Abstract
Klotho is a putative age-suppressing gene whose over-expression in mice results in extension of life span. The klotho gene encodes a single-pass transmembrane protein whose extracellular domain is shed and released into blood, urine, and cerebrospinal fluid, potentially functioning as a humoral factor. The extracellular domain of Klotho has an activity that increases the expression of anti-oxidant enzymes and confers resistance to oxidative stress in cultured cells and in whole animals. The transmembrane form of the Klotho protein directly binds to multiple fibroblast growth factor receptors and modifies their ligand affinity and specificity. The purpose of the present study was to determine the precise cellular localization of Klotho in the mouse brain. Using light microscopic immunohistochemical methods, we found the highest levels of Klotho immunoreactivity in two brain regions: the choroid plexus, and cerebellar Purkinje cells. In the choroid plexus cells, Klotho was found not only on the plasma membrane but also in large amounts near the nuclear membrane. Likewise, in the Purkinje cell Klotho was found throughout the cell including dendrites, axon and soma with large amounts near the nuclear membrane. Using immunoelectron microscopy, we found Klotho in the cell membrane, but the highest concentration was localized in the peripheral portion of the nucleus and the nucleolus in both cell types. This new finding suggests that in addition to Klotho being secreted from cells in brain, it also has a nuclear function.
Keywords: choroid plexus, electron microscopy, immunohistochemistry, Purkinje cells
1. Introduction
Deletion of the klotho gene in mice causes a syndrome that resembles human ageing. Operating in an autosomal recessive manner, klotho deficient mice exhibit a shortened life span, arteriosclerosis, osteoporosis, endothelial dysfunction, Parkinsonian gait, impaired hearing and cognitive impairment (Kuro-o et al., 1997; Saito et al., 1998; Kamemori et al., 2002; Masuda et al., 2005; Nagai et al., 2000, 2003;). The klotho gene encodes a type-I single-pass transmembrane protein and is expressed most abundantly in the kidney, where it functions as a necessary co-receptor for fibroblast growth factor-23 (FGF23), and a bone-derived hormone that regulates the phosphate, calcium and vitamin D balance. Defects in either Klotho or FGF23 cause phosphate retention and a premature-ageing syndrome in the mouse (Kurosu et al., 2006; Urakawa et al., 2006). The extracellular domain of Klotho is subject to ectodomain shedding and is secreted into blood, urine, and cerebrospinal fluid (Chen et al., 2007; Block et al., 2009). The secreted Klotho regulates the activities of multiple ion channels (Cha et al., 2008, 2009; Hu et al., 2010) and growth factors including insulin, insulin-like growth factor IGF-I and Wnt, and protects cells and tissues from oxidative stress through an unknown mechanism (Chang et al., 2005; Yamamoto et al., 2005; Kurosu et al., 2005; Mitobe et al., 2005; Ikushima et al., 2006; Liu et al., 2007). Recent work indicates that the intracellular form of Klotho inhibits the retinoic-acid gene-I-induced expression of inflammatory factors IL-6 and IL-8, and suggests this anti-inflammatory effect is related to its anti-aging effect (Liu et al., 2011).
Klotho protein has been detected in the choroid plexus and in the brain (Kuro-o, et al., 1997; Li et al., 2004). No detailed protein studies of the brain have been reported; however, mouse brain regions containing Klotho mRNA have been mapped (Allen Brain Atlas; www.brain-map.org). The purpose of the present study was to define the precise localization of Klotho protein in the mouse brain using both light and electron microscopy.
2. Materials and methods
2.1 Transgenic mice
Adult male 129S1SvImJ mice, Klotho knockout (kl/kl) mice, and Klotho overexpressing transgenic mice were used for all experiments. Details of these animals have been published elsewhere (Kuro-o et al., 1997). Animals were deeply anesthetized and were perfusion-fixed with 4% paraformaldehyde for light microscopic studies, and for EM studies with 0.1% glutaraldehyde in PBS, (pH 7.4) and immersion-fixed in 0.1% glutaraldehyde with 2% paraformaldehyde in the same buffer at 4 °C overnight.
