. Parapelvic cysts, hyperdense cysts, angiomyolipomas, enhancing masses, and cysts with concerning features were also characterized in this population. All renal cysts and masses, regardless of type, are associated with older age.
Supplementary Material
Acknowledgments
Support: This study was supported with funding from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK090358, P30 DK090728, P50 083007 and K23 DK078229).
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Item S1. Multidetector computed tomography angiogram protocol.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Descriptive Text for Online Delivery
Hyperlink: Supplementary Item S1 (PDF)
About: Multidetector computed tomography angiogram protocol.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrew D. Rule, Division of Nephrology and Hypertension, Department of Medicine, Mayo Clinic
Kewalee Sasiwimonphan, Department of Radiology, Mayo Clinic
John C. Lieske, Division of Nephrology and Hypertension, Department of Medicine, Mayo Clinic
Mira T. Keddis, Division of Nephrology and Hypertension, Department of Medicine, Mayo Clinic
Vicente E. Torres, Division of Nephrology and Hypertension, Department of Medicine, Mayo Clinic
Terri J. Vrtiska, Department of Radiology, Mayo Clinic
References
- 1.Levine E. Acquired cystic kidney disease. Radiologic clinics of North America. 1996;34:947–964. [PubMed] [Google Scholar]
- 2.Chin HJ, Ro H, Lee HJ, Na KY, Chae DW. The clinical significances of simple renal cyst: Is it related to hypertension or renal dysfunction? Kidney international. 2006;70:1468–1473. doi: 10.1038/sj.ki.5001784. [DOI] [PubMed] [Google Scholar]
- 3.Al-Said J, Brumback MA, Moghazi S, Baumgarten DA, O’Neill WC. Reduced renal function in patients with simple renal cysts. Kidney international. 2004;65:2303–2308. doi: 10.1111/j.1523-1755.2004.00651.x. [DOI] [PubMed] [Google Scholar]
- 4.Zerem E, Imamovic G, Omerovic S. Simple renal cysts and arterial hypertension: does their evacuation decrease the blood pressure? Journal of hypertension. 2009;27:2074–2078. doi: 10.1097/HJH.0b013e32832f1458. [DOI] [PubMed] [Google Scholar]
- 5.Lorenz EC, Vrtiska TJ, Lieske JC, et al. Prevalence of renal artery and kidney abnormalities by computed tomography among healthy adults. Clin J Am Soc Nephrol. 2010;5:431–438. doi: 10.2215/CJN.07641009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawashima A, Vrtiska TJ, LeRoy AJ, Hartman RP, McCollough CH, King BF., Jr CT urography. Radiographics. 2004;24(Suppl 1):S35–54. doi: 10.1148/rg.24si045513. discussion S55–38. [DOI] [PubMed] [Google Scholar]
- 7.Patel NS, Poder L, Wang ZJ, et al. The characterization of small hypoattenuating renal masses on contrast-enhanced CT. Clinical imaging. 2009;33:295–300. doi: 10.1016/j.clinimag.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Israel GM, Bosniak MA. An update of the Bosniak renal cyst classification system. Urology. 2005;66:484–488. doi: 10.1016/j.urology.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet. 1994;343:824–827. doi: 10.1016/s0140-6736(94)92026-5. [DOI] [PubMed] [Google Scholar]
- 10.Mosteller RD. Simplified calculation of body-surface area. The New England journal of medicine. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 11.Murata K, Baumann NA, Saenger AK, Larson TS, Rule AD, Lieske JC. Relative Performance of the MDRD and CKD-EPI Equations for Estimating Glomerular Filtration Rate among Patients with Varied Clinical Presentations. Clin J Am Soc Nephrol. 2011 doi: 10.2215/CJN.02300311. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crocker JF, McDonald AT. Effects of lithium chloride and ethacrynic acid on experimental polycystic kidney disease. Clinical and investigative medicine. 1988;11:16–21. [PubMed] [Google Scholar]
- 13.Schwarz A, Lenz T, Klaen R, Offermann G, Fiedler U, Nussberger J. Hygroma renale: pararenal lymphatic cysts associated with renin-dependent hypertension (Page kidney). Case report on bilateral cysts and successful therapy by marsupialization. The Journal of urology. 1993;150:953–957. doi: 10.1016/s0022-5347(17)35660-4. [DOI] [PubMed] [Google Scholar]
- 14.Baert L, Steg A. Is the diverticulum of the distal and collecting tubules a preliminary stage of the simple cyst in the adult? The Journal of urology. 1977;118:707–710. doi: 10.1016/s0022-5347(17)58167-7. [DOI] [PubMed] [Google Scholar]
- 15.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossetti S, Kubly VJ, Consugar MB, et al. Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney international. 2009;75:848–855. doi: 10.1038/ki.2008.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vujic M, Heyer CM, Ars E, et al. Incompletely penetrant PKD1 alleles mimic the renal manifestations of ARPKD. J Am Soc Nephrol. 2010;21:1097–1102. doi: 10.1681/ASN.2009101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schindera ST, Torrente JC, Ruder TD, et al. Decreased detection of hypovascular liver tumors with MDCT in obese patients: a phantom study. Ajr. 2011;196:W772–776. doi: 10.2214/AJR.10.5351. [DOI] [PubMed] [Google Scholar]
- 19.Johnson S, Rishi R, Andone A, et al. Determinants and functional significance of renal parenchymal volume in adults. Clin J Am Soc Nephrol. 2011;6:70–76. doi: 10.2215/CJN.00030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang CC, Kuo JY, Chan WL, Chen KK, Chang LS. Prevalence and clinical characteristics of simple renal cyst. J Chin Med Assoc. 2007;70:486–491. doi: 10.1016/S1726-4901(08)70046-7. [DOI] [PubMed] [Google Scholar]
- 21.Nascimento AB, Mitchell DG, Zhang XM, Kamishima T, Parker L, Holland GA. Rapid MR imaging detection of renal cysts: age-based standards. Radiology. 2001;221:628–632. doi: 10.1148/radiol.2213010178. [DOI] [PubMed] [Google Scholar]
- 22.Tada S, Yamagishi J, Kobayashi H, Hata Y, Kobari T. The incidence of simple renal cyst by computed tomography. Clinical radiology. 1983;34:437–439. doi: 10.1016/s0009-9260(83)80238-4. [DOI] [PubMed] [Google Scholar]
- 23.Carrim ZI, Murchison JT. The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clinical radiology. 2003;58:626–629. doi: 10.1016/s0009-9260(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 24.Eknoyan G. A clinical view of simple and complex renal cysts. J Am Soc Nephrol. 2009;20:1874–1876. doi: 10.1681/ASN.2008040441. [DOI] [PubMed] [Google Scholar]
- 25.Gabr AH, Gdor Y, Roberts WW, Wolf JS., Jr Radiographic surveillance of minimally and moderately complex renal cysts. BJU international. 2009;103:1116–1119. doi: 10.1111/j.1464-410X.2008.08171.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.