2.2 Light microscopic studies
Brain tissue was sectioned at 30 μm thickness in the coronal plane. Free-floating sections were processed with an anti-mouse Klotho affinity purified antibody (R&D Systems, Inc.; Minneapolis MN) using the ABC method (Vector Laboratories, Inc., Burlingame CA). In brief, sections were washed in phosphate buffered saline (PBS), pH 7.4, treated with 1% hydrogen peroxide in PBS, and blocked in 5% bovine serum albumin (BSA) in 0.3% Triton X-100 in PBS. On day 1, sections were incubated with diluted primary antibody (1:20) with 1% normal serum in Triton X-100 in PBS overnight at room temperature. On day 2, the sections were incubated with secondary antibodies (1.5 μg/ml of biotinylated goat anti-mouse immunoglobulin G; Vector Labs, Burlingame, CA) for 30 minutes and with an avidin/ biotin/peroxidase reagent (1:250 dilution; ABC Elite, Vector Labs) for 1 hour. Sections were reacted in an acetate buffer (pH 6.0) containing 0.035% diaminobenzidine tetrahydrochloride and 0.001% hydrogen peroxide for 5 to 10 minutes. All incubations were done on a shaker table, at room temperature, and with 3 × 10-minute washes in PBS. Sections were mounted on coated slides, dehydrated, and coverslipped. To be certain of the specificity of the immunoreactivity, the primary antibody step was omitted in the staining procedure, and tissues from kl/kl mice were employed. These procedures both markedly reduced the specific immunostaining intensity. The immunohistochemical staining procedure was run on tissue sections from transgenic (n=2), kl/kl (n=2) and wild-type controls (n=2) at the same time to control for antibody concentration, reaction times, etc., because these variables influence staining intensity.
2.3 Electron microscopy immunohistochemistry
We used both biotinylated and non-biotinylated affinity-purified antibodies against the N-terminal region of the mouse Klotho protein (R&D Systems, Inc., Minneapolis, MN). The brain sections were permeabilized in 0.1% Triton X-100 for 30 min, and blocked in 4% BSA for 30 min. For the present illustrations, the primary incubation was with the biotinylated anti-Klotho antibody (1:200 dilution). After washing, sections were incubated with the secondary antibody conjugated with 1.4 nm gold particles (1:100 dilution; Nanoprobes, Yaphank, NY) for 24 hours, and immunogold signal was enhanced with the HQ silver enhancement kit (Nanoprobes, Yaphank, NY). Sections were further fixed with 0.5% osmium tetroxide and dehydrated through a graded series of ethanol to 100%, finally embedded in Poly/Bed 812 epoxy resin (Polysciences Inc., Warrington, PA). Ultrathin sections (65 nm) were stained with 5% uranyl acetate solution and examined under a FEI Tecnai transmission electron microscope at 120 kV accelerating voltage. Sections were examined through the cerebellar cortex and the choroid plexus in 2 wild type mice.
3. Results
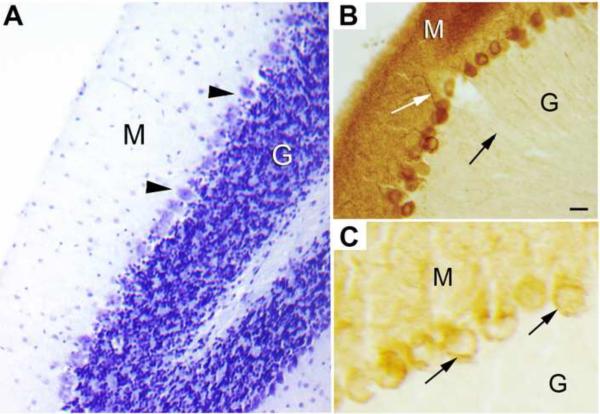
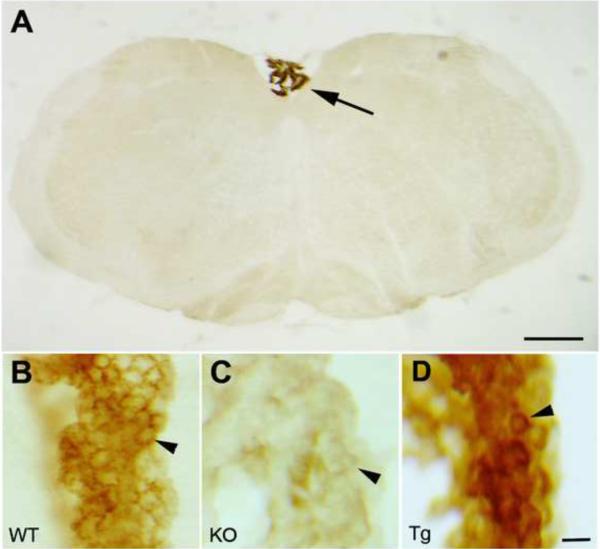
At the light microscopic level, mouse brain sections immunostained for Klotho were examined from the forebrain to the brainstem. Only two areas exhibited above background levels of immunostaining: the choroid plexus and cerebellar Purkinje cells. Purkinje cells were strongly stained and the protein was observed within the dendrites, cell body and within the axons (Figure 1). Near the nucleus of the cell, Klotho was also evident (Figure 1, panel C). Strong staining was also observed in the choroid plexus cells, which reside within the ventricular system (Figure 2A), both in the wild-type mouse (panel B) and especially in the Klotho over-expressing mouse (panel D), but not in the kl/kl mouse (panel C).
Figure 1. Light microscopic localization of Klotho in cerebellar Purkinje cells.
(A) Purkinje cells (arrowheads) form a row of large cell somas at the border of the molecular (M) layer and the granular (G) cell layer, shown in a Nissl-stained section. (B) The Purkinje cells exhibit klotho immunostaining in the cell body, dendrites (white arrow) which extend into the M layer and axons (black arrow) which exit into the G cell layer. (C) The Klotho immunostaining is often located close to the nuclear envelop. Arrows point to intense immunostaining located near the nucleus of two Purkinje cells. Bar in panel B = 25 μm for both panels A and B; and for panel C = 11 μm.
Figure 2. Light microscopic localization of Klotho in choroid plexus cells.
(A) Low power micrograph through an entire brainstem section showing the lack of immunostaining except for the choroid plexus cells (arrow) within the fourth ventricle. Choroid plexus cells reside within the ventricular system in general. (B) Choroid plexus cells stain for Klotho in the wild-type (WT) mouse, and very intensely in klotho transgenic (Tg) mouse (panel D), but only faintly in Klotho knockout (KO) mouse (panel C). Arrowheads in panels B–D point to individual choroid plexus cells. Bar in panel A = 450 μm; and for panels B–D = 15 μm.
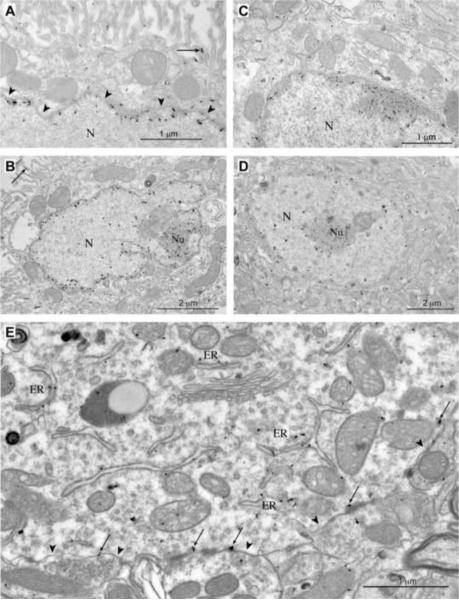
At the electron microscopic level, Klotho was identified in several cellular compartments. In the choroid plexus, some protein was found associated with the cell surface (polypoid border). A small amount of protein was also found throughout cytoplasm within the endoplasmic reticulum. The protein was highly concentrated within nuclei, specifically in the nucleolus and peripheral chromatin (Figure 3A & B). It was not present on the nuclear envelop or in the nuclear pore structure. Likewise, in Purkinje cells the protein was localized in the nucleus (Figure 3C & D), and in some cases it appeared to be associated with the dense fibrillar component, and in the peripheral chromatin. Within the soma, it was also present in the rough endoplasmic reticulum (Figure 3E), but not associated with organelles such as Golgi apparatus or mitochondria. There was also labeling on the plasma membrane (Figure 3E). Small amounts of protein were found in dendrites and axons, but failed to localize to the cell membrane or particular intracellular structures. Finally, there was no positive labeling in synapses.
Figure 3. Immuno-EM localization of Klotho.
A–B. Klotho immunogold particles indicate strong signals at the inner portion of the nuclear envelop and the nucleolus of choroid plexus cells. The less-intense immunoreactive signal is also observed at the cell surface (arrows in A and B). Arrowheads point to the nuclear membrane in panel A. C–D. Immunogold particles label Klotho in the peripheral portion of the nucleus and in the nucleolus of Purkinje cells. E. Immunogold particles label Klotho in the cell membrane of a Purkinje cell. The cell membrane is identified with arrowheads, and the Klotho-labeled particles are shown at the arrows. Also notice the immunogold particles associated with the endoplasmic reticulum (ER). N = nucleus, Nu = nucleolus.
4. Discussion
Klotho is most highly localized within the brain to two markedly different cell types: choroid plexus and Purkinje cells. Klotho's localization to the choroid plexus has been described previously (Li et al., 2004); however, this is the first report of detailed localization for the Purkinje cells. It is unclear whether Li et al. (2004) examined the cerebellum in their Klotho brain localization paper. In addition to the choroid plexus and cerebellar Purkinje cells, Klotho mRNA has been found in many brain regions, and it is moderately abundant in the cortex, hippocampus, olfactory bulb and medulla (Allen Brain Atlas). Why these latter brain regions do not exhibit immunostaining for Klotho suggests that the protein levels in these regions are too low for detection, as compared with the choroid plexus and Purkinje cells, or that the protein is not synthesized in these other neurons. The choroid plexus is present in all components of the ventricular system, except for the cerebral aqueduct, and it is composed of numerous capillaries. Fluid filters through the cells from the blood to become cerebrospinal fluid. There is also active transport of substances into, and out of the cerebrospinal fluid during its production. Thus, the choroid plexus acts as a filtration system for removing metabolic waste, foreign substances, and excess neurotransmitters from the cerebrospinal fluid. As expected, klotho is secreted into the cerebrospinal fluid (Imura et al., 2004). The Purkinje cells are among the largest cells in the brain and they are the output cells of the cerebellum. They contain a very high content of the calcium-binding protein calbindin-D28k, a vitamin D-dependent protein. It is interesting that calbindin, like Klotho, is found throughout the Purkinje cell, even in the nucleus where only the nucleolus is unstained (German et al., 1997), but a link between these two proteins is currently unknown.
Klotho protein forms constitutive binary complexes with FGF receptors on the plasma membrane to increase their affinity selectively to FGF23, a member of the endocrine FGFs that was originally isolated from a brain cDNA library (Yamashita et al., 2000; Kurosu et al., 2006; Goetz et al., 2007). FGF23 has an activity that suppresses expression of 1α-hydroxylase that converts an inactive form of vitamin D (25-hydroxyvitamin D3) to the active form (1, 25-dihydroxyvitamin D3) (Shimada et al., 2004).
Klotho may function in ribosomal RNA synthesis and ribosome assembly in the nuclei of both choroid plexus and Purkinje cells. Rough ER localization of Klotho in Purkinje cells may also imply its linkage to ribosome and protein synthesis. Finding Klotho in the nucleus of both cell types, and also finding FGF receptor-1 in the nucleus of cells (Stachowiak et al., 1996), suggests that Klotho and FGF receptors play an important role in mediating nuclear responses to cell stimulation. Also, Klotho is an amyloid precursor protein (APP) and APP-like protein 2 (APLP2) dependent gene that is regulated by the large secreted ectodomain fragment soluble APPsβ (Li et al., 2010). Perhaps Klotho plays a role in the development of the age-related disease, Alzheimer's disease.
In conclusion, the putative anti-ageing protein Klotho is localized in large amounts within two cell populations in the brain: the choroid plexus and cerebellar Purkinje cells. The klotho mRNA is present within neurons in other brain regions, based upon in-situ hybridization studies, suggesting that the protein is present in these cells but in lower amounts than in the Purkinje cells or choroid plexus cells. At the ultrastructural level, besides its cell membrane localization in the choroid plexus cells, which may represent the precursor to the secreted form of the protein, Klotho is found within the nucleus of these brain cells. This nuclear localization has been suggested previously using light microscopic methods in cells outside the brain (Wolf et al., 2008; Hu et al., 2010), and using different klotho antibodies than used in the present study. The function of Klotho within the nucleus is presently unknown, but it is known to interact with FGF receptor 1, which has a nuclear localization, and with APP-related proteins, further pointing to an age-related function for this protein. Future studies are needed to understand the function of Klotho within the nucleus of brain cells.
Acknowledgments
Disclosure statement
This work was supported in part by grants from NIA AG019712, AG025326 and The Ellison Medical Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest All authors state they have no potential or actual conflict of interest.
References
- Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, Kaether C. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett. 2009;583:3221–3224. doi: 10.1016/j.febslet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha SK, Ortega D, Kurosu H, Rosenblatt KP, Kuro-o M, Huang CL. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc. Natal. Acad. Sci. U S A. 2008;105:9805–9810. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha SK, Hu MC, Kurosu H, Kuro-o M, Moe O, Huang CL. Regulation of ROMK1 channel and renal K+ excretion by Klotho. Mol. Pharmacol. 2009;76:38–46. doi: 10.1124/mol.109.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The β- glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc. Natal. Acad. Sci. U S A. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DC, Ng MC, Liang C-L, McMahon A, Iacopino AM. Calbindin-D28k in nerve cell nuclei. Neurosci. 1997;81:735–743. doi: 10.1016/s0306-4522(97)00206-6. [DOI] [PubMed] [Google Scholar]
- Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, Eliseenkova AV, Xu C, Neubert TA, Zhang R, Linhardt RJ, Yu X, White KE, Inagaki T, Kliewer SA, Yamamoto M, Kurosu H, Ogawa Y, Kuro-o M, Lanske B, Razzaque MS, Mohammadi M. Molecular insights into the klotho-dependent, endocrine mode of action of FGF19 subfamily members. Mol. Cell. Biol. 2007;27:3417–3428. doi: 10.1128/MCB.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Shawkat R, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24:3438–3450. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikushima M, Rakugi H, Ishikawa K, Maekawa Y, Yamamoto K, Ohta J, Chihara Y, Kida I, Ogihara T. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem. Biophys. Res. Commun. 2006;339:827–832. doi: 10.1016/j.bbrc.2005.11.094. [DOI] [PubMed] [Google Scholar]
- Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- Kamemori M, Ohyama Y, Kurabayashi M, Takahashi K, Nagai R, Furuya N. Expression of Klotho protein in the inner ear. Hear. Res. 2002;171(1–2):103–110. doi: 10.1016/s0378-5955(02)00483-5. [DOI] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor- 23 signaling by klotho. J. Biol. Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wang B, Wang Z, Guo Q, Tabuchi K, Hammer RE, Südhof TC, Zheng H. Soluble amyloid precursor protein (APP) regulates transthyretin and Klotho gene expression without rescuing the essential function of APP. Proc. Natal. Acad. Sci. U S A. 2010;107(40):17362–17367. doi: 10.1073/pnas.1012568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S-A, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct. Funct. 2004;29:91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- Liu F, Wu S, Ren H, Gu J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nature Cell. Biol. 2011;13:254–262. doi: 10.1038/ncb2167. [DOI] [PubMed] [Google Scholar]
- Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- Masuda H, Chikuda H, Suga R, Kawaguchi H, Kuro-o M. Regulation of multiple ageing-like phenotypes by inducible klotho gene expression in klotho mutant mice. Mech. Ageing Dev. 2005;126:1274–1283. doi: 10.1016/j.mad.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Mitobe M, Yoshida T, Sugiura H, Shirota S, Tsuchiya K, Nihei H. Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron Exp. Nephrol. 2005;101:e67–74. doi: 10.1159/000086500. [DOI] [PubMed] [Google Scholar]
- Nagai R, Saito Y, Ohyama Y, Aizawa H, Suga T, Nakamura T, Kurabayashi M, Kuroo M. Endothelial dysfunction in the klotho mouse and downregulation of klotho gene expression in various animal models of vascular and metabolic diseases. Cell. Mol. Life Sci. 2000;57:738–746. doi: 10.1007/s000180050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Yamada K, Kim HC, Kim YS, Noda Y, Imura A, Nabeshima Y, Nabeshima T. Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J. 2003;17:50–52. doi: 10.1096/fj.02-0448fje. [DOI] [PubMed] [Google Scholar]
- Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, Matsumura Y, Masuda H, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R. Klotho protein protects against endothelial dysfunction. Biochem. Biophys. Res. Commun. 1998;248:324–329. doi: 10.1006/bbrc.1998.8943. [DOI] [PubMed] [Google Scholar]
- Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- Stachowiak MK, Maher PA, Joy A, Mordechai E, Stachowiak EK. Nuclear accumulation of fibroblast growth factor receptors is regulated by multiple signals in adrenal medullary cells. Mol. Biol. Cell. 1996;7:1299–1317. doi: 10.1091/mbc.7.8.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- Wolf I, Levanon-Cohen S, Bose S, Ligumsky H, Sredni B, Kanety H, Kuro-o M, Karlan B, Kaufman B, Koeffler HP, Rubinek T. Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene. 2008;27:7094–7105. doi: 10.1038/onc.2008.292. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-o M. Regulation of oxidative stress by the anti-aging hormone klotho. J. Biol. Chem. 2005;280:38029–38034. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem. Biophys. Res. Commun. 2000;277:494–498. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]